Introduction
Platelets are key in the normal hemostatic process
due to injuries to blood vessels, and are important contributors to
the pathogenesis of thrombotic disorders resulting from improperly
regulated formation of a platelet, or hemostatic, plug (1). Disruption of the endothelium by
trauma or by disease, including atherosclerosis, leads to platelets
adhering to exposed subendothelial structures and platelet
activation. Previous studies have determined that those irregularly
activated platelets provide a surface on which coagulation factors
assemble and initiate the clotting cascade resulting in thrombin
production (2,3). Thus, anti-platelet and anti-coagulant
compounds are useful agents for various thrombotic circulatory
diseases. However, current anti-platelet and anti-coagulant
therapeutic agents have considerable limitations with weak efficacy
and associated side effects and more effective therapeutic agents
with fewer side effects are required (4–7).
Mautan cortex of Paeonia suffruticosa Andrews
is an anti-pyretic, anti-inflammatory agent in traditional Chinese
medicine, which has been used for centuries to treat liver disease
in China, Japan, and Korea (8–10).
The major active compound of Mautan cortex, paeonol
(2′-hydroxy-4′-methoxyacetophenone), has been demonstrated to
inhibit platelet aggregation in animal models (8,11).
Furthermore, previous studies have also identified the inhibitory
effect of paeonol on the expression of intercellular adhesion
molecule-1 and the activation of the Akt, which contribute to
recovery of angiogenesis-associated disease (12,13).
Although paeonol has potential as an anti-platelet agent, its
application remains limited as the mechanism by which the thrombus
is recanalized requires elucidation.
Vascular endothelial growth factor (VEGF) is a
specific endothelial cell mitogen and chemotaxin that stimulates
in vivo angiogenesis in peripheral and myocardial ischemia
(14). Previous studies have
demonstrated that injection of a single bolus of VEGF165
protein or gene delivery in an expression plasmid directly into the
thrombus shortly following its formation results in increased
recanalization and enhanced organization (14,15).
Notably, Lee et al (16)
have reported the interaction between a paeonol derivative, paeonol
oxime (PO), and VEGF. A negative effect was reported to be exerted
on VEGF165 by PO, thus, the present study aimed to
investigate the potential of paeonol, which is similarly structured
to PO, in regulating the expression of VEGF165.
In the current study, the effect of paeonol on
thrombus recanalization using animal and cell models was
investigated. The quantity of fibronectin (FN), fibrinogen (FIB),
D-dimer (D-D), 6-keto-prostaglandinF1α
(6-keto-PGF1α), thromboxane B2
(TXB2), and VEGF165 in the serum of Sprague
Dawley (SD) rats was analyzed by a sandwich enzyme-linked
immunosorbent assay (ELISA). The cytotoxicity of paeonol on HUVEC
cultures was estimated by 3-(4,5
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
and the possible signaling pathway involved in the interaction
between paeonol and VEGF165 was evaluated using western
blotting. The present study aimed to elucidate the underlying
molecular mechanism of paeonol inhibiting platelet aggregation and
facilitate the clinical application of this traditional Chinese
medicine in improving thrombus recanalization.
Materials and methods
Materials
Paeonol (2-hydroxy 4-methoxy acetophenone) (purity,
>99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The
following antibodies were used: Anti-ERK1/2 (rabbit polyclonal;
V1141; 1:1,000; Promega Corporation, Madison, WI, USA),
phosphorylated (p)-ERK1/2 (rabbit polyclonal; ab50011; 1:800;
Abcam, Cambridge, MA, USA), glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; rabbit polyclonal; sc-25778; 1:1,200; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), VEGF165
(rabbit polyclonal; sc-13083; 1:1,000). The ERK1/2 inhibitor,
PD98059 was obtained from EMD Millipore (Billerica, MA, USA). The
human umbilical vein endothelial cell (HUVEC) line was purchased
from the American Type Culture Collection (Manassas, VA, USA) and
grown in Medium 199 supplemented with 20% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 2 mM
L-glutamine (Sigma-Aldrich), 5 U/ml heparin (Sigma-Aldrich), 100
IU/ml penicillin (Sigma-Aldrich), 10 μg/ml streptomycin
(Sigma-Aldrich) and 50 μg/ml endothelial cell growth
supplement (American Type Culture Collection). Cells were cultured
in a humidified 5% CO2 incubator at 37°C and used for
further experiments between passage 3 and 6.
Grouping of model animals and gavage
experiment
The current study was approved by the Ethics
Committee of Nanyang Institute of Technology (Nanyang, China). Male
SD rats (weight, 350–400 g; age, 18 weeks) were provided by the
Laboratory Animal Center of the First Affiliated Hospital of Sun
Yat-Sen University (Guangzhou, China) as rat models of thrombosis
and housed in cages (10 rats/cage) at room temperature with food
and water available ad libitum, and were maintained under at
12 h light/dark cycle. The rats (n=30) were randomly grouped into
three equal groups (10 in each group), as follows: i) Control
group, ii) paeonol group, and iii) aspirin group. In the control
group, SD rats received 2 ml/kg normal saline (Sigma-Aldrich) by
gavage every two days for two weeks; in the paeonol group, rats
received 1.25 mg/kg paeonol by gavage every two days for two weeks;
and in the aspirin group, rats received 50 mg/kg aspirin by gavage
every two days for two weeks. All the animal experiments were
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of Health
(17).
Serum sample collection and investigation
of prothrombotic state (PTS)
The rats were anesthetized at the end of the two
weeks with 10% chloral hydrate (0.3 ml/100 g; Sigma-Aldrich) via
intraperitoneal injection. Blood samples were collected from the
abdominal aorta by opening the abdominal cavity, and immediately
centrifuged at 2,200 × g at 4°C for 20 min to separate the serum,
which was stored at −80°C for further use. The levels of FN, FIB,
D-D, 6-keto-PGF1α, and TXB2, which are
associated with PTS, were measured by ELISA. The level of
VEGF165 was also detected. All ELISAs were conducted
using the FN (H140), FIB (F010), D-D (E029),
6-Keto-PGF1α (H214), TXB2 (R001) and
VEGF165 (H044) kits according to the manufacturer's
protocols (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China): Briefly, plates were coated and incubated overnight at 4°C
with 3.4 mg/ml nonbiotinylated 3D5 primary antibody (100
μl/well) in 200 mM NaHCO3 (Sigma-Aldrich) at pH
9.6, and then washed 4 times with phosphate-buffered saline with
0.05% Tween 20 (PBST; Sigma-Aldrich). Following incubation with 150
μl/well blocking buffer (PBST containing 2.5% gelatin) for 2
h at 37°C, the plates were washed 4 times with PBST, and 100
μl serum samples (diluted 1:1 with PBS) were added to each
well. The plates were incubated at 37°C for 2 h. Following washing
4 times with PBST, 100 μl 1 μg/ml biotinylated 3D5
secondary antibody in blocking buffer was added to each well, and
incubated at 37°C for a further 2 h. The wells were washed 4 times
with PBST and incubated with 100 μl/well ExtrAvidin-Alkaline
phosphatase (Sigma-Aldrich) in blocking buffer (dilution, 1:5,000)
and incubated for 1 h at 37°C. Following another 4 washes with
PBST, the enzyme substrate alkaline phosphatase yellow
(Sigma-Aldrich) was added to each well (100 μl/well) and
incubated for 30 min at 37°C for color development. Optical density
(OD) values were recorded using a microplate reader at 450 nm
(Multiskan MK3; Thermo Fisher Scientific, Inc.). The content was
estimated using a standard curve from serial dilutions.
Cytotoxicity and cell proliferation
assay
The MTT assay was performed to determine the
cytotoxicity of paeonol on HUVECs. Exponentially growing HUVECs
from three to six passages (50 μl; 2×105
cells/ml) were seeded into a 96-well plate in triplicate. The cells
were treated with increasing concentrations of paeonol [0
(control), 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, and 50
μmol/l] for 24 h and each concentration was repeated in
triplicate. Following paeonol treatment, 5 mg/ml MTT was added to
each well and incubated for 4 h at 37°C. MTT is converted into
purple-colored formazan in living cells, which was then solubilized
with dimethylsulfoxide (Invitrogen; Thermo Fisher Scientific,
Inc.), and the OD values in the wells were recorded using the
microplate reader at 450 nm. The survival rates (%) of different
treatments were calculated as: (OD value in treatment group - OD
value in blank control group) / (OD value in negative control group
- OD value in blank control group) ×100%.
The highest concentration with the greatest number
of cells surviving was 0.5 μmol/l, thus, the cell
proliferation assay was conducted at this concentration. HUVECs
treated with 5 μmol/l paeonol were incubated for different
times (0, 24, 48, 72 and 96 h). Normal HUVECs served as a control
to determine the effect of paeonol on the viability of HUVECs over
time. The detection of cell proliferation was conducted as
described above and the OD values in different wells were recorded
using the microplate reader at 450 nm.
Effect of paeonol on expression levels of
VEGF165 and associated signaling pathways in HUVECs
HUVECs were separated into three groups, as follows:
Normal HUVECs; HUVECs incubated with 0.5 μmol/l paeonol; and
HUVECs incubated with 0.5 μmol/l paeonol + 50 μmol/l
PD98059. For each treatment group, the protein expression levels of
VEGF165, ERK1/2, and pERK1/2 were detected in cell
samples after 24 h incubation. GAPDH served as a loading control
for western blot analysis. Total proteins were extracted using
sodium dodecyl sulfate (SDS) lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) on ice for 30 min and the protein
concentration was determined using the Bicinchoninic Protein Assay
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). All the
extracts were boiled with loading buffer for 5 min prior to
separation with SDS-polyacrylamide gel electrophoresis on 10% gels
at 160 V for 60 min. Proteins were transferred onto polyvinylidene
difluoride membranes. The membranes were washed with Tris-buffered
saline with Tween 20 (TBST; Sigma-Aldrich) for 20 min, and the
procedure was repeated three times. The membranes were incubated
with primary antibodies overnight at room temperature. Following
three additional washes with TBST, the horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibodies
(Beyotime Institute of Biotechnology) were added and the membrane
was incubated for 4 h at 37°C. Following three final washes with
TBST, the blots were developed using BeyoECL Plus reagent (Beyotime
Institute of Biotechnology) and the results were detected in the
Gel Imaging system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
All the data were expressed as the mean ± standard
deviation. Multiple comparisons were conducted using Fisher's Least
Significant Difference method. All the statistical analyses were
conducted using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
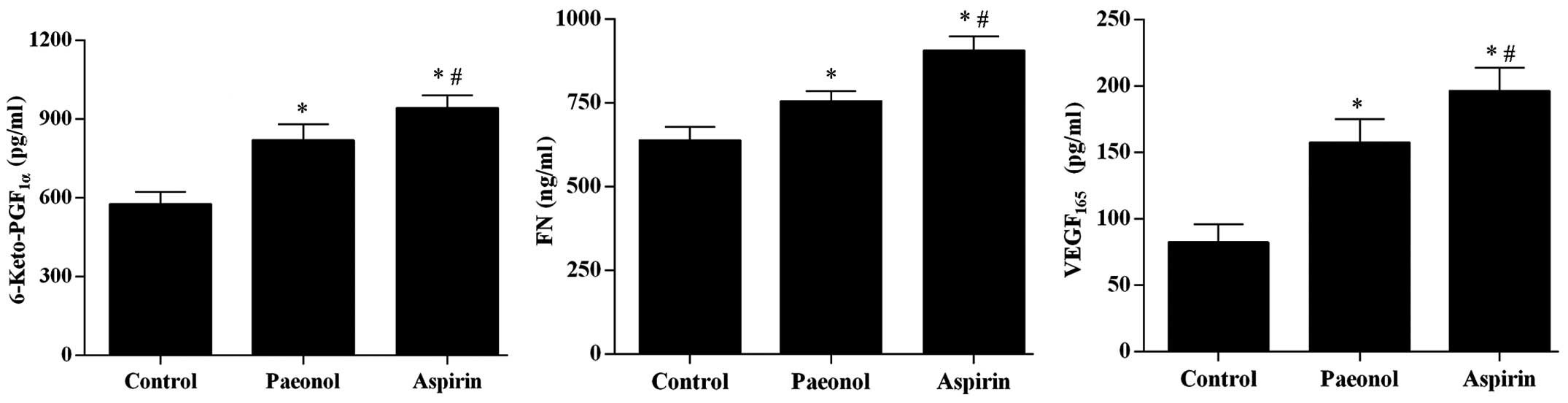
Paeonol improves the PTS and increases
VEGF expression in rat models of thrombosis
Using an ELISA, the levels of
6-keto-PGF1α, FN, and VEGF165 in serum were
determined to be significantly upregulated by treatment with
paeonol (P<0.05), however, the effect was smaller than that of
aspirin treatment (Fig. 1). The
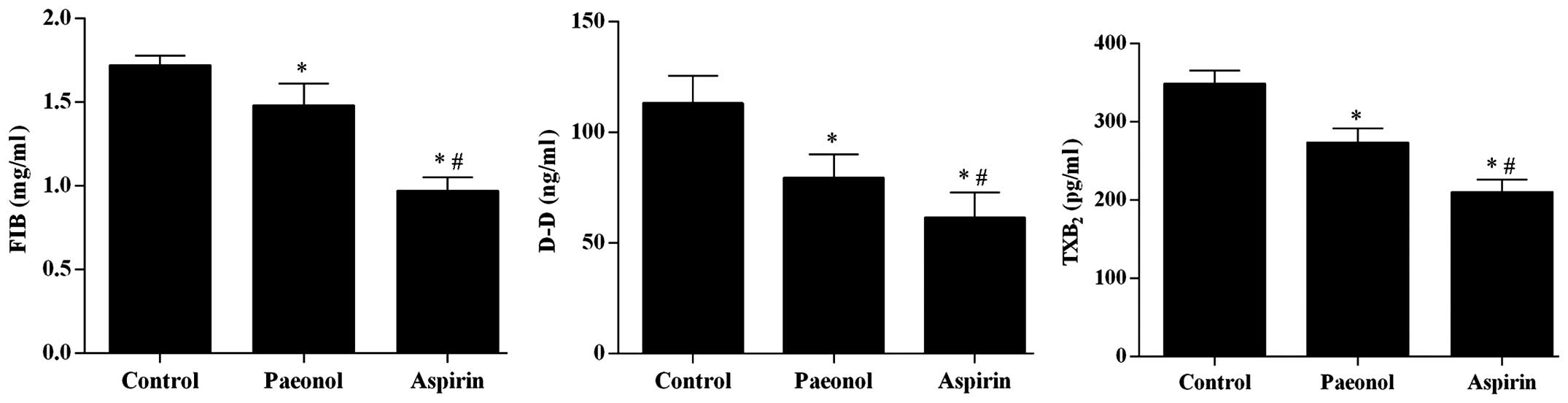
levels of FIB, D-D, and TXB2 were significantly
downregulated by paeonol (P<0.05), however, the effect was also
smaller than that of aspirin treatment (Fig. 2).
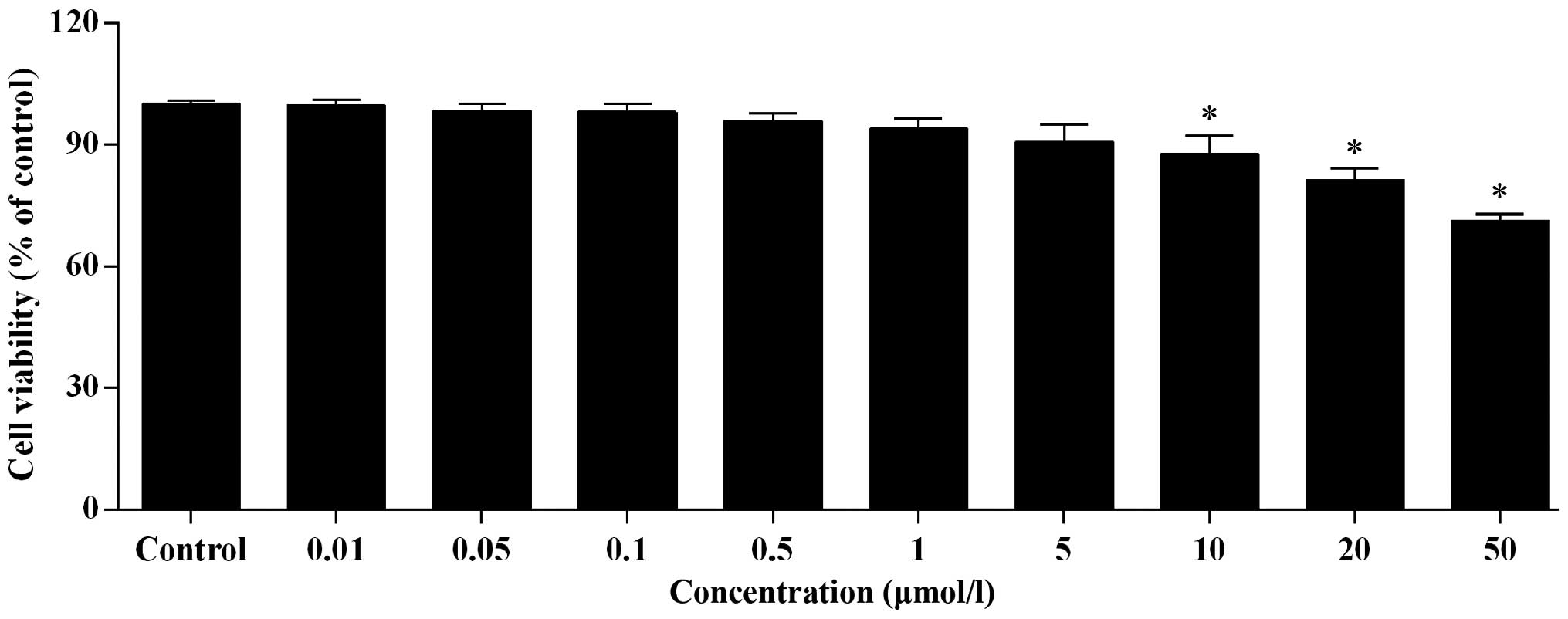
Paeonol exerts a weak cytotoxic effect on
HUVECs and improves cell proliferation
With increased concentration of paeonol, the cell
viability of HUVECs decreased gradually (Fig. 3). No significant difference was
detected for paeonol concentration <0.5 μmol/l. However,
at concentrations >0.5 μmol/l, a significant difference
in cell viability was observed (P<0.05; Fig. 3). At a concentration of 50
μmol/l paeonol, the cell viability was >70%, which
indicates a safe result.
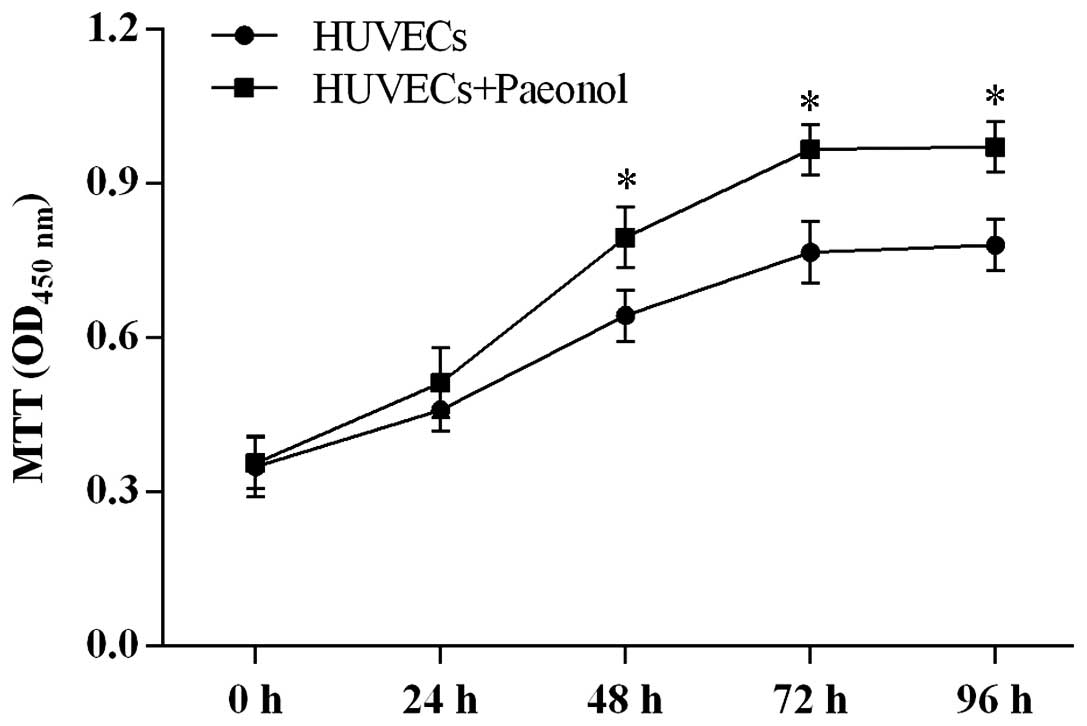
Based on the results of the MTT assay, 0.5
μmol/l was determined as the suitable concentration for
further experiments. Paeonol at this concentration significantly
improved the cell proliferation ability in a time-dependent manner
compared with normal HUVECs (P<0.05; Fig. 4).
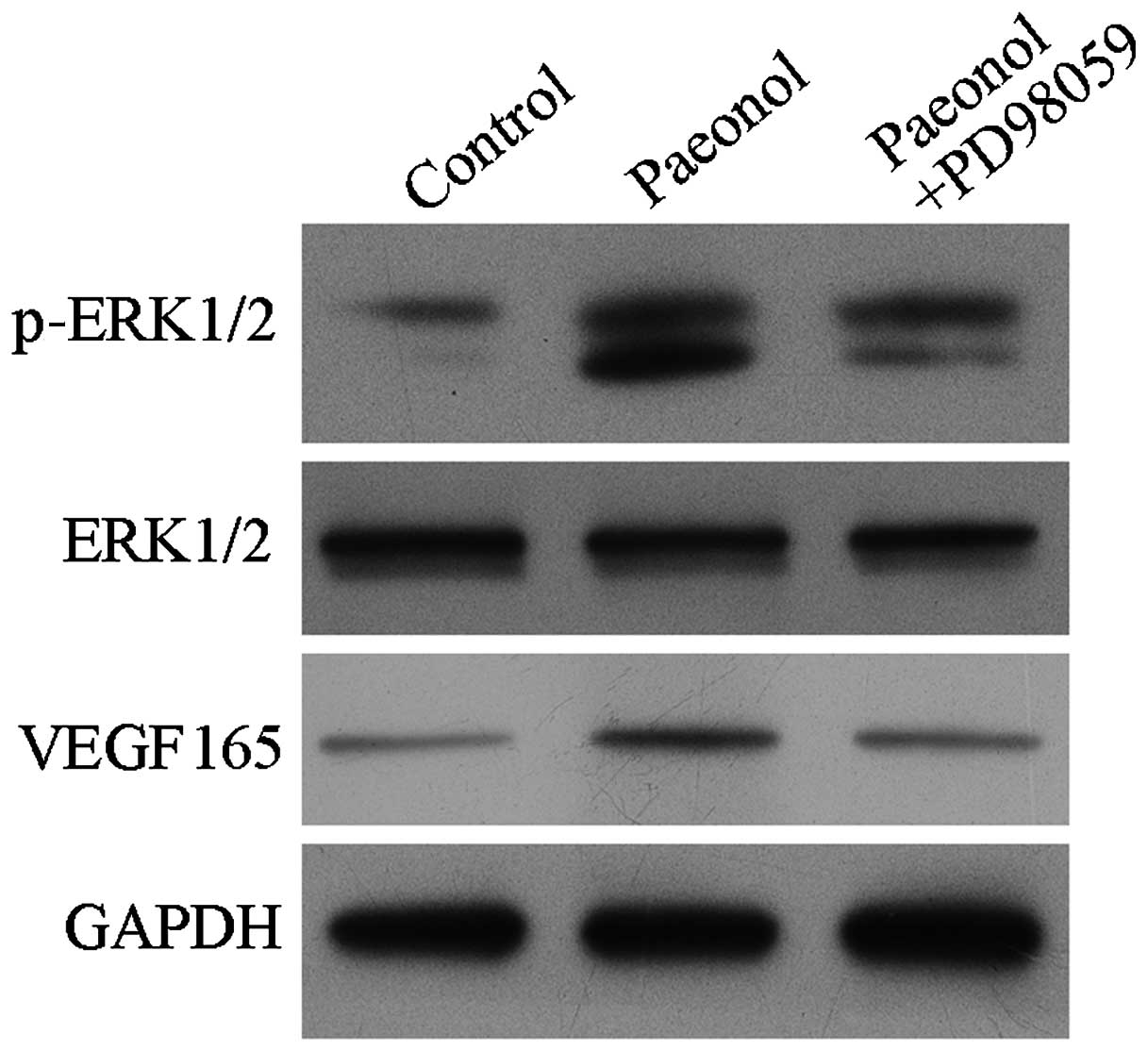
Treatment with paeonol activated the
phosphorylation of ERK1/2 and upregulated the expression of
VEGF165
To elucidate the signaling pathway involved in the
anti-thrombotic effect exerted by paeonol, the effect of paeonol on
the expression levels of ERK1/2, p-ERK1/2, and VEGF165
was investigated. As presented in Fig.
5, the expression levels of p-ERK1/2 and VEGF165
were upregulated in HUVECs. Treatment with the ERK1/2 signaling
pathway inhibitor, PD98059 markedly blocked the effect of paeonol
on p-ERK1/2 and VEGF165.
Discussion
Platelet activation and thrombus formation are
important in pathophysiology of ischemic events, thus,
anti-platelet therapeutic strategies are useful in preventing acute
thromboembolic artery occlusion. Anti-platelet therapeutic agents,
including aspirin, ticlopidine, and dipyridamole are clinically
used, however, these therapeutic agents result in a number of side
effects (18) including stent
thrombosis and acute myocardial infarction (19). Paeonol is a nonsteroidal
anti-inflammatory, with a structure similar to aspirin. Previous
studies have reported beneficial effects of paeonol as an
anti-angiogenic, anti-metastatic and anti-platelet agent (8,11).
However, more investigation into the underlying mechanism of
paeonol in recanalizing thrombi is required.
In the present study, the anti-platelet effect of
paeonol was investigated in SD rats. The ELISA assays demonstrate
improved PTS by changes in expression of FN, FIB, D-D,
6-keto-PGF1α, and TXB2 in the paeonol-treated
group. Although treatment with aspirin was observed to be more
effective, the concentration of aspirin was higher than that of
paeonol. As paeonol has demonstrated fewer side effects, the
concentration of paeonol used clinically may be adjusted to a level
with comparable or stronger effects than aspirin. However, more
comprehensive animal and clinical studies are required to be
conducted in the future. The cytotoxicity of paeonol on HUVECs was
assessed by MTT assay. It was demonstrated that the proliferative
ability of the cells was improved by paeonol with little
cytotoxicity. Although paeonol suppressed the cell proliferation in
a concentration-dependent manner when the concentration was >0.5
μmol/l, at a paeonol concentration of 50 μmol/l, cell
viability of HUVECs remained at >70%.
Gene therapy with VEGF has been used to promote
revascularization in the ischemic heart and in peripheral vascular
disease with some success (20–22).
VEGF165 release by transfection of a plasmid containing
the VEGF165 gene to improve thrombus recanalization has
also been reported (23). Lee
et al (16) focused on the
interaction between a derivative of paeonol and VEGF, PO was
demonstrated to suppress the expression of VEGF at a concentration
of 70 μmol/l. Negative effects of this derivative are
hypothesized to be due to the high concentration used in the
previous study. Thus, in the current study, based on the results of
the MTT assay, a more suitable concentration was selected for the
assessment of the effects of paeonol on anti-thrombosis associated
signaling pathways. At a concentration of 0.5 μmol/l,
paeonol activated the phosphorylation of ERK1/2 and upregulated the
expression levels of VEGF. However, exposure to paeonol had no
observable effect on the expression levels of ERK1/2 in HUVECs.
The role of ERK in transcriptional and
post-transcriptional regulation of VEGF is well-defined (24). A previous study demonstrated that
the ERK signaling pathway is also activated during hypoxia in human
endothelial cells (25). Notably,
the results from the present study were contrary to the previous
study of Nizamutdinova et al (26), which demonstrated an inhibitory
effect of paeonol on the ERK1/2 signaling pathway. The
concentration of paeonol used in the present and previous studies
was compared, and it was identified that in the MTT assay conducted
in the current study, the cell viability was significantly
decreased by paeonol at concentrations >1 μmol/l, and in
Nizamutdinova et al (26),
the expression levels of p-ERK1/2 was inhibited by paeonol >1
μmol/l. In the two studies, the expression levels of ERK1/2
were not influenced by paeonol. In addition, Lee et al
(16) showed that the expression
levels of VEGF were not down-regulated by PO at concentration up to
34.5 μmol/l. Thus, the ERK signaling pathway may also
activate other transcription factors that may mediate VEGF
induction by paeonol. Furthermore, the concentration of paeonol in
clinical application should be carefully determined, accounting for
the expression levels of anti-thrombosis associated factors and
cell viability.
In conclusion, paeonol may improve the PTS and
recanalize thrombi in animal models, which may be by the
upregulation of VEGF165 via the ERK1/2 MAPK signaling
pathway. However, this positive effect depended on the
concentration of paeonol used, a unsuitably high concentration of
the compound resulted in a negative effect on the anti-thrombosis
signaling pathways. The present study aimed to elucidate the
underlying mechanism of paeonol in recanalizing thrombi, and more
comprehensive animal and clinical studies should be conducted in
order to determine a practical concentration of paeonol for
clinical applications to improve the anti-platelet and
anti-coagulant therapeutic strategies for various thrombotic
circulatory diseases.
References
|
1
|
Packham MA: Role of platelets in
thrombosis and hemostasis. Can J Physiol Pharmacol. 72:278–284.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dupont AG, Gabriel DA and Cohen MG:
Antiplatelet therapies and the role of antiplatelet resistance in
acute coronary syndrome. Thromb Res. 124:6–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konkle BA, Simon D and Schafer AI:
Hemostasis, thrombosis, fibrinolysis and cardiovascular disease.
Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.
Libby P, Bonow RO, Mann DL and Zipes DP: 8th edition. Saunders
Elsevier; Philadelphia, PA: pp. 2049–2078. 2008
|
|
4
|
Schrör K: Antiplatelet drugs. A
comparative review. Drugs. 50:7–28. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni H and Freedman J: Platelets in
hemostasis and thrombosis: Role of integrins and their ligands.
Transfus Aphe Sci. 28:257–264. 2003. View Article : Google Scholar
|
|
6
|
Barrett NE, Holbrook L, Jones S, Kaiser
WJ, Moraes LA, Rana R, Sage T, Stanley RG, Tucker KL, Wright B and
Gibbins JM: Future innovations in anti-platelet therapies. Br J
Pharmacol. 154:918–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bird JE, Giancarli MR, Allegretto N,
Barbera F, Wong P, Schumacher WA, Ogletree ML and Seiffert D:
Prediction of the therapeutic index of marketed anti-coagulants and
anti-platelet agents by guinea pig models of thrombosis and
hemostasis. Thromb Res. 123:146–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin HC, Ding HY, Ko FN, Teng CM and Wu YC:
Aggregation inhibitory activity of minor acetophenones from Paeonia
species. Planta Med. 65:595–599. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda T, Kon R, Nakazawa T and Ohsawa K:
Metabolism of paeonol in rats. J Nat Prod. 62:1142–1144. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou TC: Anti-inflammatory and analgesic
effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J
Pharmacol. 139:1146–1152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koo YK, Kim JM, Koo JY, Kang SS, Bae K,
Kim YS, Chung JH and Yun-Choi HS: Platelet anti-aggregatory and
blood anti-coagulant effects of compounds isolated from Paeonia
lactiflora and Paeonia suffruticosa. Pharmazie. 65:624–628.
2010.PubMed/NCBI
|
|
12
|
Lin C, Lin HY, Chen JH, Tseng WP, Ko PY,
Liu YS, Yeh WL and Lu DY: Effects of paeonol on
anti-neuroinflammatory responses in microglial cells. Int J Mol
Sci. 16:8844–8860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen FS, Zhao HW, Jin XG and Yu HZ: Effect
of paeonol on intercellular adhesion molecule 1 expression after
cerebral ischemia reperfusion in rats. Chin J Clin Rehabil.
8:3792–3793. 2004.
|
|
14
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
15
|
Waltham M, Burnand KG, Collins M,
McGuinness CL, Singh I and Smith A: Vascular endothelial growth
factor enhances venous thrombus recanalisation and organisation.
Thromb Haemost. 89:169–176. 2003.PubMed/NCBI
|
|
16
|
Lee HJ, Kim SA, Lee HJ, Jeong SJ, Han I,
Jung JH, Lee EO, Zhu S, Chen CY and Kim SH: Paeonol oxime inhibits
bFGF-induced angiogenesis and reduces VEGF levels in fibrosarcoma
cells. PLoS One. 5:e123582010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 7th edition. National Academy
Press; Washington, DC: 1996
|
|
18
|
Lee KS, Oh KW, Bae KH, Kim YH, Lee MY, Cho
MR, Jin YR and Yun YP: Inhibitory effects of moutan cortex radicis
extracts and paeonol on rabbit platelet aggregation. Journal of
Food Hygiene and Safety. 19:167–170. 2004.
|
|
19
|
Kovacic JC, Lee P, Karajgikar R, Baber U,
Narechania B, Suleman J, Moreno PR, Sharma SK and Kini AS: Safety
of temporary and permanent suspension of anti-platelet therapy
after drug eluting stent implantation in contemporary “real-world”
practice. J Interv Cardiol. 25:482–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folkman J: Angiogenic therapy of the human
heart. Circulation. 97:628–629. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Folkman J: Therapeutic angiogenesis in
ischemic limbs. Circulation. 97:1108–1110. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baumgartner I, Pieczek A, Manor O, Blair
R, Kearney M, Walsh K and Isner JM: Constitutive expression of
phVEGF165 after intramuscular gene transfer promotes collateral
vessel development in patients with critical limb ischemia.
Circulation. 97:1114–1123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waltham M, Burnand K, Fenske C, Modarai B,
Humphries J and Smith A: Vascular endothelial growth factor naked
DNA gene transfer enhances thrombus recanalization and resolution.
J Vasc Surg. 42:1183–1189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milanini J, Richard DE, Berra E, Gothié E,
Viñals F and Pouysségur J: Signaling angiogenesis via p42/p44 MAP
kinase cascade. Ann NY Acad Sci. 902:187–200. 2000.PubMed/NCBI
|
|
25
|
Minet E, Arnould T, Michel G, Roland I,
Mottet D, Raes M, Remacle J and Michiels C: ERK activation upon
hypoxia: Involvement in HIF-1 activation. FEBS Lett. 468:53–58.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nizamutdinova IT, Oh HM, Min YN, Park SH,
Lee MJ, Kim JS, Yean MH, Kang SS, Kim YS, Chang KC and Kim HJ:
Paeonol suppresses intercellular adhesion molecule-1 expression in
tumor necrosis factor-alpha-stimulated human umbilical vein
endothelial cells by blocking p38, ERK and nuclear factor-kappaB
signaling pathways. Int Immunopharmacol. 7:343–350. 2007.
View Article : Google Scholar : PubMed/NCBI
|