Introduction
The anti-Müllerian hormone (AMH) is produced in the
ovarian granulosa cells (GCs). Through the specific receptor, AMH
receptor 2 (AMHR2), AMH limits the formation of primary follicles
and inhibits excessive follicular recruitment by follicle
stimulating hormone (FSH) (1,2). The
expression levels of AMH have been used as the indexes to predict
ovarian reserves and ovarian response in assisted reproductive
technology (3,4). In human ovaries, early stage antral
follicles contribute markedly to the serum AMH concentration
(5). AMHR2 is a single
transmembrane serine/threonine kinase receptor of the transforming
growth factor β (TGFβ) receptor family, which is expressed on
ovarian GCs (6). The presence of
AMHR2 is also observed in ovarian cancer cell lines that respond
positively to treatment with recombinant AMH (7). Thus, the receptor and ligand may be
important diagnostic factors or therapeutic tools.
The human ovary is a highly vascularized organ. As a
key regulator of vascularization, vascular endothelial growth
factor (VEGF) is crucial in regulating follicular growth, corpus
luteum development and maintaining ovarian functions (8). VEGF is expressed in the ovarian
granulosa, theca and granulosa lutein cells (9) and acts by binding to the tyrosine
kinase receptors, VEGFR1 and 2 in the ovary. Marked production and
secretion of follicular VEGF occur in response to gonadotrophin
stimulation (10). Furthermore,
hypersecretion of VEGF is frequently observed in patients with
polycystic ovarian syndrome (PCOS) (11,12).
It has been proposed that VEGF, by regulating vascular
permeability, induces blood vessel leaking in ovaries exhibiting
ovarian hyperstimulation syndrome (OHSS) (13). Notably, in a transgenic mouse model
with engineered inducible repression of VEGF, mice were observed to
become infertile upon VEGF repression (14). By digital gene expression assays,
831 uterus-specific and 2,398 VEGF-regulated differentially
expressed genes were identified in the mouse uterus, which
indicated that VEGF exerts a regulatory role in gene expression in
the uterus.
The majority of studies regarding AMH focus on the
early stage of follicle development. In a study on marmosets, AMH
levels were decreased in early preantral follicles as a result of
the suppression of gonadotrophin secretion and VEGF inhibition
(15). The regulatory role of VEGF
with regard to AMH and AMHR2 signaling has remained
to be elucidated in mature follicles. In the present study, VEGF,
VEGFR2, FSH receptor (FSHR), AMH, AMHR2 and TGFβ expression changes
were analyzed following FSH and/or VEGF treatment in human primary
GCs, which were isolated from IVF/ICSI patients. Furthermore, the
elevation of AMHR2 expression levels by VEGF in the ovarian
granulosa-like KGN cells was identified.
Materials and methods
The current study was performed on cultured GCs,
which were derived from human ovarian follicular fluid. The female
participants provided written informed consent, and the protocol of
the study was approved by the ethical committee of Jilin Province
People's Hospital (Changchun, China).
Primary ovarian GCs
GCs were isolated at the time of oocyte pick-up from
18 IVF/ICSI patients with male factors only (age, 31.3±4.5 years)
between April 2013 and December 2014. Eighteen follicular fluid
samples were collected and the follicular aspirates obtained from
individual patients were centrifuged at 900 × g at room
temperature. Blood contaminants were removed from GCs by
Histopaque® 1077 (Sigma-Aldrich, St. Louis, MO, USA)
gradient centrifugation at 800 × g for 20 min at room temperature.
The GC pellet was resuspended in a 20-ml volume of medium
[Dulbecco's modified Eagle's medium/Ham's Nutrient Mixture F-12
(Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10%
fetal bovine serum (Beyotime Institute of Biotechnology, Inc.,
Haimen, China)] and washed by a further 10-min centrifugation at
500 × g. The GC pellet was resuspended in 1 ml GC preparation
medium containing 0.02% (w/v) EDTA and gentle repeated pipetting
was performed to break up any cellular clumps. Following further
washing, the cell stock was resuspended in the GC culture medium.
The cells were seeded in six-well cell culture plates at a density
of 100,000 cells/well in medium. Following an overnight incubation
(37°C, 5% CO2), cells were treated with 30 ng/ml human
recombinant FSH (450 IU; GONAL-F; Merck Biopharma China, Beijing,
China) and/or 100 ng/ml VEGF-A (CB055-0231; ExCell Biotech,
Shanghai, China) for 24 or 48 h. Phosphate-buffered saline (PBS)
was added to the control group as a vehicle.
Cell line
The human granulosa tumor-derived cell line, KGN was
obtained from Riken BioResource Center (Riken Cell Bank, Tsukuba,
Japan). The KGN cells used in these experiments were passages 8-12.
The cell line was validated by short tandem repeat polymorphism
analysis, which was performed by Riken Biosource Center.
Gene expression analysis
Total RNA was isolated from primarily cultured cells
according to previous methods (16). First-strand cDNA synthesis was
performed using 1 µg total RNA in a 20-µl volume.
Random primers and 200 units Moloney murine leukemia virus reverse
transcriptase (Takara Biotechnology Co., Ltd., Dalian, China) were
used for reverse transcription (RT). The SYBR Green PCR master mix
(Roche Diagnostics, Shanghai, China) was used as a double-stranded
DNA-specific fluorescent dye. Gene expression was assessed by
RT-quantitative polymerase chain reaction (qPCR) using the Applied
Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific,
Inc.). PCR conditions were as follows: 2 min at 50°C and 10 min at
95°C, followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec,
and 72°C for 30 sec. The specificity of amplification was confirmed
by melting curve analysis and gel electrophoresis. All results were
normalized to the levels of 18S ribosomal (r)RNA, and relative
quantification was calculated using relative quantification (ΔΔCq)
values for each biological replicate (16,17).
Values are expressed as the mean ± standard error of the mean (SEM;
triplicate samples and three repeats). The following primer
sequences (Genewiz, Suzhou, China) were used for RT-PCR: Forward,
CGA AGT GGT GAA GTT CATGG and reverse, GTA CTC GAT CTC ATC AGG GT
for VEGF; forward, CAC TGG CTT CTA CAG CTG CA and reverse, CGA AAG
GTC TAC CTG TAT C for VEGFR1; forward, CGG TCA ACA AAG TCG GGA GA
and reverse, CAG TGC ACC ACA AAG ACA CG for VEGFR2; forward, GGA
ACA TCA TAG TGC TAGTG and reverse, CCA GTC AAT GGC ATA GTTGT for
FSHR; forward, GTC CTA CAC CTG GAG GAA GT and reverse, AGC CCT CGT
CAC AGT GAC CT for AMH; forward, GAT TTG AGG CCT GAC AGC AG and
reverse, GCC AGG TGG ATG GGA TGT AG for AMHR2; forward, ACT ACT ACG
CCA AGG AGGTC and reverse, CGG AGC TCT GAT GTG TTG AA for TGFβ; and
forward, CAT TCG AAC GTC TGC CCT AT and reverse, GAT GTG GTA GCC
GTT TCT CA for 18S rRNA.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde
(Sigma-Aldrich) for 30 min. Cells were blocked with 5% bovine serum
albumin (Sigma-Aldrich) in PBS for 1 h at room temperature and
incubated overnight with rabbit anti-human FSHR antibody (cat. no.
BA2317; Beijing Bioss Biosynthesis Biotechnology Co., Ltd.,
Beijing, China) or rabbit anti-human MIS antibody (cat. no.
sc-6886; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary
antibodies (dilution, 1:500) at 4°C and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary
antibody (cat. no. SA1028; Beijing Bioss Biosynthesis Biotechnology
Co., Ltd.) (dilution, 1:1,000) for 2 h at room temperature,
followed by 3,3′-diaminobenzidine and hematoxylin staining (Beijing
Chemical Co., Beijing, China). Washes with PBS (three times for 3
min each) were performed between incubations. A microscope (CX31;
Olympus Corp., Tokyo, Japan) and Image J software (version 1.46r;
National Institutes of Health, Bethesda, MD, USA) were used to
perform analyses.
Protein analysis
For western blot analysis, cells were lysed in
radioimmunoprecipitation assay buffer [0.5% Nonidet P-40, 0.1%
sodium deoxycholate, 150 mM NaCl, 50 mM Tris-Cl (pH 7.5); Beijing
Chemical Co.]. The lysates were resolved by 10% SDS-PAGE (Pierce
Biotechnology, Inc., Rockford, IL, USA), transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA) at 10 V over 2 h and probed with rabbit anti-human FSHR,
rabbit anti-human MIS and mouse anti-human GAPDH antibody (cat. no.
sc-20357; Santa Cruz Biotechnology, Inc.) at 1:500 dilution at 4°C
overnight. The secondary antibody conjugated to HRP (goat
anti-rabbit IgG-HRP; cat. no. sc-2030 or goat anti-mouse IgG-HRP;
cat. no. sc-2031; Santa Cruz Biotechnology, Inc.) was applied
successively at 1:1,000 dilution followed by incubation at room
temperature for 2 h. Blots were developed using the
Chemiluminescence WB solution ABC kit (DW101-01; Beijing TransGen
Biotech, Beijing, China).
Statistical analysis
Values are expressed as the mean ± SEM. Statistical
analysis was performed using an unpaired t-test with
GraphPad Prism software (version 5.1; GraphPad Inc., La Jolla, CA,
USA). Grouped data were analyzed by one-way analysis of variance
and P<0.05 was considered to indicate a statistically
significant difference.
Results
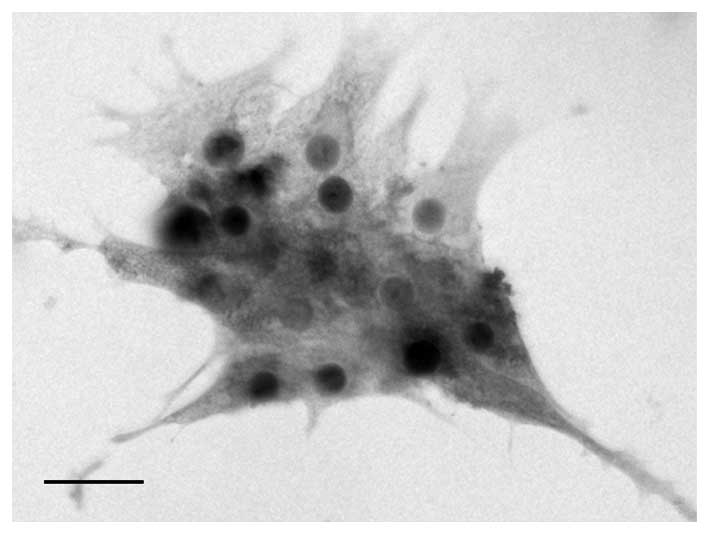
AMHR2 expression in primary GCs
Human luteinized GCs were isolated from the follicle
fluid of IVF/ICSI patients. FSHR expression is considered to be the
biological marker of ovarian GCs, thus, the isolated cells were
identified by immunocytochemistry staining of FSHR (Fig. 1). The negative controls were
established by incubation with an equal quantity of non-immunized
rabbit IgG.
FSH- and VEGF-induced gene expression
varies in primary GCs
Gonadotrophin contributes to GC growth and
proliferation (15). To
investigate the gonadotrophin- and VEGF-induced gene expression
changes, RT-qPCR was performed on the primary GCs, and human
recombinant FSH and VEGF were used to treat the cells. The mRNA
expression of VEGF was significantly elevated as a result of
treatment with FSH, and FSH + VEGF by 2.8-(P<0.05) and 2.3-fold
(P<0.01), respectively; however, no significant difference was
noted in the VEGF only group (Fig.
2A). VEGFR2 mRNA expression levels were significantly
increased in the VEGF and VEGF plus FSH groups by ~3-fold
(P<0.01) and in the FSH group by ~1.5-fold (P<0.05) (Fig. 2B), whereas AMH mRNA
expression levels were significantly decreased in all groups
(P<0.01) (Fig. 2C). Compared
with the control, the AMHR2 mRNA expression levels increased
as a result of VEGF, and FSH + VEGF treatment by 2.4- and 3.2-fold,
respectively (P<0.01); however, the increase observed in the FSH
only treatment group was not significant (Fig. 2D). FSHR mRNA expression
levels increased due to FSH treatment, by 6.2-fold (P<0.001) and
significantly decreased following VEGF treatment, by 0.7-fold
(P<0.05) (Fig. 2E). TGFβ
mRNA expression levels increased significantly as a result of VEGF
treatment (P<0.01; Fig.
2F).
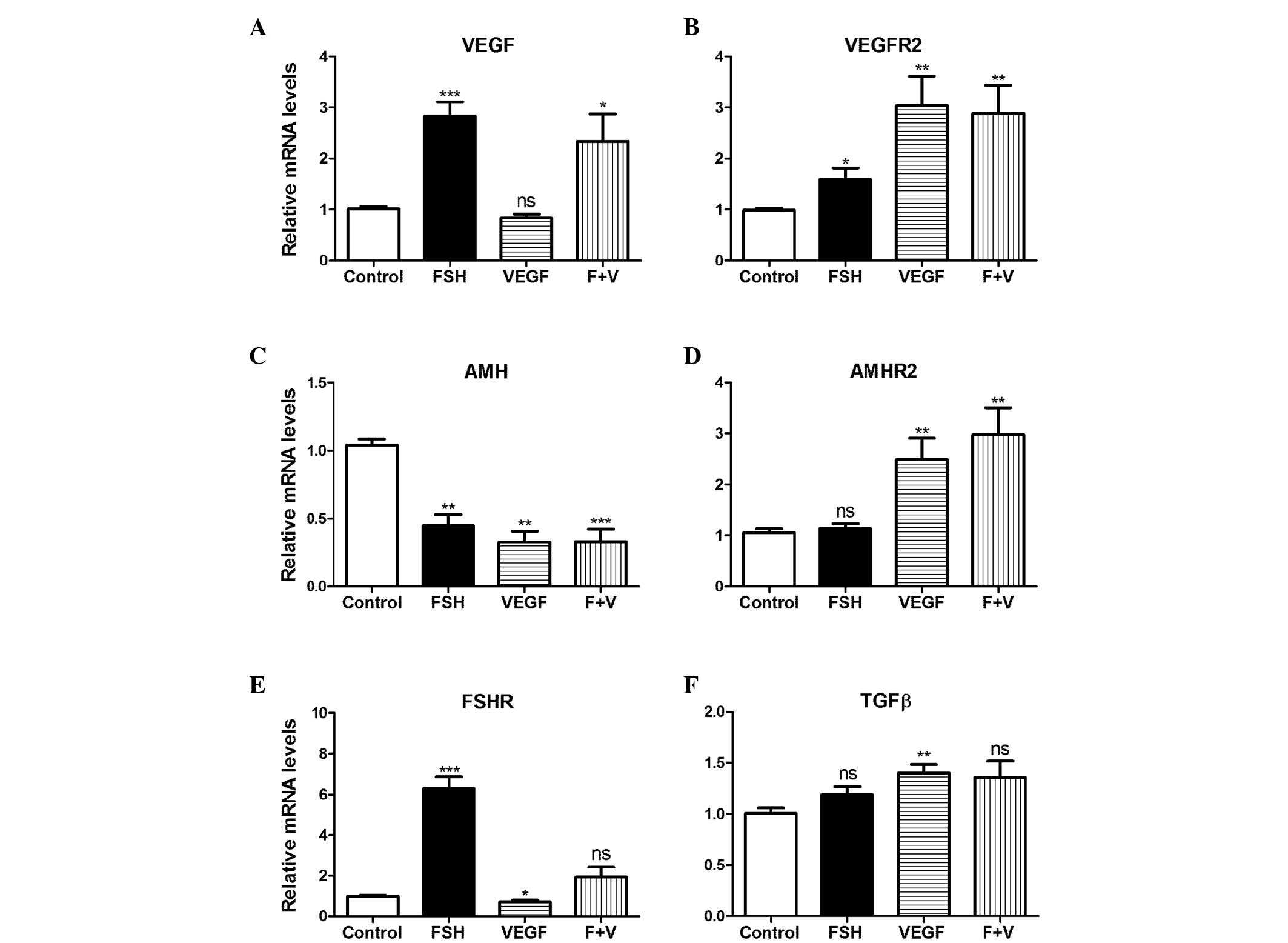 | Figure 2VEGF treatment induced changes at the
mRNA level of primary granulosa cells. rhFSH (30 ng/ml), rhVEGF
(100 ng/ml), and rhFSH (30 ng/ml) and rhVEGF (100 ng/ml; F + V)
were used to treat the primary granulosa cells. Gene expression
levels of (A) VEGF, (B) VEGFR2, (C) AMH, (D)
AMHR2, (E) FSHR and (F) TGFβ were analyzed by
reverse transcription quantitative polymerase chain reaction (n=6).
Data are presented as means ± standard error of the mean.
*P<0.05, **P<0.01,
***P<0.001 compared with the vehicle control. ns, no
significance; VEGF, vascular endothelial growth factor; rh,
recombinant human; AMH, anti-Müllerian hormone; FSHR,
follicle-stimulating hormone receptor; TGFβ, transforming growth
factor β. |
FSH- and VEGF-induced protein expression
varies in primary GCs
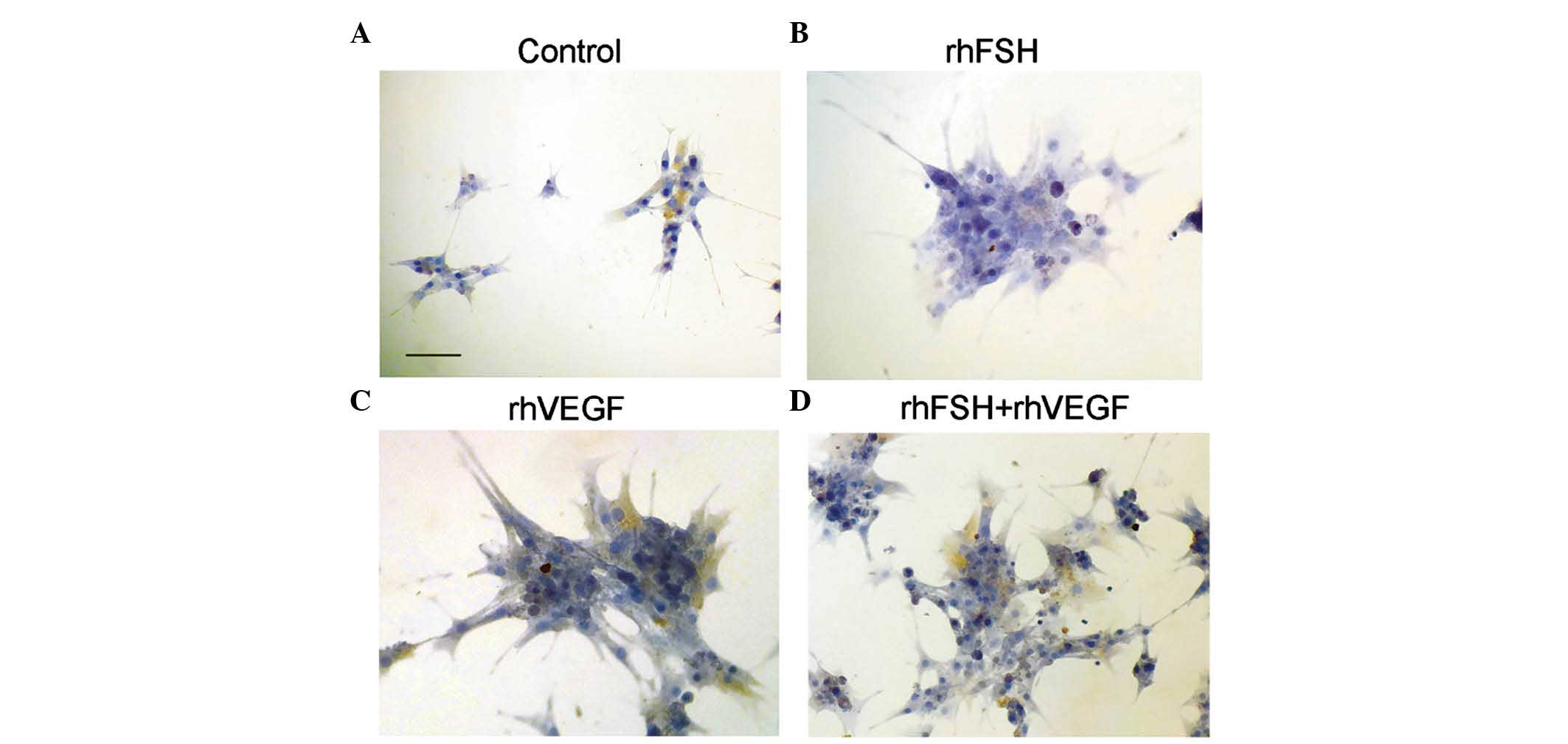
AMHR2 expression was observed in the primary ovarian
GCs by immunocytochemical staining (Fig. 3). Expression of the FSHR and AMHR2
protein was analyzed by western blotting. FSHR protein expression
was significantly increased by FSH stimulation (P<0.001), and
significantly decreased by VEGF and VEGF + FSH treatment (Fig. 4A and B). Conversely, AMHR2 protein
expression was significantly increased as a result of VEGF and VEGF
+ FSH treatment (P<0.01). Although the AMHR2 expression was
increased as a result of FSH treatment, the difference was not
significant (Fig. 4C).
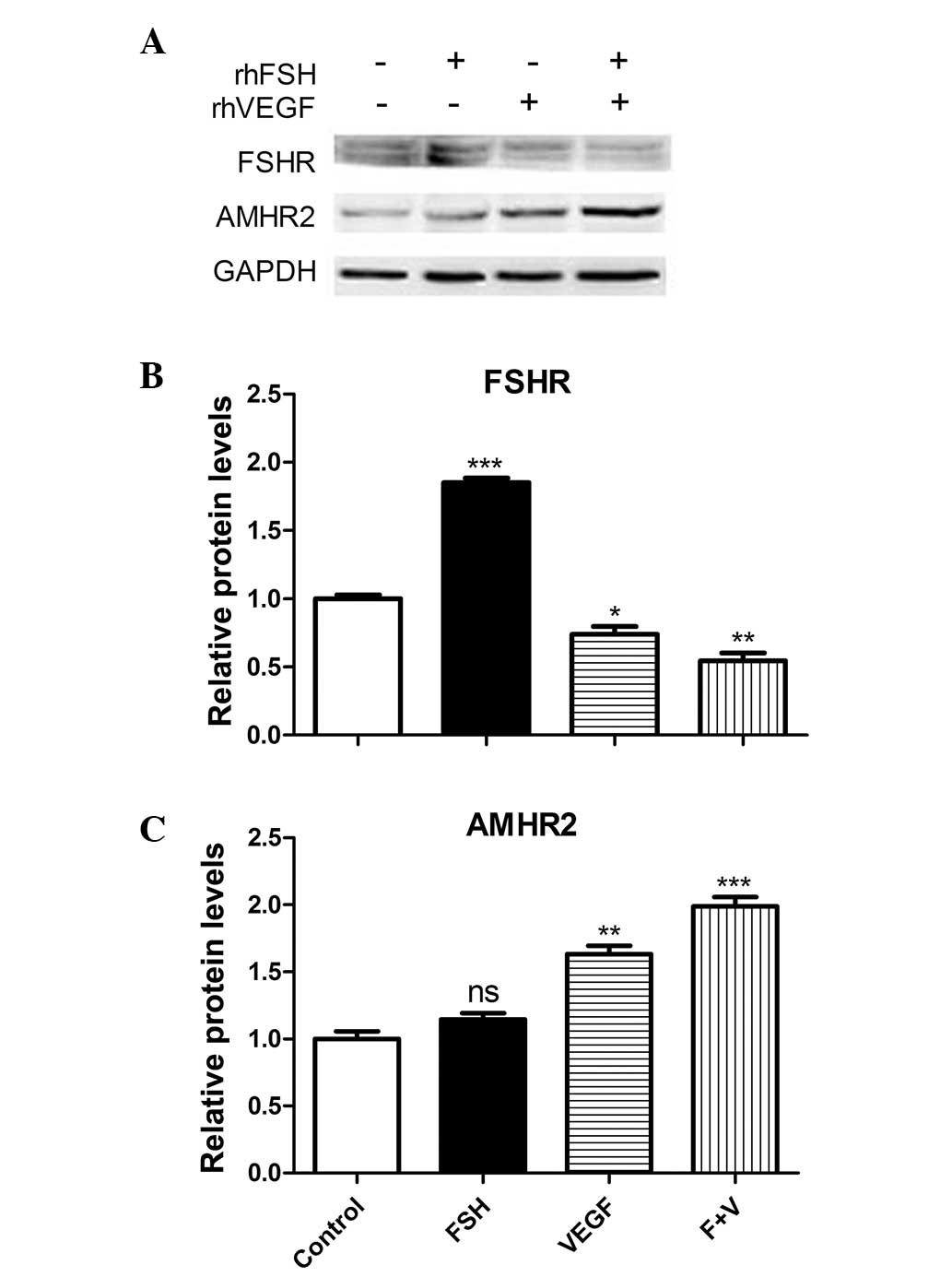 | Figure 4VEGF treatment induces changes in mRNA
expression levels in primary granulosa cells. Primary cells were
treated with rhFSH (30 ng/ml), rhVEGF (100 ng/ml), and rhFSH (30
ng/ml) and VEGF (100 ng/ml; F + V). (A) Representative western blot
analysis and the quantification of (B) FSHR and (C) AMHR2
expression levels (n=3). Values are expressed as the mean ±
standard error of the mean. *P<0.05,
**P<0.01, ***P<0.001 vs. control. rh,
recombinant human; FSH, follicle-stimulating hormone; VEGF,
vascular endothelial growth factor; AMHR2, anti-Müllerian hormone
receptor 2, ns, no significance. |
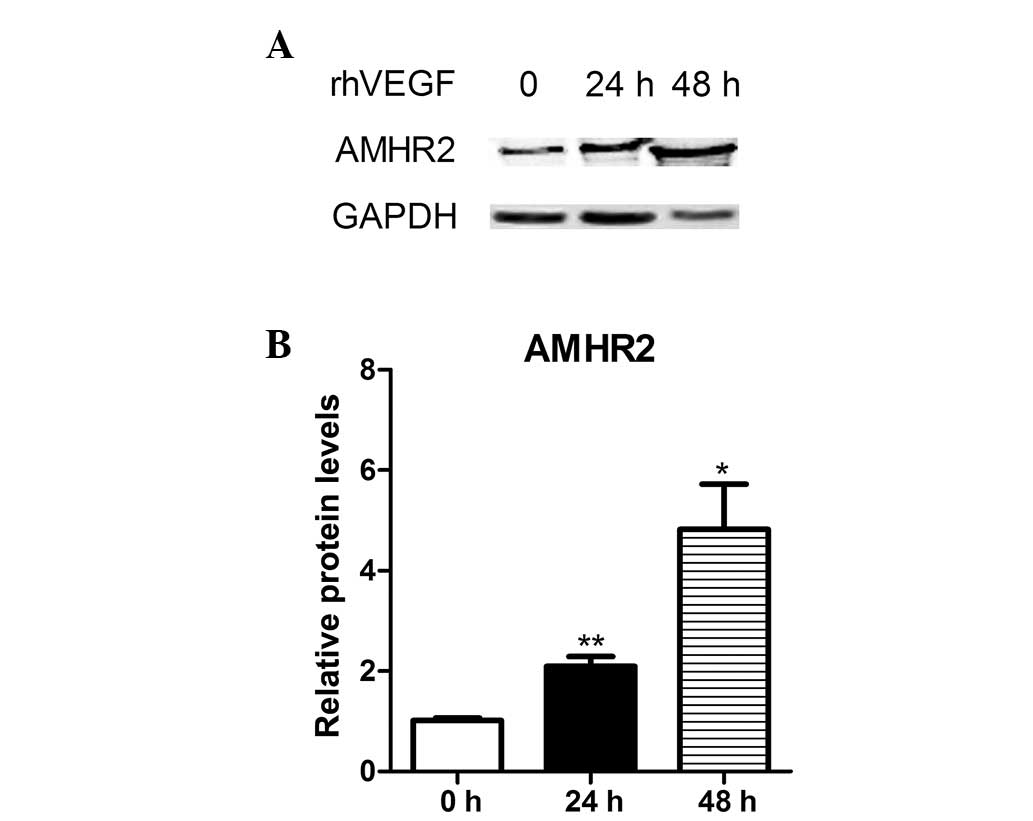
VEGF stimulated AMHR2 overexpression in
KGN cells
The human granulosa-like cell line, KGN is an
ovarian cancer cell line that expresses FSHR (18). The KGN GC tumor cells were derived
from a patient who presented with a recurrent, metastasized
granulosa cell tumor in the pelvic region. The cell line maintains
ovarian GC features, such as expression of the FSH receptor and
production of estrogen in response to FSH (7). In the present study, the expression
levels on primary cells were varied in specific individuals. To
demonstrate the induction of AMHR2, KGN cells were treated with
VEGF. The cells were treated with 100 ng/ml VEGF for 24 and 48 h,
and the representative AMHR2 protein levels increased by 2- and
5-fold, respectively (Fig. 5A and
B).
Discussion
VEGF is important in maintaining and regulating the
functions of the female reproductive system. White adipose tissue,
which is the major organ of VEGF secretion, surrounds the human
ovaries and uterus and assists with maintaining their functions.
VEGF has been detected in the follicular fluid of PCOS females
undergoing controlled ovarian hyper-stimulation, which modulates
the effects of gonadotrophins in GCs (8,10).
AMH is exclusively produced by ovarian GCs of the developing
preantral and antral follicles (19,20).
Ovarian function and reserve can be assessed by serum AMH levels to
evaluate infertility. Age-specific serum AMH levels and the antral
follicle count (AFC) baseline are evaluated to predict ovarian
reserve, particularly in females with PCOS (21-23).
AMH acts via its type II receptor, AMHR2, which has
recently been increasingly investigated. In a previous study, AMHR2
expression was evaluated in the mouse uterus (13). Genetic variants of AMHR2
appear to be associated with unexplained infertility and PCOS risk
(24). A primary study
demonstrated that antral follicles (size, 5–8 mm) contribute
markedly to serum AMH levels, and the AMHR2 gene is
co-expressed with AMH in the GCs of small antral follicles;
however, the correlation between AMHR2 gene expression level
and follicle fluid AMH concentration was not identified to be
significant (5). In the present
study, FSHR and AMHR2 were detectable in the ovarian follicular
fluid-derived GCs of the patients. AMH mRNA levels were
decreased in response to FSH and/or VEGF treatment. Furthermore,
TGFβ is important in controlling cell proliferation and exerts a
regulatory role in ovarian angiogenesis (25). TGFβ expression levels were
increased in response to VEGF treatment in the current study. Thus,
VEGF may have prompted the maturation of GCs and the reduction in
AMH expression.
In the present study, AMHR2 expression levels were
increased when the AMH expression level was repressed following
VEGF exposure. The in vitro study of KGN cells indicated
that the induction of AMHR2 by VEGF was time-dependent. VEGF and
its receptor, VEGFR2 are highly expressed in ovarian GC tumors. In
the present study, AMHR2 protein expression was increased in
response to VEGF stimulation in the KGN cells. In a previous study,
high levels of circulating VEGF were observed in the serum of
patients with primary GC tumors (26). Thus, elevated VEGF levels increase
AMHR2 levels in carcinoma tissues, which may contribute to the
malignancy of the tumor.
In conclusion, AMH exerts paracrine and hormonal
actions, and expression levels of the specific receptor, AMHR2 also
varies in different cells or tissues. VEGF misregulation increases
AMHR2 expression, which may result in binding of AMH from the
circulation, leading to paracrine AMH binding and attenuation of
the maturation of follicles in individuals using assisted
reproductive technology. AMH may exert other actions in
reproduction-associated organs; however, this requires further
investigation.
Acknowledgments
The present study was supported by grants from the
Jilin Provincial Science and Technology department (grant nos.
20150414023GH and 20150520038JH) and the China Postdoctoral Science
Foundation (grant no. 2014M551176).
References
|
1
|
Pellatt L, Rice S, Dilaver N, Heshri A,
Galea R, Brincat M, Brown K, Simpson ER and Mason HD:
Anti-Mullerian hormone reduces follicle sensitivity to
follicle-stimulating hormone in human granulosa cells. Fertil
Steril. 96:1246–1251.e1. 2011. View Article : Google Scholar
|
|
2
|
Durlinger AL, Gruijters MJ, Kramer P,
Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA
and Themmen AP: Anti-Müllerian hormone inhibits initiation of
primordial follicle growth in the mouse ovary. Endocrinology.
143:1076–1084. 2002.PubMed/NCBI
|
|
3
|
Gleicher N, Kim A, Kushnir V, Weghofer A,
Shohat-Tal A, Lazzaroni E, Lee HJ and Barad DH: Clinical relevance
of combined FSH and AMH observations in infertile women. J Clin
Endocrinol Metab. 98:2136–2145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iliodromiti S, Kelsey TW, Wu O, Anderson
RA and Nelson SM: The predictive accuracy of anti-Müllerian hormone
for live birth after assisted conception: A systematic review and
meta-analysis of the literature. Hum Reprod Update. 20:560–570.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeppesen JV, Anderson RA, Kelsey TW,
Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N,
Campbell BK and Yding Andersen C: Which follicles make the most
anti-Mullerian hormone in humans? Evidence for an abrupt decline in
AMH production at the time of follicle selection. Mol Hum Reprod.
19:519–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imhoff FM, Yang D, Mathew SF, Clarkson AN,
Kawagishi Y, Tate WP, Koishi K and McLennan IS: The type 2
anti-Müllerian hormone receptor has splice variants that are
dominant-negative inhibitors. FEBS Lett. 587:1749–1753. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu D, Lv X, Hua G, He C, Dong J, Lele SM,
Li DW, Zhai Q, Davis JS and Wang C: YAP regulates cell
proliferation, migration and steroidogenesis in adult granulosa
cell tumors. Endocr Relat Cancer. 21:297–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaya A, Atabekoglu CS, Kahraman K, Taskin
S, Ozmen B, Berker B and Sonmezer M: Follicular fluid
concentrations of IGF-I, IGF-II, IGFBP-3, VEGF, AMH and inhibin-B
in women undergoing controlled ovarian hyperstimulation using GnRH
agonist or GnRH antagonist. Eur J Obstet Gynecol Reprod Biol.
164:167–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abramovich D, Irusta G, Bas D, Cataldi NI,
Parborell F and Tesone M: Angiopoietins/TIE2 system and VEGF are
involved in ovarian function in a DHEA rat model of polycystic
ovary syndrome. Endocrinology. 153:3446–3456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doyle LK, Walker CA and Donadeu FX: VEGF
modulates the effects of gonadotropins in granulosa cells. Domest
Anim Endocrinol. 38:127–137. 2010. View Article : Google Scholar
|
|
11
|
Peitsidis P and Agrawal R: Role of
vascular endothelial growth factor in women with PCO and PCOS: A
systematic review. Reprod Biomed Online. 20:444–452. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Pietro M, Parborell F, Irusta G,
Pascuali N, Bas D, Bianchi MS, Tesone M and Abramovich D: Metformin
regulates ovarian angiogenesis and follicular development in a
female polycystic ovary syndrome rat model. Endocrinology.
156:1453–1463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar P, Sait SF, Sharma A and Kumar M:
Ovarian hyperstimulation syndrome. J Hum Reprod Sci. 4:70–75. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji Y, Lu X, Zhong Q, et al:
Transcriptional profiling of mouse uterus at pre-implantation stage
under VEGF repression. PLoS One. 8:e572872013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas FH, Telfer EE and Fraser HM:
Expression of anti-Mullerian hormone protein during early
follicular development in the primate ovary in vivo is influenced
by suppression of gonadotropin secretion and inhibition of vascular
endothelial growth factor. Endocrinology. 148:2273–2281. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu X, Ji Y, Zhang L, Zhang Y, Zhang S, An
Y, Liu P and Zheng Y: Resistance to obesity by repression of VEGF
gene expression through induction of brown-like adipocyte
differentiation. Endocrinology. 153:3123–3132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I,
Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al: Establishment
and characterization of a steroidogenic human granulosa-like tumor
cell line, KGN, that expresses functional follicle-stimulating
hormone receptor. Endocrinology. 142:437–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peluso C, Fonseca FL, Rodart IF,
Cavalcanti V, Gastaldo G, Christofolini DM, Barbosa CP and Bianco
B: AMH: An ovarian reserve biomarker in assisted reproduction. Clin
Chim Acta. 437:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlsson IB, Scott JE, Visser JA, Ritvos
O, Themmen AP and Hovatta O: Anti-Müllerian hormone inhibits
initiation of growth of human primordial ovarian follicles in
vitro. Hum Reprod. 21:2223–2227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui Y, Shi Y, Cui L, Han T, Gao X and Chen
ZJ: Age-specific serum antimüllerian hormone levels in women with
and without polycystic ovary syndrome. Fertil Steril.
102:230–236.e2. 2014. View Article : Google Scholar
|
|
22
|
Li HW, Lee VC, Lau EY, Yeung WS, Ho PC and
Ng EH: Role of baseline antral follicle count and anti-Mullerian
hormone in prediction of cumulative live birth in the first in
vitro fertilisation cycle: A retrospective cohort analysis. PLoS
One. 8:e610952013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panchal S and Nagori C: Comparison of
anti-mullerian hormone and antral follicle count for assessment of
ovarian reserve. J Hum Reprod Sci. 5:274–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rigon C, Andrisani A, Forzan M, D'Antona
D, Bruson A, Cosmi E, Ambrosini G, Tiboni GM and Clementi M:
Association study of AMH and AMHRII polymorphisms with unexplained
infertility. Fertil Steril. 94:1244–1248. 2010. View Article : Google Scholar
|
|
25
|
Kuo SW, Ke FC, Chang GD, Lee MT and Hwang
JJ: Potential role of follicle-stimulating hormone (FSH) and
transforming growth factor (TGFβ1) in the regulation of ovarian
angiogenesis. J Cell Physiol. 226:1608–1619. 2011. View Article : Google Scholar
|
|
26
|
Färkkilä A, Anttonen M, Pociuviene J,
Leminen A, Butzow R, Heikinheimo M and Unkila-Kallio L: Vascular
endothelial growth factor (VEGF) and its receptor VEGFR-2 are
highly expressed in ovarian granulosa cell tumors. Eur J
Endocrinol. 164:115–122. 2011. View Article : Google Scholar
|