Introduction
Multicentric reticulohistiocytosis (MRH) is a rare,
multi-system inflammatory disease, which is characterized by
cutaneous nodules and destructive polyarthritis. It can affect any
organs or tissues, however, the most common clinical manifestations
are papulonodular eruptions and symmetric inflammatory
polyarthritis. It is possible to observe constitutional symptoms,
including fever, weight loss and malaise, which may be associated
with joint and skin symptoms. There are no specific laboratory
tests for the diagnosis of MRH, and its current diagnosis is
predominantly dependent on histopathological evaluation (1,2).
According to tissue biopsies of the affected areas, ground-glass
opacity with increased quantities of periodic acid Schiff-positive
materials can be observed, which indicates the infiltration of
typical mononuclear histiocytes and multinucleated giant cells
(1). In the case of MRH,
immunohistochemical analyses are usually positive for CD45, CD68
and HLA-DR, but are negative for S-100, a Langerhans dendritic cell
marker, and HHF-35 actin, a fibroblast marker (1,3). In
addition, it has been found that serum levels of cytokines,
including tumor necrosis factor (TNF)-α and interleukins (ILs),
including IL-1β, IL-6 and IL-8, are increased in MRH and decreased
upon successful treatment (4).
Infliximab is a chimeric IgG1κ monoclonal antibody, which is
specific for human TNF-α. It is widely used for the treatment of
rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic
arthritis, Crohn's disease and ulcerative colitis (5). Previously, following the
demonstration of increased levels of TNF-α in patients with MRH,
anti-TNF-α treatment has been adopted with promising results
(4,6).
The present study reported on a case of a patient
with MRH, whose arthralgia and skin eruptions significantly
regressed following a treatment regimen combining infliximab,
prednisolone and methotrexate (MTX). This outcome demonstrated the
effectiveness of anti-TNF-α therapy for MRH. A systematic review of
available literature was also performed to evaluate the efficacy of
anti-TNF-α agents in the treatment of MRH.
Materials and methods
The present study was approved by the ethics
committee of Xiangya Hospital, Central South University (Changsha,
China). In 2013, a 48-year-old female diagnosed with MRH, who had a
12-month history of weakness, polyarthralgia, morning stiffness and
papulonodular skin eruption was recruited. Her past medical history
was unremarkable. According to the results of a biopsy, her skin
eruption exhibited dermal infiltration with histiocytes and
multinucleated giant cells.
Immunohistochemical staining was performed on
samples from the left face cutaneous nodules. Samples were fixed
using 10% formalin, embedded in paraffin and cut into 0.25–0.30 mm
sections. Immunohistochemical staining was conducted at room
temperature on a shaker. To enhance tissue penetration by
antibodies, sections were incubated with ethanol for 30 min and
rinsed with phosphate-buffered saline (PBS) 3 times for 5 min then
blocked to prevent nonspecific primary antibody reactions with 10%
normal donkey serum (NDS; OriGene Technologies, Inc., Beijing,
China). Tissue sections were incubated overnight in anti-S-100
(cat. no. MAB-0697) and CD68 (cat. no. MAB-0041) primary antibodies
(OriGene Technologies, Inc.). After reaction completion, tissues
were rinsed with PBS (3 times for 5 min), treated with NDS for 15
min, and incubated with goat anti-mouse fluorescein
isothiocyanate-conjugated secondary antibody (cat. no. PV-6000;
OriGene Technologies, Inc.) for 3 h, rinsed with PBS, and mounted
with Vectashield. The dilutions used were optimal, according to the
manufacturer's recommendations. Images were acquired using a cooled
CCD camera attached to a light microscope The results of
immunnohistochemical staining indicated positivity for CD68. The
patient was treated with combination therapy of infliximab
(intravenous infusion of 200 mg and subsequent infusion at weeks 2
and 6, followed by an infusion once every 8 weeks; Cilag AG,
Schaffhausen, Switzerland), prednisolone (oral administration; 30
mg/day; Zhejiang Xianju Pharmaceutical Co., Ltd., Zhejiang, China)
and MTX (15 mg/week; Shanghai Sine Pharmaceutical Laboratories
Co.,Ltd., Shanghai, China).
In addition to the above-mentioned patient, a
systematic review was performed on the therapeutic application of
anti-TNF-α agents in MRH. This involved the analysis of articles
published in the PubMed database (www.ncbi.nlm.nih.gov/pubmed) between January 2003 and
April 2014, and additional references cited in these articles were
cross-checked. The search strategy involved the use of a
combination of key words, including 'Multicentric
reticulohistiocytosis', 'Infliximab', 'Etanercept' and
'Adalimumab'.
Results
Case study
In the case of the female patient recruited in the
present study, physical examination revealed an erythematous
papulonodular rash, which had developed across her face, anterior
chest, back neck, forearms and the dorsum of her fingers, with
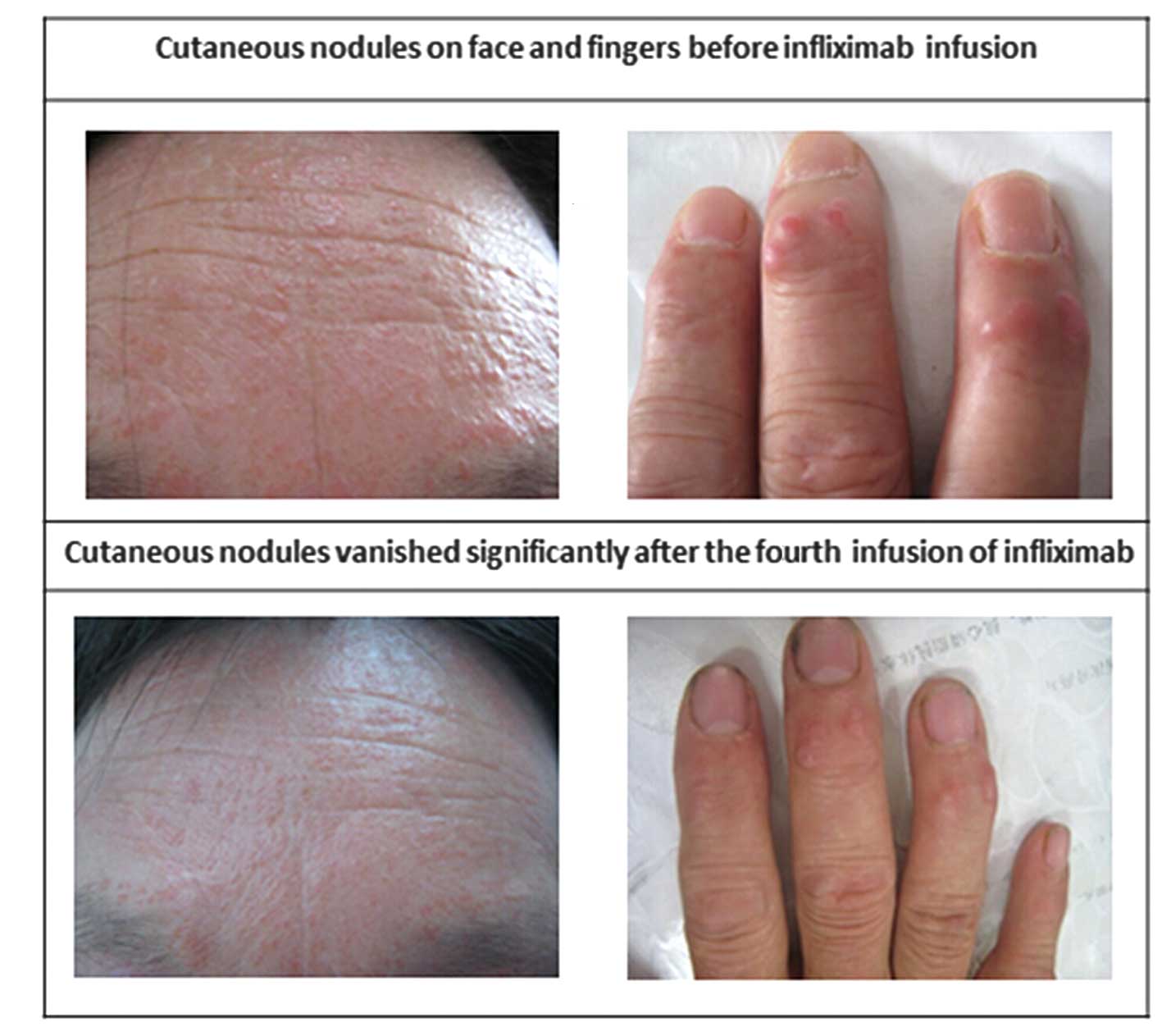
sizes ranging between 3 and 8 mm in diameter (Fig. 1). Musculoskeletal examination
revealed swelling and tenderness at the joints of the patient's
hands, elbows and knees. Initial investigations revealed normal
full blood counts, blood lipids, C-reactive protein and erythrocyte
sedimentation rate. Anti-nuclear antibodies, rheumatoid factor,
anti-neutrophil cytoplasmic antibodies, tumor markers [cancer
antigen (CA)199, CA125, CA242, CA153, carcinoembryonic antigen,
neuron-specific enolase and α-fetoprotein] and tuberculosis
antibodies were all negative. In addition, gynecological
examination and breast ultrasound were performed to exclude the
possibility of gynecological malignancy. Bone marrow aspiration was
also performed to rule out the possibility of hematologic
neoplasms. Hand X-ray revealed marginal erosions in certain areas
of the proximal interphalangeal joints, accompanied with mild
osteoporosis and knee osteoarthritis. The chest X-ray findings
suggested the possibility of tuberculosis, which was excluded by
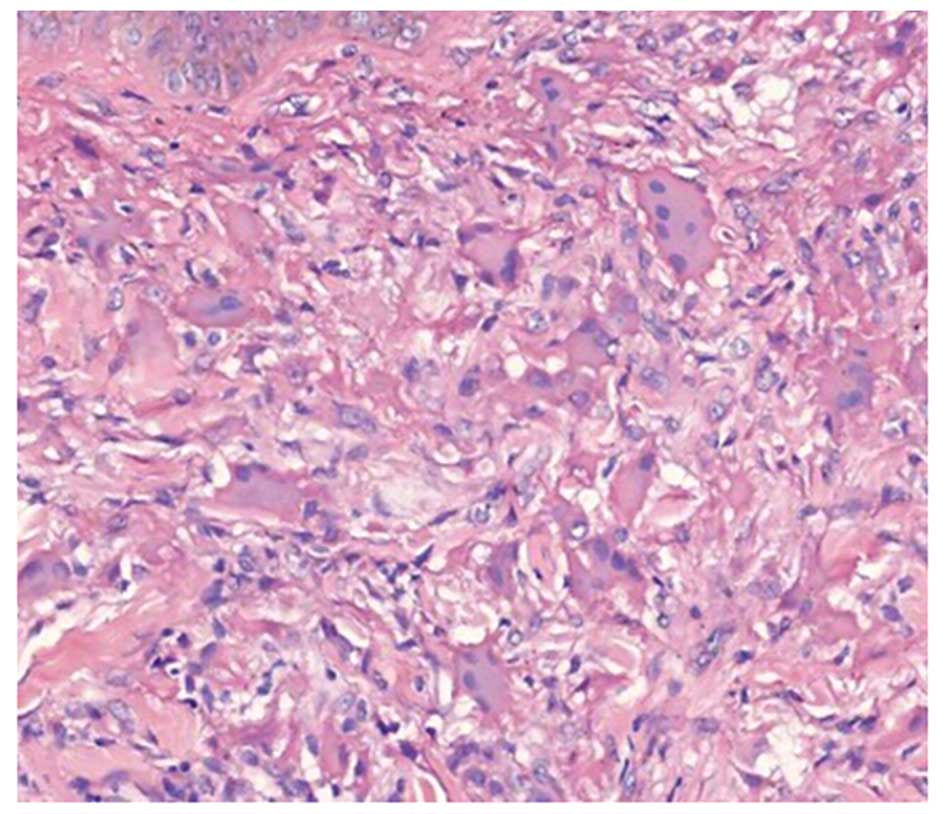
high-resolution computed tomography scan. Biopsy samples from two
of the skin nodules exhibited dermal infiltration with histiocytes
and multinucleated giant cells (Fig.
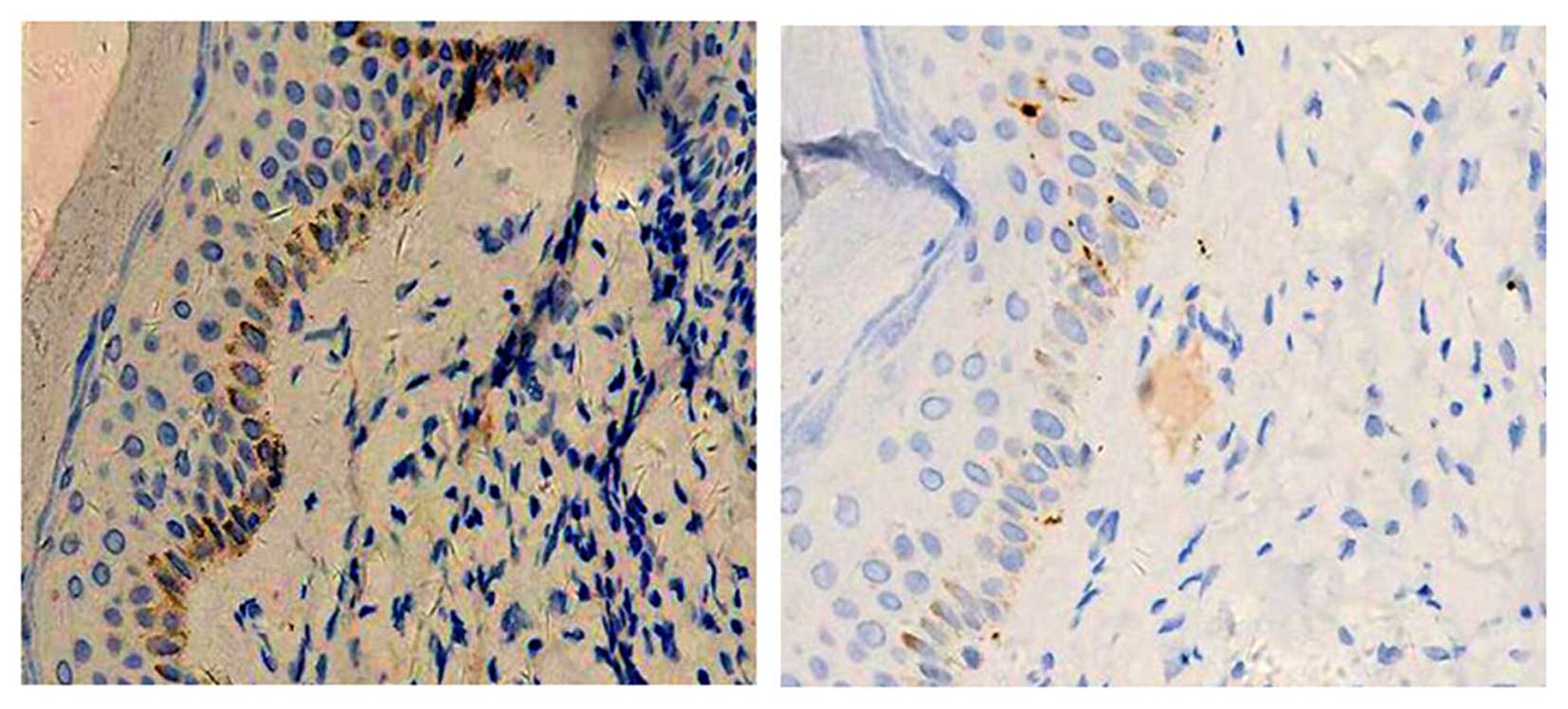
2). Immunohistochemical staining showed suspected positivity
for CD68, but negativity for S-100 (Fig. 3). These findings were particularly
indicative of MRH.
The patient was initially treated with an infliximab
infusion of 200 mg, and subsequent infusions were administered at
weeks 2 and 6, followed by an infusion once every 8 weeks. The
infliximab infusion was combined with oral prednisolone (30
mg/day), leflunomide (20 mg/day), hydroxychloroquine (HCQ; 200
mg/day), and intravenous MTX (15 mg/week). Diacerein (50 mg/day)
was added to the regimen due to osteoarthritis. The patients
symptoms improved following treatment for 3 days. Gradual remission
of the erythematous papules and nodules were noted prior to the
second infusion of infliximab, and polyarthralgia and stiffness
were markedly reduced. Following the fifth infusion, the skin signs
had regressed markedly (Fig. 1),
and symptoms of arthralgia were no longer present.
Literature review
In the present study, the following key words were
used as search terms in PubMed: 'Multicentric
reticulohistiocytosis', 'Infliximab', 'Etanercept', 'Adalimumab'
and 'Tumor necrosis factor inhibition', from which 16 articles were
found. According to the these reviewed articles and the results
from the case described above, a total of 17 patients were treated
with anti-TNF-α therapy, and none of the cases were excluded due to
incomplete data. The present study analyzed the outcomes reported
in the reviewed articles, based on the patients' responses to
treatment and the reductions in steroid dosage (Table I). The data of these patients are
summarized in Table I. The
patients comprised 10 (62.5%) women and six (37.5%) men, with a
median age of 47.5 years and age range of 3–76 years. All the
patients developed arthritis and articular manifestations, as well
as a maculopapular rash. Prior to the initiation of treatment with
anti-TNF-α agents, the majority of the reported MRH cases had
included the use of corticosteroids in their treatment, with the
exception of a case reported by Iwata et al (7). Combination treatments were
administered in 16 (94.1%) patients in the advent of relapse and
unmitigated progression of the disease. Therapeutic regimens varied
in the different reports due to the absence of standardized
treatment protocols. A total of 13 (76.5%) patients received MTX,
four (23.5%) received cyclosporine A and eight (47.1%) were treated
with HCQ. Cyclophosphamide (CTX) was used in four cases (23.5%) and
azathioprine was used in five cases (29.4%). A total of six
patients (35.3%) were treated with non-steroidal anti-inflammatory
drugs, whereas leflunomide was used in two cases (11.8%), and
mycophenolate mofetil was used in one (5.9%) case, as was
sulfasalazine (5.9%). A combination of chlorambucil and cariolysine
was used in three cases (17.6%). Different treatment modalities
were used with little or no success prior to treatment of the
patients with anti-TNF-α agents. Alopecia, hypoleucocytosis,
pruritus and other side effects appeared following the application
of immunosuppressive agents, whereas no adverse effects were
reported following the use of anti-TNF-α agents. In the previous
literature, anti-TNF-α agents were administered in combination with
glucocorticoids in all patients with promising results, with the
exception of the single case reported by Iwata et al
(7). Following the initiation of
anti-TNF-α treatment, the number of patients suffering from
constitutional symptoms was relatively low. Improvements in skin
lesions and arthralgia were observed upon receiving anti-TNF-α
treatment, which indicated a positive clinical response. Only minor
manifestations were found: Two (11.8%) patients had fever, two
(11.8%) patients presented with weight loss, two (11.8%) patients
experienced fatigue, one patient (5.9%) presented with night
sweats, one patient (5.9%) presented with stiffness and one patient
(5.9%) presented with muscle aches.
 | Table IReported cases of patients with MRH
treated with anti-TNF-α agents. |
Table I
Reported cases of patients with MRH
treated with anti-TNF-α agents.
| Case (refs.) | Age/gender | Disease duration
(months) | Skin biopsy | Radiography | Clinical
features | Laboratory tests | Previous
treatment | Anti-TNF agents | Concomitant
therapies | Outcome | IHC |
|---|
| Matejicka et
al (10) | 22/F | 36 | Multinucleated
histiocytes; abundant dense pink cytoplasm | Progressive erosions;
pencil-in-cup deformities | Erythematous rash;
papular lesions; polyarthritis | Normal | GC, CyA, MTX, HCQ,
CTX, naproxen | ETA
50 mg/W | GC, MTX, CTX,
HCQ | Skin lesions and
arthralgia relieved; radiography-no progression | NA |
| Kovach et al
(8)a | 46/M | 12 | Histocytes and
multinucleated giant cells; ground glass cytoplasm; fine
PAS-positive granules | Erosive articular
damage in hands and right hip | Skin lesions;
progressive inflammatory ployarthritis | pANCA positive | MTX, GC, HCQ,
chlorambucil | ETA
50 mg/W | GC, MTX, LEF, | Improvement in skin
and joint symptoms | NA |
| Lee et al
(15) | 53/F | 2 | Densely packed giant
cells and histiocytes; Predominantly mononuclear cytoplasm
abundant; PAS-positive | No abnormality | Polyarthalgia; Red
confluent patches; small erythematous papules | normal | NA | IFN
5 mg/kg | GC, MTX | Rapid regression of
papulonodules; no new lesions; arthralgias decreased | CD68 (+)
S100 (−)
CD1a |
| Sellam et al
(14) | 37/F | 24 | Multinucleated
histiocytes; abundant dense, pink, cytoplasm | Several
erosions | Ployarthritis; red
rash, brown-reddish nodules | ANA
(1:320) | GC, Cariolysine,
HCQ, MTX | IFN | MTX, AZA,
NSAIDs | Macular rash/nodule
decrease; ployarthritis unchanged; | NA |
| Sellam et al
(14) | 53/F | 42 | Typical pattern of
MRH | Bilateral
erosions | Polyathritis;
pruritic rash with nodules | ANA
(1:640)
ESR
(28 mm/h)
SSA positive | GC, MTX HCQ, CTX,
Chlorambucil, CyA, LEF, AZA | IFN, ETA, | AZA | Skin lesions
improved; nodules decreased; ployarthritis unchanged | NA |
| Lovelace et
al (11) | 42/M | 24 | Nodular
interstitial histiocytic infiltrate; multinucleated histiocytes;
eosinophilic granular cytoplasms | NA | Red-brown
dome-shaped papules and nodules; distal arthritis | NA | NA | ETA, (100
mg/W) | GC | Minimal improvement
of pain and skin lesions | NA |
| Shannon et
al (6) | 37/F | 4 | Mild hyperplasia of
synovial cells; scattered monocytes; occasional giant cells | Symmetric erosion
of DIP and first IP joints | Fine flesh- color
nodules, clustered; large painful boggy DIP joints | Normocytic
anemia
ESR (100 mm/h)
CRP (33 mg/dl)
ANA and RF negative | CyA, MMF, GC,
simvastatin, tramadol, NSAIDs | ADA, 40 mg | CyA, MMF, GC | Improved
significantly; no evidence of synovitis | CD68
CD3
CD45 (+)
S-100 CD1
CD30 (−) |
| Kalajian et
al (19) | 63/F | 12 | Histopathologic
dermal infiltration; multinucleated giant cells; amorphous
eosinophilic ground-glass-appearing cytoplasm varied density of
infiltration | NA | Asymptomatic
cutaneous lesions; progressively destructive arthritis; purified
protein derivative (+);episodic fevers, night sweats, weight
loss | CK, CRP
ESR elevated; ANCA, ACL
RF, ANA, AdsDNA and HCVAb negative | GC, isoniazid,
MTX | ETA, IFN | GC, MTX | Condition
fluctuations No new cutaneous lesions | NA |
| Chiba et al
(16) | 76/F | 3 | Multinucleated
giant cells | Marginal
erosions | Ployarthritis; red
maculopapuplar rash; fever | CRP, ESR
RF and CCP negative; ANA (1:320) | NA | IFN | GC, MTX | Erythematous
papules; polyarthritis disappeared | CD68 (+)
CD68 (+) |
| De Knop et
al (17) | 47/M | 120 | Multinucleated
giant cells; eosinophilic ground-glass cytoplasm | Erosions | Symmetric
polyarthritis; papulonodular rash | SSA, SSB, dsDNA, RF
and ANA positive; CRP, ESR and CCP negative | MTX, SSZ
tenoxicam
HCQ, CTX, GC, AZA | IFN | MTX | Improved morning
stiffness; tender and swollen joints | |
| Chauhan et
al (12) | 74/F | 72 | Dense histiocytic
infiltrate; abundant eosinophilic cytoplasm; multinucleation | Marginal erosive
changes | Arthralgias
erythematous nodules; papular lesions fatigue weight-loss | ESR elevated;
Anemia, RF, ANA and ENA negative; CCP positive | GC, plaquenil | ETA | NA | Skin changes
regressed; arthiritic symptoms improved | CD68 (+) |
| Matiz et al
(20) | 3/F | 6 | Dome-shaped lesion;
foamy histiocyte dermal infiltrate; admixed lymphocytes;
CD1a-stained intraepidermis, rare dermal cells; Factor
XIIIa-staining of scattered cells | Mild diffuse
osteopenia; soft tissue swelling | Papular skin
eruption; significant arthralgia | ESR and CRP normal;
ANA and RF negative | Naproxen
MTX, HCQ GC | ETA, IFN | MTX, GC | Partial initial
response to etanercept; all xanthomas disappeared; no further
synovitis improvement | CD68 (+)
CD1a |
| Broadwell et
al (9)a | 55/M | 120 | Significant healing
of hand erosions | NA | Polyarthritis;
multiple skin lesions | NA | MTX, GC | CTX, LEF, ETA | NA | Remained
asymptomatic | NA |
| Iwata et al
(7) | 44/M | 8 | Infiltration of
multinucleated giant cells and histiocytes with eosinophilic
ground-glass cytoplasm | NA | Asymptomatic; firm
and flesh-colored erythematous cutaneous papules | WBC
normal
TNF-α
MCP-1 elevated | NA | IFN | NA | Skin lesions and
arthritis gradually improved | CD68
MCP (+)
CD1a
S100 (−) |
| Yeter et al
(21) | 55/M | 12 | Intradermal
histiocytic proliferation; majority of cells mononuclear; no foam
cells | Chest
unremarkable | Red rash, muscle
aching and stiffness in shoulders, progressed to right
hand/knees/thighs swelling of right wrist | CCP, ESR, CRP, SSB,
AdsDNA, Sm negative; ANA, RF SSA positive | MTX | ETA, ADA | MTX, GC,
minocycline | Skin lesions
significantly
Improved shoulder pain, morning stiffness; weaned off steroids;
cutaneous manifestiations quiet; arthralgia improved | NA |
| Saba et al
(13) | 54/F | 120 | Histiocytic
infiltration with multinucleated giant cells | Severe diffuse
destruction Periarticular osteoporosis; new bone formation | Multiple
non-pruritic reddish-brown papulonodular lesions; severe diffuse
arthritis | Anemia; CRP
elevated ANA RF, CCP normal | Ibuprofen, AzA | ADA | MTX | Symptomatic relief;
no resolution of irreversible arthritic deformities | CD68 (+) |
| Macía-villa et
al (18) | 50/M | 48 | Non-langerhans
cutaneous histiocytosis suggests early-phase reticulohistiocytosis
subtype; Papular lesions infiltrated by histiocyteappearing cells
with macrophage monocytic features | Marginal erosions
in interphalangeal joints; loss of joint space and swan finger
deformity; X-rays of feet show hammer toes and joint space
narrowing | Symmetrical
deforming arthritis of interphalangeal joints, knees and ankles;
pruritic brown nodules in both; indurated nodules in hands | Normal RBC WBC,
ESR, RF and CRP; C3, C4, anti-CCP, anti-mitochondria; anti-thyroid;
ANA, anti- DNA and anti-ENA negative | Prednisone,
alendronate, MTX, hydoxychloroquin | IFN | Prednisone,
alendronate, MTX, hydoxychloroquine | Skin lesions
improved; complete remission of arthritis and improvement of
arthralgia; arthritic deformities failed to resolve | CD68 (+)
Factor XIII
CD10 (+)
S100 (−)
CD1a (−) |
| Zhao et al
(present) | 48/F | 12 | Dermal infiltration
with histiocytes and multinucated giant cells | Marginal erosions;
mild osteoporosis; narrowed joint space | Ployarthritis,
stiffness and weakness; papulonodular skin eruptions | ESR, CRP
RF and ANA normal | Meloxicam, GC | IFN | GC, LEF
HCQ, MTX diacerein | Erythematous
papules and nodule, and polyarthritis disappeared | CD68 (+)
S-100 (−) |
Among the cases reported in the previous studies, 10
cases included the use of etanercept for the treatment of MRH.
Among these, five cases responded well to treatment (8–12),
three cases reported the replacement of etanercept treatment with
another anti-TNF-α agent, including infliximab and adalimumab. The
remaining two cases reported the initiation of etanercept treatment
in replacement of adalimumab (13)
and infliximab (14).
In the previous literature, eight cases reported the
application of infliximab for the treatment of patients with MRH,
six of which reported successful treatment with infliximab
(5,14,15–18).
The remaining two cases reported the replacement of etanercept with
infliximab (19,20). In one case, reported by Sellam
et al (14), there was
concern regarding the replacement of infliximab with
etanercept.
The use of adalimumab for the treatment of patients
with MRH was reported in three cases, and one case was treated
successfully. Adalimumab was used in place of etanercept in one
case (21), whereas two cases
replaced adalimumab with etanercept (13).
Discussion
MRH is a rare and debilitating systemic inflammatory
disease of unknown etiology. In the case of MRH,
immunohistochemical analysis of synovial tissue shows positive
staining of TNF-α, IL-1β, IL-6 and IL-12, suggesting the presence
of these inflammatory cytokines in affected areas, as reported by
Gorman et al (3). In 2010,
Tashiro et al (22)
demonstrated the abundant accumulation of CD10 in the cytoplasm of
ground-glass-like multinucleated giant cells in two patients with
MRH. Of note, in a case reported by Bennàssar et al
(4), increases in serum cytokine
levels, namely of TNF-α, IL-1β, IL-6 and IL-8, were observed, which
decreased following treatment.
TNF antagonists have been widely used for the
treatment of rheumatoid arthritis, ankylosing spondylitis,
psoriasis, psoriatic arthritis, Crohn's disease and ulcerative
colitis (5,23–26).
Due to the fact that high levels of TNF-α are expressed in patients
with MRH, anti-TNF-α therapy has become a viable option and widely
used in the treatment of MRH in previous decades.
TNF-α antagonists are biological agents comprised of
fusion proteins or antibodies foreign to the patient. For patients,
immunogenicity and sensitization of TNF-α antagonists are of
particular concern. The presentation of neutralized antibodies to
TNF-α drugs can potentially cause inactivation and increased rates
of clearance, thus affecting treatment outcome (27). Therefore, there were no reports
pertaining to the presence of antiglobulins towards the anti-TNF-α
agents, infliximab, etanercept and adalimumab, among the MRH cases
included in the present review. In addition, there are several
adverse effects of anti-TNF-α agents, including infusion-associated
reactions, allergic reactions, increased susceptibility towards
infection, demyelinating diseases and worsening of cardiovascular
disease. These side effects are often mild, self-limiting and often
do not necessitate the discontinuation of therapy (28). It is worth noting that observations
or reports of these adverse effects were rare when anti-TNF-α
treatment was used in the MRH patients in this review.
Matejicka et al performed an initial trial
involving the application of anti-TNF-α agents in a patient with
MRH in 2003. This resulted in the successful treatment of a
22-year-old female college student using a combination of
etanercept (25 mg twice a week subcutaneously), MTX, prednisolone,
HCQ and CTX. The patient experienced remission of skin lesions and
arthralgias 6 weeks following treatment (10). The following year, Kovach et
al also reported a successful case of treating MRH with the
combination of etarnercept, leflunamide and prednisolone (8).
In 2004, Lee et al (15) reported an effective combination of
infliximab, prednisolone and MTX in treating MRH. Treatment
involved the use of infliximab (5 mg/kg/day) in combination with
MTX (7.5 mg/week) and prednisolone (30 mg/day) following
establishment of the diagnosis of MRH. There was a noticeable
regression of dermal nodules following the first infusion, and
polyarthralgia were alleviated within 3 months. Several subsequent
cases of successful treatment with TNF-α inhibitors have been
reported since, as summarized in Table
I, which were effective in alleviating symptoms, although there
was with disparity in responses to cutaneous and articular
manifestations.
According to a review by Kalajian, a trend was noted
in treatment modalities comprising TNF inhibition, with
prednisolone and MTX having higher success rates (19,29).
Among the anti-TNF-α agents, infliximab has been reported to be
more efficient than etanercept, which can be explained by the fact
that infliximab has a higher association rate and lower
dissociation rate, compared with etanercept (30). Infliximab is reported to be able to
irreversibly bind to TNF without partial inhibition, thus allowing
complete neutralisation of TNF (30). This may also explain the case
reported by Sellam et al (14), in which no further improvement of
symptoms was observed following the replacement of infliximab with
etanercept. Based on the reported effectiveness of infliximab in
the treatment of MRH, the patient in the present study was treated
with infliximab at the beginning of treatment, which was found to
be efficacious. As the patient was also receiving treatment with
pednisone, MTX, leflunomide and HCQ at the same time, the
combination of which has been confirmed to be effective (3,4,29,31),
it was not possible to independently evaluate the efficacy of
infliximab in this patient (32).
Thus, rather than exclusively attributing the success of treatment
to infliximab, it was suggested that the inclusion of infliximab in
a treatment regimen appears to be a viable option (33–35).
As a result of the notable effectiveness of infliximab in treating
MRH, according to previous literature, anti-TNF-α agents,
particularly infliximab, may be advocated as an efficacious
approach for the treatment of MRH.
The present study had certain limitations,
predominantly due to the rarity of the disease and the low number
of patients reviewed. In addition, the administration of these
TNF-α antagonists has often been included in various treatment
regiments, however, there has been no systematic comparison between
cases, and no independent evaluation of its efficiency.
Furthermore, the majority of the case reports focussed partly on
favorable responses, and those with poor outcomes have been rarely
reported. All these limitations restrict the comprehensiveness of
the analysis of anti-TNF-α treatment in the present study. In
conclusion, as stated above, TNF antagonists offer a relatively
safe and well-tolerated treatment option, and may be recommended in
refractory MRH as they induce remission and allow a reduction in
steroid dosage. It may be administered in accordance with the
sequence of therapy used in the management of rheumatoid arthritis.
However, further prospective investigations are required to improve
and standardize its application, in terms of dosage and duration,
in the treatment of patients with MRH.
Acknowledgments
This study was supported by the Hunan province
Science and Technology Program (grant no. 2012SK3202).
References
|
1
|
Islam AD, Naguwa SM, Cheema GS, Hunter JC
and Gershwin ME: Multicentric reticulohistiocytosis: A rare yet
challenging disease. Clin Rev Allergy Immunol. 45:281–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baek IW, Yoo SH, Yang H, Park J, Kim KJ
and Cho CS: A case of multicentric reticulohistiocytosis. Mod
Rheumatol. 11:1–4. 2014. View Article : Google Scholar
|
|
3
|
Gorman JD, Danning C, Schumacher HR,
Klippel JH and Davis JC Jr: Multicentric reticulohistiocytosis:
Case report with immunohistochemical analysis and literature
review. Arthritis Rheum. 43:930–938. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bennàssar A, Mas A, Guilabert A, Julià M,
Mascaró-Galy JM and Herrero C: Multicentric reticulohistiocytosis
with elevated cytokine serum levels. J Dermatol. 38:905–910.
2011.PubMed/NCBI
|
|
5
|
de Vries HS, van Oijen MG, Driessen RJ, de
Jong EM, Creemers MC, Kievit W and de Jong DJ: Appropriate
infliximab infusion dosage and monitoring: Results of a panel
meeting of rheumatologists, dermatologists and gastroenterologists.
Br J Clin Pharmacol. 71:7–19. 2011. View Article : Google Scholar :
|
|
6
|
Shannon SE, Schumacher HR, Self S and
Brown AN: Multicentric reticulohistiocytosis responding to tumor
necrosis factor-alpha inhibition in a renal transplant patient. J
Rheumatol. 32:565–567. 2005.PubMed/NCBI
|
|
7
|
Iwata H, Okumura Y, Seishima M and Aoyama
Y: Overexpression of monocyte chemoattractant protein. 1 in the
overlying epidermis of multicentric reticulohistiocytosis lesions:
A case report. Int J Dermatol. 51:492–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kovach BT, Calamia KT, Walsh JS and
Ginsburg WW: Treatment of multicentric reticulohistiocytosis with
etanercept. Arch Dermatol. 140:919–921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broadwell AW, Calamia KT, Kransdorf MJ and
Ginsburg WW: Healing of erosive disease in multicentric
reticulohistiocytosis. J Rheumatol. 37:1366–1367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matejicka C, Morgan GJ and Schlegelmilch
JG: Multicentric reticulohistiocytosis treated successfully with an
anti-tumor necrosis factor agent: Comment on the article by Gorman
et al. Arthritis Rheum. 48:864–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lovelace K, Loyd A, Adelson D, Crowson N,
Taylor JR and Cornelison R: Etanercept and the treatment of
multi-centric reticulohistiocytosis. Arch Dermatol. 141:1167–1168.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chauhan A, Mikulik Z and Hackshaw KV:
Multicentric reticu-lohistiocytosis with positive anticyclic
citrullinated antibodies. J Natl Med Assoc. 99:678–680.
2007.PubMed/NCBI
|
|
13
|
Saba R, Kwatra SG, Upadhyay B, Mirrakhimov
AE and Khan FN: Multicentric reticulohistiocytosis presenting with
papulonodular skin lesions and arthritis mutilans. Case Rep
Rheumatol. 2013:2015632013.PubMed/NCBI
|
|
14
|
Sellam J, Deslandre CJ, Dubreuil F, Arfi S
and Kahan A: Refractory multicentric reticulohistiocytosis treated
by infliximab: Two cases. Clin Exp Rheumatol. 23:97–99.
2005.PubMed/NCBI
|
|
15
|
Lee MW, Lee EY, Jeong YI, Choi JH, Moon KC
and Koh JK: Successful treatment of multicentric
reticulohistiocytosis with a combination of infliximab,
prednisolone and methotrexate. Acta Derm Venereol. 84:478–479.
2004.
|
|
16
|
Chiba E, Oda A, Tsutsumi T, Yabe H, Kamiya
Y, Sakurai T, Moriguchi M, Momomura S and Terai C: Case report; a
case with multicentric reticulohistiocytosis successfully treated
with infliximab. Nihon Naika Gakkai Zasshi. 100:483–486. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Knop KJ, Aerts NE, Ebo DG, Van Offel
JF, Stevens WJ and De Clerck LS: Multicentric reticulohistiocytosis
associated arthritis responding to anti-TNF and methotrexate. Acta
Clin Belg. 66:66–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macía-Villa CC and Zea-Mendoza A:
Multicentric reticulohistiocytosis: Case report with response to
infliximab and review of treatment options. Clin Rheumatol. Apr
15–2014.Epub ahead of print.
|
|
19
|
Kalajian AH and Callen JP: Multicentric
reticulohistiocytosis successfully treated with infliximab: An
illustrative case and evaluation of cytokine expression supporting
anti-tumor necrosis factor therapy. Arch Dermatol. 144:1360–1366.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matiz C, Ferguson PJ, Zaenglein A, Groh B
and Bingham CA: Papular xanthomas and erosive arthritis in a 3 year
old girl, is this a new MRH variant? Pediatr Rheumatol Online J.
7:152009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeter KC and Arkfeld DG: Treatment of
multicentric reticulohistiocytosis with adalimumab, minocycline,
methotrexate. Int J Rheum Dis. 16:105–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tashiro A, Takeuchi S, Nakahara T, Oba J,
Tsujita J, Fukushi J, Kiryu H, Oda Y, Xie L, Yan X, et al: Aberrant
expression of CD10 in ground-glass-like multinucleated giant cells
of multicentric reticulohistiocytosis. J Dermatol. 37:995–997.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X and Xu S: TNF inhibitor therapy for
rheumatoid arthritis. Biomed Rep. 1:177–184. 2013.
|
|
24
|
Lubrano E, Spadaro A, Amato G, Benucci M,
Cavazzana I, Chimenti MS, Ciancio G, D'Alessandro G, Angelis RD,
Lupoli S, et al: Tumour necrosis factor alpha inhibitor therapy and
rehabilitation for the treatment of ankylosing spondylitis: A
systematic review. Semin Arthritis Rheum. 44:542–550. 2015.
View Article : Google Scholar
|
|
25
|
Behrens F, Cañete JD, Olivieri I, van
Kuijk AW, McHugh N and Combe B: Tumour necrosis factor inhibitor
monotherapy vs combination with MTX in the treatment of PsA: A
systematic review of the literature. Rheumatology (Oxford).
54:915–926. 2015. View Article : Google Scholar
|
|
26
|
Schreiber S: An update on biosimilar drugs
for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol.
9(Suppl 1): 1–3. PubMed/NCBI
|
|
27
|
Jung SM, Kim HS, Kim HR, Kim NY, Lee JH,
Kim J, Kwok SK, Park KS, Park SH, Kim HY and Ju JH: Immunogenicity
of anti-tumour necrosis factor therapy in Korean patients with
rheumatoid arthritis and ankylosing spondylitis. Int
Immunopharmacol. 21:20–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scheinfeld N: A comprehensive review and
evaluation of the side effects of the tumor necrosis factor alpha
blockers etanercept, infliximab and adalimumab. J Dermatolog Treat.
15:280–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trotta F and Colina M: Multicentric
reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res
Clin Rheumatol. 26:543–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ehlers S: Tumor necrosis factor and its
blockade in granulo-matous infections: Differential modes of action
of infliximab and etanercept? Clin Infect Dis. 41(Suppl 3):
S199–S203. 2005. View
Article : Google Scholar
|
|
31
|
Lonsdale-Eccles AA, Haworth AE, McCrae FC
and Young-Min SA: Successful treatment of multicentric
reticulohistiocytosis with leflunomide. Br J Dermatol. 161:470–472.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thalayasingam N and Isaacs JD: Anti-TNF
therapy. Best Pract Res Clin Rheumatol. 25:549–567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pascual-Salcedo D, Plasencia C, Ramiro S,
Nuño L, Bonilla G, Nagore D, Ruiz Del Agua A, Martínez A, Aarden L,
Martín-Mola E and Balsa A: Influence of immunogenicity on the
efficacy of long-term treatment with infliximab in rheumatoid
arthritis. Rheumatology (Oxford). 50:1445–1452. 2011. View Article : Google Scholar
|
|
34
|
Finckh A, Dudler J, Wermelinger F, Ciurea
A, Kyburz D, Gabay C and Bas S; physicians of SCQM: Influence of
anti-infliximab antibodies and residual infliximab concentrations
on the occurrence of acquired drug resistance to infliximab in
rheumatoid arthritis patients. Joint Bone Spine. 77:313–318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenblum H and Amital H: Anti-TNF
therapy: Safety aspects of taking the risk. Autoimmun Rev.
10:563–568. 2011. View Article : Google Scholar : PubMed/NCBI
|