Introduction
Psoriasis and dermatitis eczema are common
dermatological diseases. They are considered to be two different
diseases due to their mechanisms, pathological changes and
treatments (1–3). However, clinically the normal skin of
patients with psoriasis will develop psoriatic lesions subsequent
to elicitation, which is known as the Koebner phenomenon (4). Similarly, if irritated, the plaque
lesions of patients with psoriasis will develop dermatitis
eczematous changes and lose their inherent characteristics of
psoriasis. A previous histology study determined that epidermal
thickening and intra- and inter-cellular edema may occur, instead
of extended furcella, dermal papilla and Munro's microabscess,
which are pathologically described as psoriatic dermatitis
(5). Therefore, these two diseases
may be associated and may follow the same or overlapping
inflammatory pathways (1).
Traditionally, psoriasis is considered as a T helper
type 1 (Th1)-dominated skin disease, and an imbalance of Th1 and
Th2 cells has been demonstrated in psoriasis patients (6,7).
With the discovery of Th17 cells, psoriasis was thought to be
mediated by Th1 and Th17 (8). In a
previous study, a large number of Th17 cells and cytokines were
detected in the skin lesions and serum of psoriasis patients
(7,9). The interleukin (IL)-23/IL-17 axis is
considered to be important for the development of psoriasis
(10,11).
Contact hypersensitivity (CHS) is a delayed-type
hypersensitivity mediated by T cells, which consits of
sensitization and elicitation phases, similar to the human allergic
contact dermatitis (ACD) model (12–15).
Exposure to different types of chemical sensitization may
selectively activate Th1 or Th2 cells, and induce different types
of immune response. Exposure to dinitrofluorobenzene (DNFB) can
result in Th1-type CHS with high levels of IFN-γ; however,
relatively low levels of Th2 cytokines, IL-4, -5, and -10. Exposure
to fluorescein isothiocyanate (FITC) can induce Th2-type CHS
accompanied by infiltration of Th2 cells (16,17).
The importance of Th17 in inflammatory diseases is being
increasingly recognized, with Th17 cells and cytokines detected in
CHS mouse models and patients with ACD (18–20).
There is a long-standing debate on the
susceptibility and intensity to different contact sensitizations in
patients with psoriasis and the relationship between psoriasis and
atopic disease. Previous studies have presented varied, even
contradictory results (1,21,22).
The discovery of Th17 provides a novel perspective of psoriasis and
ACD, which allows for the examination of the link between the two
diseases. Both the diseases involve Th17 and its cytokines.
Therefore, the present study aimed to determine whether the
IL-23/IL-17 axis facilitates interaction between the two diseases.
In order to achieve this, the compound mouse model of combination
CHS with imiquimod (IMQ)-induced psoriasis-like inflammation was
established. This model was used to explore the influence of
different types of CHS on psoriasis-like inflammation, and the the
importance of the Th17-associated pathway.
Materials and methods
Experimental animals
A total of 72 female Balb/c mice (6–8 weeks; 17±1.5
g) were purchased from the Guangdong Medical Laboratory Animal
Center (Guangdong, China). The animals were housed at 24°C with
40–60% humidity on a 12-h dark/light cycle, and provided with food
and water ad libitum. The current study was approved by the
Laboratory Animal Care and Use Committee of Dalian Medical
University (Dalian, China). All protocols used in the present study
were in compliance with the Dalian Medical University Guidelines
for the proper care and use of laboratory animals.
Reagents and kits
IMQ (5%) cream was purchased from Sichuan Med-Shine
Pharmaceutical Co., Ltd., (Sichuan, China). Dibutyl phthalate (DBP;
>99%) and FITC were purchased from Sigma-Aldrich (St. Louis, MO,
USA). DNFB was purchased from Shanghai Jonln Reagent Co., Ltd.
(Shanghai, China). The other chemicals were of analytical grade.
Mouse enzyme-linked immunosorbent assay (ELISA) kits for IL-17 and
IL-23 were purchased from eBioscience, Inc. (San Diego, CA, USA).
Mouse ELISA kits for IL-22 and interferon (IFN)-γ were purchased
from Dakewe Biotech Co., Ltd. (Shenzhen, China).
Establishment of compound mouse models of
Th1 type or Th2 type CHS combined with IMQ-induced psoriasis-like
inflammation
Establishment of classic murine models of
IMQ-induced psoriasis and CHS was based on previous studies
(23,24). Mice received daily doses of 62.5 mg
5% IMQ cream on their shaven backs for 15 consecutive days.
Th1-type CHS was induced by the application of 25 µl 0.5%
DNFB on the shaven abdomens in the sensitization phase (day 7 and
8), and 20 µl 0.25% DNFB on each ear in the stimulation
phase (day 13) (13). Th2-type CHS
was induced by the application of 200 µl 1% FITC on the
shaven abdomens in the sensitization phase (day 7 and 8), and 20
µl 0.5% FITC on each ear in the stimulation phase (day 13)
(25). The solvent for DNFB was
acetone and olive oil (aOO, 4:1 ratio), and for FITC was acetone
and DBP (aDBP, 1:1 ratio). In a total of 72 female Balb/c mice, 36
mice were randomly selected to establish the compound mouse model
of DNFB-induced Th1-type CHS combined with IMQ-induced
psoriasis-like inflammation (Th1-type compound mouse model). The
other 36 mice were used to establish the compound mouse model of
FITC-induced Th2-type CHS combined with IMQ-induced psoriasis-like
inflammation (Th2-type compound mouse model). The mice were divided
into 6 groups (n=6/group) in order to establish the following
compound mouse models: Group I, control; group II, solvent; group
III, Th1 or Th2; group IV, IMQ; group V, IMQ + solvent; group VI,
IMQ + Th1 or IMQ + Th2. The detailed grouping and protocol are
presented in Fig. 1.
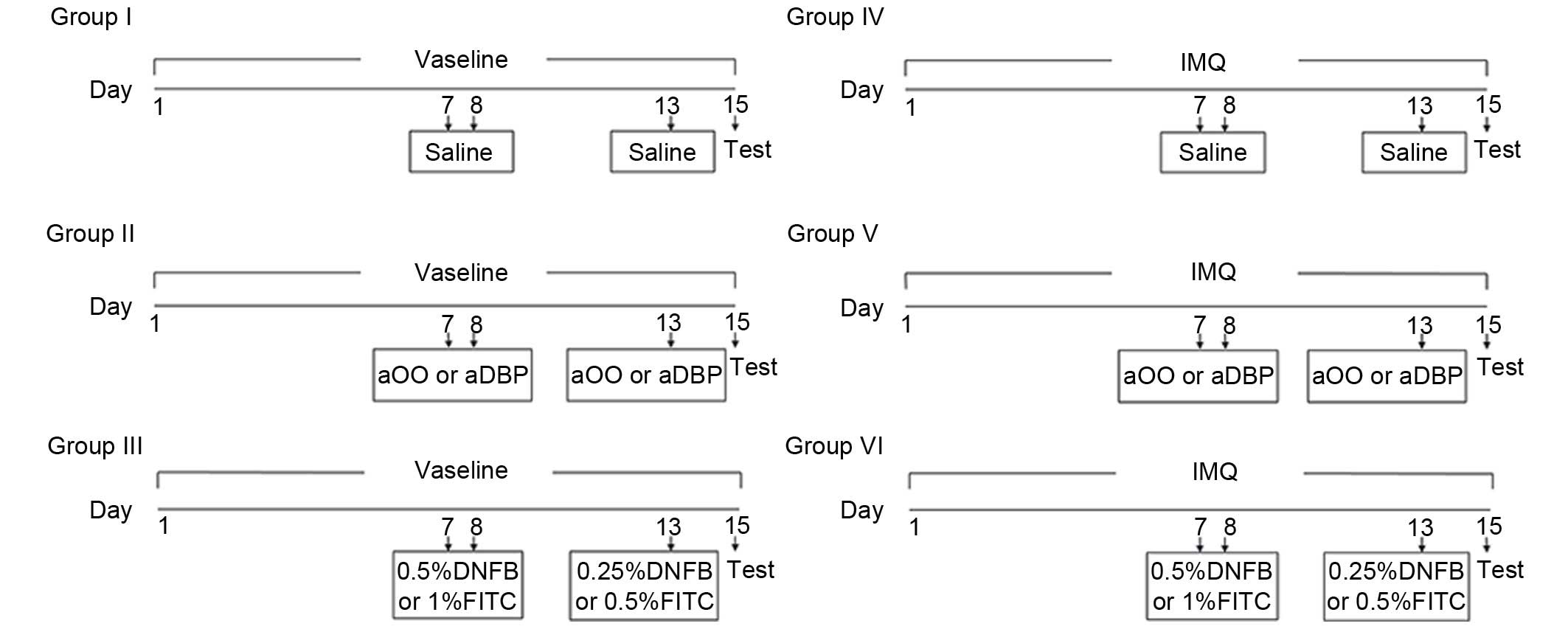 | Figure 1Grouping schedule. Establishment of
Th1-type compound murine model or Th2-type compound murine model.
Vaseline was applied on the shaven backs of the mice in groups I,
II and III for 15 days. IMQ was applied on the shaven backs of mice
in groups IV, V and VI for 15 days. The sensitization was performed
on days 7 and 8 and elicitation on day 13 by applying saline,
solvent, DNFB or FITC solution on the shaven abdomens of mice,
depending on treatment groups. Th1, T helper type 1 cells; Th2, Th
type 2 cells; IMQ, imiquimod; DNFB, dinitrofluorobenzene; FITC,
fluorescein isothiocyanate; aOO, acetone and olive oil; aDBP,
acetone and dibutyl phthalate. |
Scoring severity of skin
inflammation
Based on a previous study (23), the Psoriasis Area and Severity
Index (PASI) was used as a reference for the scoring of IMQ-induced
psoriasis model. The back skin area of the mice treated with IMQ
was excluded from the overall score. Erythema, scaling and
thickening were scored separately on a scale from 0–4: 0, none; 1
slight; 2 moderate; 3 marked; and 4 very marked. The cumulative
score (scale: 0–12), including erythema, scaling and thickening was
used to determine the severity of the inflammation.
Ear swelling and ear weight
calculations
Ear swelling was determined using vernier calipers.
Ear edema was expressed as (R + L) / 2 − (R0 + L0) / 2, where R0
and L0 stand for the thickness of the right and left ear,
respectively, prior to elicitation. R and L stand for the thickness
at 48 h after elicitation. The ears were punched with a trephine of
4 mm diameter, and weighed immediately. Increase in ear thickness
and ear weight was used to indicate the extent of inflammation.
Histology
Following the treatment, after 48 h the mice were
anesthetized using pentobarbital sodium (100 mg/kg,
intraperitoneally; Sigma-Aldrich). Blood samples were collected
from the retro-orbital plexus and the mice were sacrificed by
cervical dislocation, then the ears and back skin were collected
and fixed overnight in 10% formalin at room temperature. The
tissues were then embedded in paraffin, cut into 6 µm
sections, and stained with hematoxylin and eosin. Epidermal
thickness (acanthosis) and the number of infiltrating cells were
assessed using Photoshop CS4 analysis software (Adobe Systems,
Inc., San Jose, CA, USA).
Tissue sample preparation
The ears of the mice were cut and homogenized in 10
ml/g of PBS 48 h after the end of the treatment. Following
centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was
collected and IL-17 levels were detected by ELISA.
Enzyme-linked immunosorbent assay
Serum samples were obtained by centrifugation of the
collected blood samples (5,000 x g, 15 min, 24°C). Serum levels of
IL-17, IL-22, IL-23 and IFN-γ were determined using an ELISA kits
according to the manufacturer's protocols.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mice ears and dorsal
skin biopsies frozen in liquid nitrogen using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
SuperScript III First-Strand Synthesis System (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for cDNA synthesis. The reactions
were performed on a ViiA7 Real Time PCR System (Thermo Fisher
Scientific, Inc.) using FastStart Universal SYBR Green Master
(Roche Diagnostics, Basel, Switzerland). The qPCR reaction was
conducted as follows: 95°C for 10 min; 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The data were normalized to β-actin and the
relative mRNA levels calculated using the 2−ΔΔCq method
(26). Primer sequences used are
indicated in Table I.
 | Table IPrimer sequences used for polymerase
chain reaction. |
Table I
Primer sequences used for polymerase
chain reaction.
| Name | Forward
(5′–3′) | Reverse
(5′–3′) |
|---|
| IFN-γ |
GAAAATCCTGCAGAGCCAGATT |
TGATGGCCTGATTGTCTTTCAA |
| IL-4 |
CCCCAGCTAGTTGTCATCCTG |
CAAGTGATTTTTGTCGCATCCG |
| IL-17 |
CTCCAGAAGGCCCTCAGACTAC |
AGCTTTCCCTCCGCATTGACACAG |
| IL-22 |
CAGCTCCTGTCACATCAGCGGT |
AGGTCCAGTTCCCCAATCGCCT |
| IL-23 |
TCCTCCAGCCAGAGGATCACCC |
AGAGTTGCTGCTCCGTGGGC |
| IL-6 |
CACAAGTCCGGAGAGGAGAC |
CAGAATTGCCATTGCACAAC |
| TNF-α |
GAACTGGCAGAAGAGGCACT |
AGGGTCTGGGCCATAGAACT |
| RORγt |
CCGCTGAGAGGGCTTCAC |
TGCAGGAGTAGGCCACATTACA |
| AHR |
GCCCTTCCCGCAAGATGTTAT |
TCAGCAGGGGTGGACTTTAAT |
| T-bet |
CACTAAGCAAGGACGGCGAA |
CCACCAAGACCACATCCACA |
| β-actin |
TGGAATCCTGTGGCATCCATGAAAC |
TAAAACGCAGCTCAGTAACAGTCCG |
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA, USA).
Data are expressed as the mean ± standard deviation. The
differences between experimental groups were analyzed by one-way
analysis of variance followed by the Student-Newman-Keuls post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of the compound mouse model
of Th1-type CHS combined with IMQ-induced psoriasis-like
inflammation. Ear changes
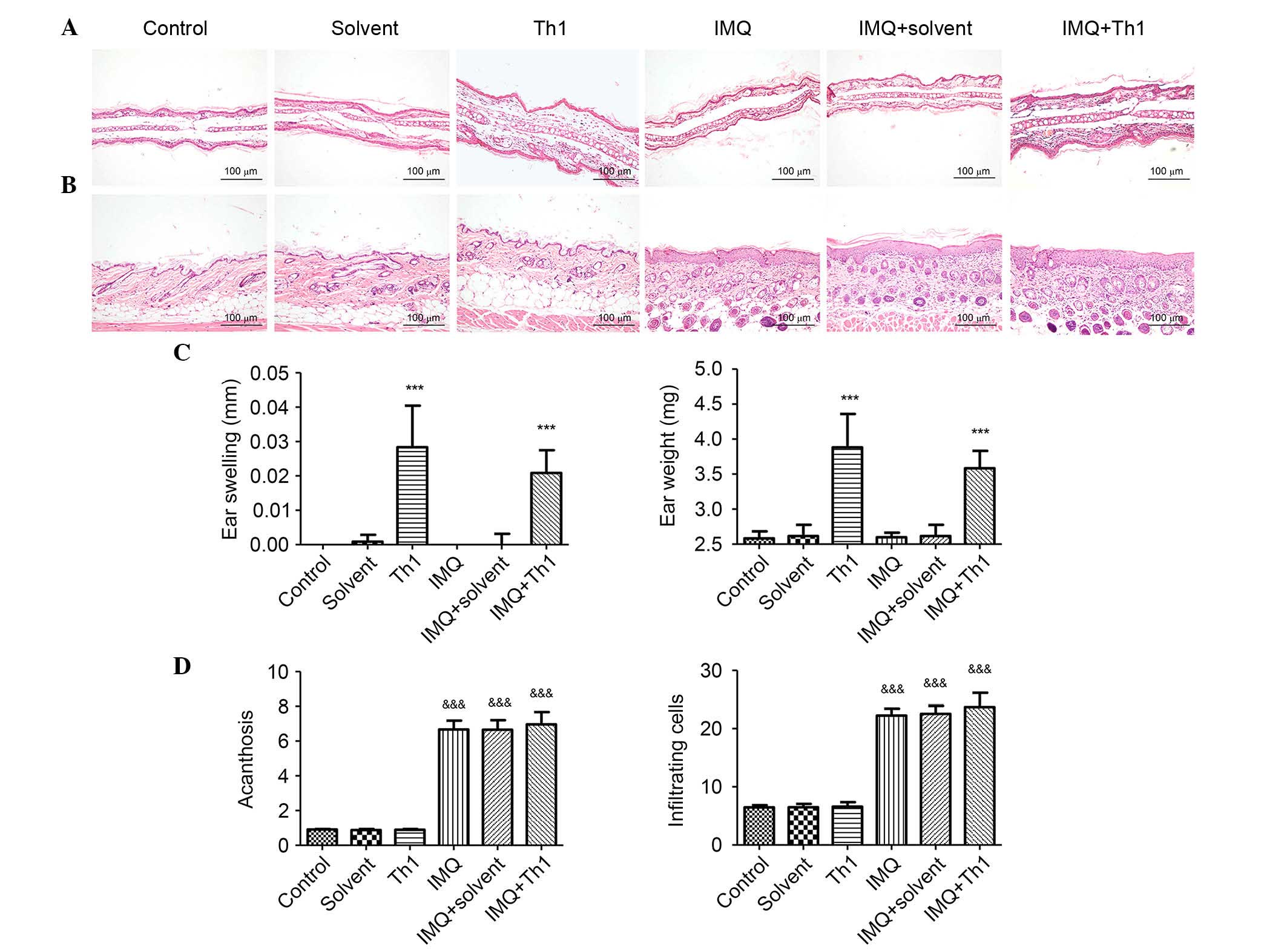
Following elicitation, signs of ear inflammation
such as swelling, capillary expansion and inflammatory cells
infiltration were observed in Th1 and IMQ + Th1 groups. Mice in the
Th1 and IMQ + Th1 groups had similar degrees of ear inflammation.
No obvious changes were observed in the control, solvent, IMQ and
IMQ + solvent groups (Fig. 2). The
ear swelling and ear weight of Th1 and IMQ+Th1 groups were
significantly greater when compared with the control, solvent, IMQ
and IMQ+solvent groups (Fig. 2C;
P<0.001). There was no significant difference between Th1 and
IMQ + Th1 groups (Fig. 2C).
Back skin changes
Similar to human psoriasis, the back skin lesions of
mice in the IMQ, IMQ + solvent and IMQ + Th1 groups presented
acanthosis, extended furcella, hyperkeratosis, parakeratosis and
infiltration of inflammatory cells. The control, solvent and Th1
groups did not demonstrate any signs of inflammation (Fig. 2B). Acanthosis and the number of
infiltrating inflammatory cells in IMQ, IMQ + solvent and IMQ + Th1
groups were significantly increased when compared with the control,
solvent and Th1 groups (Fig. 2D;
P<0.001). There were no significant differences in acanthosis
and the number of infiltrating inflammatory cells between IMQ and
IMQ + Th1 groups. No significant difference was identified between
IMQ, IMQ + solvent and IMQ + Th1 groups (Fig. 2D).
Establishment of the compound mouse model
of Th2-type CHS combined with IMQ-induced psoriasis-like
inflammation. Ear changes
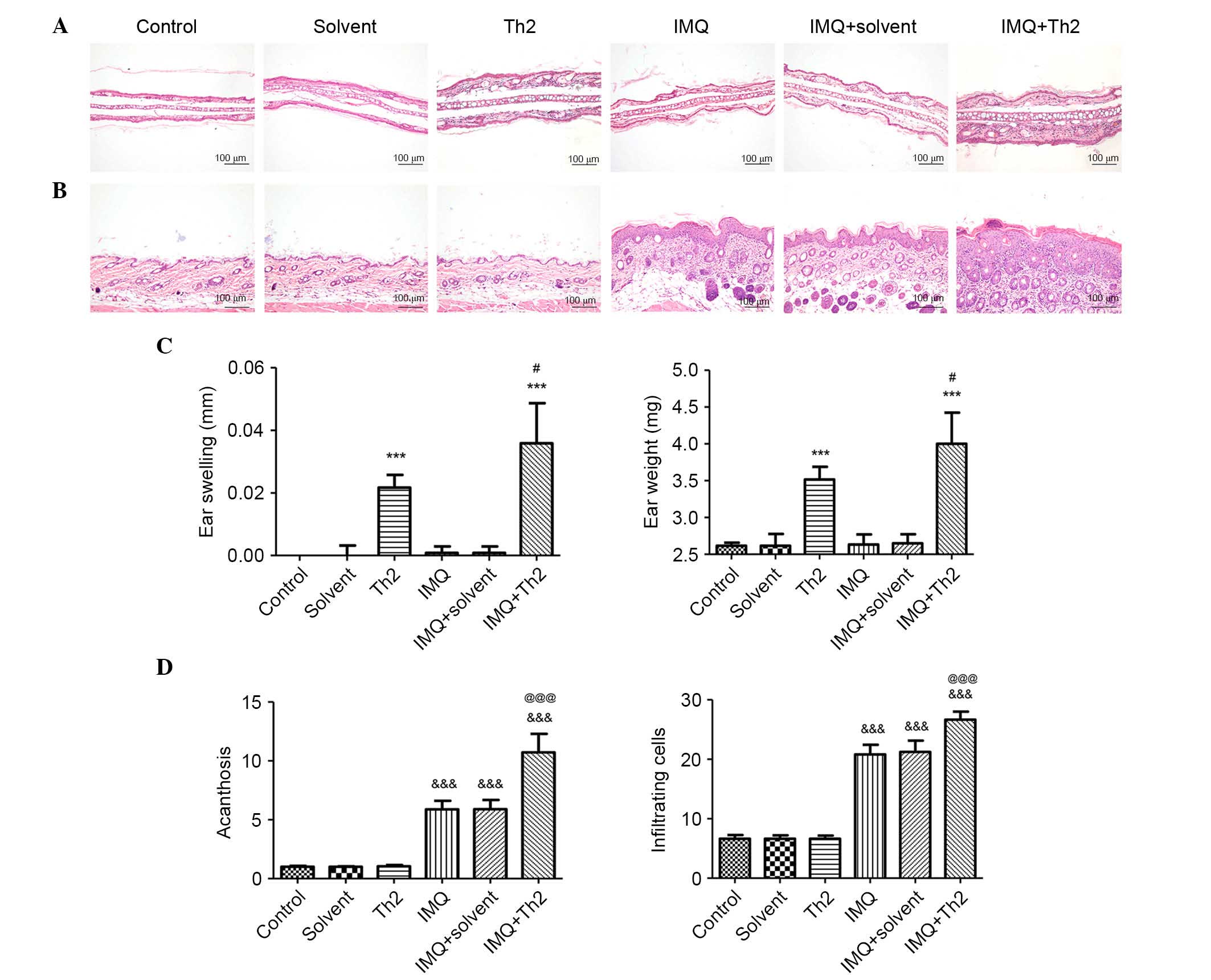
Mice in Th2 and IMQ + Th2 groups did not exhibit ear
inflammatory changes. The ear inflammation in the IMQ+Th2 group was
elevated when compared with the Th2 group (Fig. 3). There were no obvious changes in
the control, solvent, IMQ and IMQ+solvent groups (Fig. 3A). The mice in the Th2 and IMQ+Th2
groups showed increased ear swelling and ear weight when compared
with the control, solvent, IMQ and IMQ+solvent groups (Fig. 3C; P<0.001). The mice in the IMQ
+ Th2 group had enhanced ear swelling and ear weight when compared
with the Th2 group (Fig. 3C;
P<0.05;).
Back skin changes
The IMQ, IMQ + solvent and IMQ + Th2 groups
exhibited psoriasis-like inflammation, whilst the control, solvent
and Th2 groups had no obvious changes (Fig. 3B). Acanthosis and the number of
infiltrating inflammatory cells in the IMQ, IMQ + solvent and
IMQ+Th2 groups were significantly different when compared with the
control, solvent and Th1 groups (Fig.
3D; P<0.001). The IMQ + Th2 group had increased acanthosis
and infiltrating inflammatory cells when compared with the IMQ
group (Fig. 3D; P<0.05).
Comparison of ear inflammation in Th1 and
Th2-type compound mouse models
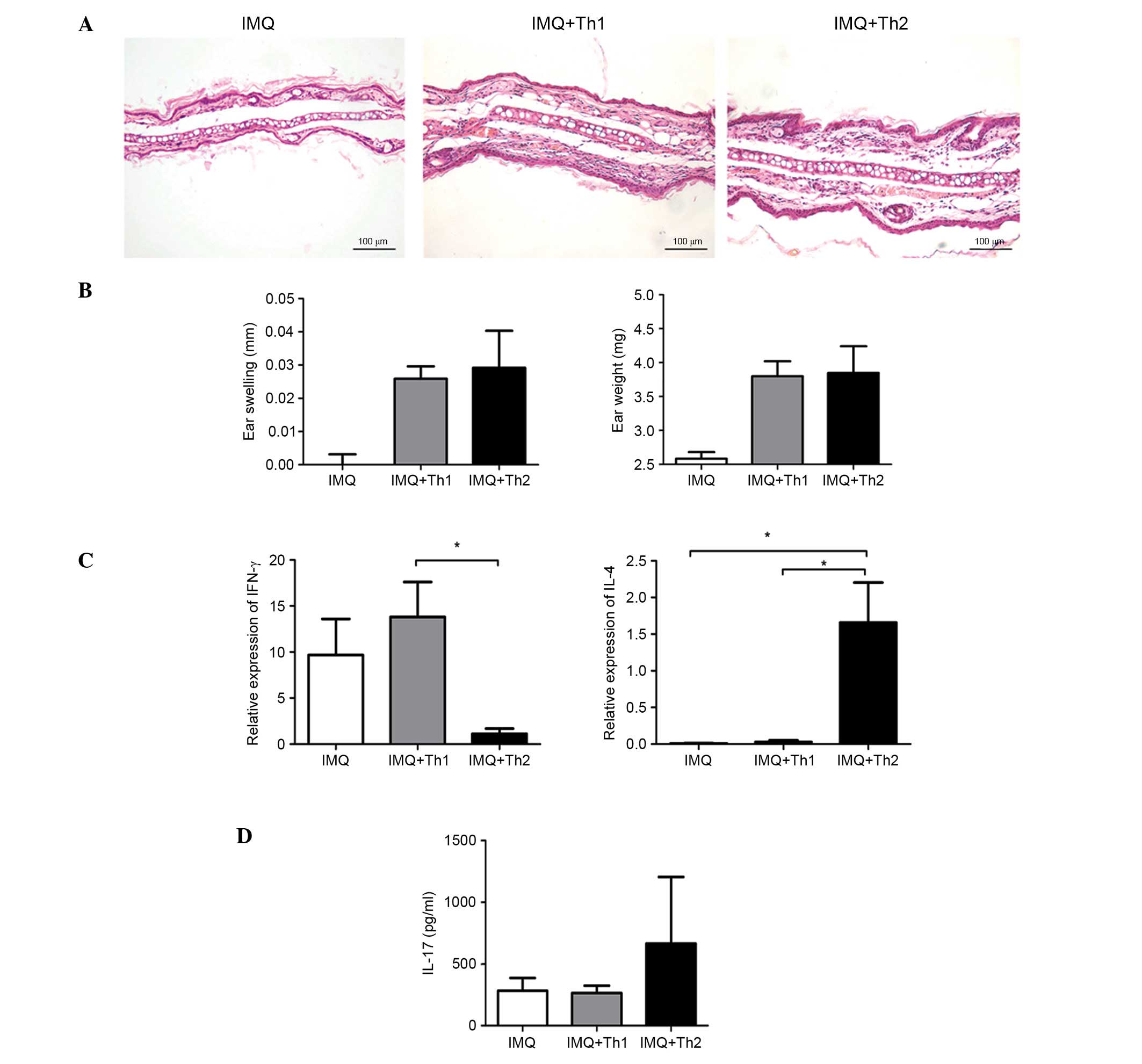
Histological examination indicated that the ear
inflammatory responses in the IMQ + Th1 and IMQ + Th2 groups were
comparable (Fig. 4A). There was no
significant difference in the ear swelling and ear weight between
the two groups (Fig. 4B). The
relative mRNA expression level of IFN-γ in the IMQ + Th1 group was
elevated and significantly higher compared with the IMQ + Th2 group
(Fig. 4C; P<0.05). However, the
difference between the IMQ and IMQ + Th1 groups was not significant
(Fig. 4C). The relative mRNA
expression level of IL-4 in the IMQ + Th2 group was significantly
higher compared with the IMQ and IMQ + Th1 groups (Fig. 4C; P<0.05). The IL-17 level in
the ears of the IMQ + Th2 group was higher compared with the IMQ
and IMQ + Th1 group; however, the difference was not statistically
significant (Fig. 4D).
Comparison of back skin lesions in Th1
and Th2-type compound mouse models
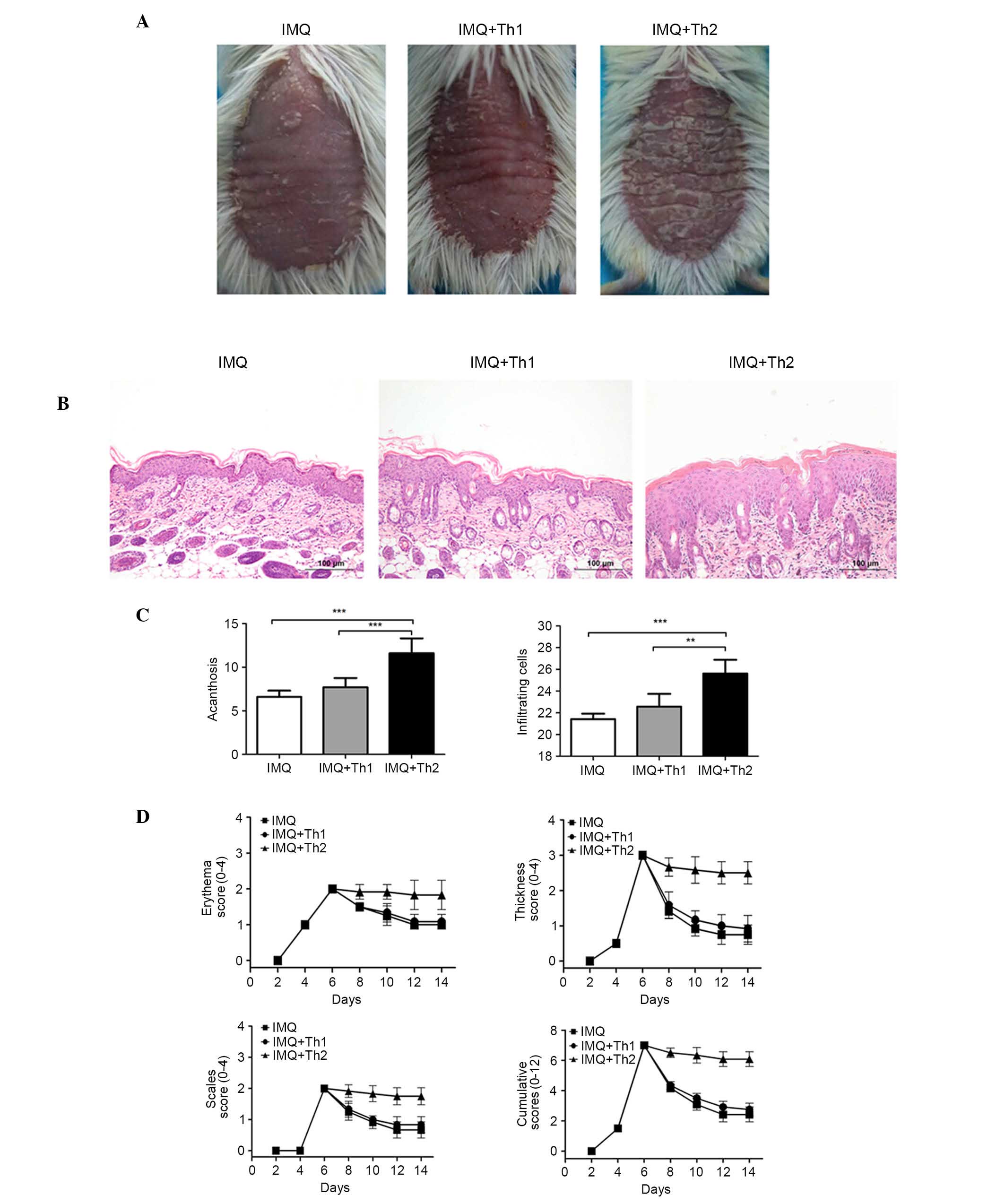
Overall, the back skin of the mice began to resemble
human psoriasis at three days following IMQ application. The
independent and cumulative scores are presented in Fig. 5. The scores of the IMQ, IMQ + Th1
and IMQ + Th2 groups were consistent, and peaked following the
application of IMQ for six consecutive days. Subsequent to the
sensitization at day 7 and 8, the scores of the IMQ and IMQ + Th1
groups began to decline; however, the IMQ + Th2 group still
presented a severe inflammatory response, and high scores (Fig. 5D). The IMQ + Th2 group presented
more severe erythema, scaling and thickness in overall appearance
compared with the IMQ and IMQ + Th1 groups, with increased
acanthosis and inflammatory cell infiltration observed
microscopically (Fig. 5A–C;
P<0.01~0.001). There were no significant differences between the
IMQ and IMQ + Th1 groups.
Comparison of serum cytokine levels in
Th1 and Th2-type compound mouse models
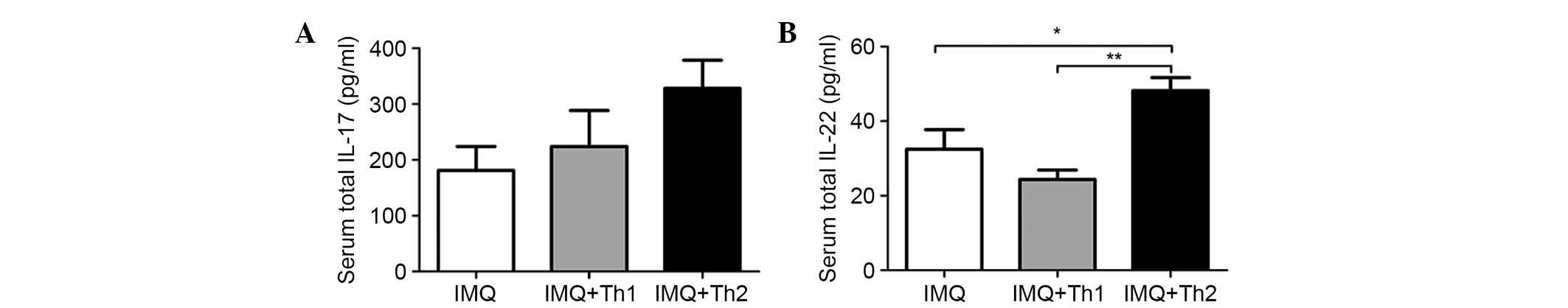
Serum levels of IL-17 and IL-22 in the IMQ + Th2
group were elevated when compared with the IMQ and IMQ + Th1
groups. However, the difference was not significant for the serum
IL-17 levels between the IMQ and IMQ + Th1 groups (Fig. 6A). Serum IL-22 levels in the IMQ +
Th2 group were significantly higher when compared with the IMQ and
IMQ + Th1 groups (Fig. 6B,
P<0.05-0.01). There was no significant difference between the
IMQ and IMQ + Th1 groups. IL-23 and IFN-γ were not detected in
serum.
Comparison of relative mRNA levels of
cytokines and transcription factors in back skin lesions of Th1 and
Th2-type compound mouse models
Relative expression mRNA levels of IL-17, IL-22 in
back skin lesions of the IMQ + Th2 group were significantly higher
compared with the IMQ and IMQ + Th1 groups (Fig. 7A; P<0.05), and there were no
significant differences between the IMQ and IMQ + Th1 groups
(Fig. 7A). Relative mRNA
expression levels of IL-23 and TNF-α were significantly higher in
the IMQ + Th2 group compared with the IMQ group (Fig. 7A; P<0.05). There were no
significant differences identified between the IMQ + Th1 and IMQ +
Th2 groups (Fig. 7A). There was no
significant difference in relative mRNA expression levels of IFN-γ
and IL-6 between the IMQ, IMQ + Th1 and IMQ + Th2 groups (Fig. 7A). Relative mRNA expression levels
of nuclear hormone receptor retinoic acid-related orphan receptor
γt (RORγt), which is a transcription factor specific to Th17 cells,
were significantly elevated in the IMQ + Th2 group when compared
with the IMQ and IMQ + Th1 groups (Fig. 7B; P<0.01~0.001). The relative
mRNA level of RORγt was significantly higher in the IMQ group when
compared with the IMQ + Th1 group (Fig. 7B; P<0.05). The relative mRNA
level of aryl hydrocarbon receptor (AHR), which is the key
transcription factor in Th22 cells, was significantly elevated in
the IMQ + Th2 group compared with the IMQ + Th1 group. There was no
significant difference between the IMQ and IMQ + Th2 groups
(Fig. 7B). There was no
significant difference in the relative mRNA level of T-bet, which
controls IFN-γ production and Th1 cell differentiation, between the
IMQ, IMQ + Th1 and IMQ + Th2 groups (Fig. 7B).
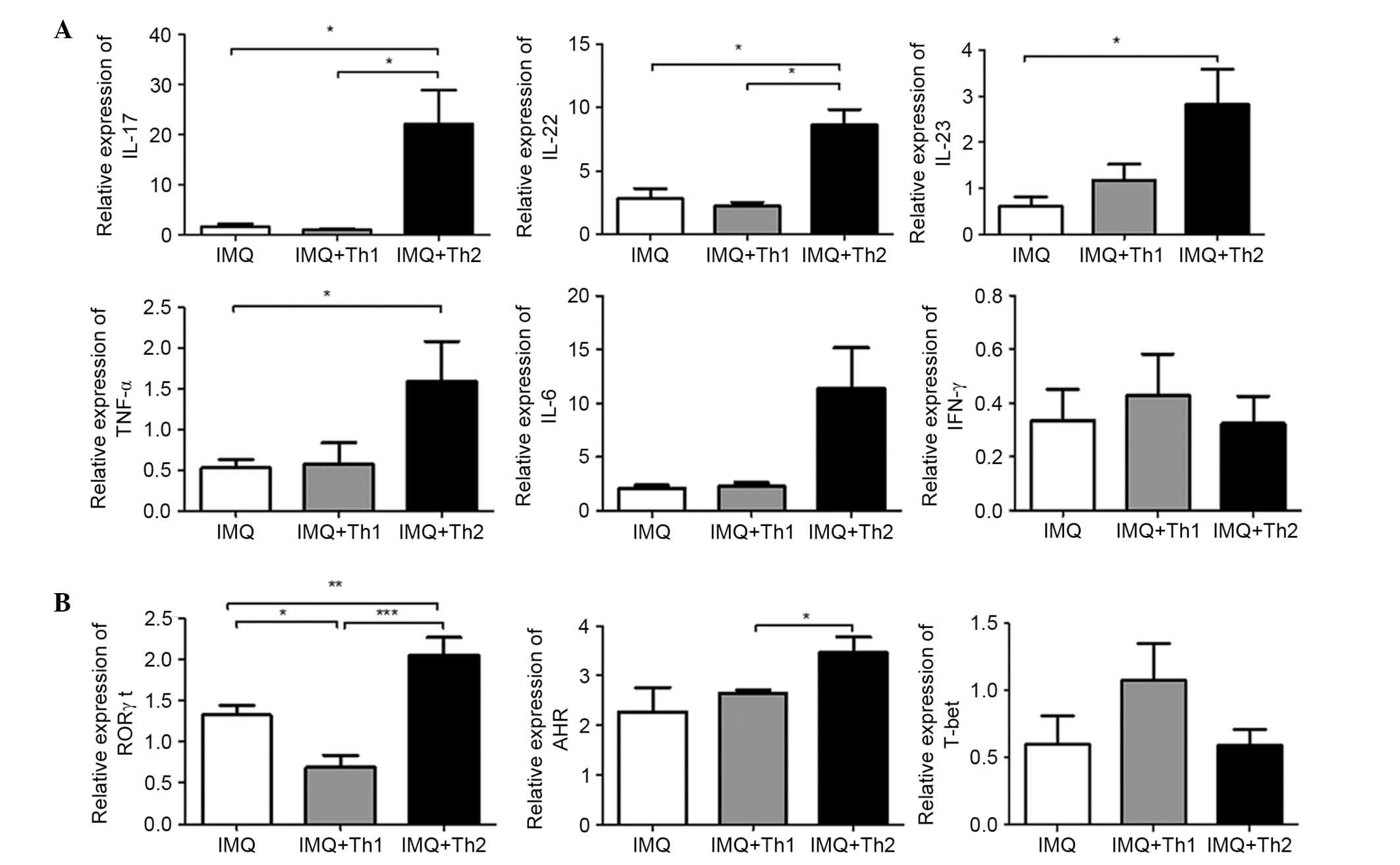 | Figure 7Differences in relative mRNA levels
of cytokines and transcription factors in back skin lesions of Th1
and Th2-type compound mouse models. (A) Relative mRNA levels of
IL-17, IL-22, IL-23, TNF-α, IL-6 and IFN-γ. (B) Relative mRNA
levels of RORγt, AHR and T-bet. *P<0.05,
**P<0.01, ***P<0.001. Th1, T helper
type 1; Th2, Th type 2; IL, interleukin; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ; RORγt, nuclear hormone receptor
retinoic acid-related orphan receptor γ t; AHR, aryl hydrocarbon
receptor; T-bet, T-cell-specific T-box transcription factor; IMQ,
imiquimod. |
Discussion
Based on stable psoriasis-like pathological changes
induced by IMQ, mice were sensitized and challenged with DNFB and
FITC, which can induce the classic Th1 or Th2-type CHS. A total of
six subgroups were established to control for the use of saline,
vaseline and solvent in the treatments. Through the detection of
alterations in morphology, histopathology and cytokines in ear
tissues, it was determined that the Th1 or Th2-type CHS mice
combined with IMQ-induced psoriasis-like inflammation had a
comparable degree of ear inflammation. In the two compound mouse
models, CHS was induced by DNFB or FITC and still maintained the
inherent inflammatory characteristics. High mRNA expression levels
of the Th1 cytokine IFN-γ and low mRNA levels of the Th2 cytokine
IL-4 were detected in the ears of mice from the combined Th1-type
CHS and IMQ-induced psoriasis-like inflammation group. The high
mRNA level of IL-4 and low mRNA level of IFN-γ were detected in the
ears of mice from the combined Th2-type CHS and IMQ-induced
psoriasis-like inflammation group. Back skin of the mice also
exhibited psoriasis-like inflammation. The histological examination
detected epidermal thickening and the infiltration of inflammatory
cells in the derma. These results indicate that the clinical and
pathological features of psoriasis and allergic contact dermatitis
were successfully simulated in the same mouse.
Th17 cells are a novel subset of T helper cells,
mainly producing IL-17 and IL-22 (20). Th17 cells are not only responsible
for the development of autoimmune diseases but also participate in
the pathogenesis of allergic diseases (19,20,27).
Previous studies have demonstrated that Th1 cytokines were
pathogenic in the development of psoriasis, while Th2 cytokines
were protective (28–31). Additionally, studies have indicated
that psoriasis is more inclined to be mediated by Th17 than Th1
(7,32). Th17 and the IL-23/IL-17 axis are
important for the pro-inflammatory response by coordinating various
inflammatory mediators, including IL-6 and TNF-α (33,34).
A previous study reported that IL-17A induced Th2 inflammation in
acute disease, and Th2 differentiation from naive T cells was
promoted in vitro by the addition of IL-17A (14). The present study indicated that the
mice with combined Th2-type CHS and IMQ-induced psoriasis-like
inflammation had marked ear inflammation, histological changes,
increased intra- and inter-cellular edema, and greater infiltration
of inflammatory cells compared with mice with Th2-type CHS. This
may be due to Th2-type CHS being exacerbated in the Th17
inflammatory microenvironment presented by IMQ-induced
psoriasis-like inflammation.
Previous studies have confirmed that IL-4 is
protective in psoriasis, and participates in the negative
regulation of Th17 cells (28,35).
Therefore, it was hypothesized that Th2-type CHS may relieve
psoriasis-like inflammation. By contrast, the results of the
present study indicate that Th2 CHS exacerbated the psoriasis-like
inflammation induced by IMQ. Although IL-4 participates in the
downregulation of Th17, and inhibits the differentiation of Th17
cells, it has also been determined that Th17 cells may develop
despite robust Th2 responses (20). The current study determined that
the psoriasis-like inflammation was more serious in the mice
treated with IMQ combined with Th2-type CHS when compared with mice
treated with IMQ + saline. Following sensitization with FITC in the
abdomen, the IMQ-treated skin exhibited sustained inflammatory
responses and high scores, which demonstrated the deteriorating
effect of Th2-type CHS on psoriasis-like inflammation initiated
from the sensitization phase. The histological examination
indicated that the mice treated with IMQ combined with Th2-type CHS
presented thicker epidermis and high infiltration of inflammatory
cells compared with mice treated with IMQ + saline, which indicated
the persistence and amplification of the psoriasis-like
inflammation. High mRNA levels of the Th17 transcription factor,
RORγt, and the associated cytokines, IL-17, IL-22, IL-23 and TNF-α,
were detected in the psoriasis-like skin lesions. High levels of
IL-17 and IL-22 were detected in the serum. However, no obvious
changes in mRNA levels of Th1 cell transcription factors, T-bet and
IFN-γ, were observed. This confirmed that the IL-23/IL-17 axis of
IMQ-induced psoriatic inflammation was activated by combining with
Th2-type CHS, resulting in the exacerbation and amplification of
the psoriasis-like inflammation. In a previous study, high levels
of thymic stromal lymphopoietin (TSLP), a Th2-polarization
cytokine, were detected in the skin lesions of psoriasis patients.
TSLP was identified to act synergistically with CD40L and aggravate
psoriasis via the promotion of the production of IL-23 (36). Therefore, it is possible that the
Th2-associated allergic inflammatory response and psoriasis are not
antagonistic. On the contrary, a certain degree of synergistic
relationship is observed between Th17 and the IL-23/IL-17 axis;
however, the mechanisms remain to be elucidated.
DNFB-induced Th1 CHS has no evident influence on
IMQ-treated psoriasis-like inflammation. No differences were
observed in the psoriasis-like skin lesions between the mice
treated with IMQ + saline and the mice treated with IMQ combined
with Th1-type CHS. They had similar skin thickness and comparable
levels of infiltration of inflammatory cells. Compared to the the
mice treated with IMQ + saline, the mice treated with IMQ combined
with Th1-type CHS did not exhibit increased mRNA levels of Th17
transcription factors and associated cytokines in the
psoriasis-like lesions or elevated IL-17 and IL-22 levels in serum.
Compared with the IMQ + saline group, the IL-23/IL-17 axis was not
influenced, indicating that psoriasis is inclined to be mediated by
Th17, and Th1 does not served a pivotal role compared with Th17.
There was no alteration to the IFN-γ/Th1 response, which may be due
to the neutralization of cytokine interactions.
Previous epidemiological studies have reported
negative associations between atopy and multiple sclerosis,
rheumatoid arthritis and type 1 diabetes (1,27).
Autoimmune inflammatory responses tend to suppress the development
of atopy, and atopy may suppress the severity of autoimmunity
(27). With respect to psoriasis,
previous studies have determined that T lymphocyte function and
hapten sensitization were impaired, and the susceptibility to
develop contact allergy were decreased in patients with psoriasis
(1,37,38).
By contrast, previous studies have demonstrated that contact
dermatitis was common in patients with psoriasis and there were no
statistically significant differences in the contact
hypersensitivity and its frequency between plaque psoriasis
patients and control groups (21,22).
An increased incidence of contact allergy has been observed in
patients with palmar-plantar psoriasis and atopy was associated
with the protection against the development of psoriatic arthritis
(39,40). The results of the current study
indicate that different types of CHS differentially influence
psoriasis-like inflammation. DNFB-induced Th1-type CHS had no
obvious impact on IMQ-treated psoriasis-like inflammation. However,
FITC-induced Th2-type CHS exacerbated IMQ-treated psoriasis-like
inflammation. Therefore, solely relying on the Th1/Th2 paradigm to
explain the relationship between psoriasis and atopic disease is an
oversimplification of the disease. It is possible that different
sensitizers affect different inflammatory pathways, and
differentially influence psoriasis. Dhingra et al (15) identified unique pathways that were
preferentially activated by different allergens, which are relevant
clinical sensitizers in humans, and determined that exposure to
fragrance, and to a lesser extent rubber, resulted in mixed and
classic 'allergic' Th2 polarization. Fragrance mix had 100% current
relevance as an aggravating factor of psoriasis, and patients with
psoriasis had significant sensitivity to fragrance compared with
general dermatology outpatients (41,42).
Taken together, these previous studies support the current
findings. Therefore, it is recommended that patients with psoriasis
should avoid contact with specific sensitizers, such as fragrance
and rubber products. We hypothesize that psoriasis and contact
dermatitis are not completely independent diseases. They have
intrinsic links and Th17-associated pathways may be a point of
convergence between the two diseases.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant nos. 30872271
and 81171507).
Abbreviations:
|
ACD
|
allergic contact dermatitis
|
|
IMQ
|
imiquimod
|
|
CHS
|
contact hypersensitivity
|
|
PASI
|
psoriasis area and severity index
|
|
DNFB
|
dinitrofluorobenzene
|
|
FITC
|
fluorescein isothiocyanate
|
|
IL
|
interleukin
|
References
|
1
|
Bangsgaard N, Engkilde K, Thyssen JP,
Linneberg A, Nielsen NH, Menné T, Skov L and Johansen JD: Inverse
relationship between contact allergy and psoriasis: Results from a
patient- and a population-based study. Br J Dermatol.
161:1119–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guttman-Yassky E, Nograles KE and Krueger
JG: Contrasting pathogenesis of atopic dermatitis and
psoriasis-part I: Clinical and pathologic concepts. J Allergy Clin
Immunol. 127:1110–1118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guttman-Yassky E, Nograles KE and Krueger
JG: Contrasting pathogenesis of atopic dermatitis and
psoriasis-part II: Immune cell subsets and therapeutic concepts. J
Allergy Clin Immunol. 127:1420–1432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Camargo CM, Brotas AM, Ramos-e-Silva M and
Carneiro S: Isomorphic phenomenon of Koebner: Facts and
controversies. Clin Dermatol. 31:741–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurz C, Wunderlich S, Spieler D, Schwaiger
BJ, Andres C, Traidl-Hoffmann C and Ilg R: Acute transverse
myelitis and psoriasiform dermatitis associated with Sjoegren's
syndrome: A case report. BMC Res Notes. 7:5802014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain S, Kaur IR, Das S, Bhattacharya SN
and Singh A: T helper 1 to T helper 2 shift in cytokine expression:
An autoregulatory process in superantigen-associated psoriasis
progression? J Med Microbiol. 58:180–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin DA, Towne JE, Kricorian G, Klekotka
P, Gudjonsson JE, Krueger JG and Russell CB: The emerging role of
IL-17 in the pathogenesis of psoriasis: Preclinical and clinical
findings. J Invest Dermatol. 133:17–26. 2013. View Article : Google Scholar
|
|
8
|
Lynde CW, Poulin Y, Vender R, Bourcier M
and Khalil S: Interleukin 17A: Toward a new understanding of
psoriasis pathogenesis. J Am Acad Dermatol. 71:141–150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michalak-Stoma A, Bartosińska J, Kowal M,
Juszkiewicz-Borowiec M, Gerkowicz A and Chodorowska G: Serum levels
of selected Th17 and Th22 cytokines in psoriatic patients. Dis
Markers. 35:625–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakajima K: Critical role of the
interleukin-23/T-helper 17 cell axis in the pathogenesis of
psoriasis. J Dermatol. 39:219–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Works MG, Yin F, Yin CC, Yiu Y, Shew K,
Tran TT, Dunlap N, Lam J, Mitchell T, Reader J, et al: Inhibition
of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like
dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J Immunol.
193:3278–3287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Honda T, Egawa G, Grabbe S and Kabashima
K: Update of immune events in the murine contact hypersensitivity
model: Toward the understanding of allergic contact dermatitis. J
Invest Dermatol. 133:303–315. 2013. View Article : Google Scholar
|
|
13
|
Sugaya M, Kuwano Y, Suga H, Miyagaki T,
Ohmatsu H, Kadono T, Okochi H, Blauvelt A, Tamaki K and Sato S:
Lymphatic dysfunction impairs antigen-specific immunization, but
augments tissue swelling following contact with allergens. J Invest
Dermatol. 132:667–676. 2012. View Article : Google Scholar
|
|
14
|
Nakajima S, Kitoh A, Egawa G, Natsuaki Y,
Nakamizo S, Moniaga CS, Otsuka A, Honda T, Hanakawa S, Amano W, et
al: IL-17A as an inducer for Th2 immune responses in murine atopic
dermatitis models. J Invest Dermatol. 134:2122–2130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dhingra N, Shemer A, Correa da Rosa J,
Rozenblit M, Fuen-tes-Duculan J, Gittler JK, Finney R, Czarnowicki
T, Zheng X, Xu H, et al: Molecular profiling of contact dermatitis
skin identifies allergen-dependent differences in immune response.
J Allergy Clin Immunol. 134:362–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hopkins JE, Naisbitt DJ, Kitteringham NR,
Dearman RJ, Kimber I and Park BK: Selective haptenation of cellular
or extra-cellular protein by chemical allergens: Association with
cytokine polarization. Chem Res Toxicol. 18:375–381. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogawa A, Yoshizaki A, Yanaba K, Ogawa F,
Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, et
al: The differential role of L-selectin and ICAM-1 in Th1-type and
Th2-type contact hypersensitivity. J Invest Dermatol.
130:1558–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Larsen JM, Bonefeld CM, Poulsen SS,
Geisler C and Skov L: IL-23 and T (H)17-mediated inflammation in
human allergic contact dermatitis. J Allergy Clin Immunol.
123:486–492. 2009. View Article : Google Scholar
|
|
19
|
van Beelen AJ, Teunissen MB, Kapsenberg ML
and de Jong EC: Interleukin-17 in inflammatory skin disorders. Curr
Opin Allergy Clin Immunol. 7:374–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Louten J, Boniface K and de Waal Malefyt
R: Development and function of TH17 cells in health and disease. J
Allergy Clin Immunol. 123:1004–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jovanović M, Boza P, Karadaglić D, Brkić
S, Petrović A, Mimica-Dukić N, Anackov G and Poljacki M: Contact
sensitivity in patients with psoriasis in Vojvodina. Int Arch
Allergy Immunol. 148:311–320. 2009. View Article : Google Scholar
|
|
22
|
Pigatto PD: Atopy and contact
sensitization in psoriasis. Acta Derm Venereol Suppl (Stockh).
19–20. 2000. View Article : Google Scholar
|
|
23
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin S, Wen J, Bai XC, Chen TY, Zheng RC,
Zhou GB, Ma J, Feng JY, Zhong BL and Li YM: Endogenous n-3
polyunsaturated fatty acids protect against imiquimod-induced
psoriasis-like inflammation via the IL-17/IL-23 axis. Mol Med Rep.
9:2097–2104. 2014.PubMed/NCBI
|
|
25
|
Onoue A, Kabashima K, Kobayashi M, Mori T
and Tokura Y: Induction of eosinophil- and Th2-attracting epidermal
chemokines and cutaneous late-phase reaction in tape-stripped skin.
Exp Dermatol. 18:1036–1043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Rabin RL and Levinson AI: The nexus
between atopic disease and autoimmunity: A review of the
epidemiological and mechanistic literature. Clin Exp Immunol.
153:19–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghoreschi K, Weigert C and Röcken M:
Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol.
25:574–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai Y, Fleming C and Yan J: New insights
of T cells in the pathogenesis of psoriasis. Cell Mol Immunol.
9:302–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Robaee AA, Al-Zolibani AA, Al-Shobili
HA, Kazamel A and Settin A: IL-10 implications in psoriasis. Int J
Health Sci (Qassim). 2:53–58. 2008.
|
|
31
|
Weigert C, Röcken M and Ghoreschi K:
Interleukin 4 as a potential drug candidate for psoriasis. Expert
Opin Drug Discov. 3:357–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishimoto S, Kotani H, Tsuruta S, Shimizu
N, Ito M, Shichita T, Morita R, Takahashi H, Amagai M and Yoshimura
A: Th17 cells carrying TCR recognizing epidermal autoantigen induce
psoriasis-like skin inflammation. J Immunol. 191:3065–3072. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh TP, Schön MP, Wallbrecht K,
Michaelis K, Rinner B, Mayer G, Schmidbauer U, Strohmaier H, Wang
XJ and Wolf P: 8-methoxypsoralen plus ultraviolet A therapy acts
via inhibition of the IL-23/Th17 axis and induction of
Foxp3+regulatory T cells involving CTLA4 signaling in a
psoriasis-like skin disorder. J Immunol. 184:7257–7267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowes MA, Russell CB, Martin DA, Towne JE
and Krueger JG: The IL-23/T17 pathogenic axis in psoriasis is
amplified by keratinocyte responses. Trends Immunol. 34:174–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakagawa R, Yoshida H, Asakawa M, Tamiya
T, Inoue N, Morita R, Inoue H, Nakao A and Yoshimura A: Pyridone 6,
a pan-JAK inhibitor, ameliorates allergic skin inflammation of
NC/Nga mice via suppression of Th2 and enhancement of Th17. J
Immunol. 187:4611–4620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Volpe E, Pattarini L, Martinez-Cingolani
C, Meller S, Donnadieu MH, Bogiatzi SI, Fernandez MI, Touzot M,
Bichet JC, Reyal F, et al: Thymic stromal lymphopoietin links
keratinocytes and dendritic cell-derived IL-23 in patients with
psoriasis. J Allergy Clin Immunol. 134:373–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moss C, Friedmann PS and Shuster S:
Impaired contact hypersensitivity in untreated psoriasis and the
effects of photochemotherapy and dithranol/UV-B. Br J Dermatol.
105:503–508. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bangsgaard N, Engkilde K, Menné T,
Løvendorf M, Jacobsen GK, Olsen J and Skov L: Impaired hapten
sensitization in patients with autoimmune disease. Clin Exp
Immunol. 165:310–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hajdarbegovic E, Nijsten T, Westgeest A,
Habraken F, Hollestein L and Thio B: Decreased prevalence of atopic
features in patients with psoriatic arthritis, but not in psoriasis
vulgaris. J Am Acad Dermatol. 68:270–277. 2013. View Article : Google Scholar
|
|
40
|
Caca-Biljanovska N, V'Lckova-Laskoska M,
Balabanova-Stefanova M and Grivceva-Panovska V: Frequency of
delayed-type hypersensitivity to contact allergens in palmo-plantar
psoriasis. Prilozi. 26:131–141. 2005.
|
|
41
|
Krupashankar DS and Manivasagam SR:
Prevalence and relevance of secondary contact sensitizers in
subjects with psoriasis. Indian Dermatol Online J. 3:177–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Malhotra V, Kaur I, Saraswat A and Kumar
B: Frequency of patch-test positivity in patients with psoriasis: A
prospective controlled study. Acta Derm Venereol. 82:432–435. 2002.
View Article : Google Scholar
|