Introduction
Diabetes-mediated renal interstitial fibrosis is an
important event in the development of diabetic kidney disease (DKD)
(1). High glucose (HG) promotes
the excessive accumulation of extracellular matrix (ECM) proteins
and expression of fibrotic factors in mesangial cells (MCs), which
leads to subsequent diabetic renal dysfunction (2). A variety of signaling molecules and
pathways are associated with mesangial matrix proliferation,
including ras homolog family member A (RhoA), a small GTPase
protein. RhoA enhances actin cytoskeleton reorganization and
endothelial cell barrier permeability (3,4).
Several studies have suggested that RhoA and Rho kinases are
critical in the pathogenesis of DKD through glomerular sclerosis
and ECM deposition, however the precise mechanism remains poorly
understood (3,4).
The Rho GTPases RhoA, ras-related C3 botulinum toxin
substrate 1 (Rac1) and cell division cycle 42 (Cdc42) are small
molecular switches that contribute to the control of cell
morphology and motility (5). RhoA
is known to regulate actomyosin-based contractility and retraction
through the Rho-kinase pathway, and is also associated with cell
apoptosis and proliferation (6).
Previous reports have observed that RhoA is localized to the plasma
membrane microdomains, caveolae, in cardiomyocytes and endothelial
cells (7). The activation of RhoA
and its downstream mediator Rho-kinase is a crucial step of the
strain-induced production and secretion of fibronectin (FN) matrix
protein in MCs, which depends on functional caveolae (8).
Caveolae are a specialized subset of lipid rafts
that are most abundant in terminally differentiated cell types
(9). Caveolin-1, the principal
residual protein of the caveolae structure, functions as a
scaffolding protein and directly interacts with various signaling
kinases (10). Endogenous
caveolin-1 depletion is associated with reduced v-akt murine
thymoma viral oncogene homolog (AKT) and extracellular regulated
mitogen-activated protein kinase 1/2 (ERK1/2) phosphorylation, and
impaired tube formation in vascular endothelial and smooth muscle
cells (11). Additionally, high
glucose has been demonstrated to alter the caveolar protein
localization of RhoA, and increase the activation of
phosphatidylinositol 3-kinase/Akt, mitogen-activated protein kinase
(MAPK), and AMP-activated protein kinase signaling cascades in
cardiomyocytes (12). However, the
association of caveolae/caveolin-1 with HG-induced dysfunction of
MCs has not been assessed. Therefore, the current study explored
the potential function of caveolae in RhoA signaling activation by
HG and its importance in ECM accumulation in MCs.
Materials and methods
Cell culture
This study was approved by the ethics committee of
Zhejiang Provincial People's Hospital (Hangzhou, China). A total of
10 Sprague-Dawley rats (weight, 230–250 g) were bred in the
Zhejiang Key Laboratory of Experimental Animal and Safety
Evaluation (Hangzhou, China). These rats were maintained in a room
with controlled temperature (22°C) and a reverse 12-h light/dark
cycle. They were supplied with food and water ad libitum.
Primary MCs were obtained from the glomeruli of the rats by
differential sieving as previously described (13) and cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 20% fetal calf serum (FCS;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
streptomycin (100 µg/ml; Dalian Meilun Biotech Co., Ltd.,
Dalian, China) and penicillin (100 U/ml; Dalian Meilun Biotech Co.,
Ltd.) at 37°C in 95% air, 5% CO2. Experiments were
performed using cells between passages 6 and 15. Following
pre-incubation in DMEM supplemented with 0.1% FCS for 24 h, the
confluent MCs were treated with HG (40 mM; Sigma-Aldrich, St.
Louis, MO, USA). Pharmacological inhibitors were purchased from
Sigma-Aldrich and added at the following concentrations and times
prior to HG stimulation: HL07, 10 µM for 60 min; filipin
III, 2.5 µg/ml for 10 min; and SU6656, 10 µM for 30
min.
Western blotting
The experiments for the immunoblotting were
performed at least 3 times. MCs were lysed with
radioimmunoprecipitation assay (Beyotime Institute of
Biotechnology, Haimen, China) lysis buffer containing
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology) protease inhibitor. Protein concentrations were
determined using the bicinchoninic acid method (Beyotime Institute
of Biotechnology, Haimen, China). Proteins from total cell lysates
were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), transferred to a polyvinylidene
fluoride (PVDF) membrane, blocked in 5% non-fat milk in
Tris-buffered saline with Tween-20 (Sigma-Aldrich) at 25°C for 2 h.
The antibodies used mouse monoclonal anti-FN (cat. no. 610077;
1:5,000) and mouse anti-phospho-caveolin-1 Y14 (cat. no. 611339,
1:1,000) (BD Biosciences, Franklin Lakes, NJ, USA); mouse
monoclonal anti-RhoA (cat. no. sc-418; 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); mouse monoclonal
anti-caveolin-1 (cat. no. 05-762 clone 7C8; 1:1,000; Upstate
Biotechnology, Inc., Lake Placid, NY, USA); polyclonal
phospho-SrcY416 (1:1,000; cat. no. 2102) and polyclonal Src
(1:1,000; cat. no. 2109) (Cell Signaling Technology, Inc., Danvers,
MA, USA); and mouse monoclonal anti-β-actin (cat. no. A5441;
1:5,000; Sigma-Aldrich). Actin and total RhoA were used as the
internal reference to calculate the relative intensity of objective
protein bands. Goat anti-mouse IRDye 680LT (926-68050) and goat
anti-rabbit IRDye 800CW (925-32211) secondary antibodies (LI-COR
Biotechnology, Lincoln, NE, USA) were diluted 1:20,000 with
blocking buffer with 0.1% Tween-20 and incubated in the dark at
25°C for 1 h. Finally, the PVDF membrane was observed using the
Odyssey Classic imager (LI-COR Biotechnology) and associated Image
Pro analysis 3.1.4 software (Media Cybernetics Rockville, MD,
USA).
RhoA pulldown assay
MCs were lysed in hypertonic buffer (30 mM HEPES,
1.5 mM MgCl2, 450 mM NaCl, 0.3 mM EDTA and 10%
glycerol), and RhoA-GTP was immunoprecipitated from the cleared
lysate with 25 µl glutathione-S-transferase-tagged
rhotekin-RhoA-binding domain bound to glutathione-agarose
(Cytoskeleon, Inc., Denver, CO, USA). Beads were washed, and the
immunoprecipitate was resolved with 15% SDS-PAGE. Membranes were
probed with monoclonal anti-RhoA antibody (1:500, Santa Cruz
Biotechnology, Inc.). The lysate (40 µg) was also probed for
RhoA to ensure equal loading across conditions.
Transfection
Rat MCs were transiently transfected with caveolin-1
siRNA (sc-29942; Santa Cruz Biotechnology, Inc.) using the
X-tremeGene siRNA transfection reagent (Roche Applied Science,
Penzberg, Germany) according to the manufacturer's protocol. The
transfected MCs were assayed 24–48 h post-transfection.
The rat caveolin-1 coding sequence was amplified
from MC cDNA. Briefly, MC RNA was extracted using TRIzol reagent
(Takara Bio Inc., Otsu, Japan) and 2 µg was
reverse-transcribed with Superscript II (Takara Bio Inc.) according
to the manufacturer's instructions. Samples were incubated at 95°C
for 3 min, followed by 30 cycles of 95°C for 1 min, 57°C for 1 min
and 72°C for 1 min. The resulting cDNA was used for
semiquantitative PCR amplification of caveolin-1, using β-actin as
an internal control. The sequence was inserted into the vector
pEGFP-C1 using the restriction sites HindIII-BamHI
(6084-1, Clontech Laboratories Inc., Mountain View, CA, USA) with
an NH2-terminal enhanced green fluorescent protein (EGFP) for use
as caveolin-1 rescue. Using the plasmid as a template, Y14 of
caveolin-1 was mutated to alanine with the QuikChange II
Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara,
CA, USA). Templates were incubated at 95°C for 1 min, then for 20
cycles of 95°C for 1 min, 65°C for 4 min, and 68°C for 12 min. The
muta-genic primers were as follows: Sense,
5′-TCGGAGGGACATCTCGCCACCGTTCCCATCCG-3′ and anti-sense,
5′-CGGATGGGAACGGTGGCGAGATGTCCCTCCGA-3′. Rat MCs were transfected
with empty vector or pEGFP-Cav-1Y14A using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). At 18–24 h
post-transfection, the medium was changed to 0% FCS and the
experiment continued.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Comparing
several groups was conducted using analysis of variance with
Fisher's Least Significant Difference test. P<0.05 was
considered to indicate a statistically significant difference. Data
represent the mean ± standard error of three independent
experiments.
Results
HG-induced FN upregulation requires
RhoA/Rho kinase activation
Exposure of rat glomerular MCs to HG was
demonstrated to increase the protein expression level of
intracellular FN at all time points compared with the 0 h control
(P<0.05; Fig. 1A). The current
study also analyzed FN secretion following HG stimulation. The
levels of extracellular FN were increased significantly following
stimulation at all time points, compared with the 0 h control
(P<0.05; Fig. 1A). The
increased FN secretion was accompanied by the activation of RhoA
and an increase in total RhoA in rat MCs, compared with untreated
control cells (P<0.05; Fig.
1B). To investigate the activity of RhoA on HG-induced FN
secretion, rat MCs were pretreated with RhoA inhibitor, HL07. It
was observed that HL07 treatment decreased the FN protein levels
compared with HG treatment (P<0.05), demonstrating that RhoA is
required for HG-induced FN upregulation in MCs (Fig. 1B).
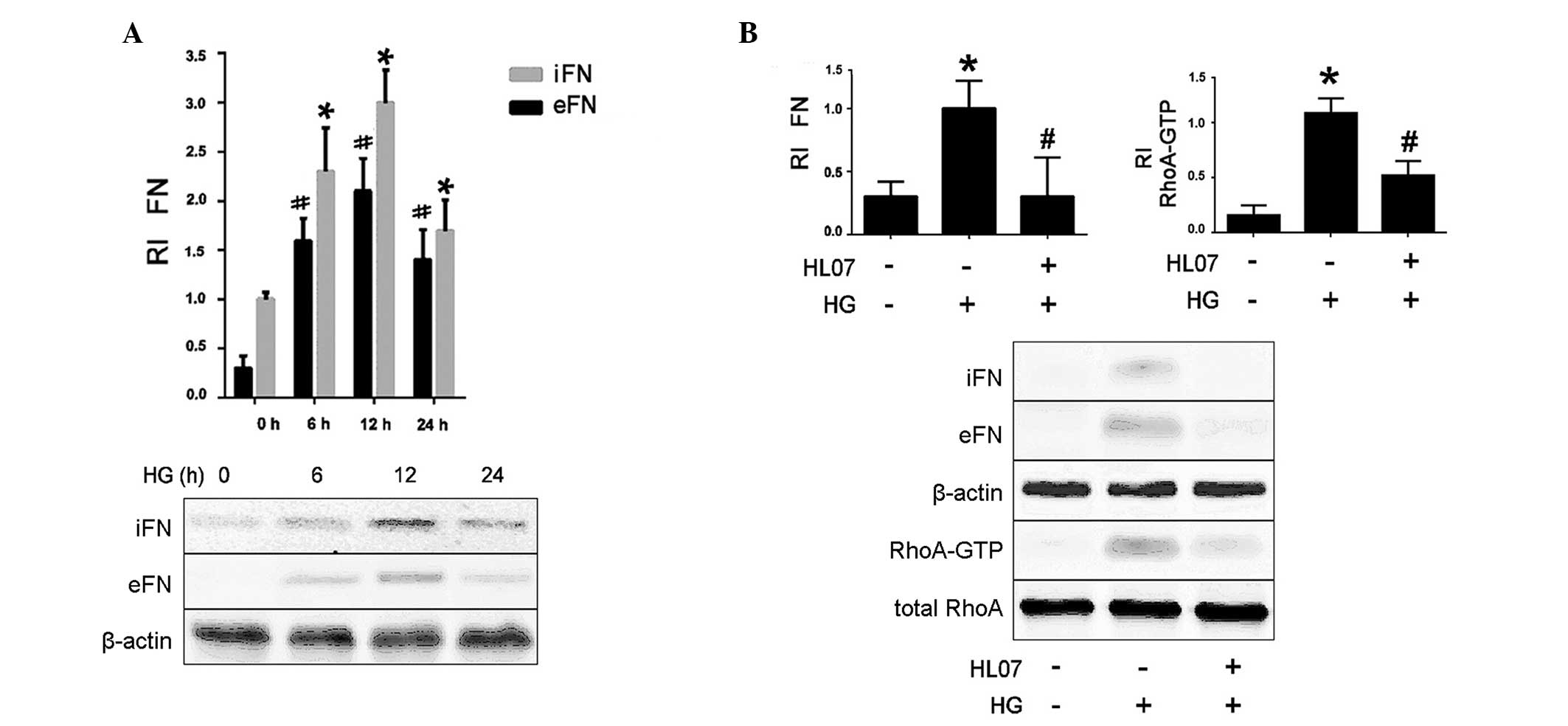 | Figure 1HG induces a significant increase in
FN protein levels via RhoA/Rho kinase activation. Data represent
the mean ± standard error of three independent experiments. (A) MCs
were treated for the indicated times with 40 mM HG, then FN protein
levels were assessed by western blotting, with β-actin used as a
loading control. *P<0.05 vs. iFN control (0 h);
#P<0.05 vs. eFN control (0 h). (B) MCs were
pretreated with a RhoA kinase inhibitor HL07 (10 µM, 60 min)
and subsequently treated with 40 mM HG for 6 h, FN protein and
RhoA-GTP protein levels were assessed by western blotting, with
β-actin used as the internal reference to calculate the relative
intensity of FN and total RhoA used as loading control.
*P<0.05 vs. control (no HG treatment);
#P<0.05 vs. HG treatment. HG, high glucose; FN,
fibronectin; RI, relative intensity; iFN, intracellular FN; eFN,
extracellular FN; RhoA-GTP, Ras homolog family member A-GTP; MC,
mesangial cell. Data are representative of three independent
experiments. |
HG induces caveolin-1 upregulation and
phosphorylation via Src kinases
RhoA has been has been demonstrated to localize to
the caveolae in MCs (13).
Therefore, the current study explored whether HG influences
caveolin-1 expression in rat MCs. It was observed that caveolin-1
Y14 phosphorylation was increased following 5, 10 and 30 min HG
treatment in a time-dependent manner, compared with the 0 min
time-point (P<0.05; Fig. 2A).
There was a trend toward increased total caveolin-1 protein levels
following HG treatment, however, this was not statistically
significant (P>0.05; Fig.
2A).
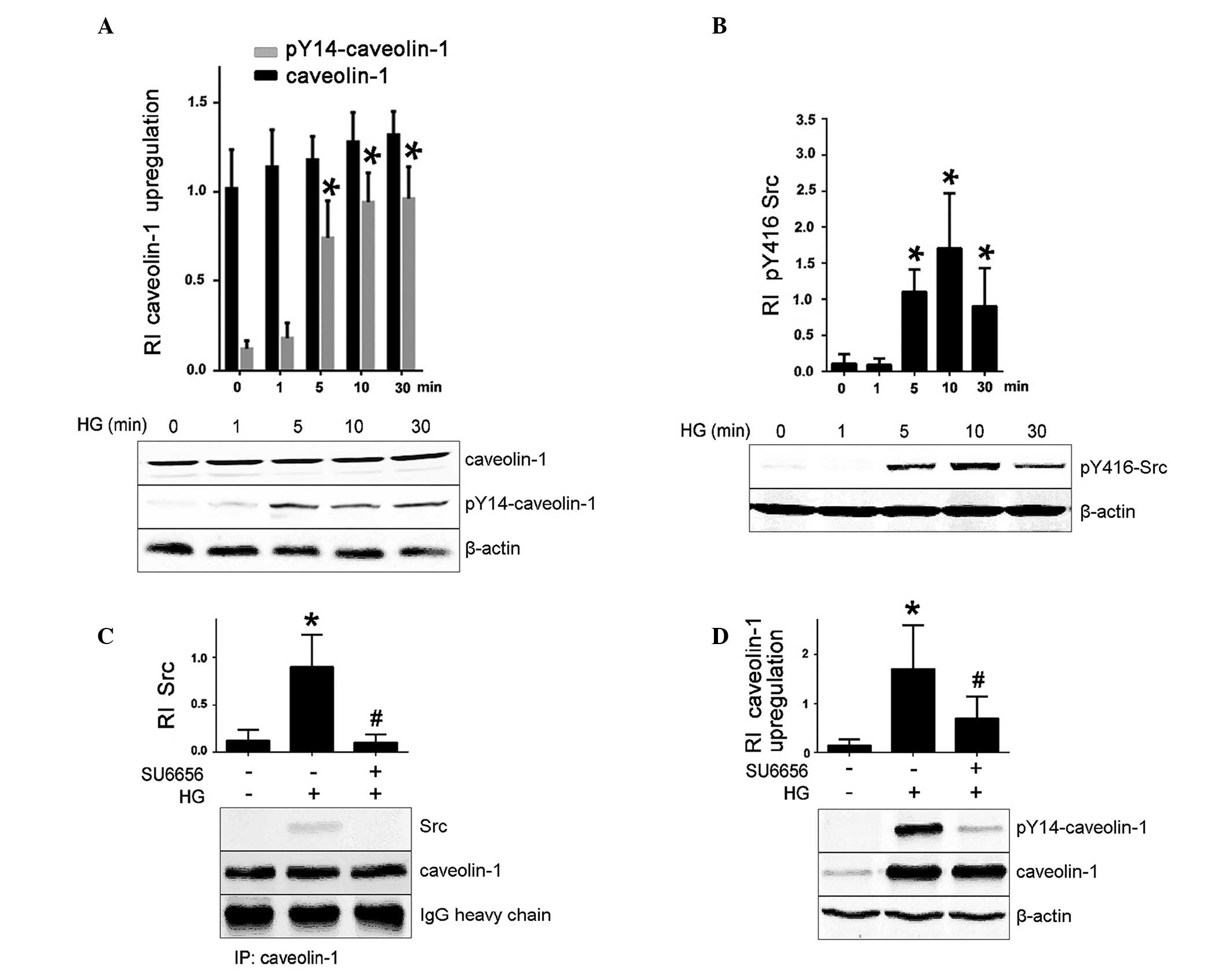 | Figure 2HG-induced caveolin-1 protein level
and caveolin-1 Y14 phosphorylation upregulation via Src kinase
activation. Data represent the mean ± standard error of three
independent experiments. (A) MCs were treated for the indicated
times with HG and caveolin-1 protein level and phosphorylation at
Y14 were assessed by western blotting. *P<0.05 vs.
pY416-caveolin-1 control (0 min). (B) MCs were treated for the
indicated times with HG and Src protein level and phosphorylation
at Y416 were assessed by western blotting. *P<0.05
vs. control (0 min). (C) MCs were pretreated with a Src kinase
inhibitor SU6656 (10 µM, 30 min) and treated with 40 mM HG
for 10 min, IP of caveolin-1 was conducted, and its association
with Src was assessed by western blotting. *P<0.05,
vs. control (no treatment); #P<0.05 vs. HG treatment.
(D) MCs were pretreated with a Src kinase inhibitor SU6656 (10
µM, 30 min) and treated with 40 mM HG for 10 min, and
caveolin-1 protein level and caveolin-1 Y14 phosphorylation were
assessed by western blotting. *P<0.05 vs. control (no
treatment); #P<0.05, vs. HG treatment. β-actin was
used as loading control for western blotting to calculate the
relative intensity of Src and caveolin-1. Data are representative
of three independent experiments. HG, high glucose; MC, mesangial
cell; RI, relative intensity; IP, immunoprecipitation. |
Src kinases are the only kinases known to
phosphorylate caveolin-1 at Y14 (3). The present study confirmed that
HG-induced caveolin-1 Y14 phosphorylation was mediated by Src in
rat MCs. As illustrated in Fig. 2B and
C, HG treatment resulted in the autophosphorylation of Src Y416
and increased association between Src and caveolin-1 compared with
untreated control cells. Treatment of MCs with SU6656 Src inhibitor
prevented the HG-induced caveolin-1 Y14 phos-phorylation, whereas,
it exhibited no effect on HG-induced upregulation of caveolin-1
protein expression (Fig. 2D).
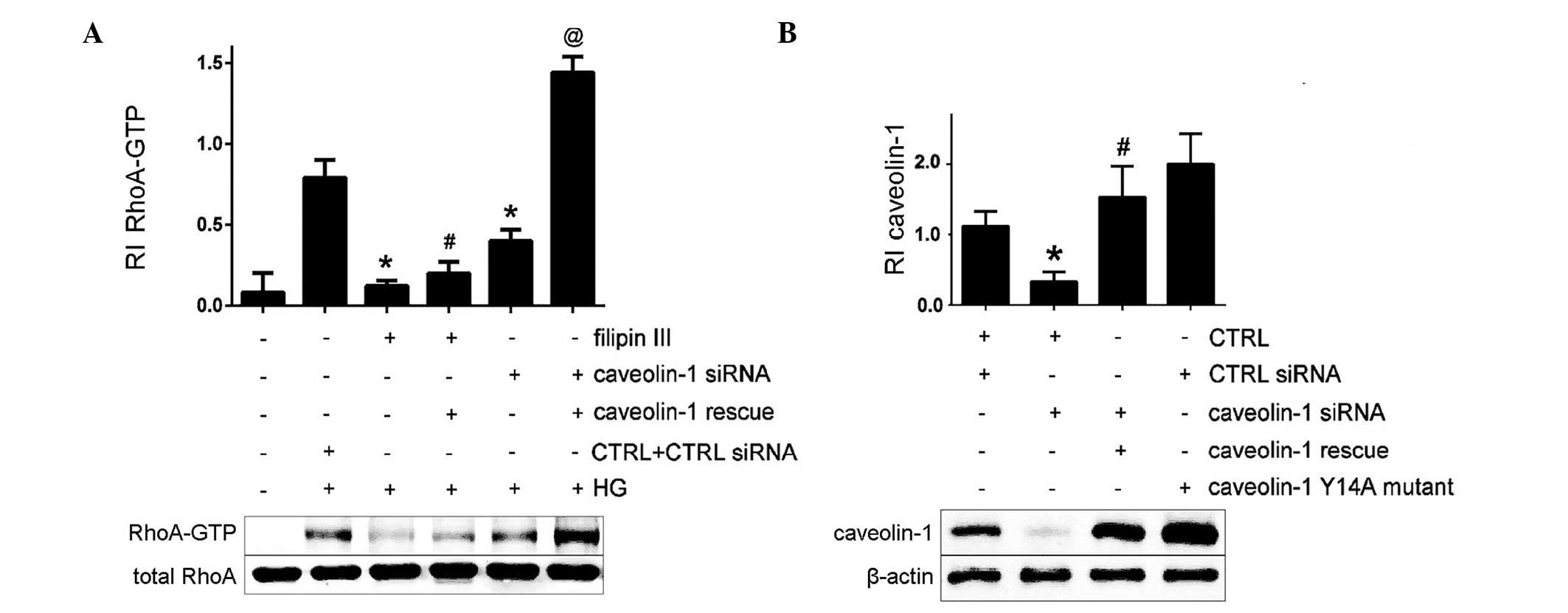
HG-induced RhoA activation requires
intact caveolae
The current study examined the effects of caveolar
disruption on HG-induced RhoA activation and FN upregulation. The
membrane-permeable agent filipin III and caveolin-1 siRNA were used
to perturb the formation of caveolae. It was observed that filipin
III and caveolin-1 siRNA prevented HG-induced RhoA activation
compared with HG-only treatment (Fig.
3A). The present study additionally investigated whether the
effects of caveolar disruption could be reversed by expressing a
non-targetable caveolin-1 complementary DNA (rescue). Exogenous
caveolin-1 expression reversed the effects of the caveolin-1 siRNA
on RhoA activation in MCs (P<0.05; Fig. 3A). However, it exhibited no
significant effect on RhoA activity inhibition in MCs pretreated
with filipin III and HG. These data suggest that HG-induced RhoA
activation requires caveolar structural integrity in MCs.
Caveolin-1 Y14A prevents HG-induced RhoA
activation and FN secretion
Caveolin-1 Y14 phosphorylation by Src kinases is
associated with RhoA activation (3). As the current study had observed that
the HG-induced RhoA activation in MCs requires caveolae, a
caveolin-1 Y14A mutant in which the tyrosine is replaced by the
non-phosphorylatable alanine was constructed to establish the
definitive role of caveolin-1 Y14 phosphorylation in this setting
(Fig. 3B). HG-induced FN secretion
was abrogated in MCs expressing the cave-olin-1 Y14A mutant
(Fig. 4A). Caveolin-1 Y14A failed
to interact with RhoA and effectively prevented HG-induced RhoA
activation (Fig. 4B and C). These
results demonstrate the importance of caveolin-1 Y14
phosphorylation for the HG-induced RhoA activation and increased FN
secretion in MCs.
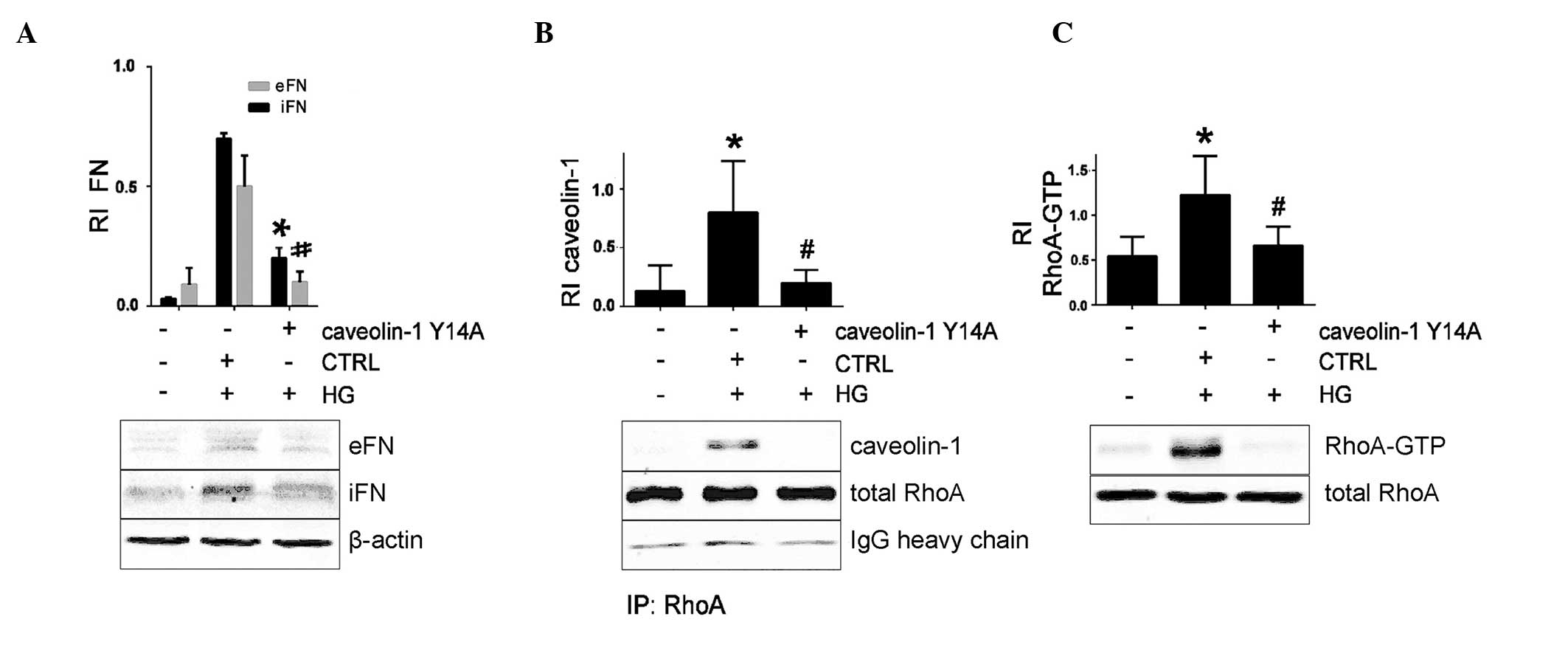 | Figure 4Caveolin-1 Y14A mutant prevents
HG-induced FN upregulation and RhoA activation, assessed by western
blotting. Data represent the mean ± standard error of three
independent experiments. MCs were pretreated with caveolin-1 Y14A
mutant and treated with HG. (A) FN protein levels, with β-actin
used as a loading control to calculate the RI.
*P<0.05 vs. iFN HG + CTRL siRNA;
#P<0.05 vs. eFN HG + CTRL siRNA. (B) Caveolin-1 was
immunoprecipitated, and its association with RhoA was assessed.
*P<0.05 vs. control (no HG); #P<0.05
vs. HG + CTRL siRNA. (C) RhoA-GTP protein level, with total RhoA
used as loading control to calculate the RI of RhoA-GTP.
*P<0.05 vs. control (no HG); #P<0.05
vs. HG + CTRL siRNA. HG, high glucose; FN, fibronectin; eFN,
extracellular FN; iFN, intracellular FN; RI, relative intensity;
CTRL, control siRNA; RhoA, Ras homolog family member A; MC,
mesangial cell; IP, immunoprecipitation. Data are representative of
three independent experiments. |
Discussion
The induction of caveolin-1 expression by high
concentrations of glucose is associated with the upregulation of FN
expression in rat MCs (14). The
current study observed that HG treatment results in a physical
association between caveolin-1 and RhoA. It was additionally
demonstrated that the caveolar integrity is important in allowing
caveolin-1/RhoA interaction and activation, which depend on the
Src-mediated phosphorylation of caveolin-1 at Y14 (8,15,16).
These data suggest that the disruption of caveolin-1 action may
attenuate the renal tissue deterioration induced by HG.
A previous study demonstrated that HG treatment
stimulated caveolar-localized protein tyrosine phosphorylation in
the isolated plasma membrane of various cell types, and resultant
MAPK/ERK activation was dependent on intact caveolae (17). The current study demonstrated that
RhoA activation requires caveolae integrity, which further
characterizes these microdomains as important transducers of
Src/phospho-caveolin-1/RhoA signaling (8). The data of the present study also
indicated that HG treatment regulated the caveolin-1 protein levels
and the caveolar structural formation in rat MCs. However, the
mechanisms of caveolar localization and how interaction with
caveolin-1 facilitates RhoA activation remains to be further
elucidated.
Caveolin-1 Y14 N-terminal phosphorylation was first
identified in v-Src-transformed cells, and the caveolin-1
phosphorylation response appeared to be cell type and
stimulus-specific (3,13,18,19).
In the current study, HG treatment stimulated a sustained increase
in Src Y416 auto-phosphorylation and caveolin-1 Y14
phosphorylation, which subsequently enabled RhoA activation. Using
SU6656 Src inhibitor and the non-phosphorylatable mutant caveolin-1
Y14A, the present study demonstrated that caveolin-1 Y14
phosphorylation, mediated by Src, is required for the downstream FN
production and secretion in MCs (20,21).
However, the initial mechanisms involved in HG-dependent
phosphorylation of caveolin-1 by Src kinase and the relationships
between these signaling components induced by HG remain obscure,
and require further research.
In summary, the present study highlights the
importance of caveolin-1 and caveolae in HG-induced ECM
accumulation. The requirement for Src kinase activity demonstrates
the importance of caveolin-1 Y14 phosphorylation in the subsequent
activation of RhoA and fibronectin upregulation. These findings
serve to elucidate a mechanism that may contribute to the
progression of DKD and control of cave-olin-1 in renal tissue, and
may be an alternative treatment possibility for delaying the renal
dysfunction induced by excessive HG.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Zhejiang Province, China (no. LQ15H050003)
and the National Science Foundation of China (no. 30670810).
References
|
1
|
Zou X, Zhang XX, Liu XY, Li R, Wang M, Wu
WJ, Sui Y and Zhao HL: Renal kallikrein activation and
renoprotection after dual blockade of renin-angiotensin system in
diet-induced diabetic nephropathy. J Diabetes Res. 2015:3106452015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo CS, Chang SY, Chenier I, Filep JG,
Ingelfinger JR, Zhang SL and Chan JS: Heterogeneous nuclear
ribonucleoprotein F suppresses angiotensinogen gene expression and
attenuates hypertension and kidney injury in diabetic mice.
Diabetes. 61:2597–2608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu SZ, Peng FF, Li JL, Ye F, Lei SQ and
Zhang BF: Akt and RhoA activation in response to high glucose
require caveolin-1 phosphorylation in mesangial cells. Am J Physiol
Renal. 306:F1308–F1317. 2014. View Article : Google Scholar
|
|
4
|
Xie X, Peng J, Chang X, Huang K, Huang J,
Wang S, Shen X, Liu P and Huang H: Activation of RhoA/ROCK
regulates NF-κB signaling pathway in experimental diabetic
nephropathy. Mol Cell Endocrinol. 369:86–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao Y and Finnemann SC: Regulation of
phagocytosis by Rho GTPases. Small GTPases. 6:88–99. 2015.
View Article : Google Scholar
|
|
6
|
Perry NA, Vitolo MI, Martin SS and
Kontrogianni-Konstantopoulos A: Loss of the obscurin-RhoGEF
downregulates RhoA signaling and increases microtentacle formation
and attachment of breast epithelial cells. Oncotarget. 5:8558–8568.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawamura S, Miyamoto S and Brown JH:
Initiation and transduction of stretch-induced RhoA and Rac1
activation through caveolae: Cytoskeletal regulation of ERK
translocation. J Biol Chem. 278:31111–31117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng F, Wu D, Ingram AJ, Zhang B, Gao B
and Krepinsky JC: RhoA activation in mesangial cells by mechanical
strain depends on caveolae and caveolin-1 interaction. J Am Soc
Nephrol. 18:189–198. 2007. View Article : Google Scholar
|
|
9
|
Stary CM, Tsutsumi YM, Patel PM, Head BP,
Patel HH and Roth DM: Caveolins: Targeting pro-survival signaling
in the heart and brain. Front Physiol. 3:3932012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shankar J, Boscher C and Nabi IR:
Caveolin-1, galectin-3 and lipid raft domains in cancer cell
signalling. Essays Biochem. 57:189–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang JW and Lee SM: Impaired expression of
caveolin-1 contributes to hepatic ischemia and reperfusion injury.
Biochem Biophys Res Commun. 450:1351–1357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balteau M, Van Steenbergen A, Timmermans
AD, Dessy C, Behets-Wydemans G, Tajeddine N, Castanares-Zapatero D,
Gilon P, Vanoverschelde JL, Horman S, et al: AMPK activation by
glucagon-like peptide-1 prevents NADPH oxidase activation induced
by hyperglycemia in adult cardiomyocytes. Am J Physiol Circ
Physiol. 307:H1120–H1133. 2014. View Article : Google Scholar
|
|
13
|
Wu T, Zhang B, Ye F and Xiao Z: A
potential role for caveolin-1 in VEGF-induced fibronectin
upregulation in mesangial cells: Involvement of VEGFR2 and Src. Am
J Physiol Renal Physiol. 304:F820–F830. 2013. View Article : Google Scholar
|
|
14
|
Zhang Y, Peng F, Gao B, Ingram AJ and
Krepinsky JC: High glucose-induced RhoA activation requires
caveolae and PKCβ1-mediated ROS generation. Am J Physiol Renal
Physiol. 302:F159–F172. 2012. View Article : Google Scholar
|
|
15
|
Jansen M, Pietiainen VM, Polonen H,
Rasilainen L, Koivusalo M, Ruotsalainen U, Jokitalo E and Ikonen E:
Cholesterol substitution increases the structural heterogeneity of
caveolae. J Biol Chem. 283:14610–14618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Radeva G, Perabo J and Sharom FJ:
P-Glycoprotein is localized in intermediate-density membrane
microdomains distinct from classical lipid rafts and caveolar
domains. FEBS J. 272:4924–4937. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karlsson M, Thorn H, Danielsson A,
Stenkula KG, Ost A, Gustavsson J, Nystrom FH and Stralfors P:
Colocalization of insulin receptor and insulin receptor substrate-1
to caveolae in primary human adipocytes. Cholesterol depletion
blocks insulin signalling for metabolic and mitogenic control. Eur
J Biochem. 271:2471–2479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G,
Minshall RD, Malik AB and Hu G: Caveolin-1 Tyr14 phosphorylation
induces interaction with TLR4 in endothelial cells and mediates
MyD88-dependent signaling and sepsis-induced lung inflammation. J
Immunol. 191:6191–6199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H, Volonte D, Galbiati F, Iyengar P,
Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez Boumediene
Bouzahza R, Pestell RG, Scherer PE and Lisanti MP: Constitutive and
growth factor-regulated phosphorylation of caveolin-1 occurs at the
same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7
signaling cassette. Mol Endocrinol. 14:1750–1775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SH, Lee YJ, Park SW, Kim HS and Han
HJ: Caveolin-1 and integrin beta1 regulate embryonic stem cell
proliferation via p38 MAPK and FAK in high glucose. J Cell Physiol.
226:1850–1859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JH and Ryu JM: Role of FAK, RhoA,
PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol. 226:267–275. 2011.
View Article : Google Scholar
|