Introduction
Following the first report by Friedenstein et
al (1), mesenchymal stem cells
(MSCs) have become a point of investigation due to their plasticity
(2), potential use for tissue
engineering (3) and marked
immunomodulatory properties (4),
and have become a widely used therapeutic method. The ability of
MSCs to migrate to injury sites is a key step in their curative
effect; however, various studies have demonstrated MSCs are present
at low levels in the majority of target organs in non-injury
models, thus, limiting their use (5,6). In
order to improve MSC efficacy, the underlying mechanism of MSC
homing to injury sites requires investigation.
Tumor necrosis factor-α (TNF-α) is an important
pro-inflammatory cytokine and a potent regulator of MSC migration
in vivo (7,8). Vascular cell adhesion molecule-1
(VCAM-1) is a vital cell surface adhesion molecule expressed by
various types of cell, including MSCs (9). In a previous report, Teo et al
(10) suggested that, similar to
leukocytes, human bone marrow-derived MSCs preferentially adhere
to, and migrate across, the TNF-α-activated endothelium in a VCAM-1
and G protein-coupled receptor signaling-dependent manner.
TNF-α-induced MSCs exhibit increased adhesion to the cardiac
microvascular endothelium (CMVE) and more efficient cardiac homing,
whereas TNF-α-induced adhesion is suppressed by pretreatment with
anti-VCAM-1 monoclonal antibodies in MSCs or CMVE, but not by
intercellular adhesion molecule-1 (ICAM-1) (11). Thus, VCAM-1 is important for the
adhesion of MSCs and CMVE, although the mechanism by which TNF-α
stimulates MSCs to produce VCAM-1 remains unclear. In human
umbilical vein endothelial cells (HUVECs), TNF-α induces expression
of ICAM-1 and VCAM-1 via the extracellular signal-regulated kinase
(ERK)/nuclear factor-κB (NF-κB) signaling pathway (12). Choi et al (13) demonstrated that c-Jun N-terminal
kinase (JNK) inhibition increases TNF-α-induced NF-κB activation
and ICAM-1 activation. Based on these findings, it was hypothesized
that, in a wound, locally produced TNF-α exerts a chemotactic
effect on MSCs, prompting them to migrate to the site while
increasing surface VCAM-1 expression, thus facilitating MSC
transmigration to the target area and adhesion to the endothelium.
The current study investigated the effect of TNF-α on MSC surface
VCAM-1 expression and adhesion to endothelial cells. In addition,
the activation of NF-κB, ERK and JNK was examined to improve the
understanding of these signal transduction pathways.
Materials and methods
Cells and culture conditions
Human MSCs were obtained from the Department of
Hematology, General Hospital of Guangzhou Military Command of
Chinese PLA (Guangzhou, China). The use of human samples was
approved by the institution's Human and Animal Ethics Committee.
Bone marrow samples were obtained with the informed consent of
patients undergoing orthopedic surgery from July, 2012 to July,
2013. MSCs were isolated and cultured from bone marrow. Briefly,
cells were harvested, centrifuged at 1,000 × g for 5 min and plated
at a density of 6×105 cells/cm2 in α-minimum
essential medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% (v/v) fetal calf serum (Hyclone;
Thermo Fisher Scientific, Inc., Logan, UT, USA), 20 mol/l
L-glutamine (Gibco; Thermo Fisher Scientific, Inc.) and 100
units/ml penicillin G (Gibco; Thermo Fisher Scientific, Inc.).
Cells were incubated at 37°C in 95% humidified air with 5%
CO2. After 24 h, non-adherent cells were removed by
replacing the culture medium and the medium was subsequently
changed twice a week. Upon reaching 90% confluence, the layer of
adherent cells was detached using 0.25% trypsin-EDTA (Invitrogen;
Thermo Fisher Scientific, Inc.), resuspended in culture medium and
seeded into flasks (Costar; Corning Incorporated, Corning, NY,
USA). The MSCs were used at passage 3 in all experiments.
HUVECs were provided by Sun Yat-Sen University
(Guangzhou, China) and cultured in M199 essential medium (Gibco;
Thermo Fisher Scientific, Inc.).
Cell treatment
Prior to each experiment, the medium was replaced
with serum-free medium and the MSCs were incubated overnight. MSCs
were divided into four groups: i) Without intervention; ii)
stimulant alone, 50 ng/ml TNF-α (ProSpec-Tany TechnoGene, Ltd.,
East Brunswick, NJ, USA); iii) inhibitors alone, 10 µM ERK
inhibitor, U0126 (Cell Signaling Technology, Inc., Danvers, MA,
USA), 1 mM NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC;
Merck Millipore, Darmstadt, Germany) or 20 µM JNK inhibitor,
SP600125 (Merck Millipore); and iv) inhibitor + stimulant,
inhibitor stimulation (as above) followed by 50 ng/ml TNF-α. TNF-α
treatment was applied for 24 h and inhibitor treatment for 1 h. All
follow-up experiments were performed in the same groups.
Measurement of VCAM-1 in MSCs by flow
cytometry
The cells were cultured in media containing TNF-α
and inhibitors within the experimental groups described. After 24
h, the cells were harvested, fixed with 4% paraformaldehyde
(Invitrogen; Thermo Fisher Scientific, Inc.), blocked with 0.5%
bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and
incubated with a monoclonal mouse anti-human VCAM-1
phycoerythrin-conjugated antibody (cat. no. 12-1069-42;
eBioscience, Inc., San Diego, CA, USA), using 5 µl antibody
per 105-108 cells at 4°C for 30 min.
Following two washes with phosphate-buffered saline, flow cytometry
was performed and analyzed using a MACS Quant flow cytometer and
MACSQuantify software (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany). Each sample was measured in three different wells and the
experiment was performed twice.
Reverse transcription-polymerase chain
reaction (RT-qPCR)
Total RNA was extracted from cells using a standard
TRIzol (Takara Bio, Inc., Otsu, Japan) RNA isolation method. RT of
1 ng RNA was performed with the Takara RT Master Mix kit (Takara
Bio, Inc.). RNA and cDNA quality was assessed
spectrophotometrically using a Nanodrop 2000 (Thermo Fisher
Scientific, Inc.) and β-actin served as an internal control. The
following primers were used: Forward, 5′-GGCGCCTATACCATCCGAAA-3′
and reverse 5′-AGAGCACGAGAAGCTCAGGAGAA-3′ for VCAM-1; and forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ for β-actin. RT-pPCR was performed
using a SYBR Premix Ex Taq kit (Takara Bio, Inc.) according to the
manufacturer's instructions. The total reaction volume was 25
µl, and 100 ng cDNA was used as the template. Fluorescence
was detected on a Rotor-Gene Q (Qiagen GmbH, Hilden, Germany). Data
were analyzed by the quantitative 2−ΔΔCq method
(14) with β-actin as the
endogenous control. Experiments were performed 3 times.
Western blotting
Harvested cells were lysed by incubation with
radioimmunoprecipitation assay buffer containing 1% protease and
phosphatase inhibitors (Thermo Fisher Scientific, Inc.) for 15 min
at 4°C. Lysates were centrifuged for 15 min at 12,000 × g and 4°C,
then the supernatants were collected and frozen at −80°C or used
immediately. Protein concentrations were determined by
bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL,
USA). Equal quantities of proteins (20–50 µg) were separated
by 7.5% SDS-PAGE and transferred to a nitrocellulose membrane
(Merck Millipore, Darmstadt, Germany). Following blocking with 3%
bovine serum albumin (for the phosphorylated antibodies; Thermo
Fisher Scientific, Inc.) or 0.5% skimmed milk (for other
antibodies) for 1.5 h at room temperature, membranes were incubated
at 4°C overnight with monoclonal rabbit anti-NF-κB (cat. no. 8242),
monoclonal rabbit anti-phosphorylated (p)-NF-κB (cat. no. 4806),
anti-ERK (cat. no. 9903), monoclonal rabbit anti-p-ERK (cat. no.
4370), polyclonal rabbit anti-JNK (cat. no. 9252), polyclonal
rabbit anti-p-JNK (cat. no. 9251) or monoclonal rabbit anti-GAPDH
(cat. no. 3683) primary antibodies (1:1,000; Cell Signaling
Technology, Inc.), and then incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:10,000;) for 1 h. Immune complexes were detected with enhanced
chemiluminescence reagent (Merck Millipore) and imaged with the
FluorChem Q system (ProteinSimple, San Jose, CA, USA). Equal
protein loading was confirmed by measuring the GAPDH or total
protein expression. The results were analyzed using Image J2x
software (version 2.1.4.7; www.rawak.de/rs2012/). Western blot analysis was
performed three times.
Cell adhesion assay
Cells were treated with control, TNF-α alone (50
ng/ml) or TNF-α with inhibitor (1 mM PDTC, 10 µM U0126 or 20
mM SP600125). MSC adherence to HUVECs was measured using
fluorescent carbocyanine CM-Dil (Invitrogen; Thermo Fisher
Scientific, Inc.) to label the cell membranes. Minor modifications
were made to the manufacturer's protocol. Briefly, prior to
transplantation, MSCs were labeled with 2 µg/ml CM-Dil for
~30 min at 37°C, then washed three times with phosphate-buffered
saline (Thermo Fisher Scientific, Inc.) and transferred to a 6-well
plate containing HUVECs for 12 h. Non-adherent cells were removed
by washing with medium. The number of adherent cells were counted
in 5 fields of each sample (n=4) at x4 magnification (BX51M;
Olympus Corporation, Tokyo, Japan).
Statistical analysis
The data represent a minimum of three independent
experiments and are expressed as means ± standard deviation. Data
analysis was performed with SPSS software version 13.0 (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance followed by Tukey's
test were performed and P<0.05 was considered to indicate a
statistically significant difference.
Results
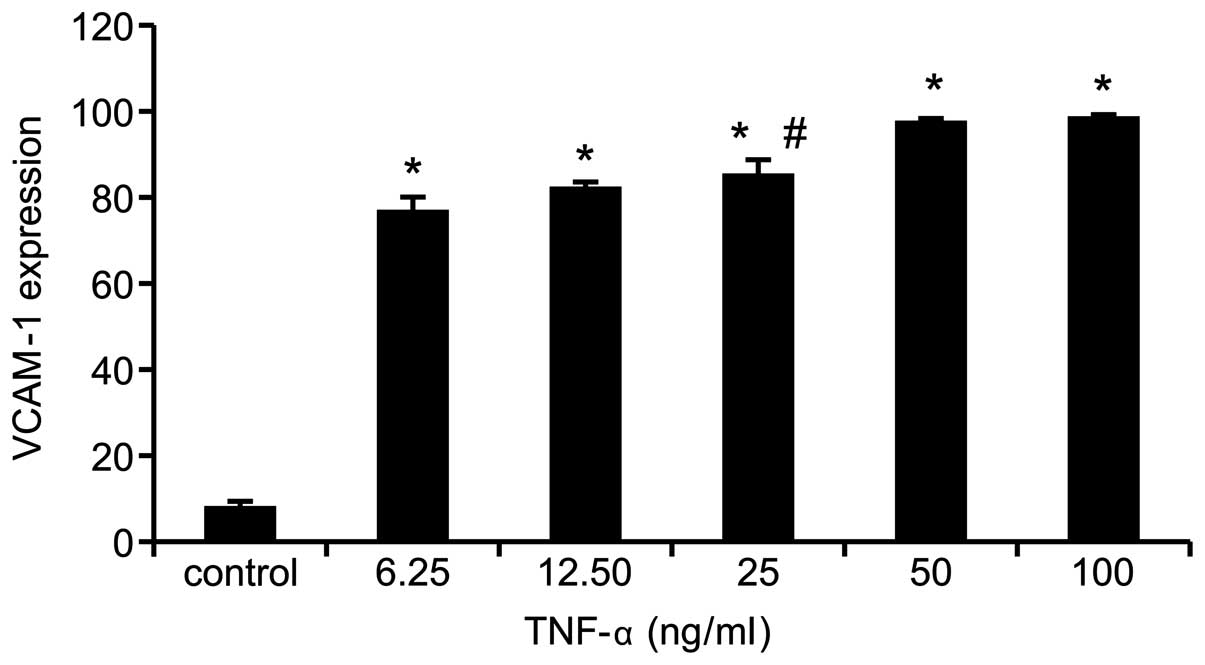
TNF-α upregulates VCAM-1 expression in
MSCs in a dose-dependent manner
The effect of TNF-α on VCAM-1 expression in MSCs was
investigated by flow cytometry. The analysis demonstrated that,
compared with the control, the VCAM-1 expression level was
increased by TNF-α in a dose-dependent manner (P=0.000). VCAM-1
expression levels peaked at 50 ng/ml TNF-α treatment. Thus, 50
ng/ml TNF-α was selected as the stimulating concentration for the
subsequent experiments (Fig.
1).
PDTC, U0126 and SP600125 inhibit
induction of VCAM-1 by TNF-α
To determine whether PDTC, U0126, or SP600125
influenced the VCAM-1 expression level, flow cytometry and RT-qPCR
were used to measure VCAM-1 protein and mRNA expression levels,
respectively. MSCs were pre-incubated with the inhibitors and
subsequently stimulated with TNF-α. Under these conditions, surface
VCAM-1 expression was significantly reduced in MSCs treated with
the inhibitors when compared with those that received TNF-α
treatment alone (P=0.000). Notably, MSCs treated with inhibitors
alone demonstrated no difference in VCAM-1 expression level when
compared with the control group (Fig.
2A–J).
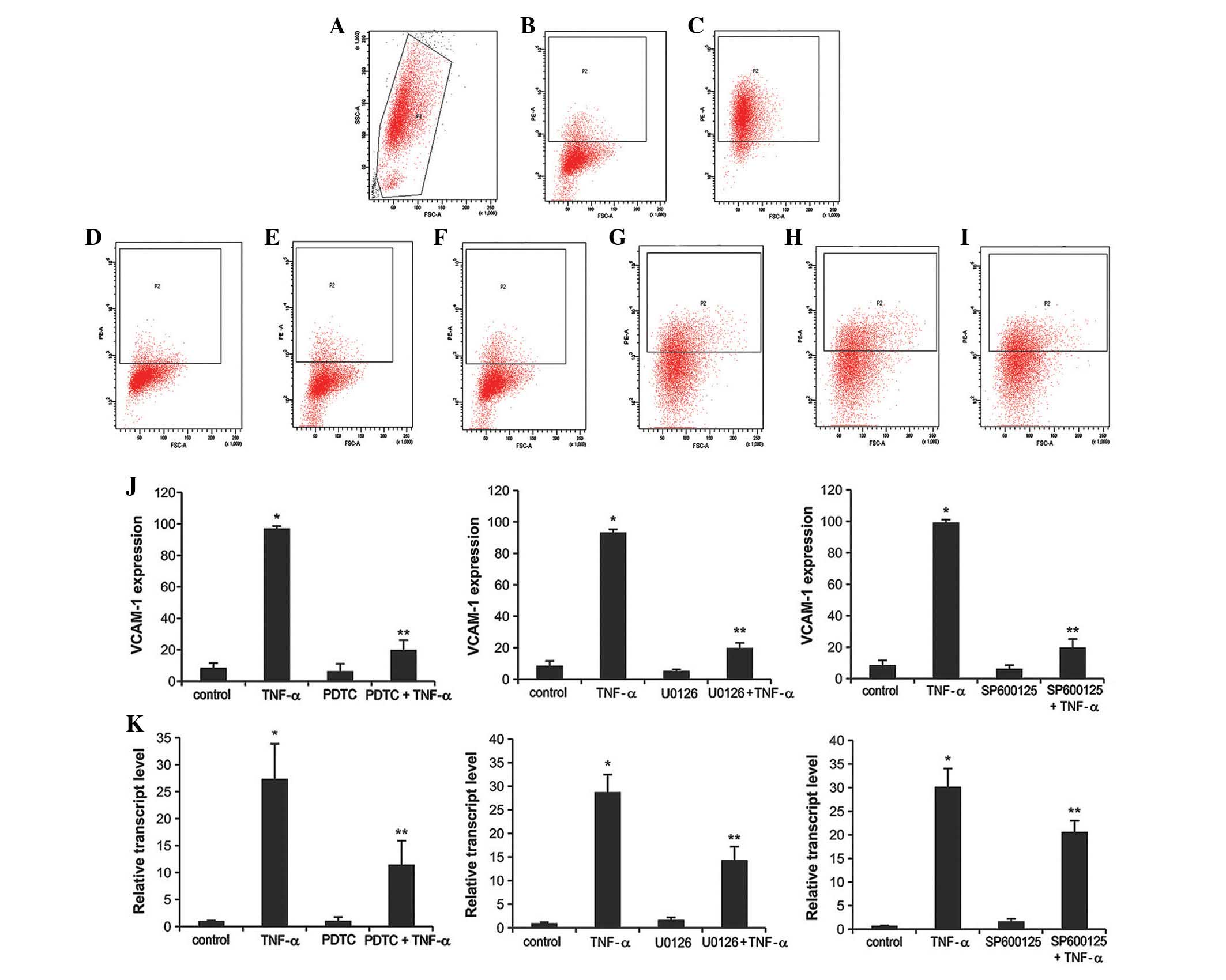 | Figure 2PDTC, U0126, and SP600125 blocked
TNF-α-induced VCAM-1 expression in MSCs. (A) The appropriate active
MSCs were analyzed by flow cytometry. (B) VCAM-1 expression of MSCs
in control cells measured by flow cytometry. The effects of (C)
TNF-α or (D) nuclear factor-κB inhibitor, PDTC, (E) ERK inhibitor,
U0126, (F) JNK inhibitor, SP600125 on VCAM-1 expression levels were
measured by flow cytometry. Additionally, the combined effect of
TNF-α and (G) PDTC, (H) U0126 and (I) SP600125 on VCAM-1 expression
were determined by flow cytometry and (J) quantified. (K) Reverse
transcription-quantitative polymerase chain reaction was performed
to measure the VCAM-1 mRNA expression levels from the same
treatment groups. Data were obtained from three independent
experiments and are presented as means ± standard deviation.
*P<0.05 vs. control, **P<0.05 vs. TNF-α
group. PDTC, pyrrolidine dithiocarbamate; TNF-α, tumor necrosis
factor-α; VCAM-1, vascular cell adhesion molecule 1; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase. |
Similarly, these trends were reflected in the VCAM-1
mRNA level (Fig. 2K). The groups
treated with TNF-α and inhibitors demonstrated a significant
decrease in the VCAM-1 mRNA expression level when compared with the
TNF-α group (PDTC, P=0.005; U0126, P=0.001; SP6000125, P=0.004). By
contrast, the inhibitors alone exhibited no apparent suppressive
effect. Thus, the changes observed by flow cytometry were more
obvious than those demonstrated by VCAM-1 mRNA expression.
Effects of TNF-α on the phosphorylation
of NF-κB, ERK and JNK
To examine the TNF-α regulated activation of the
NF-κB, ERK or JNK signaling pathways, western blot analysis was
used to detect protein phosphorylation in each pathway. MSCs were
divided into three groups: Control, TNF-α, and MSCs stimulated with
inhibitor + TNF-α. It was demonstrated that TNF-α significantly
increased the activation of all three signaling pathways (NF-κB,
ERK and JNK) compared with the control (P=0.001, P=0.001 and
P=0.032, respectively); however, the increased activation was
reduced by pretreatment with inhibitors. Furthermore, treatment
with inhibitor + TNF-α significantly reduced the phosphorylation of
NF-κB and ERK when compared with the TNF-α alone group (P=0.046 and
P=0.036, respectively; Fig.
3).
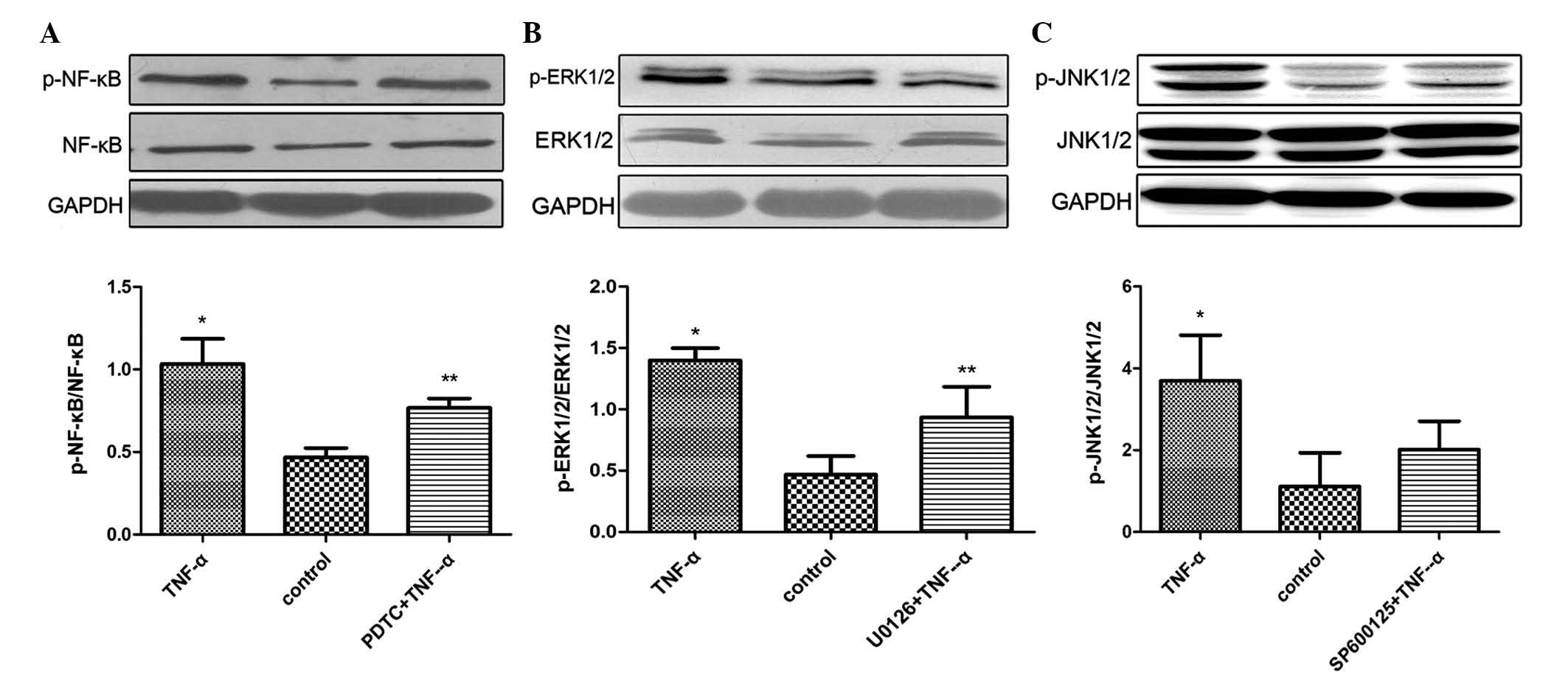 | Figure 3Activity of NF-κB, ERK, or JNK
signaling in MSCs treated with TNF-α + inhibitors and TNF-α alone.
(A) Effect of TNF-α and NF-κB inhibitor, PDTC on NF-κB signaling.
(B) Effect of TNF-α and ERK inhibitor, U0126 on ERK signaling. (C)
Effect of TNF-α and JNK inhibitor, SP600125 on the JNK signaling
pathway. Data were obtained from three independent experiments and
are presented as means ± standard deviation. *P<0.05
vs. control, **P<0.05 vs. TNF-α group. p,
phosphorylated; NF-κB, nuclear factor-κB; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; TNF-α, tumor
necrosis factor-α; MSCs, mesenchymal stem cells. |
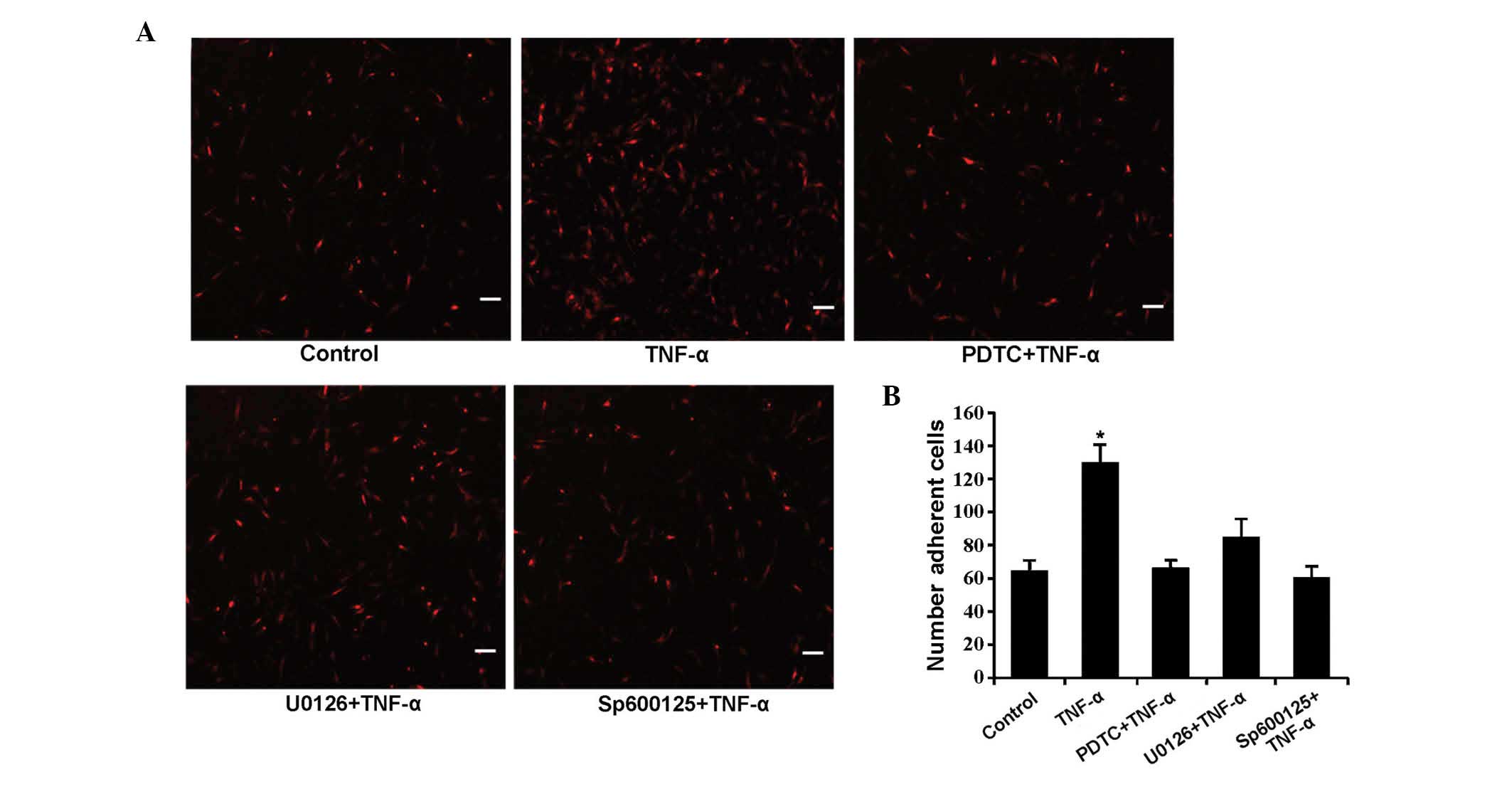
Effects of TNF-α, PDTC, U0126 and
SP600125 on MSC adhesion to HUVECs
Finally, the effects of TNF-α and the inhibitors on
MSC adhesion were investigated. The number of MSCs was
significantly increased in the TNF-α-induced group compared with
the control (P=0.000). However, the combined treatment of signaling
pathway inhibitors and TNF-α did not demonstrate a marked effect on
MSC adhesion when compared with control (Fig. 4).
Discussion
MSCs have been applied in the treatment of a variety
of diseases, however, the precise mechanisms underlying MSC homing
to injured tissues are not fully understood (15–19).
The current study demonstrated various important findings regarding
the effect of TNF-α on VCAM-1 expression in human bone
marrow-derived MSCs. The present study demonstrated that TNF-α
treatment increased the level of VCAM-1 expression in MSCs in a
dose-dependent manner. Additionally, the signaling pathway
inhibitors, PDTC, U0126 and SP600125 suppressed VCAM-1 expression
induced by TNF-α. Furthermore, TNF-α augmented the activities of
NF-κB, ERK and JNK, and promoted MSC adhesion to HUVECs, while the
signaling pathway inhibitors exhibited no observable effect on
adhesion.
Following intravenous injection, MSCs circulate in
the blood stream and adhesion to endothelial cells at sites of
injury is the first step in homing of MSCs to target organs.
Inflammatory cytokines, including TNF-α and interleukin-1β, enhance
the adhesion of MSCs to CMVE in vitro and in vivo
(11). TNF-α activates MSCs by
binding to surface receptors. There are two major receptors, TNF
receptor I and II, expressed in human MSCs (20). TNF-α interacts with its receptors
and activates downstream intracellular signaling pathways. In rat
MSCs, VCAM-1 expression, which was induced by platelet-derived
growth factor BB, required activation of
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), p38
mitogen-activated protein kinase and NF-κB (21). Furthermore, PI3K is involved in the
signal transduction of vascular endothelial growth factor-induced
migration and VCAM-1 expression of bone marrow-derived MSCs
(22). Uchibori et al
(23) demonstrated that NF-κB
signaling regulates MSC accumulation at tumor sites. The present
study demonstrated that human VCAM-1 expression in MSCs stimulated
by TNF-α was dependent on NF-κB, ERK and JNK signaling. However,
the signaling activity of cells pretreated with SP600125 and then
stimulated with TNF-α demonstrated no observable change when
compared with the control group. This indicates that the JNK
signaling pathway is not required for TNF-α-induced VCAM-1
expression in MSCs.
Notably, VCAM-1 and its major ligand, integrin α4β1,
are expressed in MSCs and the microvascular endothelium. However,
it was previously demonstrated that integrin α4β1 expression does
not change significantly following TNF-α stimulation (23). Fu et al (7) demonstrated that expression of ICAM-1
was increased by 50 µg/l TNF-α in rat MSCs, whereas VCAM-1
expression did not change. Another study demonstrated that TNF-α
induces VCAM-1 expression in rat MSCs in a concentration-dependent
manner (24). The current study
demonstrated that TNF-α increases VCAM-1 expression in a
dose-dependent manner in human MSCs. Segers et al (11) demonstrated that TNF-α-stimulated
adhesion of CMVE or MSCs is blocked by pretreatment with
anti-VCAM-1 monoclonal antibodies, but not by anti-ICAM-1
antibodies. Thus, MSC adherence to the vascular endothelium is
predominantly mediated by VCAM-1 expression. The results of the
current study demonstrate that TNF-α treatment increased the
adhesion of MSCs when compared with the controls.
In conclusion, the present study demonstrated that
TNF-α upregulates VCAM-1 expression in human bone marrow-derived
MSCs and facilitates adherence to the vascular endothelium at sites
of injury. Furthermore, TNF-α-induced adhesion is partially
mediated by the NF-κB, ERK and JNK signaling pathways. In the
clinical setting, MSCs are used as therapeutic means to treat
patients with Graft versus host disease and aplastic anemia. They
improve the symptoms and the patient survival rates, however the
curative effect varies. Few MSCs settle in bone marrow, thus, it is
important to improve the homing capacity of MSCs. MSCs adherence to
HUVECs is an important step for homing. The findings of the current
study provide insight into the molecular mechanism of this process.
In future studies, TNF-α and other cytokines may be used stimulate
MSCs in vitro to upregulate their homing capacities.
Subsequently, administration of the MSCs to patients may improve
their curative effect.
Acknowledgments
The current study was supported by grants from the
National Natural Science Foundation of China (grant no. 30900645),
Guangzhou Pearl River Scientific and Technological New Star
Capitals (grant no. 2012J2200008), and Medical Science and
Technology Research of the 12th 'Five-Year' Planning's Key Project
of Chinese Army (grant no. BWS11J071).
References
|
1
|
Friedenstein AJ, Chailakhyan RK, Latsinik
NV, Panasyuk AF and Keiliss-Borok IV: Stromal cells responsible for
transferring the microenvironment of the hemopoietic tissues.
Cloning in vitro and retransplantation in vivo. Transplantation.
17:331–340. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caplan AI: Adult mesenchymal stem cells
for tissue engineering versus regenerative medicine. J Cell
Physiol. 213:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nauta AJ and Fibbe WE: Immunomodulatory
properties of mesenchymal stromal cells. Blood. 110:3499–3506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao J, Dennis JE, Muzic RF, Lundberg M and
Caplan AI: The dynamic in vivo distribution of bone marrow-derived
mesenchymal stem cells after infusion. Cells Tissues Organs.
169:12–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devine SM, Cobbs C, Jennings M,
Bartholomew A and Hoffman R: Mesenchymal stem cells distribute to a
wide range of tissues following systemic infusion into nonhuman
primates. Blood. 101:2999–3001. 2003. View Article : Google Scholar
|
|
7
|
Fu X, Han B, Cai S, Lei Y, Sun T and Sheng
Z: Migration of bone marrow-derived mesenchymal stem cells induced
by tumor necrosis factor-alpha and its possible role in wound
healing. Wound Repair Regen. 17:185–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang A, Wang Y, Ye Z, Xie H, Zhou L and
Zheng S: Mechanism of TNF-α-induced migration and hepatocyte growth
factor production in human mesenchymal stem cells. J Cell Biochem.
111:469–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bühring HJ, Treml S, Cerabona F, de Zwart
P, Kanz L and Sobiesiak M: Phenotypic characterization of distinct
human bone marrow-derived MSC subsets. Ann N Y Acad Sci.
1176:124–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teo GS, Ankrum JA, Martinelli R, Boetto
SE, Simms K, Sciuto TE, Dvorak AM, Karp JM and Carman CV:
Mesenchymal stem cells transmigrate between and directly through
tumor necrosis factor-α-activated endothelial cells via both
leukocyte-like and novel mechanisms. Stem Cells. 30:2472–2486.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segers VF, Van Riet I, Andries LJ, Lemmens
K, Demolder MJ, De Becker AJ, Kockx MM and De Keulenaer GW:
Mesenchymal stem cell adhesion to cardiac microvascular
endothelium: Activators and mechanisms. Am J Physiol Heart Circ
Physiol. 290:H1370–H1377. 2006. View Article : Google Scholar
|
|
12
|
Zhong X, Li X, Liu F, Tan H and Shang D:
Omentin inhibits TNF-α-induced expression of adhesion molecules in
endothelial cells via ERK/NF-kB pathway. Biochem Biophys Res
Commun. 425:401–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi H, Nguyen HN and Lamb FS: Inhibition
of endocytosis exacerbates TNF-α-induced endothelial dysfunction
via enhanced JNK and p38 activation. Am J Physiol Heart Circ
Physiol. 306:H1154–H1163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Xiao Y, Jiang ZJ, Pang Y, Li L, Gao Y,
Xiao HW, Li YH, Zhang H and Liu Q: Efficacy and safety of
mesenchymal stem cell treatment from related donors for patients
with refractory aplastic anemia. Cytotherapy. 15:760–766. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodrigo SF, van Ramshorst J, Hoogslag GE,
Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I,
Dibbets-Schneider P, Fibbe WE, et al: Intramyocardial injection of
autologous bone marrow-derived ex vivo expanded mesenchymal stem
cells in acute myocardial infarction patients is feasible and safe
up to 5 years of follow-up. J Cardiovasc Transl Res. 6:816–825.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huleihel L, Levine M and Rojas M: The
potential of cell-based therapy in lung diseases. Expert Opin Biol
Ther. 13:1429–1440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Undale AH, Westendorf JJ, Yaszemski MJ and
Khosla S: Mesenchymal stem cells for bone repair and metabolic bone
diseases. Mayo Clin Proc. 84:893–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weng JY, Du X, Geng SX, Peng YW, Wang Z,
Lu ZS, Wu SJ, Luo CW, Guo R, Ling W, et al: Mesenchymal stem cell
as salvage treatment for refractory chronic GVHD. Bone Marrow
Transplant. 45:1732–1740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Croitoru-Lamoury J, Lamoury FM, Zaunders
JJ, Veas LA and Brew BJ: Human mesenchymal stem cells
constitutively express chemokines and chemokine receptors that can
be upregulated by cytokines, IFN-beta and Copaxone. J Interferon
Cytokine Res. 27:53–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Cheng P, Ma JC, Xue YX and Liu YH:
Platelet-derived growth factor BB mediates the glioma-induced
migration of bone marrow-derived mesenchymal stem cells by
promoting the expression of vascular cell adhesion molecule-1
through the PI3K, P38 MAPK and NF-kB pathways. Oncol Rep.
30:2755–2764. 2013.
|
|
22
|
Gao Z, Cheng P, Xue Y and Liu Y: Vascular
endothelial growth factor participates in modulating the C6
glioma-induced migration of rat bone marrow-derived mesenchymal
stem cells and upregulates their vascular cell adhesion molecule-1
expression. Exp Ther Med. 4:993–998. 2012.PubMed/NCBI
|
|
23
|
Uchibori R, Tsukahara T, Mizuguchi H, Saga
Y, Urabe M, Mizukami H, Kume A and Ozawa K: NF-kB activity
regulates mesenchymal stem cell accumulation at tumor sites. Cancer
Res. 73:364–372. 2013. View Article : Google Scholar
|
|
24
|
Xiao Q, Wang SK, Tian H, Xin L, Zou ZG, Hu
YL, Chang CM, Wang XY, Yin QS, Zhang XH and Wang LY: TNF-α
increases bone marrow mesenchymal stem cell migration to ischemic
tissues. Cell Biochem Biophys. 62:409–414. 2012. View Article : Google Scholar
|