Introduction
Peritoneal dialysis (PD) is a common therapy for the
treatment of patients with end-stage renal disease (ESRD).
Traditional PD solutions containing high glucose (HG) are
effective, inexpensive and easily metabolized. However, the
disadvantages of glucose-based solutions include reduced pH,
increased lactate levels, the production of glucose degradation
products and hyperosmolarity (1,2).
Eventually, these changes may result in damage to the structure and
function of the peritoneal membrane, leading to ultrafiltration
failure and peritoneal fibrosis.
Apoptosis, also termed programmed cell death,
generally occurs via two basic pathways; the intrinsic and
extrinsic pathways. The B-cell lymphoma-2 (Bcl-2) gene family is
associated with the gene-regulated, intrinsic pathway. Two
important proteins of this family are Bcl-2 and Bcl-2-associated X
protein (Bax), which are anti- and proapoptotic, respectively
(3). Apoptosis is essential for
the maintenance of normal homeostasis; however, when the
physiological rate of apoptosis changes it can lead to disease
(4). It has previously been
demonstrated that HG can induce apoptosis in peritoneal mesothelial
cells (5). This effect on
peritoneal homeostasis may induce failure of peritoneal membrane
function (6,7). It is well established that HG can
cause mitochondrial oxidative stress. Overproduction of reactive
oxygen species (ROS), driven by HG metabolism, can trigger cell
death by modulating a series of intracellular signaling pathways
(5). Therefore, understanding the
regulation of apoptosis and oxidative stress may be important for
PD therapy.
The effects of 1,25(OH)2D3 and
its analogues have previously been investigated on the regulation
of cell immunomodulation, proliferation and differentiation
(8), and a previous study
demonstrated that 1,25(OH)2D3 modulates
apoptosis (9). In addition,
research has demonstrated that vitamin D decreases ROS generation
in HG-exposed monocytes (10).
Various molecular pathways have been suggested to mediate
protective and antioxidative effects against cell death, including
inhibition of glycogen synthase kinase-3 and phosphoinositide
phosphatases, enhanced activity of cell survival molecules (such as
Bcl-2), and decreased activity of proapoptotic molecules, including
Bax, mitogen-activated protein kinase (MAPK)8 (also termed JNK) and
P38 MAPK (11,12). The importance of MAPK proteins in
human peritoneal mesothelial cells (HPMCs) during HG-induced
oxidative stress and apoptosis remains unclear. The aim of the
present study was to evaluate whether
1,25(OH)2D3 protects HPMCs from HG-induced
apoptosis and oxidative stress, and to elucidate the molecular
mechanisms involved.
Materials and methods
Materials
Fetal bovine serum (FBS) and penicillin-streptomycin
were obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). 1,25(OH)2D3 and dimethyl sulfoxide
(DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rabbit polyclonal phosphorylated (p)-P38 (cat. no. sc-7975-R),
rabbit polyclonal P38 (cat. no. sc-535) and mouse monoclonal
β-actin (cat. no. sc-47778) antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit monoclonal Bax
(cat. no. 5023) and rabbit polyclonal Bcl-2 (cat. no. 2876)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). SB203580 (P38 MAPK inhibitor) was obtained from
Selleck Chemicals (Houston, TX, USA). Annexin V/fluorescein
isothiocyanate (FITC) Apoptosis Detection kit I was obtained from
BD Pharmingen (San Diego, CA, USA). Enhanced chemiluminescence
(ECL) kit was obtained from Pierce Biotechnology, Inc. (Rockford,
IL, USA). The fluorescence microplate reader (ELx-800) was from
Bio-Tek Instruments, Inc. (Winooski, VT, USA) and FACSCalibur flow
cytometer was from BD Biosciences (Franklin Lakes, NJ, USA). An
inverted microscope (Eclipse Ti; Nikon Corporation, Tokyo, Japan)
was used to detect morphological changes.
HPMC culture
HPMCs (originally established by Dr Pierre Ronco,
Department of Nephrology, Tenon Hospital, Paris, France) were
provided by Professors Na Di and Xu Huimian (The First Affiliated
Hospital of China Medical University, Shenyang, China) and cultured
in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% FBS. HPMCs were incubated in a 5%
CO2 atmosphere at 37°C, and the culture medium was
changed every 2–3 days. HPMCs were detached using trypsin-EDTA with
a subcultivation ratio between 1:3 and 1:4. Cells at passage 5–10
were used in all experiments. The HPMCs were divided into the
following four treatment groups: i) Control group; ii)
1,25(OH)2D3 group, cells received
10−7 mol/l 1,25(OH)2D3 treatment
for 24 h (9); iii) HG (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) group, cells received
126 mM HG treatment for 24 h; and iv) HG +
1,25(OH)2D3 group, cells received
10−7 mol/l 1,25(OH)2D3
pretreatment followed by 126 mM HG for 24 h. To investigate the
MAPK/P38 signaling pathway, HPMCs were also incubated with 10
µM SB203580 for 1 h and exposed to 126 mM HG in the presence
of 10−7 mol/l 1,25(OH)2D3 for 24
h.
Measurement of cell viability
HPMCs were exposed to varying doses (76, 126 and 214
mM) of HG with or without pretreatment with different doses
(10−8, 10−7 and 10−6 mol/l) of
1,25(OH)2D3 to observe the cell viability.
Viability of HPMCs cultured in 96-well plates was measured using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, following a 24-h incubation subsequent to treatment
with HG and/or 1,25(OH)2D3, 10 µl MTT
(500 µg/ml) was added to the culture medium and incubated at
37°C for 4 h. Subsequently, the supernatant was removed and 100
µl DMSO was added to each well and mixed for 15 min. The
absorbance value of the wells was measured at 570 nm using the
microplate reader. The cell viability was expressed as the ratio of
the signal obtained from treated and control groups.
Analysis of apoptosis
Apoptosis was assessed by FACSCalibur flow cytometry
using the Annexin V/FITC Apoptosis Detection kit I, according to
the manufacturer's protocol. HPMCs were cultured at
4×106 cells/ml density and seeded in 6-well plates.
Cells were trypsinized, then washed twice with cold
phosphate-buffered saline (PBS) and centrifuged at 192 × g for 5
min. Cells were resuspended in 300 µl 1X binding buffer,
then 10 µl Annexin V/FITC was added and incubated for 30 min
at room temperature in the dark. Subsequently, 5 µl
propidium iodide was added to the cells in the dark. Finally, cells
were analyzed by flow cytometry using WinMDI 2.8 software (The
Scripps Institute, La Jolla, CA, USA) was used to analyze flow
cytometry. Fluorescence-activated cell sorting data were used to
determine the percentage of apoptotic cells.
Assessment of intracellular ROS
levels
Intracellular accumulation of ROS was measured using
2,7-dichlorofluorescein diacetate (DCF-DA; Beijing Solarbio Science
& Technology Co., Ltd., Beijing,China). HPMCs were seeded in
24-well plates and pretreated with
1,25(OH)2D3 for 2 h then exposed to HG for 24
h. The medium was then removed, cells were washed with PBS and
incubated with DCF-DA for 30 min, then washed again with PBS. DCF
oxidation was measured at 485 nm excitation and 525 nm emission
wavelengths using the fluorescence microplate reader.
Western blot analysis
HPMCs were washed with PBS, incubated with
radioimmunoprecipitation assay lysis buffer and the protein
concentrations were quantified. Protein concentrations were
quantified by BCA Protein assay (Pierce Biotechnology, Inc.). Each
protein sample (50 µg) was separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and was transferred onto
a nitrocellulose membrane at 100 V for 60 min. The membranes were
subsequently blocked in blocking solution (5% non-fat dry milk) for
1 h at room temperature, and were incubated overnight at 4°C with
Bax, Bcl-2, P38, p-P38 or β-actin antibodies (1:1,000).
Subsequently, each membrane was rinsed three times with
Tris-buffered saline 0.1% Tween 20 (TBS-T) solution, then incubated
with goat anti-rabbit IgG (cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) and goat anti-mouse IgG (cat. no. sc-2005;
Santa Cruz Biotechnology, Inc.) for 60 min at room temperature
(dilution, 1:10,000). The membranes were washed with TBS-T
solution, and an enhanced chemiluminescent western blotting
detection system was used to detect specific signals. The band
densities were measured using ImageJ software v1.6.0 (National
Institutes of Health, Bethesda, Maryland, USA) and results were
normalized against the reference gene β-actin.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18; SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard error. Multiple group comparisons
were made using standard one-way analysis of variance methodology
and individual comparisons were performed using Tukey's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
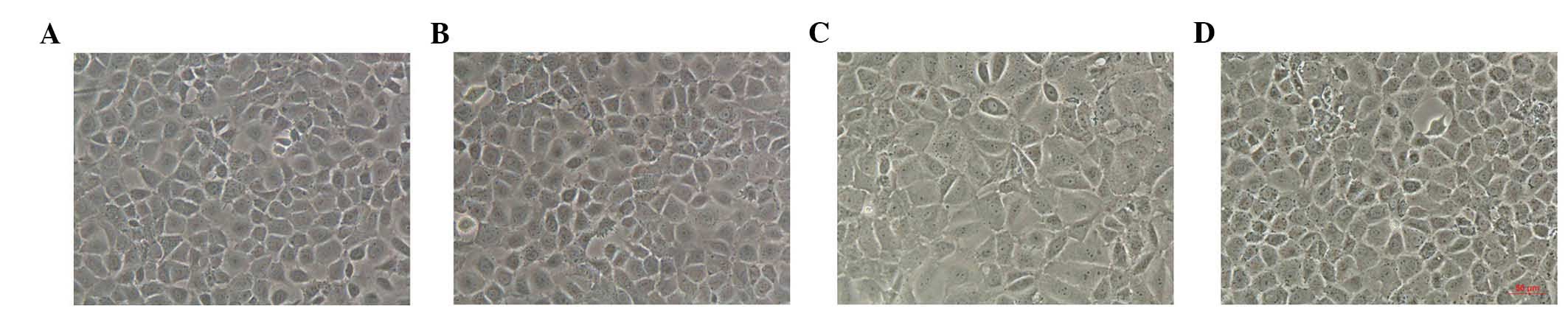
Morphological alterations of HPMCs
The control HPMCs exhibited a characteristic
cobblestone-like appearance, which was changed to a fibroblast-like
morphology after 24 h of incubation with HG (Fig. 1). Treatment with
1,25(OH)2D3(10−7 mol/l) reversed
the changes in cell morphology induced by HG. However,
1,25(OH)2D3 alone had no effect on the
morphological characteristics of HPMCs (Fig. 1).
Effects of
1,25(OH)2D3 on cell viability in
HG-stimulated HPMCs
To investigate the growth inhibitory effects of HG
on HPMCs, cell viability was measured following treatment with
different doses of HG (76, 126 and 214 mM) and
1,25(OH)2D3 (10−8, 10−7
and 10−6 mol/l). Representative doses of 126 mM HG and
10−7 mol/l 1,25(OH)2D3 were
chosen. Cells were stimulated with HG (126 mM) for 24 h, in the
presence or absence of 1,25(OH)2D3
(10−7 mol/l). Cell viability was detected by MTT
analysis. As demonstrated in Fig.
2A, HG significantly inhibited cell viability in HPMCs in a
dose-dependent manner (P<0.01). However, pretreatment with
1,25(OH)2D3 improved cell viability in a
dose-dependent manner (Fig. 2B and
C).
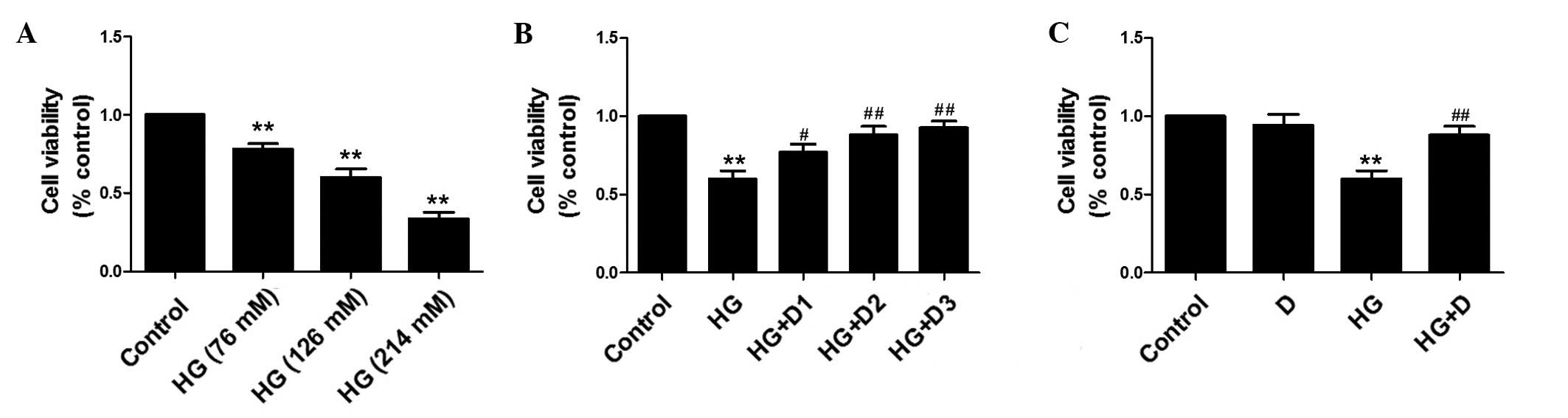 | Figure 2Effects of
1,25(OH)2D3 on cell viability in HG-treated
human peritoneal mesothelial cells. The cell viability was analyzed
by MTT assay. (A) Cells were exposed to HG at 76, 126 or 214 mM.
(B) Cells were exposed to HG (126 mM) and pretreated with
10−8, 10−7 or 10−6 mol/l
1,25(OH)2D3. (C) Cells were exposed to HG
(126 mM) and pretreated with 10−7 mol/l
1,25(OH)2D3. Data are presented as the mean ±
standard error, n=6. **P<0.01 vs. control,
#P<0.05 vs. HG, ##P<0.01 vs. HG. HG,
high glucose; D, 1,25(OH)2D3; D1,
10−8 mol/l, D2, 10−7 mol/l; D3,
10−6 mol/l. |
Effects of
1,25(OH)2D3 on HG-induced apoptosis in
HPMCs
To assess the mechanisms by which
1,25(OH)2D3 alters HG-induced apoptosis in
HPMCs, cell apoptosis was detected by FACSCalibur flow cytometry
following Annexin V/PI double staining. FACSCalibur flow cytometry
assays demonstrated that treatment with HG for 24 h markedly
increased the level of apoptotic and dead cells detected. Treatment
with 1,25(OH)2D3 markedly decreased the
population of apoptotic and dead cells (Fig. 3A).
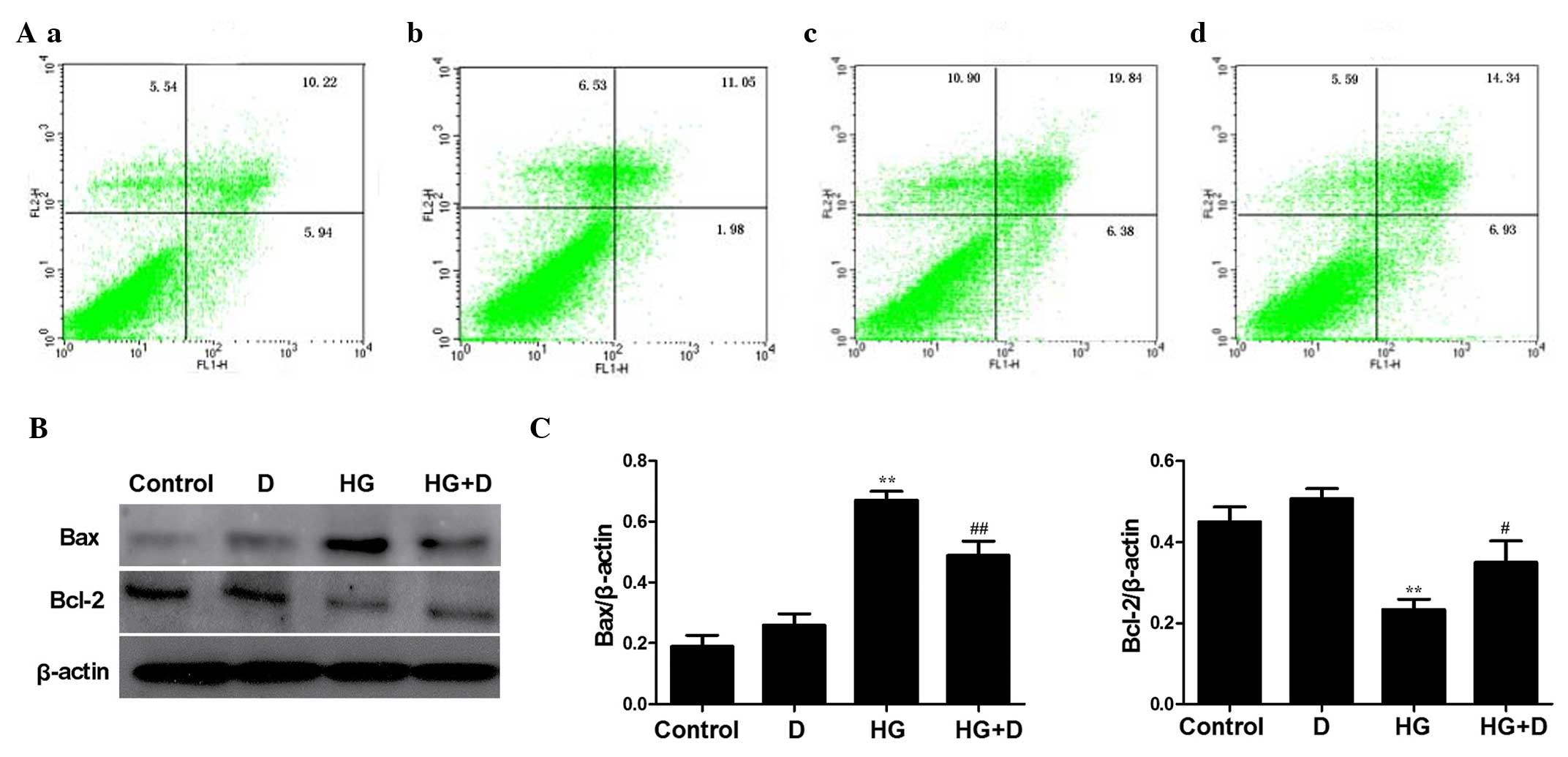 | Figure 3Effects of
1,25(OH)2D3 on HG-induced apoptosis in human
peritoneal mesothelial cells. Cells were exposed to HG (126 mM),
10−7 mol/l 1,25(OH)2D3 or both for
24 h. (A) The levels of apoptosis were determined by Annexin
V-propidium iodide double staining and flow cytometry: Top left
quadrant, necrotic cells; lower left, normal cells; top right,
early apoptotic cells; lower right, late apoptotic cells. (a)
Control group; (b) 1,25(OH)2D3
(10−7 mol/l) group; (c) HG group; (d) HG +
1,25(OH)2D3 (10−7 mol/l) group.
Flow cytometry assays demonstrated a marked increase in apoptotic
(26.22%) and necrotic (10.90%) cells treated with HG for 24 h.
However, 1,25(OH)2D3 pretreatment
significantly decreased the population of apoptotic (21.27%) and
necrotic (5.59%) cells. (B) Western blot analysis was performed and
the (C) relative expression levels of Bax and Bcl-2 were calculated
using densitometric analysis and were normalized to the loading
control. Data are presented as the mean ± standard error of the
relative intensities, n=3. **P<0.01 vs. control,
#P<0.05 and ##P<0.01 vs. HG. HG, high
glucose; D, 1,25(OH)2D3; Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2. |
To further investigate the effects of
1,25(OH)2D3 on HG-induced apoptosis, western
blot analysis was conducted to detect Bax and Bcl-2 levels. As
demonstrated in Fig. 3B and C, HG
upregulated the expression of Bax (P<0.01) and downregulated the
expression of Bcl-2 (P<0.01) compared with control cells.
Compared with the HG-treated cells, HG-induced apoptosis was
attenuated by pretreatment of HPMCs with 10−7 mol/l
1,25(OH)2D3.
Effects of
1,25(OH)2D3 on intracellular ROS production
in HG-treated HPMCs
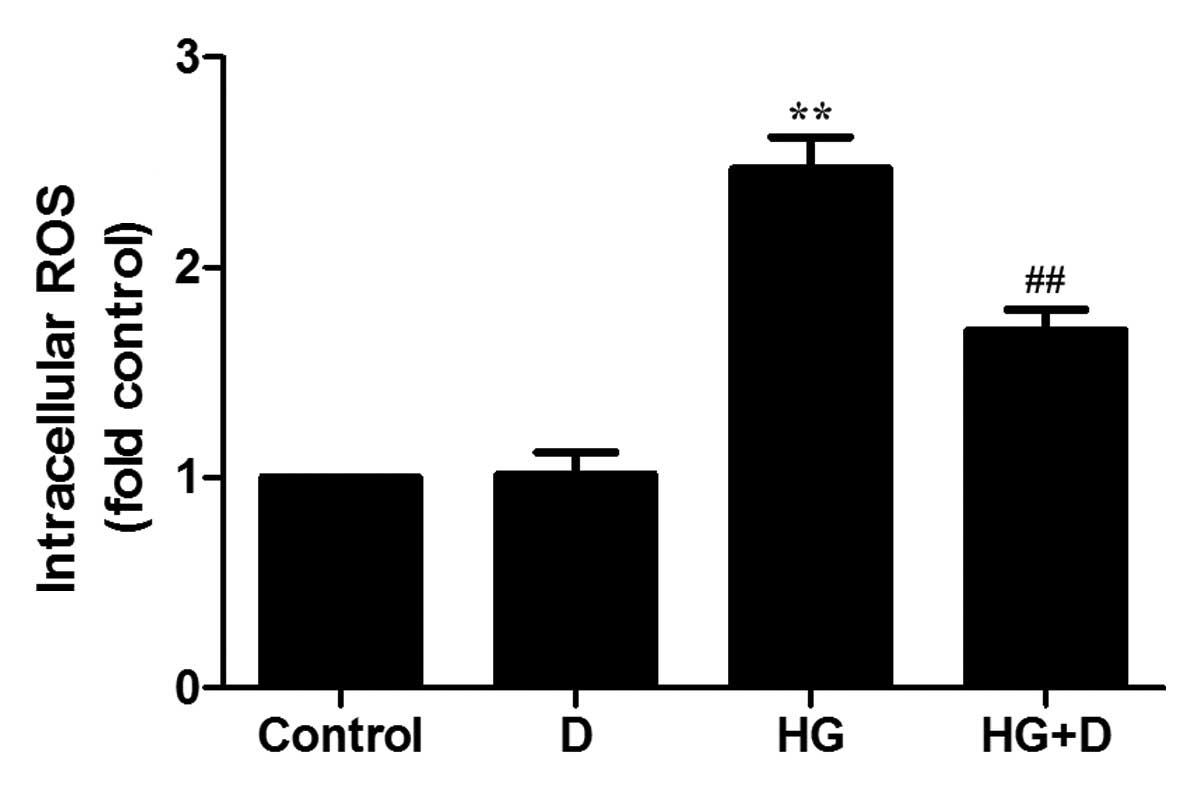
It has previously been demonstrated that ROS
contribute to HG-induced apoptosis in rat PMCs (RPMCs) (5). The aim of the present study was to
demonstrate whether 1,25(OH)2D3 influences
the elevated expression of ROS in HG-treated HPMCs. ROS levels were
detected using DCF-DA. As demonstrated in Fig. 4, exposure to 126 mM glucose for 24
h increased the DCF signal compared with the control group
(P<0.01). However, compared with HG-induced levels, pretreatment
with 10−7 mol/l 1,25(OH)2D3
inhibited increases to ROS levels (P<0.01), as measured by DCF
fluorescence.
Effects of
1,25(OH)2D3 on the MAPK/P38 pathway in
HG-treated HPMCs
MAPKs are associated with various biological
activities, including cell proliferation, differentiation,
survival, transformation, and cell death (13,14).
Extracellular-regulated kinase (ERK), P38 and JNK are three
important members of the MAPK family. To investigate the molecular
mechanism by which 1,25(OH)2D3 exerts its
anti-apoptotic effects, the MAPK/P38 pathway was examined. HPMCs
were incubated with or without 10 µM SB203580 for 1 h and
were then exposed to 126 mM HG in the presence or absence of
10−7 mol/l 1,25(OH)2D3. P38
activation was detected by western blot analysis using a p-P38
antibody. When cells were exposed to HG alone, P38 phosphorylation
was increased compared with the control (P<0.01), whereas
phosphorylation was significantly decreased by
1,25(OH)2D3 treatment compared with HG
(P<0.05; Fig. 5). In addition,
treatment of HG-stimulated HPMCs with SB203580 and
1,25(OH)2D3 inhibited P38 phosphorylation to
a greater extent compared with 1,25(OH)2D3
treatment (P<0.01; Fig. 5).
These results suggest that 1,25(OH)2D3
protects HPMCs through the MAPK/P38 pathway.
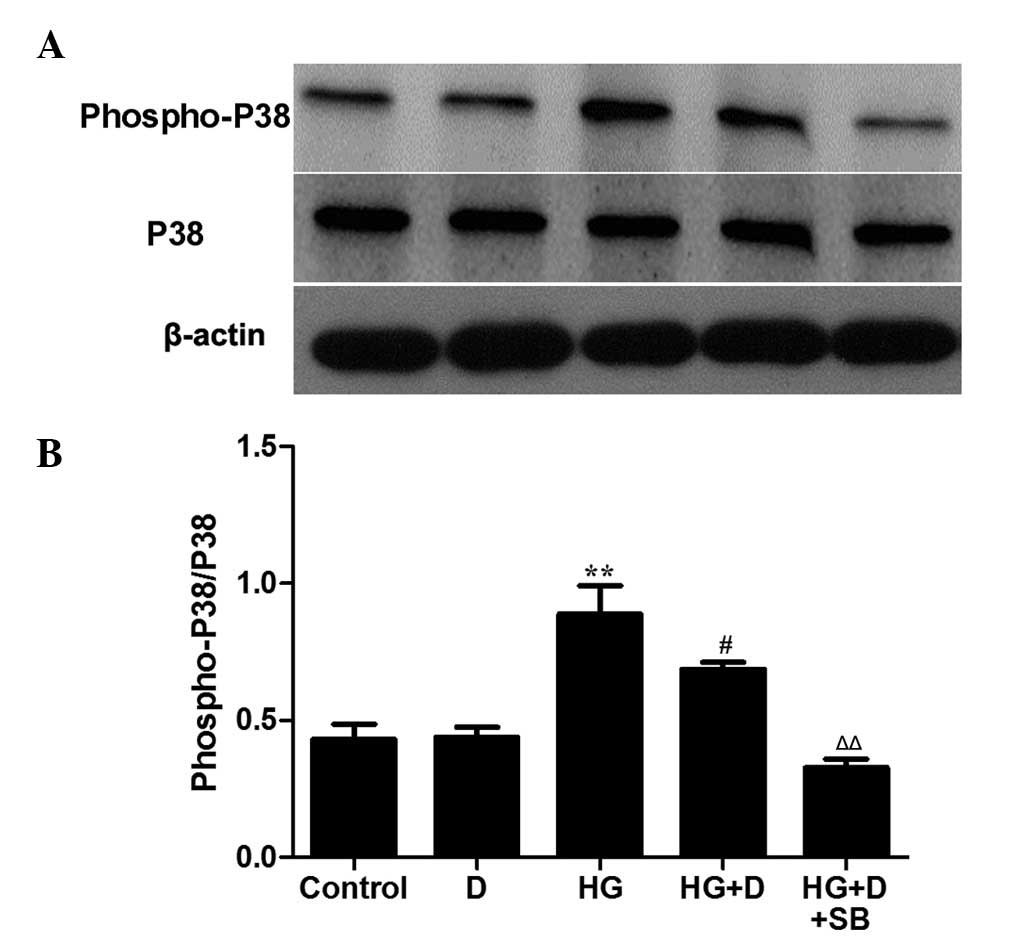 | Figure 5Effects of
1,25(OH)2D3 on the MAPK/P38 pathway in
HG-treated HPMCs. HPMCs were incubated with or without 10 µM
SB203580 for 1 h and then exposed to HG (126 mM) in the presence or
absence of 10−7 mol/l 1,25(OH)2D3.
(A) Western blot analysis was performed to detect P38 and
phospho-P38. (B) The protein levels were assessed using
densitometry and are expressed as relative intensities. Data are
presented as the mean ± standard error, n=3. **P<0.01
vs. control, #P<0.05 vs. HG, ΔΔP<0.01
vs. HG + D. HPMC, human peritoneal mesothelial cell; MAPK,
mitogen-activated protein kinase; P38, MAPK 14; D,
1,25(OH)2D3; HG, high glucose; SB,
SB203580. |
Discussion
When the peritoneum is continuously exposed to HG
the structure and function of the peritoneal membrane is altered.
HG may lead to increased peritoneal permeability, resulting in
rapid dissipation of the osmotic gradient, eventually causing
ultrafiltration failure and inadequate dialysis (15). The present study demonstrated that
HG significantly inhibits the cell viability of HPMCs, and alters
the cell morphology. However, 1,25(OH)2D3
enhanced the viability of HPMCs and reversed the observed changes
to cell morphology induced by HG.
It has previously been demonstrated that
1,25(OH)2D3 decreases apoptosis in RPMCs
(9). Plasma levels of
1,25(OH)2D3 have been observed to be reduced
in patients with ESRD. Therefore, it was hypothesized that
1,25(OH)2D3 may affect apoptosis in HPMCs.
The 1,25(OH)2D3 concentration used in the
present study was selected according to MTT assay results and a
previous investigation (9). The
present study investigated the effects of
1,25(OH)2D3 on HG-induced apoptosis in HPMCs
and aimed to elucidate the molecular mechanisms involved. It was
demonstrated that pretreatment with
1,25(OH)2D3 decreased the ratio of Bax/Bcl-2
protein expression and the rate of apoptosis induced by HG. These
findings support the results of a previous study that demonstrated
that 1,25(OH)2D3 inhibits podocyte apoptosis
via downregulating Bax and upregulating Bcl-2 expression levels,
which may result in improved podocytopenia (16).
Previous studies have demonstrated that HG induces
apoptosis via the ROS signaling pathway, and evidence suggested
that overproduction of ROS was associated with apoptotic cell death
(17,18). The present study demonstrated that
ROS production was increased by HG and that
1,25(OH)2D3 decreases HG-induced ROS
production in HPMCs. In agreement with the findings of the present
study, a previous study demonstrated that the protective activity
of 1,25(OH)2D3 was mediated by antioxidant
ROS inhibition and associated with reduced tumor necrosis factor-α
and nuclear factor-κB levels (19). It has also been previously
demonstrated that T cell apoptosis was mediated via ROS generation
in response to the downregulation of vitamin D receptor (20). However, to the best of our
knowledge, this is the first report demonstrating the protective
role of 1,25(OH)2D3 against HG-induced
apoptosis and ROS production in HPMCs.
Previous research has demonstrated that oxidative
stress stimulates the phosphorylation of MAPKs, including P38,
ERK1/2 and JNK1/2, which are important components of the signaling
pathways associated with cell death (21–25).
The association between ROS and MAPK signaling remains unknown. It
was previously demonstrated that ROS may activate apoptosis signal
regulated kinase 1, a MAPK kinase kinase that activates JNK and P38
(26). The results of the present
study revealed that pretreatment with
1,25(OH)2D3, as an early signaling factor,
inhibited HG-induced P38 phosphorylation. The results of the
present study are in accordance with previous reports indicating
that baicalein prevented apoptosis by reducing ROS generation and
deactivating MAPK/ERK/JNK/P38 signaling pathways (27).
In conclusion, the results of the present study
revealed that in HG-stimulated HMPCs,
1,25(OH)2D3 attenuated changes to cell
morphology, and reduced apoptosis and ROS production, partially
mediated by inhibition of the MAPK/P38 pathway. The results of the
present study suggested that 1,25(OH)2D3 may
be employed in the future as a promising therapeutic agent for the
prevention or treatment of peritoneal damage.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81300636).
Abbreviations:
|
HPMCs
|
human peritoneal mesothelial cells
|
|
HG
|
high glucose
|
|
ROS
|
reactive oxygen species
|
|
PD
|
peritoneal dialysis
|
|
ESRD
|
end-stage renal disease
|
|
DCF-DA
|
dichlorofluorescein diacetate
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Mortier S, De Vriese AS and Lameire N:
Recent concepts in the molecular biology of the peritoneal membrane
- implications for more biocompatible dialysis solutions. Blood
Purif. 21:14–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmad M, Shah H, Pliakogiannis T and
Oreopoulos DG: Prevention of membrane damage in patient on
peritoneal dialysis with new peritoneal dialysis solutions. Int
Urol Nephrol. 39:299–312. 2007. View Article : Google Scholar
|
|
3
|
Ghibelli L and Diederich M: Multistep and
multitask Bax activation. Mitochondrion. 10:604–613. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortiz A: Nephrology forum: Apoptotic
regulatory proteins in renal injury. Kidney Int. 58:467–485. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Liang D, Guo B, Yang L, Wang L
and Ma J: Zinc inhibits high glucose-induced apoptosis in
peritoneal mesothelial cells. Biol Trace Elem Res. 150:424–432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng ZH, Ye RG, Bergström J and Lindholm
B: Effect of dialysate composition on the apoptosis and
proliferation of human peritoneal mesothelial cells and protein
expression of Fas and c-Myc. Adv Perit Dial. 16:31–35.
2000.PubMed/NCBI
|
|
7
|
Kaifu K, Kiyomoto H, Hitomi H, Matsubara
K, Hara T, Moriwaki K, Ihara G, Fujita Y, Sugasawa N, Nagata D, et
al: Insulin attenuates apoptosis induced by high glucose via the
PI3-kinase/Akt pathway in rat peritoneal mesothelial cells. Nephrol
Dial Transplant. 24:809–815. 2009. View Article : Google Scholar
|
|
8
|
Gocek E, Kiełbiński M, Wyłób P, Kutner A
and Marcinkowska E: Side-chain modified vitamin D analogs induce
rapid accumulation of VDR in the cell nuclei proportionately to
their differentiation-inducing potential. Steroids. 73:1359–1366.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Wang J, Fan Y, Chen S, Wang L and
Ma J: Effect of 1,25(OH)2D3 on rat peritoneal
mesothelial cells treated with high glucose plus
lipopolysaccharide. Cell Immunol. 271:173–179. 2011. View Article : Google Scholar
|
|
10
|
Jain SK and Micinski D: Vitamin D
upregulates glutamate cysteine ligase and glutathione reductase,
and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion
in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun.
437:7–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiu CT and Chuang DM: Molecular actions
and therapeutic potential of lithium in preclinical and clinical
studies of CNS disorders. Pharmacol Ther. 128:281–304. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YH, Rane A, Lussier S and Andersen JK:
Lithium protects against oxidative stress-mediated cell death in
α-synuclein-overexpressing in vitro and in vivo models of
Parkinson's disease. J Neurosci Res. 89:1666–1675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kholodenko BN and Birtwistle MR:
Four-dimensional dynamics of MAPK information processing systems.
Wiley Interdiscip Rev Syst Biol Med. 1:28–44. 2009. View Article : Google Scholar
|
|
15
|
Jörres A and Witowski J: PD membrane:
Biological responses to different PD fluids. Contrib Nephrol.
150:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou MS, Yu J, Nie GM, He WS, Luo LM and Xu
HT: 1, 25-dihydroxyvitamin D3 decreases adriamycin-induced podocyte
apoptosis and loss. Int J Med Sci. 7:290–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greene DA, Stevens MJ, Obrosova I and
Feldman EL: Glucose-induced oxidative stress and programmed cell
death in diabetic neuropathy. Eur J Pharmacol. 375:217–223. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lelkes E, Unsworth BR and Lelkes PI:
Reactive oxygen species, apoptosis and altered NGF-induced
signaling in PC12 pheochromocytoma cells cultured in elevated
glucose: An in vitro cellular model for diabetic neuropathy.
Neurotox Res. 3:189–203. 2001. View Article : Google Scholar
|
|
19
|
Lan N, Luo G, Yang X, Cheng Y, Zhang Y,
Wang X, Wang X, Xie T, Li G, Liu Z and Zhong N: 25-Hydroxyvitamin
d3-deficiency enhances oxidative stress and corticosteroid
resistance in severe asthma exacerbation. PLoS One. 9:e1115992014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rehman S, Chandel N, Salhan D, Rai P,
Sharma B, Singh T, Husain M, Malhotra A and Singhal PC: Ethanol and
vitamin D receptor in T cell apoptosis. J Neuroimmune Pharmacol.
8:251–261. 2013. View Article : Google Scholar :
|
|
21
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B, Jian Z, Li Q, Li K, Wang Z, Liu L,
Tang L, Yi X, Wang H, Li C and Gao T: Baicalein protects human
melanocytes from H2O2-induced apoptosis via
inhibiting mitochondria-dependent caspase activation and the p38
MAPK pathway. Free Radic Biol Med. 53:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HJ, Noh YH, Lee DY, Kim YS, Kim KY,
Chung YH, Lee WB and Kim SS: Baicalein attenuates
6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur J
Cell Biol. 84:897–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YC, Chow JM, Lin CW, Wu CY and Shen
SC: Baicalein inhibition of oxidative-stress-induced apoptosis via
modulation of ERKs activation and induction of HO-1 gene expression
in rat glioma cells C6. Toxicol Appl Pharmacol. 216:263–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin HY, Shen SC, Lin CW, Yang LY and Chen
YC: Baicalein inhibition of hydrogen peroxide-induced apoptosis via
ROS-dependent heme oxygenase 1 gene expression. Biochim Biophys
Acta. 1773:1073–1086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagai H, Noguchi T, Takeda K and Ichijo H:
Pathophysiological roles of ASK1-MAP kinase signaling pathways. J
Biochem Mol Biol. 40:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen HM, Hsu JH, Liou SF, Chen TJ, Chen
LY, Chiu CC and Yeh JL: Baicalein, an active component of
Scutellaria baicalensis Georgi, prevents
lysophosphatidylcholine-induced cardiac injury by reducing reactive
oxygen species production, calcium overload and apoptosis via MAPK
pathways. BMC Complement Altern Med. 14:2332014. View Article : Google Scholar : PubMed/NCBI
|