Introduction
Bone graft procedures are frequently performed in
oral and maxillofacial surgery to treat defects of the maxilla or
mandible resulting from tumor resection, fractures, infection and
skeletal dysplasia (1). Currently,
the gold standard approach involves autograft bone treatment,
however, autograft bone is not readily available and is associated
with donor site morbidity (2,3).
Allograft bone and xenograft bone are feasible alternatives,
however, the risks of host rejection, disease transmission and
infections have limited their use (4).
Bone formation is a complex process, which involves
interactions between different cells, growth factors and
extracellular matrix to promote the proliferation, differentiation
and migration of osteoprogenitor cells (5,6). The
application of growth factors to induce the natural healing of bone
and promote bone tissue remodeling may be a possible strategy to
improve current approaches for bone regeneration. During laboratory
experiments and clinical trials, growth factors have exhibited
promising therapeutic potential, however, few have been approved
for clinical use (4). Bone
morphogenetic protein-2 (BMP-2) has been approved for clinical
application since 2002 (1). BMP-2
has been successfully used in non-union fractures, joint fusions,
critical bone defects and aseptic bone necrosis, which demonstrated
its use as a possible alternative to autografts (7–9).
However, soluble BMP-2 is rapidly degraded by proteolytic enzymes
in the body, making it difficult to achieve a stable maintenance
dose for treatment throughout the period of bone regeneration.
Furthermore, particularly in larger animal models and humans,
current delivery methods require high levels of BMP-2 to exert a
biological function (10). Using
of high levels of BMP-2 is associated with several issues,
including safety and the high cost of treatment (11). Several strategies are being
developed to decrease the high doses of BMP-2 required, one of
which is to develop a controlled-release delivery system, which not
only prevents the fast degradation of BMP-2, but also provides
continued release throughout the entire process of bone formation
(12–14). Other possible approaches include
augmenting bone healing using different growth factors, cytokines
or chemokines as co-therapeutics with treatments such as BMP-2
(15–17).
Stromal cell-derived factor-1 (SDF-1) belongs to the
proinflammatory CXC chemokine family (18); its receptor is CXC chemokine
receptor 4 (CXCR4) and they are expressed in various tissues
(19,20). The binding of SDF-1 to its
complementary receptor, CXCR4, induces the homing of stem cells to
the bone marrow, and previous investigations have demonstrated that
SDF-1 is an important chemokine in recruiting stem and progenitor
cells for tissue restoration following injury, including during the
acute phase of bone repair (21–24).
Several reports have also shown that SDF-1 directly regulates the
signaling during BMP-2-induced mesenchymal cell progression towards
osteogenic differentiation in vitro (25,26)
and in vivo (27–29). In our previous study, a
controlled-release system of BMP-2 cross-linked on a collagen disc
was developed. The BMP-2 was chemically conjugated onto the
collagen disc using Traut's reagent and the cross-linker,
4-(N-maleimi-domethyl) cyclohexane-1-carboxylic acid
3-sulfo-N-hydroxysuccinimide ester sodium salt (Sulfo-SMCC)
(30). This delivery system was
found to be suitable for use in bone tissue engineering.
In the present study, the chemical combination
method using Traut's reagent and the Sulfo-SMCC cross-linker was
adapted to chemically conjugate SDF-1α on collagen discs. It was
hypothesized that this method can significantly increase the
retention of SDF-1α on the collagen scaffold without decreasing its
biological activity, and reduce the rate of release. The SDF-1α
binding and release rates were determined following cross-linking
in vitro, and cell homing assays were performed to
investigate the biological function of SDF-1α chemically conjugated
on the collagen disc. It was further hypothesized that the
co-delivery of BMP-2 and SDF-1α cross-linked on collagen scaffolds
enhances bone formation, whilst requiring significantly reduced
levels of BMP-2. By evaluating ectopic bone growth 28 days
following implantation in rats, the present study investigated the
cooperative osteogenic effect of SDF-1α and a suboptimal dose of
BMP-2, which were cross-linked on separate collagen discs. The
current study aimed to investigate whether the strategy
investigated may enable reductions in BMP-2 doses, consequently
reducing the risk of side effects and the cost of therapy.
Materials and methods
Collagen discs
The collagen membranes, derived from ox leather,
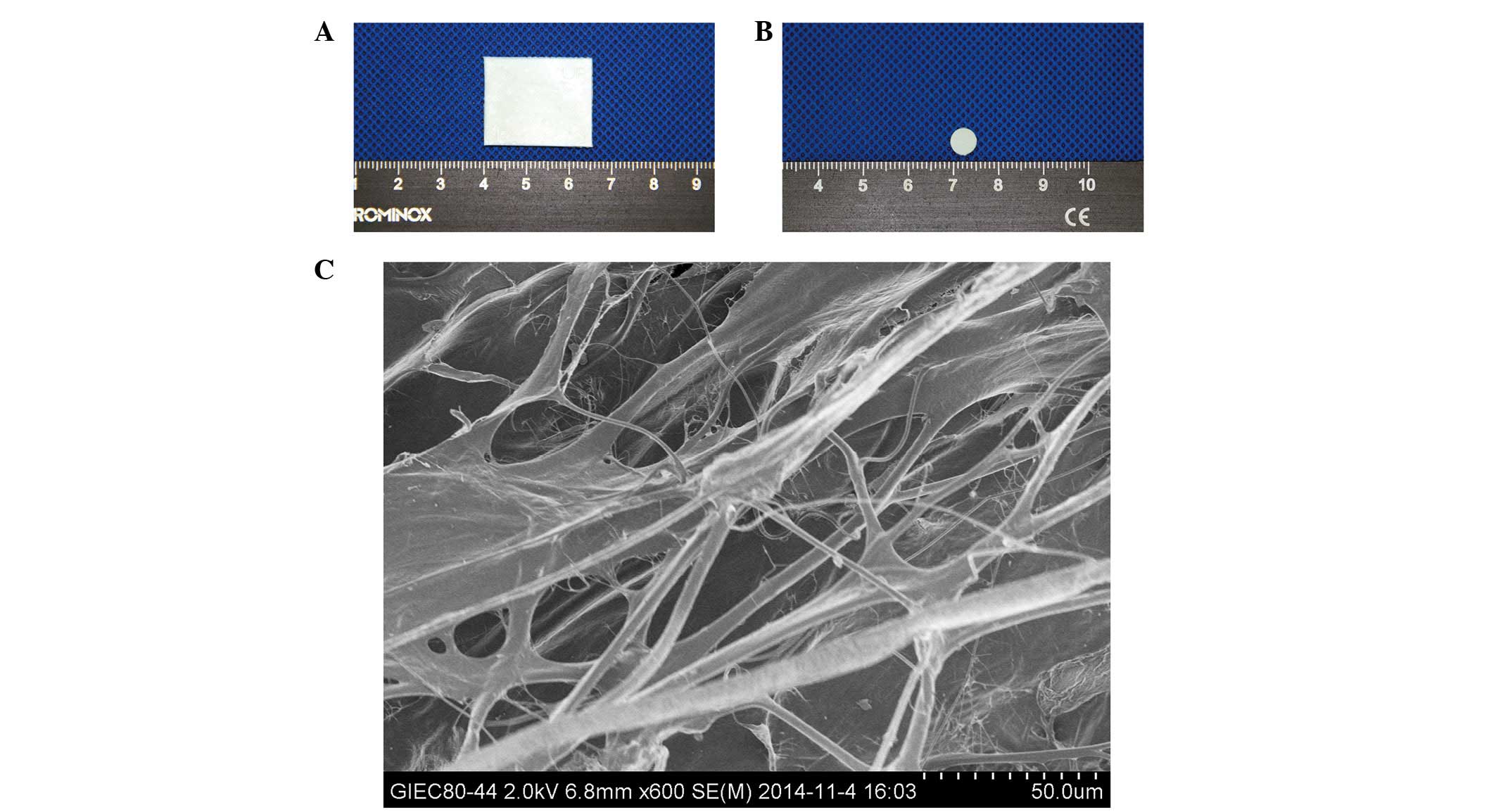
(Fig. 1A) were provided by
Zhenghai Biotechnology (Shandong, China). The uniform round
collagen discs (6.4 mm diameter; 0.5 mm thickness; Fig. 1B) were sterilized using 12 kGy
Co-60 irradiation (GM-II; Beijing Gamma High-tech Co., Ltd.,
Beijing, China) and were prepared, cut with a hole puncher into
uniform discs with a diameter of 6.4 mm and thickness of 0.5 mm, as
described previously (30,31). The collagen discs were observed
under a scanning electron microscope (SEM; model S-4800; Hitachi,
Tokyo, Japan; Fig. 1C).
Collagen scaffold modification using
Traut's reagent
Sulfhydryl (SH) groups were added to the collagen
discs, according to previously described methods (31). Briefly, Traut's reagent
(2-iminothiolane hydrochloride; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA) was dissolved in phosphate-buffered saline (PBS)
and 4 mM ethylenediamine tetraacetic acid disodium (EDTA; Guangzhou
Chemical Reagent Company, Guangzhou, China), with a final
concentration of 2.5 mg ml −1. The collagen discs were
placed into 100 μl of Traut's reagent solution in a 96-well
plate. After 12 h incubation at 4°C, the collagen-SH samples were
rinsed three times with 100 μl PBS and 4 mM EDTA (5
min/wash).
Cross-linking of SDF-1α on the collagen
disc using Sulfo-SMCC
According to the manufacturer's protocol, a 50-fold
molar overdose of Sulfo-SMCC (Sigma-Aldrich, St. Louis, MO, USA)
was mixed with SDF-1α (recombinant rat SDF-1α; PeproTech, Rocky
Hill, NJ, USA) at room temperature for 5 min. The SDF-1-SMCC
complex solution (100 μl) was then incubated with the
collagen-SH for 1 h at 4°C to form the collagen-SH-SMCC construct.
The collagen-SH-SMCC construct was rinsed three times with PBS (5
min/wash) on a shaker to remove any non-binding SDF-1α or excess
cross-linking agent.
Efficiency of SDF-1α retention on the
collagen scaffolds
The collagen discs were divided randomly into a
cross-linked collagen group and a physical adsorbed collagen group
to assess the efficiency of the conjugation of SDF-1α. The collagen
discs in the cross-linked group were modified using Traut's
reagent, as described above, and serially diluted SDF-1α (20, 10,
5, 2.5, 1.25 and 0 μgml−1) was combined with the
Sulfo-SMCC, as described above. For each SDF-1α concentration, the
collagen-SH was soaked in 100 μl of the SDF-1α-SMCC solution
for 1 h at 4ºC. The collagen discs in the physical adsorbed
collagen group were soaked in 100 μl PBS for 12 h at 4ºC,
and were then immersed in each SDF-1α concentration solution in PBS
for 1 h at 4ºC The cross-linked collagen group and the physical
adsorbed collagen group were then washed with PBS (three times; 5
min/wash) to remove any non-binding SDF-1α or excess cross-linking
agent.
The present study used the improved direct
enzyme-linked immunosorbent assay (ELISA) technique to quantify the
retention of SDF-1α on the collagen discs; this improved ELISA was
successfully used in our previous studies and those of others
(30–33). Briefly, the discs were blocked with
100 μl bovine serum albumin (50 mg ml−1; MP
Biomedicals, Solon, OH, USA) in PBS for 1 h at room temperature in
a 96-well plate. Following washing twice in PBS with 0.05% Tween-20
(PBST), the discs were incubated with 100 μl rabbit
polyclonal antibody to SDF-1α (1:1,000 in PBS; ab9797; Abcam,
Cambridge UK) for 1 h at room temperature. Following washing three
times in PBST for 5 min, 100 μl of the AffiniPure goat
anti-rabbit IgG (1:10,000 in PBS; E030220-01; EarthOx Life
Sciences, San Francisco, CA, USA) conjugated with alkaline
phosphatase (AP) was added for 1 h at room temperature. Following
washing, as above, three times, 200 μl of 2 mg
ml−1 phosphorylated-nitrophenyl phosphate hexahydrate
(Sigma-Aldrich) in AP buffer, containing 100 mM NaCl, 10 mM
MgCl2 and 100 mM Tris HCl (pH 9.6) was added for 15 min.
The bound protein was determined by removing 100 μl of the
colored substrate solution into a separate 96-well plate and
measuring at an absorbance/optical density (OD) of 405 nm (OD405)
in an ELISA reader (Infinite 2000; Tecan Group, Ltd., Männedorf,
Switzerland).
Release kinetics of SDF-1a cross-linked
on collagen scaffolds
SDF-1α (1 μg in 100 μl) was loaded
onto collagen discs using the cross-linking or physical adsorption
techniques, according to the methods described above. The collagen
discs were immersed in 100 μl PBS + 0.02% sodium azide
(Sigma-Aldrich). After 0, 12, 24, 48, 72, 96 and 120 h on a shaker
at 37ºC, the dose of SDF-1α immobilized on the collagen discs at
each time point was analyzed using ELISA, as described above.
In vitro cell-homing
The bioactivity of SDF- 1α cross-linked on collagen
was assessed by analyzing the chemotaxis of mesenchymal stem cells
(MSCs) in response to the SDF-1α stimulus using a Transwell (Boyden
chamber) migration assay. Sprague-Dawley rat bone MSCs were
isolated and purified for culture in vitro and serial
passage by the attachment culture method in basal cell culture
media. The third-generation cells were digested with 0.25% trypsin
and 0.02% EDTA to obtain a single-cell suspension. The rat MSCs
(2×104 cells in 250 μl) were placed in the upper
chamber of 24-well Transwell inserts containing polycarbonate
membranes with 8-μm pores (Corning Costar, Corning, NY,
USA). Blank collagen scaffolds and the collagen scaffolds
cross-linked or physically adsorbed with SDF-1α (1 μg) were
placed in the lower chamber of each Transwell containing 500
μl of Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific, Inc.) supplemented with 2% fetal bovin serum (Thermo
Fisher Scientific, Inc.). Following incubation at 37ºC for 24 h,
the cells were fixed in 4% paraformaldehyde (Guangzhou Chemical
Reagent Company) for 15 min. The cells retained on top of the
polycarbonate membrane of each Transwell were removed. The migrated
cells in the lower chamber of the Transwells were analyzed by
staining with 4′,6-diamidino-2-phenylindole (DAPI; Beijing Leagene
Biotechnology Co., Ltd., Beijing, China) and were detected in five
randomly selected fields (0.25 mm2; magnification, ×200;
Axiovert 40 C inverted fluorescence microscope; Zeiss, Oberkochen,
Germany). The mean numbers of cells from the five fields were
calculated. The experiments were repeated three times.
Osteogenic activity of SDF-1α and
suboptimal doses of BMP-2 cross-linked on collagen discs following
subcutaneous implantation in rats
A total of 24 male Sprague-Dawley rats (weight,
220–250 g; age, 6~8 weeks) were housed in a temperature- (24~26ºC)
and light- (12/12 h light/dark cycle) controlled environment, with
free access to food and water. The rats were used to evaluate the
osteogenic activity of SDF-1α and suboptimal doses of BMP-2
cross-linked on collagen discs. The animal experiments were
approved by the Animal Care and Research Committee of Sun Yat-sen
University (Guangzhou, China). Animal care and surgical procedures
were performed in accordance with the guidelines of Guangdong
Association on Laboratory Animal Care (Guangdong, China).
BMP-2 (2.5 μg rhBMP-2; PeproTech, Rocky Hill,
NJ, USA) and SDF-1α (1 μg rmSDF-1α; PeproTech) were loaded
onto separate sterile collagen discs in a biosafety cabinet and
transported for surgery in sterile boxes. Surgery was performed
with the animals above a heated pad. The rats were anesthetized via
an intraperitoneal injection of 30 mg kg−1 pentobarbital
sodium (Military Veterinary Institute, Changchun, China). The
dorsal surface of the implant sites were shaved and disinfected
using a 10% betadine solution (Guangzhou Chemical Reagent Company).
A total of four 0.8-cm transverse incisions were made, ~3 cm apart,
on the dorsal midline of the rat, and the skin and subcutaneous
tissues were bluntly dissected to form subcutaneous pockets. The
four groups of implants were as follows: i) Blank control group,
the implant contained two blank collagen scaffolds; ii) suboptimal
BMP-2-cross-linked group, the implant contained one blank collagen
scaffold and one collagen scaffold cross-linked with 2.5 μg
BMP-2; iii) SDF-1α/suboptimal BMP-2-physically adsorbed collagen
group, the implant contained one collagen scaffold adsorbed with 1
μg SDF-1α and one collagen scaffold adsorbed with 2.5
μg BMP-2; iv) SDF-1α/suboptimal BMP-2-cross-linked group,
the implant contained one collagen scaffold cross-linked with 1
μg SDF-1α and one collagen scaffold cross-linked with 2.5
μg BMP-2. The two overlapped discs were fixed with
absorbable sutures (Vicryl; Johnson & Johnson, Shanghai, China)
forming a compound implant. The four groups of implants were
randomly inserted into the pockets of each rat. The rats were
housed with one animal/cage and fed a normal diet and water. A
total of six animals were sacrificed 4 weeks post-implantation by
injection with an overdose of pentobarbital sodium, and the discs
were carefully removed. The specimens were fixed in 4%
paraformaldehyde for 2 days, embedded in paraffin (Guangzhou
Chemical Reagent Company) and cut into 5-μm sections. The
sections were stained with von Kossa staining (1% silver nitrate
solution and counterstained with hematoxylin and eosin staining;
Guangzhou Chemical Reagent Company). Quantitative analysis of the
percentage of calcium content was performed using Image-Pro Plus
6.0 software (Media Cybernetics, Inc., Silver Spring, MD, USA).
Statistical analysis
All statistical data were analyzed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Independent two-tailed
t-tests were used for two group comparison, and one-way
analysis of variance, followed by Tukey's test was used for
multiple group comparisons. All data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
Properties of collagen membranes
The SEM examination of the collagen membranes,
obtained from Zhenghai Biotechnology, showed that the surface
contained multiple pores (range, 50-200 μm), and collagen
fibers were loosely packed and linked with each other to form a
three-dimensional network structure (Fig. 1C). This pore size range is
conducive to the attachment of cells and growth factors (31), and the stable and mechanical
structure of the collagen were suitable for cell guidance and
attachment. Collagen has several beneficial properties, including
low antigenicity, biocompatibility and biodegradability (34,35),
which has led to it being widely used in the development and wound
repair of organs and tissues, including skin, bone and the vascular
system (36–38).
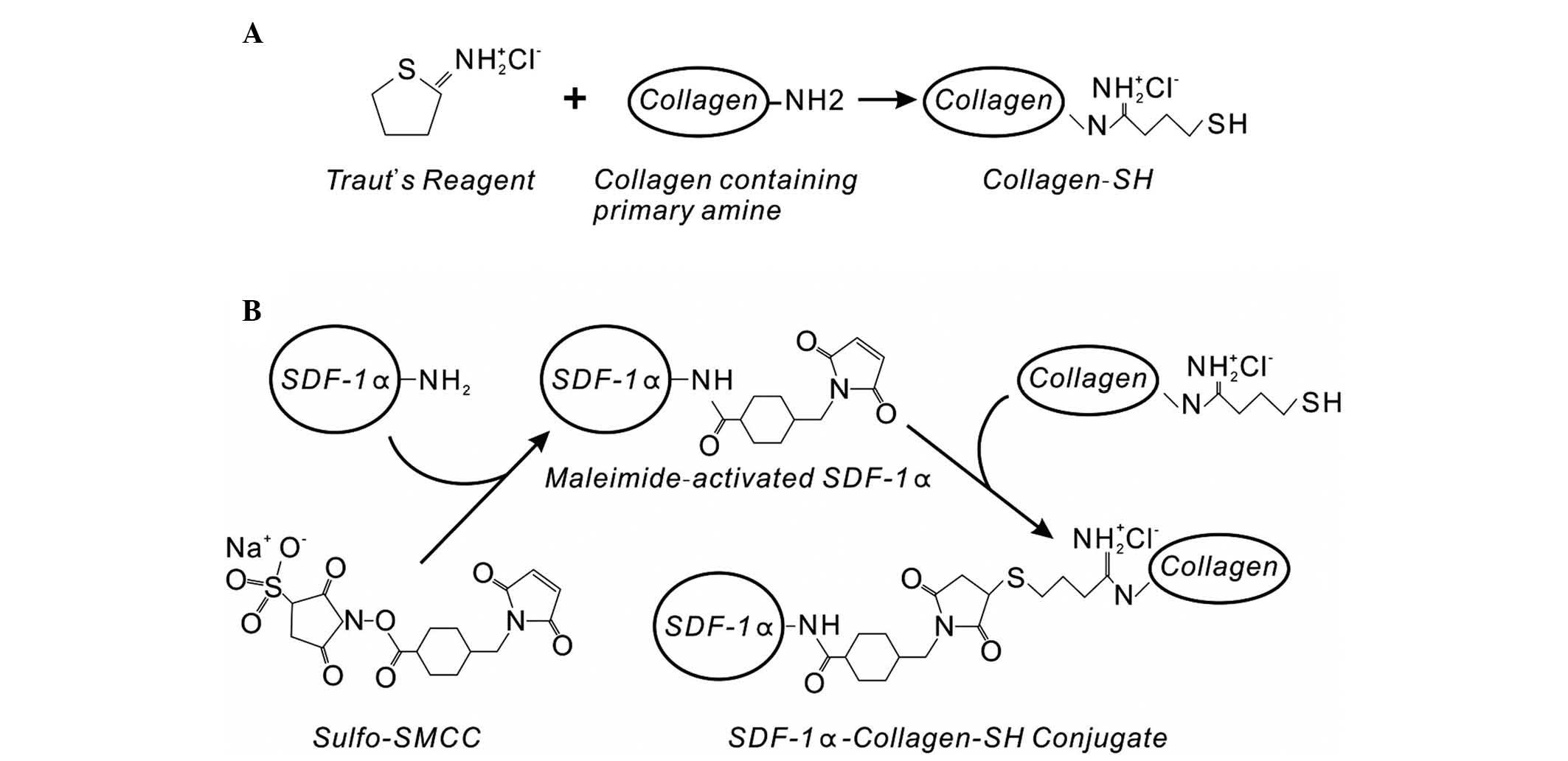
SDF-1α conjugation on collagen-SH discs
using Sulfo-SMCC
In our previous experiment, collagen-SH discs
(Fig. 2A) were formed by treating
collagen membranes with Traut's reagent, which can add SH groups to
primary amines (39). It was found
that saturation of the SH groups on the small round collagen discs
was achieved at a concentration of 2.5 mg ml−1 of
Traut's reagent (31). The
cross-linker, Sulfo-SMCC, was then used to conjugate vascular
endothelial growth factor (VEGF) or BMP-2 on the collagen-SH
scaffolds, and the biological function of these growth factors was
maintained (30,31).
In the present study, SDF-1α was cross-linked on the
collagen scaffolds using the above strategy with Traut's reagent
and Sulfo-SMCC. The same (2.5 mg ml−1) concentration of
Traut's reagent was used to form the collagen-SH scaffolds. The
Sulfo-SMCC cross-linker reagent can covalently conjugate amine- and
SH-containing molecules. The amino groups of SDF-1α can create
amide bonds with the maleimide groups of Sulfo-SMCC to form
maleimide-activated SDF-1α (Fig.
2B), which selectively reacts with the collagen-SH scaffolds
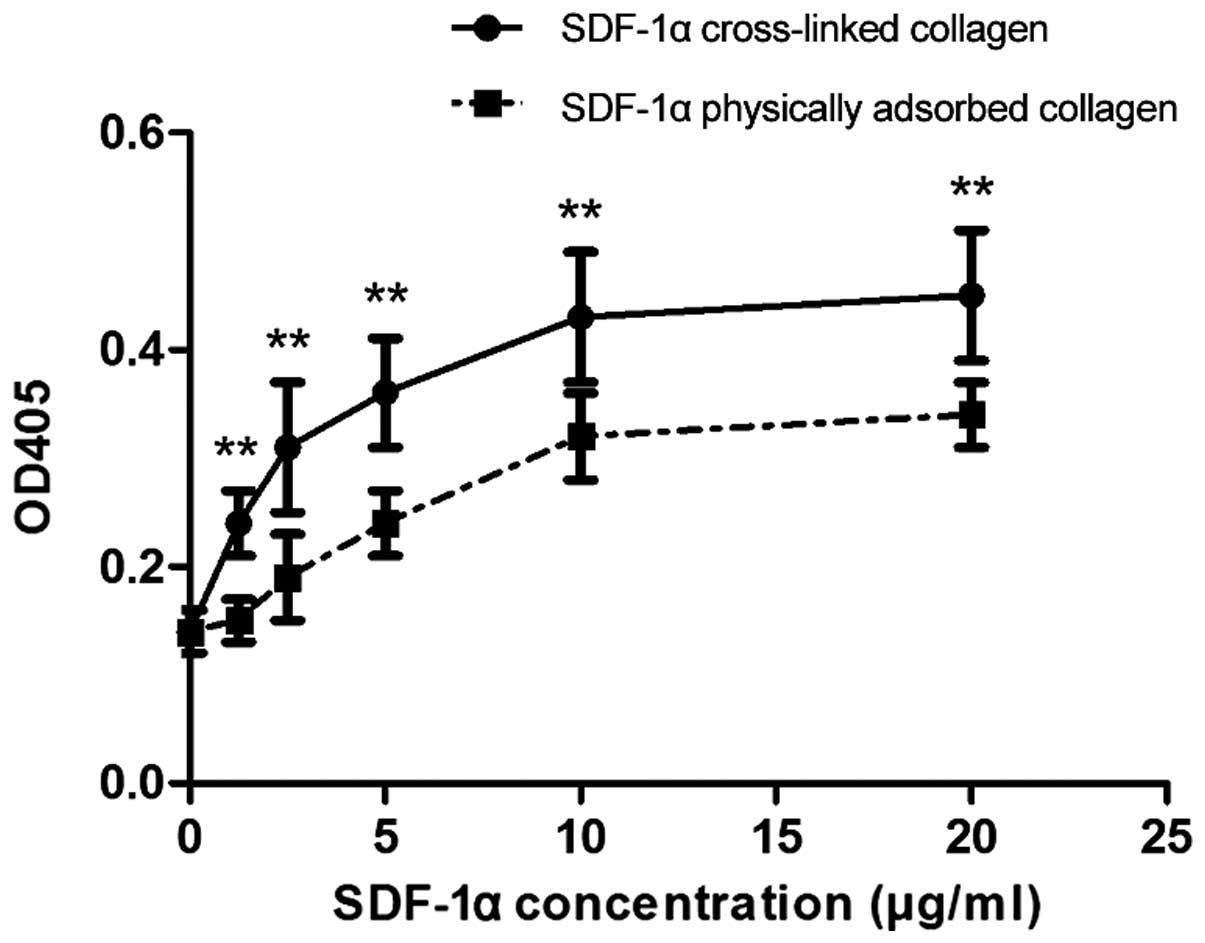
made by Traut's reagent. The results from the ELISA indicated that
SDF-1α at concentrations >1.25 μg ml−1 showed
improved immobilization on the collagen-SH disc using Sulfo-SMCC,
compared with physical adsorption (Fig. 3). The immobilized SDF-1α directly
bound to the collagen scaffold reduced its distribution and
degradation, thus reducing the concentration of SDF-1α required.
The covalently conjugated SDF-1α on the collagen scaffold was able
to maintain an effective concentration of SDF-1α at the target
location.
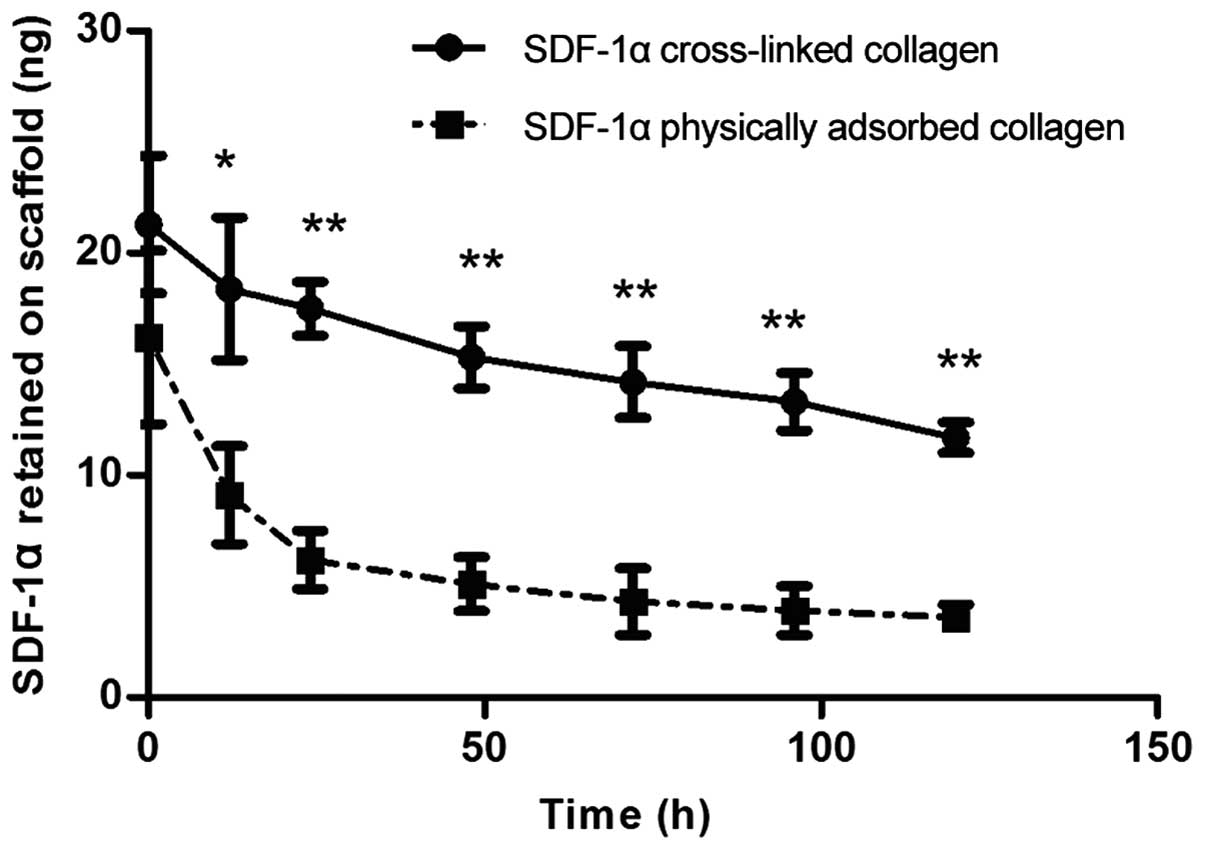
Release kinetics of SDF-1α post
cross-linkage
The release of SDF-1α cross-linked or physically
adsorbed on the collagen scaffolds within a 120 h period was
detected by analyzing the remaining protein on the collagen
scaffolds at each time point (Fig.
4). The cross-linked SDF-1α showed decreased release, compared
with the physically adsorbed SDF-1α, and this release occurred in a
controlled time-dependent manner within the 120 h period. The
physically adsorbed SDF-1α typically exhibited rapid release during
the first 24 h, releasing almost 60% of the SDF-1α adsorbed on the
collagen scaffold, compared with 20% of the SDF-1α cross-linked on
the collagen scaffold in the same period. Although equal doses of
SDF-1α were applied on the collagen scaffolds in each group, a high
level of SDF-1α remained in the cross-linked group, compared with
the physical adsorption group at 0 h, even with repeated washes
during the assessment, which may be attributed to the manner of the
binding of SDF-1α. These results verified that cross-linked
collagen scaffolds may be used for sustained chemokine-release.
Therapeutic growth factors are required to bind
non-specifically to tissue constructs, maintain their conformation,
and be released in a manner that does not affect their biological
functions. Optimal carriers of therapeutic growth factors have two
predominant properties: i) The degradation rate of the carrier is
consistent with the rate of tissue repair from several weeks to
several months, with maintained release of appropriate growth
factors; and ii) have a high surface area to volume ratio and a
sufficient number of interconnected pores to provide sufficient
space, and a conducive environment for cellular growth and
neovascularization (4). Collagen,
the predominant protein component of natural extracellular matrix,
has been extensively investigated as a natural scaffold in tissue
engineering, and its capability to deliver growth factors to induce
bone formation has been well established (40). In our previous study, it was
confirmed that the degradation rate of cross-linked collagen discs
was reduced, compared with normal collagen discs, which was of
benefit to tissue restoration (31). In the present study, the ability to
control the slow release of SDF-1α conjugated to collagen improved
its effectiveness as a cell-homing agent by reducing the
requirement for continuous replenishment of the SDF-1α, which is
constantly lost by diffusion and/or degradation by exopeptidases
and matrix metalloproteinases in an inflammatory environment in
vivo (41).
In vitro cell-homing
The bioactivity of SDF-1α cross-linked on collagen
scaffolds was evaluated using a modified Boyden chamber (Transwell
migration) assay, to determine whether the released SDF-1α
maintained its biological function as a chemoattractant for stem
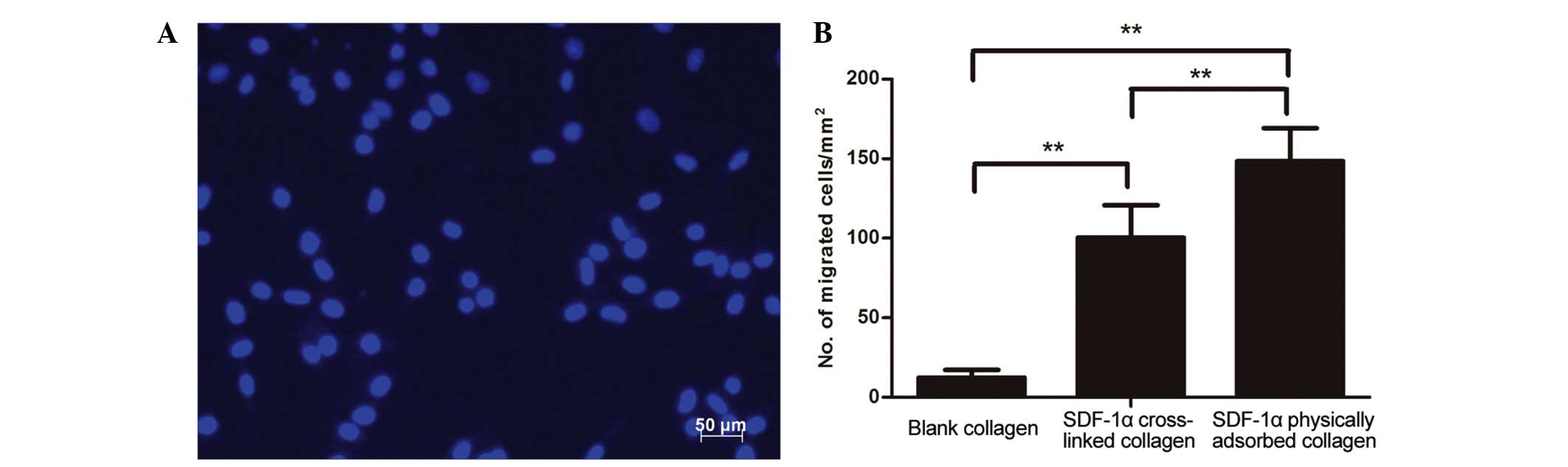
cells. As shown in Fig. 5A and B,
the nuclei of the migrated MSCs were stained blue with DAPI, and
increased MSC migration was observed from the top to the bottom of
the Transwell in the SDF-1α cross-linked collagen group, compared
with the blank collagen group. The chemotactic effect of the SDF-1α
released from chemokine-loaded collagen scaffolds caused the MSCs
to migrate from the top to the bottom of the Transwells. Compared
with the SDF-1α physically absorbed collagen group, the SDF-1α
cross-linked collagen group exhibited significantly reduced homing
effects, which may be explained by the burst release of SDF-1α in
the SDF-1α physical absorbed collagen group during the first 24 h.
It was concluded that SDF-1α retained on cross-linking collagen
scaffolds had preserved biological activity and slower release.
Ectopic bone formation in vivo
In addition to strategies, which reduce the high
levels of BMP-2 required, other approaches incorporate the
co-delivery of different growth factors, cytokines or chemokines
with treatments, including BMP-2, to augment bone healing. These
approaches simultaneously deliver two growth factors to a target
site. However, each growth factor possesses different functions to
regenerate tissues, including bone and blood vessels, requiring
appropriate combinations of growth factors for cooperative action
in regulating intact tissue regeneration (4).
In the present study, a controlled-release system
for SDF-1α and BMP-2 cross-linked on collagen scaffolds was
developed using the chemical conjugate method with Traut's reagent
and the Sulfo-SMCC cross-linker. The SDF-1α cross-linked on the
collagen disc almost reached saturation at concentrations >10
μg ml 1 SDF-1α. For the BMP-2 cross-linked on the collagen
scaffold, the same conditions were used as in our previous study,
and were those of Higashino et al with modification
(28), in which a minimum
threshold dose of rhBMP-2 (2.5 μg) as the suboptimal dose on
each collagen pellet was used in an athymic rat ectopic bone
formation model. ABMP-2 concentration of 25 μg ml 1 (2.5
μg in 100 μl) was used as the suboptimal dose for
cross-linking on the collagen scaffold. Each implant site contained
two collagen membranes fixed by absorbable sutures, and each
collagen membrane delivered one growth factor conjugated on the
collagen scaffold. This method was simple and feasible as a
delivery mechanism.
The present study analyzed the ectopic osteogenic
effects of the blank collagen group, sub-optimal BMP-2-cross-linked
collagen group, SDF-1α/suboptimal BMP-2-physically adsorbed
collagen group and SDF-1α/suboptimal BMP-2-cross-linked collagen
group using von Kossa staining. The von Kossa staining technique
can detect deposits of calcium or calcium salt, with positive
staining shown as dark brown regions, indicating the presence of
mineral content (42). The newly
formed bone was quantified by analyzing the percentage of calcified
bone tissue. Negative staining in the blank group and suboptimal
BMP-2-cross-linked group were observed. The SDF- 1α/suboptimal
BMP-2-physically adsorbed group and SDF-/suboptimal
BMP-2-cross-linked group exhibited dark brown staining, indicating
bone formation (Fig. 6). The
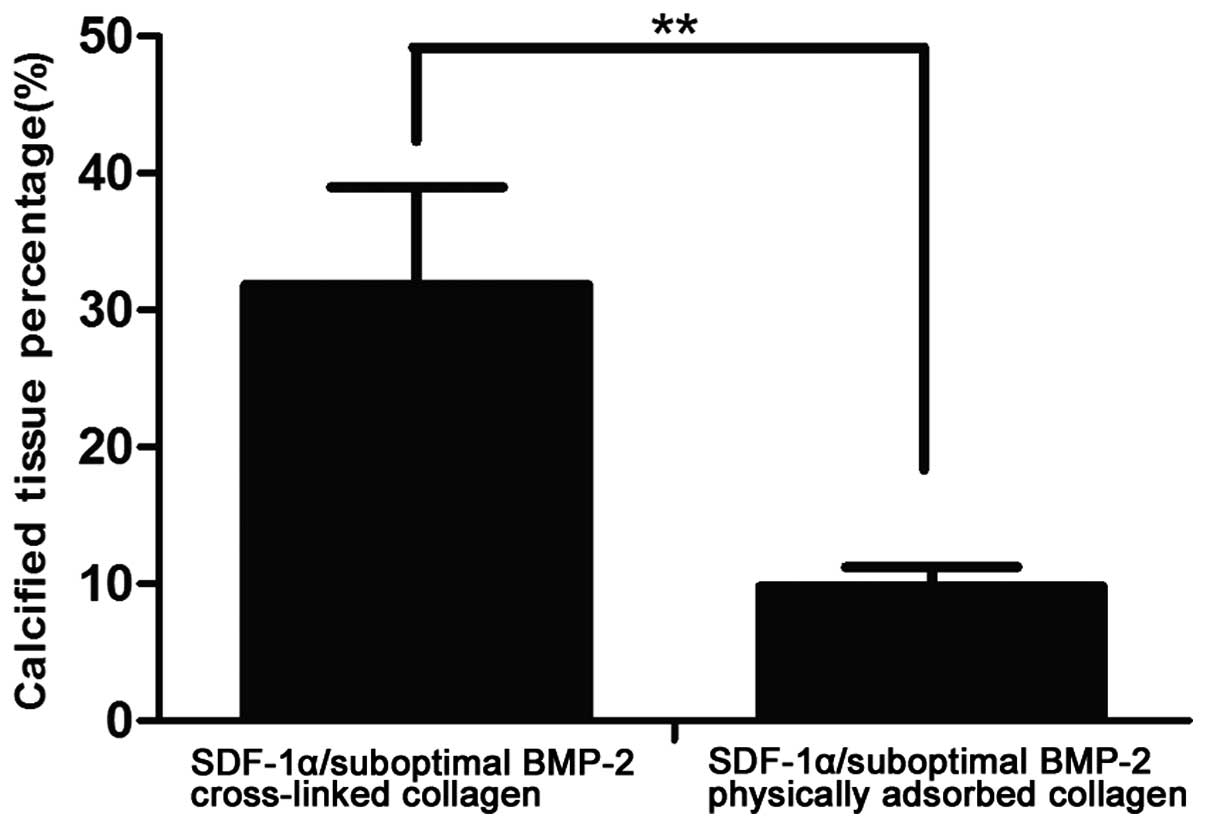
quantification of the percentages of calcified tissue showed that
the SDF-1α/suboptimal BMP-2-cross-linked group (31.86±7.10%)
induced a high level of calcium deposition, compared with the
SDF-1α/suboptimal BMP-2-physically adsorbed group (9.82±1.39%;
Fig. 7). Statistical analysis
revealed that the difference between the two groups was significant
(P<0.01). Furthermore, SDF-1α/suboptimal BMP-2 cross-linked
group showed higher calcium density, as indicated by darker
positive staining, compared with the SDF-1α/suboptimal
BMP-2-physically adsorbed group. This indicated that the rates in
the SDF-1α/suboptimal BMP-2-cross-linked group had a higher level
of ectopic bone regeneration. These findings further verified that
this delivery vehicle was effective for growth factors, and they
showed that SDF-1α potentiated suboptimal levels of BMP-2 to induce
bone regeneration, whereas suboptimal BMP-2 alone was not able to
induce bone regeneration.
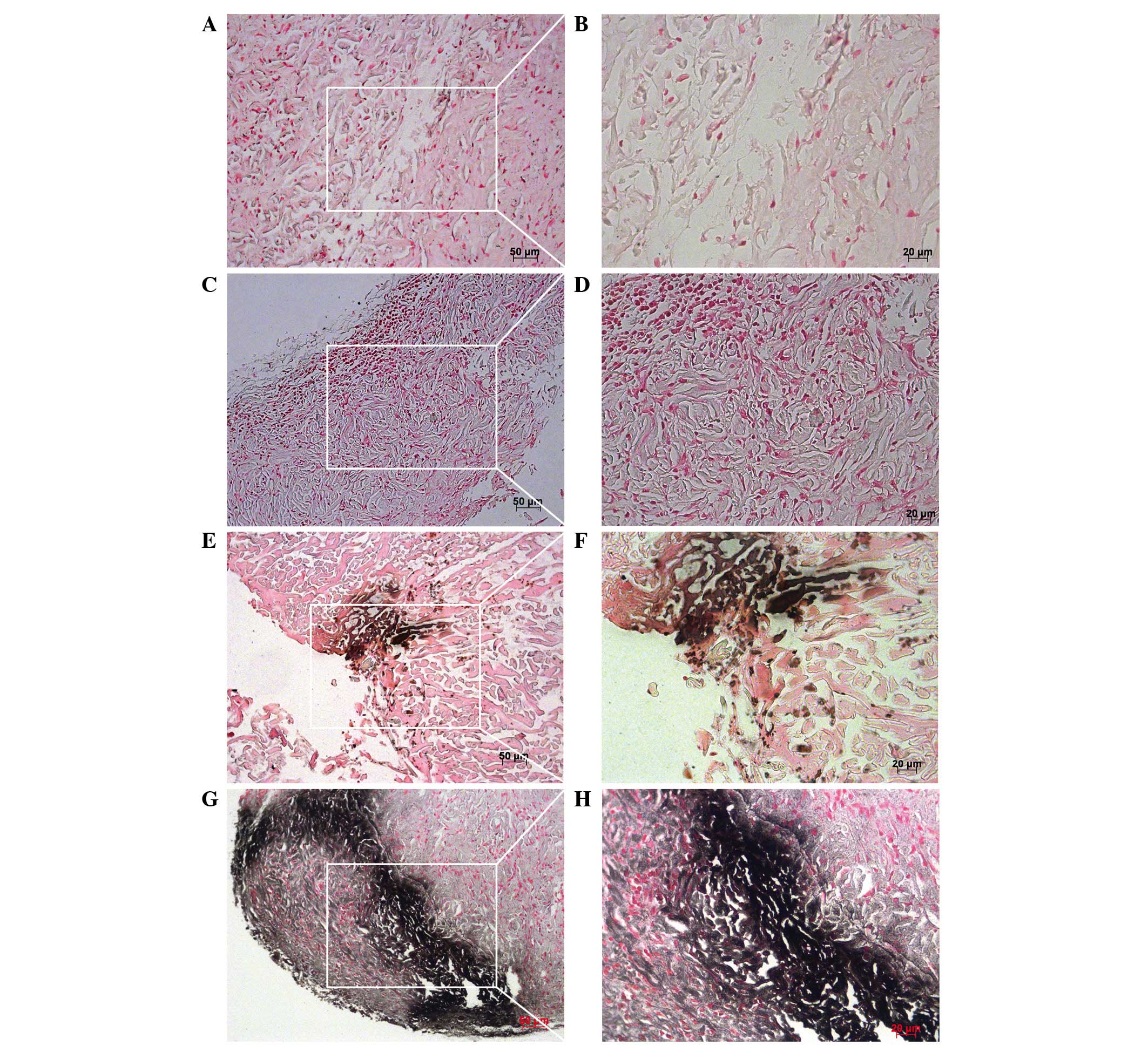 | Figure 6Von Kossa staining 4 weeks
post-implantation. The black/brown staining shows positive calcium
deposition. (A and B) The blank collagen group; (C and D) the low
dose BMP-2 cross-linked collagen group; (E and F) the SDF-1α/low
dose BMP-2-physically adsorbed collagen group; and (G and H) the
SDF-1α/low dose BMP-2 cross-linked collagen group. The white frames
indicate the magnification areas. (A, C, E, G), scale bar = 50
μm, magnification, ×200; (B, D, F, H), scale bar = 20
μm, magnification, ×400. SDF-1α, stromal cell-derived
factor-1α; BMP-2, bone morphogenetic protein-2. |
Richardson et al reported on a novel vehicle,
which allowed for the tissue-specific delivery of two or more
growth factors with distinct kinetics, and their findings showed
that the dual delivery of VEGF-165 and platelet-derived growth
factor-BB resulted in the rapid formation of a mature vascular
network (43). The cooperative
effects of the co-delivery of BMP-2 with growth factors, including
VEGF, transforming growth factor β-3 or insulin-like growth
factor-1 have been investigated in vitro and in vivo
using structural polymer scaffolds for bone tissue regeneration and
engineering utility (44–47). Previously, several studies have
focused on the cooperative effects of BMP-2 and SDF-1. Herberg
et al reported that SDF-1β had potent synergistic effects on
BMP-induced local bone formation and may be a suitable candidate
for the optimization of bone augmentation, which used significantly
lower levels of BMP-2 for spinal, orthopedic and craniofacial
treatments (27). In this previous
study, BMP-2 and SDF-1 were physically adsorbed onto a collagen
sponge by soak-loading. Higashino et al reported that the
addition of SDF-1 to implants containing a suboptimal level of
BMP-2 enhanced the mobilization and migration of MOPCs to the
implant and induced ectopic bone formation (28). BMP-2 and SDF-1 were also physically
adsorbed on the collagen pellets by soak-loading. There are certain
limitations, including higher levels of growth factors and rapid
release, in the reports on the physical adsorption of growth
factors by soak-loading. Herberg et al also reported on a
delivery system for the sustained release of a low-dose growth
factor using biopatterning technology, which assisted in the
healing of CSD injuries. It was found that SDF-1β augmented the
ability of BMP-2 to drive the healing (48). The biopatterning technology was
used to achieve the controlled release of growth factors. In the
present study, SDF-1α and BMP-2 directly cross-linked on collagen
were used to obtain slow controlled release and reduce the dose of
growth factors required. The results of the present study
conceptualize a process whereby the initial release of SDF-1α from
a collagen scaffold may stimulate stem cell migration to the
collagen scaffold, which subsequently promoted bone formation in
the presence of suboptimal levels of BMP-2.
The findings from the present study indicated that a
higher level of SDF-1α was retained on the collagen disc applying
Traut's reagent and the Sulfo-SMCC cross-linker, with intact
biological function. Accordingly, the SDF-1α cross-linked collagen
group showed more marked binding ability and improved controlled
release, compared with the SDF- 1α physically adsorbed collagen
group. Co-therapy with SDF-1α and suboptimal levels of BMP-2
chemically conjugated on collagen scaffolds enhanced ectopic bone
formation. This strategy may enable decreases in doses of BMP-2,
consequently reducing the risk of side effects and the costs of
therapy.
Acknowledgments
The authors would like to thank all the members of
Guangdong Provincial Key Laboratory of Stomatology (Guangdong,
China) for their technical assistance. This study was supported by
the National Natural Science Foundation of China (grant no.
81100734) and the Natural Science Foundation of Guangdong of China
(grant no. S2013010015805).
References
|
1
|
Dimitriou R, Jones E, McGonagle D and
Giannoudis PV: Bone regeneration: Current concepts and future
directions. BMC Med. 9:662011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz CE, Martha JF, Kowalski P, Wang
DA, Bode R, Li L and Kim DH: Prospective evaluation of chronic pain
associated with posterior autologous iliac crest bone graft harvest
and its effect on postoperative outcome. Health Qual Life Outcomes.
7:492009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim DH, Rhim R, Li L, Martha J, Swaim BH,
Banco RJ, Jenis LG and Tromanhauser SG: Prospective study of iliac
crest bone graft harvest site pain and morbidity. Spine J.
9:886–892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vo TN, Kasper FK and Mikos AG: Strategies
for controlled delivery of growth factors and cells for bone
regeneration. Adv Drug Deliv Rev. 64:1292–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schindeler A, McDonald MM, Bokko P and
Little DG: Bone remodeling during fracture repair: The cellular
picture. Semin Cell Dev Biol. 19:459–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnes GL, Kostenuik PJ, Gerstenfeld LC
and Einhorn TA: Growth factor regulation of fracture repair. J Bone
Miner Res. 14:1805–1815. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nauth A, Ristevski B, Li R and Schemitsch
EH: Growth factors and bone regeneration: How much bone can we
expect? Injury. 42:574–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannoudis PV and Einhorn TA: Bone
morphogenetic proteins in musculoskeletal medicine. Injury.
40(Suppl 3): S1–S3. 2009.
|
|
9
|
Lieberman JR, Daluiski A and Einhorn TA:
The role of growth factors in the repair of bone. Biology and
clinical applications. J Bone Joint Surg Am. 84-A:1032–1044.
2002.PubMed/NCBI
|
|
10
|
Seeherman H and Wozney JM: Delivery of
bone morphogenetic proteins for orthopedic tissue regeneration.
Cytokine Growth. Factor Rev. 16:329–345. 2005. View Article : Google Scholar
|
|
11
|
Argintar E, Edwards S and Delahay J: Bone
morphogenetic proteins in orthopaedic trauma surgery. Injury.
42:730–734. 2011. View Article : Google Scholar
|
|
12
|
Kroese-Deutman HC, Ruhé PQ, Spauwen PH and
Jansen JA: Bone inductive properties of rhBMP-2 loaded porous
calcium phosphate cement implants inserted at an ectopic site in
rabbits. Biomaterials. 26:1131–1138. 2005. View Article : Google Scholar
|
|
13
|
Ishibe T, Goto T, Kodama T, Miyazaki T,
Kobayashi S and Takahashi T: Bone formation on apatite-coated
titanium with incorporated BMP-2/heparin in vivo. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 108:867–875. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang HS, La WG, Bhang SH, Lee TJ, Lee M
and Kim BS: Apatite-coated collagen scaffold for bone morphogenetic
protein-2 delivery. Tissue Eng Part A. 17:1–12. 2011. View Article : Google Scholar
|
|
15
|
Fu TS, Chang YH, Wong CB, Wang IC, Tsai
TT, Lai PL, Chen LH and Chen WJ: Mesenchymal stem cells expressing
baculovirus-engineered BMP-2 and VEGF enhance postero-lateral spine
fusion in a rabbit model. Spine J. 15:2036–2044. 2015. View Article : Google Scholar
|
|
16
|
Huang RL, Chen G, Wang W, Herller T, Xie
Y, Gu B and Li Q: Synergy between IL-6 and soluble IL-6 receptor
enhances bone morphogenetic protein-2/absorbable collagen
sponge-induced bone regeneration via regulation of BMPRIA
distribution and degradation. Biomaterials. 67:308–322. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang HD, Lee JT, Koh JT, Jung HM, Lee HJ
and Kwon TG: Sequential treatment with SDF-1 and BMP-2 potentiates
bone formation in calvarial defects. Tissue Eng Part A.
21:2125–2135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bleul CC, Farzan M, Choe H, Parolin C, Cla
rk-Lewis I, Sodroski J and Springer TA: The lymphocyte
chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1
entry. Nature. 382:829–833. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng Y, Broder CC, Kennedy PE and Berger
EA: HIV-1 entry cofactor: Functional cDNA cloning of a
seven-transmembrane, G protein-coupled receptor. Science.
272:872–877. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iannone M, Ventre M, Pagano G, Giannoni P,
Quarto R and Netti PA: Defining an optimal stromal derived factor-1
presentation for effective recruitment of mesenchymal stem cells in
3D. Biotechnol Bioeng. 111:2303–2316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naderi-Meshkin H, Bahrami AR, Bidkhori HR,
Mirahmadi M and Ahmadiankia N: Strategies to improve homing of
mesenchymal stem cells for greater efficacy in stem cell therapy.
Cell Biol Int. 39:23–34. 2015. View Article : Google Scholar
|
|
23
|
Granero-Moltó F, Weis JA, Miga MI, Landis
B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP and
Spagnoli A: Regenerative effects of transplanted mesenchymal stem
cells in fracture healing. Stem Cells. 27:1887–1898. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otsuru S, Tamai K, Yamazaki T, Yoshikawa H
and Kaneda Y: Circulating bone marrow-derived osteoblast progenitor
cells are recruited to the bone-forming site by the CXCR4/stromal
cell-derived factor-1 pathway. Stem Cells. 26:223–234. 2008.
View Article : Google Scholar
|
|
25
|
Hosogane N, Huang Z, Rawlins BA, Liu X,
Boachie-Adjei O, Boskey AL and Zhu W: Stromal derived factor-1
regulates bone morphogenetic protein 2-induced osteogenic
differentiation of primary mesenchymal stem cells. Int J Biochem
Cell Biol. 42:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu W, Boachie-Adjei O, Rawlins BA,
Frenkel B, Boskey AL, Ivashkiv LB and Blobel CP: A novel regulatory
role for stromal-derived factor-1 signaling in bone morphogenic
protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J
Biol Chem. 282:18676–18685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Herberg S, Susin C, Pelaez M, Howie RN,
Moreno de Freitas R, Lee J, Cray JJ Jr, Johnson MH, Elsalanty ME,
Hamrick MW, et al: Low-dose bone morphogenetic protein-2/stromal
cell-derived factor-1β cotherapy induces bone regeneration in
critical-size rat calvarial defects. Tissue Eng Part A.
20:1444–1453. 2014. View Article : Google Scholar :
|
|
28
|
Higashino K, Viggeswarapu M, Bargouti M,
Liu H, Titus L and Boden SD: Stromal cell-derived factor-1
potentiates bone morphogenetic protein-2 induced bone formation.
Tissue Eng Part A. 17:523–530. 2011. View Article : Google Scholar
|
|
29
|
Ratanavaraporn J, Furuya H, Kohara H and
Ta bat a Y: Synergistic effects of the dual release of stromal
cell-derived factor-1 and bone morphogenetic protein-2 from
hydrogels on bone regeneration. Biomaterials. 32:2797–2811. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Q, He QF, Zhang TH, Yu XL, Liu Q and
Deng FL: Improvement in the delivery system of bone morphogenetic
protein-2: A new approach to promote bone formation. Biomed Mater.
7:450022012. View Article : Google Scholar
|
|
31
|
He Q, Zhao Y, Chen B, Xiao Z, Zhang J,
Chen L, Chen W, Deng F and Dai J: Improved cellularization and
angiogenesis using collagen scaffolds chemically conjugated with
vascular endothelial growth factor. Acta Biomater. 7:1084–1093.
2011. View Article : Google Scholar
|
|
32
|
Chen L, He Z, Chen B, Zhao Y, Sun W, Xiao
Z, Zhang J, Yang M, Gao Z and Dai J: Direct chemical cross-linking
of platelet-derived growth factor-BB to the demineralized bone
matrix improves cellularization and vascularization.
Biomacromolecules. 10:3193–3198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen B, Lin H, Wang J, Zhao Y, Wang B,
Zhao W, Sun W and Dai J: Homogeneous osteogenesis and bone
regeneration by demineralized bone matrix loading with
collagen-targeting bone morphogenetic protein-2. Biomaterials.
28:1027–1035. 2007. View Article : Google Scholar
|
|
34
|
Kolácná L, Bakesová J, Varga F, Kostáková
E, Plánka L, Necas A, Lukás D, Amler E and Pelouch V: Biochemical
and biophysical aspects of collagen nanostructure in the
extracellular matrix. Physiol Res. 56(Suppl 1): S51–S60.
2007.PubMed/NCBI
|
|
35
|
Venugopal J and Ramakrishna S:
Biocompatible nanofiber matrices for the engineering of a dermal
substitute for skin regeneration. Tissue Eng. 11:847–854. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Feng Q, Liu X, Dong W and Cui F:
Collagen-based implants reinforced by chitin fibres in a goat shank
bone defect model. Biomaterials. 27:1917–1923. 2006. View Article : Google Scholar
|
|
37
|
Boccafoschi F, Habermehl J, Vesentini S
and Mantovani D: Biological performances of collagen-based
scaffolds for vascular tissue engineering. Biomaterials.
26:7410–7417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SN, Kim JK and Suh H: Evaluation of
antibiotic-loaded collagen-hyaluronic acid matrix as a skin
substitute. Biomaterials. 25:3689–3698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jue R, Lambert JM, Pierce LR and Traut RR:
Addition of sulfhydryl groups to Escherichia coli ribosomes by
protein modification with 2-iminothiolane (methyl
4-mercaptobutyrimidate). Biochemistry. 17:5399–5406. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geiger M, Li RH and Friess W: Collagen
sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev.
55:1613–1629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McQuibban GA, Butler GS, Gong JH, Bendall
L, Power C, Clark-Lewis I and Overall CM: Matrix metalloproteinase
activity inactivates the CXC chemokine stromal cell-derived
factor-1. J Biol Chem. 276:43503–43508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gomes ME, Sikavitsas VI, Behravesh E, Reis
RL and Mikos AG: Effect of flow perfusion on the osteogenic
differentiation of bone marrow stromal cells cultured on
starch-based three-dimensional scaffolds. J Biomed Mater Res A.
67:87–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Richardson TP, Peters MC, Ennett AB and
Mooney DJ: Polymeric system for dual growth factor delivery. Nat
Biotechnol. 19:1029–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kanczler JM, Ginty PJ, White L, Clarke NM,
Howdle SM, Shakesheff KM and Oreffo RO: The effect of the delivery
of vascular endothelial growth factor and bone morphogenic
protein-2 to osteoprogenitor cell populations on bone formation.
Biomaterials. 31:1242–1250. 2010. View Article : Google Scholar
|
|
45
|
Chen FM, Chen R, Wang XJ, Sun HH and Wu
ZF: In vitro cellular responses to scaffolds containing two
microencapulated growth factors. Biomaterials. 30:5215–5224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Patel ZS, Young S, Tabata Y, Jansen JA,
Wong ME and Mikos AG: Dual delivery of an angiogenic and an
osteogenic growth factor for bone regeneration in a critical size
defect model. Bone. 43:931–940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Simmons CA, Alsberg E, Hsiong S, Kim WJ
and Mooney DJ: Dual growth factor delivery and controlled scaffold
degradation enhance in vivo bone formation by transplanted bone
marrow stromal cells. Bone. 35:562–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Herberg S, Kondrikova G,
Periyasamy-Thandavan S, Howie RN, Elsalanty ME, Weiss L, Campbell
P, Hill WD and Cray JJ: Inkjet-based biopatterning of SDF-1β
augments BMP-2-induced repair of critical size calvarial bone
defects in mice. Bone. 67:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|