Introduction
It has previously been suggested that interactions
between intestinal neuroendocrine peptides/amines and the immune
system have a significant role in the pathophysiology of
inflammatory bowel disease (IBD) (1–12).
Understanding this role may shed light on the etiology of IBD and
lead to potential therapeutic strategies for the treatment of IBD,
involving the use of agonists or antagonists to these
peptides/amines.
Abnormalities in the enteroendocrine cells of
patients with IBD and in animal models of human IBD have previously
been reported (6,10,11,13–29).
Some of the alterations observed in enteroendocrine cells are
similar in ulcerative colitis (UC), Crohn's disease (CD) and
microscopic colitis (MC), including an increased density of cells
expressing serotonin, whereas others differ between these three
diseases. A recent study involving an animal model of human CD,
namely trinitrobenzene sulfonic acid (TNBS)-induced colitis,
demonstrated that abnormalities in enteroendocrine cells were
strongly correlated with changes in immune cells (30). These observations support the
presence of interactions between intestinal hormones and the immune
system, as represented by immune cells in IBD (1–12).
3-[(Dodecylthiocarbonyl)-methyl]-glutarimide
(DTCM-G) is an inhibitor of activator protein (AP)-1, which has
been reported to exhibit potent anti-inflammatory activity in
animal experiments (31,32). Dehydroxymethylepoxyquinomicin
(DHMEQ) is a novel inhibitor of nuclear factor (NF)-κB with a low
molecular weight (33,34). Similar to DTCM-G, DHMEQ has been
reported to exert potent anti-inflammatory effects in experimental
animal models of human IBD (32,35).
The aim of the present study was to identify the effects of these
two novel anti-inflammatory agents on inflammation-induced
alterations to enteroendocrine cells.
Materials and methods
Rats
A total of 48 male Wistar rats (Hannover GALAS™;
Taconic Europe A/S, Lille Skensved, Denmark) with a mean body
weight of 202 g (range, 166–249 g) were housed in Macrolon III
cages with ad libitum access to food and water. The rats
were fed a standard diet (B&K Universal, Nittedal, Norway) and
were maintained at a temperature of 20–22°C, a relative humidity of
50–60%, and under a 12/12-h light/dark cycle. The rats were allowed
to acclimate in the animal house for at least 7 days prior to
experimentation, and were divided into the following four groups
(n=12 rats/group): Control, TNBS-induced colitis only (TNBS group),
TNBS-induced colitis with DTCM-G treatment (DTCM-G group), and
TNBS-induced colitis with DHMEQ treatment (DHMEQ group).
The present study was performed in accordance with
the Directive for the Protection of Vertebrate Animals used for
Experimental and other Scientific Purposes (86/609/EEC), in
compliance with the Helsinki Declaration. The local ethical
committee for experimental animals at the University of Bergen
(Bergen, Norway) approved the protocols used in the present
study.
Induction of colitis using TNBS
TNBS-colitis was induced in the TNBS, DTCM-G and
DHMEQ groups as previously described (36). The dose of TNBS chosen in the
present study induces severe inflammation in rats (36). Briefly, after a 24 h fast, a single
dose of TNBS (Sigma-Aldrich Produktions GmbH, Steinheim, Germany)
was administered to the colon of each rat (25 mg/animal in 50%
ethanol solution; 0.5 ml/rat), followed by 2 ml of air, at 8 cm
from the anal margin via an 8.5-cm-long, 2.5-mm-diameter
round-tipped Teflon feeding tube (AngTheo, Lidingö, Sweden). The
rats were anesthetized by isoflurane inhalation (Merck
Pharmaceuticals, Kenilworth, NJ, USA) during the procedure. The
animals were kept prone with their hind legs raised for 2–3 min
following administration of TNBS. The rats were supervised until
recovery and were subsequently monitored several times daily. The
control group received the same treatment as the TNBS group, except
that 0.9% saline was introduced into the colon instead of TNBS.
DTCM-G and DHMEQ treatments
A total of 3 days following administration of TNBS,
the rats were treated as follows: The control and TNBS groups
received 0.5 ml vehicle (0.5% carboxymethyl cellulose; CMC),
respectively; the DTCM-G group received DTCM-G (20 mg/kg body
weight) in 0.5% CMC; and the DHMEQ group received DHMEQ (15 mg/kg
body weight) in 0.5% CMC. All injections were performed
intraperitoneally twice daily for 5 days. The doses of DTCM-G and
DHMEQ used here were the same as those earlier reported to
ameliorate TNBS-induced colitis in rats (32). The synthesis of DTCM-G and DHMEQ is
described in previous studies (31,37–41).
The rats were checked twice daily, and any animals exhibiting signs
of pain were given a subcutaneous injection of 1 ml 0.3-g/ml
Temgesic solution (Merck Pharmaceuticals).
At the end of the experiments, the rats were
sacrificed by CO2 inhalation and a post-mortem
laparotomy was carried out. Tissue samples obtained from the colon
were examined histopathologically and immunohistochemically.
Histopathological and immunohistochemical
examinations
The colonic tissues were fixed in 4% buffered
paraformaldehyde overnight, embedded in paraffin, and cut into 5-µm
sections. The sections were routinely stained with hematoxylin and
eosin in the pathology laboratory. Inflammation was evaluated using
the scoring system as described by Hunter et al (42), in which the total score was
calculated as the summation of four parameter scores: Inflammatory
infiltration (0–3), the number of gut walls engaged (0–3), damage
to the mucosal architecture (0–3) and edema (0 or 1). The total
score of this scale ranged between 0 and 10.
The sections were also immunostained and visualized
using the ultraView Universal DAB Detection kit (version 1.02.0018;
Ventana Medical Systems, Inc., Basel, Switzerland) and the
BenchMark Ultra IHC/ISH staining module (Ventana Medical Systems,
Inc.). The sections were incubated with one of the following
primary antibodies for 32 min at 37°C: Monoclonal mouse antibody
raised against the N-terminal of purified chromogranin A (CgA; no.
M869; Dako, Glostrup, Denmark), monoclonal mouse anti-serotonin
(no. M0758; Dako), polyclonal rabbit anti-porcine peptide YY (PYY;
no. PYY 11A; Alpha Diagnostic International, San Antonio, TX, USA),
polyclonal rabbit anti-synthetic-human pancreatic polypeptide (PP;
no. #114; Diagnostic Biosystems Inc., Pleasanton, CA, USA),
polyclonal rabbit anti-porcine oxyntomodulin 'glicentin/glucagon'
(no. BP508; Acris Antibodies GmbH, Herford, Germany), and
polyclonal rabbit anti-synthetic-human somatostatin (no. A566;
Dako); these antibodies were used at dilutions of 1:1,000, 1:1,500,
1:1,000, 1:800, 1:400 and 1:200, respectively.
Quantification of endocrine cells
The endocrine cells were quantified using
image-analysis software (version 1.7, cellSens; Olympus
Corporation, Tokyo, Japan). The number of endocrine cells in the
epithelium was counted manually in each field by pointing and
clicking the computer mouse. The area of the epithelial cells was
determined by manually drawing an enclosed region using the
computer mouse. Cell counting was conducted in ten randomly chosen
microscopic fields, as observed using a 40x objective, for which
each field represented a tissue area of 0.035 mm2. The
data are presented as the numbers of endocrine cells per square
millimeter of epithelium, and measurements were made by the same
person (M.E-S.), who was not aware of the identities of the
slides.
Statistical analysis
Differences between the control, TNBS, DTCM-G and
DHMEQ groups were analyzed using the Kruskal-Wallis nonparametric
test followed by Dunn's post-hoc test. Data are presented as the
mean ± standard error of the mean. Statistical analysis was
conducted using Prism 6 (GraphPad Software, Inc., La Jolla, CA,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Body weight and mortality
The initial body weight did not differ between the
control, TNBS, DTCM-G and DHMEQ groups (P=0.08); however, there
were differences in reductions in body weight at the end of the
experiment (P<0.0001): 0.0±0.0, 21.5±1.1, 1.7±0.6 and 1.6±0.8%,
respectively. Multiple comparisons indicated that the body weight
reductions differed significantly between the TNBS group, and the
DTCM-G and DHMEQ groups (P<0.001). Three animals succumbed in
the TNBS group: Two due to spontaneous mortality and one was
sacrificed due to animal welfare reasons. There were no cases of
mortality in the other three groups.
Histopathological and immunohistochemical
examinations
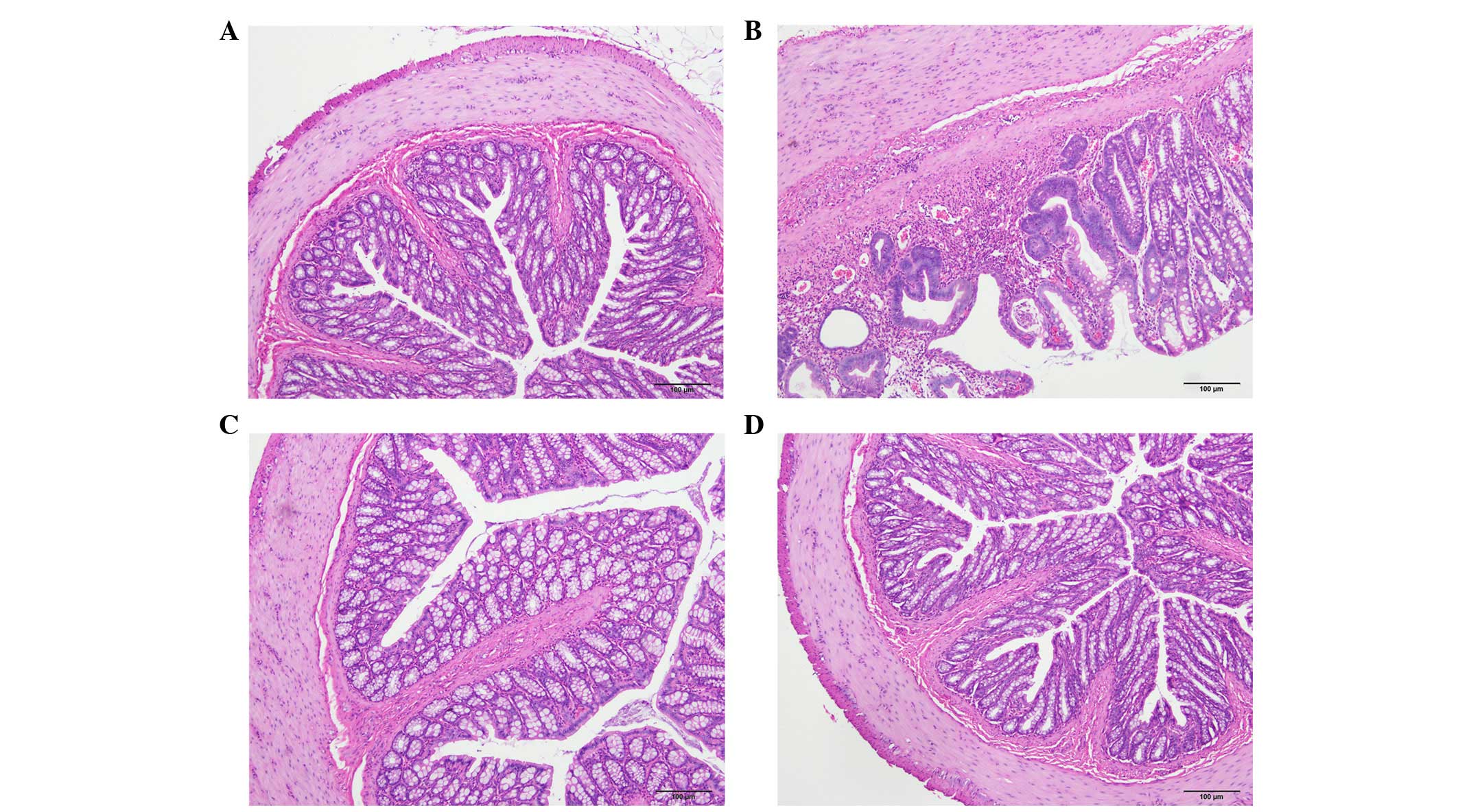
The histopathological inflammation scores were
6.6±0.9, 2.0±1.0 and 2.2±0.6 in the TNBS, DTCM-G and DHMEQ groups,
respectively (Fig. 1). Multiple
comparisons revealed a statistically significant difference between
the three groups (P=0.002). The scores differed between the TNBS
group, and the DTCM-G and DHMEQ groups (P=0.03 and P=0.01,
respectively).
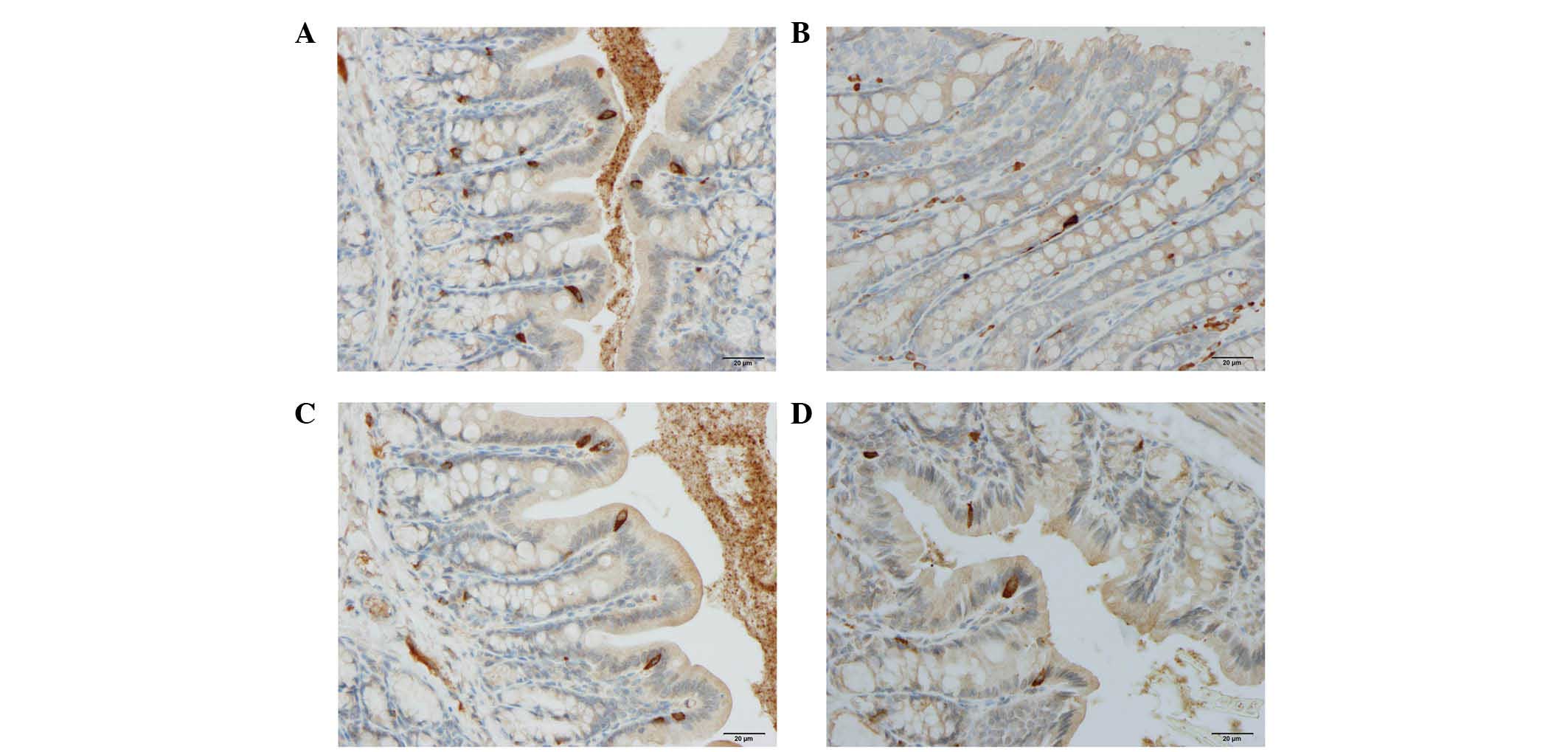
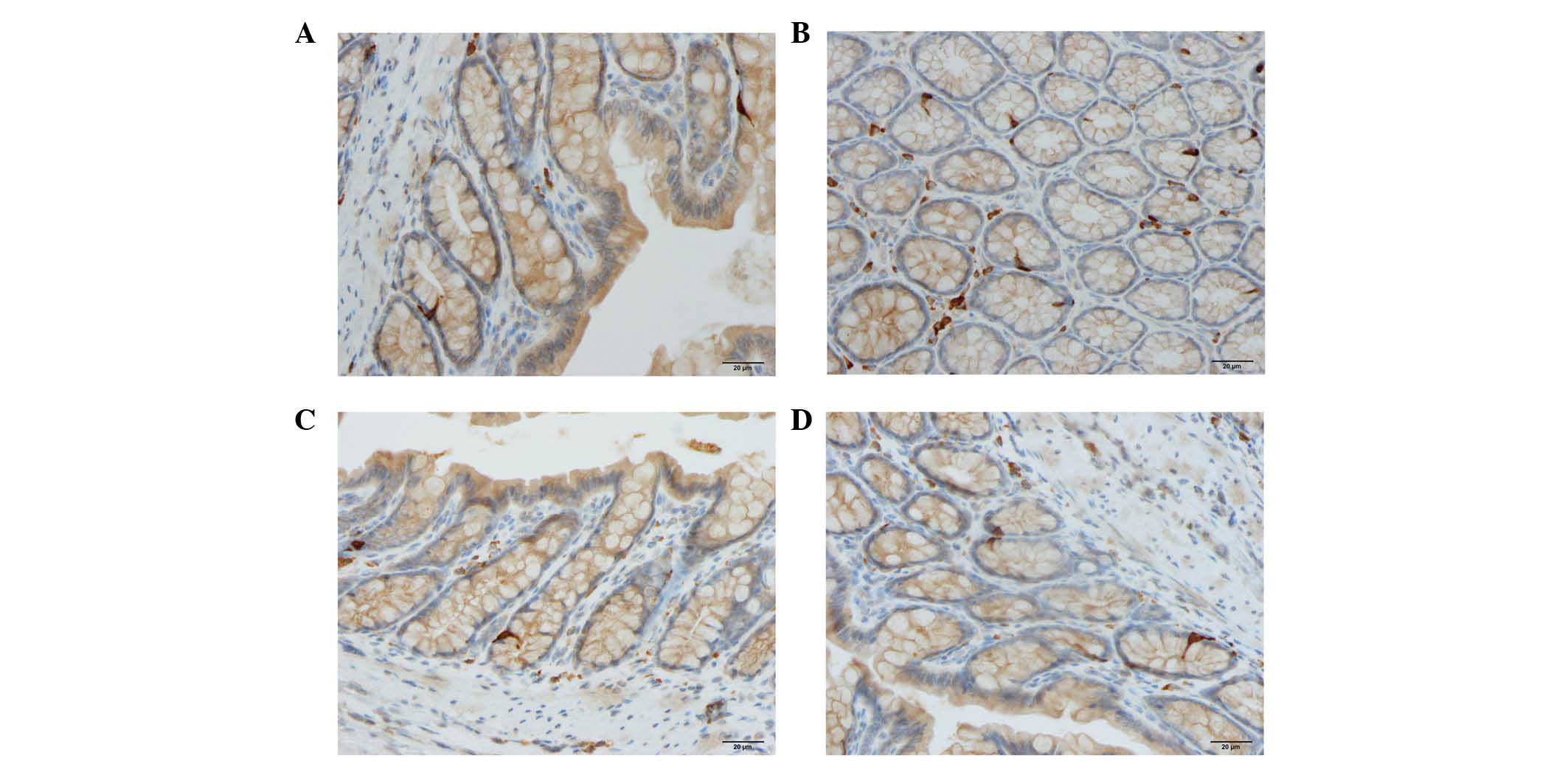
Cells expressing CgA, serotonin, PYY, oxyntomodulin,
PP and somatostatin were detected in all colonic tissues from all
of the groups. The endocrine cells were predominantly located at
the crypts of Lieberkühn. These cells were flask-shaped and
occasionally contained a long basal cytoplasmic process, often
known as a 'neuropod' (43–46)
(Figs. 2 and 3).
Quantification of endocrine cells
Results of the quantification of the various types
of endocrine cells in all four experimental groups are presented in
Fig. 4.
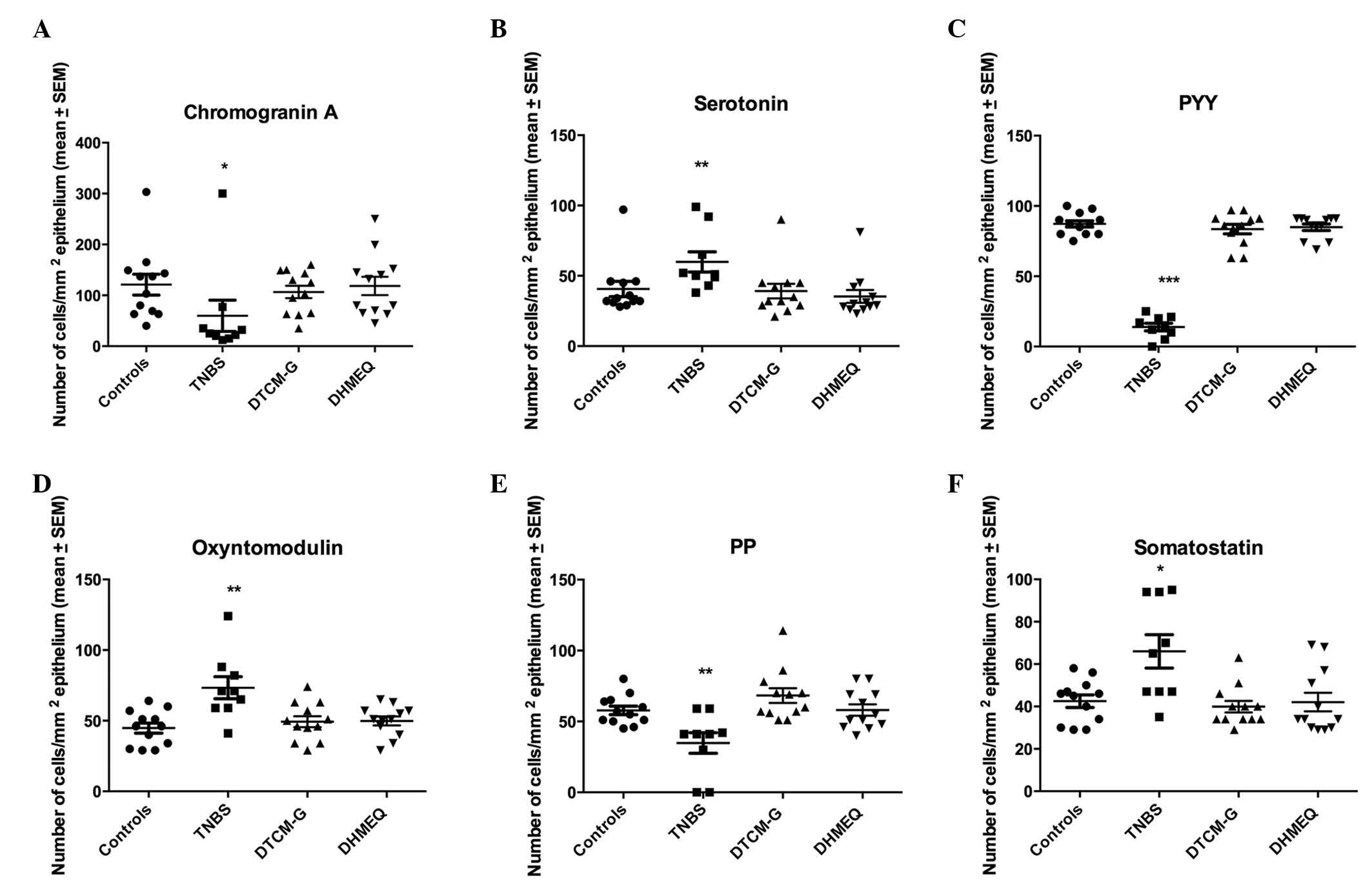 | Figure 4Densities of different colonic
endocrine cell types in the control, trinitrobenzene sulfonic acid
(TNBS), 3-[(dodecylthiocarbonyl)-methyl]-glutarimide (DTCM-G) and
dehydroxymethylepoxyquinomicin (DHMEQ) groups. Immunostaining was
conducted to detect (A) chromogranin A, (B) serotonin, (C) peptide
YY (PYY), (D) oxyntomodulin, (E) pancreatic polypeptide (PP) and
(F) somatostatin. *P<0.05, **P<0.01,
***P<0.001 vs. the controls, as determined by Dunn's
post-hoc test. SEM, standard error of the mean. |
CgA-expressing cells
The density of cells expressing CgA was 121±20.4,
59.8±30.7, 106.6±12.2 and 118.4±18.0 cells/mm2
epithelium in the control, TNBS, DTCM-G and DHMEQ groups,
respectively. The Kruskal-Wallis test revealed a significant
difference among the four experimental groups (P=0.01). The density
of cells expressing CgA was significantly lower in the TNBS group
compared with in the control group (P=0.01); however, there was no
significant difference between the control, DTCM-G and DHMEQ
groups.
Serotonin-expressing cells
The density of cells expressing serotonin was
40.7±5.5, 59.9±7.7, 39.2±5.2 and 35.3±4.7 cells/mm2
epithelium in the control, TNBS, DTCM-G and DHMEQ groups,
respectively. The Kruskal-Wallis test revealed a significant
difference among the four experimental groups (P=0.002). The
density of cells expressing serotonin was higher in the TNBS group
compared with in the control group (P=0.003); however, there was no
significant difference between the control, DTCM-G, and DHMEQ
groups.
PYY-expressing cells
The density of cells expressing PYY was 87.3±2.2,
13.9±2.7, 83.6±3.3 and 84.9±2.3 in the control, TNBS, DTCM-G and
DHMEQ groups, respectively. The Kruskal-Wallis test revealed a
significant difference among the four experimental groups
(P<0.0001). The density of cells expressing PYY was
significantly lower in the TNBS group compared with in the control
group (P<0.001); however, there was no significant difference
between the control, DTCM-G and DHMEQ groups.
Oxyntomodulin-expressing cells
The density of cells expressing oxyntomodulin in the
control, TNBS, DTCM-G and DHMEQ groups was 44.8±3.6, 73.3±7.9,
49.3±3.9 and 49.8±3.2 cells/mm2 epithelium,
respectively. The Kruskal-Wallis test revealed a statistically
significant difference among the four experimental groups
(P=0.006). The density of cells expressing oxyntomodulin was
significantly higher in the TNBS group compared with in the control
group (P=0.001).
PP-expressing cells
The density of cells expressing PP in the control,
TNBS, DTCM-G and DHMEQ groups was 57.8±3.0, 34.8±7.2, 68.3±5.2 and
58.1±4.0 cells/mm2 epithelium, respectively. The
Kruskal-Wallis test revealed a significant difference among the
four experimental groups (P=0.002). The density of cells expressing
PP was significantly lower in the TNBS group compared with in the
control group (P=0.003); however, there was no significant
difference between the DTCM-G, DHMEQ and control groups.
Somatostatin-expressing cells
The density of cells expressing somatostatin was
42.9±2.9, 66.0±7.9, 39.9±2.7 and 42.1±4.4 cells/mm2
epithelium in the control, TNBS, DTCM-G and DHMEQ groups,
respectively. The Kruskal-Wallis test revealed a significant
difference among the four experimental groups (P=0.002). The
density of cells expressing somatostatin was significantly higher
in the TNBS group compared with in the control group (P=0.01);
however, there was no significant difference between the control,
DTCM-G and DHMEQ groups.
Discussion
TNBS-induced colitis resulted in alterations in the
densities of all colonic endocrine cells. The density of cells
expressing CgA, PYY and PP decreased, whereas the density of those
expressing serotonin, oxyntomodulin and somatostatin increased.
These changes occurred rapidly, 3 days after the induction of
inflammation. Explaining the underlying mechanisms requires
consideration of the recently published findings, which have
reported that mature intestinal endocrine cells are capable of
expressing up to seven hormones (47,48).
It may be hypothesized that the inflammatory substances produced
during inflammation, including cytokines, affect the endocrine cell
expression of certain hormones, thus upregulating the expression of
some hormones whilst downregulating others. The change in the
density of particular endocrine cell types may therefore depend on
switching hormone expression rather than changing the actual number
of these cells. In support of this assumption are L-cells, which
are known to express both PYY and oxyntomodulin (49,50).
In the present study it seems that L-cells exhibited downregulated
expression of PYY, whereas the expression of oxyntomodulin was
upregulated.
Comparisons of the findings of the present study
with those of previous studies reveal both discrepant and
consistent results. The decreased density of CgA-expressing cells
observed in the present study disagrees with previously reported
increases in CgA-expressing cells in UC and CD (13,23);
however, the increased density of serotonin-expressing colonic
cells in TNBS-induced colitis in the present study is in agreement
with previously published findings in patients with UC, CD and MC,
and in animal models of colitis (13,15,51–53).
In addition, the present observation of a decrease in the density
of cells expressing PYY disagrees with previous observations in UC
and interleukin (IL)-2 gene knockout mice (13,52).
Furthermore, whereas the increased density of cells expressing
oxyntomodulin detected in the present study is in concordance with
previous findings in IL-2 knockout mice, it disagrees with the
findings of a previous study, which demonstrated that the density
of oxyntomodulin-expressing cells was unchanged in UC (13,52).
The decreased density of cells expressing PP observed in the
present study is in concordance with what has previously been
reported in UC and CD (13).
Conversely, the density of cells expressing somatostatin has been
reported to be decreased in the colon of patients with UC and CD,
and in animal models of induced colitis (28,29,54);
however, the present study detected an increase in the density of
cells expressing somatostatin in TNBS-induced colitis. When
considering all of these differences, it should be kept in mind
that the type of inflammation differs between UC, CD and MC, and
that animal models of colitis are not identical to human UC and
CD.
Treatment with either DTCM-G or DHMEQ for 5 days
ameliorated TNBS-induced inflammation, as indicated by the reduced
histopathological inflammation scores for the treated rats. This
finding is in concordance with the results of previous studies in
TNBS- and dextran sodium sulfate-induced colitis in rats and mice
(3,32,35).
In the present study, the attenuation of inflammation was
associated with restoration of the normal densities of all colonic
endocrine cell types to normal levels. DTCM-G affects inflammation
by inhibiting AP-1, whereas the anti-inflammatory effects of DHMEQ
are due to the inhibition of NF-κB. The effects of these agents on
the density of colonic endocrine cells are most likely to be
attributed to their effects on inflammation. Furthermore, it may be
hypothesized that attenuation of inflammation by treatment with
anti-inflammatory agents that reduce the secretion of inflammatory
substances may result in restoration of normal expression levels of
hormones in colonic cells.
CgA peptides and serotonin are considered to have
proinflammatory actions (54–66),
whereas somatostatin exerts anti-inflammatory effects (4,67–82).
The exact roles of PYY, oxyntomodulin and PP in inflammation remain
to be elucidated. Serotonin stimulates gastric and intestinal
motility, modulates visceral sensitivity, and stimulates intestinal
secretion (83,84), whereas PYY delays gastric emptying
and mediates the ileal brake. In addition, PYY inhibits gastric and
pancreatic secretion, and stimulates the absorption of water and
electrolytes (6,83,85).
The increase in serotonin and decrease in PYY detected in
TNBS-induced colitis in the present study may lead to accelerated
gastrointestinal motility and an increase in intestinal secretion,
giving rise to diarrhea, which is the cardinal symptom of
colitis.
In conclusion, TNBS-induced colitis affected the
density of all colonic endocrine cell types investigated in the
present study. The observed changes in these densities may have
been caused by switching the expression from one hormone to
another, rather than changing the actual number of cells. Treatment
with anti-inflammatory agents, AP-1 and NF-κB inhibitors, restored
the density of these endocrine cells to normal. These results
support the hypothesis that interactions between the immune system
and enteroendocrine cells have a significant role in the
pathophysiology of IBD and in the development of the clinical
symptoms of colitis.
Acknowledgments
The present study was supported by grants from
Helse-Vest (grant no. 911978) and Helse-Fonna (grant no.
40515).
References
|
1
|
Khan WI and Ghia JE: Gut hormones:
Emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
2
|
Margolis KG and Gershon MD: Neuropeptides
and inflammatory bowel disease. Curr Opin Gastroenterol.
25:503–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bampton PA and Dinning PG: High resolution
colonic manometry-what have we learnt?-A review of the literature
2012. Curr Gastroenterol Rep. 15:3282013. View Article : Google Scholar
|
|
4
|
Ameri P and Ferone D: Diffuse endocrine
system, neuroendocrine tumors and immunity: What's new?
Neuroendocrinology. 95:267–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farzi A, Reichmann F and Holzer P: The
homeostatic role of neuropeptide Y in immune function and its
impact on mood and behaviour. Acta Physiol (Oxf). 213:603–627.
2015. View Article : Google Scholar
|
|
6
|
Vona-Davis LC and McFadden DW: NPY family
of hormones: Clinical relevance and potential use in
gastrointestinal disease. Curr Top Med Chem. 7:1710–1720. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wheway J, Herzog H and Mackay F: NPY and
receptors in immune and inflammatory diseases. Curr Top Med Chem.
7:1743–1752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wheway J, Herzog H and Mackay F: The Y1
receptor for NPY: A key modulator of the adaptive immune system.
Peptides. 28:453–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wheway J, Mackay CR, Newton RA, Sainsbury
A, Boey D, Herzog H and Mackay F: A fundamental bimodal role for
neuropeptide Y1 receptor in the immune system. J Exp Med.
202:1527–1538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Salhy M, Suhr O and Danielsson A:
Peptide YY in gastrointestinal disorders. Peptides. 23:397–402.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (Review). Int J Mol Med.
31:275–282. 2013.PubMed/NCBI
|
|
12
|
El-Salhy M and Hausken T: The role of the
neuropeptide Y (NPY) family in he pathophysiology of inflammatory
bowel disease (IBD). Neuropeptides. 55:137–144. 2015. View Article : Google Scholar
|
|
13
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar
|
|
14
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Chromogranin a cell density as a diagnostic marker for
lymphocytic colitis. Dig Dis Sci. 57:3154–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: High densities of serotonin and peptide YY cells in the
colon of patients with lymphocytic colitis. World J Gastroenterol.
18:6070–6075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Salhy M, Lomholt-Beck B and Gundersen
TD: High chromogranin A cell density in the colon of patients with
lymphocytic colitis. Mol Med Rep. 4:603–605. 2011.PubMed/NCBI
|
|
17
|
Moran GW, Pennock J and McLaughlin JT:
Enteroendocrine cells in terminal ileal Crohn's disease. J Crohns
Colitis. 6:871–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moran GW, Leslie FC and McLaughlin JT:
Crohn's disease affecting the small bowel is associated with
reduced appetite and elevated levels of circulating gut peptides.
Clin Nutr. 32:404–411. 2013. View Article : Google Scholar
|
|
19
|
Besterman HS, Mallinson CN, Modigliani R,
Christofides ND, Pera A, Ponti V, Sarson DL and Bloom SR: Gut
hormones in inflammatory bowel disease. Scand J Gastroenterol.
18:845–852. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirotani Y, Mikajiri K, Ikeda K, Myotoku M
and Kurokawa N: Changes of the peptide YY levels in the intestinal
tissue of rats with experimental colitis following oral
administration of mesalazine and prednisolone. Yakugaku Zasshi.
128:1347–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tari A, Teshima H, Sumii K, Haruma K,
Ohgoshi H, Yoshihara M, Kajiyama G and Miyachi Y: Peptide YY
abnormalities in patients with ulcerative colitis. Jpn J Med.
27:49–55. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sciola V, Massironi S, Conte D, Caprioli
F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT and Piodi L:
Plasma chromogranin a in patients with inflammatory bowel disease.
Inflamm Bowel Dis. 15:867–871. 2009. View Article : Google Scholar
|
|
23
|
Bishop AE, Pietroletti R, Taat CW,
Brummelkamp WH and Polak JM: Increased populations of endocrine
cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol.
410:391–396. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manocha M and Khan WI: Serotonin and GI
Disorders: An update on clinical and experimental studies. Clin
Transl Gastroenterol. 3:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stoyanova II and Gulubova MV: Mast cells
and inflammatory mediators in chronic ulcerative colitis. Acta
Histochem. 104:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto H, Morise K, Kusugami K, Furusawa
A, Konagaya T, Nishio Y, Kaneko H, Uchida K, Nagai H, Mitsuma T and
Nagura H: Abnormal neuropeptide concentration in rectal mucosa of
patients with inflammatory bowel disease. J Gastroenterol.
31:525–532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Payer J, Huorka M, Duris I, Mikulecky M,
Kratochvílová H, Ondrejka P and Lukác L: Plasma somatostatin levels
in ulcerative colitis. Hepatogastroenterology. 41:552–553.
1994.PubMed/NCBI
|
|
28
|
Watanabe T, Kubota Y, Sawada T and Muto T:
Distribution and quantification of somatostatin in inflammatory
disease. Dis Colon Rectum. 35:488–494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koch TR, Carney JA, Morris VA and Go VL:
Somatostatin in the idiopathic inflammatory bowel diseases. Dis
Colon Rectum. 31:198–203. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Sahy M and Hatlebakk JG: Changes in
endocrine and immune cells following colitis induction by TNBS in
rats. Mol Med Rep. In press.
|
|
31
|
Takeiri M, Tachibana M, Kaneda A, Ito A,
Ishikawa Y, Nishiyama S, Goto R, Yamashita K, Shibasaki S, Hirokata
G, et al: Inhibition of macrophage activation and suppression of
graft rejection by DTCM-glutarimide, a novel piperidine derived
from the antibiotic 9-methylstreptimidone. Inflamm Res. 60:879–888.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El-Salhy M, Umezawa K, Gilja OH, Hatlebakk
JG, Gundersen D and Hausken T: Amelioration of Severe TNBS Induced
colitis by novel AP-1 and NF-κB inhibitors in rats. Scientfic World
Journal. 2014:8138042014.
|
|
33
|
Umezawa K, Ariga A and Matsumoto N:
Naturally occurring and synthetic inhibitors of NF-kappaB
functions. Anticancer Drug Des. 15:239–244. 2000.
|
|
34
|
Umezawa K: Possible role of peritoneal
NF-kappaB in peripheral inflammation and cancer: Lessons from the
inhibitor DHMEQ. Biomed Pharmacother. 65:252–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Funakoshi T, Yamashita K, Ichikawa N,
Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T,
Ichihara S, et al: A novel NF-kappaB inhibitor,
dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic
injury in mice. J Crohns Colitis. 6:215–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Salhy M, Wendelbo IH, Gundersen D,
Hatlebakk JG and Hausken T: Evaluation of the usefulness of
colonoscopy with mucosal biopsies in the follow-up of TNBS-induced
colitis in rats. Mol Med Rep. 8:446–450. 2013.PubMed/NCBI
|
|
37
|
Ota E, Takeiri M, Tachibana M, Ishikawa Y,
Umezawa K and Nishiyama S: Synthesis and biological evaluation of
molecular probes based on the 9-methylstreptimidone derivative
DTCM-glutarimide. Bioorg Med Chem Lett. 22:164–167. 2012.
View Article : Google Scholar
|
|
38
|
Ishikawa Y, Tachibana M, Matsui C, Obata
R, Umezawa K and Nishiyama S: Synthesis and biological evaluation
on novel analogs of 9-methylstreptimidone, an inhibitor of
NF-kappaB. Bioorg Med Chem Lett. 19:1726–1728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueki S, Yamashita K, Aoyagi T, Haga S,
Suzuki T, Itoh T, Taniguchi M, Shimamura T, Furukawa H, Ozaki M, et
al: Control of allograft rejection by applying a novel nuclear
factor-kappaB inhibitor, dehydroxymethylepoxyquinomicin.
Transplantation. 82:1720–1727. 2006. View Article : Google Scholar
|
|
40
|
Matsumoto N, Ariga A, To-e S, Nakamura H,
Agata N, Hirano S, Inoue J and Umezawa K: Synthesis of NF-kappaB
activation inhibitors derived from epoxyquinomicin C. Bioorg Med
Chem Lett. 10:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Umezawa N, Matsumoto N, Iwama S, Kato N
and Higuchi T: Facile synthesis of peptide-porphyrin conjugates:
Towards artificial catalase. Bioorg Med Chem. 18:6340–6350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hunter MM, Wang A, Hirota CL and McKay DM:
Neutralizing anti-IL-10 antibody blocks the protective effect of
tapeworm infection in a murine model of chemically induced colitis.
J Immunol. 174:7368–7375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pang XH, Li TK, Xie Q, He FQ, Cui de J,
Chen YQ, Huang XL and Gan HT: Amelioration of dextran sulfate
sodium-induced colitis by neuropeptide Y antisense
oligodeoxynucleotide. Int J Colorectal Dis. 25:1047–1053. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bohorquez DV, Chandra R, Samsa LA, Vigna
SR and Liddle RA: Characterization of basal pseudopod-like
processes in ileal and colonic PYY cells. J Mol Histol. 42:3–13.
2011. View Article : Google Scholar
|
|
45
|
Bohórquez DV, Samsa LA, Roholt A,
Medicetty S, Chandra R and Liddle RA: An enteroendocrine
cell-enteric glia connection revealed by 3D electron microscopy.
PloS one. 9:e898812014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bohórquez DV, Shahid RA, Erdmann A, Kreger
AM, Wang Y, Calakos N, Wang F and Liddle RA: Neuroepithelial
circuit formed by innervation of sensory enteroendocrine cells. J
Clin Invest. 125:782–786. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ghia JE, Li N, Wang H, Collins M, Deng Y,
El-Sharkawy RT, Côté F, Mallet J and Khan WI: Serotonin has a key
role in pathogenesis of experimental colitis. Gastroenterology.
137:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dryden S, Wang Q, Frankish HM, Pickavance
L and Williams G: The serotonin (5-HT) antagonist methysergide
increases neuropeptide Y (NPY) synthesis and secretion in the
hypothalamus of the rat. Brain Res. 699:12–18. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Spångeus A, Forsgren S and el-Salhy M:
Does diabetic state affect co-localization of peptide YY and
enteroglucagon in colonic endocrine cells? Histol Histopathol.
15:37–41. 2000.PubMed/NCBI
|
|
50
|
Pyarokhil AH, Ishihara M, Sasaki M and
Kitamura N: The developmental plasticity of colocalization pattern
of peptide YY and glucagon-like peptide-1 in the endocrine cells of
bovine rectum. Biomed Res. 33:35–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bertrand PP and Bertrand RL: Serotonin
release and uptake in the gastrointestinal tract. Auton Neurosci.
153:47–57. 2010. View Article : Google Scholar
|
|
52
|
Qian BF, El-Salhy M, Melgar S, Hammarström
ML and Danielsson A: Neuroendocrine changes in colon of mice with a
disrupted IL-2 gene. Clin Exp Immunol. 120:424–433. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Oshima S, Fujimura M and Fukimiya M:
Changes in number of serotonin-containing cells and serotonin
levels in the intestinal mucosa of rats with colitis induced by
dextran sodium sulfate. Histochem Cell Biol. 112:257–263. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Spiller R: Serotonin and GI clinical
disorders. Neuropharmacology. 55:1072–1080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Egger M, Beer AG, Theurl M, Schgoer W,
Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J,
Patsch JR, et al: Monocyte migration: A novel effect and signaling
pathways of catestatin. Eur J Pharmacol. 598:104–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feistritzer C, Mosheimer BA, Colleselli D,
Wiedermann CJ and Kähler CM: Effects of the neuropeptide
secretoneurin on natural killer cell migration and cytokine
release. Regul Pept. 126:195–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ferrero E, Magni E, Curnis F, Villa A,
Ferrero ME and Corti A: Regulation of endothelial cell shape and
barrier function by chromogranin A. Ann N Y Acad Sci. 971:355–358.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang H, Steeds J, Motomura Y, Deng Y,
Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK and Khan
WI: CD4+ T cell-mediated immunological control of enterochromaffin
cell hyperplasia and 5-hydroxytryptamine production in enteric
infection. Gut. 56:949–957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cloez-Tayarani I and Changeux JP: Nicotine
and serotonin in immune regulation and inflammatory processes: A
perspective. J Leukoc Biol. 81:599–606. 2007. View Article : Google Scholar
|
|
60
|
Stefulj J, Cicin-Sain L, Schauenstein K
and Jernej B: Serotonin and immune response: Effect of the amine on
in vitro proliferation of rat lymphocytes. Neuroimmunomodulation.
9:103–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Betten A, Dahlgren C, Hermodsson S and
Hellstrand K: Serotonin protects NK cells against oxidatively
induced functional inhibition and apoptosis. J Leukoc Biol.
70:65–72. 2001.PubMed/NCBI
|
|
62
|
Laberge S, Cruikshank WW, Beer DJ and
Center DM: Secretion of IL-16 (lymphocyte chemoattractant factor)
from serotonin-stimulated CD8+ T cells in vitro. J Immunol.
156:310–315. 1996.PubMed/NCBI
|
|
63
|
Soga F, Katoh N, Inoue T and Kishimoto S:
Serotonin activates human monocytes and prevents apoptosis. J
Invest Dermatol. 127:1947–1955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Di Sabatino A, Volta U, Salvatore C,
Biancheri P, Caio G, De Giorgio R, Di Stefano M and Corazza GR:
Small amounts of gluten in subjects with suspected nonceliac gluten
sensitivity: A randomized, double-blind, placebo-controlled,
cross-over trial. Clin Gastroenterol Hepatol. 13:1604–1612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Macia L, Yulyaningsih E, Pangon L, Nguyen
AD, Lin S, Shi YC, Zhang L, Bijker M, Grey S, Mackay F, et al:
Neuropeptide Y1 receptor in immune cells regulates inflammation and
insulin resistance associated with diet-induced obesity. Diabetes.
61:3228–3238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
De la Fuente M, Bernaez I, Del Rio M and
Hernanz A: Stimulation of murine peritoneal macrophage functions by
neuropeptide Y and peptide YY. Involvement of protein kinase C.
Immunology. 80:259–265. 1993.PubMed/NCBI
|
|
67
|
Ferone D, Resmini E, Boschetti M, Arvigo
M, Albanese V, Ceresola E, Pivonello R, Albertelli M, Bianchi F,
Giusti M and Minuto F: Potential indications for somatostatin
analogues: Immune system and limphoproliferative disorders. J
Endocrinol Invest. 28(11 Supply International): 111–117. 2005.
|
|
68
|
ten Bokum AM, Hofland LJ and van Hagen PM:
Somatostatin and somatostatin receptors in the immune system: A
review. Eur Cytokine Netw. 11:161–176. 2000.PubMed/NCBI
|
|
69
|
Ferone D, Pivonello R, Kwekkeboom DJ,
Gatto F, Ameri P, Colao A, de Krijger RR, Minuto F, Lamberts SW,
van Hagen PM and Hofland LJ: Immunohistochemical localization and
quantitative expression of somatostatin receptors in normal human
spleen and thymus: Implications for the in vivo visualization
during somatostatin receptor scintigraphy. J Endocrinol Invest.
35:528–534. 2012.
|
|
70
|
Ferone D, Pivonello R, Van Hagen PM, Dalm
VA, Lichtenauer-Kaligis EG, Waaijers M, Van Koetsveld PM, Mooy DM,
Colao A, Minuto F, et al: Quantitative and functional expression of
somatostatin receptor subtypes in human thymocytes. Am J Physiol
Endocrinol Metab. 283:E1056–E1066. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dalm VA, van Hagen PM, van Koetsveld PM,
Achilefu S, Houtsmuller AB, Pols DH, van der Lely AJ, Lamberts SW
and Hofland LJ: Expression of somatostatin, cortistatin and
soma-tostatin receptors in human monocytes, macrophages and
dendritic cells. Am J Physiol Endocrinol Metab. 285:E344–E353.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lichtenauer-Kaligis EG, Dalm VA, Oomen SP,
Mooij DM, van Hagen PM, Lamberts SW and Hofland LJ: Differential
expression of somatostatin receptor subtypes in human peripheral
blood mononuclear cell subsets. Eur J Endocrinol. 150:565–577.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Armani C, Catalani E, Balbarini A, Bagnoli
P and Cervia D: Expression, pharmacology and functional role of
somatostatin receptor subtypes 1 and 2 in human macrophages. J
Leukoc Biol. 81:845–855. 2007. View Article : Google Scholar
|
|
74
|
Taniyama Y, Suzuki T, Mikami Y, Moriya T,
Satomi S and Sasano H: Systemic distribution of somatostatin
receptor subtypes in human: An immunohistochemical study. Endocrine
J. 52:605–611. 2005. View Article : Google Scholar
|
|
75
|
Hagströmer L, Emtestam L, Stridsberg M and
Talme T: Expression pattern of somatostatin receptor subtypes 1–5
in human skin: An immunohistochemical study of healthy subjects and
patients with psoriasis or atopic dermatitis. Exp Dermatol.
15:950–957. 2006. View Article : Google Scholar
|
|
76
|
Talme T, Ivanoff J, Hägglund M, Van
Neerven RJ, Ivanoff A and Sundqvist KG: Somatostatin receptor
(SSTR) expression and function in normal and leukaemic T-cells.
Evidence for selective effects on adhesion to extracellular matrix
components via SSTR2 and/or 3. Clin Exp Immunol. 125:71–79. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rosskopf D, Schürks M, Manthey I, Joisten
M, Busch S and Siffert W: Signal transduction of somatostatin in
human B lymphoblasts. American journal of physiology. Cell
physiology. 284:C179–C190. 2003. View Article : Google Scholar
|
|
78
|
Casnici C, Lattuada D, Perego C, Franco P
and Marelli O: Inhibitory effect of somatostatin on human T
lymphocytes proliferation. Int J Immunopharmacol. 19:721–727. 1997.
View Article : Google Scholar
|
|
79
|
Radosević-Stasić B, Trobonjaca Z, Lucin P,
Cuk M, Polić B and Rukavina D: Immunosuppressive and
antiproliferative effects of somatostatin analog SMS 201–995. Int J
Neurosci. 81:283–297. 1995. View Article : Google Scholar
|
|
80
|
Sirianni MC, Annibale B, Fais S and Delle
Fave G: Inhibitory effect of somatostatin-14 and some analogues on
human natural killer cell activity. Peptides. 15:1033–1036. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Helyes Z, Elekes K, Németh J, Pozsgai G,
Sándor K, Kereskai L, Börzsei R, Pintér E, Szabó A and Szolcsányi
J: Role of transient receptor potential vanilloid 1 receptors in
endotoxin-induced airway inflammation in the mouse. Am J Physiol
Lung Cell Mol Physiol. 292:L1173–L1181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Helyes Z, Pintér E, Sándor K, Elekes K,
Bánvölgyi A, Keszthelyi D, Szoke E, Tóth DM, Sándor Z, Kereskai L,
et al: Impaired defense mechanism against inflammation,
hyperalgesia and airway hyperreactivity in somatostatin 4 receptor
gene-deleted mice. Proc Natl Acad Sci USA. 106:13088–13093. 2009.
View Article : Google Scholar
|
|
83
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: The role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012.
|
|
84
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome: Diagnosis, pathogenesis and
treatment options. Nova Science Publishers; Inc, New York: 2012
|
|
85
|
El-Salhy M, Gundersen D, Gilja OH,
Hatlebakk JG and Hausken T: Is irritable bowel syndrome an organic
disorder? World J Gastroenterol. 20:384–400. 2014. View Article : Google Scholar : PubMed/NCBI
|