Introduction
Aristolochic acids (AAs), a medicinal plant extract
of Aristolochia clematitis and Asarum species
(1), is widely used for the
treatment of snake bites, diarrhoea, rheumatoicd arthritis and
gastrointestinal disturbances in Asia (2–4), and
are in the homeopathic pharmacopeia in Germany (5). It has been reported in several
studies that the ingestion of AA remedies has resulted in AA
nephropathy (AAN), which is associated with urothelial malignancy
(6–8). AAI, the major component in AAs, is
responsible for the nephrotoxicity and the development of
urothelial carcinoma (5,9). Additionally, it is well documented
that the micromechanism of AAN resulting in renal ischemia combined
with impaired regeneration and the apoptosis of proximal tubular
epithelial cells, may due to the accumulation of AAI following
long-term oral administration (10,11).
However, the etiology of AAI-induced renal tubular atrophy and the
mechanisms underlying the therapeutic effect of AAI remain to be
fully elucidated. As AAI has been documented to be remedial and
nephrotoxic, the mechanism of AAI in traditional Chinese medicine
(TCM) theory requires investigation.
In TCM theory, food and water is received by the
stomach, following which it is transported and transformed by the
spleen to enable the substance to be transformed, absorbed and
distributed to all areas of the body. The body performs metabolism
constantly in association with the spleen, centered through the
normal functions of other organs: The nutrients nourish the whole
body, whereas the waste products are naturally excreted from the
body. The Chinese medicine classic, Jing Mai Bie Lun of Su Wen
(Plain Questions), states that, in the first stage, food enters the
stomach and moves towards the spleen, which distributes the
nutrients upwards to the lungs and directs the waste downwards to
the bladder; the nutrients are distributed to the whole body and
the waste is excreted from the bladder following metabolism
(12). Based on the above concept,
the spleen is the predominant organ involved in the transportation
and distribution of substances, which indicates that the spleen is
pivotal in substance metabolism. In TCM, the spleen is
comprehensive in structure and function, and is involved in
digestion, absorption, energy conversion and immune function
(13). Huang et al
(14,15) reported that the spleen is also
involved in the pharmacokinetics hypothesis, which assumes that the
spleen is responsible for the absorption, distribution, metabolism
and excretion of drugs. Therefore, the Spleen-Stomach Doctrine
(16) was introduced in order to
provide a possible approach for the safety assessment of AAI.
The nature and the utilization of AAI indicates that
metabolites of AAI are waste products for the body. It is necessary
to obtain scientific evidence to verify the traditional theory
based on the transportation and distribution of waste products,
including AAI. Several previous studies have demonstrated that
aquaporins may be associated with water metabolism (17,18);
however, few studies have focused on waste metabolites in TCM. It
is accepted that organic anion transporting peptides (oatps) can
regulate the transportion of endobiotics, xenobiotics and drugs, by
which a steady state in the body can be maintained (19–21).
A member of a subfamily of oatps, oatp2a1, was first identified in
rats in 1995 (22), and is widely
expressed in the lung, stomach, intestine, liver and kidney. Of
note, these organs are efficient in transporting metabolites and
drugs, and they are reported to function in the expression of
antilipemic and antineoplastic agents (23–25).
The present study hypothesized that these drugs and their
metabolites are similar to the waste products of water metabolism.
Thus, the expression levels and pattern of oatp2a1 may reflect the
transformation and transportation of waste products, including
AAI.
The present study was performed to investigate the
metabolism of AAI and the expression levels of oatp2a1 in spleen
deficiency groups and blank groups without spleen deficiency, in
order to confirm the role of the spleen in transformation and
transportation. In addition, the present study aimed to
preliminarily examine the possible mechanism involved, including
other organs, in metabolizing AAI.
Materials and methods
Animal handling and sampling
A total of 48 male 10-week-old (250–300 g)
Sprague-Dawley (SD) rats, obtained from the Laboratory Animals
Central of Sun Yat-Sen University (Guangzhou, China). Rats were
housed under a 12-h dark/light cycle, 24±1°C humidity-controlled
environment. The rats had free access to food and water ad
libitum. The rats were treated in accordance with the National
Animal Treatment Guidelines (26).
All the animal experiments were conducted in strict accordance with
the ethical principles that were approved and supervised by the
Ethics Committee of the First Affiliated Hospital of Sun Yat-sen
University (approval no. IACUC-2011-0701).
Experimental design
The 48 male SD rats were randomly divided into six
groups: A blank group without spleen deficiency (BS), two groups
without spleen deficiency administered with 20 mg/kg−1
AAI for 5 min (BS5) (Delta Pharmaceutical Technology Co., Ltd.,
Wuhu, China) and 60 min (BS60), a spleen deficiency group (RS), and
two groups with spleen deficiency administered with 20
mg/kg−1 AAI for 5 min (RS5) and 60 min (RS60). For the
establishment of the spleen deficiency model, the rats were
injected subcutaneously with 0.5 mg/kg−1 reserpine
(Jinyao Amino Acids Co., Ltd., Tianjin, China) (27), with 0.9% saline used as a control,
for 16 consecutive days with an unrestricted diet, activity and
sleep. General characteristics of the rats, including the quantity
of food and water consumed, body weight, growth, excrement,
behavior and activity were closely observed on each day of the
experiment.
The experiment was divided into two parts.
Experiment 1 involved examination of the metabolism of AAI in the
BS and RS groups (n=6). Blood samples (0.3 ml) from the rats were
collected at scheduled time points of 5, 15, 30, 45 and 60 min
following the oral administration of 20 mg/kg−1 AAI. The
collected blood samples were immediately centrifuged at 1,006 × g
at 4°C for 10 min, and the clear plasma was stored at −80°C for
subsequent analysis. Experiment 2 investigated the expression
levels of oatp2a1 in tissue samples from six organs (lung, liver,
stomach, kidney, small intestine and large intestine). Rats
received an overdose of 0.3 ml/100 g weight of 10% chloral hydrate
(Sigma-Aldrich, St. Louis, MO, USA) administered by intraperitoneal
injection. Samples (~5×5 mm) of lung, stomach, liver, spleen,
kidney, large intestine and small intestine were removed 5 or 60
min after oral administration of 20 mg/kg−1 AAI. The
samples were placed in freezing tubes and preserved within 15 min,
following which they were placed in a −80°C refrigerator.
Drugs and reagents
AAI was purchased from Delta Pharmaceutical
Technology, Co. Ltd (Wuhu, China) at 20 mg/pc (cat. no. 20101126)
and reserpine was purchased from the First Affiliated Hospital of
Guangzhou University of Chinese Medicine (Guangzhou, China) at 1
mg/pc (cat. no. 101016). Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) mouse monoclonal antibody, poly-L-lysine was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). TRIzol was
purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA), and the 5X reverse transcription (RT) buffer, 5X
quantitative polymerase chain reaction (qPCR) buffer, dNTPs, mMLV
and Taq enzyme were purchased from Takara Bio, Inc. (Otsu, Japan).
The PVDF membrane was purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). The protein extraction liquid was obtained
from the Beyotime Institute of Biotechnology (Shanghai, China).
Acrylamide, N-methylene bisacrylamide, Tris alkali and tween-20
were purchased from Sigma-Aldrich. 3,3-diaminobenzidine was
purchased from Novolink Biotech Co., Ltd. (Shenzhen, China). The
residual immunohistochemical kits were purchased from Golden Bridge
Biotechnology Co., Ltd. (Beijing, China). Ammonium persulfate,
methanol and glycerol were purchased from Guangzhou Chemical
Reagent Factory (Guangzhou, China).
Instruments
The intelligent artificial climate box (BD-PRX) was
purchased from Beidi Experimental Instrument Co., Ltd. (Nanjing,
China). The 3900 tabletop high flux DNA synthesizer and 7500
Fluorescence Quantitative PCR instrument were purchased from
Applied Biosystems; Thermo Fisher Scientific, Inc.). The
qualitative PCR instrument, and the vertical electrophoresis and
transfer system were purchased from Bio-Rad Laboratories, Inc. The
5417R high-speed refrigerated tabletop centrifuge was purchased
from Eppendorf (Hamburg, Germany). The MDF-U4084S ultra-low
temperature freezer was purchased from Sanyo (Osaka, Japan). The
ultrasonic cell disruption equipment was purchased from Touching
Technology Co., Ltd. (Shanghai, China). The decolorization table
was purchased from Rotomix Company (Shanghai, China). The X-ray
photography magazine was purchased from Yijia Biotechnology Co.,
Ltd. (Guangzhou, China). The RM2235 microtome and ASP300 dehydrator
were purchased from Leica (Wetzlar, Germany). The BX41 microscope
and BX40F4 digital microcamera were purchased from Olympus
Corporation (Tokyo, Japan). The DHG-9123A drying machine was
purchased from Jinghong Technology Co., Ltd. (Shanghai, China).
High performance liquid chromatography
(HPLC) and pharmacokinetic analysis of AAI
The concentrations of AAI in the above-mentioned
treatment groups were measured using HPLC, based on an established
method, with AAI used as an internal standard. The mixture, which
consisted of a 100-µl aliquot of sample in a 2.2-ml
eppendorf tube, an internal standard (AAI; 50 µg ml/1) in a
50-µl aliquot of trichloroacetic acid and a 50-µl
aliquot of 0.2 M phosphate buffer (pH 7.0), was then extracted with
1 ml diethylether. Subsequently, the organic layer was transferred
into a clean eppendorf tube and evaporated under a gentle stream of
nitrogen gas at 50°C. The residue was reconstituted in a
125-µl aliquot of the mobile phase, and a 50-µl
aliquot was injected directly into a reversed-phase (C8) HPLC
column. The mobile phase of phosphate buffer (0.2 M
KH2PO4; pH 7.0): acetonitrile (77:23; v/v)
was run at a flow rate of 1.4 ml/min, with the column effluent
monitored using an ultraviolet detector set at 302 nm. The
retention times of AA and the internal standard were ~9.8 and 9.8
min, respectively. The detection limits of the concentrations in
the rat plasma and tissue samples were 0.05 µg/ml and 0.31
ng/g, respectively. The coefficients of variation of the
concentrations in hte plasma and tissue samples were <2.81 and
4.55%, respectively.
Total RNA extraction and RT-qPCR analysis
of oatp2a1
The extraction of total RNA from the six tissue
samples was performed using TRIzol, according to the manufacturer's
protocol. The total RNA was reverse-transcribed, and cDNA was
synthesized from 4 µg of total RNA using primers and
Superscript Reverse Transcriptase II (Invitrogen; Thermo Fisher
Scientific, Inc.). The final cDNA was used for the subsequent qPCR
analysis. The primers were predesigned in the coding DNA sequence
of the mRNA sequence of the target gene in GenBank (www.ncbi.nlm.nih.gov/). The primers were designed to
amplify DNA fragments, which crossed exon/exon boundaries. The
sequence of the forward primer was 5′-CAG CTA CTG CTC CCG TCC AT-3′
and the sequence of the reverse primer was 5′-CAC GCG AAG GAC CAT
CATG-3′. The sequence of the forward primer for the housekeeping
gene, GAPDH, was 5′-TGG TCT ACA TGT TCC AGT ATG ACT-3′ and the
sequence of the reverse primer was 5′-CCA TTT GAT GTT AGC GGG ATC
TC-3′. The expression was evaluated on a 1% agarose gel following
PCR amplification. The mRNA levels of oatp2a1 were calculated
relative to the signal for the target transcript in human tissue
samples, which served as a standard. Target mRNA levels were
normalized to the internal control gene, GAPDH, according to the
following equation: Relative target mRNA level = 2−∆∆Cq,
where ∆∆Cq = (CqTarget –
CqGAPDH)Tissue1 – (CqTarget –
CqGAPDH)Tissue2 (28).
Western blot analysis of oatp2a1
The total protein was extracted form the rat
tissues, according to the instructions of the KGP total protein
extraction kit. The tissues, which had been cut into sections (5×5
mm) prior to homogenization, were then extracted by
ultracentrifugation at 4°C, 335 × g for 5 min. The concentrations
of the proteins were measured using Coomassie brilliant blue
staining. In the subsequent step, the separated proteins (20
µl) underwent 5% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and were transferred to polyvinylidene
difluoride membranes. The membrane was blocked with 5% skimmed milk
for 2 h. Membranes were then incubated with goat polyclonal
anti-Oatp2a1 (cat no. sc-103085; 1:500; Santa Cruz Biotechnology
Inc., Dallas, TX, USA) at 25°C for 2 h. The membrane was washed
with Tris-buffered saline with Tween 20 6 times (5 min per wash).
They were then incubated with a horseradish peroxidase
(HRP)-conjugated rabbit anti-goat IgG (cat. no. ZB-2306) or
HRP-conjugated goat anti-mouse (H+L) IgG (cat. no. ZB-2305)
(ZSGB-BIO Biotechnology Co., Ltd., Beijing, China; 1:5,000). at
25°C for 1 h. The bands were analyzed using enhanced
chemiluminescence (ECL) with the SuperSignal Chemiluminescent HRP
Substrates (Thermo Fisher Scientific Inc.). The ECL was detected by
films [MXG-1, Kodak (China) Co., Ltd., Shanghai, China]. The
results were analyzed by Image J software (Version 1.50b, National
Institutes of Health, Bethesda, MD, USA). GAPDH was detected using
mouse monoclonal anti-GAPDH (cat. no. AC002, ABclonal Biotech Co.,
Ltd., Wobrun, MA, USA), the dilution is 1:5000 utilized as a
reference protein to assess the expression levels of the target
protein.
Immunohistochemical analysis of
oatp2a1
The six tissues were fixed in neutral-buffered
formalin for 24 h at 4°C. Following routine gradient alcohol
dehydration, the tissues were sectioned into 6-µm sections
following being embedded in paraffin. On poly-L-lysine-coated
slides, the sections were incubated with dry milk solution for 1 h
at room temperature in order to avoid nonspecific staining. On
poly-L-lysine-coated slides, the primary anti-Oatp2a1 antibodies
were diluted with dry milk solution to 1:200 and incubated at 37°C
for 1 h. The slides were then incubated with HRP-conjugated rabbit
anti-goat IgG diluted 1:1,000 at 37°C for 30 min. Finally, images
of histological changes were captured using an Olympus microscope,
which revealed depositions of brown granular materials in the
cytoplasm and nuclei of the cells.
Statistical analysis
The data were analyzed using Statistical Product and
Service Solutions (SPSS) 17.0 software (SPSS, Inc., Chicago, IL,
USA). The continuous measurement data are expressed as the mean ±
standard deviation and were analyzed using Student's unpaired
t-test; P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of general
characteristics
The rats with spleen deficiency treated with
reserpine presented with inactivity, somnolence, grouping, loose
stools, loss of appetite and anorexia, with lackluster, loose and
disorderly fur, which are considered standard characteristics of
the spleen deficiency rat model. As shown in Table I, 16 days following spleen
deficiency establishment, the weights and the quantities of food
and water consumed by the rats in the RS group were significantly
lower, compared with those of the rats in the BS group (P<0.01).
This result demonstrated that the spleen deficiency model had been
successfully established by reserpine.
 | Table IWeight, diet and water consumption of
rats of the blank group and spleen deficiency groups. |
Table I
Weight, diet and water consumption of
rats of the blank group and spleen deficiency groups.
| Group | Weight (g) | Food consumption
(g) | Water consumption
(ml) |
|---|
| Spleen deficiency
(n=24) |
236.88±6.62a,b |
8.14±1.70a,b |
9.17±2.03a,b |
| Blank (n=24) | 317.19±4.68 | 25.56±0.98 | 24.20±1.11 |
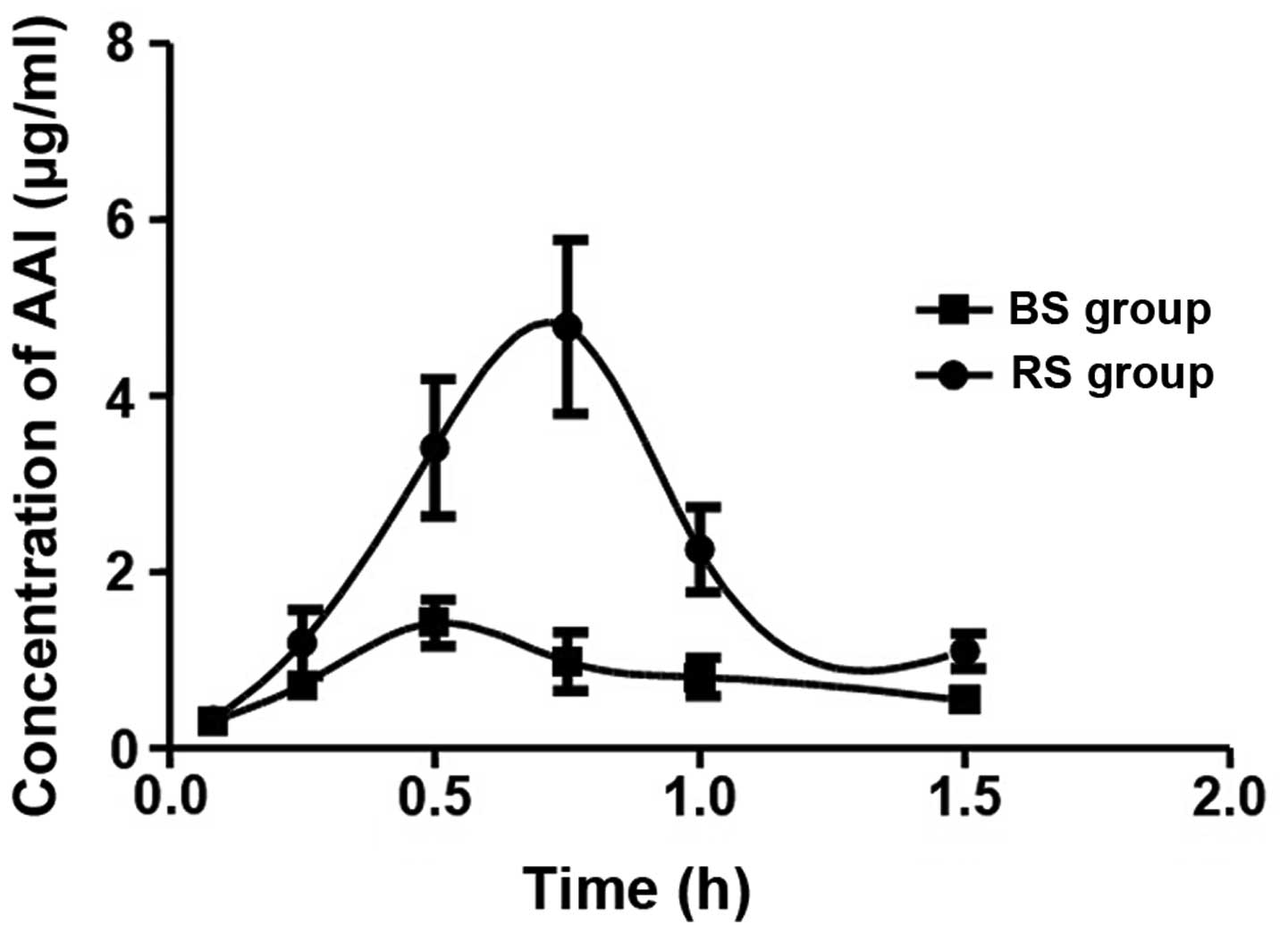
AAI pharmacokinetics
As shown in Fig. 1,
the concentration-time curves of AAI in the rats following oral
administration of a single 20 mg/kg−1 dose of AAI were
constructed to examine the intrinsic differences in the metabolism
of AAI in the BS and RS groups. The results are presented in the
form of separate curves, which showed that the AAI concentrations
in the rats of the RS group were higher, compared with those in the
BS group between 5 and 60 min, indicating that the metabolic
process of AAI in the rats of the RS group was altered. According
to the concentration-time curve of AAI in the RS group, there was a
significant increase in the peak concentration, compared with the
BS group, with peaking occurring at 45 min (Cmax 4.78
µg/ml), which was 15 min later than that in the BS group
(Cmax 1.42 µg/ml), indicating that the metabolism of AAI in
RS group was delayed, possibly due to the dysfunction of the
spleen.
mRNA expression levels of oatp2a1
The transcription levels of the transporter,
oatp2a1, were detected in the lung, liver, stomach, kidney, small
intestine and large intestine tissues of the rats using RT-qPCR
analysis. The pairwise comparisons of the results for these tissues
were as follows: The mRNA transcription levels of oatp2a1 in the
small intestine tissue was downregulated in the BS60 group,
compared with the corresponding level in the BS group (Fig. 2A), whereas no difference was
observed between the BS5 group and the BS group. However, the mRNA
levels of oatp2a1 in the lung and liver tissues were downregulated
in the RS5 group, compared with those of the RS group (Fig. 2B), whereas the level in the kidney
was upregulated in the RS60 group, compared with that in the RS
group (Fig. 2C).
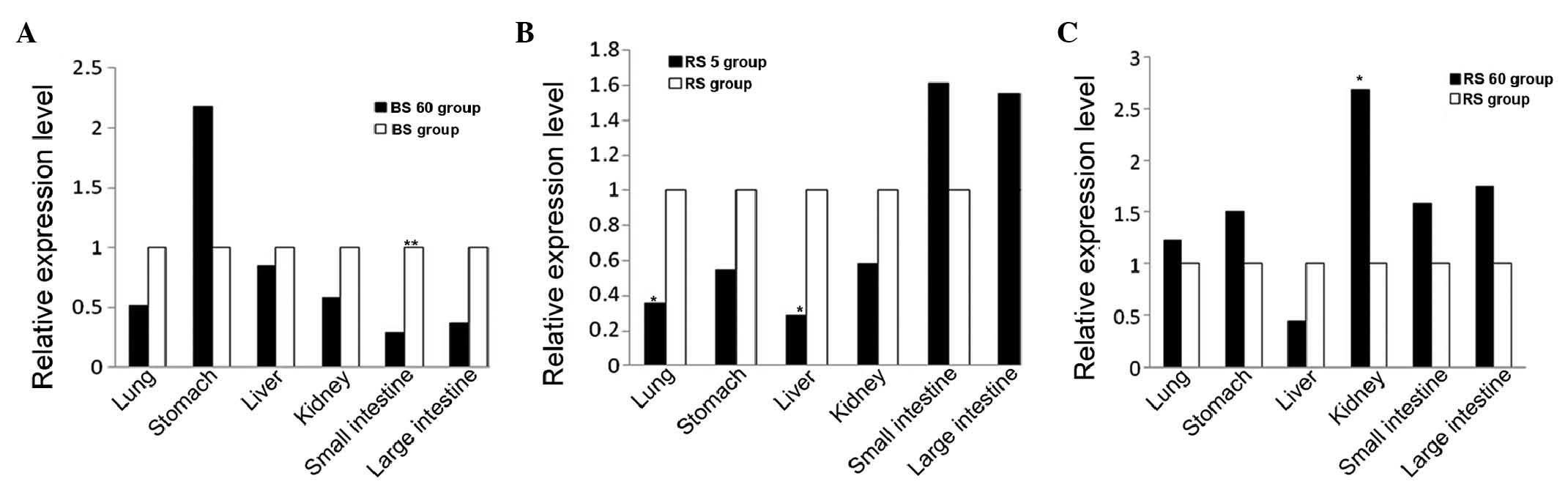 | Figure 2mRNA expression levels of oatp2a1.
Reverse transcription-quantitative polymerase chain reaction
analysis was performed to determine the mRNA expression levels of
oatp2a1 in tissues of the lung, stomach, liver, kidney, small
intestine and large intestine. The levels were compared between the
(A) BS60 and BS, (B) RS5 and RS, and (C) RS60 and RS groups. All
transcript levels are relative to the levels in the respective
tissues of the other treatment group (white bars). The values were
normalized with those of the housekeeping gene, glyceraldehyde
3-phosphate dehydrogenase. *P<0.05 and
**P<0.01, compared with the same organ in the other
tratment group. oatp2a1, organic anion transporting peptide 2a1;
BS, blank (without spleen deficiency; RS, spleen deficiency. |
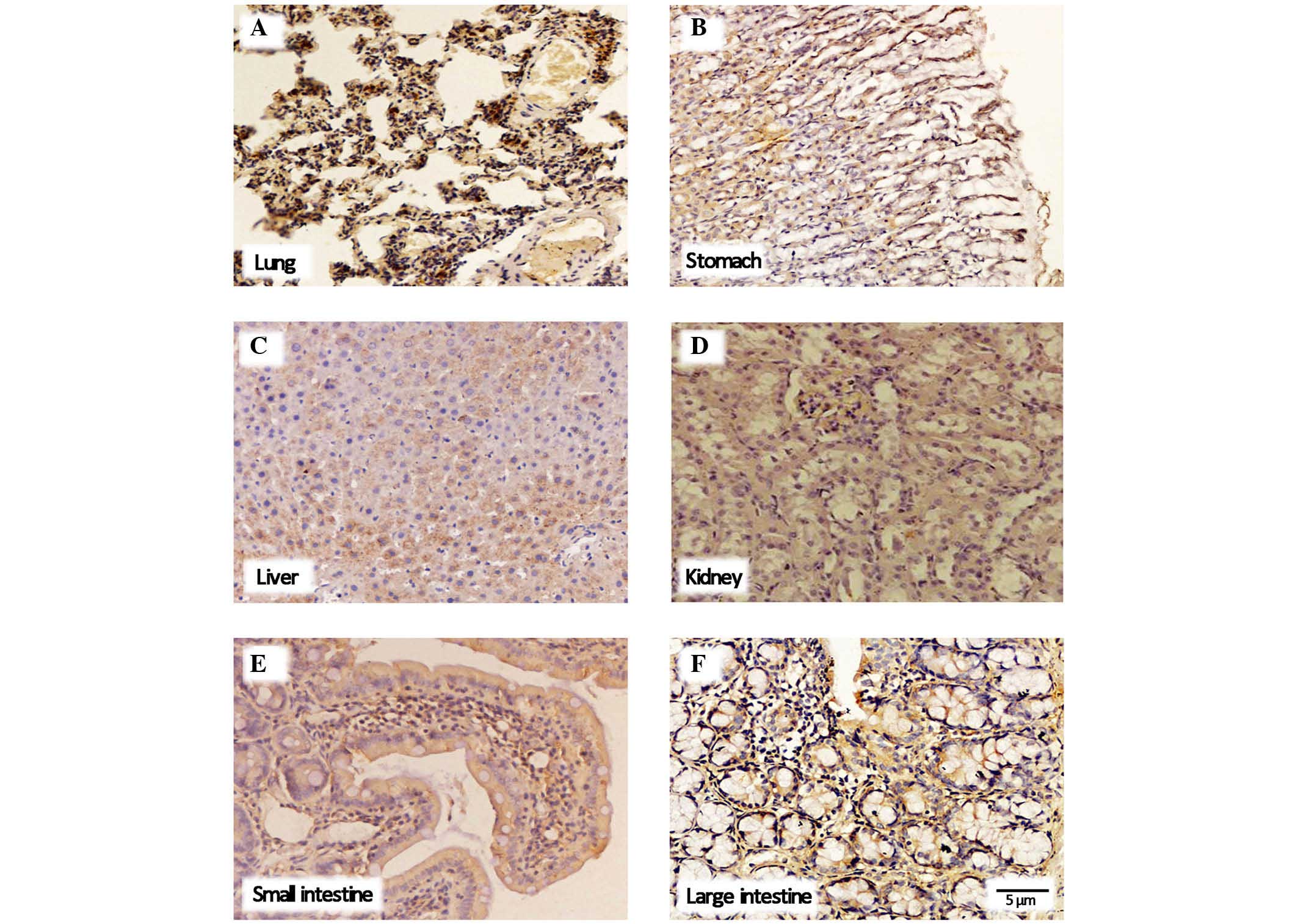
Expression and localization of the
oatp2a1 protein
As shown in Fig. 3,
the oatp2a1 protein was generally expressed in the six tissue
samples from the rats, including the lung, liver, stomach, kidney,
small intestinal and large intestine. Furthermore, as shown in
Fig. 4, the patterns of protein
distribution observed in the membranes of various tissues samples
were similar, including those of the stomach, lung and large
intestine. By contrast, the expression patterns differed in the
liver, kidney and small intestine tissues, in which staining was
observed in the membrane and cytoplasm. The immunoreactivity of
oatp2a1 was detected in different areas of the tissues, and intense
staining was observed in the epithelial cells of the alveoli in the
lung and blood sinus, and macrophages in the liver, stomach and
renal collecting tubule.
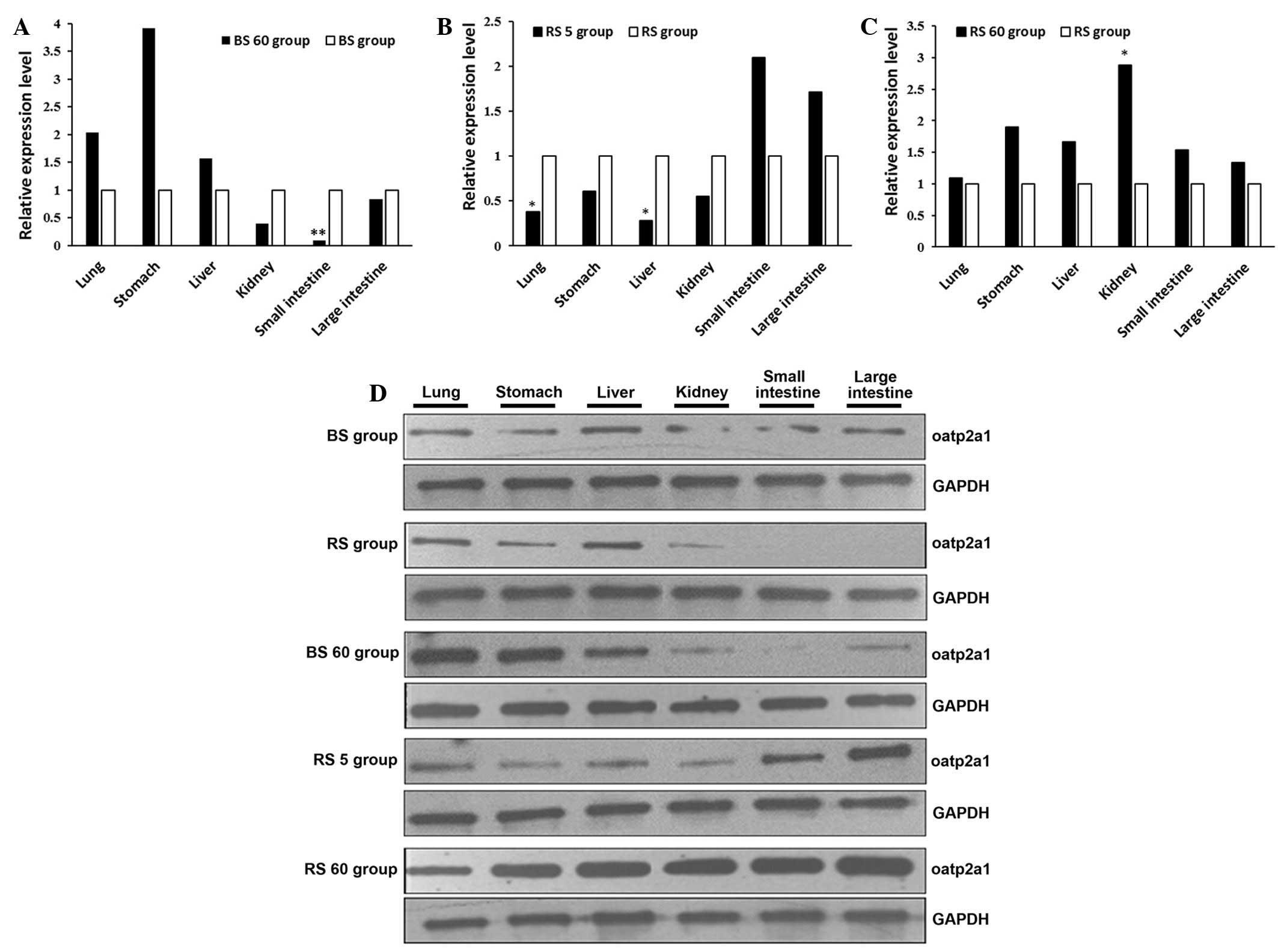 | Figure 3Western blot analysis of the protein
expression of oatp2a1 in tissues of the lung, liver, stomach,
kidney, small intestine and large intestine of rats. The protein
expression of GAPDH was determined as an internal standard. (A)
BS60 and BS, (B) RS5 and RS, (C) RS60 and RS groups and (D) western
blot results of all groups. *P<0.05 and
**P<0.01, compared with the same organ in the other
treatment group. Oatp2a1, organic anion transporting peptide 2a1;
AAI aristolochic acid I; BS, blank (without spleen deficiency); RS,
spleen deficiency; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Discussion
The spleen is a functional unit for substance
transformation and transportation in TCM. Due to its importance and
complexity, researchers have focused on the properties of the
spleen. It is well documented that the spleen is not only an
anatomical concept, but is also associated with the digestive
system (29,30) in particular, in addition to the
immune (31), circulatory
(32) and endocrine systems
(33). Previously, Huang et
al (14,15) suggested that the spleen was
involved in the pharmacokinetics hypothesis, which assumes that the
spleen is responsible for the absorption, distribution, metabolism
and excretion of drugs. However, the role of the spleen has only
been demonstrated in absorption (14), and there is no evidence focusing on
drug distribution, transportation or excretion. Therefore, the
present study aimed to determine whether the spleen affects the
entire metabolic process of AAI, which may be associated with the
nephrotoxicity of AAI.
In the present study, a widely-accepted rat model of
spleen deficiency was introduced, which was characterized by
pathological changes, including loss of appetite, anorexia,
listlessness, somnolence, loose stools and weight loss. As a method
for the investigation of spleen deficiency, this animal model has
been confirmed to exhibit long-lasting spleen deficiency, which can
lead to the dysfunction of digestion and absorption (13).
Orally administered AAI in rats represents an
exogenous toxin due to its nephrotoxic, carcinogenic and mutagenic
effects (34,35). As shown in Fig. 1, the present study revealed that,
when a single dose of 20 mg kg−1 was administrated, the
concentration of AAI in the blood of animals in the RS group was
higher, compared with that in the BS group for a period of 5–60
min, indicating an abnormally high concentration of AAI with
increased toxicity, possibly due to the dysfunction of the spleen.
However, the peak concentration of AAI in the spleen deficiency
group was observed at 45 min, which was 15 min later than that in
the blank group, following which the concentration decreased
rapidly Thus, the absorption, metabolism and excretion of AAI were
delayed in the RS group, although the body may accelerate the
excretion of AAI by regulating the internal mechanism to maintain
homeostasis.
In the present study, the interaction between the
heart, spleen, liver, lung and kidney during AAI metabolism was
investigated based on the TCM classic principle Jing yue quan shu
(Jingyue's Complete Works) (36).
It is generally considered that the spleen is pivotal in the
transportation and transformation of substances among the other
five organs examined in the present study, however, few studies
have focused on the transport activities of these organs. Spleen
deficiency affected the normal physiological function of other
organs, thus the absorption of exogenous organic anions and AAI
were altered, as were the metabolism and excretion processes, and
the blood concentration of these compounds. In our preliminary
study (37), it was demonstrated
that the liver is important and associated with the transportation
and transformation function of the spleen via the expression level
of oatps, however, the expression levels were not compared among
these transportation-associated organs.
It has been reported that the absorption,
distribution and elimination of endobiotics and xenobiotics in
various organs can be regulated by the oatp superfamilies (19,20).
Specifically, the function of oatp in the process of drug
transportation is well characterized as tissue-specific
accumulation and elimination (38,39).
In addition, previous studies have found that several members of
the oatp family, including oatp2a1, oatp2b1 and oatp4a1, are widely
distributed (37,40), and the liver and small intestine
are also important in spleen transportation. Among these
transporters, oatp2a1 is expressed at higher levels than the other
two oatps. In the present study, analyses of the mRNA and protein
expression levels of oatp2a1 revealed that the changes in mRNA
expression levels were observed, not only in the liver and small
intestine, but also in the lung, stomach and kidney, and expression
was prominent in the blood sinus, macrophage cells of the liver,
the stomach, the cytoplasm and membrane of renal tubules and the
small intestine. Based on these findings, it was hypothesized that
oatp2a1 regulates the transport and excretion of endobiotics and
xenobiotics through transmembrane transportation, and may be the
mechanism of AAI metabolism, indicated by AAI plasma
concentrations.
To clarify the mechanisms and the toxicities of AAI,
the present study monitored the process of AAI metabolism, based on
the expression of oatp2a1 in six tissue samples. It was
demonstrated that the mRNA expression of oatp2a1 remained unchanged
in the BS5 group, but was downregulated in the small intestine in
BS60 group, compared with the BS group, which is where absorption
occurred. These altered absorption levels suggested that the body
may decrease the rate of uptake of AAI in the small intestine and
increase its excretion to maintain the stability of the environment
in rats without spleen deficiency, which may be explained by the
hypothesis that the small intestine is important in AAI transport
and the maintenance of homeostasis.
In the present study, 5 min following the oral
administration of AAI, when the plasma concentration began to
change, the expression levels of oatp2a1 were downregulated in the
liver and lung tissues of the RS group, but remained unchanged in
the BS group. The expression levels of oatp2a1 in the liver cell
membranes and the liver macrophages were decreased, predominantly
due to the decline in AAI absorption, transportation and phagocytic
clearance, respectively. Similarly, the lower expression level in
the pulmonary alveolar epithelial cells, which are involved in
transportation, indicated a reduction in AAI transportation. Thus,
in the state of spleen deficiency, the body reduced the
transportation of AAI in the liver and lung, at the beginning of
AAI metabolism, which confirmed the importance of the spleen in
transporting AAI. At 60 min following the oral administration of
AAI, the plasma concentrations were significantly decreased in the
two groups, particularly in the RS group. Of note, a novel
observation was recorded in the RS group, in that no change in
expression was observed in the small intestine, however, the
expression level of oatp2a1 was increased in the kidney at the same
time. These findings suggested that the kidney excreted more AAI,
which may increase the burden on the kidney, and this pathological
process may lead to potential renal impairment.
In conclusion, the present study provided
experimental evidence of the effects of oatp2a1 on the
transformation and transportation of AAI. In addition, the findings
suggested the possibility that the function of the spleen involved
not only the observation process, but also the distribution and
excretion processes of AAI, which may be associated with the
function of liver, lung and kidney, and supports the hypothesis
that spleen is important among the five organs. Finally, a novel
hypothesis was suggested that spleen deficiency accelerates the
accumulation of the AAI in the kidney, which may lead to
AAI-induced toxicity.
Investigations involving the monitoring of
sufficient timepoints are currently in progress to investigate the
entire cycle of AAI metabolism. Other approaches, including in
vivo labeling are required to examine the mechanism of AAI in
TCM.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81373500, 81072806
and 81102581) and the Natural Science Foundation of Guangdong
Province (grant no. S2012010009277).
References
|
1
|
Cheung TP, Xue C, Leung K, Chan K and Li
CG: Aristolochic acids detected in some raw Chinese medicinal herbs
and manufactured herbal products-a consequence of inappropriate
nomenclature and imprecise labelling? Clin Toxicol (Phila).
44:371–378. 2006. View Article : Google Scholar
|
|
2
|
Wang Y and Chan W: Determination of
aristolochic acids by high-performance liquid chromatography with
fluorescence detection. J Agric Food Chem. 62:5859–5864. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schaneberg BT and Khan IA: Analysis of
products suspected of containing Aristolochia or Asarum species. J
Ethnopharmacol. 94:245–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adeyemi OO, Aigbe FR and Badru OA: The
antidiarrhoeal activity of the aqueous root extract of Aristolochia
ringens (Vahl.) Aristolochiaceae. Nig Q J Hosp Med. 22:29–33.
2012.PubMed/NCBI
|
|
5
|
Nitzsche D, Melzig MF and Arlt VM:
Evaluation of the cytotoxicity and genotoxicity of aristolochic
acid I-a component of Aristolochiaceae plant extracts used in
homeopathy. Environ Toxicol Pharmacol. 35:325–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SM, Lai MN, Chen PC, Pu YS, Lai MK,
Hwang JS and Wang JD: Increased upper and lower tract urothelial
carcinoma in patients with end-stage renal disease: a nationwide
cohort study in Taiwan during 1997–2008. Biomed Res Int.
2014:1497502014.
|
|
7
|
Chau W, Ross R, Li JY, Yong TY, Klebe S
and Barbara JA: Nephropathy associated with use of a Chinese herbal
product containing aristolochic acid. Med J Aust. 194:367–368.
2011.PubMed/NCBI
|
|
8
|
Vanherweghem JL, Depierreux M, Tielemans
C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D,
Verbeelen D and Vanhaelen-Fastre R: Rapidly progressive
interstitial renal fibrosis in young women: Association with
slimming regimen including Chinese herbs. Lancet. 341:387–391.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibutani S, Dong H, Suzuki N, Ueda S,
Miller F and Grollman AP: Selective toxicity of aristolochic acids
I and II. Drug Metab Dispos. 35:1217–1222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai MN, Lai JN, Chen PC, Hsieh SC, Hu FC
and Wang JD: Risks of kidney failure associated with consumption of
herbal products containing Mu Tong or Fangchi: A population-based
case-control study. Am J Kidney Dis. 55:507–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmeiser HH, Nortier JL, Singh R, Gamboa
da Costa G, Sennesael J, Cassuto-Viguier E, Ambrosetti D, Rorive S,
Pozdzik A, Phillips DH, et al: Exceptionally long-term persistence
of DNA adducts formed by carcinogenic aristolochic acid I in renal
tissue from patients with aristolochic acid nephropathy. Int J
Cancer. 135:502–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan FL: On English translation of name of
sections in 'huangdi neijing suwen' with a comment on the English
translation of the section names in two books of English version.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 24:265–8. 2004.In Chinese.
PubMed/NCBI
|
|
13
|
Xiong B and Qian H: Effects of Sijunzi
decoction and Yupingfeng powder on expression of janus
kinase-signal transducer and activator of transcription signal
pathway in the brain of spleen-deficiency model rats. J Tradit Chin
Med. 33:78–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Ren P, Wen AD, Wang LL, Zhang L
and Gao F: Pharmacokinetics of traditional Chinese syndrome and
recipe: A hypothesis and its verification (I). World J
Gastroenterol. 6:384–391. 2000. View Article : Google Scholar
|
|
15
|
Ren P, Huang X, Li SQ, Xu SY, Wan MH, Zhou
YX, Zhou YW and Tang WF: Pharmacokinetic characteristics of ferulic
acid in patients with different syndromes of deficiency of spleen
qi, stagnation of liver qi and spleen deficiency, and excess of
stomach heat. Zhong Xi Yi Jie He Xue Bao. 4:147–151. 2006.In
Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu XN: Current concept of Spleen-Stomach
theory and Spleen deficiency syndrome in TCM. World J
Gastroenterol. 4:2–6. 1998. View Article : Google Scholar
|
|
17
|
Ouyang S, Chen W and Kuang XB: Effects of
perindopril on expression of kidney aquaporin-2 and urine
aquaporin-2 excretion in chronic heart failure rats. Zhonghua Xin
Xue Guan Bing Za Zhi. 41:276–281. 2013.In Chinese. PubMed/NCBI
|
|
18
|
Zhao WX, Gao J, Shi YY and Xu SQ: Effects
of different fluid resuscitation regimes on lung injury and
expression of pulmonary aquaporin 1 and aquaporin 5 in uncontrolled
hemorrhagic shock in rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
21:282–285. 2009.In Chinese. PubMed/NCBI
|
|
19
|
Wu LX, Guo CX, Qu Q, Yu J, Chen WQ, Wang
G, Fan L, Li Q, Zhang W and Zhou HH: Effects of natural products on
the function of human organic anion transporting polypeptide 1B1.
Xenobiotica. 42:339–348. 2012. View Article : Google Scholar
|
|
20
|
Csanaky IL, Lu H, Zhang Y, Ogura K,
Choudhuri S and Klaassen CD: Organic anion-transporting polypeptide
1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated
bile acids: Studies in Oatp1b2-null mice. Hepatology. 53:272–281.
2011. View Article : Google Scholar
|
|
21
|
Meyer zu Schwabedissen HE, Ware JA,
Finkelstein D, Chaudhry AS, Mansell S, Leon-Ponte M, Strom SC,
Zaher H, Schwarz UI, Freeman DJ, et al: Hepatic organic anion
transporting polypeptide transporter and thyroid hormone receptor
interplay determines cholesterol and glucose homeostasis.
Hepatology. 54:644–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff
AW and Schuster VL: Identification and characterization of a
prostaglandin transporter. Science. 268:866–869. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mandery K, Bujok K, Schmidt I, Wex T,
Treiber G, Malfertheiner P, Rau TT, Amann KU, Brune K, Fromm MF and
Glaeser H: Influence of cyclooxygenase inhibitors on the function
of the prostaglandin transporter organic anion-transporting
polypeptide 2A1 expressed in human gastroduodenal mucosa. J
Pharmacol Exp Ther. 332:345–351. 2010. View Article : Google Scholar
|
|
24
|
Nomura T, Chang HY, Lu R, Hankin J, Murphy
RC and Schuster VL: Prostaglandin signaling in the renal collecting
duct: Release, reuptake and oxidation in the same cell. J Biol
Chem. 280:28424–28429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu ZY: Disposition pathway-dependent
approach for predicting organic anion-transporting
polypeptide-mediated drug-drug interactions. Clin Pharmacokinet.
52:433–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shirasu T, Koyama H, Miura Y, Hoshina K,
Kataoka K and Watanabe T: Nanoparticles effectively target
rapamycin delivery to sites of experimental aortic aneurysm in
rats. PLoS One. 11:e01578132016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao N, Zhang W, Guo Y, Jia H, Zha Q, Liu
Z, Xu S and Lu A: Effects on neuroendocrinoimmune network of
Lizhong Pill in the reserpine induced rats with spleen deficiency
in traditional Chinese medicine. J Ethnopharmacol. 133:454–459.
2011. View Article : Google Scholar
|
|
28
|
Corona-Meraz FI, Navarro-Hernández RE,
Ruíz-Quezada SL, Madrigal-Ruíz PM, Castro-Albarrán J,
Chavarría-Ávila E, Guzmán-Ornelas MO, Gómez-Bañuelos E, Petri MH,
Ramírez-Cedano JI, Aguilar-Aldrete ME, Ríos-Ibarra C and
Vázquez-Del Mercado M: Inverse relationship of the CMKLR1 relative
expression and chemerin serum levels in obesity with dysmetabolic
phenotype and insulin resistance. Mediators Inflamm.
2016:30853902016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li ZH, Wang J, Cai RL, Wang YW and Hu JP:
Establishment and evaluation of a rat model of ulcerative colitis
with syndrome of dampness stagnancy due to spleen deficiency. Zhong
Xi Yi Jie He Xue Bao. 10:918–924. 2012.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li LS, Qu RY, Wang W and Guo H:
Significance of changes of gastrointestinal peptides in blood and
ileum of experimental spleen deficiency rats. World J
Gastroenterol. 9:553–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Sun BG, Zhang SJ, Chen ZX, Hardi
CF and Xiang T: Observations of TCRVβ gene expression in rats with
dampness syndrome. Evid Based Complement Alternat Med.
2014:3736082014. View Article : Google Scholar
|
|
32
|
Sponaas AM, Freitas do Rosario AP, Voisine
C, Mastelic B, Thompson J, Koernig S, Jarra W, Renia L, Mauduit M,
Potocnik AJ and Langhorne J: Migrating monocytes recruited to the
spleen play an important role in control of blood stage malaria.
Blood. 114:5522–5531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grant SJ, Schnyer RN, Chang DH, Fahey P
and Bensoussan A: Interrater reliability of chinese medicine
diagnosis in people with prediabetes. Evid Based Complement
Alternat Med. 2013:7108922013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fuchs TC, Mally A, Wool A, Beiman M and
Hewitt P: An exploratory evaluation of the utility of
transcriptional and urinary kidney injury biomarkers for the
prediction of aristolochic acid-induced renal injury in male rats.
Vet Pathol. 51:680–694. 2014. View Article : Google Scholar
|
|
35
|
McDaniel LP, Elander ER, Guo X, Chen T,
Arlt VM and Mei N: Mutagenicity and DNA adduct formation by
aristolochic acid in the spleen of Big Blue® rats.
Environ Mol Mutagen. 53:358–368. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J and Liu BY: Evaluation of clinical
therapeutic effect in Jing yue quan shu (Jingyue's Complete Works).
Zhonghua Yi Shi Za Zhi. 39:59–61. 2009.In Chinese. PubMed/NCBI
|
|
37
|
Pan AZ, Dong XA, Zhang SJ, Xiang T, Chen
ZX and Lin YW: Study on mRNA and protein expressions of organic
anion transporting polypeptide (oatp2b1) in rats with high fat diet
and overstrain induced Pi deficiency syndrome. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 33:953–957. 2013.In Chinese. PubMed/NCBI
|
|
38
|
Takanohashi T, Kubo S, Arisaka H, Shinkai
K and Ubukata K: Contribution of organic anion transporting
polypeptide (OATP) 1B1 and OATP1B3 to hepatic uptake of nateglinide
and the prediction of drug-drug interactions via these
transporters. J Pharm Pharmacol. 64:199–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi MK, Shin HJ, Choi YL, Deng JW, Shin
JG and Song IS: Differential effect of genetic variants of Na
(+)-taurocholate co-transporting polypeptide (NTCP) and organic
anion-transporting polypeptide 1B1 (OATP1B1) on the uptake of
HMG-CoA reductase inhibitors. Xenobiotica. 41:24–34. 2011.
View Article : Google Scholar
|
|
40
|
Dong X, Pan AZ and Sun BG: Organic anion
transporting polypeptide (oatp4a1) mRNA and protein expressions in
high fat and over-fatigue impairing Pi rats. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 32:1223–1226. 2012.In Chinese. PubMed/NCBI
|