Introduction
Ellipticine
[5,11-dimethyl-6H-pyrido(4,3-b)carbazole] is a naturally occurring
alkaloid isolated from the leaves of Apocyanaceae plants (1). Ellipticine and its analogues have
demonstrated potent anti-cancer activity in a phase II study of
advanced breast cancer (2) and
several other types of cancer (3).
The main reason why ellipticine and its derivatives have become
noteworthy is its high efficiency against cancer, its rather
limited toxic side effects, and its limited intrinsic toxicity
(4). The chemopreventive activity
of ellipticine is likely to be associated with its ability to
modulate pathways involved in cell cycle progression (5) and apoptotic cell death (6–8).
However, its efficacy in bladder cancer cells and the associated
mechanisms of action are not completely understood.
Bladder cancer is the fourth commonest male
malignancy and is associated with significant morbidity and
mortality (9). Approximately 90%
of all bladder cancer cases are classified as urothelial cell
carcinomas (UCC) which are originated from epithelial cells lining
the interior of the urothelial organ (10). Therapeutic options for bladder
cancer include surgical resection, intravascular chemotherapy,
radiation, immunotherapy and system chemotherapy. Despite the
low-grade cases (good differentiation) having an excellent
prognosis, high-grade cases (medium and poor differentiation)
progress to invasion, metastases and death (11). Therefore, identifying novel and
alternative therapeutic strategies is critical for prolonging
survival.
On the basis of its effectiveness in other types of
cancer, ellipticine could be a potential candidate for the therapy
of bladder cancer. The present study investigated whether
ellipticine reduces the proliferation and migration abilities of
bladder cancer cells and its underlying molecular mechanisms.
Materials and methods
Reagents and cell culture
Ellipticine (≥99% pure; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was dissolved in dimethylsulfoxide
(DMSO) to make 10 mM stock solutions and was stored at −20°C.
Primary antibodies against phosphorylated (p-)ATM (cat. no. 5883;
1:1,000), M-phase inducer phosphatase 3 (Cdc25) (cat. no. 4688,
1:1,000), p-Cdc25C (Ser-216) (cat. no. 4901; 1:1,000), checkpoint
kinase 1 (Chk1) (cat. no. 2360; 1:1,000), p-Chk1 (Ser-345) (cat.
no. 2348, 1:1,000), Cyclin B1 (cat. no. 12231, 1:1,000), cyclin
dependent kinase 1 (Cdk1) (cat. no. 7519; 1:1,000), and secondary
antibodies (cat. no. 7074; 1:5,000) were purchased from Cell
Signaling Technology (Danvers, MA, USA). The bicinchoninic acid
protein assay kit was purchased from Pierce; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The human bladder cancer cell
line T-24, obtained from the Shanghai Institute of Cell Biology,
Chinese Academy of Sciences (Shanghai, China), was cultured in
RPMI-1640 medium (HyClone; GE Healthcare, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
Millipore), 100 U/ml penicillin and 100 mg/ml streptomycin, and was
grown in an incubator with 5% CO2 at 37°C.
Cell viability assay
The effect of ellipticine on the viability of T-24
cells was evaluated by Cell Counting Kit-8 (CCK-8) assay.
Approximately 10×104 T-24 cells were seeded in 96-well plates.
Following an overnight incubation, T-24 cells were treated with
either 1 µl/ml DMSO (vehicle control) or 1, 2, 4, 8 or 16 µM
ellipticine for 24 h. Following incubation, 10% CCK-8, diluted in
normal culture medium, was added to each well and incubated at 37°C
until color conversion occurred. Absorbance at 450 nm was then
measured using a MRX II absorbance reader (Dynex Technologies,
Chantilly, VA, USA). Results were displayed as a percentage of
growth, with 100% representing control cells treated with DMSO
alone.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from T-24 cells treated with
2, 4 or 8 µM ellipticine or 1 µl/ml DMSO using RNAiso Plus (Takara
Biotechnology Co, Ltd., Dalian, China) and transcribed into cDNA
using the PrimeScript RT Reagent Kit (Takara Biotechnology Co,
Ltd.). qPCR was performed using an ABI 7500 FAST Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and a
SYBR-Green PCR kit (Takara Biotechnology Co, Ltd.). The relative
expression level of mRNA was quantified with the 2-∆∆Cq
method (12) following
normalization with the endogenous reference, GAPDH. The primers
used were as follows: ATM, forward 5′-TTACGGGTGTTGAAGGTGTCT-3′ and
reverse 5′-GGATTCATGGTCCAGTCAAAG-3′; and GAPDH, forward
5′-GCTGAACGGGAAGCTCACTG-3′ and reverse
5′-GTGCTCAGTGTAGCCCAGGA-3′.
In vitro motility assays
T-24 cells were seeded in a 6-well plate at a
density of 8×104 cells/well. Following overnight incubation, cells
were treated with 0.2, 0.4 or 0.8 µM ellipticine for 24 h, then
harvested by centrifugation at 800 × g for 5 min at 20°C. Cells
were resuspended in growth medium at a concentration of 4×105
cells/ml, and 0.2 ml of each was added to the top chamber of each
well (24-well insert, 8 µm pore size; Merck Millipore), and growth
medium containing 20% FBS was added to the lower chamber of each
well to act as a chemoattractant. The cells were allowed to migrate
to the lower chamber for 24 h in an incubator at 37°C and those
that did not invade through the membrane were removed with a cotton
swab by scraping the upper surface of the membrane. Cells that had
migrated to the lower surface of the membrane were fixed for 15 min
in 100% methanol and stained with 0.1% crystal violet for
observation and counting. Experiments were performed in
triplicate.
Cell cycle assay
Cells were seeded in 6-well culture dishes at
concentrations determined to output 60–70% confluence within 24 h,
and then treated with 1, 2, 4, 8, or 16 µM ellipticine. Following
24 h incubation, cells were washed with PBS and fixed using
pre-cooled 70% ethanol at 4°C overnight. The cells were washed and
subjected to propidium iodide (PI)/RNase staining for 30 min at
37°C in the dark. Cell cycle distribution was then analyzed using
the FC500 flow cytometer (Beckman Coulter Inc., Brea, CA, USA) and
BD FACSDiva software (version 6.1.3, BD Biosciences, Franklin
Lakes, NJ, USA).
Western blot analysis
Cells were harvested at 24 h following ellipticine
treatment, washed with PBS, and lysed with lysis buffer at 4°C for
45 min [10 mmol/l Tris-HCl, 0.25 mol/l sucrose, 5 mmol/l EDTA, 50
mmol/l NaCl, 30 mmol/l sodium pyrophosphate, 50 mmol/l NaF, 1
mmol/l Na3VO4, 1 mmol/l phenylmethylsulfonyl
fluoride, and 2% cocktail (protease inhibitor, pH 7.5; Servicebio,
Wuhan, China) and then centrifuged at 1,200 × g, for 15 min at 4°C.
The protein concentrations were measured by bicinchoninic acid
assay and equalized to 4 µg/µl using lysis buffer. Each sample was
supplemented with 4X loading buffer and boiled for 5 min.
Appropriate amounts of protein (20–30 µg) were resolved by
electrophoresis in 10–12% Tris-glycine polyacrylamide gels and
transferred onto nitrocellulose membranes. Membranes were blocked
with 5% nonfat milk in Tris-buffered saline solution (TBS)
containing 0.05% Tween [TBST; 10 mM Tris-Cl (pH 7.4), 150 mM NaCl,
0.1% −20], then hybridized overnight at 4°C with the appropriate
primary antibody. They were then washed with TBST and incubated
with horseradish peroxidase-conjugated secondary antibody at
1:1,000 dilution in TBST by gentle agitation at room temperature
for 2 h, and subsequently washed. The signal density was visualized
using an enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.). The results were quantitated using ImageJ
version 1.48 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The data analyzed were from three independent
experiments. Statistical significance was assessed between various
treatment groups and controls using analysis of variance (ANOVA).
Least-Significant Difference (LSD) was used to compare individual
groups following ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
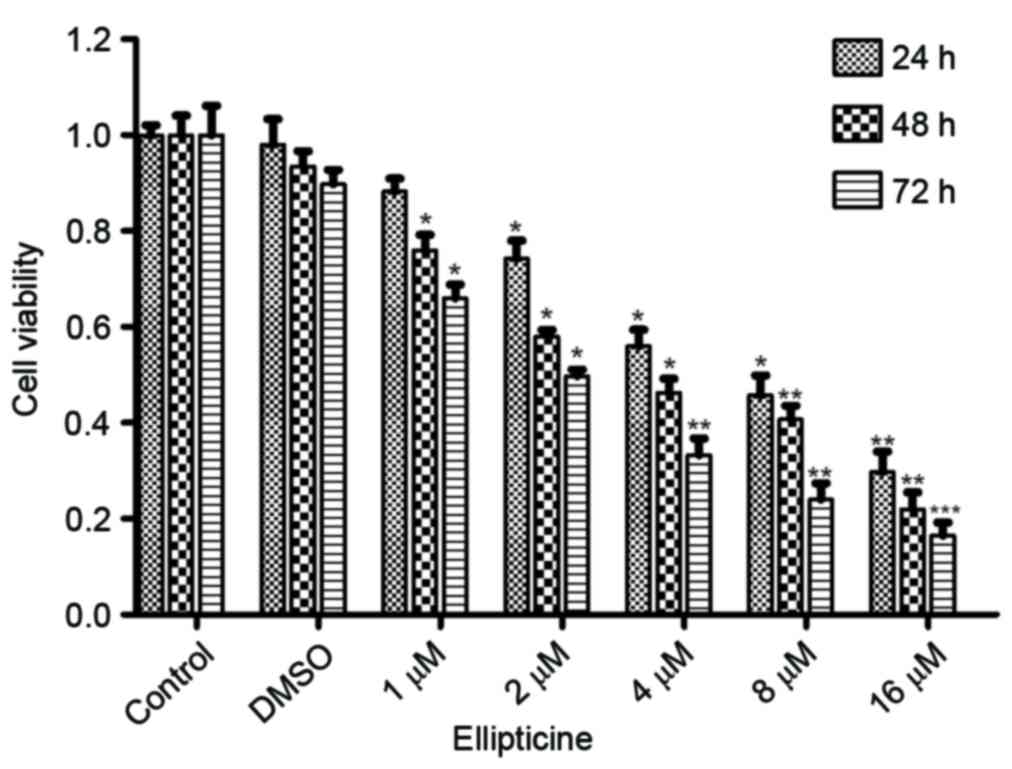
Ellipticine affects cell growth in
T-24 cells
The CCK-8 assay revealed that compared to untreated
and DMSO controls, ellipticine treatment induced a dose- and
time-dependent inhibition of T-24 cell growth (Fig. 1). There was no significant
difference between untreated control and DMSO control, which
indicated that the vehicle, DMSO, did not affect the proliferation
ability of T-24 cells (Fig. 1).
Compared to untreated cells, ellipticine treatment resulted in a
significant reduction in cell viability with increasing
concentration and time, particularly at the concentration of 8 and
16 µM (Fig. 1). The 50% growth
inhibitory concentration (IC50) for ellipticine
treatment was estimated to be 4.3, 3.8, and 2.0 µM for 24, 48, and
72 h, respectively (Fig. 1). These
results, therefore, demonstrate that ellipticine has a significant
inhibitory effect on T-24 cell proliferation.
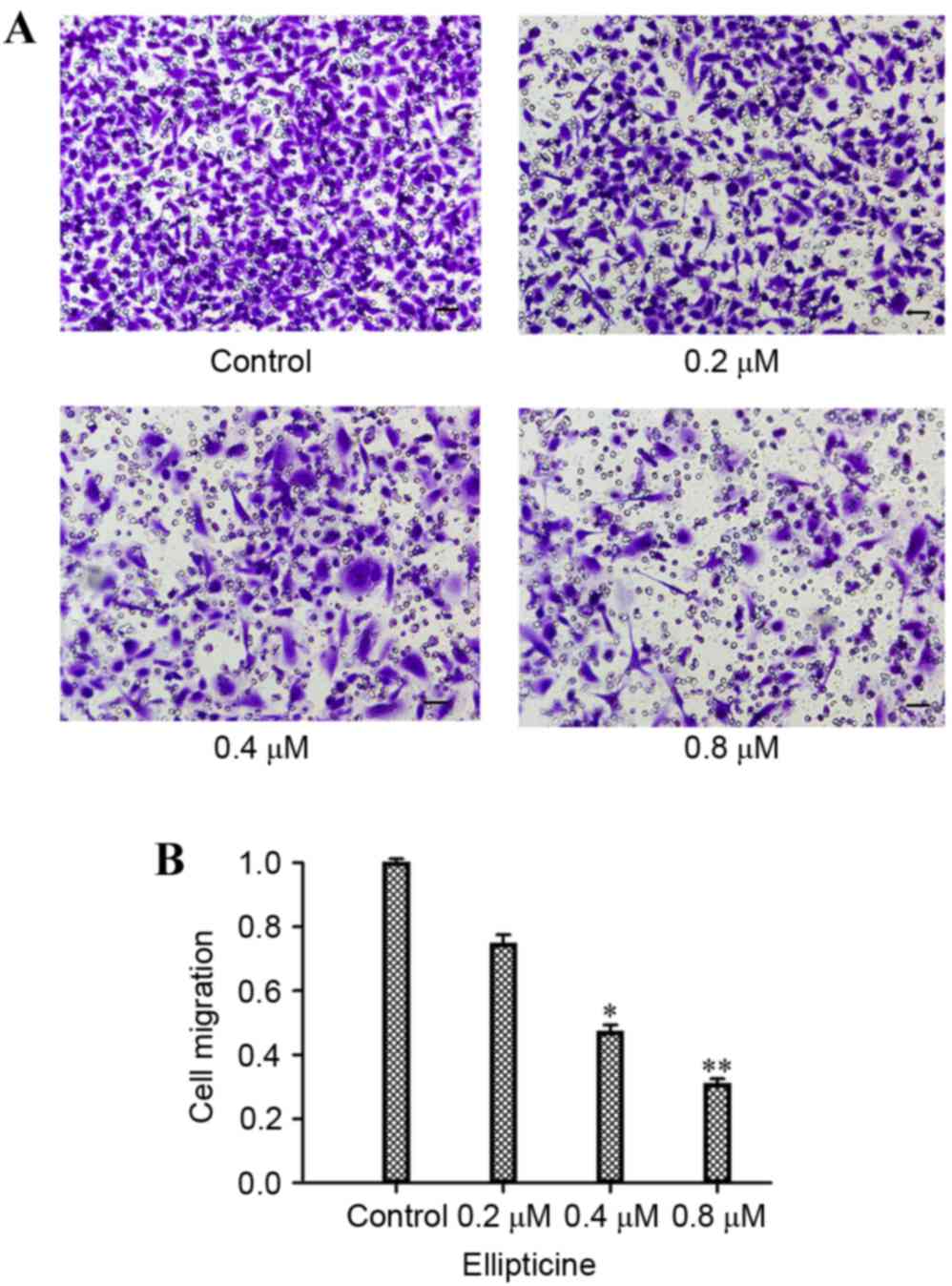
Ellipticine inhibits T-24 cell
migration
As demonstrated in Fig.
1, when 1 µM ellipticine was added for 24 h, the viability of
the cells was not significantly altered. Hence, the concentrations
of 0.2–0.8 µM and the time of 24 h were selected to perform the
migration assay and examine the effect of ellipticine on the
migration ability of T-24 cells. As demonstrated in Fig. 2, treatment with 0.4 and 0.8 µM
ellipticine resulted in a significant reduction in motility
compared with untreated control (P<0.05 and P<0.01,
respectively; Fig. 2).
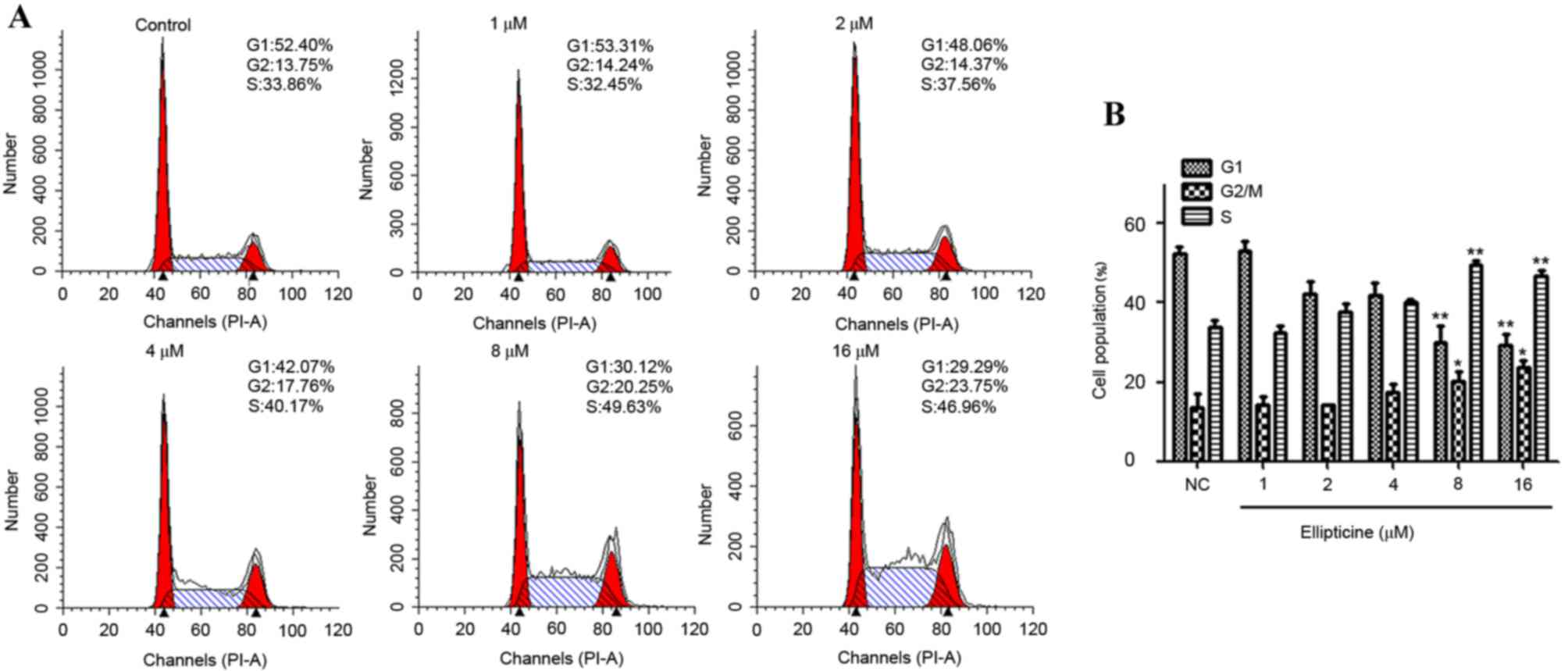
Ellipticine induces G2/M phase cell
cycle arrest
To test the hypothesis that an arrest of cells at a
specific cell cycle check point may be involved, the effect of
ellipticine on cell cycle perturbations was assessed. Compared with
the untreated controls, ellipticine treatment induced a significant
arrest of T-24 cells in the G2/M phase of the cell cycle. Cell
cycle analysis suggested that the percentage of G2/M phase-arrested
cells in the control was 13.75%, while the percentage of G2/M
phase-arrested cells increased significantly following 24 h
treatment with 8 and 16 µM ellipticine compared with the untreated
control (20.25 and 23.75%, respectively; P<0.05 and P<0.05
respectively; Fig. 3A).
Concomitant with the increased G2/M phase cell population,
ellipticine treatment resulted in a reduction in cell numbers
arrested in the G1 phase of the cell cycle compared with the
untreated control (Fig. 3B).
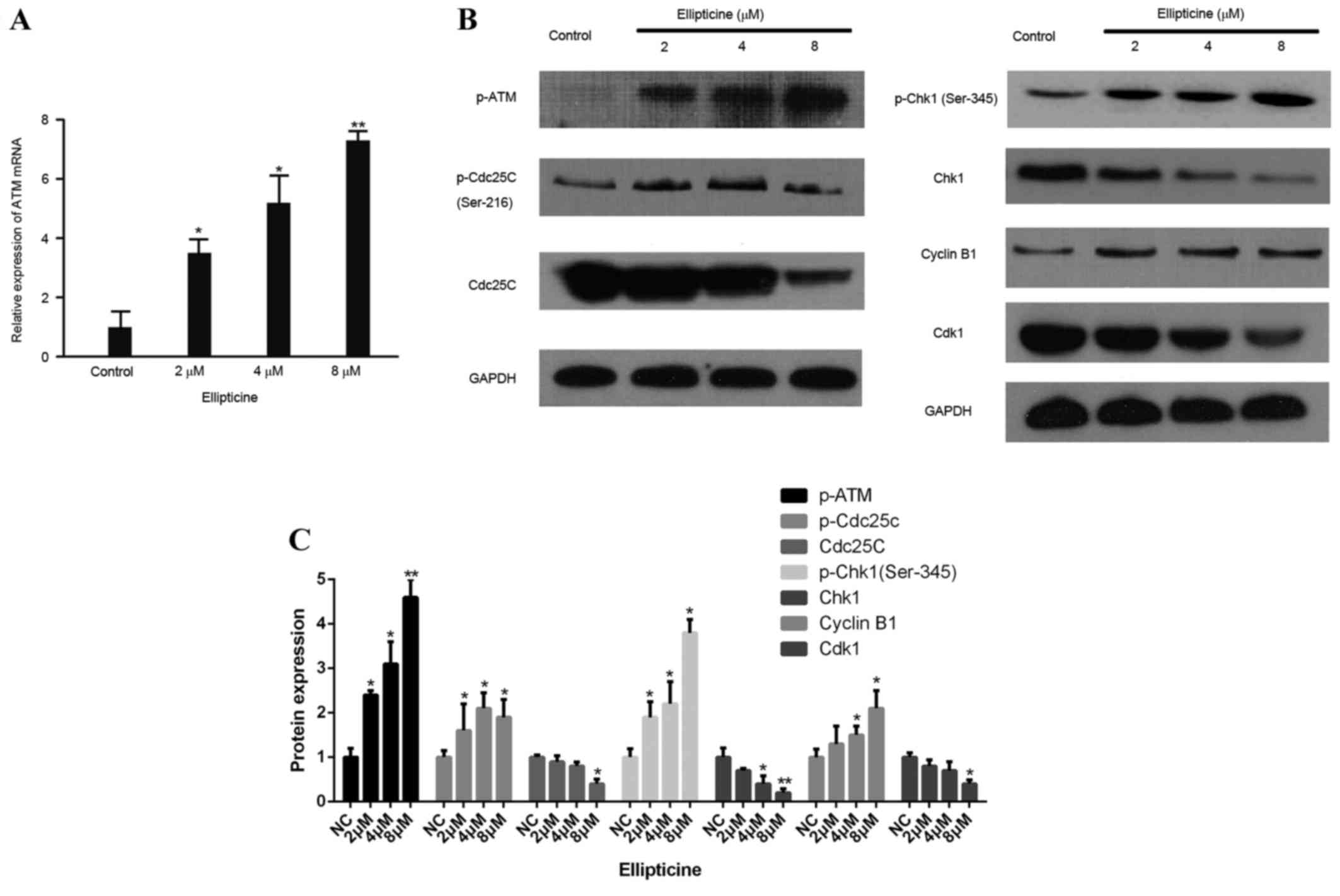
Ellipticine modulates
ATM-Chk1-Cdc25C-Cdk1 pathway
To explore the molecular events leading to
ellipticine mediated G2/M phase cell cycle arrest, the signaling
cascade responsible for G2/M checkpoint control was examined
further. As cellular responses to DNA damage during cell cycle are
coordinated primarily by activated ATM kinase and its following
signaling cascades, mRNA expression levels of ATM in T-24 cells
treated with different concentrations of ellipticine were tested.
As demonstrated in Fig. 4A, the
expression of ATM mRNA was significantly upregulated following
treatment with 2, 4 and 8 µM ellipticine compared with control
(P<0.05, P<0.05 and P<0.01, respectively). Next, the
effect of ellipticine on protein expression of p-ATM, Chk1 and
p-Chk1 (Ser345) were examined. Compared with control, p-ATM and
p-Chk1 protein levels were significantly increased, while Chk1
total protein was reduced, following 24 h treatment with 2, 4, and
8 µM ellipticine (Fig. 4B). Chk1
activation has been demonstrated to phosphorylate Cdc25C on Ser-216
in vitro (13). The effect
of ellipticine on total Cdc25C and p-Cdc25C (Ser-216) was,
therefore, further examined. Following ellipticine treatment, total
Cdc25C protein significantly decreased while p-Cdc25C was
upregulated (Fig. 4B), which
suggests that activation of Chk1 by ellipticine may be due to the
inactivation of Cdc25C. Downstream components of Cdc25C, Cdk1 and
Cyclin B1, were also down- and upregulated, respectively (Fig. 4B). These results suggest that the
ATM-Chk1-Cdc25C-Cdk1 cascade is involved in the G2/M arrest of
bladder cancer cells via checkpoint activation following
ellipticine treatment.
Discussion
The present study revealed that ellipticine, a
naturally occurring alkaloid, exhibits an inhibitory function on
T-24 bladder cancer cell proliferation and migration. The
chemopreventive/therapeutic potential of ellipticine against
bladder cancer was demonstrated to be mediated by induction of cell
cycle arrest and inhibition of migration. The antiproliferative
effect of ellipticine on cancer cells has been previously reported
(3). Based on its action on other
reported cancer types, the present study hypothesized that
ellipticine could significantly inhibit T-24 cell proliferation in
a dose- and time- dependent manner. Previous studies suggested that
the mechanism underlying the cytotoxicity of ellipticine is related
to covalent binding of ellipticine to DNA in neuroblastoma cell
lines (14) and the interaction of
ellipticine with a human DNA sequence derived from the telomeric
DNA region (15). Low
concentrations (1–4 µM) of ellipticine have been demonstrated to
exhibit considerable cytotoxicity, which, combined with its limited
intrinsic toxicity (4), suggests
that ellipticine could be a promising therapeutic molecule against
bladder cancer.
Loss of key cell cycle checkpoints is a hallmark of
cancer, inducing abnormal proliferation and subsequent oncogenic
transformation (16). Among
phosphoinositide kinase (PIK)-related proteins family members, ATM
is one of the key kinases responding to DNA damage during cell
cycle checkpoints. A series of downstream substrates including
Chk1, p53, nijmegen breakage syndrome 1 and p38 mitogen-activated
protein kinase are targets of ATM, participating in modulating the
cell cycle. Among these substrates, Chk1 is the most conserved
during evolution (17). p-ATM
leads to self-activation through disjoining ATM dimer into monomer,
which further activates Chk1 by phosphorylation of Ser-345 sites,
thereby causing deactivation of p-Cdc25C (18). Cdk1 is a cyclin-dependent kinase,
which bonds to cyclin B1, and the complex facilitates dividing
cells to enter mitosis from G2 phase (13). By means of deactivated Cdc25C, Cdk1
cannot be dephosphorylated by Cdc25C, which leads to a deactivated
status of Cdk1-cyclinB1 complex (19). In conclusion, activated Chk1 leads
to Cdk1 inhibition by deactivation of Cdc25 through phosphorylation
which results in G2-arrest (20).
Previous studies have suggested that ellipticine is a potent
inhibitor of cell-cycle progression in several different cell lines
(6,21). The present study has focused on the
effect of ellipticine on the T-24 cell cycle and demonstrated that
ellipticine causes a G2/M phase arrest. Similar results were
observed in human endometrial and breast carcinomas (21,22).
The present study demonstrated that ellipticine treatment increased
the percentage of cells in G2/M phase from 13.75 to 23.75%, in a
dose-dependent manner, which suggests that the ellipticine-induced
cell growth inhibition is related to cell cycle arrest. The
ATM-Chk1-Cdc25C-Cdk1 pathway was examined, which revealed that
treatment with ellipticine induces G2/M arrest via activation of
the ATM-Chk1-Cdc25C-Cdk1 pathway. In addition, as demonstrated in
Fig. 3, the percentage of cells in
S-phase was 33.86, 32.45, 37.56, 40.17, 49.63 and 46.96% with
ellipticine concentrations of 0, 1, 2, 4, 8 and 16 µM,
respectively. Unlike the G2/M phase, the S-phase revealed a
non-dose dependent reduction, therefore suggesting that this was
not a specific change caused by ellipticine. Fang et al
(8) demonstrated that Akt is also
the target of ellipticine in lung cancer. In the present study, ATM
was confirmed to be a target of ellipticine in bladder cancer, but
further research is needed to test for other targets. In addition,
the mechanisms by which the ATM-Chk1-Cdc25C-Cdk1 pathway was
activated by ellipticine treatment are not clear and need to be
explored further.
To the best of our knowledge, the present study is
the first to evaluate the motility of bladder cancer cells
following ellipticine treatment in vitro. Using the T-24
bladder cancer cell line, a significant and dose-dependent decrease
in motility was demonstrated following ellipticine treatment.
Although a single cell line was used to substantiate these
findings, T-24 is a typical bladder epithelial cell line which is
hypothesized to accurately represent the features of bladder
cancer. In preliminary experiments, the proliferation of T-24,
5637, UM-UC-3 and J82 cell lines treated with ellipticine was
assessed by CCK-8 assay, which revealed that the inhibitory effect
on T-24 cells was the most significant (data not shown). Finally,
although an effect of ellipticine in the motility of bladder cancer
cells was demonstrated in the present study, the molecular
mechanisms underlying this reduction remain unknown and future
studies will be required to address this.
In conclusion, the present study demonstrated that
ellipticine induces cell death and G2/M cell cycle arrest and
diminishes the migration ability of T-24 bladder cancer cells in a
dose- and time-dependent manner. As demonstrated by western blot
analysis of the ATM-Chk1-Cdc25C-Cdk1 pathway, ellipticine may
inhibit cellular proliferation by generating a cell cycle arrest at
the G2/M phase in T-24 bladder cancer cells via the ATM pathway.
Further investigation of the molecular events underlying
ellipticine-triggered cancer cell inhibition is required to fully
assess the potential of ellipticine as a therapeutic in bladder
cancer.
References
|
1
|
Goodwin S, Smith AF and Horning EC:
Alkaloids of ochrosia elliptica labill. J Am Chem Soc.
81:1903–1908. 1959. View Article : Google Scholar
|
|
2
|
Rouësse JG, Le Chevalier T, Caille P,
Mondesir JM, Sancho-Garnier H, May-Levin F, Spielmann M, De Jager R
and Amiel JL: Phase II study of elliptinium in advanced breast
cancer. Cancer Treat Rep. 69:707–708. 1985.PubMed/NCBI
|
|
3
|
Stiborová M, Bieler CA, Wiessler M and
Frei E: The anticancer agent ellipticine on activation by
cytochrome P450 forms covalent DNA adducts. Biochem Pharmacol.
62:1675–1684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Auclair C: Multimodal action of antitumor
agents on DNA: The ellipticine series. Arch Biochem Biophys.
259:1–14. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martinkova E, Maglott A, Leger DY, Bonnet
D, Stiborova M, Takeda K, Martin S and Dontenwill M: alpha5beta1
integrin antagonists reduce chemotherapy-induced premature
senescence and facilitate apoptosis in human glioblastoma cells.
Int J Cancer. 127:1240–1248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuo PL, Hsu YL, Kuo YC, Chang CH and Lin
CC: The anti-proliferative inhibition of ellipticine in human
breast mda-mb-231 cancer cells is through cell cycle arrest and
apoptosis induction. Anticancer Drugs. 16:789–795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo YC, Kuo PL, Hsu YL, Cho CY and Lin CC:
Ellipticine induces apoptosis through p53-dependent pathway in
human hepatocellular carcinoma HepG2 cells. Life Sci. 78:2550–2557.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang K, Chen SP, Lin CW, Cheng WC and
Huang HT: Ellipticine-induced apoptosis depends on Akt
translocation and signaling in lung epithelial cancer cells. Lung
Cancer. 63:227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chavan S, Bray F, Lortet-Tieulent J,
Goodman M and Jemal A: International variations in bladder cancer
incidence and mortality. Eur Urol. 66:59–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amsellem-Ouazana D, Bièche I, Tozlu S,
Botto H, Debre B and Lidereau R: Gene expression profiling of ERBB
receptors and ligands in human transitional cell carcinoma of the
bladder. J Urol. 175:1127–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pliarchopoulou K, Laschos K and Pectasides
D: Current chemotherapeutic options for the treatment of advanced
bladder cancer: A review. Urol Oncol. 31:294–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw
AS and Piwnica-Worms H: Mitotic and G2 checkpoint control:
Regulation of 14–3-3 protein binding by phosphorylation of Cdc25C
on serine-216. Science. 277:1501–1505. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stiborova M, Poljakova J, Eckschlager T,
Kizek R and Frei E: Analysis of covalent ellipticine- and
doxorubicin-derived adducts in DNA of neuroblastoma cells by the
32P-postlabeling technique. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 156:115–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh S, Kar A, Chowdhury S and Dasgupta
D: Ellipticine binds to a human telomere sequence: An additional
mode of action as a putative anticancer agent? Biochemistry.
52:4127–4137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Helt CE, Cliby WA, Keng PC, Bambara RA and
O'Reilly MA: Ataxia telangiectasia mutated (ATM) and ATM and
Rad3-related protein exhibit selective target specificities in
response to different forms of DNA damage. J Biol Chem.
280:1186–1192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thanasoula M, Escandell JM, Suwaki N and
Tarsounas M: ATM/ATR checkpoint activation downregulates CDC25C to
prevent mitotic entry with uncapped telomeres. EMBO J.
31:3398–3410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Graves PR, Lovly CM, Uy GL and
Piwnica-Worms H: Localization of human Cdc25C is regulated both by
nuclear export and 14-3-3 protein binding. Oncogene. 20:1839–1851.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JY, Lee SG, Chung JY, Kim YJ, Park JE,
Koh H, Han MS, Park YC, Yoo YH and Kim JM: Ellipticine induces
apoptosis in human endometrial cancer cells: The potential
involvement of reactive oxygen species and mitogen-activated
protein kinases. Toxicology. 289:91–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuo PL, Hsu YL, Chang CH and Lin CC: The
mechanism of ellipticine-induced apoptosis and cell cycle arrest in
human breast MCF-7 cancer cells. Cancer Lett. 223:293–301. 2005.
View Article : Google Scholar : PubMed/NCBI
|