Introduction
Chronic obstructive pulmonary disease (COPD) and
obstructive sleep apnea syndrome (OSAS) represent two of the most
prevalent chronic respiratory disorders in clinical practice.
Respiratory overlap syndrome (OS), defined as the coexistence of
these two diseases, occurs in ~1% of adults and leads to the
development of increased nocturnal oxygen desaturation compared
with mono-COPD or OSAS (1–3). The possible coexistence of COPD with
OSAS is notable, as systemic inflammation develops in each disorder
(3,4) and may contribute to the pathogenesis
of associated comorbidities. Hypoxia induces systemic inflammatory
reactions and acts as an aggravating factor of liver injury
(5,6). It has been hypothesized that, in
patients with mono-COPD or OSAS, inflammatory mediators generated
in the lung may ‘spill over’ into the bloodstream and promote the
release of inflammatory proteins from the liver, which may cause
damage to target organs and act as a source of systemic
inflammation (7,8).
Inflammation modulates the expression of hepatic
metabolizing enzymes. Cytochrome p450 enzymes (CYPs) catalyze the
oxidative metabolism of numerous drugs, and the level of CYP
expression may affect drug efficacy, toxicity and consequently,
therapeutic outcome (9). Previous
studies demonstrated that the blood concentration of theophylline
was above the normal range when the expression and activity of
CYP1A2 was inhibited (10,11). A further previous study
demonstrated that sleep hypoxia combined with emphysema
synergistically enhanced hepatic inflammation and produced a more
apparent liver-derived inflammatory state (12). It has been reported that
inflammatory challenges may suppress the expression of major CYPs
(13). Underexpression of the
nuclear receptors pregnane X receptor (PXR) and constitutive
androstane receptor (CAR), as upstream regulatory molecules, may
inhibit the transcription of CYPs (11–13)
by attenuating nuclear translocation (14,15).
It is hypothesized that severe inflammation in the liver tissues of
patients with OS alters hepatic metabolism via nuclear
receptors.
In the present study, a previously-published rat
model of OS was developed by exposing rats to intermittent hypoxia
(IH) and cigarette smoke (CS). Gene expression analysis of liver
samples with reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was used to evaluate the effect of IH with CS on
inflammatory cytokines and CYPs. The present study may provide a
molecular mechanism to explain adverse drug reactions in patients
with OS.
Materials and methods
Ethics statement
Rats were used in accordance with the protocol
approved by the Animal Care Committee of Tianjin Medical University
(permit no. 2010-0002).
Animals and treatments
Rats were provided by the Model Animal Center of the
Radiological Medicine Research Institute, Chinese Academy of
Medical Science (Beijing, China), and housed in standard laboratory
cages (5 rats/cage) with food and water available ad libitum. As
described previously (12), a
total of 30 male Wistar rats weighing 180±20 g at age 6 weeks were
divided into two groups of 15 according to exposure condition, as
follows: i) Control group; ii) IH with CS experimental group. For
IH, the rats were treated in a 120 sec cycle, comprising 30 sec
nitrogen followed by 90 sec air, between 9:00 a.m. and 5:00 p.m.
daily. For CS exposure, the rats underwent whole-body exposure to
the smoke of five unfiltered cigarettes (Daqianmen, Yunnan, China;
≤15 mg tar, ≤1.1 mg nicotine and ≤13 mg CO) for 30 min twice-daily
(before 9:00 a.m. and after 5:00 p.m.), 7 days/week for 14 weeks,
inside a 0.6 m3 custom-made plexiglas chamber (16,17).
Measurement of serum liver
enzymes
Blood samples from control and experimental rats
were centrifuged at 600 g for 15 min at 4°C, and the serum was
stored at −80°C prior to being assayed. The serum alanine
aminotransferase (ALT) and aspartate aminotransferase (AST)
concentrations were quantified using a transaminase CII test,
according to the manufacturer's protocol (Wako Pure Chemical
Industries, Ltd., Osaka, Japan).
Liver tissue sampling
Following treatment, 10 rats from the control and
experimental groups were anesthetized with 10% chloral hydrate (0.3
ml/100 g body weight) and sacrificed. For gene expression analysis
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), liver tissues were excised, rinsed in ice-cold PBS,
frozen in liquid nitrogen and stored at −80°C prior to analysis.
For hematoxylin and eosin staining, liver tissues were fixed in 10%
formalin at 4°C overnight, embedded in paraffin, and sliced into
5-µm-thick sections. Sections were then stained with 1% hematoxylin
and eosin solution for 5 min at room temperature, according to the
manufacturer's protocol.
Preparation of RNA from tissue
samples
RNA was extracted from liver tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The extract yield and quality were
determined by measuring the absorbance at 260 and 280 nm using the
Maestro Nano Micro-Volume Spectrophotometer (Maestrogen, Inc.,
Hsinchu, Taiwan). The absorbance ratio at 260:280 nm was between
1.8 and 2.0. The RNA was subsequently reverse-transcribed into
cDNA.
RT-qPCR
mRNA (3 µg) was reverse-transcribed into cDNA using
oligo(dT) primers for 1 h at 50°C, with the TIAN Script RT kit
(Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocol. The cDNA served as a template for qPCR,
which was performed using SYBR Green PCR core reagents (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Specific gene primers were
designed using the PrimerQuest SM software (http://sg.idtdna.com/Primerquest/Home/Index;
Integrated DNA Technologies, Inc., Coralville, IA, USA) and
commercially produced (BGI Tech, Shenzen, China) (Table I). DNA amplification was performed
using a CFX96 Real-Time System (Bio-Rad Laboratories, Inc.) with
the following reaction conditions: An initial heating cycle of 95°C
for 2 min; 40 cycles, alternating between denaturation at 95°C for
25 sec and primer annealing at 60°C for 25 sec; and final extension
at 72°C for 20 sec. Melt curves were used to clarify the identity
of the amplicons and the housekeeping gene GAPDH served as an
internal control. The relative mRNA expression of targeted genes
was calculated using the comparative Cq (threshold cycle) method
and normalized to GAPDH mRNA in the same sample (18). The specific ΔCq was calculated as
follows: [ΔCq=(CqGAPDH)-(Cqtarget)]; relative
expression was defined as 2−ΔΔCq.
 | Table I.Primer sequences for the present
study. |
Table I.
Primer sequences for the present
study.
| Genes | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| IL-1β |
TCCCTGAACTCAACTGTGAAATA |
GGCTTGGAAGCAATCCTTAATC |
| IL-6 |
GAAGTTAGAGTCACAGAAGGAGTG |
GTTTGCCGAGTAGACCTCATAG |
| TNF-α |
ACCTTATCTACTCCCAGGTTCT |
GGCTGACTTTCTCCTGGTATG |
| NF-κB |
AGACATCCTTCCGCAAACTC |
TAGGTCCATCCTGCCCATAA |
| PXR |
GAAGATCATGGCTGTCCTCAC |
CGTCCGTGCTGCTGAATAA |
| CAR |
GAGACCATGACCAGTGAAGAAG |
AGTCAGGGCATGGAAATGATAG |
| GR |
CAGCAGTGAAATGGGCAAAG |
GGGCAAATGCCATGAGAAAC |
| CYP1A2 |
GACAAGACCCTGAGTGAGAAG |
GAGGATGGCTAAGAAGAGGAAG |
| CYP2C9 |
CCCAAGGGCACAACCATATTA |
CTTTCTGGATGAAGGTGGCA |
| CYP2C19 |
CCCAAGGGCACAACCATATTA |
TTTGACCCTCGTCACTTTCTG |
| CYP2D4 |
CCTTTCAGCCCTAACACTCTAC |
ATGAAGCGTGGGTCATTGT |
| CYP3A2 |
GGAAACCCGTCTGGATTCTAAG |
GAAGTGTCTCATAAAGCCCTGT |
| GAPDH |
ACTCCCATTCTTCCACCTTTG |
AATATGGCTACAGCAACAGGG |
Statistical analysis
The numerical data are presented as the mean ±
standard error of the mean. The statistical significance of the
differences between the two groups was assessed using Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
Microsoft Excel software version 2007 (Microsoft Corporation,
Redmond, WA, USA).
Results
IH with CS exposure causes elevated
expression of liver enzymes, upregulated mRNA expression of
inflammatory cytokines and hepatocyte damage
Consistent with a previous study (12), IH with CS exposure resulted in
apparent emphysemic alterations in rat lungs and decreased blood
gas concentration, indicating that the rat model of IH with
emphysema was successfully established (data not presented).
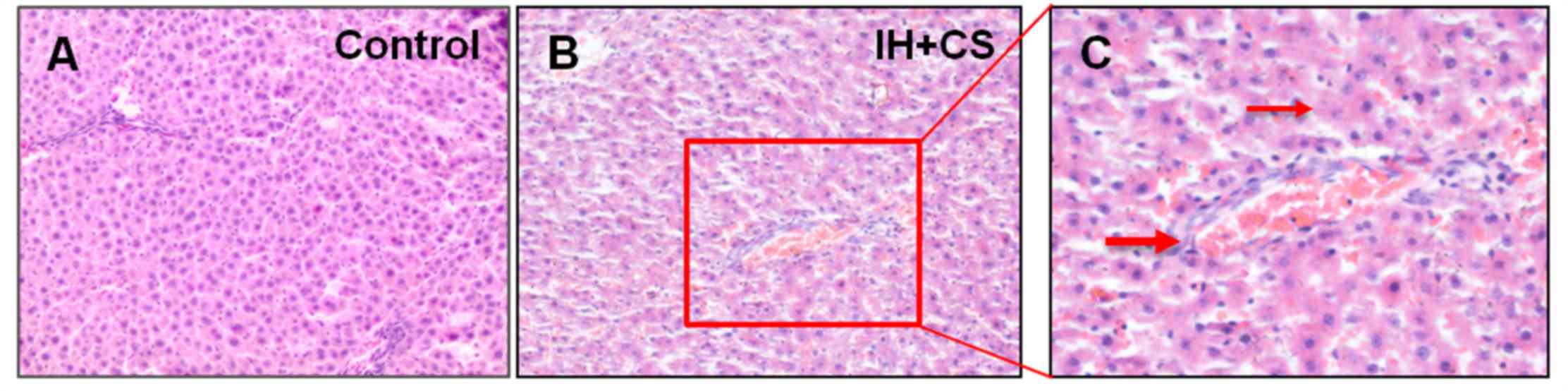
Hematoxylin and eosin staining of the liver demonstrated that
various hepatic lesions were observed in the IH with CS group, and
not in the control group (Fig. 1A and
B). Compared with the control group, hepatic lobules in the IH
with CS group exhibited partial clarity and integrity, sinusoids
were broadened, inflammatory cell infiltration was observed in the
periportal space and various foci of lobular inflammatory cell
accumulation were noted. Inflammatory cell infiltrates were
observed in the portal area and light staining of the cytoplasm of
liver cells suggested cytoplasmic loss (Fig. 1C).
The concentration of serum ALT and AST in the
experimental group was increased compared with the control group
(P<0.05; Table II), which
suggested a more severe impairment of hepatocyte function.
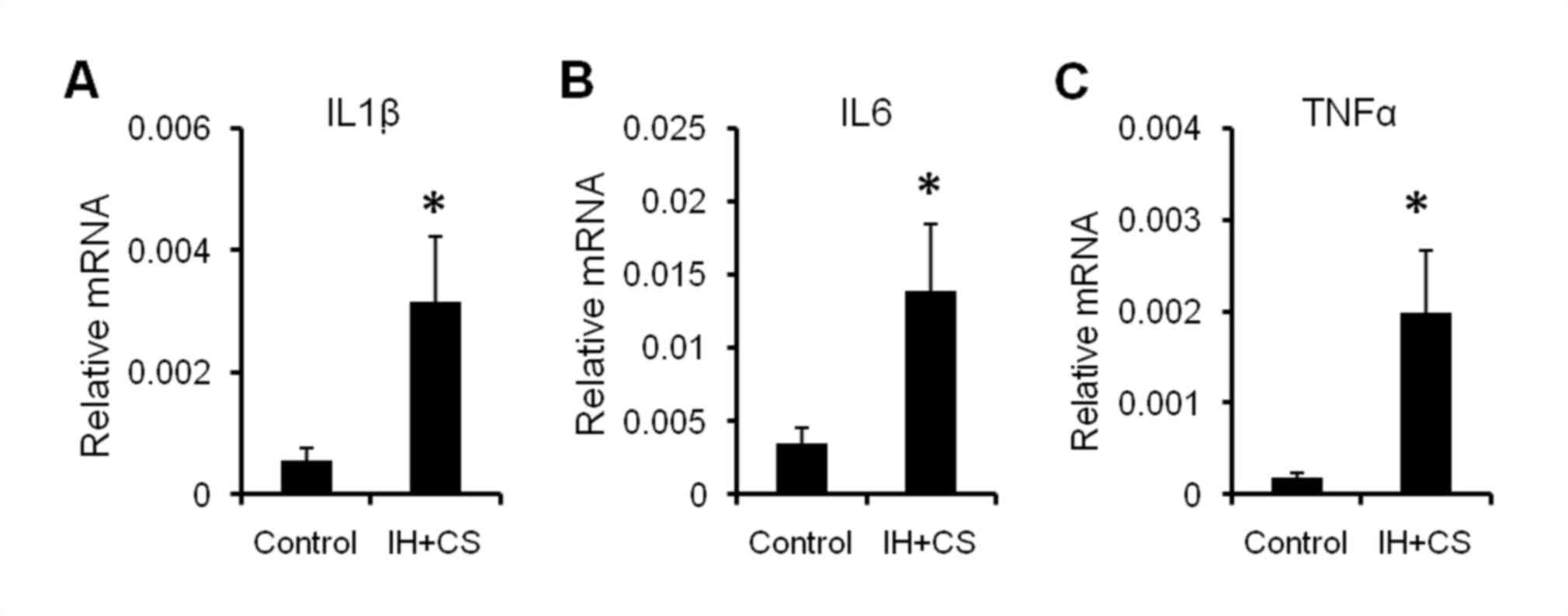
Additionally, the mRNA expression levels of interleukin (IL)-1β,
IL-6, and tumor necrosis factor (TNF)-α in the livers of the
experimental rats were significantly increased compared with the
control group (P<0.05; Fig. 2).
The results of the present study suggested that early-phase
inflammation and mild hepatocyte damage had occurred.
 | Table II.Serum ALT and AST levels in the
liver. |
Table II.
Serum ALT and AST levels in the
liver.
| Group | n | ALT, U/l | AST, U/l |
|---|
| Contro | 15 | 21.0±5.3 l | 17.8±3.0 |
| IH+CS | 15 |
50.5±2.1a |
26.0±3.2a |
Underexpressed mRNA levels of CYPs in
the injured liver
Expression of each CYP is influenced by a unique
combination of mechanisms and factors, including genetic
polymorphisms, xenobiotic induction, regulation by cytokines,
hormones and disease states, in addition to sex, age, and others
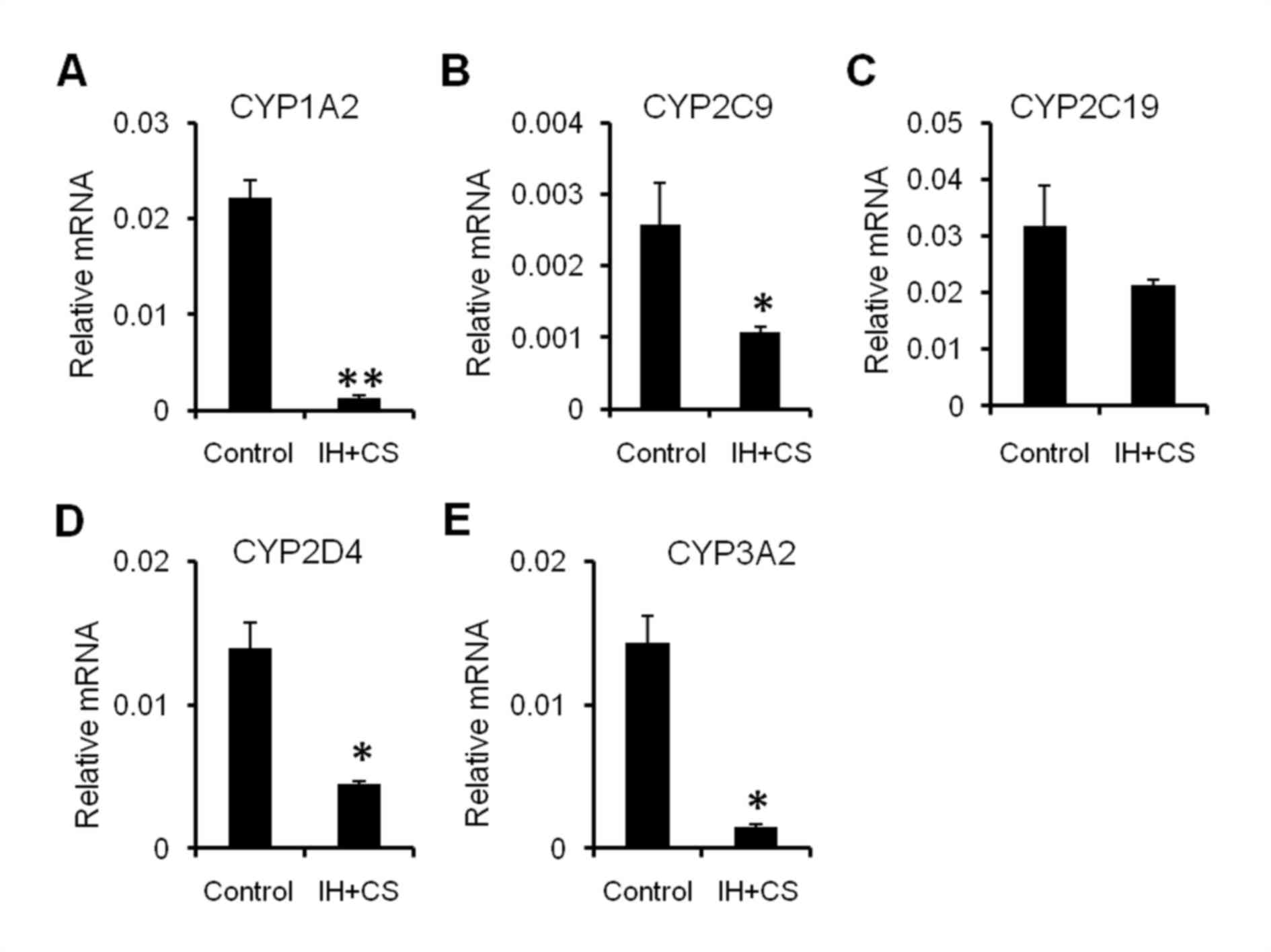
(13). Compared with the control
group, the IH with CS group exhibited markedly increased mRNA
expression of CYP1A2, CYP2C9, CYP2C19, CYP2D4, and CYP3A2 (Fig. 3). The results of the present study
demonstrated that liver inflammation and hepatocyte damage affect
the transcription of major CYPs in a rat model of IH with
emphysema, which was consistent with the previous literature
suggesting inflammation may induce downregulation of CYP expression
(19,20).
Upregulation of nuclear factor (NF)-κB and
subsequent downregulation of nuclear receptors in the liver.
Transcription factor NF-κB serves a role in inflammatory reactions
and oxidative stress (21,22). CYP3A expression is modulated by
nuclear receptors, including PXR and CAR (23–25).
If the nuclear translocation of these receptors is decreased, CYP3A
expression will consequently decrease. In addition, the synthesis
and nuclear translocation of these receptors is
negatively-associated with NF-κB nuclear translocation (14,26).
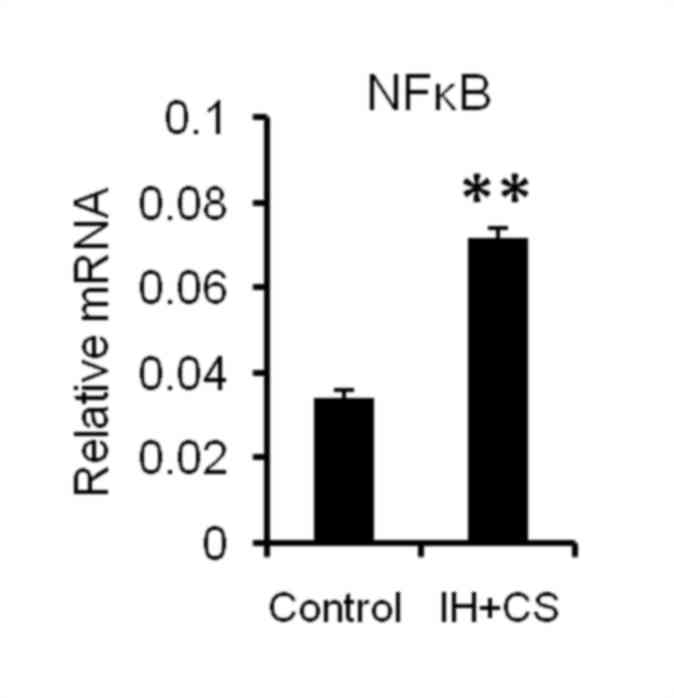
Consequently, NF-κB expression was significantly increased in the
IH with CS rats, compared with the control group (P<0.05;
Fig. 4). Additionally, in the IH
with CS group, the hepatic mRNA expression of PXR, CAR and the
glucocorticoid receptor (GR) was significantly decreased compared
with that in the control group (P<0.05; Fig. 5). The results of the present study
demonstrated that upregulated NF-κB may be involved in reduced
hepatic CYP expression, by negatively impacting the synthesis and
nuclear translocation of PXR, CAR and GR.
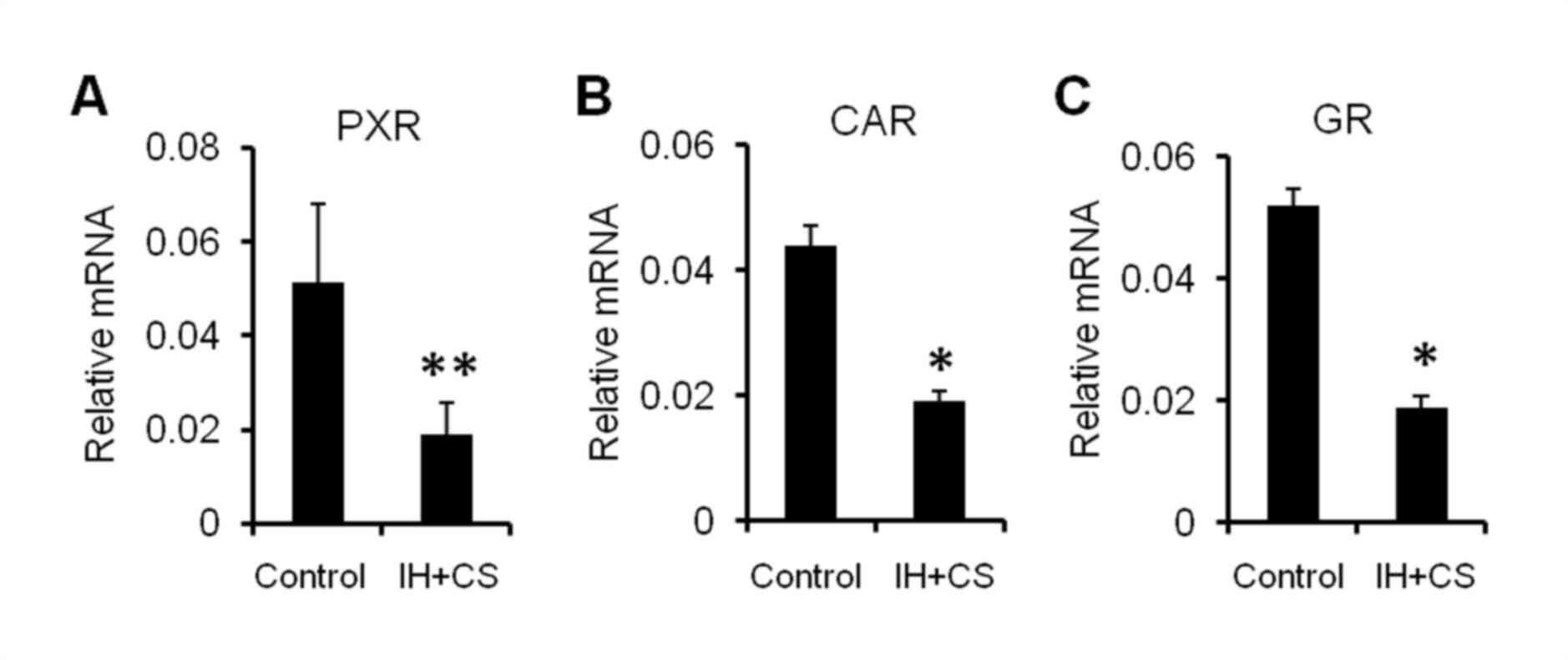 | Figure 5.mRNA expression levels of (A) PXR,
(B) CAR, and (C) GR in the liver. After established successfully
model, the liver was removed, and the mRNA expression levels of
PXR, CAR, and GR were measured using the reverse
transcription-quantitative polymerase chain reaction. GAPDH was
used as an internal reference. The data are presented as the mean ±
standard error of the mean, and were obtained from ten rats per
group. Student's t-test; *P<0.05, **P<0.01 vs. the control.
PXR, pregnane X receptor; CAR, constitutive androstane receptor;
GR, glucocorticoid receptor; IH, intermittent hypoxia. |
Discussion
The emerging pathophysiology of OS encompasses
intermittent hypoxia and emphysema (27). In the present study, a rat model of
intermittent hypoxia with emphysema was developed. The results of
the present study suggested that an early phase of inflammation and
mild hepatocyte damage had occurred in the liver. The results of
the present study were consistent with previous studies, which
stated that hypoxia contributes to liver injury (28,29).
Alterations in hepatic CYP expression, and the mechanisms
underlying these alterations, were analyzed in the present study
using the IH with CS rat model. In the IH with CS group, the
hepatic mRNA expression levels of a number of CYP molecular
species, specifically CYP1A2, CYP2C9, CYP2C19, CYP2D4 and CYP3A2,
were markedly decreased compared with the control group.
The present study sought to analyze why the
expression of hepatic CYPs decreased in the rat model of
intermittent hypoxia with emphysema. It is known that CYPs are
generated in damaged hepatocytes, where the degree of damage may
affect the quantity and quality of CYPs. Previous studies have
demonstrated that the severity of liver injury is
positively-associated with levels of hypoxia and inflammation
(30,31). Compared with patients with only
COPD or OSAS, nocturnal hypoxemia, hypoxia and hypercapnia are more
severe in patients with OS (32),
and the present study demonstrated that intermittent hypoxia and
emphysema interact synergistically. The mechanism of the decrease
in CYP expression was analyzed by focusing on hepatic inflammation,
as the pathological conditions of early-phase hepatic inflammation
and mild hepatocyte damage were detected in the present
experimental model. Hepatic inflammation is promoted by the binding
of cyclic pro-inflammatory factors to Toll-like receptor 4 in
hepatic Kupffer cells. The activated Kupffer cells subsequently
initiate the secretion of inflammatory cytokines, which promote the
activity of NF-κB (21). Activated
NF-κB dissociates from the inhibitor of NF-κB and translocates to
the nucleus (33). Nuclear NF-κB
forms a complex with the transcription factor GR and prevents the
binding of GR to GR-responsive elements, thereby inhibiting the
transcription of PXR and CAR (14,15).
The decrease in PXR and CAR expression causes a decrease in nuclear
translocations (26,34). Additionally, the decrease in the
expression of PXR and CAR, which bind to the responsive elements of
DNA, results in the inhibition of CYP3A transcription and a
subsequent decrease in its expression (35–37).
Hepatic NF-κB nuclear translocation was examined in the present
study, and was observed to be increased in the experimental group
compared with the control group. By contrast, the hepatic mRNA
expression of PXR, CAR and GR was decreased in the experimental
group. The results of the present study demonstrated that, in a rat
model of intermittent hypoxia with emphysema, hepatic inflammatory
cytokines activate NF-κB, which inhibits the transcription of PXR
and CAR. This inhibited transcription subsequently leads to a
decrease in nuclear translocation, which inhibits the transcription
of CYP3A.
The transcription of CYP2C is modified by CAR
(38,39). Therefore, it is hypothesized that
the mechanism underlying the decrease in the expression level of
CYP2C is identical to that underlying the decrease in the CYP3A
expression level. However, the transcription of CYP1A and CYP2D is
not primarily regulated by PXR and CAR (40,41).
Therefore, the decrease in the nuclear translocation of these CYP
molecular species cannot be explained by the decrease in the
nuclear translocation of PXR and CAR. However, it has been
previously demonstrated that the expression levels of these CYP
molecular species are downregulated by inflammatory cytokines
(42). It is therefore
hypothesized that the decrease in the hepatic expression levels of
CYP1A and CYP2D in the IH with CS group, in the present study, may
be triggered by inflammatory cytokines, as is the case with other
CYP molecular species.
In conclusion, early-phase hepatic inflammation and
mild hepatocyte damage was detected in the rat model of
intermittent hypoxia with emphysema generated in the present study;
additionally, hepatic CYP expression was markedly decreased. Based
on the results of the present study, it is hypothesized that,
during the development of OS, systematic inflammatory reactions may
downregulate hepatic CYP gene expression via the NF-κB signaling
pathway. The results of the present study may provide an important
molecular mechanism to explain adverse drug reactions in patients
with OS.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Tianjin City (grant nos. 13JCYBJC22400,
14JCYBJC25700 and 13JCYBJC40000) and the National Natural Science
Foundation of China (grant nos. 31471121 and 81270144).
References
|
1
|
McNicholas WT: Chronic obstructive
pulmonary disease and obstructive sleep apnea: Overlaps in
pathophysiology, systemic inflammation and cardiovascular disease.
Am J Respir Crit Care Med. 180:692–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ioachimescu OC and Teodorescu M:
Integrating the overlap of obstructive lung disease and obstructive
sleep apnoea: OLDOSA syndrome. Respirology. 18:421–431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wouters EF: Local and systemic
inflammation in chronic obstructive pulmonary disease. Proc Am
Thorac Soc. 2:26–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thunström E, Glantz H, Fu M,
Yucel-Lindberg T, Petzold M, Lindberg K and Peker Y: Increased
inflammatory activity in nonobese patients with coronary artery
disease and obstructive sleep apnea. Sleep. 38:463–471. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez NC and Wood JG: Alveolar
hypoxia-induced systemic inflammation: What low PO (2) does and
does not do. Adv Exp Med Biol. 662:27–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuhrmann V, Kneidinger N, Herkner H, Heinz
G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Kitzberger
R, Warszawska J, et al: Hypoxic hepatitis: Underlying conditions
and risk factors for mortality in critically ill patients.
Intensive Care Med. 35:1397–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Eeden SF and Sin DD: Chronic
obstructive pulmonary disease: A chronic systemic inflammatory
disease. Respiration. 75:224–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dopp JM, Moran JJ, Abel NJ, Wiegert NA,
Cowgill JB, Olson EB and Sims JJ: Influence of intermittent hypoxia
on myocardial and hepatic P-glycoprotein expression in a rodent
model. Pharmacotherapy. 29:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wrighton SA, Schuetz EG, Thummel KE, Shen
DD, Korzekwa KR and Watkins PB: The human CYP3A subfamily:
Practical considerations. Drug Metab Rev. 32:339–361. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan X, Wang P, Hu N, Liu L, Liu X, Xie L
and Wang G: A physiologically based pharmacokinetic model
characterizing mechanism-based inhibition of CYP1A2 for predicting
theophylline/antofloxacin interaction in both rats and humans. Drug
Metab Pharmacokinet. 26:387–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wang Y, Yang Y, Luo Y and Chen P:
Effect of chronic intermittent hypoxia on the expression of
fractalkine in rat liver. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
38:984–990. 2013.(In Chinese). PubMed/NCBI
|
|
12
|
Feng J, Wang QS, Chiang A and Chen BY: The
effects of sleep hypoxia on coagulant factors and hepatic
inflammation in emphysematous rats. PLoS One. 5:e132012010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zanger UM and Schwab M: Cytochrome P450
enzymes in drug metabolism: Regulation of gene expression, enzyme
activities, and impact of genetic variation. Pharmacol Ther.
138:103–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Assenat E, Gerbal-Chaloin S, Larrey D,
Saric J, Fabre JM, Maurel P, Vilarem MJ and Pascussi JM:
Interleukin 1beta inhibits CAR-induced expression of hepatic genes
involved in drug and bilirubin clearance. Hepatology. 40:951–960.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Widén C, Gustafsson JA and Wikström AC:
Cytosolic glucocorticoid receptor interaction with nuclear
factor-kappa B proteins in rat liver cells. Biochem J. 373:211–220.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magalang UJ, Cruff JP, Rajappan R, Hunter
MG, Patel T, Marsh CB, Raman SV and Parinandi NL: Intermittent
hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin
Endocrinol Diabetes. 117:129–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng J, Chiang AA, Wu Q, Chen BY, Cui LY,
Liang DC, Zhang ZL and Yao W: Sleep-related hypoxemia aggravates
systematic inflammation in emphysematous rats. Chin Med J (Engl).
123:2392–2399. 2010.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slaviero KA, Clarke SJ and Rivory LP:
Inflammatory response: An unrecognised source of variability in the
pharmacokinetics and pharmacodynamics of cancer chemotherapy.
Lancet Oncol. 4:224–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aitken AE, Richardson TA and Morgan ET:
Regulation of drug-metabolizing enzymes and transporters in
inflammation. Annu Rev Pharmacol Toxicol. 46:123–149. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ivanenkov YA, Balakin KV and Lavrovsky Y:
Small molecule inhibitors of NF-kB and JAK/STAT signal transduction
pathways as promising anti-inflammatory therapeutics. Mini Rev Med
Chem. 11:55–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandireddy R, Yerra VG, Areti A,
Komirishetty P and Kumar A: Neuroinflammation and oxidative stress
in diabetic neuropathy: Futuristic strategies based on these
targets. Int J Endocrinol. 2014:6749872014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pondugula SR, Dong H and Chen T:
Phosphorylation and protein-protein interactions in PXR-mediated
CYP3A repression. Expert Opin Drug Metab Toxicol. 5:861–873. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chai X, Zeng S and Xie W: Nuclear
receptors PXR and CAR: Implications for drug metabolism regulation,
pharmacogenomics and beyond. Expert Opin Drug Metab Toxicol.
9:253–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah P, Guo T, Moore DD and Ghose R: Role
of constitutive androstane receptor in Toll-like receptor-mediated
regulation of gene expression of hepatic drug-metabolizing enzymes
and transporters. Drug Metab Dispos. 42:172–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghose R, Omoluabi O, Gandhi A, Shah P,
Strohacker K, Carpenter KC, McFarlin B and Guo T: Role of high-fat
diet in regulation of gene expression of drug metabolizing enzymes
and transporters. Life Sci. 89:57–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaouat A, Weitzenblum E, Krieger J,
Ifoundza T, Oswald M and Kessler R: Association of chronic
obstructive pulmonary disease and sleep apnea syndrome. Am J Respir
Crit Care Med. 151:82–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanné F, Gagnadoux F, Chazouillères O,
Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R and Serfaty L:
Chronic liver injury during obstructive sleep apnea. Hepatology.
41:1290–1296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Savransky V, Nanayakkara A, Vivero A, Li
J, Bevans S, Smith PL, Torbenson MS and Polotsky VY: Chronic
intermittent hypoxia predisposes to liver injury. Hepatology.
45:1007–1013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebert EC: Hypoxic liver injury. Mayo Clin
Proc. 81:1232–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian JL, Zhang Y and Chen BY: Sleep apnea
hypopnea syndrome and liver injury. Chin Med J (Engl). 123:89–94.
2010.PubMed/NCBI
|
|
32
|
Verbraecken J and McNicholas WT:
Respiratory mechanics and ventilatory control in overlap syndrome
and obesity hypoventilation. Respir Res. 14:1322013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baeuerle PA and Baltimore D: I kappa B: A
specific inhibitor of the NF-kappa B transcription factor. Science.
242:540–546. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ogura J, Terada Y, Tsujimoto T, Koizumi T,
Kuwayama K, Maruyama H, Fujikawa A, Takaya A, Kobayashi M, Itagaki
S, et al: The decrease in farnesoid X receptor, pregnane X receptor
and constitutive androstane receptor in the liver after intestinal
ischemia-reperfusion. J Pharm Pharm Sci. 15:616–631. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beigneux AP, Moser AH, Shigenaga JK,
Grunfeld C and Feingold KR: Reduction in cytochrome P-450 enzyme
expression is associated with repression of CAR (constitutive
androstane receptor) and PXR (pregnane X receptor) in mouse liver
during the acute phase response. Biochem Biophys Res Commun.
293:145–149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu X, Ke S, Liu D, Sheng T, Thomas PE,
Rabson AB, Gallo MA, Xie W and Tian Y: Role of NF-kappaB in
regulation of PXR-mediated gene expression: A mechanism for the
suppression of cytochrome P-450 3A4 by proinflammatory agents. J
Biol Chem. 281:17882–17889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang YM, Ong SS, Chai SC and Chen T: Role
of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin
Drug Metab Toxicol. 8:803–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Ferguson SS, Negishi M and
Goldstein JA: Identification of constitutive androstane receptor
and glucocorticoid receptor binding sites in the CYP2C19 promoter.
Mol Pharmacol. 64:316–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wortham M, Czerwinski M, He L, Parkinson A
and Wan YJ: Expression of constitutive and rostane receptor,
hepatic nuclear factor 4 alpha and P450 oxidoreductase genes
determines interindividual variability in basal expression and
activity of a broad scope of xenobiotic metabolism genes in the
human liver. Drug Metab Dispos. 35:1700–1710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aleksunes LM and Klaassen CD: Coordinated
regulation of hepatic phase I and II drug-metabolizing genes and
transporters using AhR-, CAR-, PXR-, PPARα- and Nrf2-null mice.
Drug Metab Dispos. 40:1366–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai Y, Konishi T, Han G, Campwala KH,
French SW and Wan YJ: The role of hepatocyte RXR alpha in
xenobiotic-sensing nuclear receptor-mediated pathways. Eur J Pharm
Sci. 15:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdulla D, Goralski KB, Del Busto Cano EG
and Renton KW: The signal transduction pathways involved in hepatic
cytochrome P450 regulation in the rat during a
lipopolysaccharide-induced model of central nervous system
inflammation. Drug Metab Dispos. 33:1521–1531. 2005. View Article : Google Scholar : PubMed/NCBI
|