Introduction
Specific differentiation protocols induce
mesenchymal stem cells (MSCs) to differentiate into various types
of mature target cells, and MSCs are a suitable option for adult
stem cell transplantation (1).
MSCs have been used in numerous studies that involve the
regeneration of cardiac cells post-myocardial infarction, and are
capable of enhancing myocardial perfusion and cardiac function in
the ischemic hearts of patients with acute or chronic heart
diseases (2–4). However, the culture of MSCs from the
bone marrow of humans or animals, or embryonic and adult tissues
has a number of limitations, including the possibility of
carcinoma, short survival time and difficulties in isolation.
Therefore, the identification of additional types of suitable stem
cells is required. Amniotic fluid-derived MSCs (AFMSCs) exhibit a
higher proliferation rate, are easier to isolate and extraction
process does not injure the mother or the embryo. Therefore, AFMSCs
may be a favorable source of cardiomyocytes due to their
differentiation potential and characteristics (5). A focus of cardiovascular regenerative
medicine is to improve the efficiency of directed differentiation
of AFMSCs. In a previous preliminary study, high-quality AFMSCs
were successfully isolated (6). To
the best of our knowledge, the present study provides novel results
demonstrating that combined transforming growth factor β1 (TGFβ1)
and 5-azacytidine (5Aza) treatment may be used to improve the
efficiency of AFMSC differentiation.
Materials and methods
Isolation and culture of AFMSCs
Female New Zealand white rabbits (weight: 4.5–5.5
kg, age: 1-1.5y; Song Lian Laboratory Animal Farms, Shanghai,
China; production license no. SCXK2007-0011) at 16–18 days
gestation were narcotized via the ear vein with pentobarbitone
(concentration: 30 mg/ml, dosage: 2 ml/kg), the uterus excised, the
muscle removed and amniotic fluid obtained with an injector. This
was then centrifuged at 152.9 × g) for 15 min at 37°C, resuspended
in amniocyte-specific medium (AmnioMAX-C100; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and plated in 6-cm
dishes at 37°C and 5% CO2 in a fully humidified
atmosphere. Following 7–10 days of culture, the specific medium was
replaced with normal medium DMEM (cat. no. 31600034; Invitrogen;
Thermo Fisher Scientific, Inc.) + 10% fetal bovine serum to remove
non-adherent cells and this was refreshed every 3 days thereafter.
When cells reached 80% confluence, they were treated with 0.25%
trypsin (Invitrogen; Thermo Fisher Scientific, Inc.) for 1 min.
Trypsin digestion was then inhibited and cells were subcultured at
a ratio of 1:2, and cultured under the same aforementioned
conditions. Animal experiments performed in the present study were
approved by the Ethics Committee of Xinhua Hospital (Affiliated
Hospital of Shanghai Jiaotong University, Shanghai, China).
Western blot analysis of
octamer-binding transcription factor 4 (OCT4) expression
Stem cells at passage 3 were washed with
phosphate-buffered saline (PBS) and protein extracts were obtained
following treatment of cells with radioimmunoprecipitation assay
(RIPA) lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology, Shanghai, China), then centrifuged at 4°C, 152.9 × g
for 15 min. A BCA kit (cat. no. P0010S; Beyotime Institute of
Biotechnology) was used to quantify the protein samples. Samples
(20 µl) were loaded onto a precast gel (15% SDS-PAGE kit; cat. no.
P0012A, Beyotime Institute of Biotechnology) and run at 80 V,
followed by an increase to 120 V on ice. Proteins were subsequently
transferred to polyvinylidene difluoride membranes for 45 min at
280 mA in transfer buffer. Membranes were blocked for 2 h with 5%
skimmed milk powder in PBS at room temperature and subsequently
incubated overnight at 4°C with mouse anti-rabbit OCT4 antibodies
(cat. no. ab52110; 1:500; Abcam, Cambridge, UK). Following washing
by PBS, the membranes were treated with goat anti-mouse horseradish
peroxidase (HRP)-conjugated secondary antibodies (cat. no. ab6789;
1:1,000; Abcam) for 2 h at 37°C. GAPDH (cat. no. ab9483; 1:1,000;
Abcam) was used as the loading control. Membranes were subsequently
washed and enhanced by BeyoECL Plus reagent (cat. no. P0018A;
Beyotime Institute of Biotechnology) and used for protein
identification by GelDocXR+ system (model 1708195;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
AFMSC tumorigenicity experiment
A total of 20 female BALB/C nude mice (age: 3–4
weeks, 9.8–14.2 g weight, 12-h light/dark cycle, humidity: 45–55%,
in rat cages, 32–34°C) were divided into 2 groups at random, with
10 mice in the control group and 10 in the experimental group.
OCT4-positive stem cells at passage 3 were treated with 0.25%
trypsin, before digestion was inhibited and the cells were
resuspended in PBS at a density of 5×106/cm3.
A 0.2 ml cell suspension was injected subcutaneously into the necks
of the mice in the experimental group, while 0.2 ml PBS was
injected in the same manner in the control group. The 2 groups were
observed for 8 weeks, then all mice were dissected and the neck,
axillary cavity and groin examined for signs of tumor growth or
hyperplasia in the injection site or lymphatic distribution
area.
Induction of AFMSC
differentiation
OCT4-positive cells were divided into 4 groups,
including the control group (group A), TGFβ1-induced group (group
B), 5Aza-induced group (group C), and the combined TGFβ1 and
5Aza-induced group (group D). When the cells reached 70%
confluence, TGFβ1 (cat. no. 100-21, PeproTech, Suzhou, China)
and/or 5Aza (cat. no. A3656, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) were added to the media of the appropriate groups as
follows: PBS in group A for 24 h; 5 ng/ml TGFβ1 for 24 h in group
B; 10 µmol/l 5Aza for 24 h in group C; and 5 ng/ml TGFβ1 + 10
µmol/l 5Aza for 24 h in group D. All groups were cultured at 37°C
and 5% CO2 in a fully humidified atmosphere. Cells were
harvested when they reached 80% confluence.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR analysis of GATA binding protein
4 (GATA4) expression
Following AFMSC differentiation, total RNA was
extracted from cultured cells each week for 4 weeks using TRIzol
reagent (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. The sequences of the GATA4 primers
(GeneBank Reference Sequence: XM_002717998.1; Sangon Biotech Co.,
Ltd., Shanghai, China) were as follows: Forward,
cagtgagagccttcctcctac (5′-3′), and reverse, catagccttgtggggacag
(5′-3′). The sequences of the GAPDH primers (GeneBank Reference
Sequence: NM_001082253.1; Sangon Biotech Co., Ltd.) were as
follows: Forward, atggtgaaggtcggagtgaa (5′-3′), and reverse,
tgggtggaatcatactggaac (5′-3′). A cDNA synthesis kit (cat. no.
6110A, Takara Bio, Inc., Otsu, Japan) was used to reverse
transcribe total RNA in accordance with the manufacturer's
protocols. PCR was performed at 85°C for 30 sec and 37°C for 15 min
to reverse transcribe to cDNA, then qPCR was subsequently performed
with a SYBR Premix Ex Taq GC kit (cat. no. DRR041A; Takara Bio,
Inc.) and 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) used to analyze the
expression of GATA4 (7). Briefly,
the reaction volume of 20 µl comprised SYBR Premix Ex Taq (10 µl),
PCR Forward Primer (10 µM; 0.4 µl), PCR Reverse Primer (10 µM; 0.4
µl), ROX Reference Dye II (0.4 µl), cDNA (2 µl) and dH2O
(6.8 µl) mixed on ice, and was then used on the 7500 with the
following steps: 1 cycle at 95°C for 30 sec, 40 cycles at 95°C for
5 sec and 60°C for 34 sec, then 1 cycle at 95°C for 15 sec, 60°C
for 1 h, and finally 95°C for 15 sec.
Immunofluorescence analysis of cardiac
troponin T (cTnT) expression
Cells in the 4 experimental groups were cultured in
6-well culture plates (5×106/cm3), and the
cytomembranes were permeabilized for 30 min with 0.3% Triton X-100
at 37°C. Following fixation with 4% paraformaldehyde for 12 h at
4°C, cells were blocked with 5% bovine serum albumin (FBS+PBS) for
2 h at room temperature, and incubated overnight at 4°C with mouse
anti-rabbit cTnT antibodies (1:200; cat. no. ab10214; Abcam,
Cambridge, UK). Subsequent to washing with PBS, cells were
incubated with goat anti-mouse antibodies (cat. no. ab6789;
1:1,000; Abcam) for 2 h in the dark at room temperature. They were
then washed with PBS and stained with DAPI for 5 min in the dark at
room temperature, and subsequently observed under a fluorescence
microscope.
Western blot analysis of connexin 43
expression
Cells (5×106/cm3) in each
experimental group were washed with PBS and protein extracts were
obtained following treatment with RIPA lysis buffer. Samples,
prepared as for OCT4, above, were loaded onto a 15% precast gel and
run at 80 V followed by an increase to 120 V. Proteins were then
transferred to polyvinylidene fluoride membranes for 45 min at 280
mA in transfer buffer. Membranes were subsequently blocked with 5%
skimmed milk powder in PBS for 2 h at room temperature and
incubated overnight at 4°C with mouse anti-rabbit connexin 43
primary antibodies (cat. no. ab79010; 1:500; Abcam). GAPDH (cat.
no. ab9483; 1:1,000; Abcam) was used as the loading control.
Following washing with PBS, membranes were treated with
HRP-conjugated goat anti-mouse secondary antibodies (dilution,
1:1,000; cat. no. ab6789; Abcam). The membranes were washed and ECL
reagent (cat. no. P0018A; Institute of Biotechnology) was used for
protein imaging with the GelDocXR+ system (model
1708195; Bio-Rad Laboratories, Inc.) was used for analysis and the
number of replicates per group was 28.
Transmission electron microscopy
Following AFMSC differentiation, cells were treated
with 0.25% trypsin, washed with PBS, fixed with 4% glutaraldehyde
and 1% osmic acid at 4°C for 12 h and embedded in Epon 812 epoxy
resin following graded ethanol dehydration at room temperature.
Samples were cut at 60–70 nm with an ultramicrotome and
double-stained with uranyl acetate for 5–10 min and lead citrate
for 5 min all on ice. A transmission electron microscope was used
to observe the ultrastructure of differentiated cells in each
group.
Statistical analysis
Data were presented as mean + standard deviation and
differences between any 2 groups were compared using one-way
analysis of variance by SPSS version 19.0 (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and culture of AFMSCs
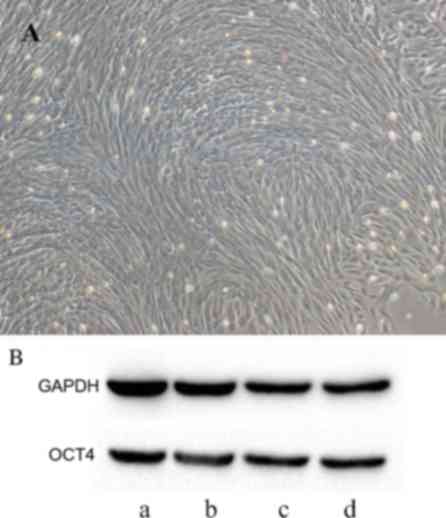
Amniotic fluid samples were first obtained from
rabbits. Following AFMSC isolation for 7–10 days, the proliferative
capacity of cells increased (data not shown) and the cells were
elongated and formed numerous discontiguous colonies that displayed
a circinate alignment pattern and began to proliferate more rapidly
(Fig. 1A). Red blood cells
disappeared from the culture medium following several media
changes.
Western blot analysis of OCT4
expression
Western blot analysis demonstrated positive OCT4
expression in AFMSCs (Fig. 1B).
AFMSCs exhibited characteristics similar to embryonic stem cells;
the cell colonies displayed a circinate alignment pattern and began
to proliferate more rapidly (Fig.
1A).
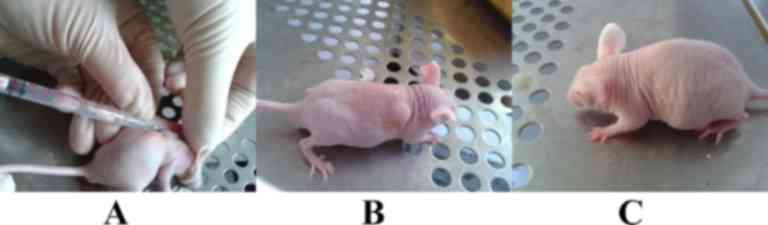
AFMSC tumorigenicity experiment
The tumorigenicity experiment demonstrated that nude
mice in the control and OCT4-positive AFMSC-injected groups
(Fig. 2A and B) remained alive and
did not develop tumors (Fig.
2C).
Induction of AFMSC
differentiation
Following induction of AFMSC differentiation, no
marked alterations in cell morphology were observed in group A
cells, which maintained their morphology following passage 6
(Fig. 3A). However, cells in group
B proliferated rapidly and were more elongated following TGFβ1
treatment when compared with group A cells (Fig. 3B). Cytomixis was observed in group
C cells, which were elongated following 5Aza treatment when
compared with group A cells (Fig.
3C). Following combined TGFβ1 and 5Aza treatment (group D),
cytomixis was observed and cells were elongated and larger when
compared with group A cells (Fig.
3D).
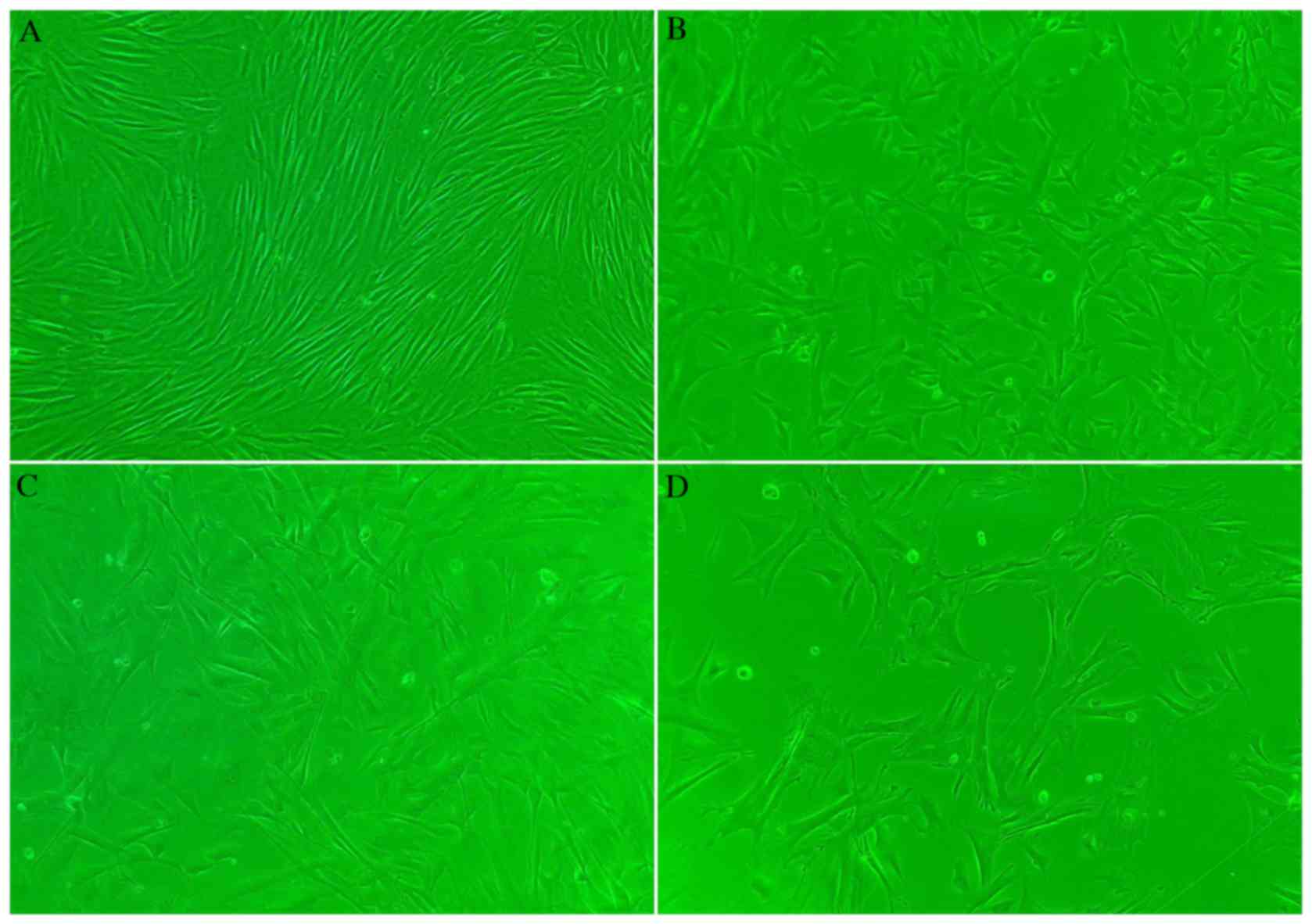 | Figure 3.Morphology of AFMSCs in
differentially-treated groups following induction of
differentiation (magnification, ×5). (A) Group A cells maintained
their morphology, and kept their distribution characteristics. (B)
Cells in group B proliferated rapidly and became elongated. (C)
Cytomixis and elongation was observed in group C cells. (D)
Cytomixis was observed in group D cells, which were elongated and
larger in size compared with other groups. AFMSCs, amniotic
fluid-derived mesenchymal stem cells; group A, untreated control;
group B, TGFβ1-treated; group C, 5Aza-treated; group D, TGFβ1 +
5Aza-treated; TGFβ1, transforming growth factor β1; 5Aza,
5-azacytidine. |
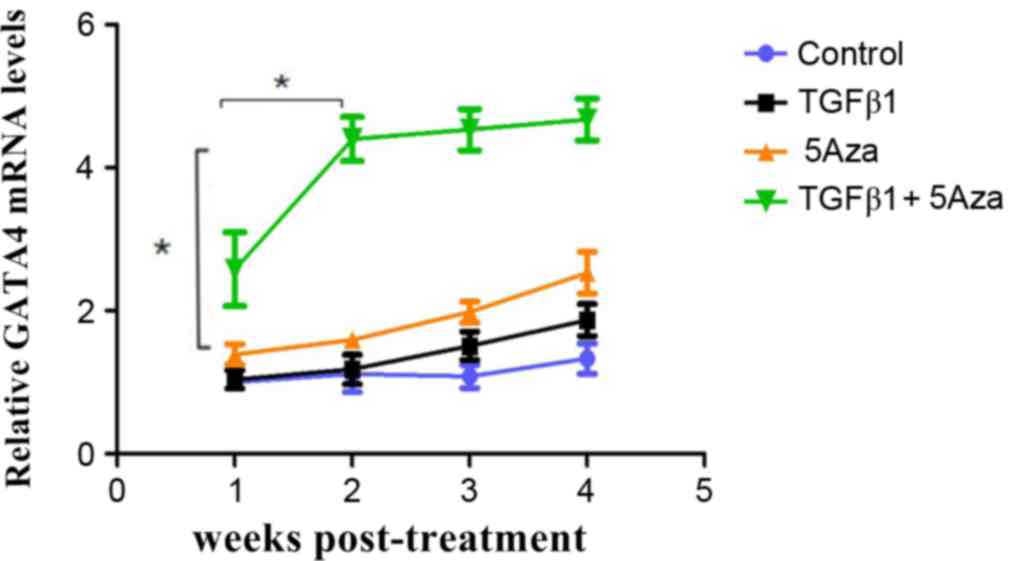
RT-qPCR analysis of GATA4
expression
RT-qPCR was performed to analyze GATA4 expression in
AFMSCs among the 4 experimental groups and all groups were treated
for 4 weeks. No increase in GATA4 expression was observed in group
A at 4 weeks following treatment when compared with 1 week
following treatment (Fig. 4).
However, GATA4 expression was increased in group B cells at 4 weeks
following treatment, in group C cells at 3 weeks following
treatment, and in group D cells at 2 weeks following treatment when
compared with 1 week following treatment. GATA4 expression was
highest in group D and this difference was statistically
significant when compared with the other groups (Fig. 4). These results indicated that
GATA4 was expressed earlier and at a higher level in group D cells,
which were treated with a combination of TGF1β and 5Aza (Fig. 4).
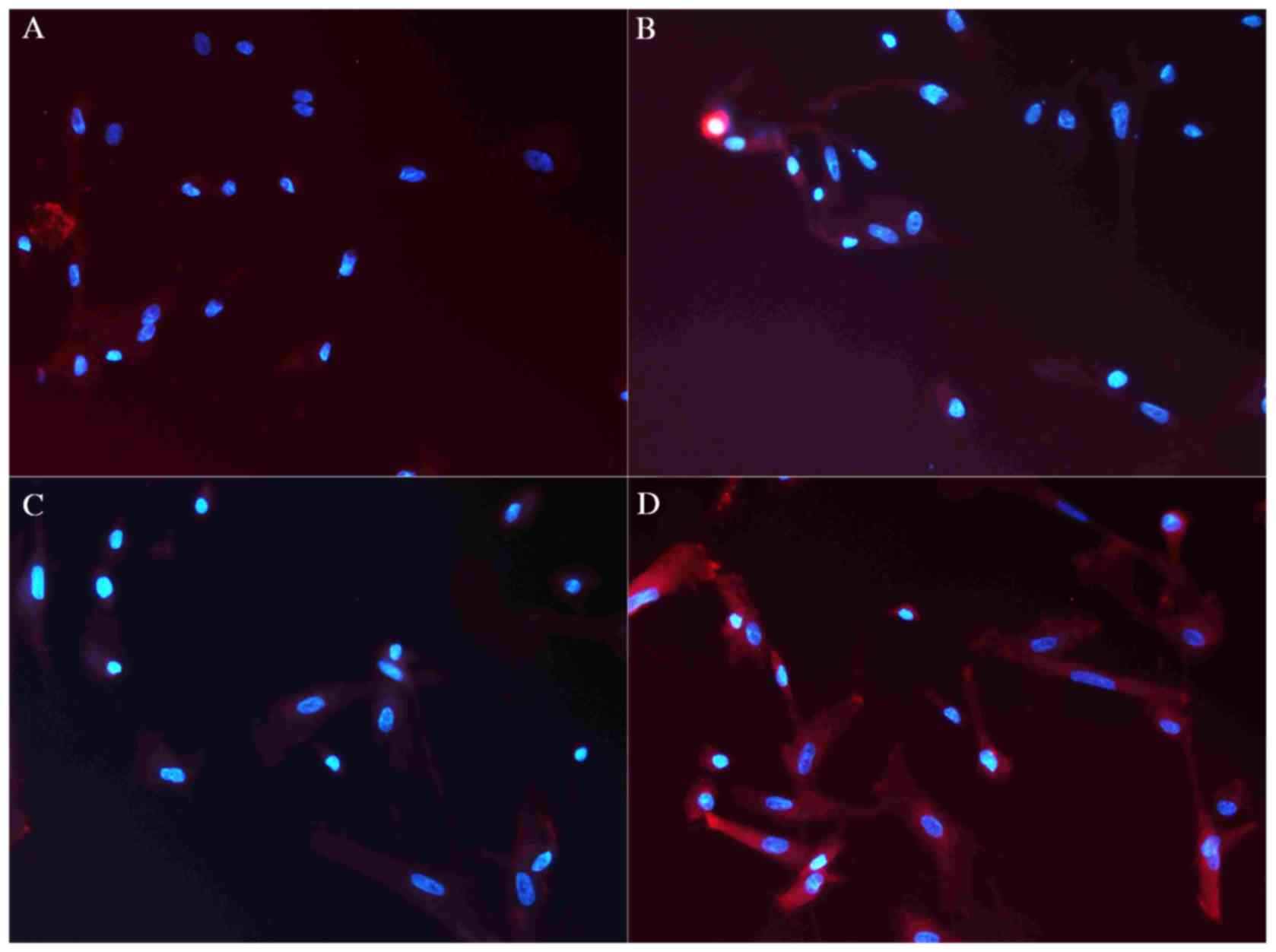
Immunofluorescence analysis of cTnT
expression
Immunofluorescence was performed to analyze the
expression of cTnT. At 2 weeks following treatment, low cTnT
expression was observed in group A cells, while marginally higher
levels were observed in groups B and C (Fig. 5A-C). However, cTnT expression in
group D was increased when compared with groups A, B and C
(Fig. 5D), with more red
fluorescing cells in group D (P<0.05) when compared with groups
A, B and C. After 2 weeks, the expression of cTnT in all groups was
stable.
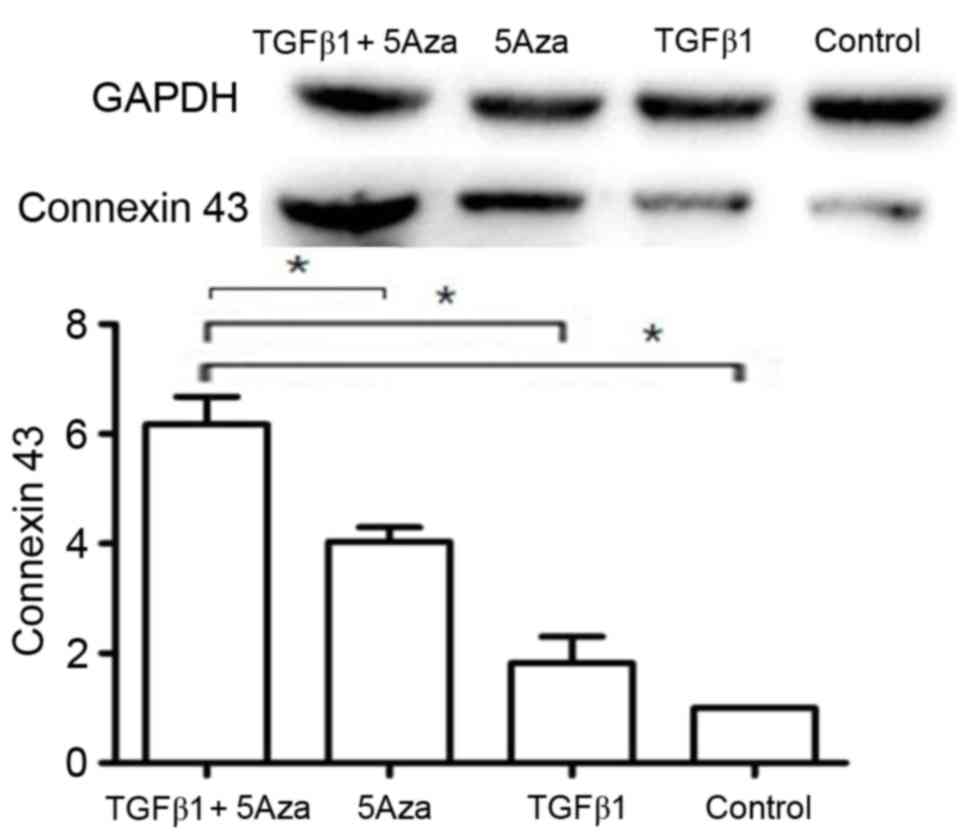
Western blot analysis of connexin 43
expression
Western blot analysis of connexin 43 expression
revealed low expression in group A (Fig. 6). By contrast, a marked increase in
connexin 43 expression was observed in groups B and C when compared
with group A (Fig. 6). Notably,
connexin 43 expression was significantly (P<0.05) higher in
group D when compared with groups A, B and C (Fig. 6).
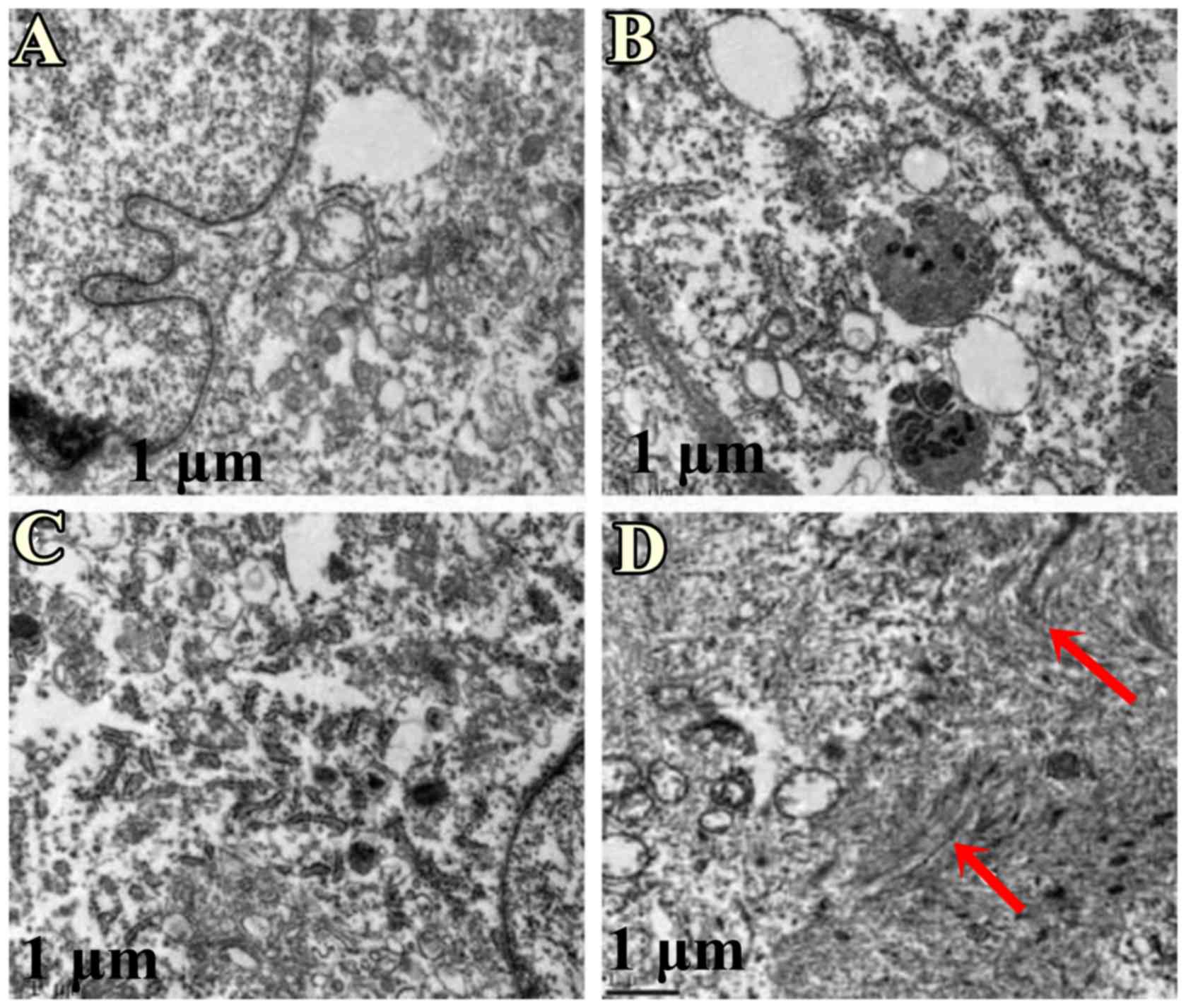
Transmission electron microscopy
analysis
Following AFMSC differentiation, cells were observed
under a transmission electron microscope. A myofilament-like
structure, which is a characteristic feature of cardiomyocytes, was
observed in group D cells (Fig.
7), however this was not observed in the other groups.
Discussion
The development of molecular biological techniques
and stem cell research has provided promising progress in the
regeneration of necrotic myocardium. In 2001, Orlic et al
(8) were the first to report that
bone marrow cells were capable of regenerating infarcted
myocardium. This previous study demonstrated that bone marrow cells
were able to regenerate myocardial cells, which may improve the
prognosis of coronary artery disease. These results were the first
to demonstrate that stem cells may be used to regenerate infarcted
myocardium, thus representing a novel approach for the regeneration
and therapy of myocardial infarction. Therefore, the focus of
research into stem cell treatment and regenerative medicine, is to
identify an alternative source of stem cells. Recently, AFMSCs have
been used for several applications, including prenatal diagnostics,
tissue engineering, gene therapy, cell transplantation, as well as
additional cell-based procedures, such as neuro-regeneration, and
myocardial infarction therapy (3,9).
Researchers have recently started to investigate the
differentiation of AFMSCs into myocardial cells. In 2007,
Chiavegato et al (4)
assayed the phenotypic conversion of AFMSCs using
cardiovascular-specific induction media or co-culture with rat
neonatal cardiomyocytes. AFMSCs exhibited a cardiomyocyte phenotype
following co-culture with rat neonatal cardiomyocytes. In 2011,
Guan et al (10)
demonstrated that human MSCs may be a potential source of cells for
cardiac cell therapy. This previous study induced the
differentiation of hAFS along the cardiac lineage by incubation
with 5Aza for 24 h. Evidence for this differentiation included
morphological alterations, upregulation of cardiac-specific genes
and redistribution of connexin 43. Thus, hMSC differentiated into a
cardiomyocyte-like phenotype and established functional
communication when co-cultured with neonatal rat
cardiomyocytes.
Once suitable cells for seeding are obtained,
controlling the direction of differentiation and improving the
efficiency of differentiation are important. 5Aza promoted the
generation of myocardial sarcomeres and the production of myosin
and muscle protein (11). Xing
et al (12) demonstrated
that mouse bone marrow MSCs transformed into cardiomyocyte-like
cells following induction with 5Aza in vitro. However, the
differentiation cycle induced by 5Aza alone was lengthy (13), and such a long duration of
treatment reduces the rate of cell proliferation (14). Therefore, an alternative and
effective method for the induction of stem cell differentiation is
required.
TGFβ is a multifunctional cytokine that regulates
cell growth, differentiation and death, and has emerged as an
important factor in the self-renewal and maintenance of stem cells
(15). There are 3 subtypes of
TGFβ, and the TGFβ1 signaling pathway serves an important role in
the regulation of cell growth, differentiation, tissue repair and
carcinogenesis. TGFβ1 has been previously implicated in a number of
cardiac diseases, such as post-myocardial infarction ventricular
remodeling, and it stimulates the proliferation of mouse and human
myofibroblasts (16). Huntgeburth
et al (17) reported that
transgenic mice lacking TGFβ1 displayed increased fibrosis and
myocyte hypertrophy. An additional study indicated that TGFβ1
affected the regulation of MSCs at transcriptional and
post-transcriptional levels, and promoted MSC differentiation
(1).
Improving the efficiency of directional
differentiation of AFMSCs is one of the technological challenges
for cardiovascular stem cell therapy. Therefore, the present study
used 5Aza combined with TGFβ1 to induce AFMSC differentiation more
effectively. Taking the results of previous studies into account
(18–20), the optimum concentration was
determined to be 10 µmol/l for 5Aza and 5 ng/ml of TGFβ1, with each
group treated for 24 h, and the effectiveness of induction was
subsequently observed.
GATA4 is a cardiac marker that is highly expressed
in cardiac muscle cells throughout embryonic development, postnatal
growth and adulthood. During this process, it functions as a
critical regulator of cardiac differentiation (19), and regulates the expression of
genes that are critical for cardiac contraction. GATA4 controls the
expression of genes involved in cardiac structure and is essential
for the cardiovascular system; the quantity of its expression has a
decisive effect (21). Thus, the
present study selected GATA4 as a target to investigate whether
AFMSCs transformed into cardiomyocytes.
Cardiac troponin includes proteins T, I, and C. cTnT
is considered to be a reliable biomarker with sufficient
sensitivity and specificity for cardiac injury in the majority of
laboratory animals (22), and it
is the ‘gold-standard’ biomarker of myocardial injury in humans
(23).
Connexin 43 controls the migration and proliferation
of smooth muscle cells, and the expression of Connexin 43 is
increased according to the synthetic state of these cells, which
develop in early atherosclerotic lesions (24). Delmar and Makita (25) demonstrated that connexin 43 serves
an important role in cardiac conduction and heart disease, and
Thimm et al (26) reported
that connexin 43 was functionally associated with calcium, which
regulated its open/closed conformations. Antunes et al
(27) demonstrated that connexin
43 was an important component of ventricles and cardiac muscle.
Therefore, the present study selected cTnT, connexin 43 and GATA4
as indicators of AFMSC-to-cardiomyocyte differentiation.
In the present study, AFMSCs were successfully
isolated and cultured from amniotic fluid using the direct
adherence method. Western blot analysis demonstrated the expression
of OCT4, which confirmed that AFMSCs are capable of multipotent
differentiation. Furthermore, the tumorigenicity experiment
demonstrated that AFMSCs were not tumorigenic. Following combined
treatment with 5Aza and TGFβ1, AFMSCs exhibited positive expression
of GATA4, cTnT and connexin 43, and a myofilament-like structure
was observed under transmission electron microscopy. The results of
the present study demonstrated that AFMSCs exhibited
cardiomyocyte-like characteristics following differentiation,
indicating that transformation into cardiomyocyte-like cells had
occurred. A small number of cardiomyocyte-like cells were observed
following treatment with 5Aza or TGFβ1 alone, however, an increased
number of cardiomyocyte-like cells were observed following combined
treatment with 5Aza and TGFβ1. These results indicated that
combined induction may improve the directional differentiation
efficiency of AFMSCs. The current study provides an efficient and
practical method for the directional differentiation of AFMSCs,
increases the effectiveness of the transformation of
cardiomyocyte-like cells in vitro, and presents a promising
strategy for the regeneration of myocardial cells.
Acknowledgements
The present study was supported by the Nature
Science Foundation of China (grant no. 81170124/H0203).
References
|
1
|
Zhao L and Hantash BM: TGF-β1 regulates
differentiation of bone marrow mesenchymal stem cells. Vitam Horm.
87:127–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohanty S, Bose S, Jain KG, Bhargava B and
Airan B: TGFβ1 contributes to cardiomyogenic-like differentiation
of human bone marrow mesenchymal stem cells. Int J Cardiol.
163:93–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Liu X, Goldstein S, Li Y, Ge J,
He B, Fei X, Wang Z and Ruiz G: Role of the JAK/STAT signaling
pathway in the pathogenesis of acute myocardial infarction in rats
and its effect on NF-κB expression. Mol Med Rep. 7:93–98. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiavegato A, Bollini S, Pozzobon M,
Callegari A, Gasparotto L, Taiani J, Piccoli M, Lenzini E, Gerosa
G, Vendramin I, et al: Human amniotic fluid-derived stem cells are
rejected after transplantation in the myocardium of normal,
ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell
Cardiol. 42:746–759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benthien JP and Behrens P: Reviewing
subchondral cartilage surgery: Considerations for standardised and
outcome predictable cartilage remodelling: A technical note. Int
Orthop. 37:2139–2145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fei X, Jiang S, Zhang S, Li Y, Ge J, He B,
Goldstein S and Ruiz G: Isolation, culture and identification of
amniotic fluid-derived mesenchymal stem cells. Cell Biochem
Biophys. 67:689–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dubuis C, May L, Alonso F, Luca L,
Mylonaki I, Meda P, Delie F, Jordan O, Déglise S, Corpataux JM, et
al: Atorvastatin-loaded hydrogel affects the smooth muscle cells of
human veins. J Pharmacol Exp Ther. 347:574–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan X, Delo DM, Atala A and Soker S: In
vitro cardiomyogenic potential of human amniotic fluid stem cells.
J Tissue Eng Regen Med. 5:220–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aseem O, Barth JL, Klatt SC, Smith BT and
Argraves WS: Cubilin expression is monoallelic and epigenetically
augmented via PPARs. BMC Genomics. 14:4052013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing Y, Lv A, Wang L and Yan X: The
combination of angiotensin II and 5-azacytidine promotes
cardiomyocyte differentiation of rat bone marrow mesenchymal stem
cells. Mol Cell Biochem. 360:279–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balana B, Nicoletti C, Zahanich I, Graf
EM, Christ T, Boxberger S and Ravens U: 5-Azacytidine induces
changes in electrophysiological properties of human mesenchymal
stem cells. Cell Res. 16:949–960. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Chu Y, Shen W and Dou Z: Effect
of 5-azacytidine induction duration on differentiation of human
first-trimester fetal mesenchymal stem cells towards
cardiomyocyte-like cells. Interact Cardiovasc Thorac Surg.
9:943–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YM, Kim J, Heo SC, Shin SH, Do EK, Suh
DS, Kim KH, Yoon MS, Lee TG and Kim JH: Proteomic identification of
ADAM12 as a regulator for TGF-β1-induced differentiation of human
mesenchymal stem cells to smooth muscle cells. PLoS One.
7:e408202012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen LX, Yang K, Sun M, Chen Q, Wang ZH,
Hu GY and Tao LJ: Fluorofenidone inhibits transforming growth
factor-beta1-induced cardiac myofibroblast differentiation.
Pharmazie. 67:452–456. 2012.PubMed/NCBI
|
|
17
|
Huntgeburth M, Tiemann K, Shahverdyan R,
Schlüter KD, Schreckenberg R, Gross ML, Mödersheim S, Caglayan E,
Müller-Ehmsen J, Ghanem A, et al: Transforming growth factor
β1 oppositely regulates the hypertrophic and contractile
response to β-adrenergic stimulation in the heart. PLoS One.
6:e266282011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan Safwani WK, Makpol S, Sathapan S and
Chua KH: 5-Azacytidine is insufficient for cardiogenesis in human
adipose-derived stem cells. J Negat Results Biomed. 11:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosca AM and Burlacu A: Effect of
5-azacytidine: Evidence for alteration of the multipotent ability
of mesenchymal stem cells. Stem Cells Dev. 20:1213–1221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Zhang M, Goldstein S, Li Y, Ge J,
He B and Ruiz G: The effect of c-fos on acute myocardial infarction
and the significance of metoprolol intervention in a rat model.
Cell Biochem Biophys. 65:249–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu M, Millard RW and Ashraf M: Role of
GATA-4 in differentiation and survival of bone marrow mesenchymal
stem cells. Prog Mol Biol Transl Sci. 111:217–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wada R, Muraoka N, Inagawa K, Yamakawa H,
Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, et
al: Induction of human cardiomyocyte-like cells from fibroblasts by
defined factors. Proc Natl Acad Sci USA. 110:12667–12672. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Lemos JA: Increasingly sensitive assays
for cardiac troponins: A review. JAMA. 309:2262–2269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wildi K, Reichlin T, Twerenbold R, Mäder
F, Zellweger C, Moehring B, Stallone F, Minners J, Gimenez M
Rubini, et al: Serial changes in high-sensitivity cardiac troponin
I in the early diagnosis of acute myocardial infarction. Int J
Cardiol. 168:4103–4110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delmar M and Makita N: Cardiac connexins,
mutations and arrhythmias. Curr Opin Cardiol. 27:236–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thimm J, Mechler A, Lin H, Rhee S and Lal
R: Calcium-dependent open/closed conformations and interfacial
energy maps of reconstituted hemichannels. J Biol Chem.
280:10646–10654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antunes E, Borrecho G, Oliveira P, Brito
J, Águas A and dos Santos J Martins: Immunohistochemical evaluation
of cardiac connexin43 in rats exposed to low-frequency noise. Int J
Clin Exp Pathol. 6:1874–1879. 2013.PubMed/NCBI
|