Introduction
Contrast-induced acute kidney injury (CI-AKI) is the
third most common cause of hospital-acquired renal failure, and is
associated with increased cardiovascular and renal morbidity and
mortality (1,2). Although efforts have focused on the
treatment and prevention of the development of renal dysfunction,
its incidence is increasing (3,4).
Contrast medium (CM)-induced renal epithelial cell apoptosis is an
important underlying cause of renal failure (5), although the mechanism remains
unclear.
MicroRNAs (miRNAs/miRs) are a class of small,
non-coding RNA molecules with a length of 18–25 nucleotides that
serve an important role in normal biological functions, including
cell growth, proliferation, differentiation and apoptosis, via
post-transcriptional regulation of gene expression (6). Recent evidence has implicated miRNAs
in ischemia-reperfusion injury (IRI), in addition to
cisplatin-/cyclosporine-induced AKI (7–10).
Specifically, miR-21 was observed to regulate renal cell apoptosis
and fibrosis (11), although these
finding are controversial, with certain studies demonstrating
miR-21 upregulation (12,13) and others reporting a
downregulation, or no change, in expression levels (14,15),
depending on the tissue or disease model.
Programmed cell death protein 4 (PDCD4) is expressed
in proliferating cells where it is known to suppress tumorigenesis
and induce apoptosis, and is negatively regulated by miR-21 in
various types of cancer (16).
PDCD4 has been demonstrated to be associated with IRI (12). However, the role of PDCD4 in
CM-induced renal cell injury has yet to be fully elucidated. It may
be hypothesized that PDCD4 may be involved in renal tubular
epithelial cell apoptosis induced by CM exposure (5). In order to test this hypothesis, the
present study examined miR-21 expression levels in human renal
proximal tubular epithelial (HK-2) cells under CM treatment and in
gain- and loss-of-function experiments, to determine the
association between miR-21 and PDCD4 expression and renal cell
apoptosis. The results of the present study indicated that miR-21
protected kidney cells against CM-induced apoptosis by directly
targeting PDCD4.
Materials and methods
Cell culture
HK-2 and human embryonic kidney (HEK)-293T cells
were provided by the Stem Cell Bank, Chinese Academy of Sciences
(Shanghai, China), and were cultured in Dulbecco's modified Eagle's
medium/nutrient mixture F-12 (Gibco™; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C and
5% CO2, and were subcultured when they reached 80–85%
confluence. The cells were treated with 150 mg iodide (mgI)/ml
Ultravist CM (370 mgI/ml; Bayer AG, Leverkusen, Germany) for 2 h at
37°C.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
Cells (2×106) were washed with PBS and
fixed with 4% paraformaldehyde for 30 min at room temperature.
Following three washes with PBS, cells were treated with 0.2%
Triton X-100 for 5 min on ice, washed with PBS, and incubated with
fluorescein isothiocyanate-labeled dUTP and terminal
deoxynucleotidyl transferase for 1 h at 37°C. Following a series of
washes with PBS and nuclear staining with DAPI at room temperature
away from light, cells were analyzed with an epifluorescence
microscope in five different visual fields at ×400
magnification.
Target gene prediction
The Targetscan (http://www.targetscan.org) and microRNA.org databases (http://www.microrna.org/microrna/home.do) were used to
predict the potential target of miR-21.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Subsequent to the addition of 100%
chloroform followed by 100% isopropanol, washes with 75% ethanol,
and a series of centrifugation steps, RNA concentration and quality
were measured by spectrophotometry at 260 and 280 nm. Isolated RNA
was reverse-transcribed using M-MLV reverse transcriptase (Takara
Bio, Inc., Otsu, Japan), and the cDNA was amplified using SYBR
Premix Ex Taq (Takara Bio, Inc.) on a CFX96 Touch real-time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to a standard protocol under the following conditions:
95°C for 5 min, followed by 40 cycles of 95°C for 10 sec, 60°C for
10 sec, and 72°C for 10 sec. U6 was used as an internal control for
miRNAs. The primer for miRNA amplification was synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). The comparative
threshold cycle (2−ΔΔCq) method was used to analyze
relative changes in gene expression (17). The primers were as follows: miR-21,
forward 5′-ACGGGTAGCTTATCAGACTGA-3′ and reverse
5′-CAGTGCGTGTCGTGGAGT-3′; PDCD4, forward 5′-AACCCTGCAGAAAATGCTGG-3′
and reverse 5′-CCTTAGTCGCCTTTTTGCCTTG-3′; and U6, forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Western blotting
Cells were lysed by incubation with trypsin-EDTA
solution (Invitrogen; Thermo Fisher Scientific, Inc.) and protein
was extracted with radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.) containing protease inhibitor. Protein
concentration was quantified using a BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts of protein (20 µg) were
separated by SDS-PAGE on a 12% gel and electrophoretically
transferred to a polyvinylidene difluoride membrane, which was
incubated in blocking solution containing 5% non-fat milk in a
Tris-buffered saline/Tween 20 solution (TBST) for 60 min at room
temperature. Blocking was followed by overnight incubation at 4°C
with rabbit primary antibodies against the following proteins:
PDCD4 (cat. no. 9535; 1:1,000), the apoptosis regulators, B-cell
lymphoma 2 (Bcl-2; cat. no. 4223; 1:1,000) and Bcl-2-associated X
protein (Bax; cat. no. 5023; 1:500) (all Cell Signaling Technology,
Inc., Danvers, MA, USA); and β-actin (cat. no. ab8227; 1:1,000;
Abcam, Cambridge, UK). Following washing with TBST, bound
antibodies were detected by incubation for 60 min at room
temperature with horseradish peroxidase-labelled goat anti-rabbit
immunoglobulin G (cat. no. 7074; 1:5,000; Cell Signaling
Technology, Inc.). Enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.) was used to visualize protein bands on X-ray
film. Protein expression levels were normalized to that of β-actin.
Image J v1.48 U (National Institutes of Health, Bethesda, MD, USA)
was used for densitometric analysis.
Transfection of oligonucleotides and
small interfering (si) RNA
Cells were seeded at a density of 2×105
in 6-well plates and grown to 60–70% confluence in DMEM-F12, and
subsequently transfected with miR-21 mimic or miR-21 inhibitor,
scrambled control miR-21, siRNA against PDCD4 or negative control
siRNA (Shanghai GenePharma Co., Ltd., Shanghai, China) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The working
concentrations were 100 nM. After 48 h, cells were treated with 150
mgI/ml Ultravist CM for 2 h at 37°C. The efficiency of each mimic
or inhibitor was confirmed by RT-qPCR. Proteins were detected by
western blotting. The fraction of apoptotic cells was determined
with the TUNEL assay. The sequences were as follows: miR-21-mimics,
5′-UAGCUUAUCAGACUGAUGUUGA-3′; miR-21-inhibitors,
5′-UCAACAUCAGUCUGAUAAGCUA-3′; and siRNA-PDCD4,
5′-GCGGUUUGUAGAAGAAUGUTT-3′.
Dual luciferase reporter assay
HEK-293T (2×104) cells were seeded in
24-well plates 24 h prior to transfection. Cells were
co-transfected with wild-type (WT) or mutant (MUT) PDCD4
untranslated region (UTR) constructs (pGL3-PDCD4-3′UTR-WT and
pGL3-PDCD4-3′UTR-MUT, respectively) or the empty control vector
pGL3-promoter pRL-TK (Promega Corporation, Madison, WI, USA) or
miR-21 mimic (Guangzhou Ribobio Co., Ltd, Guangzhou, China) using
Lipofectamine 2000. Relative luciferase activity was measured 48 h
after transfection on a GloMax luminometer (Promega Corporation)
and normalized to the firefly/Renilla luciferase signal in
HEK-293T cells.
Statistical analysis
Data were described as mean ± standard deviation.
The determinations were performed at least in triplicate. An
unpaired t-test and one way-analysis of variance (Bonferroni post
hoc test for equal variances assumed; Tambane's T2 post hoc test
for equal variances not assumed) were used to compare the groups
using GraphPad Prism version 5.0 software (GraphPad Software, Inc.,
La Jolla, CA, USA) and SPSS software version 22.0 (IBM Corp.,
Armonk, NY, USA). Two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
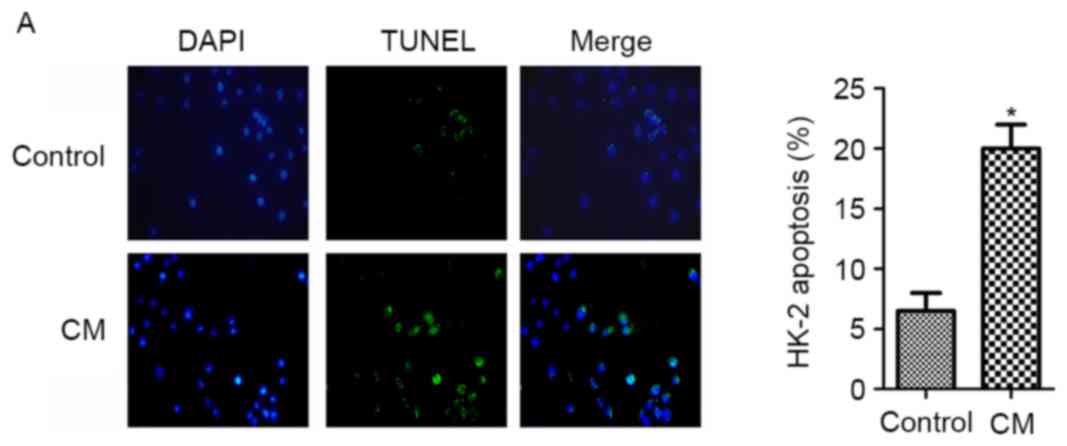
CM induces apoptosis and inhibits
miR-21 expression in HK-2 cells
HK-2 cells were treated with 150 mgI/ml Ultravist
(370 mgI/ml) for 2 h and subsequently harvested for analysis. The
rate of apoptosis was increased following CM treatment, as
determined by the TUNEL assay (Fig.
1A). Consistent with this observation, the expression of the
pro-apoptotic factor Bax was increased, whereas that of the
anti-apoptotic factor Bcl-2 was decreased under these conditions
(Fig. 1B). Additionally, compared
with untreated cells, the miR-21 level was downregulated by
treatment with CM, as determined by RT-qPCR analysis (Fig. 1C), suggesting a negative
association between miR-21 expression and HK-2 cell apoptosis in
the presence of CM.
miR-21 overexpression inhibits
CM-induced apoptosis in HK-2 cells
In order to investigate the effect of miR-21 on HK-2
cell apoptosis under CM treatment, cells were transfected with
miR-21 mimic or inhibitor, or a negative control miRNA. The miR-21
level was increased in cells transfected with mimic and reduced in
inhibitor-treated cells, demonstrating a successful transfection
(Fig. 2A). Western blot analysis
revealed that Bax expression was downregulated, whereas that of
Bcl-2 was upregulated, following transfection of the miR-21 mimic;
the converse was observed in cells transfected with miR-21
inhibitor (Fig. 2B). Additionally,
overexpression of miR-21 mimic decreased CM-induced apoptosis,
whereas miR-21 inhibitor exerted the opposite effect, as determined
by TUNEL assay (Fig. 2C). The
results of the present study demonstrated that miR-21 may protect
HK-2 cell against CM-induced apoptosis.
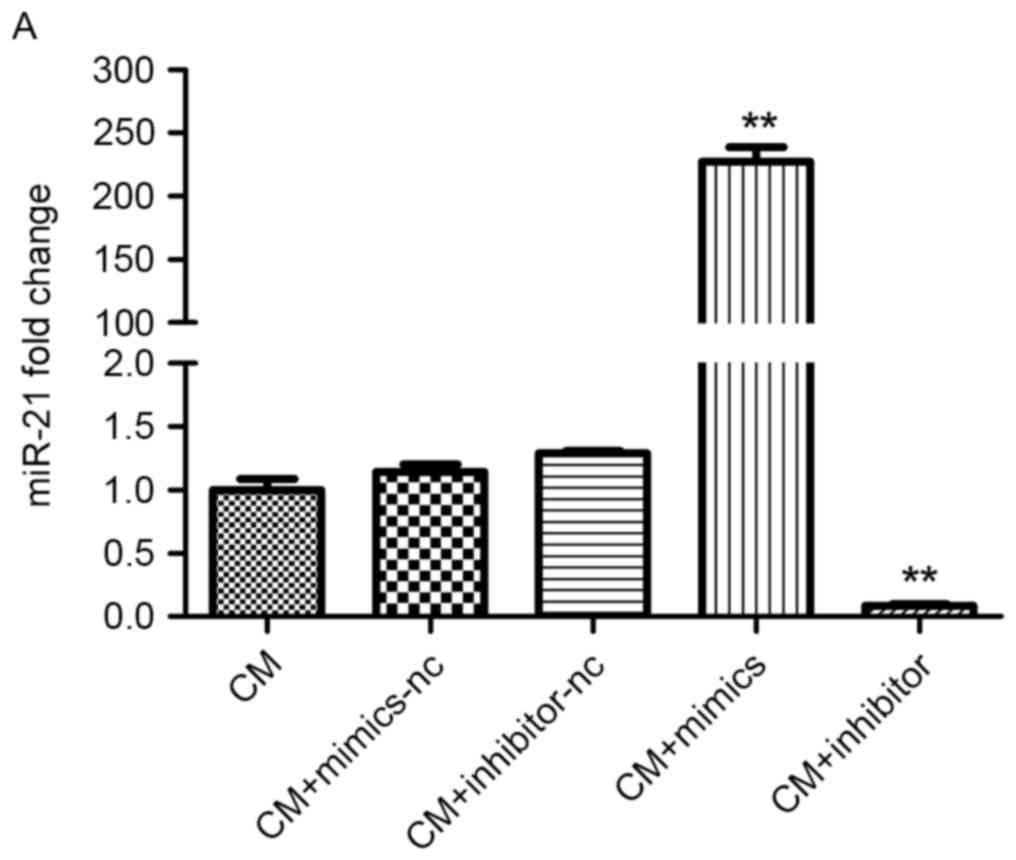 | Figure 2.Effect of miR-21 on HK-2 cell
apoptosis under CM treatment. (A) MiR-21 expression in cells
transfected with miR-21 mimic, inhibitor, or negative control miR
was detected using the reverse transcription-quantitative
polymerase chain reaction. (B) Bcl-2 and Bax protein expression in
cells transfected with miR-21 mimic, inhibitor or negative control
miR was measured by western blotting. (C) Detection of apoptosis
(green cells) with the TUNEL assay. Magnification, ×400. Cells were
treated with 150 mgI/ml Ultravist in the CM groups. *P<0.05,
**P<0.01 vs. CM group (n=3). CM, contrast medium; miR, microRNA;
HK-2, human renal proximal tubular epithelial; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick-end labeling; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein. |
miR-21 inhibits HK-2 cell apoptosis by
binding to the PDCD4 3′ UTR
Target gene prediction indicated that PDCD4 may be a
potential target of miR-21, since the PDCD4 3′ UTR harbored a
miR-21 binding site (Fig. 3A). In
order to test the possibility of a miR-21 interaction with PDCD4,
PDCD4 expression was evaluated in HK-2 cells transfected with
miR-21 under CM treatment, using RT-qPCR analysis and western
blotting. PDCD4 expression was upregulated in cells in the presence
of CM (Fig. 3B and C); however,
this effect was reversed by overexpression of miR-21 mimic,
compared with cells transfected with negative control miR-21 mimic
or those that were untransfected (Fig.
3D and E). Additionally, PDCD4 expression was increased in
cells transfected with miR-21 inhibitor compared with the CM-only
group, whereas the level was reduced upon transfection of miR-21
mimic (Fig. 3D and E), suggesting
that miR-21 may attenuate apoptosis by inhibiting PDCD4
expression.
 | Figure 3.miR-21 inhibits PDCD4 expression. (A)
Target gene analysis results, exhibiting the miR-21 binding sites
in the 3′ UTR of the human PDCD4 transcript. PDCD4 mRNA and protein
levels in HK-2 cells treated with 150 mgI/ml Ultravist were
determined by (B) reverse transcription-quantitative polymerase
chain reaction and (C) western blotting, respectively. PDCD4 mRNA
and protein expression in cells treated with 150 mgI/ml Ultravist
following transfection with miR-21 mimic, inhibitor, or negative
control miR was also measured by (D) reverse
transcription-quantitative polymerase chain reaction and (E)
western blotting, respectively. *P<0.05, **P<0.01 vs. CM
group (n=3). miR, microRNA; PDCD4, programmed cell death protein 4;
UTR, untranslated region; HK-2, human renal proximal tubular
epithelial; CM, contrast medium. |
In order to confirm this hypothesis, constructs
containing WT or MUT PDCD4 3′ UTR were generated (Fig. 4A). The luciferase activity in
HEK-293T cells transfected with pGL3-PDCD4-3′UTR-WT was reduced by
~50% relative to that in cells transfected with the MUT construct
(Fig. 4B), suggesting that miR-21
may directly bind to the PDCD4 transcript, and thereby regulate its
expression.
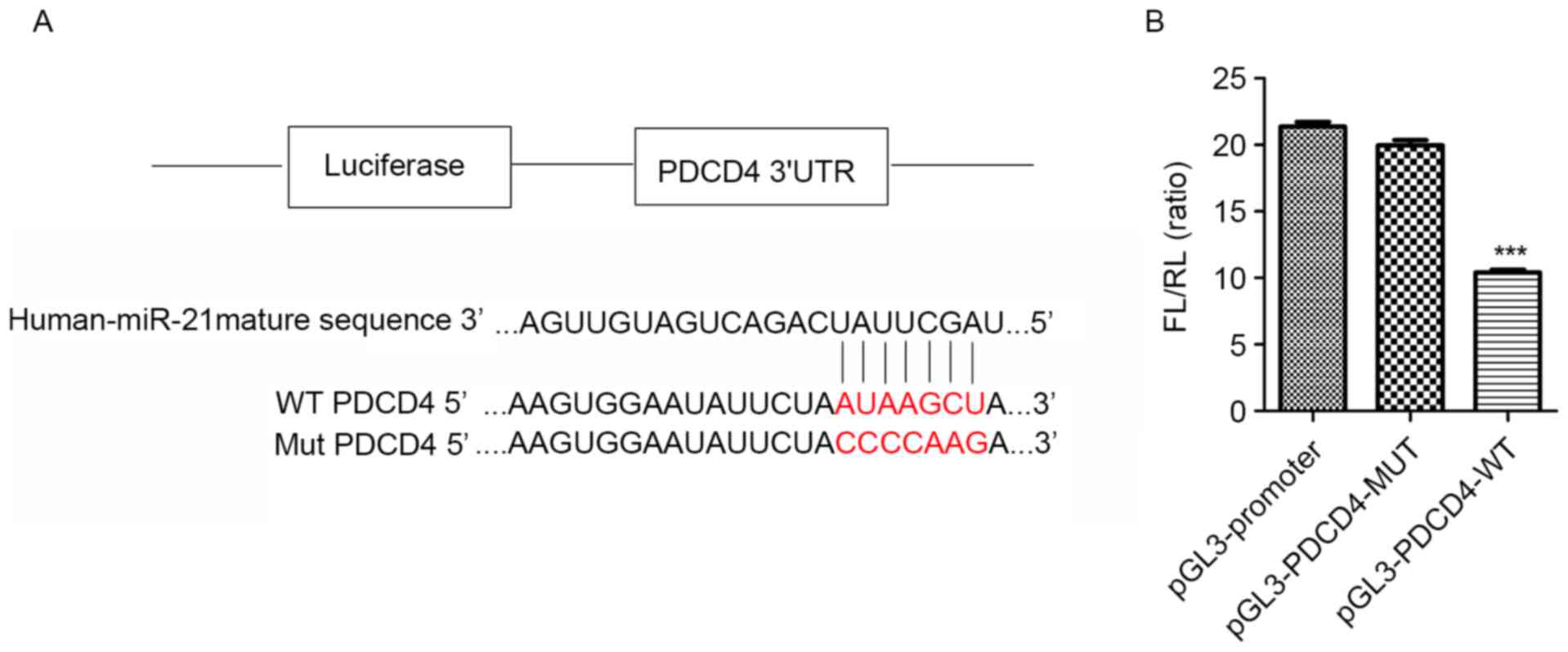 | Figure 4.miR-21 inhibits renal cell apoptosis
by directly targeting PDCD4 expression. (A) Plasmid with and
without the miR-21 binding site in the 3′ UTR sequence of PDCD4.
The WT and MUT sequences were AUAAGCU and CCCCAAG, respectively
(bold red letters). (B) Targeting of PDCD4 by miR-21, as determined
with the dual-luciferase reporter assay. ***P<0.001 vs. MUT
construct. (C) PDCD4 expression in HK-2 cells treated with siRNA
against PDCD4. (D) PDCD4, Bcl-2, and Bax expression in HK-2 cells
treated with siRNA against PDCD4, as determined by western
blotting. *P<0.05, **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. CM group (n=3).
miR, microRNA; PDCD4, programmed cell death protein 4; UTR,
untranslated region; WT, wild-type; MUT, mutant; HK-2, HK-2, human
renal proximal tubular epithelial; CM, contrast medium; siRNA,
small interfering RNA; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; nc, negative control. |
siRNA was used to knock down PDCD4 expression in
HK-2 cells, and the effect of this on apoptosis was examined. PDCD4
mRNA and protein levels were decreased upon transfection of
siRNA-PDCD4 (Fig. 4C and D). This
decreased was accompanied by a downregulation of Bax and an
upregulation of Bcl-2 compared with cells treated with CM only
(Fig. 4D). The results of the
present study demonstrated that miR-21 may protect renal cells from
CM-induced apoptosis by suppressing PDCD4 expression.
Discussion
With the increasing use of CM in the diagnosis and
treatment of coronary disease, CI-AKI has become the third leading
cause of hospital-acquired AKI (18). Although the pathogenesis of CI-AKI
is not well understood, apoptosis of renal epithelial and
glomerular cells is hypothesized to be the underlying cause
(19,20). Bax and Bcl-2 are two important
mediators of the mitochondrial apoptosis pathway (21); Bcl-2 prevents Bax activation and
thereby inhibits apoptosis. It was previously reported that Bcl-2
was downregulated in proximal renal tubular cells in AKI (22). As exhibited in the present study,
Bax and Bcl-2 were increased and decreased, respectively, with a
corresponding increase in the rate of apoptosis following CM
treatment, consistent with previous reports of CM-induced cell
apoptosis.
miRNAs contribute to kidney homeostasis and disease
(23). miR-21 has been implicated
in apoptosis, fibrosis, inflammation and IRI (24,25),
and is considered to be a biomarker of AKI (8,26).
One study demonstrated that miR-21 was upregulated 24 h subsequent
to IRI in an animal model (12);
this was confirmed by other investigators who reported that miR-21
expression was increased in renal proximal tubular cells 24 h
following hypoxia/reoxygenation or treatment with CM (27,28).
miR-21 levels have been observed to increase continuously in
proliferating cells (12,25), starting at 24 h post injury
(29). Therefore, its expression
did not increase within 24 h reperfusion subsequent to 20 min of
ischemia, or within 8 h of CM administration (13,30).
It has been demonstrated that one-half of the amount of CM in the
bloodstream may be eliminated quickly, in ~2 h (31). In addition, the structure of renal
cells exhibited moderate changes 2 h after CM administration
(32). Therefore, Neutrophil
gelatinase-associated lipocalin, a novel biomarker of CI-AKI, has
been demonstrated to begin to increase at 2 h post-CM exposure
(33), although the level of serum
creatinine did not significantly alter. In order to observe the
effect of CM on renal cells, cells were incubated with CM for 2 h
in the present study, and the expression of miR-21 was
downregulated. Future studies are required to investigate
alterations in miR-21 expression with respect to the degree of
renal injury.
miR-21 was demonstrated to be renoprotective in
animal and cellular models of IRI (27,34).
However, miR-21 has additionally been reported to exert deleterious
effects in IRI, diabetic nephropathy and renal fibrosis (35–37).
Physiological differences between models and variable times of
ischemia may account for these conflicting observations. miR-21 was
observed to regulate tumor cell proliferation, invasion, apoptosis
and migration by targeting PDCD4 and Bcl-2 (38), while PDCD4 is thought to inhibit
neoplastic transformation (39).
As demonstrated in the present study, the levels of PDCD4 were
reduced and enhanced upon transfection with a miR-21 mimic and
inhibitor, respectively. In addition, the rate of apoptosis was
decreased following PDCD4 knockdown. The results of the present
study corroborated previous findings and demonstrated that PDCD4
may be negatively regulated by miR-21; additionally, these results
suggested that the miR-21/PDCD4 pathway serves an important role in
preventing CM-induced renal tubular cell apoptosis. To the best of
our knowledge, the present study is the first to analyze the
mechanism of CM-induced renal cell apoptosis from a genetic
perspective. Whether the miR-21/PDCD4 pathway exhibits this
function in vivo will be investigated in future studies.
In conclusion, the present study demonstrated that
miR-21 protected renal cells against CM-induced apoptosis by
directly regulating PDCD4. The results of the present study may
provide a basis for the development of therapeutic strategies to
treat CI-AKI.
Acknowledgements
The present study was supported by the National
Science Foundation for Young Scientists of China (grant no.
81500520), the National Natural Science Foundation of China (grant
no. 81270286), and the Progress in Science and Technology Project
of Guangdong Province (grant nos. 2013b031800025 and
2016b020215130).
Glossary
Abbreviations
Abbreviations:
|
miR-21
|
microRNA-21
|
|
PDCD4
|
programmed cell death protein 4
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bax
|
Bcl-2-associated X protein
|
|
CM
|
contrast medium
|
|
CI-AKI
|
contrast-induced acute kidney
injury
|
|
HK-2
|
human renal proximal tubular
epithelial
|
|
HEK
|
human embryonic kidney
|
|
IRI
|
ischemia reperfusion injury
|
|
MUT
|
mutant type
|
|
WT
|
wild type
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
siRNA
|
small interfering RNA
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
References
|
1
|
Nash K, Hafeez A and Hou S:
Hospital-acquired renal insufficiency. Am J Kidney Dis. 39:930–936.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai TT, Patel UD, Chang TI, Kennedy KF,
Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC,
Rumsfeld JS and Spertus JA: Contemporary incidence, predictors, and
outcomes of acute kidney injury in patients undergoing percutaneous
coronary interventions: Insights from the NCDR Cath-PCI registry.
JACC Cardiovasc Interv. 7:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hernandez GT and Nasri H: World Kidney Day
2014: Increasing awareness of chronic kidney disease and aging. J
Renal Inj Prev. 3:3–4. 2014.PubMed/NCBI
|
|
4
|
Nicola R, Shaqdan KW, Aran K, Mansouri M,
Singh A and Abujudeh HH: Contrast-induced nephropathy: Identifying
the risks, choosing the right agent and reviewing effective
prevention and management methods. Curr Probl Diagn Radiol.
44:501–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perrin T, Descombes E and Cook S:
Contrast-induced nephropathy in invasive cardiology. Swiss Med
Wkly. 142:w136082012.PubMed/NCBI
|
|
6
|
Schickel R, Boyerinas B, Park S and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schena FP, Serino G and Sallustio F:
MicroRNAs in kidney diseases: New promising biomarkers for
diagnosis and monitoring. Nephrol Dial Transpl. 29:755–763. 2014.
View Article : Google Scholar
|
|
8
|
Du J, Cao X, Zou L, Chen Y, Guo J, Chen Z,
Hu S and Zheng Z: MicroRNA-21 and risk of severe acute kidney
injury and poor outcomes after adult cardiac surgery. PLoS One.
8:e633902013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shapiro MD, Bagley J, Latz J, Godwin JG,
Ge X, Tullius SG and Iacomini J: MicroRNA expression data reveals a
signature of kidney damage following ischemia reperfusion injury.
PLoS One. 6:e230112011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pavkovic M, Riefke B and
Ellinger-Ziegelbauer H: Urinary microRNA profiling for
identification of biomarkers after cisplatin-induced kidney injury.
Toxicology. 324:147–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YF, Jing Y, Hao J, Frankfort NC, Zhou
X, Shen B, Liu X, Wang L and Li R: MicroRNA-21 in the pathogenesis
of acute kidney injury. Protein Cell. 4:813–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Godwin JG, Ge X, Stephan K, Jurisch A,
Tullius SG and Iacomini J: Identification of a microRNA signature
of renal ischemia reperfusion injury. Proc Natl Acad Sci USA.
107:14339–14344. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaucsár T, Révész C, Godó M, Krenács T,
Albert M, Szalay CI, Rosivall L, Benyó Z, Bátkai S, Thum T, et al:
Activation of the miR-17 Family and miR-21 During murine kidney
ischemia-reperfusion injury. Nucleic Acid Ther. 23:344–354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saikumar J, Hoffmann D, Kim TM, Gonzalez
VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS and
Vaidya VS: Expression, circulation, and excretion profile of
MicroRNA-21, −155 and −18a following acute kidney injury. Toxicol
Sci. 129:256–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanki M, Moriguchi A, Sasaki D, Mitori H,
Yamada A, Unami A and Miyamae Y: Identification of urinary miRNA
biomarkers for detecting cisplatin-induced proximal tubular injury
in rats. Toxicology. 324:158–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allgayer H: Pdcd4, a colon cancer
prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol.
73:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aurelio A and Durante A: Contrast-induced
nephropathy in percutaneous coronary interventions: Pathogenesis,
risk factors, outcome, prevention and treatment. Cardiology.
128:62–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Duarte CG and Ellis S: Contrast
medium- and mannitol-induced apoptosis in heart and kidney of SHR
rats. Toxicol Pathol. 27:427–435. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Romano G, Briguori C, Quintavalle C, Zanca
C, Rivera NV, Colombo A and Condorelli G: Contrast agents and renal
cell apoptosis. Eur Heart J. 29:2569–2576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borkan SC: The Role of BCL-2 Family
Members in Acute Kidney Injury. Semin Nephrol. 36:237–250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chandrasekaran K, Karolina DS, Sepramaniam
S, Armugam A, Wintour EM, Bertram JF and Jeyaseelan K: Role of
microRNAs in kidney homeostasis and disease. Kidney Int.
81:617–627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai JY, Luo J, O'Connor C, Jing X, Nair V,
Ju W, Randolph A, Ben-Dov IZ, Matar RN, Briskin D, et al:
MicroRNA-21 in Glomerular Injury. J Am Soc Nephrol. 26:805–816.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov
D, Liu H, Fang Y, Ding X and Liang M: Delayed ischemic
preconditioning contributes to renal protection by upregulation of
miR-21. Kidney Int. 82:1167–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramachandran K, Saikumar J, Bijol V,
Koyner JL, Qian J, Betensky RA, Waikar SS and Vaidya VS: Human
miRNome Profiling Identifies MicroRNAs Differentially Present in
the Urine after Kidney Injury. Clin Chem. 59:1742–1752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W and Shu L: Upregulation of miR-21
by Ghrelin ameliorates ischemia/reperfusion-induced acute kidney
injury by inhibiting inflammation and cell apoptosis. DNA Cell
Biol. 35:417–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gutiérrez-Escolano A, Santacruz-Vázquez E
and Gómez-Pérez F: Dysregulated microRNAs involved in
contrast-induced acute kidney injury in rat and human. Ren Fail.
37:1498–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Humphreys BD, Czerniak S, DiRocco DP,
Hasnain W, Cheema R and Bonventre JV: Repair of injured proximal
tubule does not involve specialized progenitors. Proc Natl Acad Sci
USA. 108:9226–9231. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun SQ, Zhang T, Ding D, Zhang WF, Wang
XL, Sun Z, Hu LH, Qin SY, Shen LH and He B: Circulating
MicroRNA-188, −30a, and −30e as early biomarkers for
contrast-induced acute kidney injury. J Am Heart Assoc. 5(pii):
e0041382016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geenen RW, Kingma HJ and van der Molen AJ:
Contrast-induced nephropathy: pharmacology, pathophysiology and
prevention. Insights Imaging. 4:811–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tervahartiala P, Kivisaari L, Kivisaari R,
Vehmas T and Virtanen I: Structural changes in the renal proximal
tubular cells induced by iodinated contrast media. Nephron.
76:96–102. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirsch R, Dent C, Pfriem H, Allen J,
Beekman RH III, Ma Q, Dastrala S, Bennett M, Mitsnefes M and
Devarajan P: NGAL is an early predictive biomarker of
contrast-induced nephropathy in children. Pediatr Nephrol.
22:2089–2095. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia P, Teng J, Zou J, Fang Y, Zhang X,
Bosnjak ZJ, Liang M and Ding X: miR-21 contributes to
xenon-conferred amelioration of renal ischemia-reperfusion injury
in mice. Anesthesiology. 119:621–630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Hong Q, Wang Z, Yu Y, Zou X and Xu
L: MiR-21 inhibits autophagy by targeting Rab11a in renal
ischemia/reperfusion. Exp Cell Res. 338:64–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhong X, Chung AC, Chen HY, Dong Y, Meng
XM, Li R, Yang W, Hou FF and Lan HY: miR-21 is a key therapeutic
target for renal injury in a mouse model of type 2 diabetes.
Diabetologia. 56:663–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chau BN, Xin C, Hartner J, Ren S, Castano
AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, et al: MicroRNA-21
promotes fibrosis of the kidney by silencing metabolic pathways.
Sci Transl Med. 4:118ra–121ra. 2012. View Article : Google Scholar
|
|
38
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2007. View Article : Google Scholar : PubMed/NCBI
|