Introduction
An estimated 200 million patients undergo anesthesia
and surgery worldwide each year (1). Volatile anesthetics, such as
isoflurane, desflurane and sevoflurane, are usually used in general
anesthesia. The differences in the chemical structures of volatile
anesthetics result in diverse physicochemical properties leading to
different biological effects, with particularly different effects
on neuronal cells (2). Depending
on the experimental conditions used, sevoflurane can be
neuroprotective in anesthesia (3).
However, in certain conditions, particularly in animal models with
neonatal sevoflurane exposure, sevoflurane can exert relevant
neurotoxicity effects (4).
Additionally, increasing studies have demonstrated that exposure to
individual anesthetic drugs, including volatile anesthetics,
triggers significant damage in the developing brain (5). Furthermore, sevoflurane has been
reported to induce cell damage in various neuronal and non-neuronal
cells and tissues (6). Thus,
volatile anesthetic, such as sevoflurane, can be a risk for cell
injury, particularly for neuronal injury during anesthesia.
Therefore, understanding the pathological mechanisms of the
neurotoxic effects of sevoflurane is of great importance for
developing effective methods of anesthesia.
Traditional Chinese medicine has been developed in
China over 5,000 years, providing health care services to Chinese
people and worldwide. An in vitro study demonstrated that
traditional Chinese medicine may be effective for the treatment and
prevention of central nervous system diseases (7). Thus, the effect of traditional
Chinese medicine on nerve cell protection has become a major topic
of research in the Chinese neuroscience community, and an important
part of medical research worldwide.
Panax Notoginseng Saponins (PNS) is the active
ingredient of the Chinese herb Sanqui, which is predominantly
cultivated in the Yunnan and Guangxi provinces of China (8). The medicinal properties of the Panax
Notoginseng root include relieving swelling, promoting blood
clotting and alleviating pain (9).
It has been reported that PNS has a number of biological activities
including immunomodulatory effects, antioxidation and anticancer
properties (10). Additionally, it
has been demonstrated that PNS has neuroprotective effects
following stroke through reducing the apoptosis of nerve cells and
neurotoxicity (11). Another study
suggested that PNS promoted angiogenesis and the synthesis and
release of neurotrophic factors (12). However, the effect of PNS on
anesthesia-induced neurotoxicity effects remains to be
elucidated.
In the present study, nerve cells were separated
from the hippocampus of day 16 embryonic mice and used to
investigate the influence of PNS on sevoflurane-induced nerve cell
injury. By culturing the neuronal cells in a sevoflurane
environment using an anesthesia machine, it was determined that
sevoflurane significantly induced neurotoxicity by decreasing cell
growth and increasing apoptosis. Additionally, PNS treatment
inhibited the neurotoxic effect of sevoflurane. Furthermore, PNS
attenuated sevoflurane-induced neurotoxicity through the
phosphoinositide 3-kinase (PI3K)/AKT serine/threonine kinase (AKT)
pathway. Understanding the mechanism of sevoflurane-induced
neurotoxicity is essential for providing novel insights into the
action of volatile anesthetics and developing neuroprotective
strategy for anesthesia-induced neuronal injury.
Materials and methods
Animals
This study was approved by the ethical committee of
the Experimental Animal Center of Harbin Medical University
(Harbin, China). All experimental animals were purchased from the
Experimental Animal Center of Harbin Medical University. All
experiments with animals were performed according to the guidelines
of the University Ethics Committee of Harbin Medical University.
Neuronal cells were harvested from embryonic day 16 mice by
caesarean section from pregnant BALB/c mice and derived from the
hippocampus. The harvested cells were first plated on 24 or 96-well
plates pre-coated with poly-L-lysine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and cultured at 37°C with 5% CO2.
The cells were cultured in neurobasal medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with B27 (X1) and
glutamine (25 mM; Sigma-Aldrich; Merck KGaA). After 7 days, the
neuronal cells were ready to use for the following experiments.
Treatment of sevoflurane and PNS
The neuronal cells were first divided into four
groups for sevoflurane (Sigma-Aldrich; Merck KGaA) treatment:
Normal group, cells cultured in 95% O2 and 5%
CO2; and sevoflurane groups, cell cultured in 95%
O2 and 5% CO2 with 1, 2 or 3% sevoflurane.
All gases were delivered into cells using an anesthesia machine in
a sealed plastic box. For the PNS (Sigma-Aldrich; Merck KGaA)
experiments, the cell were divided into five groups: Normal group,
normal neuronal cells; 3% sevoflurane group, cells treated with 95%
O2 and 5% CO2 with 3% sevoflurane for 6 h;
PNS groups, cells cultured in 50, 100 or 200 µmol/l PNS for 6 h and
then with 3% sevoflurane for 6 h.
MTT assay
The cell growth and viability were assessed by an
MTT assay. Following the addition of growth medium containing 10% 5
mg/ml MTT (Sigma-Aldrich; Merck KGaA), the cells were seeded in a
96-well plate and cultured at 37°C overnight in 5% CO2.
Then, the formazan crystals were dissolved with dimethylsulfoxide.
Optical density was determined using a microculture plate reader
(BD Biosciences, Franklin Lakes, NJ, USA) at 490 nm.
Cells apoptosis analysis
Cell apoptosis was detected using flow cytometric
analysis using an Annexin V-FITC/propidium iodide kit
(Sigma-Aldrich; Merck KGaA). Briefly, cells were trypsinized and
washed with phosphate buffered saline. After centrifugation at 300
× g for 10 min at room temperature, the cell were resuspended in
500 µl of binding buffer, cells were incubating with 5 µl Annexin
V-fluorescein isothiocyanate and 5 µl propidium iodide
(Sigma-Aldrich; Merck KGaA) for 30 min at room temperature.
Ultimately, all specimens were analyzed on a FACScan flow cytometer
with CellQuest Pro software 5.1 (BD Biosciences).
Western blot analysis
The protein expression level was assessed by western
blot. Total protein from neuronal cells was extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Nantong, China) and quantified using a bicinchoninic
acid assay. Proteins (200 µg) were separated by 10 or 15% SDS-PAGE
and transferred to nitrocellulose membranes. Following blocking in
a 5% skimmed milk solution for 30 min at room temperature, the
target proteins were incubated overnight at 4°C with anti-capase-3
(1:500; ab13847), anti-capase-9 (1:2,000; ab202068), anti-B cell
lympoma-2 (Bcl-2; 1:10,000; ab59348), anti-Bcl-2 associated X
protein (Bax; 1:1,000; ab32503), anti-β-secretase (Bace-1; 1:1,000;
ab183612), anti-phospho-AKT (1:500; ab38449), anti-AKT (1:500;
ab8805) or anti-β-actin (1:1,000; ab8827) rabbit anti-mouse
antibodies (all from Abcam, Cambridge, UK). Membranes were
subsequently incubated with a goat anti-rabbit secondary antibody
(1:20,000; ab7090; Abcam, Cambridge, UK) for 1 h at room
temperature. The band density of each gene was normalized to the
corresponding density of β-actin and visualized using the enhanced
chemiluminescence detection system (GE Healthcare Life Sciences,
Little Chalfont, UK). Band densities were quantified using Odyssey
Image Analysis software version 4.0 (LI-COR Biosciences, Lincoln,
NE, USA).
ELISA
The content of amyloid precursor protein (APP;
E2035m; Beijing Huaxia Tech, Beijing, China) and β-amyloid peptide
(Aβ; KMB3441; Invitrogen; Thermo Fisher Scientific, Inc.)
concentrations were measured using corresponding quantification
ELISA kits according to the manufacturer's instructions. Optical
density values were read at 450 nm using a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Cell transfection
AKT siRNA was purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China). The sequences were as follows: AKT siRNA
sense, GCC AGU ACC UCA UGG AUU ATT and antisense, UAA UCC AUG AGG
UAC UGG CTT; siRNA control sense, GGA CTA TCA TAT GCT TAC CGAA and
antisense, CAG GAA ACA GCT ATG ACG. Additionally, neuronal cells
were divided into four groups. Control group, normal neuronal
cells; 3% sevoflurane group, cells treated with 95% O2
and 5% CO2 with 3% sevoflurane; 3% sevoflurane + PNS
group, cells treated with 200 µmol/l PNS and then 3% sevoflurane;
3% sevoflurane + PNS + siAKT group, cells were transfected with AKT
siRNA using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h and then treated as the PNS group.
Statistical analysis
All results were presented as the mean ± standard
deviation from a minimum of three replicates. Differences between
groups was evaluated using SPSS version 15.0 statistical software
(SPSS, Inc., Chicago. IL, USA) with one-way analysis followed by a
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PNS elevates cell proliferation in
sevoflurane-stimulated nerve cells
To explore the effect of sevoflurane on nerve cell
proliferation, the cells were treated with 1, 2 and 3% sevoflurane.
As presented in Fig. 1A, the
proliferation rates of the nerve cells were inhibited by
sevoflurane in a concentration-dependent manner. Given the
effectiveness of the sevoflurane at these concentrations (1, 2 and
3%), 3% sevoflurane was used to perform the subsequent experiments.
The nerve cells were cultured in the presence of increasing
concentrations of PNS (50, 100 and 200 µM) then treated with 3%
sevoflurane, and this led to an increase in cell proliferation in a
concentration-dependent manner (Fig.
1B); thus PNS at 200 µM was used in the subsequent experiments.
These results demonstrated that PNS alleviates reduced cell
proliferation induced by sevoflurane treatment in nerve cells.
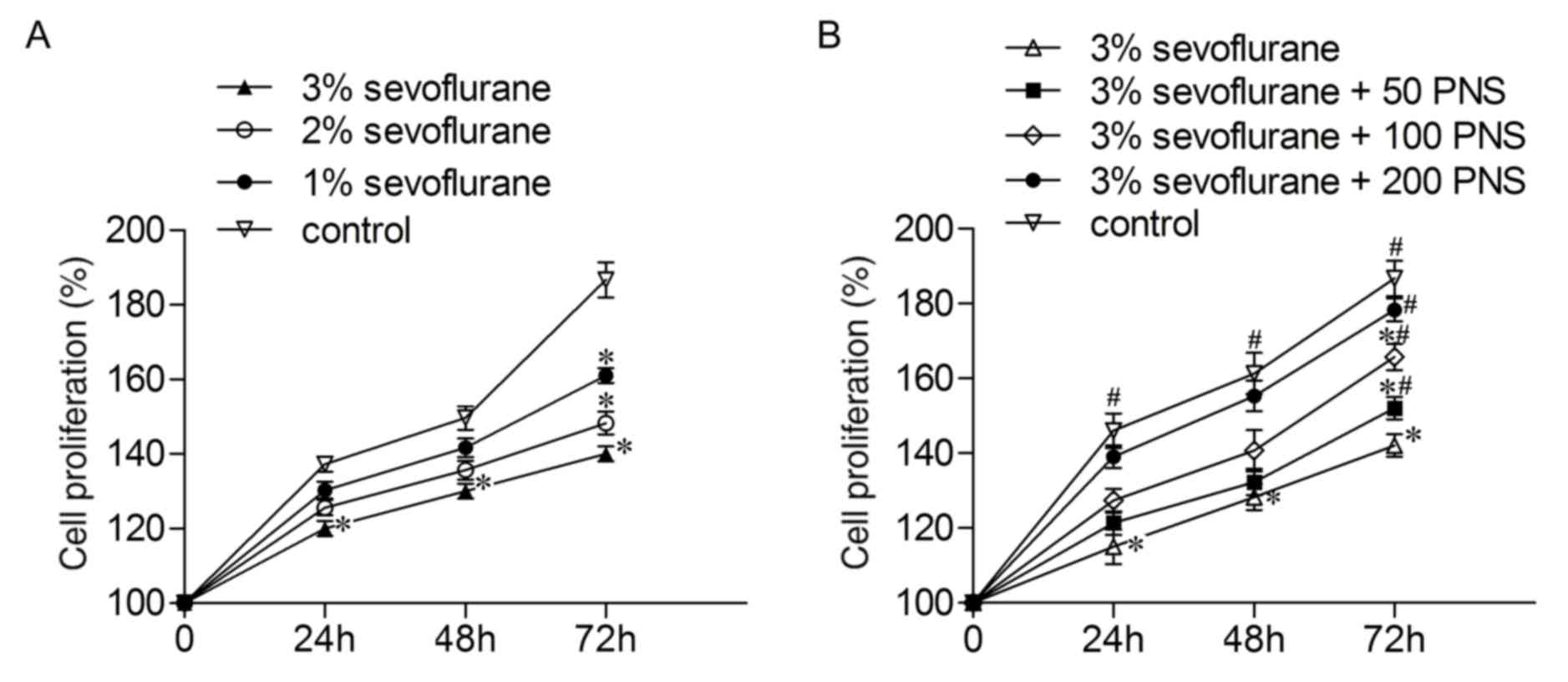 | Figure 1.PNS elevates cell proliferation in
sevoflurane-stimulated nerve cells. (A) Nerve cells were divided
into four groups. Control group, normal cells; sevoflurane groups,
cells cultured in 95% O2 and 5% CO2 with 1, 2
or 3% sevoflurane. The cell proliferation rates were measured by
MTT assay. *P<0.05 vs control group. (B) Nerve cells were
divided into five groups. Control group, normal cells; 3%
sevoflurane group, cells cultured in 95% O2, 5%
CO2 and 3% sevoflurane; 3% sevoflurane + PNS groups,
cells treated with 50, 100 or 200 µmol/l PNS for 6 h and then with
3% sevoflurane. The cell proliferation was detected by MTT assay.
*P<0.05 vs control group, #P<0.05 vs. 3%
sevoflurane group. Data are presented as the mean ± standard
deviation. PNS, Panax Notoginseng Saponins. |
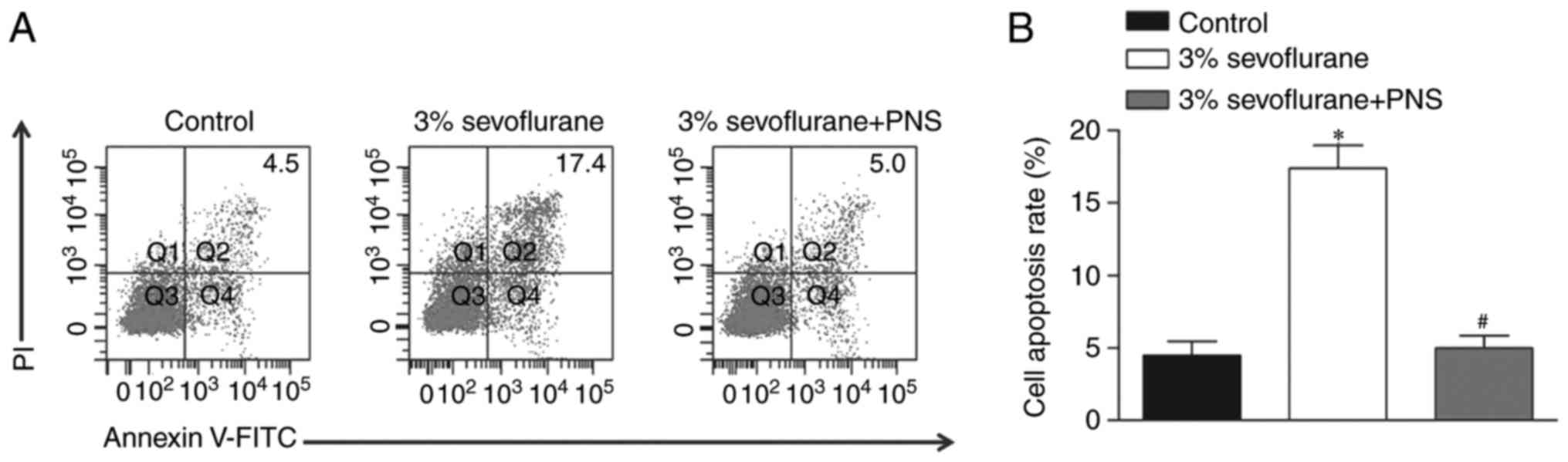
PNS protects nerve cell against
sevoflurane-induced apoptosis
To investigate the effects of PNS on
sevoflurane-stimulated nerve cells, the cell apoptosis of nerve
cells was examined using flow cytometric analysis. Following
treatment with 3% sevoflurane, the nerve cells were stimulated with
200 µM PNS. The results illustrated that the treatment with
sevoflurane induced an increase in apoptosis rate, whereas PNS
inhibited the apoptosis rate in nerve cells compared with those
treated with sevoflurane only (Fig.
2).
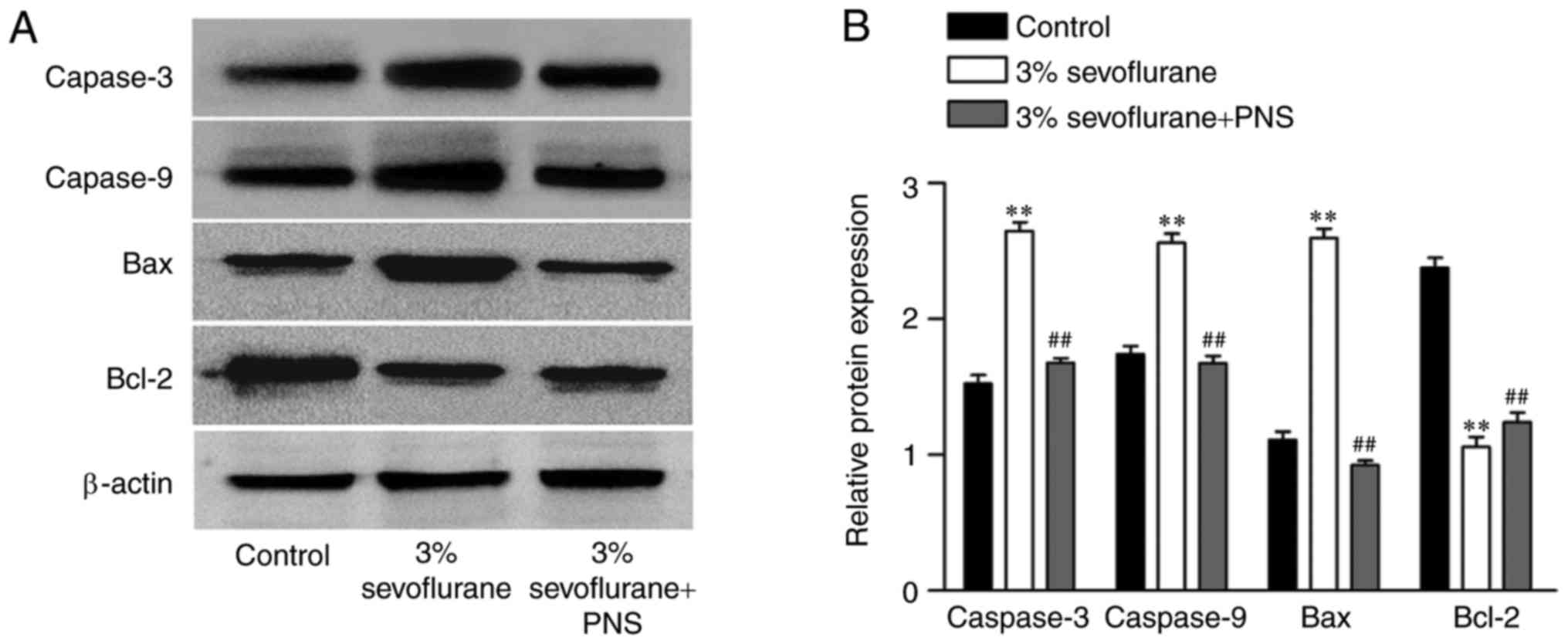
PNS regulates the expression of cell
apoptosis-associated proteins in sevoflurane-stimulated nerve
cells
To further validate the regulatory effect of PNS on
sevoflurane-induced nerve cell apoptosis, the expression level of
caspase-3, caspase-9, Bax and Bcl-2 were determined using western
blot (Fig. 3). The results
demonstrated that the expression of caspase-3, caspase-9 and Bax
were significantly increased in the sevoflurane group as compared
with control group, and decreased in sevoflurane + PNS group
compared with the sevoflurane group. Furthermore, the expression
level of Bcl-2 was decreased in the sevoflurane group compared with
control group, and elevated by PNS treatment. These results further
confirmed PNS inhibited cell apoptosis in sevoflurane-stimulated
nerve cells.
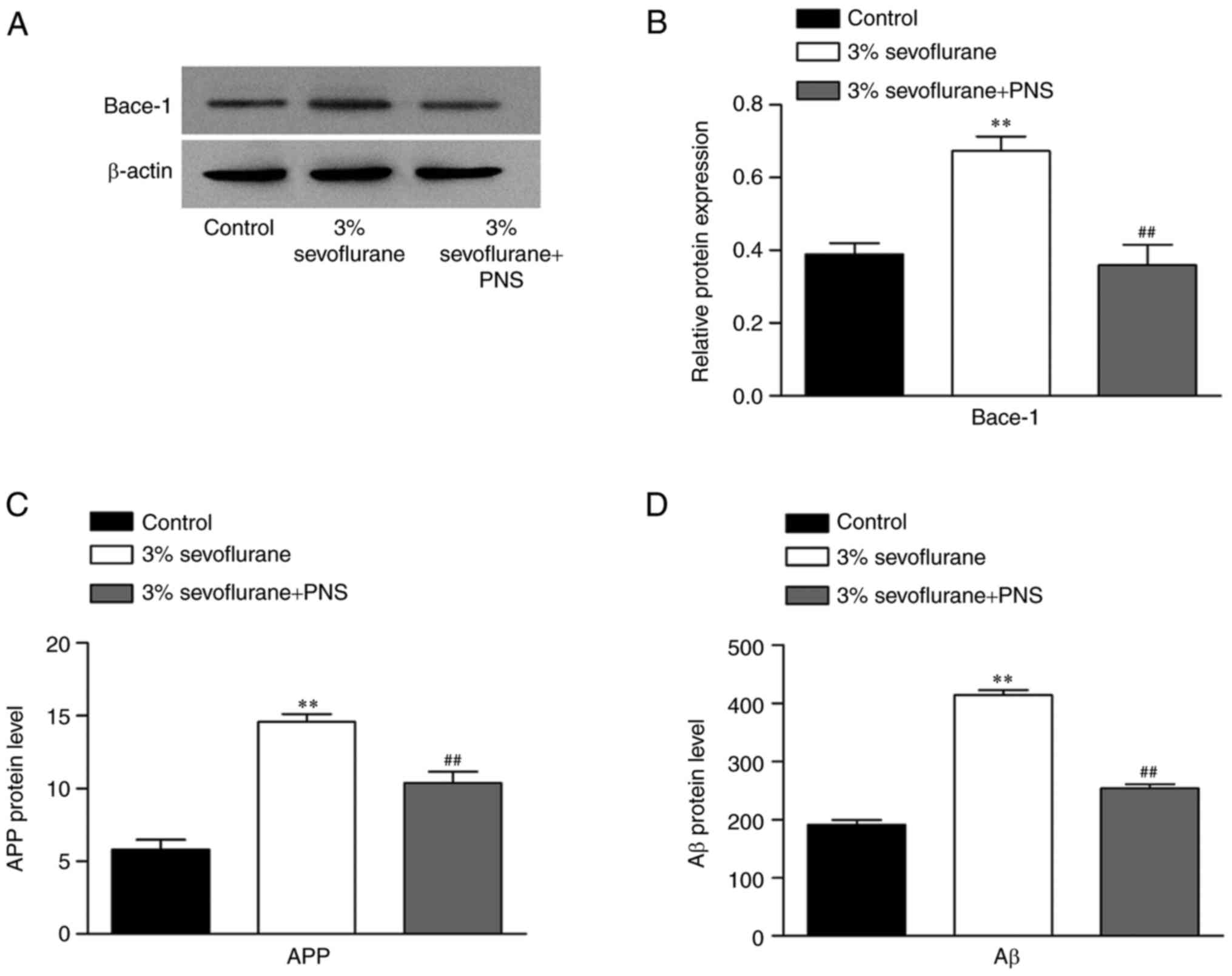
PNS suppresses the expression bace-1,
APP and Aβ in sevoflurane-stimulated nerve cells
To evaluate the effect of PNS on neurotoxicity, the
expression level of Bace-1 was detected using western blot in
sevoflurane-stimulated nerve cells. As presented in Fig. 4A and B, the expression of Bace-1
was significantly increased in the sevoflurane group as compared
with control group, and inhibited by PNS treatment compared with
the sevoflurane group. Additionally, the protein level of APP and
Aβ were measured by ELISA (Fig. 4C and
D). The results demonstrated that the protein levels of APP and
Aβ were significantly increased in the sevoflurane group, and
decreased by PNS treatment, which was consistent with the changes
in the protein expression of Bace-1.
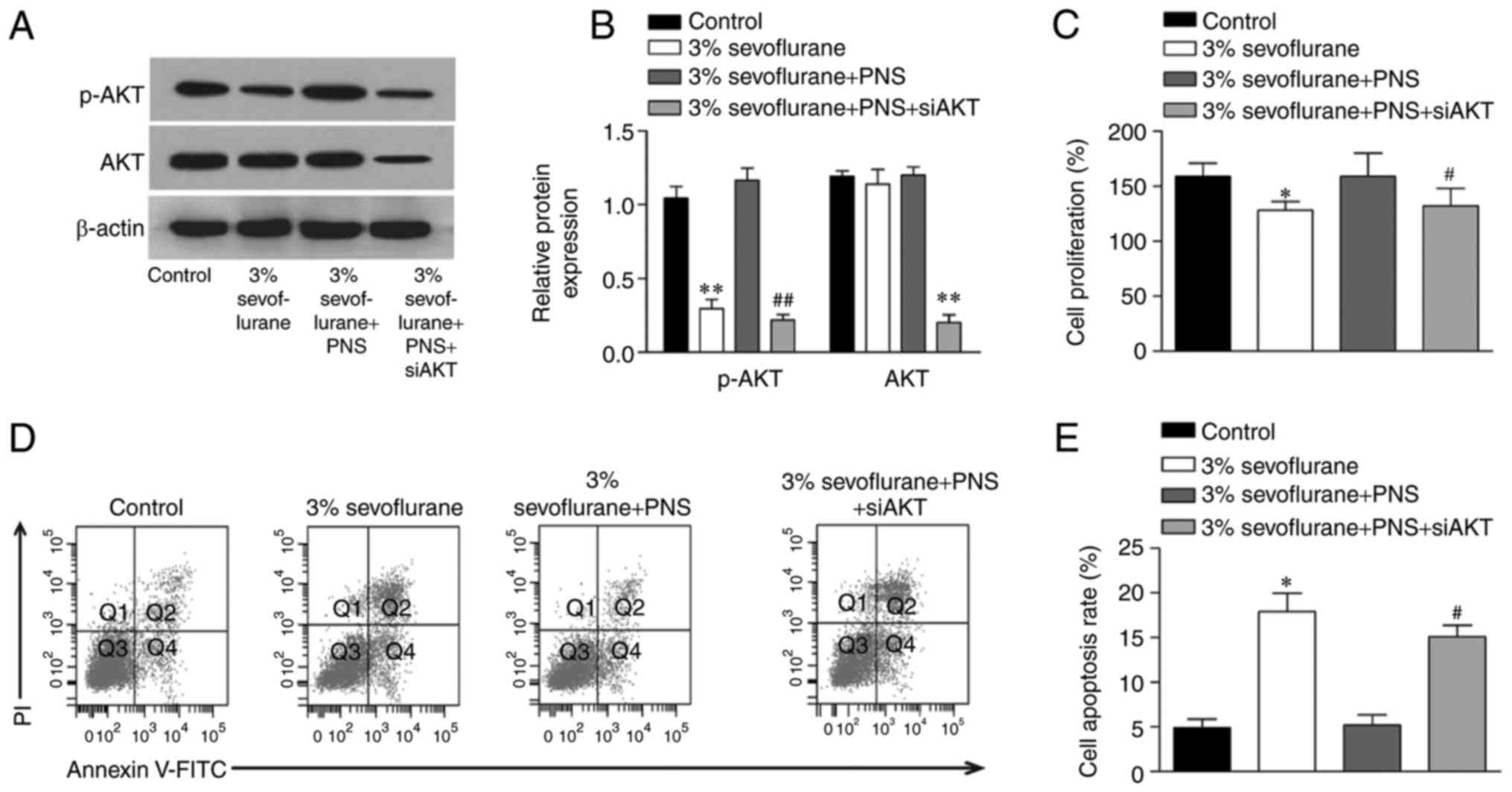
PNS elevates sevoflurane-inhibited AKT
signaling in nerve cells
In the present study, the role of AKT signaling in
sevoflurane-induced nerve cell injury and the protective effects of
PNS were examined using western blot. The results demonstrated that
the phosphorylation of AKT was significantly decreased by
sevoflurane, and the levels of total AKT showed no changes.
Additionally, the phosphorylation of AKT was restored following the
treatment with PNS. To further confirm the role of AKT, the nerve
cells were transfected with a specific siRNA targeting AKT and the
control cells were transfected with siRNA control sequences.
Moreover, the results suggested that the protective effects of PNS
were markedly diminished by AKT siRNA transfection. These results
suggested that PNS protected against sevoflurane-induced nerve cell
injury by promoting the AKT signaling pathway (Fig. 5).
Discussion
Anesthesia neurotoxicity in the developing brain has
become a major health issue of interest to the medical community
and the public (13). Sevoflurane
is a commonly used inhalation anesthetic. Previous studies have
reported that anesthesia with sevoflurane can induce neurotoxicity
in the brain tissues in adult mice, and in fetal and offspring mice
(14). PNS, extracted from
Panax notoginseng, a perennial herb of a perennial herb of
the Acanthopanax gracilistylus family, inhibits neuronal
apoptosis, inflammation and focal ischemia, therefore it may be
beneficial in the treatment of nerve injury (12). However, to the best of our
knowledge, no reports to date have investigated the effect of PNS
on sevoflurane-induced neurotoxicity. In the current study, the
neuroprotective effects of PNS against sevoflurane-induced
neurotoxicity were investigated in vitro systems. The
results demonstrated that administration of PNS protected nerve
cells against sevoflurane-induced neurotoxicity, increasing cell
proliferation and inhibiting apoptosis. Further investigating
demonstrated that PNS promoted AKT signaling in the
sevoflurane-stimulated nerve cells.
Although the underlying molecular mechanisms of
neurotoxicity are not yet fully understood, altered cell
proliferation and apoptosis have been implicated. A previous study
have demonstrated that inhalation anesthetic induces widespread
cerebral neuroapoptosis in neonatal rat pups with subsequent
long-term neurocognitive impairment of the animals (15). Another finding suggested that the
sevoflurane may induce neurotoxicity in vitro (16). A previous study reported that
anesthesia with 2.5% sevoflurane for 2 h can induce neurotoxicity
in the brain tissues of adult mice (17). In the current study, nerve cells
were treated with sevoflurane in vitro at the concentrations
of 1, 2 and 3%. The results demonstrated that the cell
proliferation was decreased by sevoflurane stimulate. Furthermore,
the neurotoxic effect was stronger at higher concentrations of
sevoflurane, and the cell growth as significantly reduced by
stimulation with 3% sevoflurane. These results were consistent with
the previous study by Satomoto et al (18). Thus, 3% sevoflurane was used to
perform subsequent experiments, and the results demonstrated that
sevoflurane significantly elevated cell apoptosis. These results
suggested that sevoflurane has a neurotoxic effect on nerve cells
in vitro by inhibiting cell proliferation and promoting cell
apoptosis.
PNS has become one of China's fastest-growing drugs
used in hospitals. Injectable preparations of Radix notoginseng,
including freeze-dried Xueshuantong and Xuesetong powders, have
been used in the clinic for maintenance treatment of acute cerebral
infarction and its complications (19). A previous study have demonstrated
the beneficial effects of PNS on central nervous system disorders
and neurodegenerative diseases, thus suggesting a neuroprotective
role of PNS (20). In the current
study, the effect of PNS on sevoflurane-stimulated nerve cells was
investigated. The results indicated that administration of PNS to
sevoflurane-induced nerve cells significantly elevated cell
proliferation. Additionally, the effect was most obvious in the 200
µM PNS group. Antioxidant, anti-inflammatory and anti-apoptotic
activities have previously been suggested as mechanisms underlying
the therapeutic effects of PNS (21). The present study demonstrated that
PNS restrained sevoflurane-induced nerve cell apoptosis, which was
consistent with previous research (21). These results suggested that PNS
treatment inhibited the neurotoxic effect of sevoflurane in nerve
cells.
It has been reported that excessive Aβ accumulation
is a major pathological hallmark of neurological disorders
(14). Aβ is produced via serial
proteolysis of the APP protein by Bace-1 enzyme. Increasing
evidence suggested that the caspase activation and apoptosis in
nerves may enhance the Bace-1 level and then facilitate APP
processing, leading to increases in Aβ levels (22). In the present study, the results
illustrated that sevoflurane increased the expression levels of Aβ,
Bace-1 and APP, while PNS treatment decreased the protein
expression levels. These results suggested that PNS treatment
inhibited sevoflurane-induced Aβ accumulation and attenuated the
progression of neurological dysfunctions.
An earlier study reported that PNS alters the
activity of PI3K/AKT signaling pathway molecules, and then
regulated the cell proliferation and apoptosis (23). In the current study, the results
indicated that nerve cells stimulated with sevoflurane had
significantly decreased phosphorylation of AKT. In addition, PNS
increased cell proliferation and inhibited cell apoptosis. However,
the protective effects of PNS were markedly diminished by a
specific siRNA targeting AKT. These results suggested that PNS
effectively protects nerve cells from sevoflurane-induced
cytotoxicity by activating the AKT signaling pathway.
In conclusion, the present study provides strong
evidence that PNS regulates neurotoxicity triggered by sevoflurane
in vitro. Experiments using cell culture revealed that PNS
acted, at least in part, by activating the AKT signaling pathway.
These findings provide a novel theory supporting current clinical
experiments aimed at assessing the beneficial effects of PNS
administration against sevoflurane-induced cytotoxicity.
Acknowledgements
The authors would like to thank members of the
Department of Anesthesiology in Cancer Hospital of Harbin Medical
University (Harbin, China) and Heilongjiang Province Hospital
(Harbin, China) for helpful discussions.
Glossary
Abbreviations
Abbreviations:
|
PNS
|
Panax Notoginseng Saponins
|
|
APP
|
amyloid precursor protein
|
|
Aβ
|
β-amyloid peptide
|
References
|
1
|
Moonesinghe SR, Mythen MG and Grocott MP:
High-risk surgery: Epidemiology and outcomes. Anesth Analg.
112:891–901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schallner N, Ulbrich F, Engelstaedter H,
Biermann J, Auwaerter V, Loop T and Goebel U: Isoflurane but not
sevoflurane or desflurane aggravates injury to neurons in vitro and
in vivo via p75NTR-NF-κB activation. Anesth Analg. 119:1429–1441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adamczyk S, Robin E, Simerabet M, Kipnis
E, Tavernier B, Vallet B, Bordet R and Lebuffe G: Sevoflurane pre-
and post-conditioning protect the brain via the mitochondrial K ATP
channel. Br J Anaesth. 104:191–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng H, Dong Y, Xu Z, Crosby G, Culley
DJ, Zhang Y and Xie Z: Sevoflurane anesthesia in pregnant mice
induces neurotoxicity in fetal and offspring mice. Anesthesiology.
118:516–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cascella M: Mechanisms underlying brain
monitoring during anesthesia: Limitations, possible improvements,
and perspectives. Korean J Anesthesiol. 69:113–120. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou YF, Wang QX, Zhou HY and Chen G:
Autophagy activation prevents sevoflurane-induced neurotoxicity in
H4 human neuroglioma cells. Acta Pharmacol Sin. 37:580–588. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Si YC, Li Q, Xie CE, Niu X, Xia XH and Yu
CY: Chinese herbs and their active ingredients for activating xue
(blood) promote the proliferation and differentiation of neural
stem cells and mesenchymal stem cells. Chin Med. 9:132014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan Y, Qiao Y, Huang J and Tang M:
Protective effects of panax notoginseng saponins against high
glucose-induced oxidative injury in rat retinal capillary
endothelial cells. Evid Based Complement Alternat Med.
2016:53263822016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang BR, Cheung KK, Zhou X, Xie RF, Cheng
PP, Wu S, Zhou ZY, Tang JY, Hoi PM, Wang YH and Lee SM:
Amelioration of acute myocardial infarction by saponins from flower
buds of Panax notoginseng via pro-angiogenesis and anti-apoptosis.
J Ethnopharmacol. 181:50–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li B, Chen D, Li W and Xiao D:
20(S)-Protopanaxadiol saponins inhibit SKOV3 cell migration. Oncol
Lett. 11:1693–1698. 2016.PubMed/NCBI
|
|
11
|
Liu L, Zhu L, Zou Y, Liu W, Zhang X, Wei
X, Hu B and Chen J: Panax notoginseng saponins promotes stroke
recovery by influencing expression of Nogo-A, NgR and p75NGF, in
vitro and in vivo. Biol Pharm Bull. 37:560–568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B and Li Y, Li XP and Li Y: Panax
notoginseng saponins improve recovery after spinal cord transection
by upregulating neurotrophic factors. Neural Regen Res.
10:1317–1320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clausen NG, Pedersen DA, Pedersen JK,
Møller SE, Grosen D, Wehby GL, Christensen K and Hansen TG: Oral
clefts and academic performance in adolescence: The impact of
anesthesia-related neurotoxicity, timing of surgery and type of
oral clefts. Cleft Palate Craniofac J. 54:371–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Zhang G, Zhang B, Moir RD, Xia W,
Marcantonio ER, Culley DJ, Crosby G, Tanzi RE and Xie Z: The common
inhalational anesthetic sevoflurane induces apoptosis and increases
beta-amyloid protein levels. Arch Neurol. 66:620–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quinn JJ, Loya F, Ma QD and Fanselow MS:
Dorsal hippocampus NMDA receptors differentially mediate trace and
contextual fear conditioning. Hippocampus. 15:665–674. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang WY, Wu XM, Jia LJ, Zhang HH, Cai F,
Mao H, Xu WC, Chen L, Zhang J and Hu SF: Beta-arrestin1 and 2
differently modulate metabotropic glutamate receptor 7 signaling in
rat developmental sevoflurane-induced neuronal apoptosis.
Neuroscience. 313:199–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoffman AN, Malena RR, Westergom BP,
Luthra P, Cheng JP, Aslam HA, Zafonte RD and Kline AE:
Environmental enrichment-mediated functional improvement after
experimental traumatic brain injury is contingent on task-specific
neurobehavioral experience. Neurosci Lett. 431:226–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
CAST, . Randomised placebo-controlled
trial of early aspirin use in 20,000 patients with acute ischaemic
stroke: CAST (Chinese Acute Stroke Trial) Collaborative Group.
Lancet. 349:1641–1649. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin DX, Zou XL, Luo W, Zhang W, Zhang HT,
Li XL, Zhang H, Wang XY and Wang TH: Expression of some
neurotrophins in the spinal motoneurons after cord hemisection in
adult rats. Neurosci Lett. 410:222–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ning N, Dang X, Bai C, Zhang C and Wang K:
Panax notoginsenoside produces neuroprotective effects in rat model
of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol.
139:504–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Dong Y, Zhang G, Moir RD, Xia W,
Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE and Xie Z: The
inhalation anesthetic desflurane induces caspase activation and
increases amyloid beta-protein levels under hypoxic conditions. J
Biol Chem. 283:11866–11875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang E, Gao B, Yang L, Wu X and Wang Z:
Notoginsenoside Ft1 promotes fibroblast proliferation via
PI3K/Akt/mTOR signaling pathway and benefits wound healing in
genetically diabetic mice. J Pharmacol Exp Ther. 356:324–332. 2016.
View Article : Google Scholar : PubMed/NCBI
|