Introduction
Acute pancreatitis (AP) is one of the most prominent
emerging diseases in the world; 15% of cases are severe AP, with an
associated mortality of ~10% (1).
Up to 20% of all mortalities induced by AP are associated with
acute lung injury, which is the predominant cause of mortality
within the first week of pancreatitis (2). Previous studies indicated that
AP-associated lung injury may be associated with systemic
inflammatory response syndrome, including activation of neutrophils
and macrophages and certain cytokines (3–5).
Furthermore, previous research has indicated that oxidative stress
resulting from an imbalance between pro-oxidants and antioxidants
also serves an important role in the pathogenesis of AP-associated
lung injury (6–8).
Danhong injection (DHI), a widely used Chinese
Medicine preparation extracted from Salvia miltiorrhiza
(Danshen in Chinese) and Carthamus tinctorius (Honghua in
Chinese), had been used extensively in the clinic to treat
cardiovascular diseases, such as coronary heart disease and
cerebral ischemia (9–11). The main components of DHI are
danshensu, protocatechuic aldehyde, savianolic acid B, rosmarinic
acid and hydroxysafflor yellow A (12–14),
and exerts anti-inflammatory, anti-oxidative and anti-fibrinolytic
properties (10,11,15–18).
In the present study, the protective effects of DHI
on AP-associated lung injury were evaluated. The effects of DHI on
lung and pancreas pathological changes, malondiadelhyde (MDA)
level, and myeloperoxidase (MPO) and superoxide dismutase (SOD)
activities were investigated. Furthermore, the influences of DHI in
the expression of nuclear factor (NF)-κB and cell adhesion
molecules in lung tissues were examined. The results demonstrated
the protective effects of DHI on AP-associated lung injury. The
mechanism may be due to the suppression of NF-κB activation and
cell adhesion molecule expression, and the reduction of neutrophil
infiltration and oxidative stress levels.
Materials and methods
Chemical and reagents
DHI was obtained from Shangdong Buchang
Pharmaceutical Co., Ltd. (Jinan, China). MPO, SOD and MDA detection
kits were purchased from Nanjing Jiancheng Bionengineering
Institute (Nanjing, China). Sodium taurocholate was from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Other reagents were
commercially available in China.
Animals and animal model
All experiments were performed according to the
protocols approved by the Animal Care Committee of Nanchang
University (Jiangxi, China). A total of 60 male Sprague-Dawley rats
(4–6 weeks, 200–220 g) were supplied by Laboratory Animal Center of
Jiangxi University of Traditional Chinese Medicine (Nanchang,
China). All rats were acclimated for 7 days prior to the
experiment, housed in standard shoebox cages in a climate
controlled environment with an ambient temperature of 23°C and a
12-h light/dark cycle, and had free access to standard laboratory
food and water. The rats were maintained under controlled
environmental conditions and fasted for 24 h with free access to
water prior to experiments. AP was induced with 3% sodium
taurocholate by retrograde injection into the pancreatic duct as
previously described (19).
Briefly, rats were anesthetized with intraperitoneal sodium
pentobarbital (Sigma-Aldrich; Merck KGaA) at a dose of 50 mg/kg.
The abdomen was opened by midline incision to allow manipulation of
the duodenum and biliopancreatic duct. The common bile duct was
occluded, and the duodenal wall was punctured on the antimesenteric
side with a 24-gauge catheter. The catheter was advanced into the
papilla vateri and fixed to the duodenal wall. For inducing AP, the
catheter was brought near the pancreatic canal and 3%
trichloroacetic acid (TCA, 0.1 ml/100 g; Sigma-Aldrich; Merck KGaA)
was infused slowly using a pump according to the retrograde ductal
injection model, followed by closure of the abdomen in two layers.
The same procedure was applied to the sham-operated group, to which
0.9% NaCl was administered instead of TCA. No mortality was
observed in the rats after AP was induced.
All animals were randomly assigned to the three
groups (n=20/group): i) Control (N), ii) AP and iii) DHI + AP (20
rats). Each group was randomly divided into two time-dependent
subgroups (A, AP group 12 h; B, DHI + AP group 12 h; C, AP group 24
h; D, DHI + AP group 24 h) after the induction of AP. In the DHI +
AP group, DHI was administered (8 ml/kg) intravenously 1 and 12 h
after inducing AP, and the other groups was subjected to the same
amount of normal saline.
Rats were sacrificed 12 or 24 h after the induction
of AP, and the blood samples were obtained via the retro-orbital
sinus using a 1.5 ml tube. After 30 min of standing, the serum was
obtained by centrifugation (1,500 × g, 15 min, 4°C) and 100 µl was
used to measure serum amylase activity. The left upper lung tissues
were dissected for determination of the wet/dry ratio immediately.
The left lower lung tissues and head of pancreas were fixed in 4%
paraformaldehyde for histopathologic analysis, and then the other
portions of lung and pancreatic tissues were removed and stored at
−70°C until use.
Determination of the wet/dry ratio of
lung
After the mice were sacrificed, the left upper lung
tissues (~1 g) were dissected, cleansed of blood with absorbent
paper, weighed to obtain the ‘wet’ weight, torrefied in an 80°C
thermostatic baking oven for 48 h, and weighed again to obtain the
‘dry’ weight. Subsequently, the ratio of wet lung to dry lung was
calculated to assess tissue edema.
Pathological analysis of lung and
pancreas
For pathological analysis, the lung tissues and
pancreas were processed by hematoxylin and eosin (HE) staining;
~4-µm thick sections were cut and then heat fixed, deparaffinized
and rehydrated through a series of xylene and graded alcohols (100,
95, 85, 75%) and merged in distilled water. Section were stained
with hematoxylin for 5 min, washed with water, and then stained
with 0.5% eosin for 1–3 min at room temperature. After a further
wash, the sections were sealed by neutral balsam. The specimens
were examined under a light microscope, and scored by two blinded
pathologists with expertise in lung and pancreatic pathology. The
score of the pancreas and lung were determined using Schmidt's
Method (20) and Tanino Method
(21), respectively.
Amylase levels, MPO activity, MDA
level and SOD activity determination of the lung and pancreas
MPO activity was used as a marker of neutrophil
infiltration. In addition, the level of MDA is an index of membrane
lipid peroxidation, and SOD activity is an index of superoxide
toxicity. Lung and pancreas tissues were frozen in liquid nitrogen
and then homogenized in PBS. The amylase levels in the tissue were
detected by an Amylase Assay kit (Abnova, Taipei, Taiwan). The MPO
activities in their homogenates were examined using a MPO
determination kit. The remaining homogenates were centrifuged at
2,000 × g for 10 min at 4°C, and the supernatants were used to
detect the level of MDA and the SOD activity by using the MDA and
SOD determination kits, respectively, according to the
manufacturer's protocol.
Reverse-transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR)
Total RNA was extracted from rat lungs according
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) (22). Total RNA was reverse
transcribed into cDNA using a ReverTraAce (Toyobo Life Science,
Osaka, Japan). The mRNA expression levels of vascular cell adhesion
protein 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1),
NF-κB p65 and β-actin were determined using a SYBR qPCR mix (Toyobo
Life Science) and the ABI PRISM 7500 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.), using the
primer sequences listed in Table
I. The PCR product was detected by 1.2% agarose gel
electrophoresis, and the amount of PCR product was estimated by a
gel imaging system (ChemiDoc XRS Image Lab™ software version 3.0,
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The intensity of
VCAM-1, ICAM-1 and NF-κB was normalized against β-actin
content.
 | Table I.Primer sequences for reverse
transcription-semi-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-semi-quantitative polymerase chain reaction.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| VCAM-1 |
TGGGAAGGTGAAGACAGAGG |
TTGGGAATAGAATCAGTTTGGT |
| ICAM-1 |
TGGGTCATAATTGTTGGTG |
CAGACCAGCAGCACTCCATC |
| NF-κB p65 |
GGCAGCACTCCTTATCAACC |
GGTGTCGTCCCATCGTAG |
| β-actin |
TCCTGTGGCATCCACGAAACT |
GAAGCATTTGCGGTGGACGAT |
Western blotting
Proteins were extracted from cells using lysis
buffer containing 50 mM Tris-HCl (pH 8.0), 50 mM KCl, 5 mM DTT, 1
mM EDTA, 0.1% SDS, 0.5% Triton X-100 and protease inhibitor
cocktail tablets (Roche Applied Science, Penzberg, Germany). The
proteins were separated by on 10% gels by SDS-PAGE. The quantity of
protein loaded onto the gels was 50 µg, and proteins were then
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were probed with antibodies
specific for VCAM-1 (cat no. 14694), ICAM-1 (cat no. 4915) and
NF-κB p65 (cat no. 8242) (Cell Signaling Technology, Inc., Danvers,
MA, USA) at a dilution of 1:1,000 and β-actin (cat no. sc-47778,
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a dilution of
1:2,000 with PBST, overnight at 4°C. Finally, the membrane was
incubated with a horseradish peroxidase conjugated secondary mouse
antibody or antibody rabbit (cat nos. sc-2314 or sc-2313, Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature.
Immunocomplexes were visualized with an enhanced chemiluminescence
system (Thermo Fisher Scientific, Inc.). ImageJ software version
3.0 (National Institutes of Health, Bethesda, MD, USA) was used to
compare the density of bands on the blots.
Statistical analysis
The data were analyzed by SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). The results are expressed as the mean ± standard
error. The statistical significance was evaluated by one-way
analysis of variance followed by Student-Newmane-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
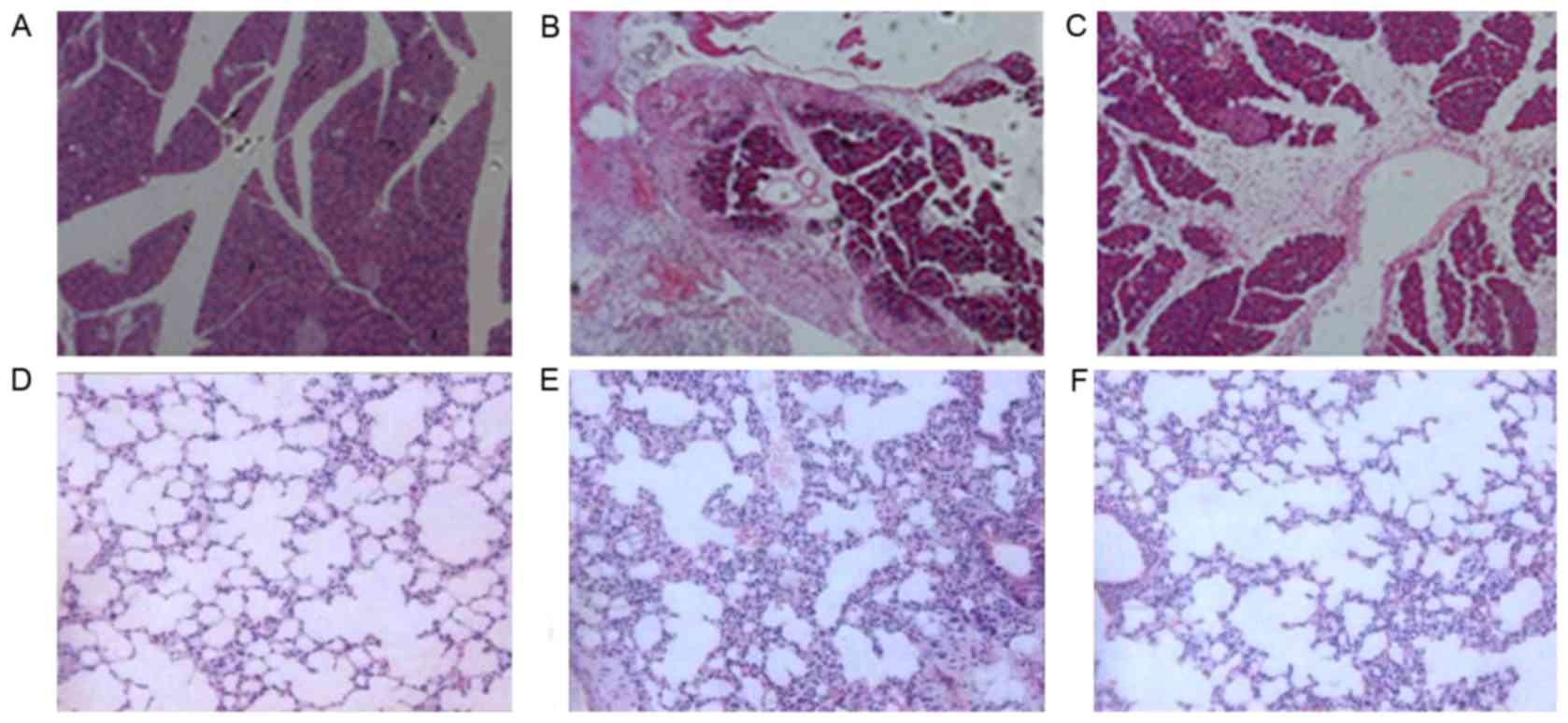
Histological examination of the
effects of DHI on pancreatic and lung injury in AP
The model of AP was induced by infusion of 5% sodium
taurocholate (1 ml/kg). Compared with control rats (Fig. 1A), AP rats pancreas had significant
morphological changes in the pancreas (Fig. 1B). Furthermore, an extensive
infiltration of leukocytes into the pancreas, beside of tissue
edema, blood vessel dilatation, and congestion vessel could be seen
in the pancreas (Fig. 1B).
Compared with the AP group, a significant reduction of acinar
necrosis, edema, and inflammatory infiltration were observed in the
DHI + AP group (Fig. 1C). No
evident histological alteration was observed in the lung of control
mice (Fig. 1D). AP resulted in
significant lung injury, evidenced by presence of interstitial
edema, alveolar thickening and extensive recruitment of neutrophils
into the alveolar spaces (Fig.
1E). These pathological changes were improved by DHI
administration (Fig. 1F). In both
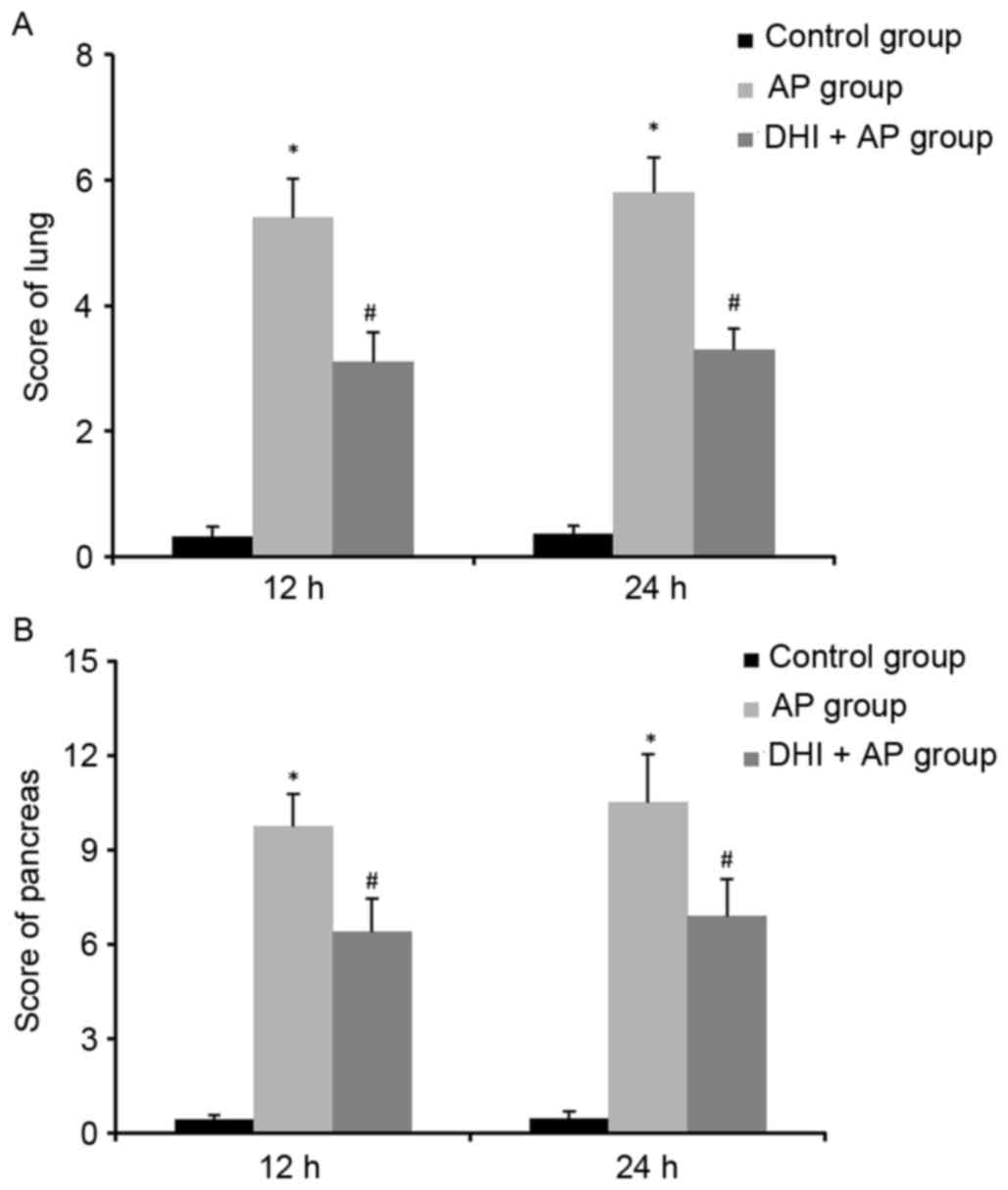
the lung (Fig. 2A) and the
pancreas (Fig. 2B), AP
significantly increased the histopathologic damage score; however,
DHI treatment reduced this score. Therefore, DHI may prevent the
pancreatic injury in AP.
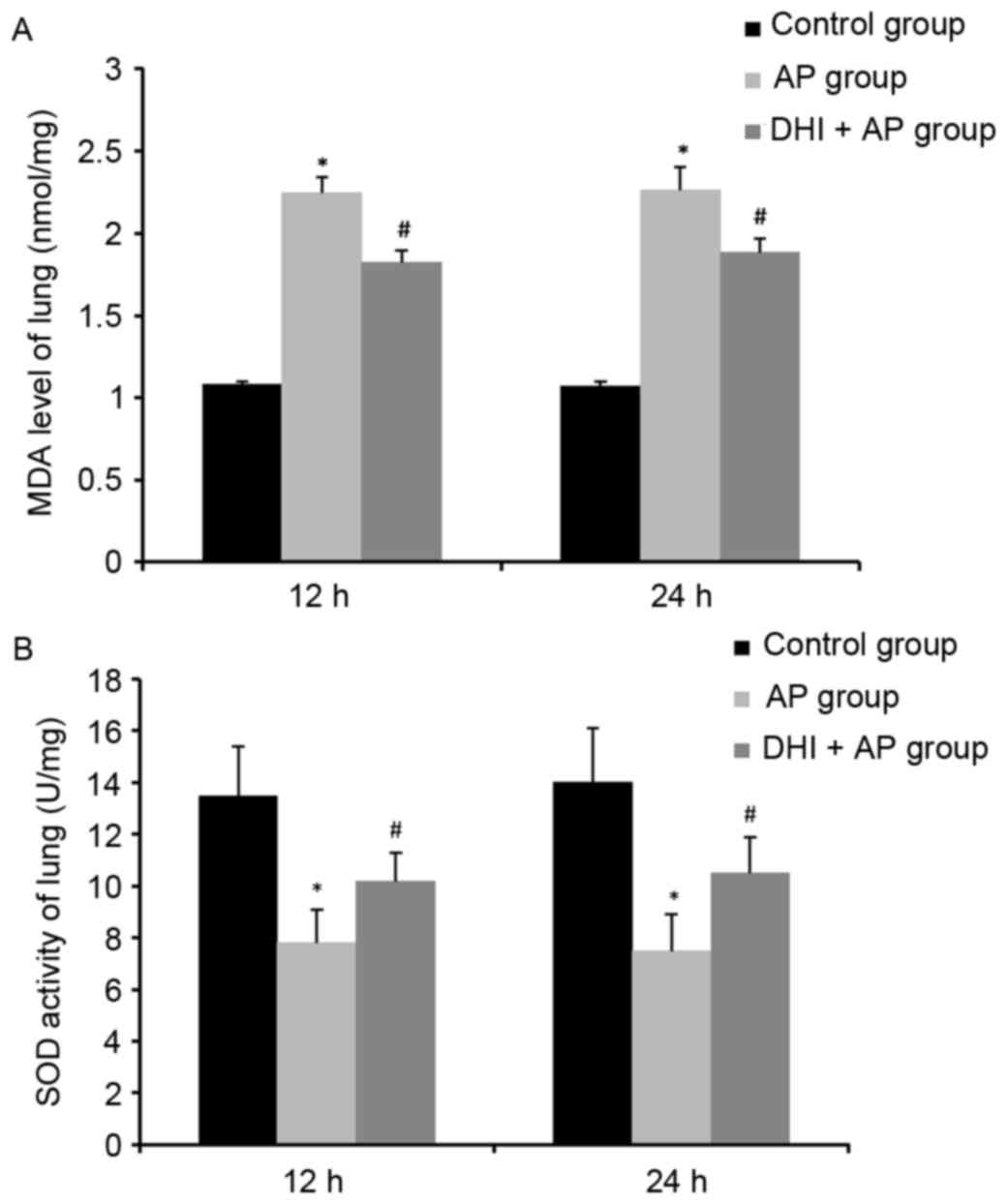
MPO activity, MDA levels and SOD
activity in the pancreas in AP following DHI treatment
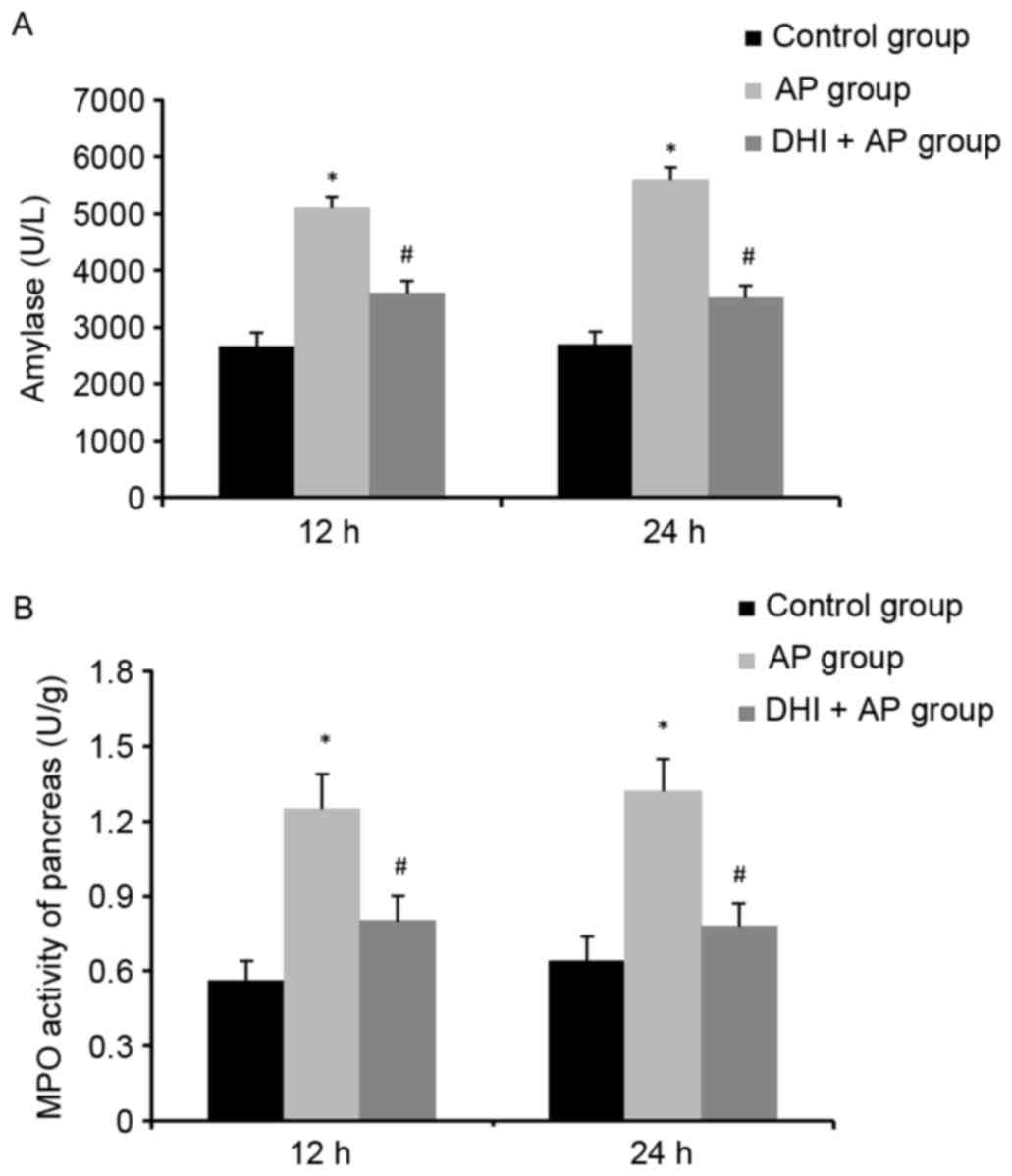
MPO activity, a marker of neutrophil function, is
used for assessing the intensity of inflammation (23). In addition to increased plasma
amylase levels (Fig. 3A), MPO
activity (Fig. 3B) and MDA levels
(Fig. 4A) were significantly
enhanced in the AP group and markedly ameliorated by DHI. However,
SOD activity was reduced by AP, but DHI significantly reversed this
effect (Fig. 4B). These results
suggested that DHI may reduce pancreatic inflammation.
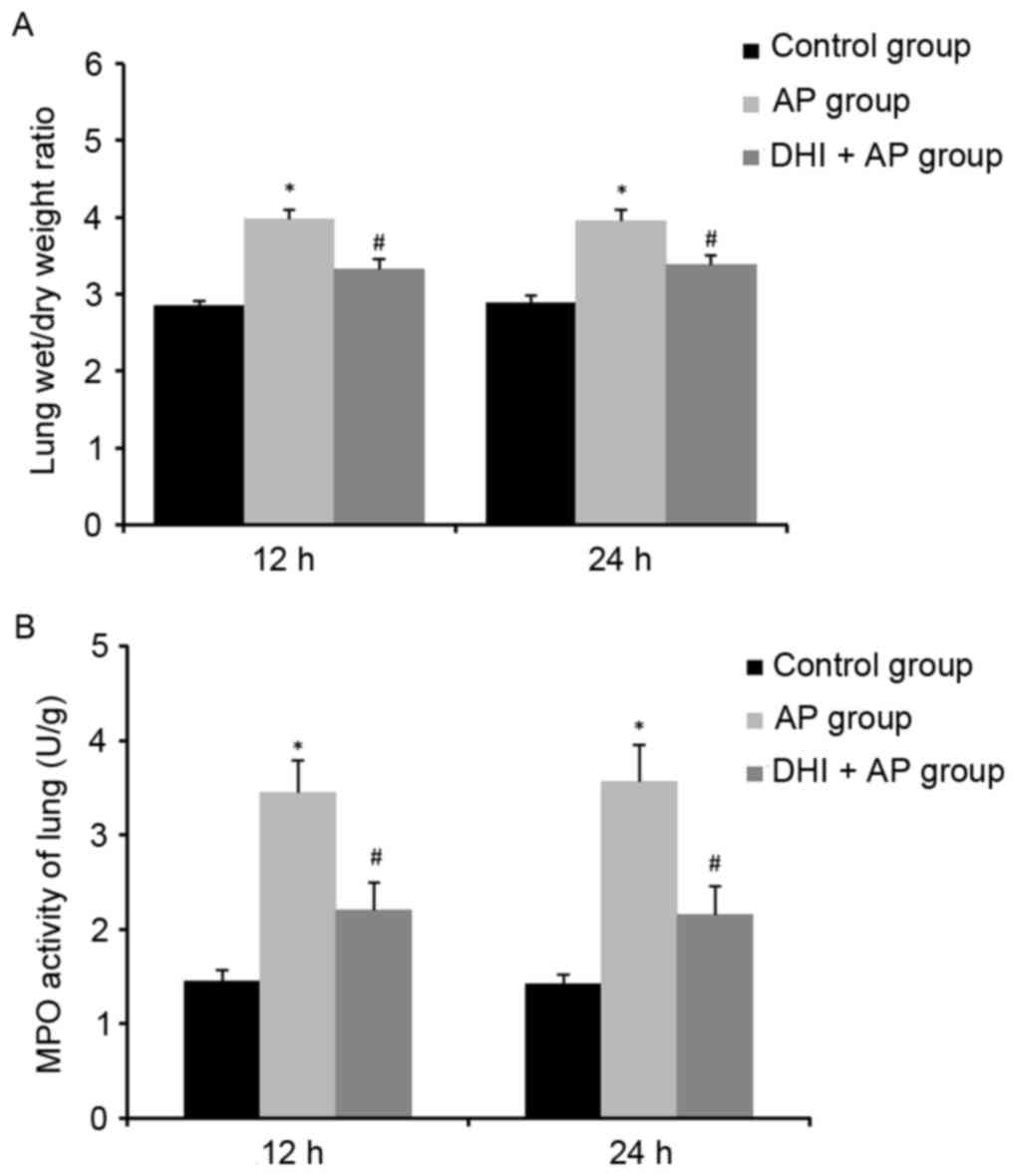
Effect of DHI on lung wet/dry ratio in
AP
Compared with the control group, the lung wet/dry
ratio in the AP group increased significantly, whereas it was
significantly attenuated in the DHI + AP group (Fig. 5A). Therefore, DHI could suppress
AP-induced lung edema.
MPO activity, MDA levels and SOD
activity in the lung in AP following DHI treatment
The role of DHI on MPO activity, MDA content and SOD
activity in lung tissues were investigated. A significant rise in
lung MPO activity (Fig. 5B) and
MDA level (Fig. 6A), and a
decrease in lung SOD activity (Fig.
6B) were observed in the AP group, indicating neutrophil
infiltration, membrane lipid peroxidation and superoxide toxicity
in lung tissue as a result of AP, respectively. Treatment with DHI
ameliorated this effect.
mRNA and protein expression level
alterations in the lung in AP following DHI treatment
In order to gain understanding into the action of
DHI on AP-associated lung injury at the molecular level, the
effects of DHI on the mRNA and protein expression levels of VCAM-1,
ICAM-1 and NF-κB p65 were assessed by RT-sqPCR and western
blotting, respectively. AP rats exhibited significantly increased
mRNA expression levels of NF-κB p65 (Fig. 7A), VCAM-1 (Fig. 7B) and ICAM-1 (Fig. 7C). The same effect was observed
from RT-sqPCR (Fig. 7D). However,
treatment with DHI significantly reversed this effect.
Representative western blot images are presented in Fig. 7E.
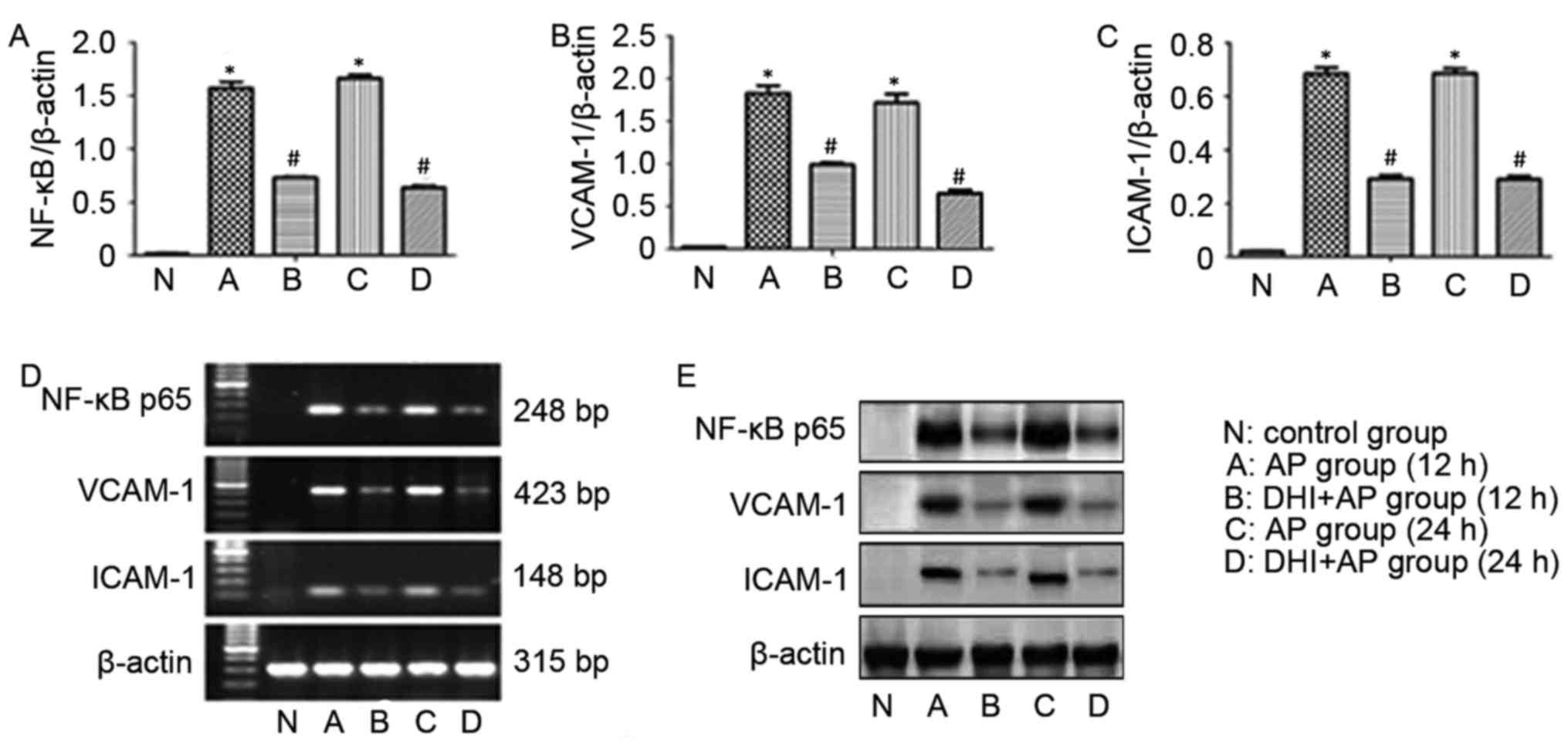 | Figure 7.Effects of DHI on expression levels of
VCAM-1, ICAM-1 and NF-κB p65 in AP rats. The mRNA expression levels
of (A) NF-κB p65, (B) VCAM-1 and (C) ICAM-1. (D) RNA and (E)
protein expression levels VCAM-1, ICAM-1, NF-κB p65. Data are
presented as the mean ± standard error (n=10/group). *P<0.01 vs.
control group, #P<0.01 vs. AP group. AP, acute
pancreatitis; DHI, Danhong injection; VCAM-1, vascular cell
adhesion protein 1; ICAM-1, intracellular adhesion molecule 1;
NF-κB, nuclear factor-κB. |
Discussion
DHI, a popular herbal medicine in China, consists of
Salvia miltiorrhiza and Carthamus tinctorius. As
described previously, DHI has served a positive role on scavenging
oxygen free radicals, preventing lipid peroxide, inhibiting
inflammatory reaction and improving microcirculation. Furthermore,
inflammatory release and the oxygen reaction serve an important
role in the pathogenesis of AP-associated multi-organ
complications. Therefore, the present study investigated the
protective effect of DHI on AP-associated lung injury.
NF-κB is as a transcription factor which regulates
various genes involved in inflammatory and immune responses
(24). Current research has
focused on the association between NF-κB and AP (25). NF-κB modulates the expression of
numerous genes, such as genes encoding for cytokines, adhesion
molecules and enzymes, which serves a pivotal role in the
initiation, promotion and progression of the inflammatory response
(26). In turn, AP could
upregulate NF-κB expression, exacerbating inflammation to AP
(27,28). In the present study, in the AP
group, NF-κB p65 was markedly increased, and the mRNA and protein
expression levels of adhesion factors (VCAM-1 and ICAM-1) were
increased. After animals were treated with DHI, the activation of
NF-κB p65 was significantly inhibited, and the expression of VCAM-1
and ICAM-1 were markedly decreased. These results demonstrated that
DHI could inhibit NF-κB p65 activation, modulate the expression of
VCAM-1 and ICAM-1, and suppress the inflammatory response in
AP.
Oxidative stress had been demonstrated to serve a
key role in causing tissue damage in AP. Abnormal generation of
reactive oxygen species occurs during the course of pancreatitis,
leading to pancreatic oxidative stress and even systemic oxidative
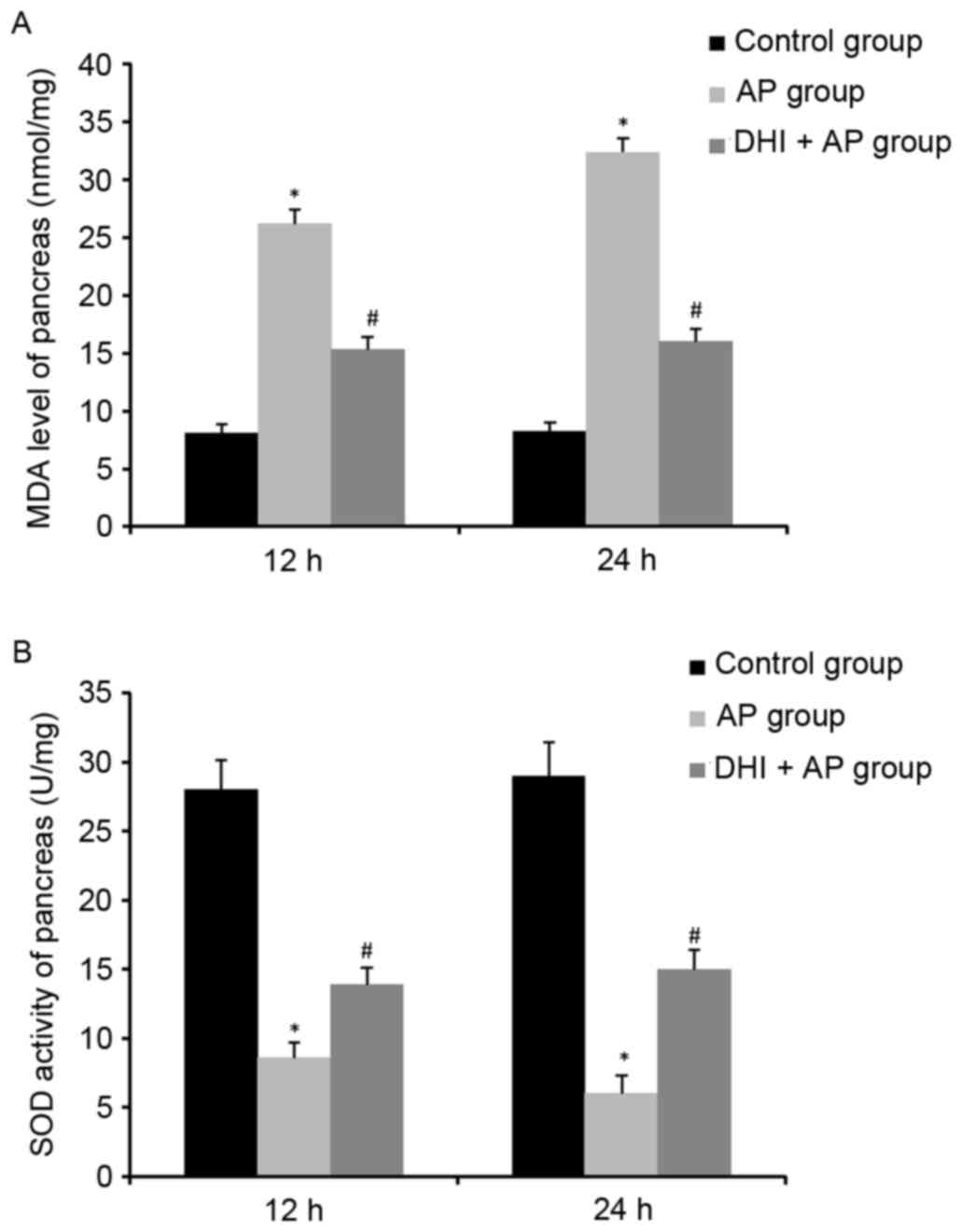
stress (29,30). Furthermore, the MDA level, an
important index of oxidative stress, could increase expression of
NF-κB (31). In the present study,
a significant rise of MDA level was observed in the AP group,
whereas this was decreased in the DHI + AP group. In addition, the
activity of SOD, an endogenous free radical scavenging agent which
can eliminate oxyradicals, was also examined. AP decreased SOD
activity in pancreas and lung tissue, while SOD activities
increased in mice treated with DHI, compared with those in the AP
group. These results indicated the DHI could effectively attenuate
oxidative stress injury in AP-associated lung injury.
In conclusion, the present study demonstrated the
protective and therapeutic effects of DHI on AP-associated lung
injury by suppressing the NF-κB activity, decreasing neutrophil
infiltration and the oxidative stress level, and reducing the
expression levels endodermis attachment proteins (VCAM-1 and
ICAM-1). Therefore, DHI may represent a potential candidate for
therapy of AP.
Acknowledgements
The present study was supported by the Program of
Science and Technology Planning of Health and Family Planning
Commission of Jiangxi Province (grant nos. 20155239 and 20161074)
and the Program of Science and Technology Planning of Nanchang
(grant no. 2012-CYH-SXHZ-YLWS-001).
References
|
1
|
Lund H, Tønnesen H, Tønnesen MH and Olsen
O: Long-term recurrence and death rates after acute pancreatitis.
Scand J Gastroentero. 41:234–238. 2006. View Article : Google Scholar
|
|
2
|
Akbarshahi H, Rosendahl AH,
Westergren-Thorsson G and Andersson R: Acute lung injury in acute
pancreatitis-awaiting the big leap. Resp Med. 106:1199–1210. 2012.
View Article : Google Scholar
|
|
3
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. J Pathol. 190:117–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norman J: The role of cytokines in the
pathogenesis of acute pancreatitis. Am J Surg. 175:76–83. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lane JS, Todd KE, Gloor B, Chandler CF,
Kau AW, Ashley SW and McFadden DW: Platelet activating factor
antagonism reduces the systemic inflammatory response in a murine
model of acute pancreatitis. J Surg Res. 99:365–370. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shabanov VV, Sarbaeva NN and Milyakova MN:
Generation of free oxygen radicals in the pathogenesis of
experimental acute reflux pancreatitis. Bull Exp Biol Med.
134:26–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eşrefoğlu M, Gül M, Ates B, Batçioğlu K
and Selimoğlu MA: Antioxidative effect of melatonin, ascorbic acid
and N-acetylcysteine on caerulein-induced pancreatitis and
associated liver injury in rats. World J Gastroentero. 12:259–264.
2006. View Article : Google Scholar
|
|
8
|
Lapidot T, Walker MD and Kanner J:
Antioxidant and prooxidant effects of phenolics on pancreatic
β-cells in vitro. J Agr Food Chem. 50:7220–7225. 2002. View Article : Google Scholar
|
|
9
|
Sun M, Zhang JJ, Shan JZ, Zhang H, Jin CY,
Xu S and Wang YL: Clinical observation of Danhong Injection (herbal
TCM product from Radix Salviae miltiorrhizae and Flos Carthami
tinctorii) in the treatment of traumatic intracranial hematoma.
Phytomedicine. 16:683–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Y, Wan H, Du Y, Bie X, Zhao T, Fu W and
Xing P: Protective effect of Danhong injection on cerebral
ischemia-reperfusion injury in rats. J Ethnopharmacol. 144:387–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao LN, Cui YL, Wang QS and Wang SX:
Amelioration of Danhong injection on the
lipopolysaccharide-stimulated systemic acute inflammatory reaction
via multi-target strategy. J Ethnopharmacol. 149:772–782. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang YM, Zhang ZJ, Guo AM, Pan LH and Li
CE: Determination of protocatechuic aldehyde in Danhong injection
by HPLC. Jie Fang Jun Yao Xue Xue Bao Bian Ji Bu. 21:467–469.
2005.(In Chinese).
|
|
13
|
Wang SM: Determination of water-solubility
component in Danhong injection by HPLC. Shi Zhen Guo Yi Guo Yao
Bian Ji Bu. 17:989–990. 2006.(In Chinese).
|
|
14
|
Liu X, Wu Z, Yang K, Ding H and Wu Y:
Quantitative analysis combined with chromatographic fingerprint for
comprehensive evaluation of Danhong injection using HPLC-DAD. J
Pharm Biomed Anal. 76:70–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan LH, Chen J, Li L, Xiong WB and Zhou
LM: Protective effects of Carthamus tinctorius injection on
isoprenaline-induced myocardial injury in rats. Pharm Biol.
49:1204–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY,
Chen JW and Chen YL: Salvianolic acid B attenuates VCAM-1 and
ICAM-1 expression in TNF-alpha-treated human aortic endothelial
cells. J Cell Biochem. 82:512–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen ZQ, Hong L and Wang H: Effect of
Danhong injection on platelet activation and inflammatory factors
in patients of acute coronary syndrome after intervention therapy.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 29:692–694. 2009.(In Chinese).
PubMed/NCBI
|
|
18
|
Wang L, Zhang X, Liu L, Cui L, Yang R, Li
M and Du W: Tanshinone II A down-regulates HMGB1, RAGE, TLR4,
NF-κappaB expression, ameliorates BBB permeability and endothelial
cell function and protects rat brains against focal ischemia. Brain
Res. 1321:143–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perides G, van Acker GJ, Laukkarinen JM
and Steer ML: Experimental acute biliary pancreatitis induced by
retrograde infusion of bile acids into the mouse pancreatic duct.
Nat Protoc. 5:335–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanino Y, Makita H, Miyamoto K, Betsuyaku
T, Ohtsuka Y, Nishihira J and Nishimura M: Role of macrophage
migration inhibitory factor in bleomycin-induced lung injury and
fibrosis in mice. Am J Physiol Lung Cell Mol Physiol.
283:L156–L162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simms D, Cizdziel PE and Chomczynski P:
TRIzol: A new reagent for optimal single-step isolation of RNA.
Focus. 15:532–535. 1993.
|
|
23
|
Yoshida M and Yamada M, Sudo Y, Kojima T,
Tomiyasu T, Yoshikawa N, Oda T and Yamada M: Myeloperoxidase
anti-neutrophil cytoplasmic antibody affinity is associated with
the formation of neutrophil extracellular traps in the kidney and
vasculitis activity in myeloperoxidase anti-neutrophil cytoplasmic
antibody-associated microscopic polyangiitis. Nephrology (Carlton).
21:624–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Medzhitov R and Horng T: Transcriptional
control of the inflammatory response. Nat Rev Immunol. 9:692–703.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pooran N, Indaram A, Singh P and Bank S:
Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of
severe acute pancreatitis. J Clin Gastroenterol. 37:263–266. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boone DL, Lee EG, Libby S, Gibson PJ,
Chien M, Chan F, Madonia M, Burkett PR and Ma A: Recent advances in
understanding NF-κappaB regulation. Inflamm Bowel Dis. 8:201–212.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi C, Zhao X, Lagergren A, Sigvardsson M,
Wang X and Andersson R: Immune status and inflammatory response
differ locally and systemically in severe acute pancreatitis. Scand
J Gastroenterol. 41:472–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng Y, Ma QY, Kou XP and Xu J: Effect of
resveratrol on activation of nuclear factor kappa-B and
inflammatory factors in rat model of acute pancreatitis. World J
Gastroenterol. 11:525–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leung PS and Chan YC: Role of oxidative
stress in pancreatic inflammation. Antioxid Redox Signal.
11:135–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu JH, Lim JW, Kim KH, Morio T and Kim H:
NADPH oxidase and apoptosis in cerulein-stimulated pancreatic
acinar AR42J cells. Free Radical Bio Med. 39:590–602. 2005.
View Article : Google Scholar
|
|
31
|
Seo JY, Kim H, Seo JT and Kim KH:
Oxidative stress induced cytokine production in isolated rat
pancreatic acinar cells: Effects of small-molecule antioxidants.
Pharmacology. 64:63–70. 2002. View Article : Google Scholar : PubMed/NCBI
|