Introduction
Propofol (2,6-diisopropylphenol) belongs to phenol
derivatives and its biosynthesis dates back to the 1970s (1). Propofol is one of the most commonly
used intravenous anesthetics at present (2–4).
However, several reports have shown that anesthetics, including
propofol, can inhibit the stress response during surgery and exert
adverse effects on the immune system (5–7).
Toll-like receptors (TLRs) are a type of innate immune receptor,
which are widely distributed in mononuclear cells,
polymorphonuclear cells, macrophages, lymphocytes, dendritic cells
and natural killer cells (8,9).
TLRs are vital in the cell immune defense process (9,10).
Firstly, TLR identifies and combines the specific highly conserved
sequence of several pathogens or pathogenic products. A series of
cell signal transduction pathways are induced and inflammatory
mediators are released, and the adaptive immune system is activated
(11–14). TLR4 was found to be a major
receptor mediating the course of the lipopolysaccharide
(LPS)-induced immune response (15–17).
The identification and development of drugs, which inhibit the TLR4
signaling pathway, has been a focus of investigations.
MicroRNAs (miRNAs) are a class of small, non-coding
RNAs (~20–25 nucleotides in length), which regulate gene expression
post-transcriptionally (18,19).
MiRNAs are involved in several aspects of growth and development,
depending on their target genes (20–22).
The present study aimed to investigate whether miRNAs are involved
in the inflammatory response induced by propofol.
Materials and methods
Cell culture and transfection
The human umbilical vein endothelial cell (HUVEC)
line was purchased from American Type Culture Collection (Manassas,
VA, USA). The cells were cultured in DMEM (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, CA, USA) containing 10% fetal
calf serum (Invitrogen; Thermo Fisher Scientific, Inc.). The cells
were grown on sterilized culture dishes (37°C, 5% CO2)
and passaged every 48 h with 0.25% trypsin (Invitrogen; Thermo
Fisher Scientific, Inc.). A mimic negative control, miR-21 mimic,
inhibitor negative control, and miR-221 inhibitor were purchased
from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The miR-221
mimic and inhibitor were transfected into HUVECs using Dharmafect1
Transfection Reagent (GE Healthcare Dharmacon, Inc., Lafayette, CO,
USA). LPS was dissolved in DMSO and added into the culture medium
at a final concentration of 100 µg/ml for 12 h. Propofol was added
to the medium to a concentration of 25, 50 and 100 µM for 24 h at
37°C.
Western blot analysis
Total protein was extracted using lysis buffer
purchased from Pierce; Thermo Fisher Scientific, Inc. Total
proteins were quantified according to the Bradford method,
following which 30 µg samples were separated using 10% SDS-PAGE.
The proteins were transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA) and incubated
overnight at 4°C with antibodies against TLR4 (1:800; cat. no.
ab22048; Abcam, Cambridge, MA, USA), CD14 (1:800; cat. no.
ab182032; Abcam), TNFα (1:2,000; cat. no. ab6671; Abcam) and GAPDH
(1:2,000; cat. no. 60004-1-lg; ProteinTech Group, Inc., Chicago,
IL, USA). The membranes were then incubated with peroxidase-coupled
anti-mouse (cat. no. 5127)/rabbit (cat. no. 58802) IgG (1:2,000;
Cell Signaling Technology, Inc., Boston, MA, USA) at 37°C for 2 h.
Finally, the proteins were visualized using
electrochemiluminescence (Pierce; Thermo Fisher Scientific, Inc.)
and detected using a DNR Bio-Imaging system (DNR Bio-Imaging
Systems, Ltd., Jerusalem, Israel). ImageJ version 1.48 u software
(National Institutes of Health, Bethesda, MA, USA) was used to
quantify the relative protein levels.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of miR-21 using the
SYBR-Green method
Total RNA was extracted from cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The total RNA was then quantified using a
Multiskan FC microplate reader (Thermo Fisher Scientific, Inc.).
The quantification of miRNA from the extracted RNA was performed
according to the SYBR-Green method. The qPCR reaction volume was 20
µl and consisted of the following: cDNA (0.01 µg/µl), 5 µl; forward
primer (5 µM), 1µl; reverse primer (5 µM), 1 µl; H2O, 3
µl; SYBR-Green Master mix, 10 µl (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling steps were as follows: 95°C
for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30
sec. Primers for miR-21 (Bulge-Loop™ miRNA qRT-PCR
primer set for has-mir-2) and U6 (snRNA qRT-PCR primer set;
Guangzhou RiboBio Co., Ltd.) were used for qPCR analysis using an
ABI 7900HT Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sequences were as follows:
Has-miR-21-5p forward primer, GGC GGT AGC TTA TCA GAC TGA TG and
reverse primer, GTG CAG GGT CCG AGG TAT TC; U6 forward primer, CTC
GCT TCG GCA GCA CA and reverse primer, AAC GCT TCA CGA ATT TGC GT.
Experiments were performed in triplicate. The relative levels of
gene expression were determined as: ΔCq = Cq gene - Cq reference.
The fold change of gene expression was calculated using the
2−ΔΔCq method (23).
RT-qPCR of target genes using the SYBR
Green method
RT-qPCR analysis was performed using SYBR Green PCR
master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on
the 7900HT Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The total volume of the PCR reaction
system was 20 µl and consisted of the following: cDNA (0.02 µg/µl),
5 µl; forward primer (10 µM), 0.5 µl; reverse primer (10 µM), 0.5
µl; H2O, 4 µl; SYBR-Green Master mix, 10 µl (Applied
Biosystems, Thermo Fisher Scientific, Inc.) and the reaction
process was as follows: 95°C for 30 sec, 40 cycles of 95°C for 5
sec, 60°C for 30 sec. A dissociation step was performed to generate
a melting curve to confirm the specificity of the amplification.
β-actin was used as the reference gene. Each PCR analysis was
performed in triplicate. The relative levels of gene expression
were determined as: ΔCq = Cq gene - Cq reference. The fold change
of gene expression was calculated using the 2−ΔΔCq
method (23). The primer sequences
were as follows: TLR4 forward, 5′-CGAATGGAATGTGCAACACCT-3′ and
reverse, 5′-ACAAGCACACTGAGGACCGAC-3′; TNFα forward,
5′-CCACGCTCTTCTGCCTGCT-3′ and reverse,
5′-GCCAGAGGGCTGATTAGAGAGA-3′; β-actin forward,
5′-GATAGCACAGCCTGGATAGCAAC-3′ and reverse,
5′-CCTGAACCCCAAGGCCAAC-3′.
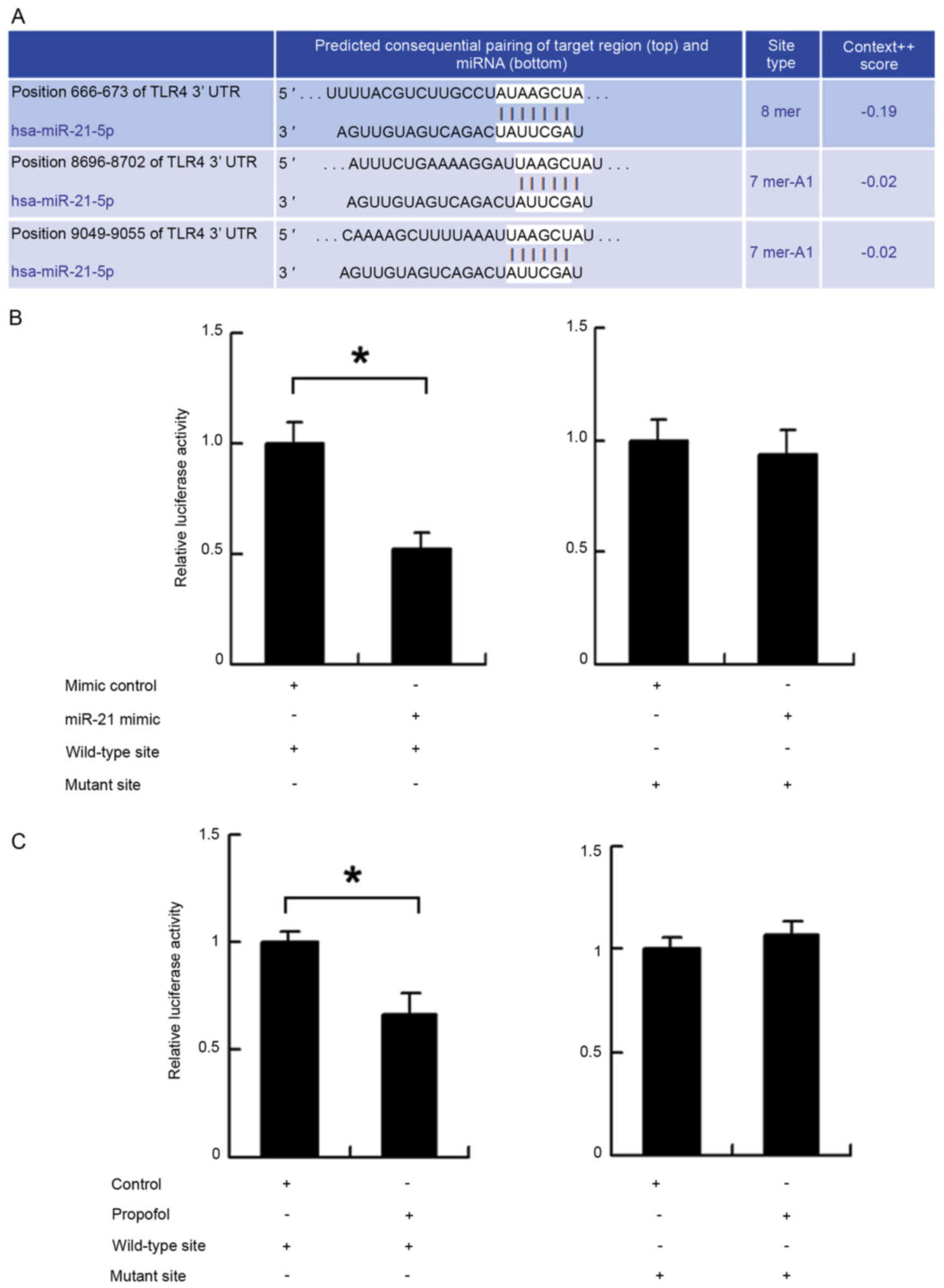
Confirmation of the interaction
between miR-21 and target genes using luciferase reporter
assays
A pmiR-Reporter vector, which was obtained from
Addgene (Cambridge, MA, USA), was used for the reporter assays to
detect interactions between miR-21 and TLR4. The wild-type miR-21
target site in the TLR4 3′-untranslated region (3′-UTR) was
AUAAGCUA, which was predicted using TargetScan (http://targetscan.org/). The mutant miR-21 target site
was AUGGGGUA. Luciferase activity was examined using the luciferase
reporter gene assay kit from Promega Corporation (Madison, WI,
USA).
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used
for all statistical analyses. Student's t-test was performed to
compare all data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Propofol downregulates the LPS-induced
expression of TLR4 in HUVECs
The expression levels of TLR4 in HUVECs treated with
LPS and propofol were detected using western blot and RT-qPCR
analyses. The results indicated that (Fig. 1) LPS upregulated the protein
expression levels of TLR4 and CD14 in the HUVECs. Propofol
inhibited the protein expression levels of TLR4 and CD14 in a
concentration-dependent manner at propofol concentrations of 25, 50
and 100 µM. The results of the RT-qPCR analysis showed the same
trend (Fig. 1B). LPS upregulated
the mRNA level of TLR4 in the HUVECs, and propofol suppressed the
mRNA level of TLR4 in a concentration-dependent manner in the
HUVECs (P<0.05 in control, vs. LPS; P<0.05 in LPS, vs. LPS+25
µM propofol). In addition, LPS treatment significantly upregulated
the mRNA and protein levels of TNFα, whereas propofol treatment
downregulated its expression (Fig. 1A
and B).
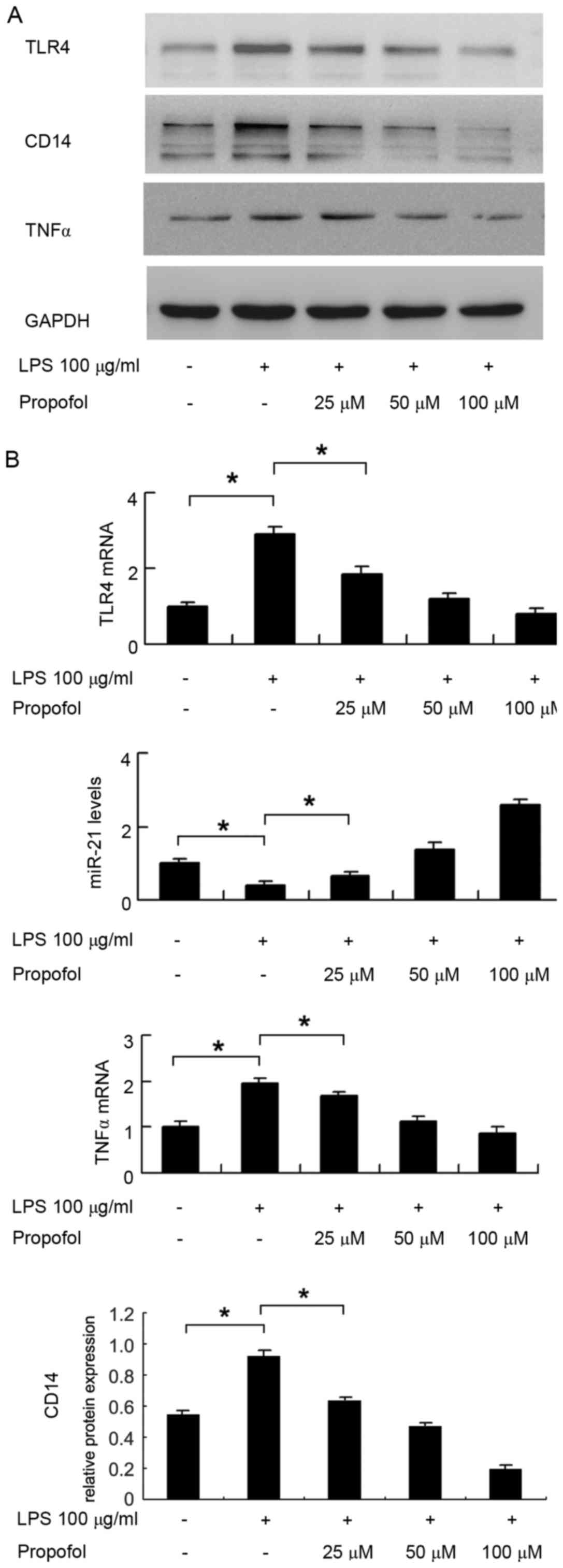 | Figure 1.Expression pattern of TLR4, CD14 and
miR-21 in HUVECs treated with LPS and propofol. (A) Western blots
show the expression levels of TLR4, CD14 and TNFα were upregulated
in HUVECs treated with LPS. Propofol inhibited the expression
levels of TLR4, CD14 and TNFα in HUVECs in a
concentration-dependent manner at propofol concentrations of 25, 50
and 100 µM. (B) Reverse transcription-quantitative polymerase chain
reaction analysis showed that LPS upregulated the expression levels
of TLR4 and TNFα, and propofol inhibited the expression of TLR4 and
TNFα in HUVECs, in a concentration-dependent manner. LPS
downregulated the expression of miR-21, whereas propofol
upregulated the expression of miR-21 in HUVECs in a
concentration-dependent manner (LPS, 100 µg/ml; propofol, 25, 50
and 100 µM). *P<0.05. HUVECs, human umbilical vein endothelial
cells; TLR4, Toll-like receptor 4; CD14, cluster of differentiation
14; TNFα, tumor necrosis factor α; LPS, lipopolysaccharide; miR,
microRNA. |
Propofol upregulates miR-21 in
HUVECs
The present study analyzed changes in the expression
of miR-21 in HUVECs using RT-qPCR analysis. As shown in Fig. 1B, LPS treatment decreased the
expression of miR-21 (P<0.05, control vs. LPS), whereas propofol
treatment increased the expression of miR-21 in a
concentration-dependent manner in the HUVECs (P<0.05 in LPS, vs.
LPS+25 µM propofol).
miR-21 downregulates TLR4 in
HUVECs
The present study examined the link between miR-21
and TLR4 in HUVECs, and found that there were binding sites between
miR-21 and the 3′-UTR of TLR4. Therefore, TLR4 may be a target gene
of miR-21 in HUVECs. In order to verify whether miR-21 targeted
TLR4 in HUVECs, the miR-21 mimic and miR-21 inhibitor were
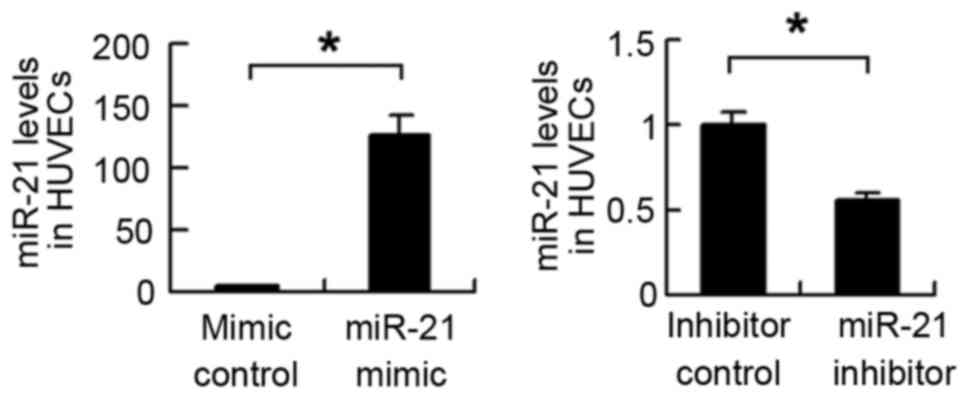
transfected into HUVECs, and the transfection efficiency was
confirmed using RT-qPCR analysis (Fig.
2). The expression of TLR4 was then examined. As shown in
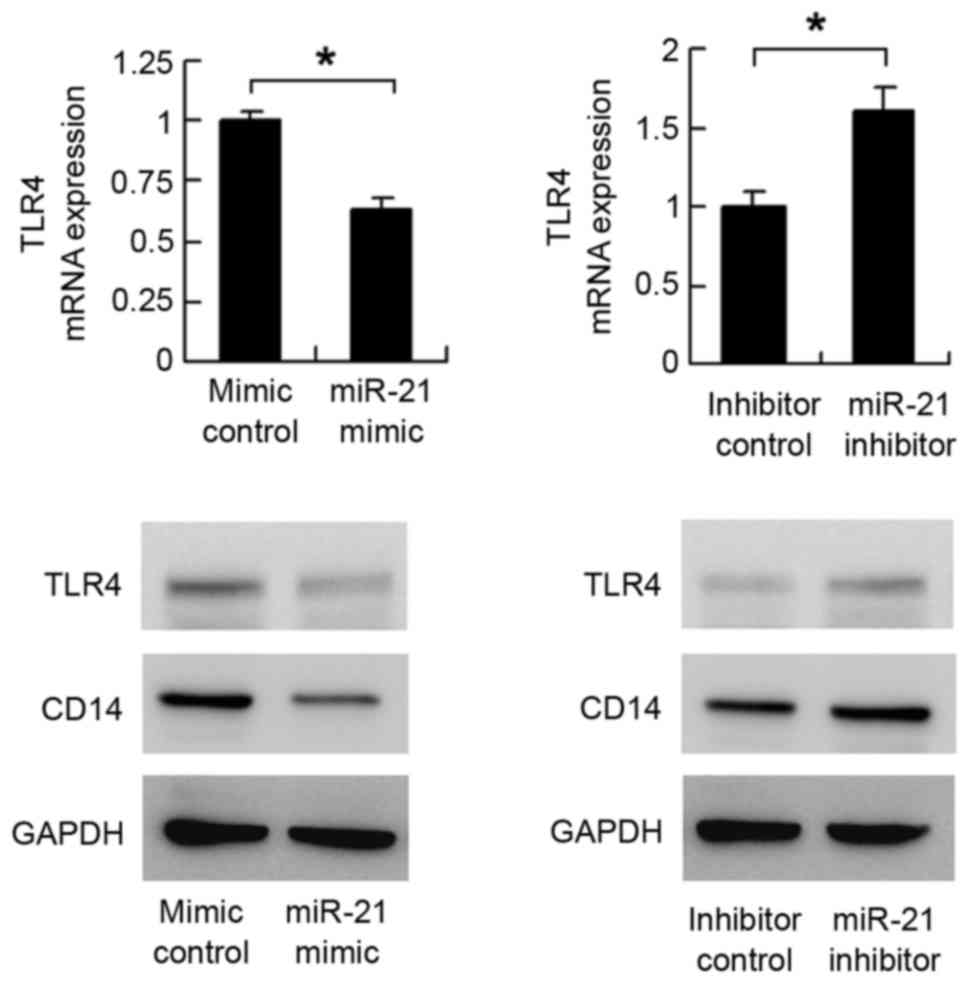
Fig. 3, the mRNA level of
TLR4decreased following miR-21 mimic transfection. The mRNA
expression of TLR4 increased in the miR-21 inhibitor-transfected
cells. The results of the western blot analysis showed that miR-21
mimic suppressed the protein expression of TLR4, whereas
transfection with the miR-21 inhibitor upregulated the protein
expression of TLR4. These results indicated that miR-21 regulated
TLR4 at the mRNA and protein levels.
Propofol regulates TLR4 through
miR-21
The above results indicated that propofol regulated
the expression of TLR4 through miR-21 in the HUVECs. Subsequently,
the present study examined whether the LPS-induced upregulation of
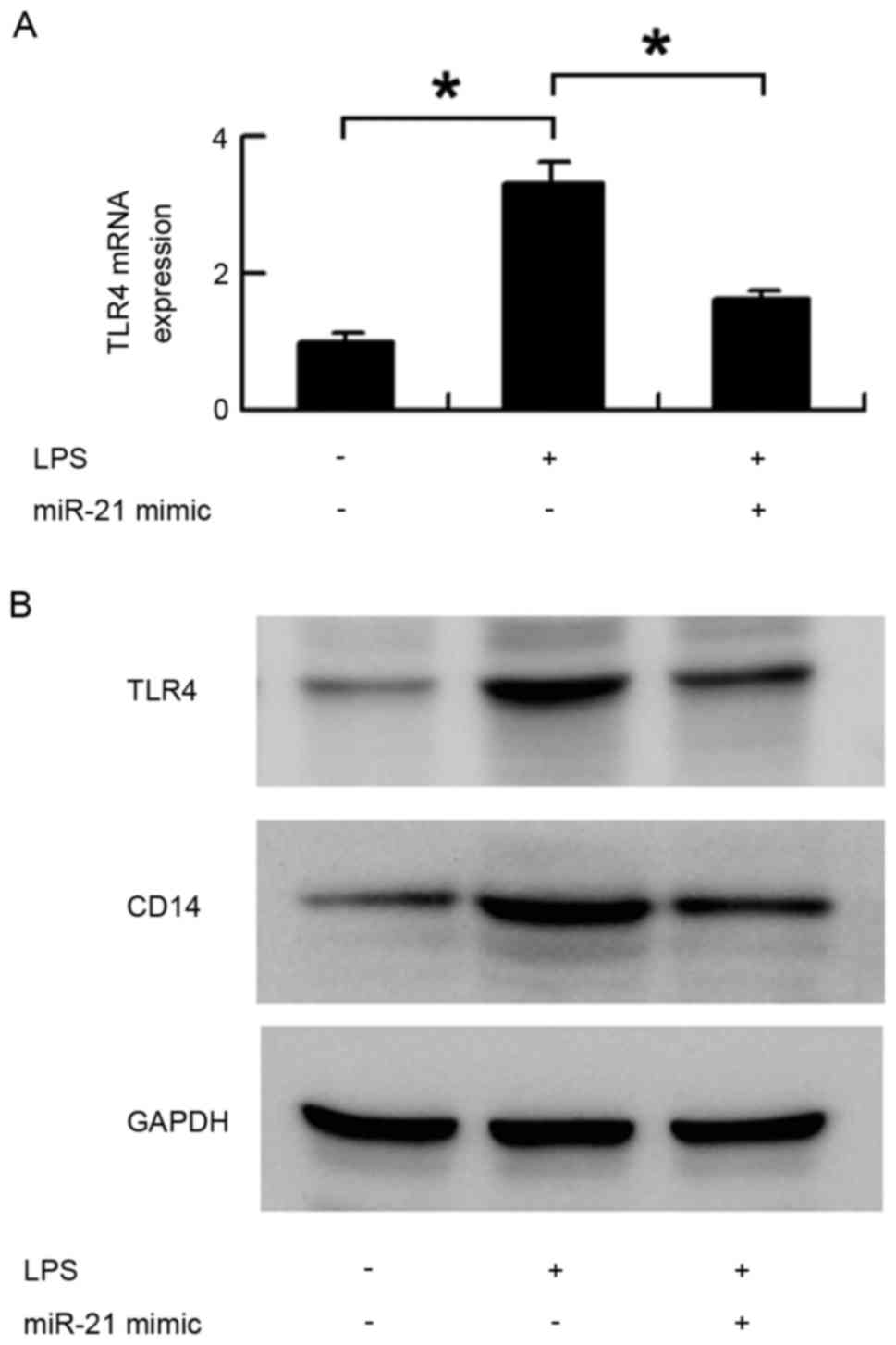
TLR4 and CD14 were reversed by the miR-21 mimic. As shown in
Fig. 4, miR-21 significantly
inhibited the protein and mRNA expression of TLR4, which were
upregulated by LPS treatment (Fig. 4A
and B). miR-21 also downregulated the protein expression of
CD14 induced by LPS (Fig. 4B).
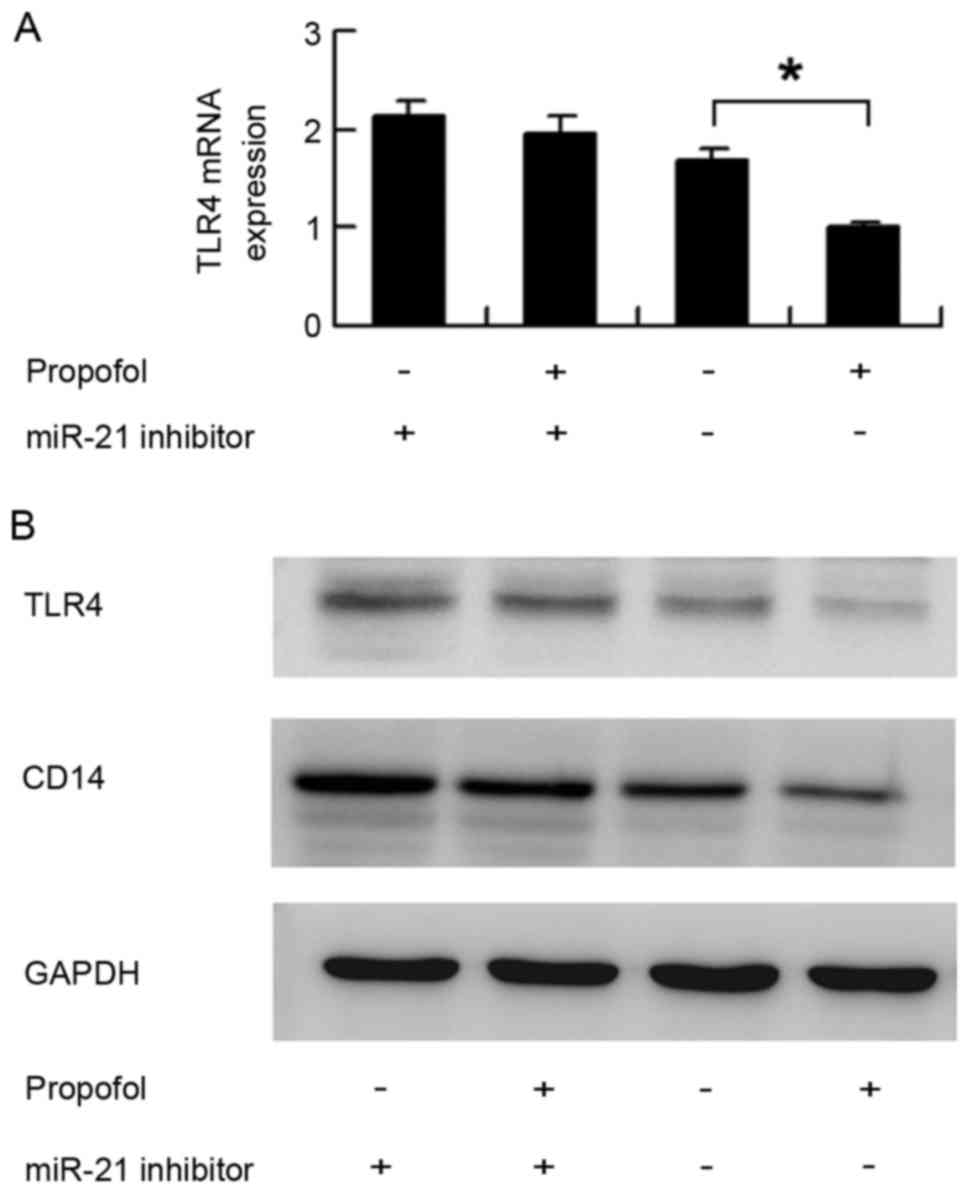
To confirm the role of miR-21 during
propofol-induced downregulation of TLR4, the miR-21 inhibitor was
transfected into HUVECs and the cells were treated with propofol.
The expression of TLR4 was determined using western blot and
RT-qPCR analyses. As shown in Fig. 5A
and B, in the cells transfected with the miRNA inhibitor
control, propofol significantly downregulated the mRNA and protein
expression levels of TLR4. In cells transfected with the miR-21
inhibitor, propofol had no significant effect on TLR4. Similar to
TLR4, the miR-21 inhibitor eliminated the downregulation of CD14
induced by propofol 4 (Fig. 5B).
These results showed that propofol regulated the expression of
TLR4/CD14 though miR-21.
TLR4 is a direct target of miR-21. To further
determine whether TLR4 was a direct target of miR-21, fluorescent
reporter assays were performed. The 3′-UTR of TLR4, containing
wild-type (AUAAGCUA) or mutant (AUGGGGUA) binding sites for miR-21,
was cloned into a reporter vector (Fig. 6A). The ratio of fluorescence
intensity for the wild-type and mutant binding sites was then
calculated. As shown in Fig. 6B,
the miR-21 mimic reduced the fluorescence intensity in cells
transfected with the vector containing the wild-type TLR4 3′-UTR,
compared with that in the controls, whereas no significant change
was observed in the cells transfected with the vector containing
the mutant binding site. In addition, a reporter assay was used to
assess the effect of propofol. As shown in Fig. 6C, propofol treatment reduced the
fluorescence intensity of cells transfected with the reporter
vector containing the wild-type TLR4 3′-UTR. These results
indicated that miR-21 binds to the TLR4 3′-UTR directly and
downregulates the mRNA expression of TLR4.
Discussion
TLR4 predominantly responds to LPS from
Gram-negative bacteria through its co-receptor (24). TLR4 contains three protein domains
(25): Extracellular,
transmembrane and intracellular. The extracellular domain consists
of a leucine-rich fragment and is involved in the identification of
pathogen-associated molecular patterns through combining with CD14.
The TLR4 signal transduction pathway is widespread and important in
inflammatory molecular signaling pathways (26–28).
The cascade of inflammatory signals triggered by TLR4 is crucial
during the development of several diseases (29). In the present study, it was found
that LPS promoted the expression of TLR4, CD14 and TNFα, suggesting
that TLR4 may serve as a mediator of inflammatory responses in
HUVEC cells. In addition, propofol suppressed the expression of
TLR4, CD14 and TNFα in a concentration-dependent manner, suggesting
that propofol reversed the inflammatory response induced by LPS.
The role of propofol on inflammation has been reported previously.
Propofol inhibits the NOD-like receptor family, pyrin
domain-containing 3 inflammasome and attenuates blast-induced
traumatic brain injury (30).
Propofol attenuates high glucose-induced superoxide anion
accumulation in HUVECs (31), and
inhibits pro-inflammatory cytokines in adult rats following
traumatic brain injury (32). In
accordance with these reports, the present study demonstrated that
propofol was able to reduce the expression of TLR4, which serves as
an important component of the inflammatory response. However, the
exact mechanism underlying the regulation of inflammation and TLR4
by propofol remains to be fully elucidated. The present study aimed
to determine how propofol downregulates the expression of TLR4, and
found miR-21 was an important mediator.
miR-21, which is highly conserved, has been found in
several vertebrates, including mammals, fish and birds (33–35).
It has been reported that the expression pattern of miR-21 is
closely associated with the development of several diseases
(36–38). However, whether and how miR-21 is
involved in the inflammatory response of cells regulated by
propofol remain to be elucidated. In the present study, it was
found that LPS treatment downregulated the expression of miR-21,
whereas propofol treatment upregulated the expression of miR-21 in
a concentration-dependent manner in HUVECs. In addition, the
present study showed that the miR-21 mimic downregulated the
expression of TLR4, whereas the miR-21 inhibitor upregulated the
expression of TLR4, indicating that TLR4 is a target of miR-21.
These results suggested that propofol regulated the
LPS-induced expression of TLR4 through miR-21 in HUVEC cells. To
confirm this hypothesis, the present study demonstrated that miR-21
reverses the effect of LPS on TLR4. The cells were also transfected
with miR-21 inhibitor and then exposed to propofol, and the results
demonstrated that the miR-21 inhibitor eliminated the
downregulatory effect of propofol on TLR4, suggesting that miR-21
is essential in the biological effect of propofol. To confirm TLR4
as a direct target of miR-21, the present study performed a
fluorescent reporter assay, which demonstrated that miR-21 was able
to bind directly to the TLR4 3′-UTR. Taken together, these results
confirmed that propofol regulated the expression of TLR4 though
miR-21.
In conclusion, the present study demonstrated that
propofol regulated the expression of TLR4 through the upregulation
of miR-21 in HUVECs, which may explain the protective effects of
propofol against inflammation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81302534) and the Doctor
Start-up Funding Program of the Science and Technology Department
of Liaoning Province (grant no. 20111098).
References
|
1
|
Ouchi K and Sugiyama K: Required propofol
dose for anesthesia and time to emerge are affected by the use of
antiepileptics: Prospective cohort study. BMC Anesthesiol.
15:342015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakanuno R, Yasuda T, Hamada H, Yoshikawa
H, Nakamura R, Saeki N and Kawamoto M: Propofol for anesthesia and
postoperative sedation resulted in fewer inflammatory responses
than sevoflurane anesthesia and midazolam sedation after
thoracoabdominal esophagectomy. Hiroshima J Med Sci. 64:31–37.
2015.PubMed/NCBI
|
|
3
|
Siampalioti A, Karavias D, Zotou A,
Kalfarentzos F and Filos K: Anesthesia management for the super
obese: Is sevoflurane superior to propofol as a sole anesthetic
agent? A double-blind randomized controlled trial. Eur Rev Med
Pharmacol Sci. 19:2493–2500. 2015.PubMed/NCBI
|
|
4
|
Chen Y, Liang M, Zhu Y and Zhou D: The
effect of propofol and sevoflurane on the perioperative immunity in
patients under laparoscopic radical resection of colorectal cancer.
Zhonghua Yi Xue Za Zhi. 95:3440–3444. 2015.(In Chinese). PubMed/NCBI
|
|
5
|
Inada T, Kubo K and Shingu K: Vaccines
using dendritic cells, differentiated with propofol, enhance
antitumor immunity in mice. Immunopharmacol Immunotoxicol.
31:150–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji X and Cao SH: The influences of
propofol on corticosteroid and immunity of rats after hemorrhagic
shock and resuscitation. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
21:278–281. 2009.(In Chinese). PubMed/NCBI
|
|
7
|
Kushida A, Inada T and Shingu K:
Enhancement of antitumor immunity after propofol treatment in mice.
Immunopharmacol Immunotoxicol. 29:477–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vogel SN: How discovery of Toll-mediated
innate immunity in Drosophila impacted our understanding of TLR
signaling (and vice versa). J Immunol. 188:5207–5209. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein M, Obermaier B, Angele B, Pfister
HW, Wagner H, Koedel U and Kirschning CJ: Innate immunity to
pneumococcal infection of the central nervous system depends on
toll-like receptor (TLR) 2 and TLR4. J Infect Dis. 198:1028–1036.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krejsek J, Kunes P, Andrýs C, Holická M,
Novosad J, Kudlová M and Kolácková M: Innate immunity, receptors
for exogenous and endogenous danger patterns in immunopathogenesis
of atherosclerosis-part II: TLR receptors, significance of genetic
polymorphism of danger signals receptors. Cas Lek Cesk Cesk
receptors, significance of genetic polymorphism of danger signals
receptors. Cas Lek Cesk. 144:790–794. 2005.(In Czech). PubMed/NCBI
|
|
11
|
Leavy O: Innate immunity: SHP regulates
TLR signalling. Nat Rev Immunol. 11:5022011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levy O, Zarember KA, Roy RM, Cywes C,
Godowski PJ and Wessels MR: Selective impairment of TLR-mediated
innate immunity in human newborns: Neonatal blood plasma reduces
monocyte TNF-alpha induction by bacterial lipopeptides,
lipopolysaccharide, and imiquimod, but preserves the response to
R-848. J Immunol. 173:4627–4634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
López CB, Moltedo B, Alexopoulou L,
Bonifaz L, Flavell RA and Moran TM: TLR-independent induction of
dendritic cell maturation and adaptive immunity by negative-strand
RNA viruses. J Immunol. 173:6882–6889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zivkovic A, Sharif O, Stich K, Doninger B,
Biaggio M, Colinge J, Bilban M, Mesteri I, Hazemi P, Lemmens-Gruber
R and Knapp S: TLR 2 and CD14 mediate innate immunity and lung
inflammation to staphylococcal Panton-Valentine leukocidin in vivo.
J Immunol. 186:1608–1617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang G, Zhuang S, Seyfert HM, Zhang K, Xu
T, Jin D, Guo J and Shen X: Hepatic TLR4 signaling is activated by
LPS from digestive tract during SARA, and epigenetic mechanisms
contribute to enforced TLR4 expression. Oncotarget. 6:38578–38590.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma B, Dohle E, Li M and Kirkpatrick CJ:
TLR4 stimulation by LPS enhances angiogenesis in a co-culture
system consisting of primary human osteoblasts and outgrowth
endothelial cells. J Tissue Eng Regen Med. 11:1779–1791. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Płóciennikowska A, Hromada-Judycka A,
Borzęcka K and Kwiatkowska K: Co-operation of TLR4 and raft
proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life
Sci. 72:557–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mannavola F, Tucci M, Felici C, Stucci S
and Silvestris F: miRNAs in melanoma: A defined role in tumor
progression and metastasis. Expert Rev Clin Immunol. 12:79–89.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Liu X, Hu Z, Wang Y, Liu M, Liu X,
Li H, Ji R, Guo Q and Zhou Y: Identification and characterization
of tumor suppressor and oncogenic miRNAs in gastric cancer. Oncol
Lett. 10:329–336. 2015.PubMed/NCBI
|
|
20
|
Xie F, Jones DC, Wang Q, Sun R and Zhang
B: Small RNA sequencing identifies miRNA roles in ovule and fibre
development. Plant Biotechnol J. 13:355–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shumin G, Yanfei D and Cheng Z: Role of
miRNA in plant seed development. Yi Chuan. 37:554–560. 2015.(In
Chinese). PubMed/NCBI
|
|
22
|
Rachagani S, Macha MA, Menning MS, Dey P,
Pai P, Smith LM, Mo YY and Batra SK: Changes in microRNA (miRNA)
expression during pancreatic cancer development and progression in
a genetically engineered KrasG12D; Pdx1-Cre mouse (KC) model.
Oncotarget. 6:40295–40309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Z, Lu J, Zhou W and Shen Y: Structural
insights into TIR domain specificity of the bridging adaptor Mal in
TLR4 signaling. PLoS One. 7:e342022012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohnishi H, Tochio H, Kato Z, Orii KE, Li
A, Kimura T, Hiroaki H, Kondo N and Shirakawa M: Structural basis
for the multiple interactions of the MyD88 TIR domain in TLR4
signaling. Proc Natl Acad Sci USA. 106:pp. 10260–10265. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rashidi N, Mirahmadian M, Jeddi-Tehrani M,
Rezania S, Ghasemi J, Kazemnejad S, Mirzadegan E, Vafaei S,
Kashanian M, Rasoulzadeh Z and Zarnani AH: Lipopolysaccharide- and
lipoteichoic acid-mediated pro-inflammatory cytokine production and
modulation of TLR2, TLR4 and MyD88 expression in human endometrial
cells. J Reprod Infertil. 16:72–81. 2015.PubMed/NCBI
|
|
27
|
Bamford S, Ryley H and Jackson SK: Highly
purified lipopolysaccharides from Burkholderia cepacia complex
clinical isolates induce inflammatory cytokine responses via
TLR4-mediated MAPK signalling pathways and activation of NFkappaB.
Cell Microbiol. 9:532–543. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calil IL, Zarpelon AC, Guerrero AT,
Alves-Filho JC, Ferreira SH, Cunha FQ, Cunha TM and Verri WA Jr:
Lipopolysaccharide induces inflammatory hyperalgesia triggering a
TLR4/MyD88-dependent cytokine cascade in the mice paw. PLoS One.
9:e900132014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma J, Mishra BB, Li Q and Teale JM:
TLR4-dependent activation of inflammatory cytokine response in
macrophages by Francisella elongation factor Tu. Cell Immunol.
269:69–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J,
Gu J, Fan K and Yu B: Propofol inhibits NLRP3 inflammasome and
attenuates blast-induced traumatic brain injury in rats.
Inflammation. 39:2094–2103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Jiang H, Wang J, Zhao Y, Zhu Y and
Zhu M: Propofol attenuates high glucose-induced superoxide anion
accumulation in human umbilical vein endothelial cells. Fundam Clin
Pharmacol. 30:511–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu F, Chen MR, Liu J, Zou Y, Wang TY, Zuo
YX and Wang TH: Propofol administration improves neurological
function associated with inhibition of pro-inflammatory cytokines
in adult rats after traumatic brain injury. Neuropeptides. 58:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB,
Luo XL, Zhu XL, Liu C, Xu FP, Luo DL, et al: PIK3R1 targeting by
miR-21 suppresses tumor cell migration and invasion by reducing
PI3K/AKT signaling and reversing EMT, and predicts clinical outcome
of breast cancer. Int J Oncol. 48:471–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Jiao J, Cermelli S, Muir K, Jung
KH, Zou R, Rashid A, Gagea M, Zabludoff S, Kalluri R and Beretta L:
miR-21 inhibition reduces liver fibrosis and prevents tumor
development by inducing apoptosis of CD24+ progenitor
cells. Cancer Res. 75:1859–1867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong G, Liang X, Wang D, Gao H, Wang L,
Wang L, Liu J and Du Z: High expression of miR-21 in
triple-negative breast cancers was correlated with a poor prognosis
and promoted tumor cell in vitro proliferation. Med Oncol.
31:572014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu
XX, Han J, Wu YC, Liu X, Zhu X, et al: miR-21 regulates tumor
progression through the miR-21-PDCD4-Stat3 pathway in human
salivary adenoid cystic carcinoma. Lab Invest. 95:1398–1408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang WK, Lee JK, Oh ST, Lee SH and Jung
CK: Stromal expression of miR-21 in T3-4a colorectal cancer is an
independent predictor of early tumor relapse. BMC Gastroenterol.
15:22015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu C, Li B, Cheng Y, Lin J, Hao J, Zhang
S, Mitchel RE, Sun D, Ni J, Zhao L, et al: MiR-21 plays an
important role in radiation induced carcinogenesis in BALB/c mice
by directly targeting the tumor suppressor gene Big-h3. Int J Biol
Sci. 7:347–363. 2011. View Article : Google Scholar : PubMed/NCBI
|