Introduction
Spastic cerebral palsy (SCP) is a common
complication in premature infants. With the rapid progression of
perinatology and technology in neonatal intensive care units, the
survival rates of premature infants have increased (1). However, ~10–20% of premature infants
are affected by sequelae in the nervous system at differing levels
of severity, placing a substantial burden on families and society
(2). Therefore, early diagnosis
and prevention is urgently required (3). In addition to premature delivery, the
occurrence of cerebral injury in premature infants is also
correlated with hypoxia-ischemia, infection, oxidative stress and
inflammatory response. Previous studies have demonstrated that
inflammatory mediators and cytokines are involved in the
pathophysiological process of SCP (2,3).
There has been an increasing focus on the changes of inflammatory
factors when SCP is present (4).
As an important component of immune responses, the
inflammatory response is closely associated with SCP (5). The inflammatory response includes the
adhesion, metastasis, infiltration and activity of inflammatory
cells, and the release of inflammatory cytokines (6). Inflammatory cytokines, including
interleukin (IL)-6 and tumor necrosis factor (TNF)-α, are involved
in the entire process of the inflammatory response. The levels of
inflammatory cytokines in children with hypoxic-ischemic brain
damage are high, indicating that infection and hypoxic-ischemic
brain damage may result in brain tissue damage through inflammatory
factors (7). Increasing studies
have demonstrated that high levels of proinflammatory cytokines in
amniotic fluid, plasma or the umbilical cord blood may be
associated with periventricular leukomalacia and subsequently lead
to the development of SCP (6).
The expression of vascular endothelial growth factor
(VEGF) is involved in the entire process of nervous system
development (8). VEGF is expressed
predominantly in surrounding regions of the neuroderm ventricle,
which decrease from the inside to the outside. With the maturity of
the central nervous system, the expression of VEGF decreases
gradually. VEGF can reduce the apoptosis of endothelial cells and
prevent the disappearance of blood capillaries. It can also induce
the relaxation of angio-smooth muscle through the release of nitric
oxide (NO) to protect ischemic brain tissues. Animal experiments
have shown that, following cerebral damage the expression of VEGF
can promote angiogenesis, resist apoptosis and protect neurocytes.
However, the animals involved in this study were adults (9).
With the continuous progression of modern
pharmacology, monomer compositions of various types of traditional
Chinese medicine have been shown to have inhibitory effects on
pulmonary interstitial fibrosis (10). Extracted from the classic and
traditional Chinese medicine salvia miltiorrhizae, tanshinone IIA
is a fat-soluble active constituent, which is used for treating
cardiovascular disease (11). With
a definite molecular structure, it is an active ingredient with the
highest stability at a higher content (11). Experiments have shown that
tanshinone IIA possesses several pharmacological activities,
including oxygen radical scavenging, and anti-inflammatory and
antitumor effects (12,13). Therefore, in the present study, the
neuroprotective effects of tanshinone IIA on the weakening of SCP
were investigated in neonatal rats. The central mechanisms involved
in the mediation or modulation of inflammation, p38
mitogen-activated protein kinase (MAPK) and VEGF were also
investigated.
Materials and methods
Animals and SCP model
A total of 26 male Sprague-Dawley
specific-pathogen-free rats (7-day-old, 15–20 g) were provided by
the Navy General Hospital Experimental Animal Center (Beijing,
China). The rats were housed at 22–23°C and 50–55% humidity with a
12-h light/dark cycle, and ad libitum access to pellet chow
and water. The animals were randomly assigned into three groups
(n=26): Sham injury (skin incision of the skull only; n=6), SCP
model (SCP rat treated with normal saline; n=10) and tanshinone IIA
group (SCP rat treated with 20 mg/kg of tanshinone IIA for 5 weeks;
n=10).
Unilateral occlusion of the carotid artery was
performed to establish the SCP animal model. The 7-day-old rats
were used as the SCP animal model to represent what occurs in
newborn humans. The rats were anesthetized via an intraperitoneal
injection of 10% chloral hydrate and placed in a supine position. A
median neck incision was performed and the left common carotid
artery was ligated. The wound was then sterilized and sutured, and
the animals were placed in cages under routine conditions. The
animal experiments were approved by the Ethics Committee of the
Naval General Hospital (Beijing, China).
Radial arm water maze test and holding
test
The eight-arm radial maze consisted of a central
platform symmetrically extended. The rats were deprived of water
for 48 h prior to assessment. A single well was placed at the outer
end of each arm and the rat was allowed to drink for 30 min. The
rat was allowed to freely explore the maze containing the
water-filled wells for 2 days prior to the spatial discrimination
test days. In the spatial discrimination test, the wells were
baited in only three arms and the sequence of angles was 135°, 90°
and 135°.
Each rat was measured over three daily sessions
consisting of five trials separated at 1-min intervals. The rat was
placed on the central platform in each trial, facing arm three, and
the experiment ended when the rat visited the three baited arms.
Three measurements were recorded: i) time taken to visit the three
baited arms; ii) number of re-entries into previously visited
baited arms; iii) number of entries into a non-baited arm. In the
holding test, the forepaws of the rat were grasped in a hollow
plastic tube, which was placed horizontally from a desk to allow
them to hold freely. The time spent suspended during 1 min was
recorded.
ELISA analysis
The hippocampus from every rat was dissected and
homogenized with ice-cold radioimmunoprecipitation (RIPA) and 0.1
mmol/l of PMSF for 30 min. The concentration of total protein was
determined using Bio-Rad protein assay reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Following determination,
l10 µg total protein was incubated with TNF-α (cat. no.
E-EL-R0019c), interleukin (IL)-1β (cat. no. E-EL-R0012c), IL-6
(cat. no. E-EL-R0015c) and monocyte chemoattractant protein 1
(MCP-1; cat. no. E-EL-R0633c) ELISA kits (Elabscience Biotechnology
Co., Ltd., Bethesda, MD, USA) at 37°C for 1 h. The absorbance was
measured using an ELISA analyzer (DNM-9606; Perlong Medical,
Jiangsu, China) at 450 nm.
Western blot analysis
The hippocampus from every rat was dissected and
homogenized with ice-cold RIPA and 0.1 mmol/l of PMSF for 30 min.
The concentration of total protein was determined using Bio-Rad
protein assay reagent, and 50 µg/lane total protein was subjected
to 8–10% SDS-polyacrylamide gel electrophoresis, following which it
was electrotransferred onto a nitrocellulose membrane. The
nitrocellulose membrane was blocked with 5% skim milk powder in
TBST and probed consecutively with the following primary polyclonal
antibodies: Nuclear factor (NF)-κB (cat. no. sc-109; 1:500; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), phosphorylated
(p)-p38MAPK (cat. no. sc-17852-R; 1:500; Santa Cruz Biotechnology,
Inc.), VEGF (cat. no. sc-13083; 1:500; Santa Cruz Biotechnology,
Inc.), p-inhibitor of NF-κB (p-IκB; cat. no. sc-101713; 1:500;
Santa Cruz Biotechnology, Inc.), inducible nitric oxide synthase
(iNOS; cat. no. sc-649; 1:500; Santa Cruz Biotechnology, Inc.),
neruronal NOS (nNOS; cat. no. sc-8309; 1:500; Santa Cruz
Biotechnology, Inc.) and β-actin (cat. no. sc-7210; 1:500; Santa
Cruz Biotechnology, Inc.) at 4°C overnight. The membrane was then
washed with TBST and incubated with the anti-rabbit immunoglobulin
G secondary antibody (1:5,000; cat. no. sc-2004; Santa Cruz
Biotechnology Inc.) at 37°C for 1 h. Protein expression was
visualized using BeyoECL Plus (Beyotime Institute of Biotechnology,
Haimen, China) and analyzed using Image_Lab_3.0 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The
experimental results were evaluated using one-way analysis of
variance by a Tukey post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Neuroprotective effect of tanshinone
IIA reduces SCP
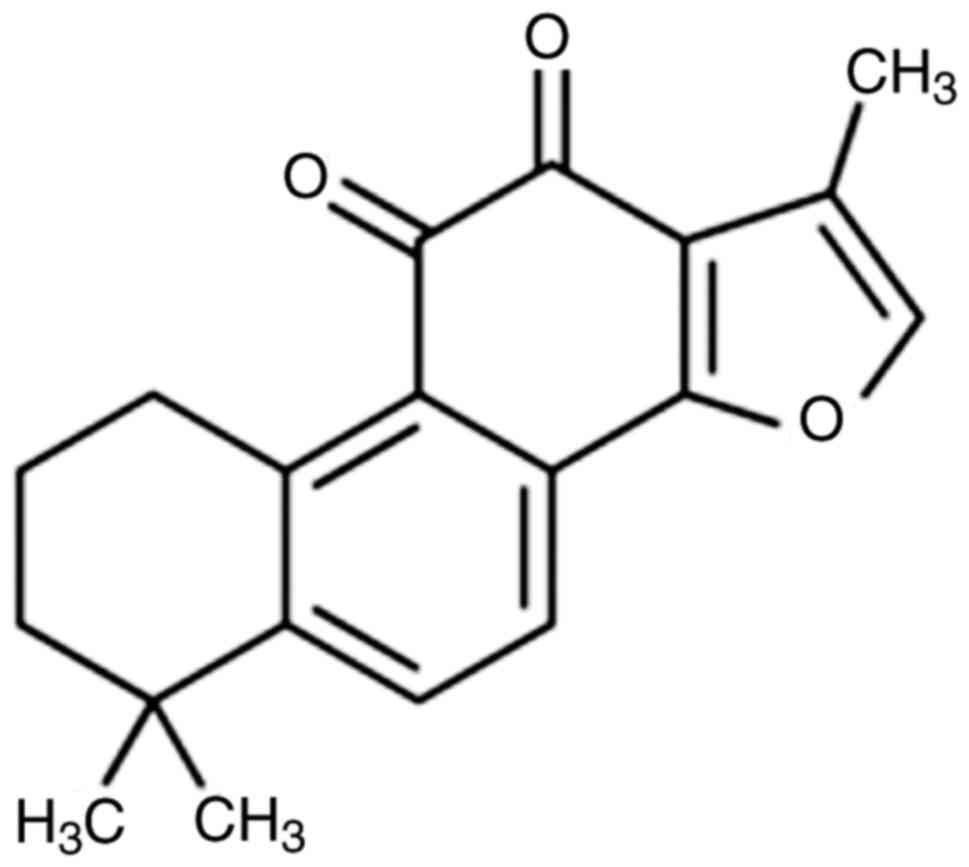
The structural formula of tanshinone IIA is shown in
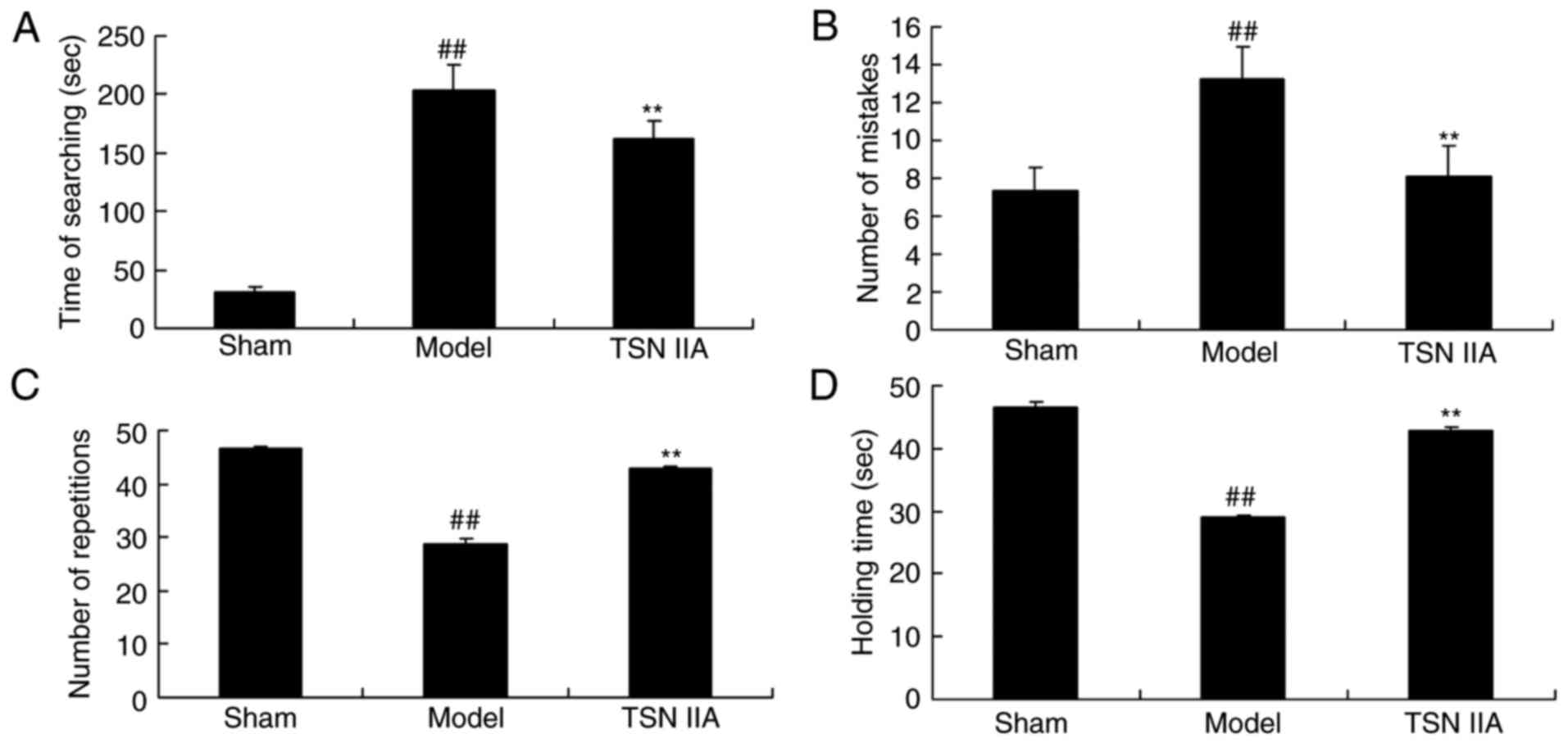
Fig. 1. As exhibited in Fig. 2A and B, there was a significant
increase in the time spent searching and number of mistakes in the
SCP model group rats, compared with the control group rats.
Significant decreases in the number of repetitions and holding
times were observed in rats of the SCP model group, compared with
those of the control group (Fig. 2C
and D). Treatment with tanshinone IIA significantly inhibited
the increased search duration and number of mistakes, and increased
the inhibited number of repetitions and holding times in the SCP
rats, compared with the rats in the SCP model group (Fig. 2A and D).
Neuroprotective effect of tanshinone
IIA reduces the expression of inflammatory factors
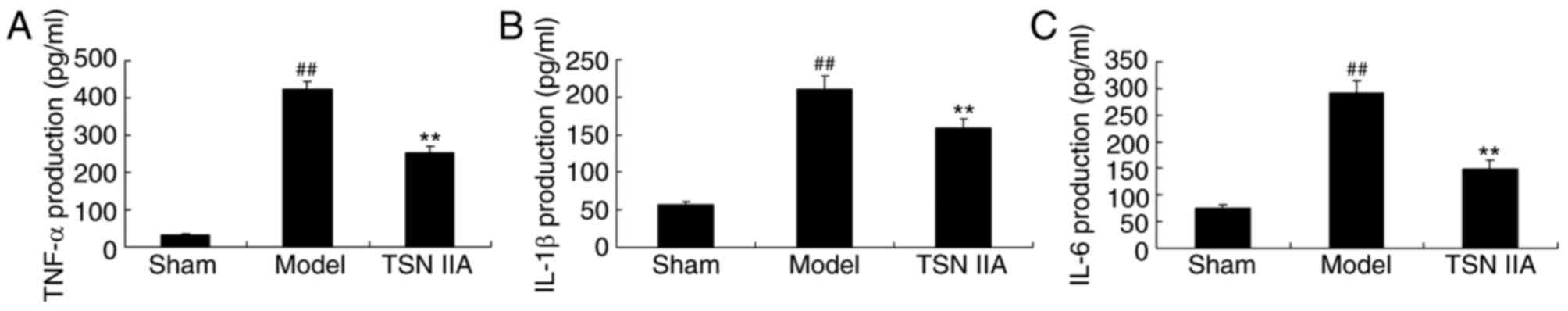
To examine the anti-inflammatory effect of
tanshinone IIA in the SCP rats, the levels of TNF-α, IL-1β and IL-6
in the hippocampal tissue samples were analyzed. The levels of
TNF-α, IL-1β and IL-6 in the SCP rats were significantly increased,
compared with those in the control group rats (Fig. 3A-C). Pre-treatment with tanshinone
IIA significantly inhibited the increased levels of TNF-α, IL-1β
and IL-6 in the SCP rats, compared with the rats in the sham
control group (Fig. 3A-C).
Neuroprotective effect of tanshinone
IIA reduces the mRNA expression of COX-2
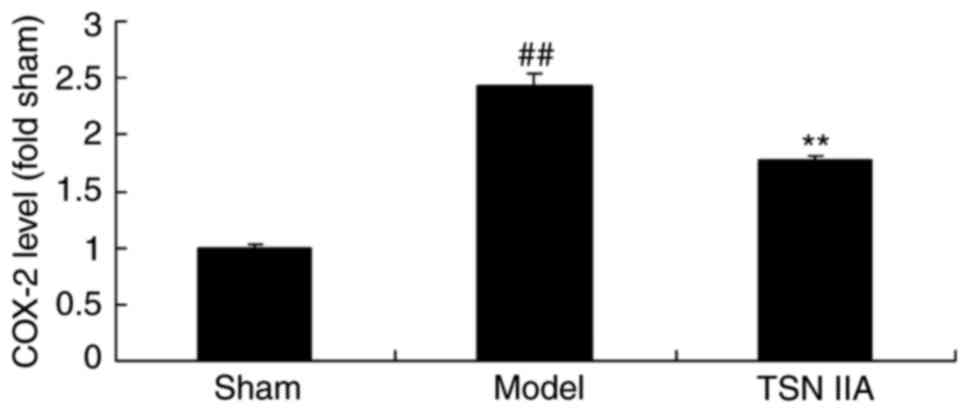
To further examine the anti-inflammatory effect of
tanshinone IIA in the SCP rats, the expression levels of COX-2 were
analyzed. The level of COX-2 was significantly increased in the SCP
model, compared with that in the sham group. The administration of
tanshinone IIA significantly inhibited the promoted expression
level of COX-2 in the SCP rats, compared with the rats in the sham
control group (Fig. 4).
Neuroprotective effect of tanshinone
IIA reduces the expression of MCP-1
To examine the anti-inflammatory effect of
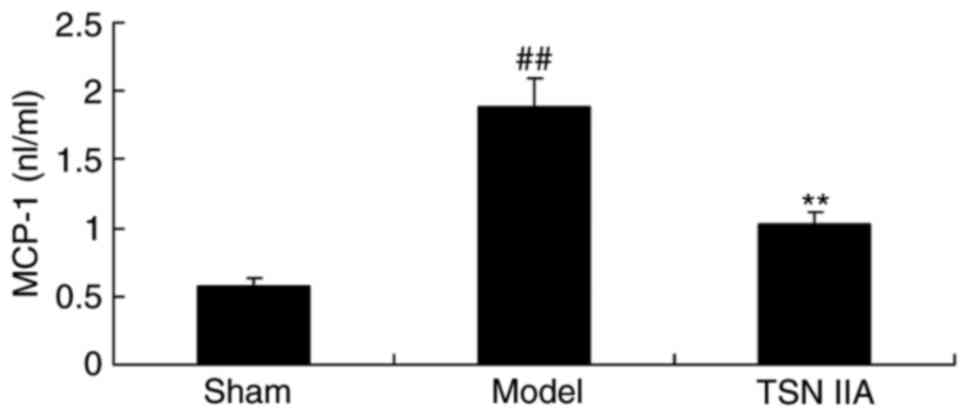
tanshinone IIA in the SCP rat, the expression of MCP-1 was also
measured. As demonstrated in Fig.
5, the expression if MCP-1 in the SCP model was significantly
increased, compared with that in the sham group. Treatment with
tanshinone IIA significantly reduced the expression of MCP-1 in the
SCP rats, compared with the rats in the SCP model group (Fig. 5).
Neuroprotective effect of tanshinone
IIA inhibits the NF-κB signaling pathway
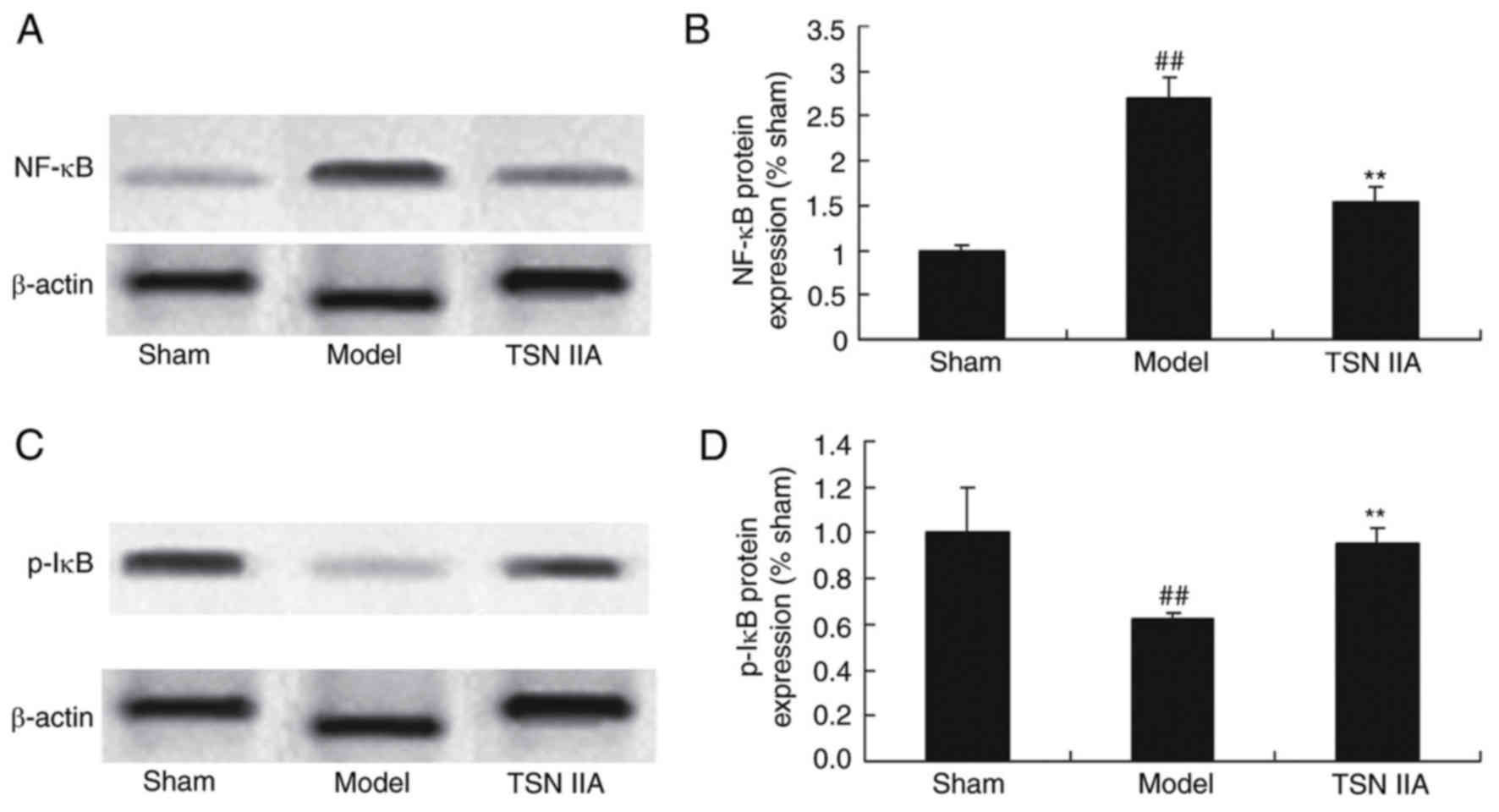
To investigate the anti-inflammatory mechanism of
tanshinone IIA in the SCP rats, the protein expression levels of
NF-κB and p-IκB were detected using western blot analysis. The
results of the western blot analysis demonstrated that the protein
expression levels of NF-κB and p-IκB were significantly increased
and decreased in the SCP model, respectively (Fig. 6A-D). Tanshinone IIA significantly
suppressed the protein expression of NF-κB and increased the
protein expression of p-IκB in the SCP rats, compared with the rats
in the SCP model group (Fig.
6A-D).
Neuroprotective effect of tanshinone
IIA reduces the expression of NO
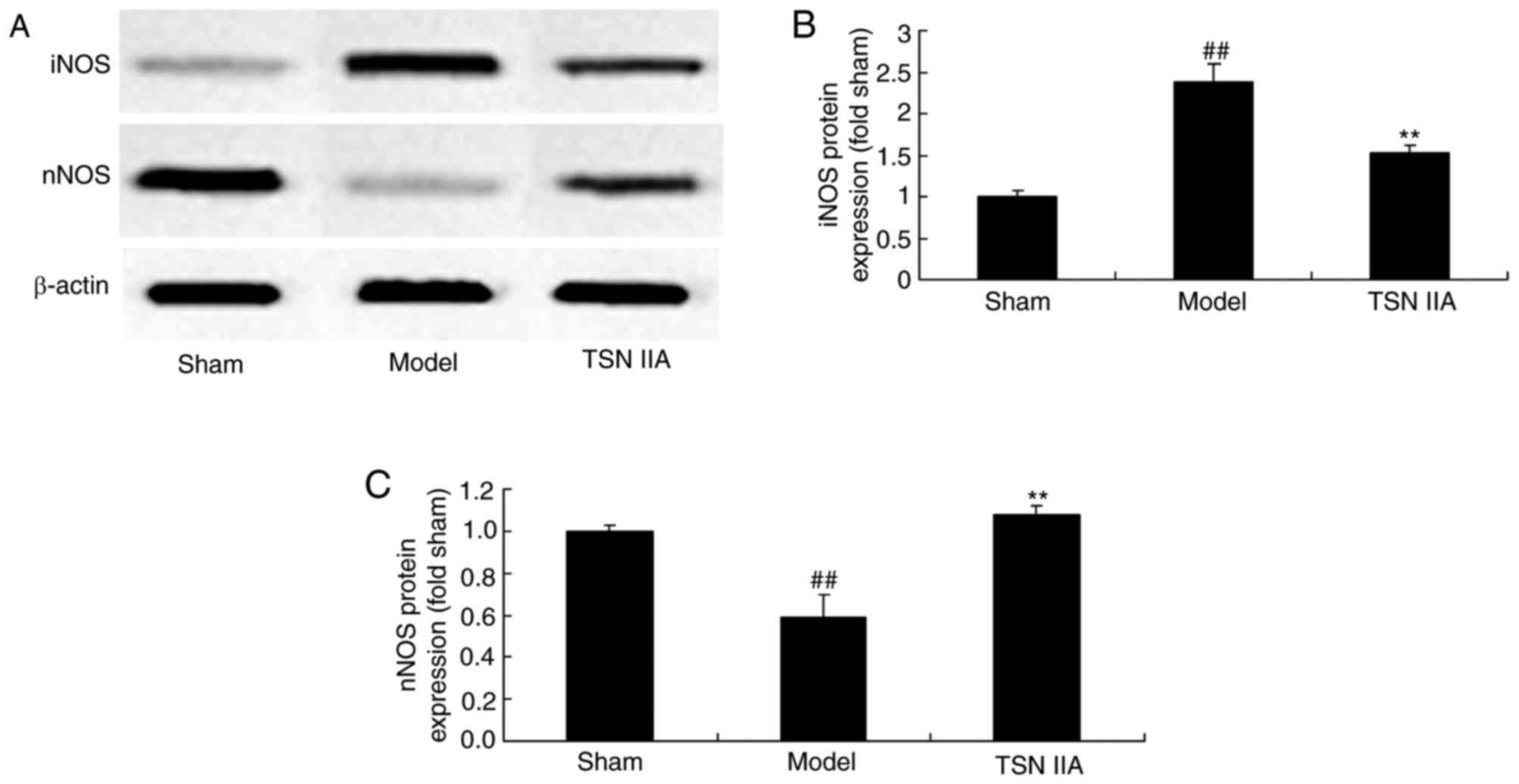
In order to examine the effect of tanshinone IIA on
changes in NO levels, the protein expression levels of iNOS and
nNOS were analyzed using western blot analysis. As exhibited in
Fig. 7A-C, the protein expression
of iNOS was increased and the protein expression of nNOS was
reduced in the SCP model group, compared with the levels in the
sham control group. Treatment with tanshinone IIA significantly
inhibited the induced protein expression of iNOS and increased the
suppressed protein expression of nNOS in the SCP rats (Fig. 7A-C).
Neuroprotective effect of tanshinone
IIA reduces the protein expression of p38MAPK
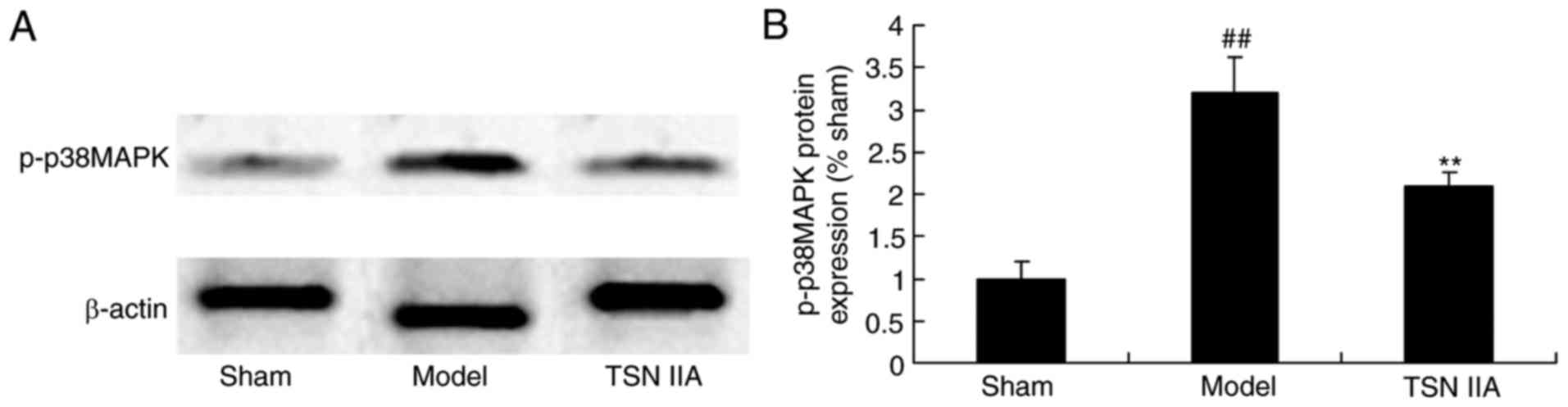
The present study further examined the mechanism
underlying the effects of tanshinone IIA in the SCP rats. p38MAPK
may be involved in the effect of tanshinone IIA on SCP. As
exhibited in Fig. 8A and B, the
expression of p-p38 of the SCP rat group was significantly
increased, compared with that in the sham group. This increased
protein expression of p-p38 in the SCP rats was significantly
suppressed by tanshinone IIA (Fig. 8A
and B).
Neuroprotective effect of tanshinone
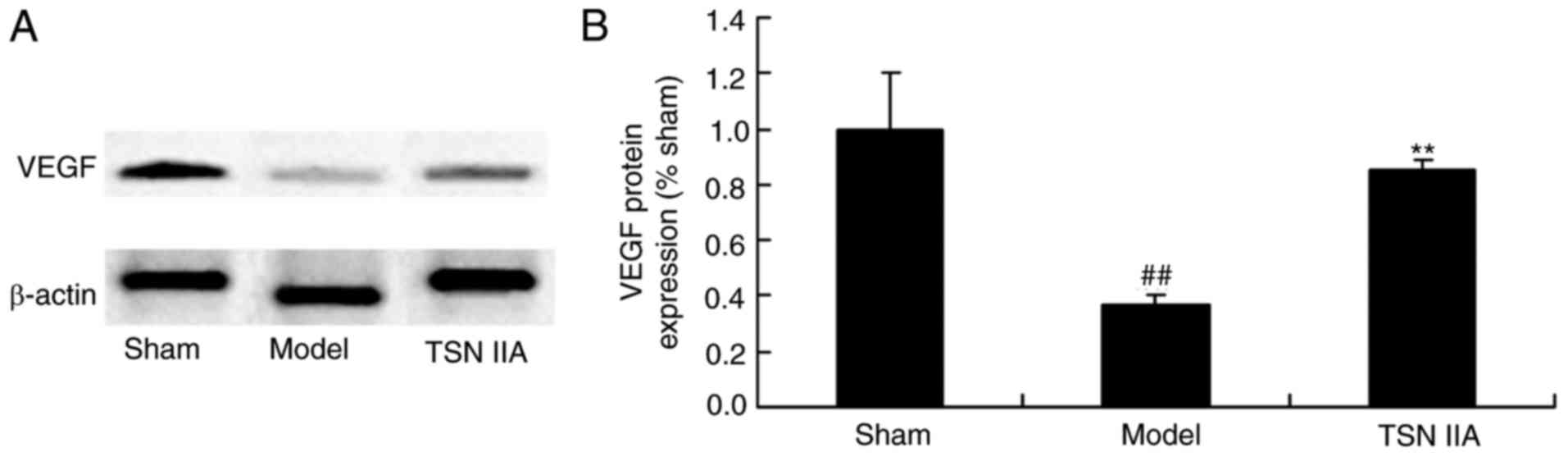
IIA reduces the protein expression of VEGF
The present study investigated whether tanshinone
IIA had an effect on blood vessels in the brain by detecting the
protein expression of VEGF. The results indicated that the protein
expression of VEGF was significantly suppressed in the SCP model
group, compared with that in the sham control group. Treatment with
tanshinone IIA significantly increased the protein expression of
VEGF in the SCP group (Fig. 9A and
B).
Discussion
SCP refers to the syndrome caused by non-progressive
cerebral damage and developmental defects occurring between
fertilization and infancy, which features dyskinesia and postural
dysfunction (14). Brain-derived
SCP, non-progressive cerebral damage and symptoms occur at infancy
caused by complications, whereas central coordination disturbance
caused by progressive disease and temporary motor retardation in
normal children are excluded (15). In the present study, the tests
performed showed that tanshinone IIA significantly inhibited the
time spent searching and number of mistakes, and increased the
number of repetitions and holding times in the SCP rats.
Similar results have been demonstrated in previous
animal experiments. When lipopolysaccharide was injected into the
uterus of pregnant rats, the expression levels of TNF-α and IL-1β
in the brains of newborn rats were dose-dependently increased
(15). Fibrillary acidic protein
was positive in the hippocampus and cortex, astrocytes were
increased, myelin basic protein was deceased, and the activities of
oligodendroglial cells showed abnormal changes (16). The results of the present study
revealed that tanshinone IIA significantly inhibited the increased
levels of TNF-α, IL-1β and IL-6 in the SCP rats. Jiang et al
also suggested that tanshinone IIA protects against folic
acid-induced acute kidney injury through inhibition of the
inflammatory response (17).
Monocytes, macrophages, endothelial cells and
contractile fiber cells can express MCP-1 (18). Major biological effects of MCP-1
include the chemotaxis of monocytes, and it can act on lymphocytes
and basophilic granulocytes, although it has no biological effects
on neutrophile granulocytes (18).
MCP-1 receptor is a member of the g-protein coupled receptor
super-family (19). Following the
combination of MCP-1 with its targeted specific receptor, it
activates the specific receptor of MCP-1 through g-protein coupling
on the cytomembrane (19). It also
triggers the release of calcium ions in the cytoplasm and induces
the activation of protein kinase C. In SCP, the injured cerebral
tissues produce various inflammatory chemokine factors (20). MCP-1 is a major inflammatory
chemokine factor, which exhibits potent chemotaxis of
monocytes/macrophages (20). The
monocytes/macrophages gather to regions of inflammation, and are
involved in the occurrence and progression of the inflammatory
response (21), which causes
damage to brain tissues (21). The
data obtained in the present study demonstrated that tanshinone IIA
significantly weakened the expression levels of MCP-1 and COX-2,
inhibited the induced protein expression of iNOS and increased the
protein expression of nNOS in the SCP rats. Tang et al
suggested that tanshinone IIA in neuropathic pain is mediated
predominantly by the downregulation of the c-Jun N-terminal
kinase/MCP-1 pathway (22).
NF-κB comprises a group of transcription factors in
eukaryocytes, which is distributed widely in the nervous system
(23). Under physiological
conditions, it does not have transcriptional activities. When the
cells are stimulated, its phosphorylation and ubiquitylation are
initiated through the secondary messenger system (24). NF-κB and IκB are activated and
shift into the nucleus from the cytoplasm (24). Studies have found that NF-κB is
activated in rats with SCP at an early stage (23,25).
Activated NF-κB can be found in the cytoplasm and cell nuclei of
neurons 24 h following injury (25). During the acute inflammatory
reaction, NF-κB is involved in the activation of macrophages and
hemameba, and controls the genetic expression of proinflammatory
factors (25). The control of this
process leads to the amplification of inflammatory responses and
tissue injury (26). The present
study observed that tanshinone IIA significantly suppressed the
protein expression of NF-κB and increased the protein expression of
p-IκB in the SCP rats. Bai et al also suggested that
tanshinone IIA induces apoptosis through the suppression of NF-κB
signaling in colon cancer cells (27).
SCP is one of the main factors resulting in neonatal
death, which is closely associated with SCP (28). VEGF functions in promoting
endothelial cell proliferation, and increasing vascular
permeability and angiogenesis (28). It has been suggested that VEGF may
have direct protective functions on endothelial cells and
astrocytes of the central nervous system. It can promote
angiogenesis, and stimulate axon growth and neuronal survival
(9,29). The present study demonstrated that
tanshinone IIA significantly induced the protein expression of VEGF
in SCP rats. Xu et al reported that tanshinone IIA protected
free flaps against hypoxic injury through VEGF and CD34 in
epithelial skin cells (30).
The activities of p38MAPK are significantly
increased in microglial cells (31). Activated p38MAPK locates in the
nucleus or endochylema, which may be associated with the functions
and status of microglial cells (32). p38MAPK is associated with
activities of NF-κB. The inhibition of p38MAPK can inhibit the
transcription of NF-κB (33).
Major biological functions following the activation of p38MAPK
include generating and activating various inflammatory cytokines,
including TNF-α, IL-1β, IL-6 and IL-8 (34). Macrophages at the ischemic core can
be found with activated P38MAPK, indicating that p38MAPK may be
involved in the inflammatory responses when cerebral ischemic
injury occurs (32). In the
present study, it was found that tanshinone IIA significantly
suppressed the protein expression of p-p38 in SCP rats. Liu et
al reported that the neuroprotective effects of tanshinone IIA
significantly suppressed the protein expression of p-p38 and
induced the protein expression of VEGF in SCP rats (35).
In conclusion, tanshinone IIA significantly
inhibited the searching time and number of mistakes made by the
rats, and increased the number of repetitions and holding times in
the SCP rats. Tanshinone IIA also weakened the increased expression
levels of TNF-α, IL-1β and IL-6, suppressed the expression levels
of MCP-1 and COX-2, reduced the protein expression of iNOS, and
induced the protein expression of nNOS in the SCP rats, which
regulated the NF-κB and p38MAPK signaling pathway.
References
|
1
|
Kerkum YL, Buizer AI, van den Noort JC,
Becher JG, Harlaar J and Brehm MA: The effects of varying ankle
foot orthosis stiffness on gait in children with spastic cerebral
palsy who walk with excessive knee flexion. PLoS One.
10:e01428782015. View Article : Google Scholar :
|
|
2
|
Kuypers E, Ophelders D, Jellema RK,
Kunzmann S, Gavilanes AW and Kramer BW: White matter injury
following fetal inflammatory response syndrome induced by
chorioamnionitis and fetal sepsis: Lessons from experimental ovine
models. Early Hum Dev. 88:931–936. 2012. View Article : Google Scholar
|
|
3
|
Colson SB, Siparsky GL, Capocelli KE, Pan
Z, Sokol RJ and Hoffenberg EJ: Inflammatory bowel disease in
pediatric patients with cerebral palsy. J Pediatr Gastroenterol
Nutr. 56:e502013. View Article : Google Scholar :
|
|
4
|
Qi YC, Xiao XJ, Duan RS, Yue YH, Zhang XL,
Li JT and Li YZ: Effect of acupuncture on inflammatory cytokines
expression of spastic cerebral palsy rats. Asian Pac J Trop Med.
7:492–495. 2014. View Article : Google Scholar
|
|
5
|
Beloosesky R, Ginsberg Y, Khatib N, Maravi
N, Ross MG, Itskovitz-Eldor J and Weiner Z: Prophylactic maternal
N-acetylcysteine in rats prevents maternal inflammation-induced
offspring cerebral injury shown on magnetic resonance imaging. Am J
Obstet Gynecol. 208(213): e1–6. 2013.
|
|
6
|
Jacobsson B: Infectious and inflammatory
mechanisms in preterm birth and cerebral palsy. Eur J Obstet
Gynecol Reprod Biol. 115:159–160. 2004. View Article : Google Scholar
|
|
7
|
Destot-Wong KD, Liang K, Gupta SK, Favrais
G, Schwendimann L, Pansiot J, Baud O, Spedding M, Lelièvre V, Mani
S and Gressens P: The AMPA receptor positive allosteric modulator,
S18986, is neuroprotective against neonatal excitotoxic and
inflammatory brain damage through BDNF synthesis.
Neuropharmacology. 57:277–286. 2009. View Article : Google Scholar
|
|
8
|
Zheng XR, Zhang SS, Yin F, Tang JL, Yang
YJ, Wang X and Zhong L: Neuroprotection of VEGF-expression neural
stem cells in neonatal cerebral palsy rats. Behav Brain Res.
230:108–115. 2012. View Article : Google Scholar
|
|
9
|
El Ghazi F, Desfeux A, Brasse-Lagnel C,
Roux C, Lesueur C, Mazur D, Remy-Jouet I, Richard V, Jégou S,
Laudenbach V, et al: NO-dependent protective effect of VEGF against
excitotoxicity on layer VI of the developing cerebral cortex.
Neurobiol Dis. 45:871–886. 2012. View Article : Google Scholar
|
|
10
|
Lin JY, Ke YM, Lai JS and Ho TF:
Tanshinone IIA enhances the effects of TRAIL by downregulating
survivin in human ovarian carcinoma cells. Phytomedicine.
22:929–938. 2015. View Article : Google Scholar
|
|
11
|
Zhang K, Li J, Meng W, Xing H and Yang Y:
Tanshinone IIA inhibits acute promyelocytic leukemia cell
proliferation and induces their apoptosis in vivo. Blood Cells Mol
Dis. 56:46–52. 2016. View Article : Google Scholar
|
|
12
|
Lu BL, Li J, Zhou J, Li WW and Wu HF:
Tanshinone IIA decreases the levels of inflammation induced by
Abeta1-42 in brain tissues of Alzheimer's disease model rats.
Neuroreport. 27:883–893. 2016. View Article : Google Scholar
|
|
13
|
Qiu S, Granet R, Mbakidi JP, Brégier F,
Pouget C, Micallef L, Sothea-Ouk T, Leger DY, Liagre B, Chaleix V
and Sol V: Delivery of tanshinone IIA and α-mangostin from
gold/PEI/cyclodextrin nanoparticle platform designed for prostate
cancer chemotherapy. Bioorg Med Chem Lett. 26:2503–2506. 2016.
View Article : Google Scholar
|
|
14
|
Huetz N, Triau S, Leboucher B, Sentilhes
L, Hanf M, Nguyen S, Flamant C, Roze JC and Gascoin G: Association
of severe placental inflammation with death prior to discharge and
cerebral palsy in preterm infants. BJOG. 123:1956–1963. 2016.
View Article : Google Scholar
|
|
15
|
Srivastava IN, Shperdheja J, Baybis M,
Ferguson T and Crino PB: mTOR pathway inhibition prevents
neuroinflammation and neuronal death in a mouse model of cerebral
palsy. Neurobiol Dis. 85:144–154. 2016. View Article : Google Scholar
|
|
16
|
Goldenberg RL and McClure EM: Placental
inflammation, neonatal death and cerebral palsy in preterm infants:
Is there a relationship? BJOG. 123:19642016. View Article : Google Scholar
|
|
17
|
Jiang C, Zhu W, Shao Q, Yan X, Jin B,
Zhang M and Xu B: Tanshinone IIA protects against folic
acid-induced acute kidney injury. Am J Chin Med. 44:737–753. 2016.
View Article : Google Scholar
|
|
18
|
Lloyd E, Somera-Molina K, Van Eldik LJ,
Watterson DM and Wainwright MS: Suppression of acute
proinflammatory cytokine and chemokine upregulation by post-injury
administration of a novel small molecule improves long-term
neurologic outcome in a mouse model of traumatic brain injury. J
Neuroinflammation. 5:282008. View Article : Google Scholar :
|
|
19
|
Ragin AB, Wu Y, Storey P, Cohen BA,
Edelman RR and Epstein LG: Monocyte chemoattractant protein-1
correlates with subcortical brain injury in HIV infection.
Neurology. 66:1255–1257. 2006. View Article : Google Scholar :
|
|
20
|
Helmy A, Guilfoyle MR, Carpenter KL,
Pickard JD, Menon DK and Hutchinson PJ: Recombinant human
interleukin-1 receptor antagonist promotes M1 microglia biased
cytokines and chemokines following human traumatic brain injury. J
Cereb Blood Flow Metab. 36:1434–1448. 2016. View Article : Google Scholar
|
|
21
|
Shein SL, Shellington DK, Exo JL, Jackson
TC, Wisniewski SR, Jackson EK, Vagni VA, Bayır H, Clark RS, Dixon
CE, et al: Hemorrhagic shock shifts the serum cytokine profile from
pro- to anti-inflammatory after experimental traumatic brain injury
in mice. J Neurotrauma. 31:1386–1395. 2014. View Article : Google Scholar :
|
|
22
|
Tang J, Zhu C, Li ZH, Liu XY, Sun SK,
Zhang T, Luo ZJ, Zhang H and Li WY: Inhibition of the spinal
astrocytic JNK/MCP-1 pathway activation correlates with the
analgesic effects of tanshinone IIA sulfonate in neuropathic pain.
J Neuroinflammation. 12:572015. View Article : Google Scholar :
|
|
23
|
Wang ZR, Li YX, Lei HY, Yang DQ, Wang LQ
and Luo MY: Regulating effect of activated NF-κB on edema induced
by traumatic brain injury of rats. Asian Pac J Trop Med. 9:274–277.
2016. View Article : Google Scholar
|
|
24
|
Su X, Wang H, Zhao J, Pan H and Mao L:
Beneficial effects of ethyl pyruvate through inhibiting
high-mobility group box 1 expression and TLR4/NF-κB pathway after
traumatic brain injury in the rat. Mediators Inflamm.
2011:8071422011. View Article : Google Scholar :
|
|
25
|
Wang CX, Xie GB, Zhou CH, Zhang XS, Li T,
Xu JG, Li N, Ding K, Hang CH, Shi JX and Zhou ML: Baincalein
alleviates early brain injury after experimental subarachnoid
hemorrhage in rats: Possible involvement of TLR4/NF-κB-mediated
inflammatory pathway. Brain Res. 1594:245–255. 2015. View Article : Google Scholar
|
|
26
|
Gao W, Zhao Z, Yu G, Zhou Z, Zhou Y, Hu T,
Jiang R and Zhang J: VEGI attenuates the inflammatory injury and
disruption of blood-brain barrier partly by suppressing the
TLR4/NF-κB signaling pathway in experimental traumatic brain
injury. Brain Res. 1622:230–239. 2015. View Article : Google Scholar
|
|
27
|
Bai Y, Zhang L, Fang X and Yang Y:
Tanshinone IIA enhances chemosensitivity of colon cancer cells by
suppressing nuclear factor-κB. Exp Ther Med. 11:1085–1089. 2016.
View Article : Google Scholar :
|
|
28
|
Dashti SR, Spalding A, Kadner RJ, Yao T,
Kumar A, Sun DA and LaRocca R: Targeted intraarterial anti-VEGF
therapy for medically refractory radiation necrosis in the brain. J
Neurosurg Pediatr. 15:20–25. 2015. View Article : Google Scholar
|
|
29
|
Jin X, Liang B, Chen Z, Liu X and Zhang Z:
The dynamic changes of capillary permeability and upregulation of
VEGF in rats following radiation-induced brain injury.
Microcirculation. 21:171–177. 2014. View Article : Google Scholar
|
|
30
|
Xu Z, Wu L, Sun Y, Guo Y, Qin G, Mu S, Fan
R, Wang B, Gao W and Zhang Z: Tanshinone IIA pretreatment protects
free flaps against hypoxic injury by upregulating stem cell-related
biomarkers in epithelial skin cells. BMC Complement Altern Med.
14:3312014. View Article : Google Scholar :
|
|
31
|
Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH
and Yune TY: Inhibition of ROS-induced p38MAPK and ERK activation
in microglia by acupuncture relieves neuropathic pain after spinal
cord injury in rats. Exp Neurol. 236:268–282. 2012. View Article : Google Scholar
|
|
32
|
Yang H, Feng GD, Liang Z, Vitale A, Jiao
XY, Ju G and You SW: In vitro beneficial activation of microglial
cells by mechanically-injured astrocytes enhances the synthesis and
secretion of BDNF through p38MAPK. Neurochem Int. 61:175–186. 2012.
View Article : Google Scholar
|
|
33
|
Lee KF, Chen JH, Teng CC, Shen CH, Hsieh
MC, Lu CC, Lee KC, Lee LY, Chen WP, Chen CC, et al: Protective
effects of Hericium erinaceus mycelium and its isolated erinacine A
against ischemia-injury-induced neuronal cell death via the
inhibition of iNOS/p38 MAPK and nitrotyrosine. Int J Mol Sci.
15:15073–15089. 2014. View Article : Google Scholar :
|
|
34
|
Su J, Zhou H, Tao Y, Guo J, Guo Z, Zhang
S, Zhang Y, Huang Y, Tang Y, Dong Q and Hu R: G-CSF protects human
brain vascular endothelial cells injury induced by high glucose,
free fatty acids and hypoxia through MAPK and Akt signaling. PLoS
One. 10:e01207072015. View Article : Google Scholar :
|
|
35
|
Liu X, Ye M, An C, Pan L and Ji L: The
effect of cationic albumin-conjugated PEGylated tanshinone IIA
nanoparticles on neuronal signal pathways and neuroprotection in
cerebral ischemia. Biomaterials. 34:6893–6905. 2013. View Article : Google Scholar
|