Introduction
Breast cancer remains the most common cancer in
women worldwide and its mortality rate is increasing. This disease
may cause serious harm to physical and mental health of women.
Surgery remains the primary treatment in most cases and entails the
complete removal of the primary tumour in the breast (1,2).
Traditional Chinese herbal medicine (CHM), as an adjunctive
treatment following surgery, radiotherapy and chemotherapy, may
prolong survival time and improve the quality of life of breast
cancer patients.
Chrysophanol
(1,8-Dihydroxy-3-methyl-9,10-anthraquinone) belongs to
anthraquinone family, which also contains emodin, aloe-emodin,
rhein, and physcion.
Derivatives of anthraquinone family are natural
products, which were mainly extracted from rhubarb and used in CHM
(3–5). As a palliative treatment, CHM was
commonly used with oral administration for different types of
cancers in China (6–8).
There were some reports indicating that rhubarb
extracts induced apoptosis or inhibit migration of cancer cells
(9,10). For example, emodin extracted from
rhubarb root has anti-cancer effects in numerous cancers such as
prostate, breast and cervical cancers (11–15).
Chrysophanol was also reported for its anti-cancer role, showing
that it inhibits viability of colon cancer cells through inhibition
of the NF-κB-mediated signaling cascades (16). In choriocarcinoma, Chrysophanol
induced cell apoptosis by regulating production of reactive oxygen
species (ROS) through AKT and ERK1/2 signaling pathways (17). Chrysophanol induces necrosis in
A549 cells through increasing ROS and decreasing the level of
mitochondrial membrane potential (3). Chrysophanol also impairs
mitochondrial ATP synthesis in Hep3B cells (4). However, whether chrysophanol play a
role in breast cancer development is still unknown. This study aims
to find out the biological effects of chrysophanol on breast cancer
and the potential mechanism.
Materials and methods
Chemicals
Chrysophanol (cat no. 01542) and Paclitaxel (cat no.
1491332) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
NF-κB inhibitor (BAY 11–7082; cat no. S1523) was purchased from
Beyotime Institute of Biotechnology (Shanghai, China).
Cell culture
Breast cancer cell line MCF-7 and MDA-MB-231 were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in RPMI-1640 (Invitrogen; Thermo Fisher
Scientific, Carlsbad, CA, USA) with 10% FBS (Invitrogen; Thermo
Fisher Scientific) and 0.01 mg/ml human recombinant insulin (cat
no. I3536; Sigma-Aldrich). The cultures were maintained at 37°C in
a humidified incubator containing 5% (v/v) CO2 in air.
Cells were seeded at a density of 1×106 cells/ml in
6-well plates.
MTT assay
MCF-7 cells (5×103/well) were plated in
96-well plates and cultured overnight. MTT solution (volume, 20 µl;
concentration, 5 mg/ml; Sigma-Aldrich) was added to each well and
then incubated for another 4 h. The supernatant was removed and
DMSO (150 µl) was added for test preparation. Absorbance was
measured at 490 nm. Data were obtained from triplicate wells per
condition.
Flow cytometry for cell cycle and
apoptosis analysis
Cells in 6-well plates were collected using 0.25%
trypsase 24 h after chrysophanol treatment. Cells were washed twice
with PBS butter and then resuspended in 250 µl of binding buffer.
Cells were fixed in 1% paraformaldehyde for 24 h and then stained
with 5 mg/ml propidium iodide for cell cycle analysis. Cells were
stained with propidium iodide and Annexin V/FITC for cell apoptosis
analysis. Stained cells were analyzed by FACS Calibur flow
cytometer (BD Biosciences, San Diego, CA, USA) after incubation in
the dark for 15 min.
Western blot analysis
Total protein of MCF-7 cells was extracted using
lysis buffer (Pierce Biotechnology, Inc., Rockford, IL, USA) and
Bradford method was used to quantify the protein. When SDS-PAGE
assay was performed, 30 µg of the protein was separated and then
transferred to polyvinylidene fluoride membranes (Millipore Corp.,
Billerica, MA, USA). For primary antibody incubation, cleaved
caspase 3, caspase 3, cleaved PARP, PARP, p-p65, p65, p-IκB, IκB,
Bcl-2 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
and GAPDH (1:2,000; Cell Signaling Technology, Inc.) were incubated
overnight at 4°C. For secondary antibody incubation,
peroxidase-coupled anti-mouse/rabbit IgG (1:1,000; Cell Signaling
Technology, Inc.) was incubated at 37°C for 2 h. Sample protein was
visualized using ECL (Pierce Biotechnology, Inc.) and detected
using a DNR BioImaging System (DNR Bio-Imaging Systems, Ltd.,
Jerusalem, Israel). Relative protein levels were quantified using
ImageJ software.
Realtime quantitative PCR
Total RNA was isolated from MCF-7 cells using TRIzol
reagent (Thermo Fisher Scientific) following the manufacturer's
instructions. Then, reverse transcription of total RNA into cDNA
was performed using the Reverse Transcription System (A3500;
Promega Corp., Madison, WI, USA) and PrimerScript RT Master Mix kit
(Takara Bio, Dalian, China). Briefly, a total 20 µl of
reverse-transcription reaction solution was prepared containing 4
µl of 5X RT Master Mix and 800 ng RNA and the mixture was reacted
at 85°C for 2 min and 37°C for 30 min. PCR was performed using 7500
Realtime PCR System (Applied Biosystems Life Technologies, Foster
City, CA, USA) and SYBR-Green master mix kit (Applied Biosystems
Life Technologies). The relative expression of target genes were
calculated as ΔCq=Cq gene-Cq reference, and the fold change of
target gene expression was calculated by the 2−ΔΔCq
method. GAPDH was used as the reference gene. Experiments were
repeated in triplicate. Primer sequences were listed as follows:
Cyclin D1 forward TGGAGGTCTGCGAGGAACA, cyclin D1 reverse
TTCATCTTAGAGGCCACGAACAT; cyclin E forward AGCCAGCCTTGGGACAATAAT,
cyclin E reverse GAGCCTCTGGATGGTGCAAT; p27 forward
CTGCAACCGACGATTCTTCTACT, p27 reverse CTTCTGAGGCCAGGCTTCTT; GAP DH
forward AAGATCATCAGCAATGCCTCCT, GAP DH reverse
TGGTCATGAGTCCTTCCACGAT.
Statistical analysis
Statistical analyses were performed using SPSS
version 16 for Windows. The Student's t-test was used to compare
differences between control and treatment groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
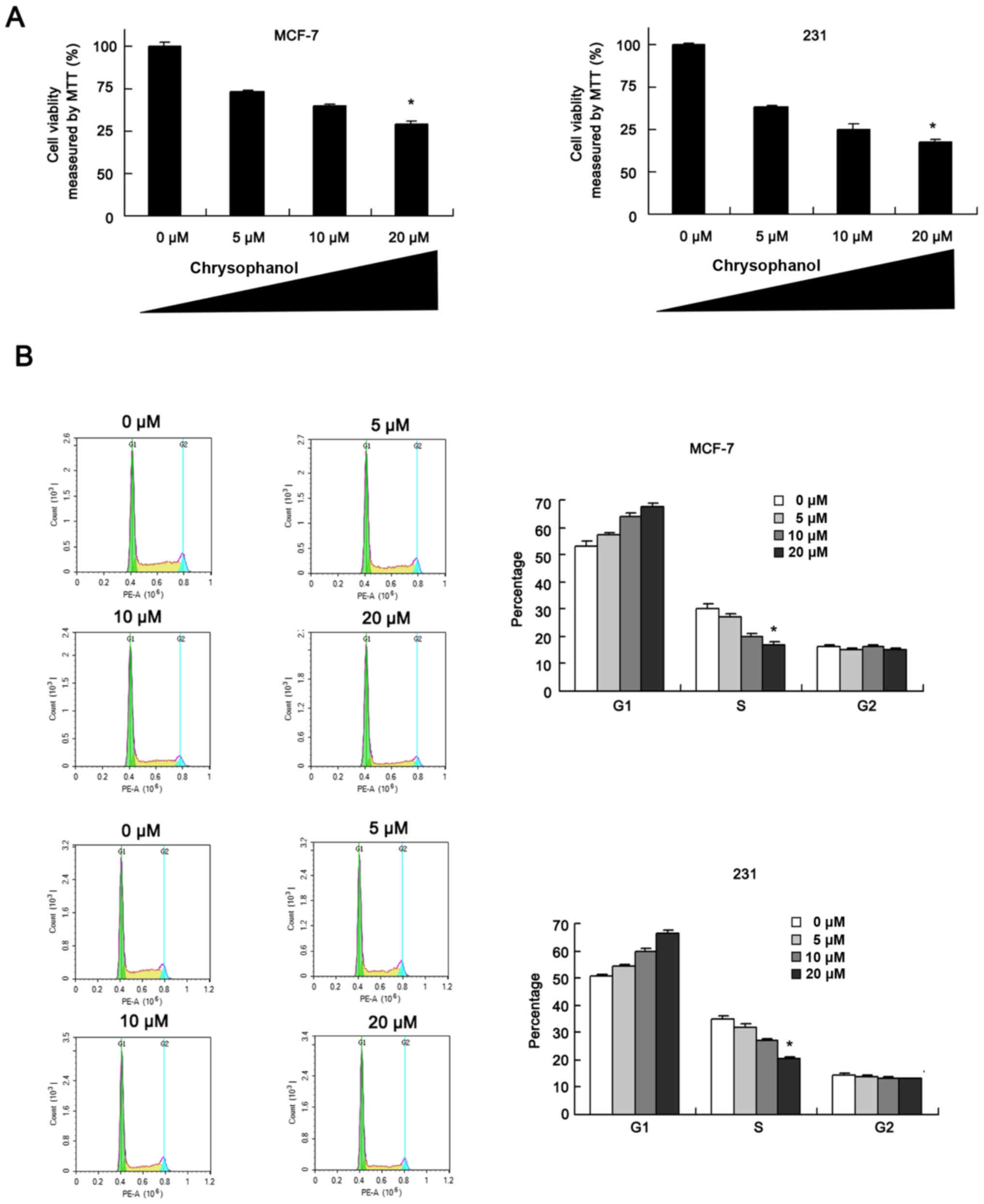
Chrysophanol inhibits breast cancer
cell proliferation and cell cycle progression
MCF-7 and MDA-MB-231 cells were employed to explore
effect of chrysophanol on breast cancer. Cells were treated with
chrysophanol at the concentrations of 0, 5, 10, 20 µM. MTT assay
and cell cycle analysis were performed. As shown in Fig. 1A, proliferation rates of MCF-7 and
MDA-MB-231 cells were decreased significantly when treated with
chrysophanol in a concentration-dependent manner after 48 h
treatment. Cycle analysis results showed that G1 percentage
increased while S percentage decreased after chrysophanol treatment
(24 h) in a concentration-dependent manner (Fig. 1B), indicating chrysophanol could
arrest breast cancer cells at G1-S cell cycle checkpoint.
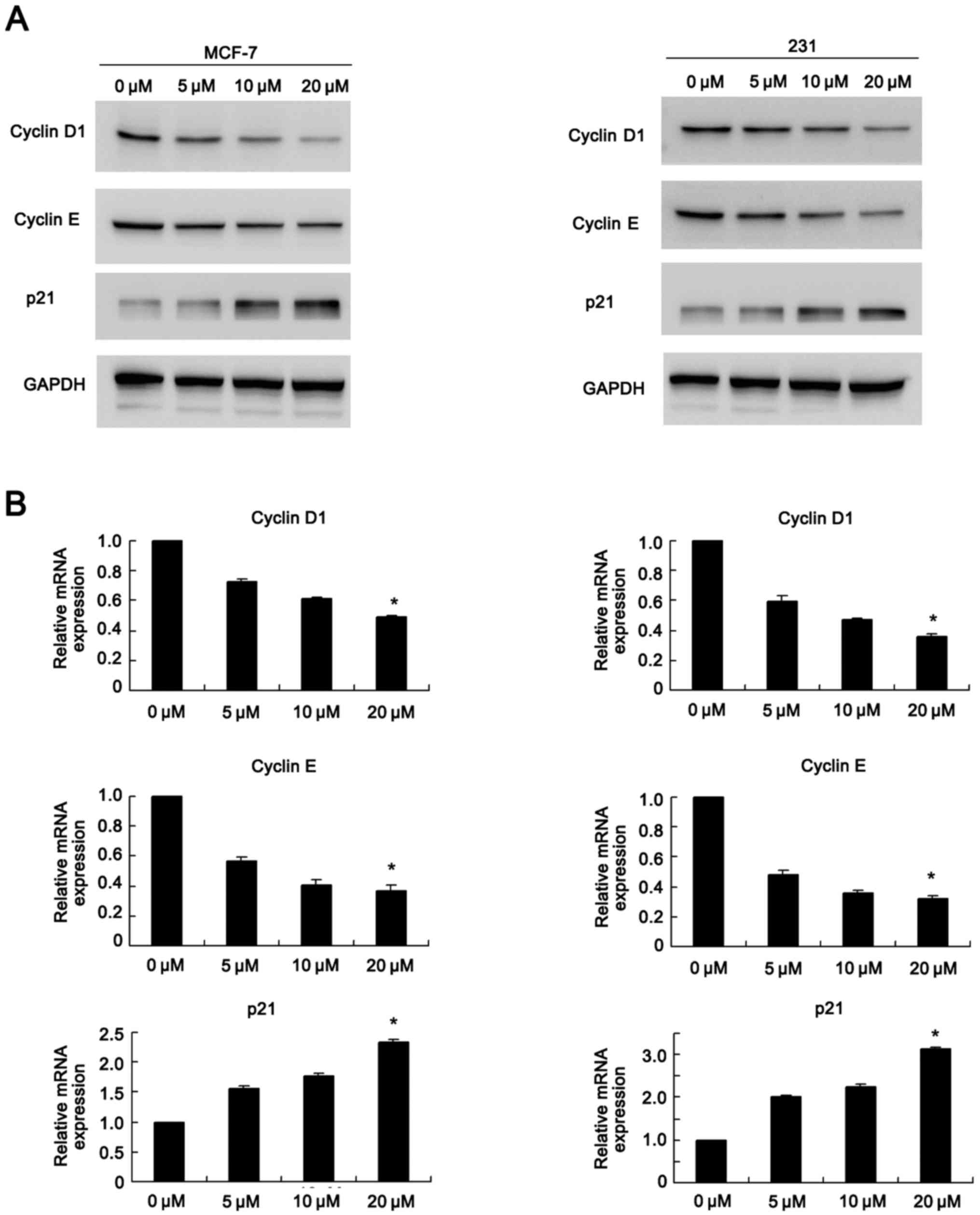
Chrysophanol regulates cell cycle
related proteins
Western blot analysis and real-time quantitative PCR
were used to determine the change of cycle related genes (Fig. 2). Western blot analysis results
showed that chrysophanol exposure dramatically inhibited expression
of cyclin D1, cyclin E while upregulated p21 levels in a
concentration dependent manner in both MCF-7 and MDA-MB-231 cell
lines (0, 5, 10, 20 µM, 24 h) (Fig.
2A). In accordance with western blot analysis results, PCR
results showed cyclin D1 and cyclin E mRNA levels decreased when
treated with chrysophanol. The mRNA expression of p27 was
upregulated in both cell lines in a dose dependent manner (0, 5,
10, 20 µM, 24 h) (Fig. 2B).
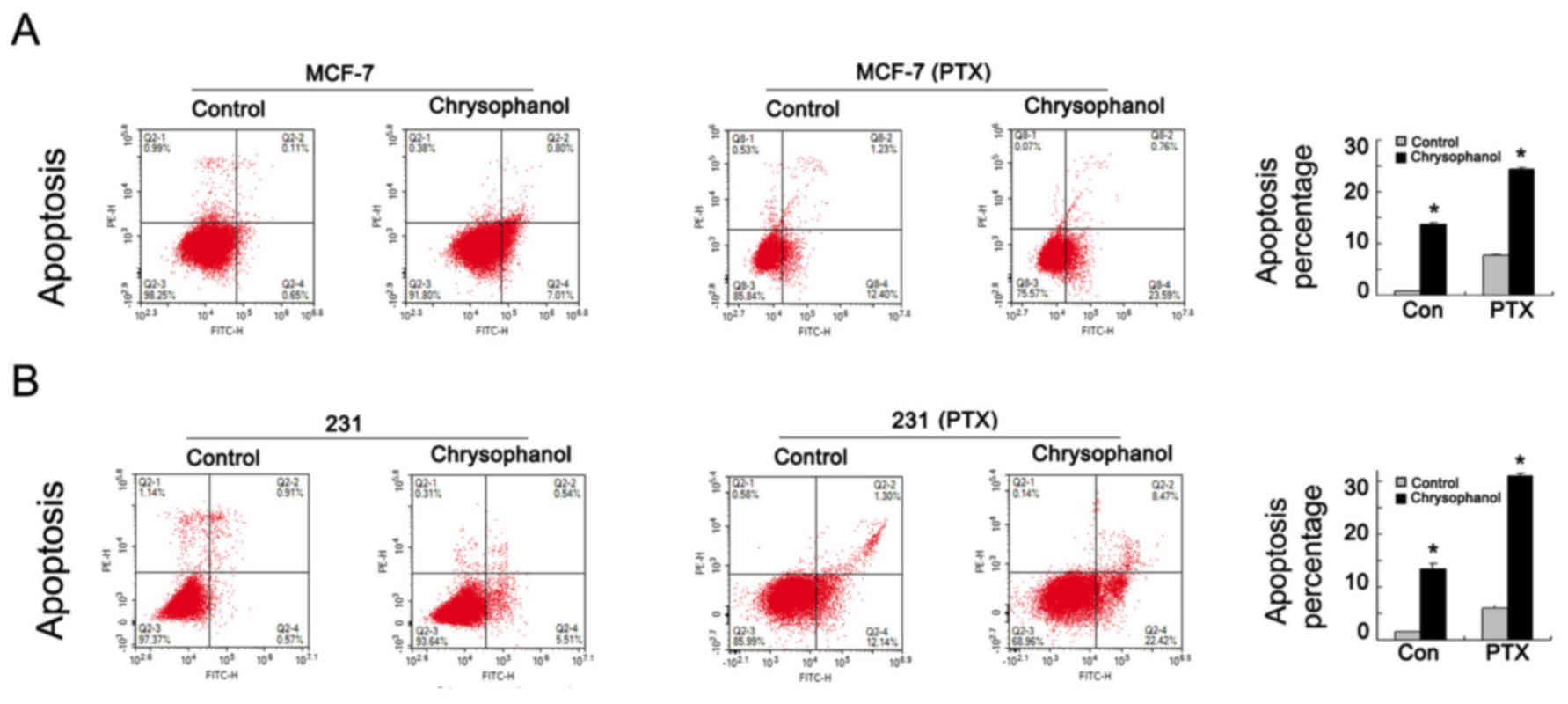
Chrysophanol regulates cell apoptosis
and related proteins
To explore effect of chrysophanol on cell apoptosis,
MCF-7 and MDA-MB-231 cells were treated with chrysophanol (20 nM,
24 h) and stained with Annexin V/PI. As shown in Fig. 3 A&B, percentage of apoptotic
cells was increased significantly when treated with chrysophanol in
both cell lines. In order to explore the role of chrysophanol on
chemosensitivity, we adopted 5 nM paclitaxel (PTX) to treat MCF-7
and MDA-MB-231 cell lines for 12 h and examined apoptosis rate
after chrysophanol (20 nM, 24 h) treatment. As shown in Fig. 3A and B, chrysophanol significantly
increased apoptosis rate in paclitaxel treated breast cancer
cells.
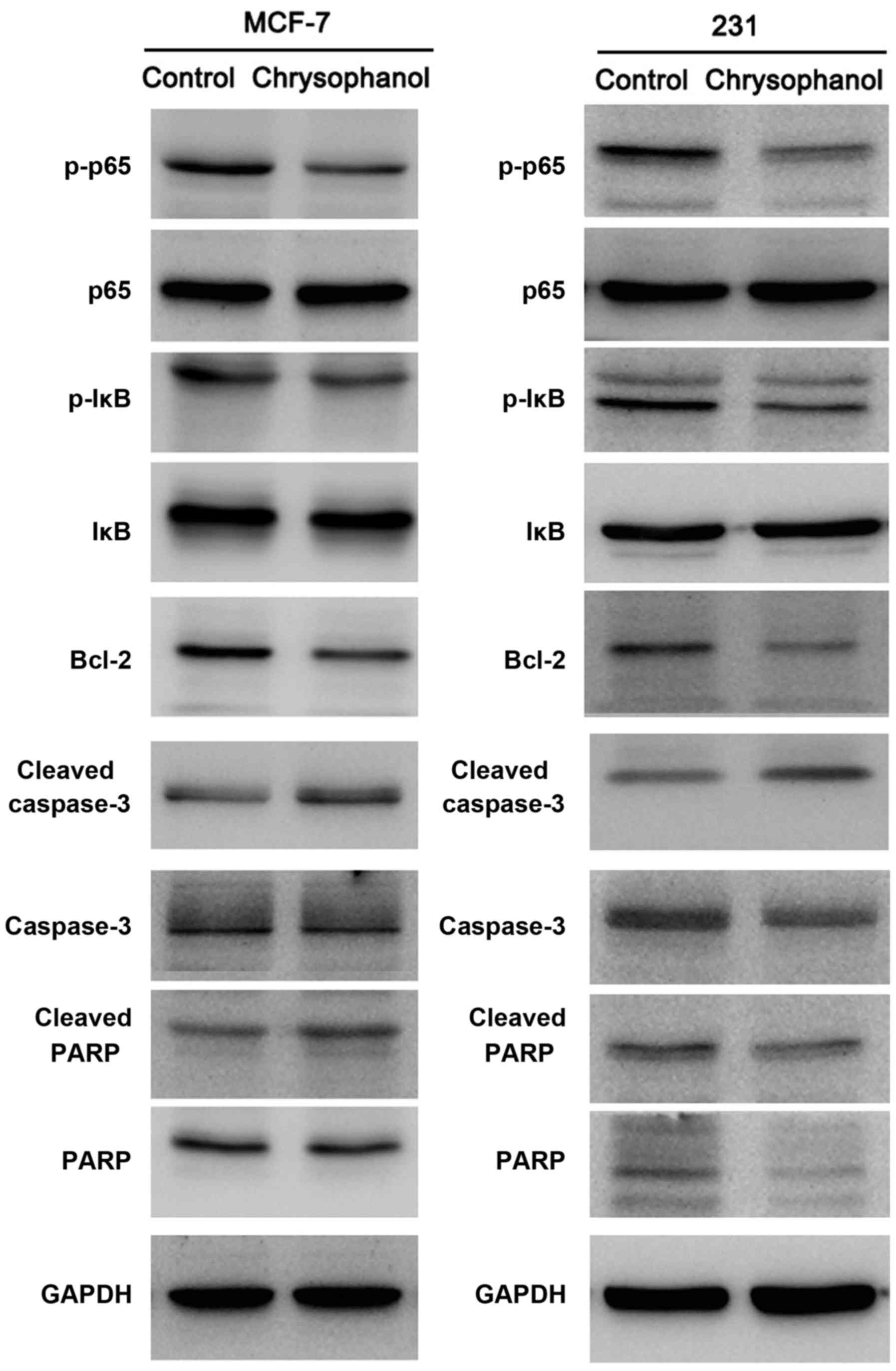
Next, expression of apoptosis related protein was
examined using western blot analysis and the results showed that
chrysophanol treatment upregulated cleaved caspase 3 and cleaved
PARP levels in both cell line (Fig.
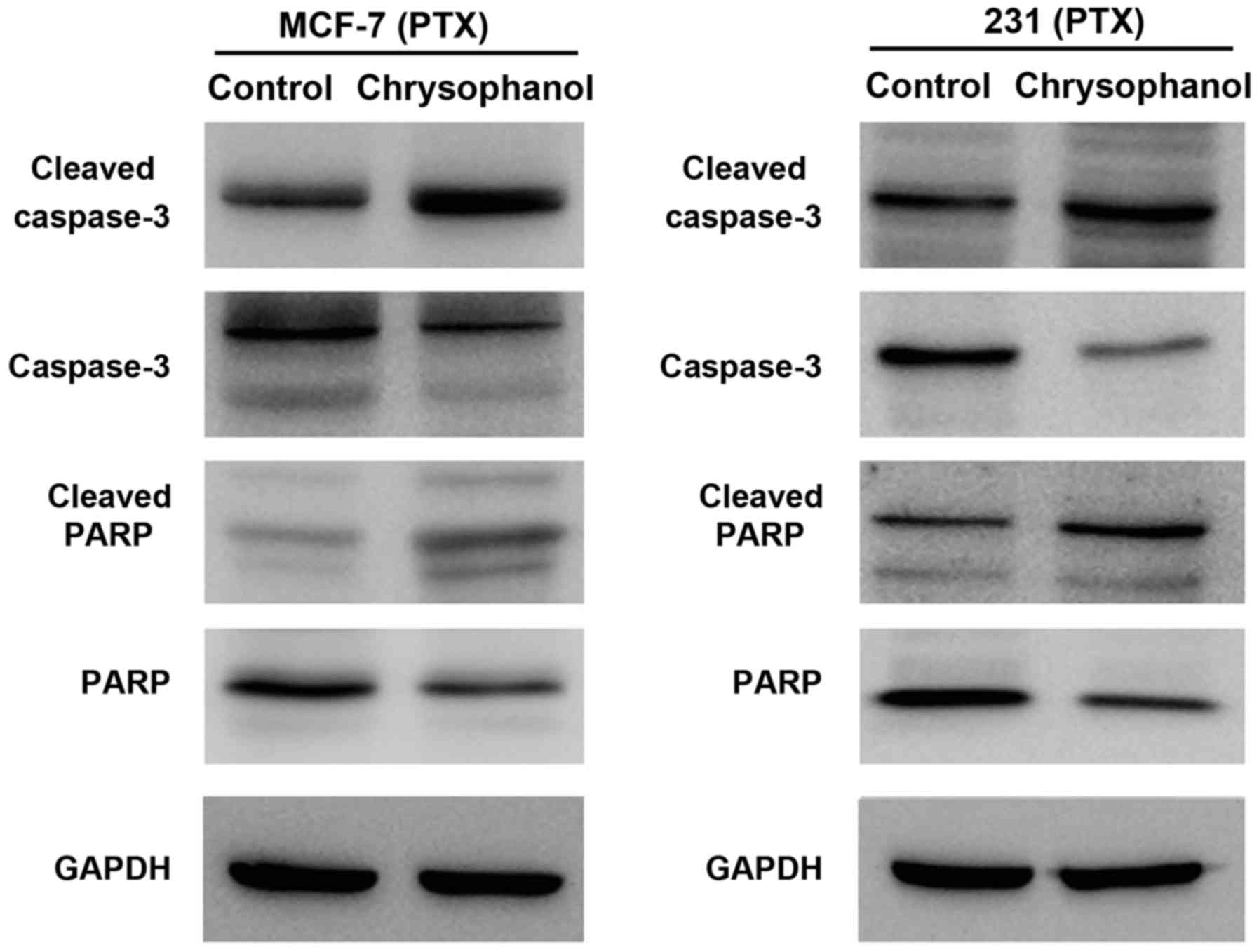
4). In cells treated with paclitaxel (5 nM, 12 h), chrysophanol
also significantly upregulated caspase 3 and PARP cleavage in both
cell lines (Fig. 5).
Chrysophanol regulates
chemosensitivity through NF-κB signaling pathway
To explore the potential mechanism of chrysophanol
in MCF-7 and MDA-MB-231 cell lines, we examined several signaling
pathways which is related to cancer cell proliferation and
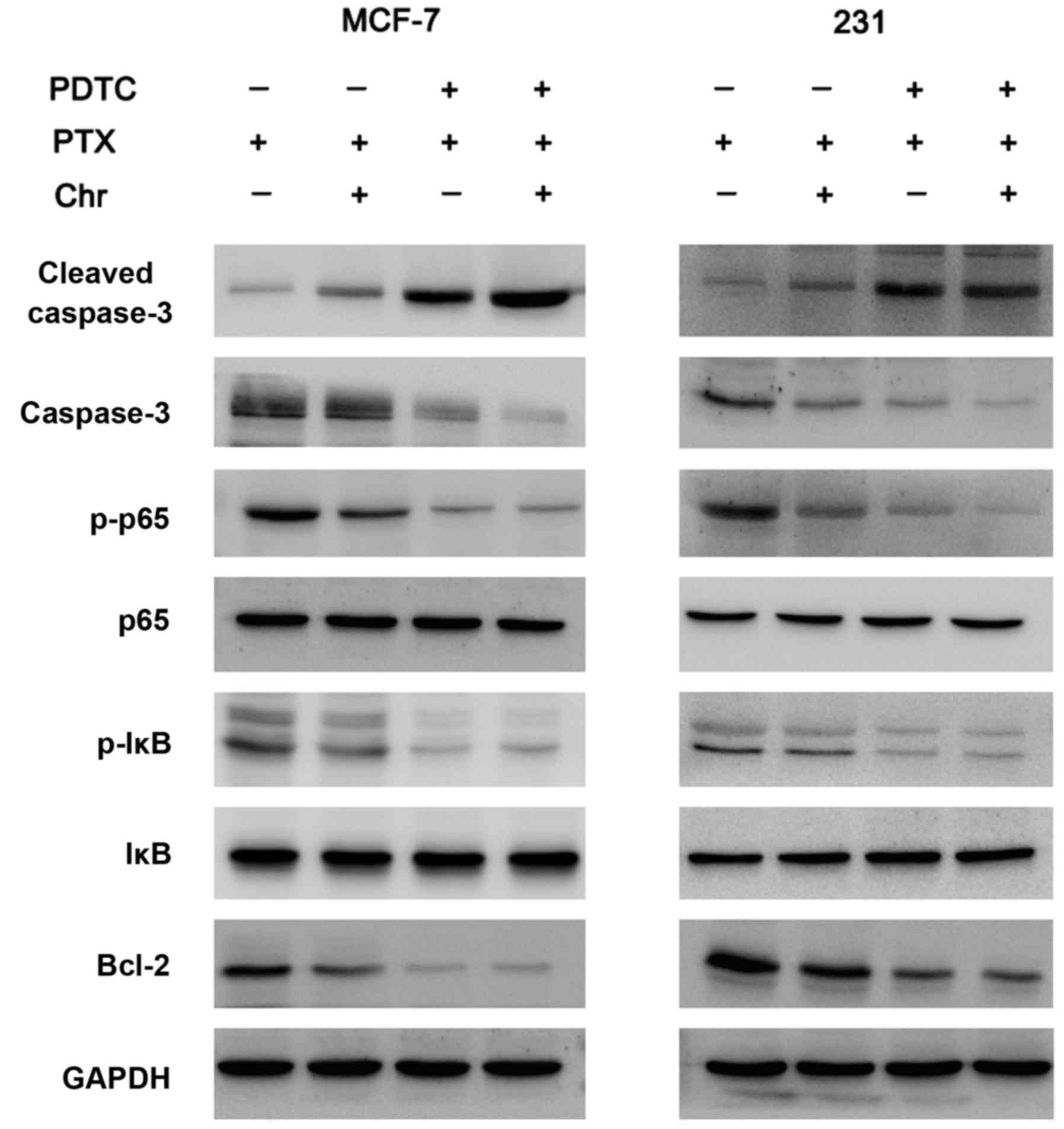
chemoresistance. Western blot analysis showed that expression of
Bcl-2, p-IκB, p-p65 expression were significantly decreased after
treatment with chrysophanol (Fig.
4).
The above results demonstrated that NF-κB activity
was suppressed after chrysophanol treatment. Bcl-2 was reported as
a downstream target of NF-κB signaling, which serves as a potent
inhibitor of apoptosis and an indicator of chemoresistance. To
confirm if chrysophanol mediated cell behavior was dependent on
NF-κB signaling pathway, cancer cells were treated with NF-κB
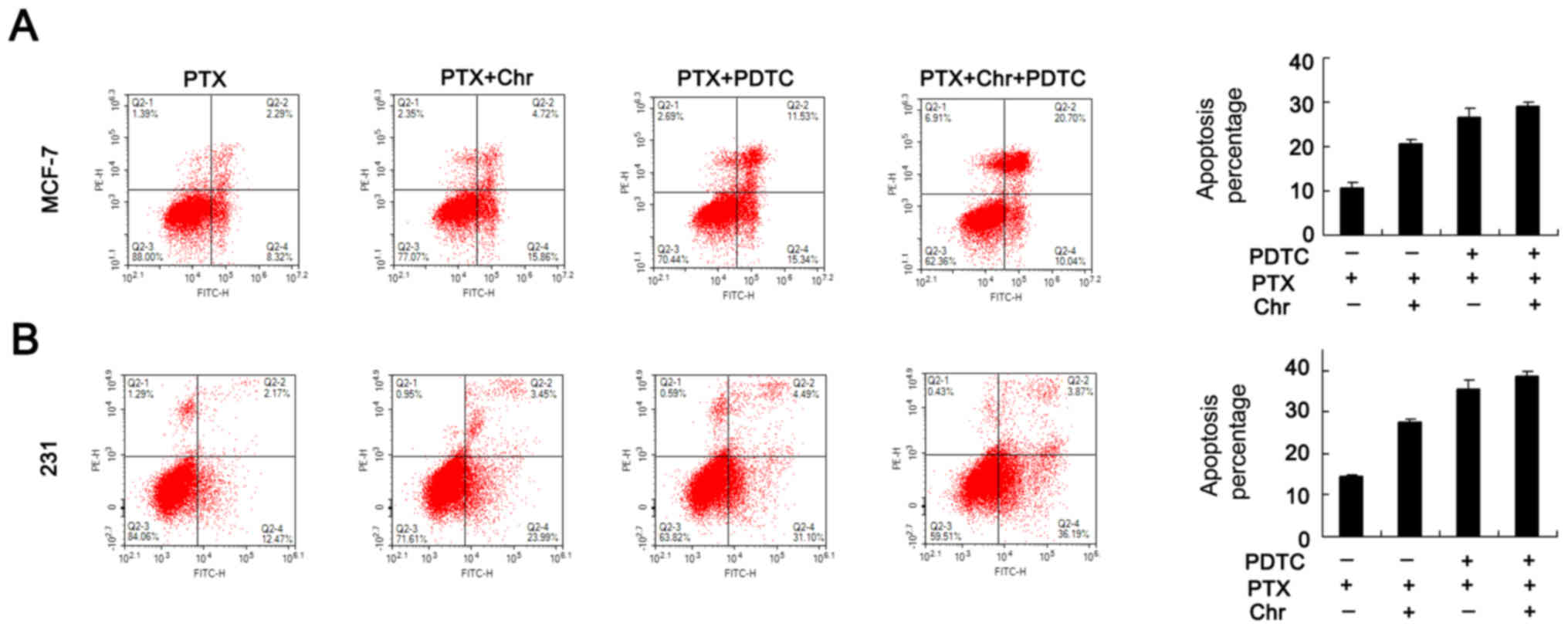
inhibitor (10 µM). Apoptosis analysis was performed and the results
showed that difference of PTX induced apoptosis rate between
control+ PDTC and chrysophanol+PDTC groups was not as significant
as that between control and chrysophanol groups (Fig. 6). As shown in Fig. 7, PDTC blocked NF-κB signaling by
reducing p-p65 and p-IκB expression. Apoptosis analysis was
performed and the results showed that difference of PTX induced
apoptosis rate between control+ PDTC and chrysophanol+PDTC groups
was not as significant as that between control and chrysophanol
groups (Fig. 6 A&B). In
addition, in PDTC treated cells, the role of chrysophanol on Bcl-2
reduction was not significant, suggesting chrysophanol induced
chemosensitivity through inhibition of NF-κB/Bcl-2 signaling
(Fig. 7).
Discussion
In this study, we used breast cancer cell lines
MCF-7 and MDA-MB-231 to examine the anti-tumor effect of
chrysophanol. MCF-7 is Luminal A subtype cell with positive ER, PR
status and negative HER2 status. MDA-MB-231 cell line is a Basal
subtype with negative ER, PR and HER2 status (Triple negative). We
chose these 2 cell lines because they represent the most common
subtype (Luminal A) and the chemotherapy resistant subtype (Triple
negative). As shown in MTT assay, chrysophanol inhibited the
proliferation of MCF-7 and MDA-MB-231 cells in a dose-dependent
manner. In addition, cell cycle progression was arrested at G1-S
point with downregulation of cyclin family proteins such as cyclin
D1 and cyclin E. When checking signaling pathways involved in
chrysophanol mediated effects, we found that chrysophanol
facilitated PTX induced apoptosis and downregulated Bcl-2.
Interestingly, we found that chrysophanol inactivated IκB and p65
phosphorylation, which are pivotal tyrosine kinases of NF-κB
(18). To confirm the involvement
of NF-κB in the anti-cancer effect of chrysophanol, we used NF-κB
inhibitor PDTC. In PDTC treated cells, the effect of chrysophanol
on Bcl-2 was not significant compared with normal MCF-7 and
MDA-MB-231 cells, suggesting chrysophanol exert its effect through
its inhibition of NF-κB activity.
First, we demonstrated that chrysophanol reduced
breast cancer cell growth, cell cycle and related proteins in a
dose dependent manner. Further study suggested chrysophanol could
inhibit NF-κB activity. NF-κB activation has been found in many
human cancers, which plays important roles in cancer proliferation,
invasion and metastasis and associates with poor survival rate
(19–21). cyclin D1 and cyclin E play pivotal
roles in cell growth of many malignant cancers (22,23).
NF-κB could upregulate its downstream molecule cyclin D1 through
phosphorylation of p65 and IκB, which induced NF-κB p65 nuclear
localization and subsequent transcriptional activation of cyclin
related proteins (24). Our result
was in accordance with these report, suggesting chrysophanol
inhibits cancer cell growth through modulation of NF-κB/cyclin
signaling.
In addition to the role of chrysophanol on NF-κB
related cancer proliferation, we showed that chrysophanol
upregulates apoptosis, which is in parallel with downregulation of
Bcl-2 protein and cleavage of caspase 3& PARP. Furthermore,
chrysophanol pretreatment enhanced the apoptosis inducing effect of
paclitaxel in breast cancer cell lines, suggesting combined
treatment would significant enhance the biological effect of PTX.
In breast cancer cells, NF-κB signaling activates Bcl-2 which plays
a central role in cancer cell survival and chemoresistance. Bcl-2
is overexpressed in breast cancers and serves as an indicator of
chemotherapy response. Many studies demonstrated that targeting
Bcl-2 inhibits tumor growth and reduces the development of
chemoresistance (25,26). Mitochondrial membrane permeability
is regulated by the Bcl-2 family of proteins. Compromised
mitochondrial membrane integrity leads to downregulated
mitochondrial potential. Thus NF-κB signaling plays an important
role during regulation of mitochondrial potential in cancer cells
(27,28). These Bcl-2 family anti-apoptotic
proteins selectively bind to Bax and block its oligomerization. Bax
can then insert into the mitochondrial membrane, compromising its
integrity and releasing cytochrome c, which leads to activation of
caspase 3 and apoptosis. PARP could be cleaved by caspase 3 in
vitro, which is involved in DNA repair in response to
environmental stress and serves as a marker of cells undergoing
apoptosis (29,30). Thus we postulate that chrysophanol
inhibits NF-κB/Bcl-2 signaling, which in turn leads to caspase
3/PARP cleavage.
To further validate the relationship between
chrysophanol, apoptosis and NF-κB/Bcl-2 signaling. NF-κB inhibitor
PDTC was adopted. In PDTC treated cells, the promoting effect of
chrysophanol on PTX induced apoptosis was not significant. The
change of Bcl-2 induced by chrysophanol was also diminished.
Together, our results revealed that chrysophanol targets
NF-κB/Bcl-2 to suppress breast cancer cell proliferation and
chemoresistance, suggesting chrysophanol could be used as a
chemotherapeutic agent towards breast cancer cells.
In conclusion, our study demonstrated that
chrysophanol inhibits malignant growth and cell cycle of breast
cancer cells by inhibiting phosphorylation of NF-κB and its
downstream NF-κB/cyclin D1 pathways. Chrysophanol also inhibits
NF-κB/Bcl-2 pathway, which facilitates PTX induced apoptosis.
Chrysophanol may serve as a novel therapeutic drug for human breast
cancer.
Acknowledgements
The present study was supported by a grant from the
National Nature Science Foundation of Liaoning Province (grant no.
201602306).
References
|
1
|
Golshan M, Cirrincione CT, Sikov WM, Carey
LA, Berry DA, Overmoyer B, Henry NL, Somlo G, Port E, Burstein HJ,
et al: Impact of neoadjuvant therapy on eligibility for and
frequency of breast conservation in stage II–III HER2-positive
breast cancer: Surgical results of CALGB 40601 (Alliance). Breast
Cancer Res Treat. 160:297–304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuyuki S, Senda N, Kanng Y, Yamaguchi A,
Yoshibayashi H, Kikawa Y, Katakami N, Kato H, Hashimoto T, Okuno T,
et al: Evaluation of the effect of compression therapy using
surgical gloves on nanoparticle albumin-bound paclitaxel-induced
peripheral neuropathy: A phase II multicenter study by the kamigata
breast cancer study group. Breast Cancer Res Treat. 160:61–67.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ni CH, Yu CS, Lu HF, Yang JS, Huang HY,
Chen PY, Wu SH, Ip SW, Chiang SY, Lin JG and Chung JG:
Chrysophanol-induced cell death (necrosis) in human lung cancer
A549 cells is mediated through increasing reactive oxygen species
and decreasing the level of mitochondrial membrane potential.
Environ Toxicol. 29:740–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ni CH, Chen PY, Lu HF, Yang JS, Huang HY,
Wu SH, Ip SW, Wu CT, Chiang SY, Lin JG, et al: Chrysophanol-induced
necrotic-like cell death through an impaired mitochondrial ATP
synthesis in Hep3B human liver cancer cells. Arch Pharm Res.
35:887–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu CC, Yang JS, Huang AC, Hsia TC, Chou
ST, Kuo CL, Lu HF, Lee TH, Wood WG and Chung JG: Chrysophanol
induces necrosis through the production of ROS and alteration of
ATP levels in J5 human liver cancer cells. Mol Nutr Food Res.
54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao L, Zhao AG, Zhao G, Xu Y, Zhu XH, Cao
ND, Zheng J, Yang JK and Xu JH: Survival benefit of traditional
chinese herbal medicine (a herbal formula for invigorating spleen)
in gastric cancer patients with peritoneal metastasis. Evid Based
Complement Alternat Med. 2014:6254932014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hun Lee J, Shu L, Fuentes F, Su ZY and
Tony Kong AN: Cancer chemoprevention by traditional chinese herbal
medicine and dietary phytochemicals: Targeting nrf2-mediated
oxidative stress/anti-inflammatory responses, epigenetics, and
cancer stem cells. J Tradit Complement Med. 3:69–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Zhao AG, Li ZY, Zhao G, Cai Y, Zhu
XH, Cao ND, Yang JK, Zheng J, Gu Y, et al: Survival benefit of
traditional chinese herbal medicine (a herbal formula for
invigorating spleen) for patients with advanced gastric cancer.
Integr Cancer Ther. 12:414–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu K, Zhang C, Wu W, Zhou M, Tang Y and
Peng Y: Rhubarb extract has a protective role against
radiation-induced brain injury and neuronal cell apoptosis. Mol Med
Rep. 12:2689–2694. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong JY, Chung HJ, Bae SY, Trung TN, Bae K
and Lee SK: Induction of cell cycle arrest and apoptosis by
physcion, an anthraquinone isolated from rhubarb (rhizomes of rheum
tanguticum), in MDA-MB-231 human breast cancer cells. J Cancer
Prev. 19:273–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Xu H, Zhang C, Gao M, Gao X, Ma C,
Lv L, Gao D, Deng S, Wang C and Tian Y: Emodin-loaded PLGA-TPGS
nanoparticles combined with heparin sodium-loaded PLGA-TPGS
nanoparticles to enhance chemotherapeutic efficacy against liver
cancer. Pharm Res. 33:2828–2843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma JW, Hung CM, Lin YC, Ho CT, Kao JY and
Way TD: Aloe-emodin inhibits HER-2 expression through the
downregulation of Y-box binding protein-1 in HER-2-overexpressing
human breast cancer cells. Oncotarget. 7:58915–58930.
2016.PubMed/NCBI
|
|
13
|
Pan FP, Zhou HK, Bu HQ, Chen ZQ, Zhang H,
Xu LP, Tang J, Yu QJ, Chu YQ, Pan J, et al: Emodin enhances the
demethylation by 5-Aza-CdR of pancreatic cancer cell
tumor-suppressor genes P16, RASSF1A and ppENK. Oncol Rep.
35:1941–1949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li KT, Duan QQ, Chen Q, He JW, Tian S, Lin
HD, Gao Q and Bai DQ: The effect of aloe emodin-encapsulated
nanoliposome-mediated r-caspase-3 gene transfection and
photodynamic therapy on human gastric cancer cells. Cancer Med.
5:361–369. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Gao M, Xu H, Guan X, Lv L, Deng S,
Zhang C and Tian Y: A promising emodin-loaded poly
(lactic-co-glycolic acid)-d-α-tocopheryl polyethylene glycol 1000
succinate nanoparticles for liver cancer therapy. Pharm Res.
33:217–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SJ, Kim MC, Lee BJ, Park DH, Hong SH
and Um JY: Anti-Inflammatory activity of chrysophanol through the
suppression of NF-kappaB/caspase-1 activation in vitro and in vivo.
Molecules. 15:6436–6451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim W, Yang C, Bazer FW and Song G:
Chrysophanol induces apoptosis of choriocarcinoma through
regulation of ROS and the AKT and ERK1/2 pathways. J Cell Physiol.
232:331–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Wu XL, Wu XY, Zhang ZH and Liu XH:
Effect of NF-κB p65 antisense oligodeoxynucleotide on
transdifferentiation of normal human lens epithelial cells induced
by transforming growth factor-β2. Int J Ophthalmol. 9:29–32.
2016.PubMed/NCBI
|
|
19
|
Hsieh SC, Hsieh WJ, Chiang AN, Su NW, Yeh
YT and Liao YC: The methanol-ethyl acetate partitioned fraction
from Chinese olive fruits inhibits cancer cell proliferation and
tumor growth by promoting apoptosis through the suppression of the
NF-κB signaling pathway. Food Funct. 7:4797–4803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng Y, Hu J, Chen Y, Yu T and Hu L:
Silencing MARCH1 suppresses proliferation, migration and invasion
of ovarian cancer SKOV3 cells via downregulation of NF-κB and
Wnt/β-catenin pathways. Oncol Rep. 36:2463–2470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thoompumkal IJ, Rehna K, Anbarasu K and
Mahalingam S: Leucine zipper down-regulated in cancer-1 (LDOC1)
interacts with Guanine nucleotide binding protein-like 3-like
(GNL3L) to modulate nuclear factor-kappa B (NF-κB) signaling during
cell proliferation. Cell Cycle. 15:3251–3267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park GH, Song HM and Jeong JB: The coffee
diterpene kahweol suppresses the cell proliferation by inducing
cyclin D1 proteasomal degradation via ERK1/2, JNK and
GKS3β-dependent threonine-286 phosphorylation in human colorectal
cancer cells. Food Chem Toxicol. 95:142–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan C, Zhu X, Han Y, Song C, Liu C, Lu S,
Zhang M, Yu F, Peng Z and Zhou C: Elevated HOXA1 expression
correlates with accelerated tumor cell proliferation and poor
prognosis in gastric cancer partly via cyclin D1. J Exp Clin Cancer
Res. 35:152016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Kady A, Sun Y, Li YX and Liao DJ:
Cyclin D1 inhibits whereas c-Myc enhances the cytotoxicity of
cisplatin in mouse pancreatic cancer cells via regulation of
several members of the NF-κB and Bcl-2 families. J Carcinog.
10:242011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fennell DA: Bcl-2 as a target for
overcoming chemoresistance in small-cell lung cancer. Clin Lung
Cancer. 4:307–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reed JC: Bcl-2 family proteins: Strategies
for overcoming chemoresistance in cancer. Adv Pharmacol.
41:501–532. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abbaspour Babaei M, Zaman Huri H,
Kamalidehghan B, Yeap SK and Ahmadipour F: Apoptotic induction and
inhibition of NF-κB signaling pathway in human prostatic cancer PC3
cells by natural compound 2,2′-oxybis (4-allyl-1-methoxybenzene),
biseugenol B, from Litsea costalis: An in vitro study. Onco Targets
Ther. 10:277–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehmood T, Maryam A, Zhang H, Li Y, Khan M
and Ma T: Deoxyelephantopin induces apoptosis in HepG2 cells via
oxidative stress, NF-κB inhibition and mitochondrial dysfunction.
Biofactors. 43:63–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Um HD: Bcl-2 family proteins as regulators
of cancer cell invasion and metastasis: A review focusing on
mitochondrial respiration and reactive oxygen species. Oncotarget.
7:5193–5203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang D, Chen MB, Wang LQ, Yang L, Liu CY
and Lu PH: Bcl-2 expression predicts sensitivity to chemotherapy in
breast cancer: A systematic review and meta-analysis. J Exp Clin
Cancer Res. 32:1052013. View Article : Google Scholar : PubMed/NCBI
|