Introduction
With the rapid development of medical technology,
the complexity of pediatric surgery has increased, as has the use
of general anesthesia (1). It is
well known in pediatric surgery that inhaled or intravenously
administered general anesthesia results in the inhibition of
synaptic transmission in the brain, thus resulting in a temporary
loss of consciousness, which is reversible. Subsequently, the
anesthetic is excreted from the body by rapid metabolism or in its
original form, without resulting in long-term damage to the
developing brain; therefore, if there are no factors leading to
cerebral hypoxia during anesthesia, the intellectual development of
infants and young children administered anesthesia will not be
affected (1,2). However, recent research has indicated
that the effects of anesthesia on central nervous system
development is not as simple as originally presumed, and the
administration of general anesthesia during the peak of nervous
system development can induce long-term neurobehavioral alterations
and cognitive function defects (3).
Ketamine (chemical formula,
C13H16ClNO), which is a white crystalline
powder at room temperature, is a similar compound to
phenylcyclidine and is a non-competitive antagonist of the
N-methyl-D-aspartate (NMDA) receptor (3). Ketamine can selectively act on the
neural pathway, and can block the pain pathway, thus resulting in
improved analgesia; therefore, ketamine is often used as an
anesthetic in minor surgery, pediatric examination or diagnostic
procedures. In addition, ketamine inhibits the functional
activities of the new cortical in relation to the hypothalamus,
stimulates the reward pathways of the limbic system, and has the
potential to induce psychological dependence (3). Ketamine initiates the generation of
strong euphoria, and continuous use for recreational purposes can
seriously damage the cognitive function of the nervous system and
mental health (4).
At present, although it has been confirmed by
numerous studies that chronic exposure to ketamine leads to
persistent cognitive impairment, such as in learning and memory,
there are relatively few studies regarding potential therapeutic
intervention, as the precise biological mechanism underlying
chronic ketamine-induced cognitive impairment remains unclear
(5,6).
Traditional Chinese medicines, including Corydalis
Tuber, are believed to possess numerous functions, including
activating blood circulation, dissipating stasis and regulating Qi.
In addition, traditional Chinese medicines may prevent platelet
aggregation, dilate small blood vessels and improve
microcirculation; these compounds are widely used to clinically
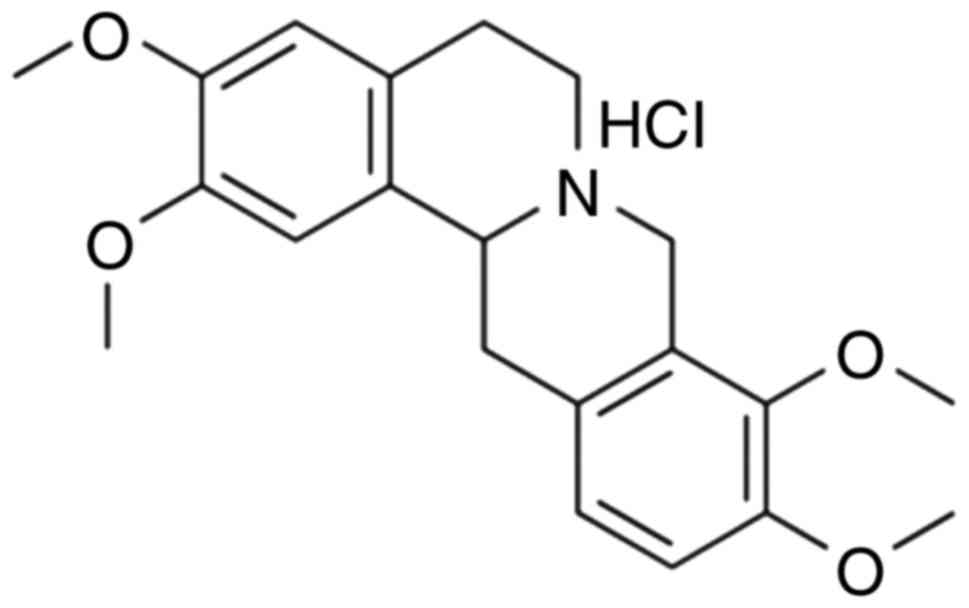
treat ischemic cerebrovascular disease (7). Tetrahydropalmatine (Fig. 1) is widely present in numerous
plants and plant extracts, such as the following: Yuan Hu,
Stephania, Hierophis viridiflavus, yellow vine and Decumbent
Corydalis Tuber. Tetrahydropalmatine is also known as Rotundine
(8). Tetrahydropalmatine is an
alkaloid that can be extracted from the Corydalis genus from
the Papaveraceae plant family. According to previous studies,
tetrahydropalmatine possesses numerous pharmacological activities,
including analgesic, anti-cerebral ischemia, anti-arrhythmic and
anti-ischemic effects; therefore, tetrahydropalmatine is used as a
sedative, analgesic, and tranquilizer in clinical practice
(8,9). The present study aimed to investigate
whether tetrahydropalmatine protects against ketamine-induced
learning and memory impairment in mice.
Materials and methods
Animals and drug administration
Male C57BL/6 mice (age, 6–8 weeks; weight, 20±2 g)
were purchased from Experimental Animal Center of Shandong
University, and housed under the following conditions: 23±2°C,
50±5% humidity, under a 12-h light/dark cycle, and were given ad
libitum access to standard chow and water. A total of 38 mice
were divided into five groups (n=6-8/group): Control group
(n=6/group), model group (n=8/group), and 20, 40 and 80 mg/kg
tetrahydropalmatine groups (n=8/group). In model or 20, 40 and 80
mg/kg tetrahydropalmatine groups, mice were intraperitoneally
(i.p.) injected with 80 mg/kg of ketamine. Then, 20, 40 and 80
mg/kg tetrahydropalmatine groups were i.p. injected at 10 ml/kg
body weight for 1 week. In control group, mice were i.p. injected
with normal saline. The present study was approved by the Animal
Administration Committee of Shandong Tumor Combat Research
Institute (Jinan, China) and were performed according to the
Guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (10).
Morris water maze test and open field
test
After 1 week of treatment with tetrahydropalmatine,
mice underwent a spatial learning test. A Morris water maze (120-cm
diameter; Shenzhen Rui Wode Life Technology Co., Ltd., Shenzhen,
China) was placed at 60 cm depth; water temperature was maintained
at 21–23°C. A cylindrical platform (14-cm diameter) was placed into
the maze (1–1.5 cm below the water surface) 35 cm from the pool
wall. Mice were allowed to remain for 90 sec and the time to find
the target recorded. The water maze test was observed for 5 days.
At the end of training, the pool was cleaned to eliminate olfactory
cues. For the open field test, an open field (36×36 cm) was created
as follows: An area of tiled floor with high plywood planks (40 cm)
was divided into 25 equal squares during the open field test. Mice
were placed in the center of the open field and the path length of
every mice was recorded for 1 min.
Biochemical analysis of brain
tissue
Following treatment with tetrahydropalmatine, mice
were sacrificed using 35 mg/kg pentobarbital sodium and the
hippocampus was dissected from each mouse onto an ice-cold plate.
Proteins were extracted from the hippocampal samples using an RIPA
assay (Beyotime Institute of Biotechnology, Haimen, China) and were
used to measure glutathione (GSH)-peroxidase (GSH-PX; A005), GSH
(A006-2), superoxide dismutase (SOD; A001-1-1), malondialdehyde
(MDA; A003-1), tumor necrosis factor (TNF)-α (H052), interleukin
(IL)-1β (H002), IL-6 (H007) and acetylcholine (ACh; A105-1) levels,
as well as acetylcholinesterase (AChE; A024), caspase-3 (G015) and
caspase-9 activity (G018) using commercially available ELISA kits
(Nanjing Jiancheng Bioengineering Research Institute, Nanjing,
China) according to the manufacturer's protocols. The absorbance
was then measured using an Infinite M200 PRO plate reader (Tecan
Group Ltd., Männedorf, Switzerland) at 450 or 405 nm.
Western blotting
The hippocampus was dissected from each mouse onto
an ice-cold plate. Proteins were extracted from the hippocampal
samples using an extraction kit (Beyotime Institute of
Biotechnology) and protein concentration was determined using BCA
assay (Beyotime Institute of Biotechnology) and an ultraviolet
spectrophotometer (UV-1601; Shimadzu Corporation, Kyoto, Japan).
Subsequently, 50 µg total protein was size-fractionated by 10%
SDS-PAGE and was immunoblotted onto polyvinylidene fluoride
membranes. The membranes were blocked with 5% non-fat milk in TBST
for 1 h at 37°C and then incubated with antibodies against iNOS
(13120; 1:2,000), GFAP (12389; 1:2,000), GDNF (3897, 1:2,000),
cytochrome c (11940; 1:2,000), PLC-γ1 (5690; 1:2,000) and GAPDH
(5174; 1:1,000; all from Cell Signaling Technology, Inc., Danvers,
MA, USA) at 4°C overnight. After washing with Tris-buffered saline
containing 0.01% Tween-20, the membranes were incubated with
biotinylated goat anti-rabbit IgG-HRP secondary antibody (sc-2030;
1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h
at 37°C, and were visualized using an enhanced chemiluminescence
kit (Amersham; GE Healthcare Life Sciences, Little Chalfont, UK)
and analyzed using Image-ProPlus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean (n=3) using SPSS version 17.0 (SPSS, Inc., Chicago, IL,
USA) and were analyzed using two-way analysis of variance and
Tukey's post hoc test with repeated measures. P<0.05 was
considered to indicate a statistically significant difference.
Results
Protective effects of
tetrahydropalmatine against ketamine-induced learning and memory
impairment in mice
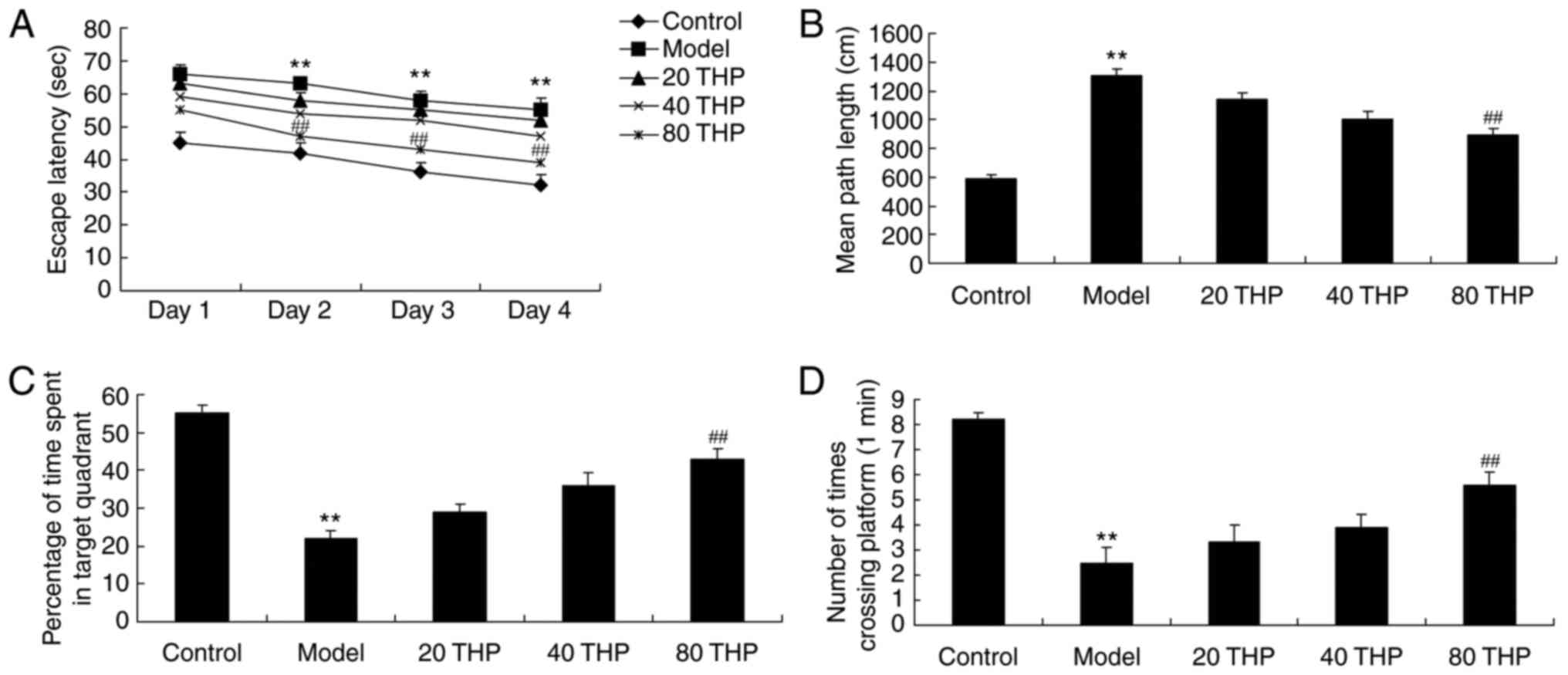
Mice treated with ketamine exhibited increased
escape latency and mean path length compared with the control mice
(Fig. 2A and B). In addition,
ketamine inhibited the percentage of time spent in the target
quadrant and the number of times crossing the platform compared
with the control mice (Fig. 2C and
D). Conversely, tetrahydropalmatine (80 mg/kg) effectively
inhibited the ketamine-induced increase in escape latency and mean
path length, and reversed the ketamine-induced decrease in the
percentage of time spent in the target quadrant and the number of
times crossing the platform (Fig.
2A-D).
Protective effects of
tetrahydropalmatine against ketamine-induced oxidative stress
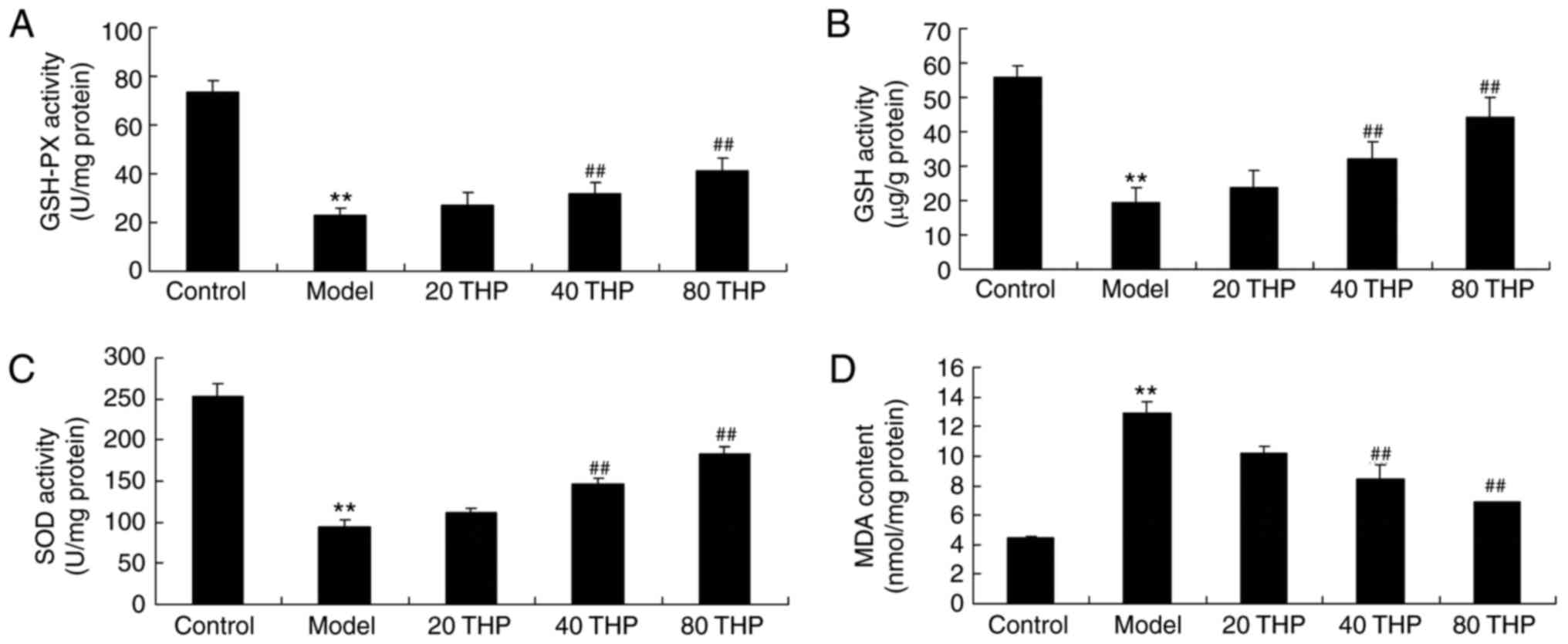
To determine the effects of tetrahydropalmatine on
ketamine-induced oxidative stress in mice, GSH-PX, GSH, SOD and MDA
activities were measured using ELISA kits. The results demonstrated
that there was a significant decrease in GSH-PX, GSH and SOD
activities, and an increase in MDA content, in ketamine-induced
mice compared with in the control mice (Fig. 3). However, treatment with
tetrahydropalmatine significantly increased GSH-PX, GSH and SOD
activities, and inhibited MDA activity, in ketamine-induced mice
(Fig. 3).
Protective effects of
tetrahydropalmatine against ketamine-induced inflammation
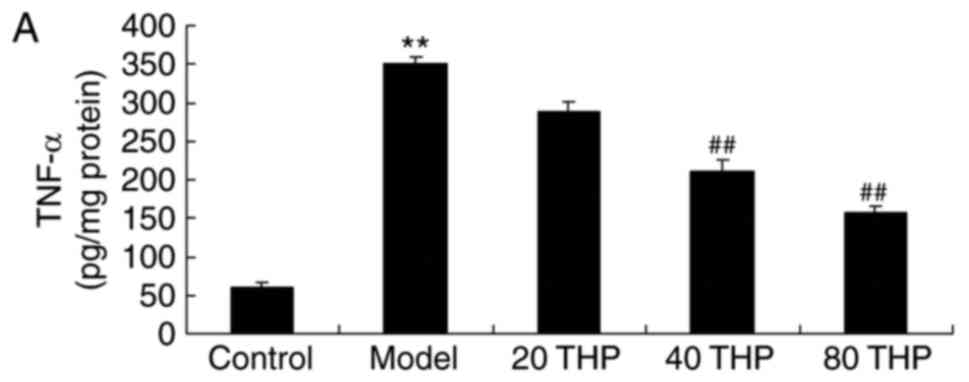
TNF-α, IL-1β and IL-6 activities were examined using
ELISA kits. As shown in Fig. 4,
there was a significant increase in TNF-α, IL-1β and IL-6
activities in ketamine-induced mice compared with in control mice.
Tetrahydropalmatine significantly decreased TNF-α, IL-1β and IL-6
activities in ketamine-induced mice (Fig. 4).
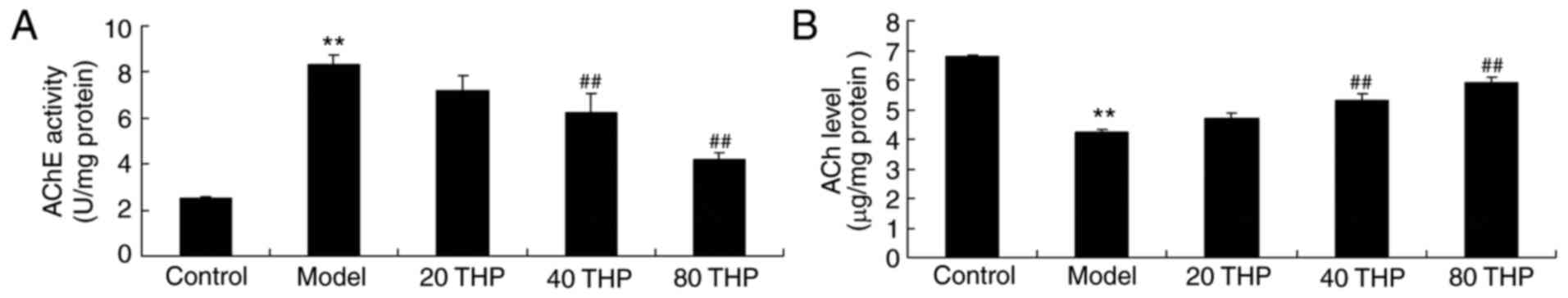
Protective effects of
tetrahydropalmatine against ACh levels and AChE activities in
ketamine-induced mice
ACh levels and AChE activities were examined using
ELISA kits. As presented in Fig.
5, AChE activity was induced and ACh levels were inhibited in
ketamine-induced mice compared with control mice. Administration of
tetrahydropalmatine significantly reduced ketamine-induced AChE
activity and increased ACh levels in ketamine-induced mice
(Fig. 5).
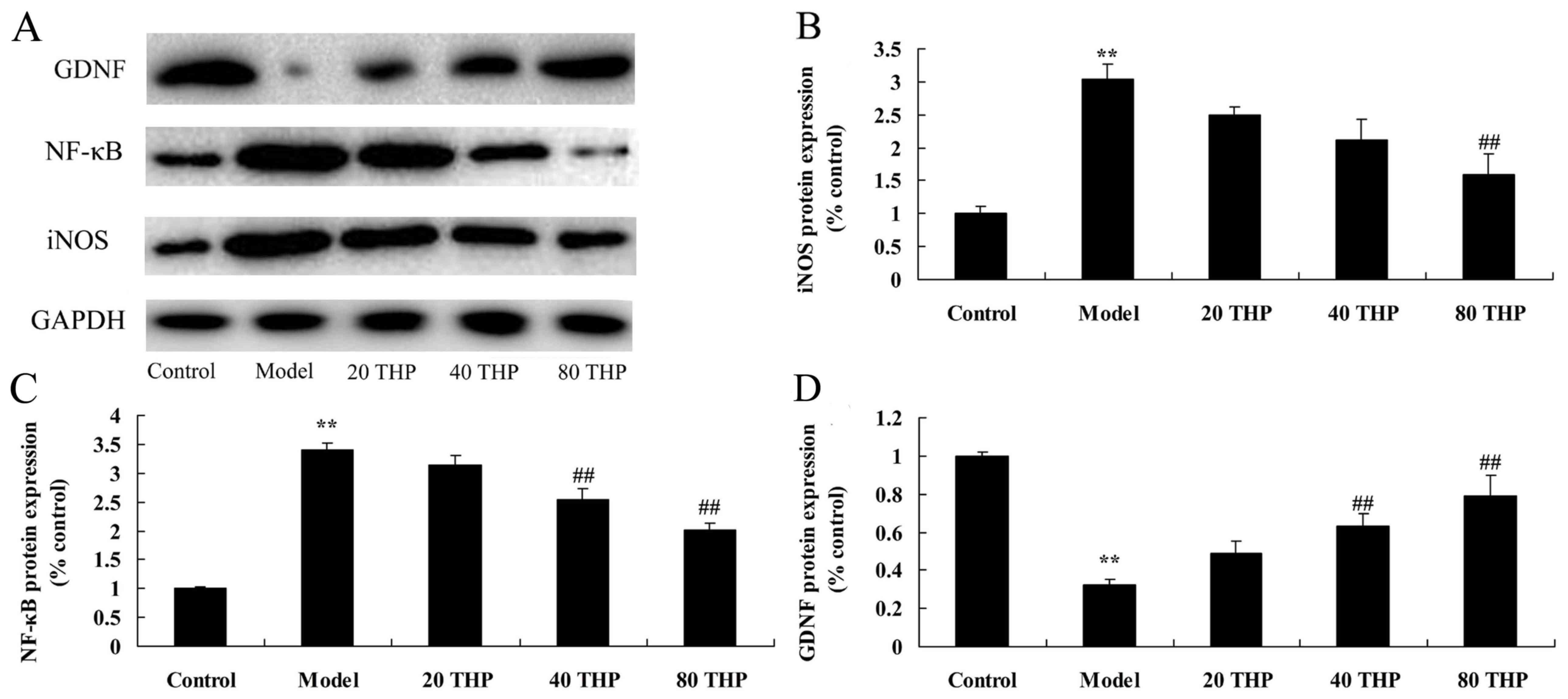
Effects of tetrahydropalmatine on
iNOS, GDNF and NF-κB protein in ketamine-induced mice
The results of a western blot analysis revealed that
ketamine significantly induced iNOS and NF-κB protein expression in
mice compared with in the control group (Fig. 6A-C). Conversely, GDNF protein
expression was significantly suppressed in the ketamine model group
compared with in the control group (Fig. 6A and D). Compared with in the
ketamine model group, treatment with tetrahydropalmatine
significantly suppressed iNOS and NF-κB protein expression, and
induced GDNF protein expression (Fig.
6).
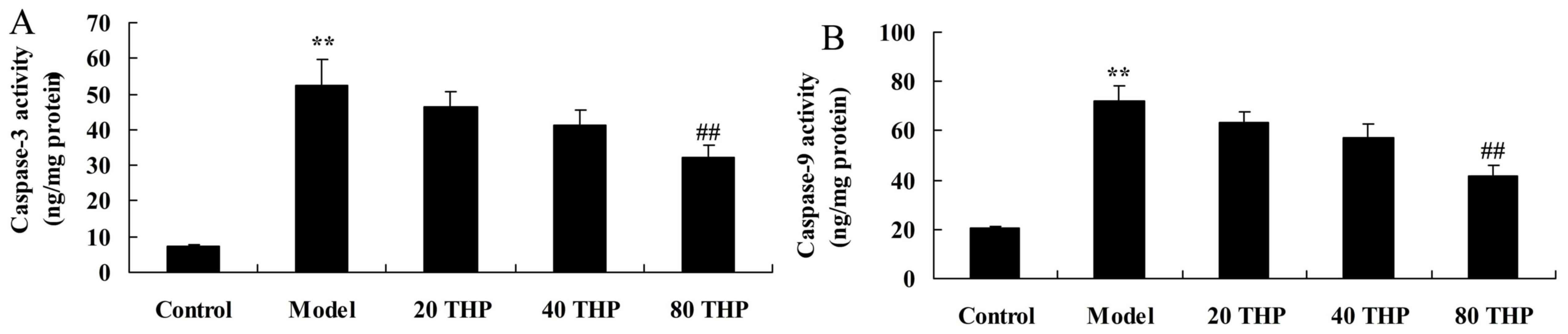
Protective effects of
tetrahydropalmatine against caspase-3 and caspase-9 activation in
ketamine-induced mice
Caspase-3 and caspase-9 activation were measured to
analyze the protective effects of tetrahydropalmatine against
learning and memory impairment. As presented in Fig. 7, activation of caspase-3 and
caspase-9 were higher in ketamine-induced mice compared with in the
control group. Conversely, tetrahydropalmatine significantly
inhibited caspase-3 and caspase-9 activation in ketamine-induced
mice (Fig. 7).
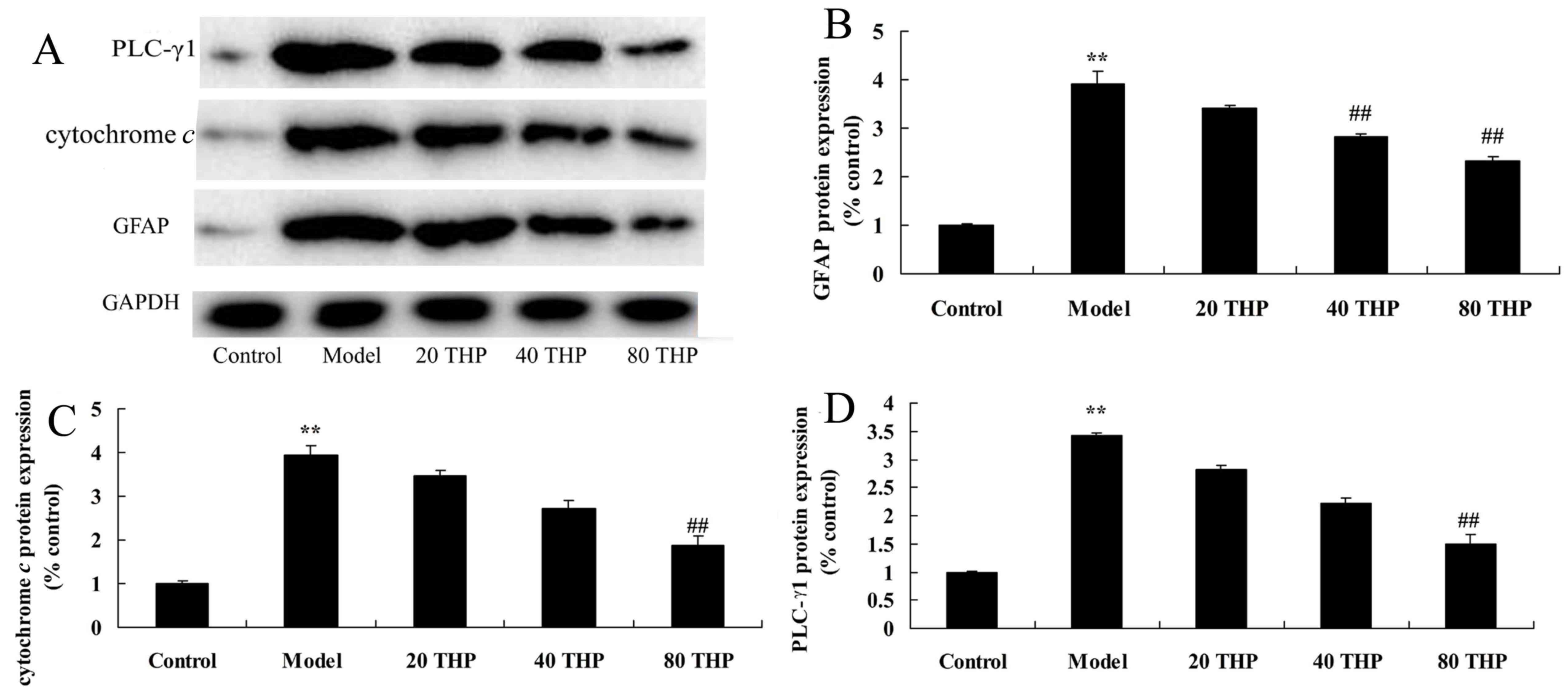
Protective effects of
tetrahydropalmatine against GFAP, cytochrome c and PLC-γ1 protein
expression in ketamine-induced mice
The present study aimed to determine the effects of
tetrahydropalmatine on GFAP, cytochrome c and PLC-γ1
expression in ketamine-induced mice. As presented in Fig. 8, the protein expression levels of
GFAP, cytochrome c and PLC-γ1 were significantly higher in
ketamine-induced mice compared with in the control group. However,
treatment with tetrahydropalmatine significantly suppressed GFAP,
cytochrome c and PLC-γ1 protein expression in
ketamine-induced mice (Fig.
8).
Discussion
A previous clinical retrospective cohort study
indicated that general anesthesia administered to children <4
years old may be considered a risk factor for long-term learning
disabilities following surgery (11). Therefore, examination of the
effects of anesthetic agents on juvenile brains during the
developmental period has great significance (12). Ketamine, which is a non-competitive
antagonist of the NMDA receptor, is a commonly used intravenous
anesthetic drug, and has been in use for nearly half a century and
is widely used in pediatric surgery (13). However, its use is controversial
due to the reported ketamine-induced reduction in juvenile learning
and memory function (14). In
addition, ketamine has been reported to induce schizophrenia-like
behaviors and oxidative damage in mice (15). The present study demonstrated that
tetrahydropalmatine effectively inhibited ketamine-induced
increases in escape latency and mean path length, and reversed the
ketamine-induced decreases in percentage of time spent in the
target quadrant and number of times crossing the platform.
Oxidative stress has been reported to be an
important cause of nerve cell damage (16). In addition, it has been
demonstrated that the abnormal deposition of amyloid-β (Aβ) may
induce oxidative stress, which leads to the loss of synaptic
function and neuronal metabolism barrier, which serves a key role
in the pathogenesis of learning and memory impairment (17). Elevated levels of oxidative stress
in brain tissues induce injury to neurons (18). The present study demonstrated that
tetrahydropalmatine significantly increased GSH-PX, GSH and SOD
activity, inhibited MDA activity, and decreased TNF-α, IL-1β and
IL-6 expression in ketamine-induced mice via the suppression of
NF-κB protein expression. Yu et al previously reported that
tetrahydropalmatine can effectively attenuate irradiation-induced
lung injury in the thoracic region through anti-apoptotic,
antifibrotic and anti-inflammatory mechanisms (8).
In neurons, choline and acetyl coenzyme A are
synthesized into ACh, which is catalyzed by choline
acetyltransferase. ACh is stored in synaptic vesicles and, in
response to stimulation, is released from cholinergic nerve endings
(19). The postsynaptic membrane
ACh receptor is known as the cholinergic receptor, of which there
are two types: Muscarinic and nicotinic receptors, which are widely
distributed in the central nervous system (20). The subsequent effects of ACh depend
on the role of AChE in the synaptic cleft; AChE can hydrolyze ACh
into choline and acetic acid, which has a very high catalytic
activity, thus ensuring that the concentration of ACh declines
rapidly (21). Therefore, AChE and
choline acetyltransferase activities in the brain can indirectly
reflect ACh content, and can thus be used to infer the functional
status of the central cholinergic system (22). Furthermore, the present study
indicated that tetrahydropalmatine significantly decreased the
ketamine-induced increase in AChE activity and reversed the
ketamine-induced decrease in ACh levels, demonstrating that
tetrahydropalmatine protected nerve cell apoptosis in
ketamine-induced mice. Qu et al clearly demonstrated that
tetrahydropalmatine may protect against D-galactose-induced memory
impairment in rats through AChE and ACh activity (23).
Learning and memory are brain functions that are
indispensable to life (24).
Learning is a neurological process that refers to the acquisition
of novel information, whereas memory refers to the process by which
obtained information is stored, organized and re-acquired by
learning experiences in the brain. These two processes are
interdependent; cognitive ability is a very important factor for
learning and memory, and also an important factor associated with
intelligence (20). The
hippocampus is the main part of the brain associated with cognitive
function. A previous study reported that the hippocampal CA1 region
serves an important role in speech recognition, and declarative
learning and memory (24). The
present study revealed that tetrahydropalmatine significantly
inhibited caspase-3 and caspase-9 activation in ketamine-induced
mice. In addition, Yu et al demonstrated that
tetrahydropalmatine may protect endothelial cells against
gamma-irradiation injury via caspase-3 activation and cytochrome
c (7). The results of the
present study demonstrated that tetrahydropalmatine may inhibit
nerve cell apoptosis in ketamine-induced mice via the caspases
signaling pathway.
B-cell lymphoma 2 (Bcl-2) and caspase-3 are two
important members of the protein family that regulates cell
apoptosis, in particular the role of these proteins in the
regulation of brain cell apoptosis has been confirmed (25). Previous studies have reported that
Bcl-2 expression is closely associated with cell survival, and an
increase in Bcl-2 expression in the brain may reduce infarct size
and protect nerve cells (25,26).
In addition, it has been demonstrated, using an
ischemia-reperfusion model, that lateral ventricle injection with
caspase-3 inhibitors not only reduces caspase-3 activity, but also
significantly reduces infarct size and apoptosis (27). Caspase-3 is a downstream regulating
protein of Bcl-2, which is the originating factor for triggering
apoptosis, and Bcl-2 overexpression can effectively inhibit
caspase-3 activation and apoptosis (27). The specific enzyme of Bcl-2
requires caspase-3 activation in the cell body for cell apoptosis;
apoptosis inducing factor and cytochrome c can activate DNA
damage, leading to cascade activation of the caspase family, which
induces apoptosis (28). The
results of the present study suggested that tetrahydropalmatine may
significantly suppress cytochrome c protein expression in
ketamine-induced mice. These data are consistent with the results
of Yu et al (29), which
indicated that tetrahydropalmatine protects rat pulmonary
endothelial cells from irradiation-induced apoptosis by inhibiting
cytochrome c and PLC-γ1.
GDNF is a newly-discovered neurotrophic factor,
which was initially detected in rat glioblastoma. GDNF is a member
of the transforming growth factor β superfamily, which is mainly
secreted by glial cells, and is expressed in the granule cells of
the striatum, thalamic nuclei, hippocampus, cingulate gyrus and
olfactory bulb, where it exerts a wide range of nutritional
functions in various central nerve cells (30). At present, it is the only
biological factor that can both resist neuronal apoptosis and
prevent tissue atrophy of the nerve cell body (31). In addition, GDNF serves an
important role in the cognitive functions of learning and memory;
in a mouse model in which GDNF expression was knocked down,
hippocampal synaptic transmission was abnormal and water maze
performance was impaired (32). In
this study, it was demonstrated that tetrahydropalmatine
significantly increased GDNF protein expression in ketamine-induced
mice.
GFAP is an important cytoskeletal protein for
astrocyte synthesis, which is now recognized as a characteristic
astrocyte marker (33). Diabetes
can affect astrocytes, resulting in alterations in GFAP expression.
A previous study revealed that in diabetic rats, the expression of
GFAP is decreased in the rat cortex, hippocampus and cerebellum,
resulting in a decrease in the generation of blood vessels, the
blood-brain barrier and the change in LTP, eventually leading to
learning and memory dysfunction (33). It has also been reported that with
the long-term stimulation of hyperglycemia, learning and memory
functions in rats are gradually decreased, accompanied by the
increased expression of GFAP in hippocampus; these results
indicated that astrocytes are associated with anesthesia-induced
cognitive dysfunction (34,35).
The results of the present study indicated that tetrahydropalmatine
significantly reduced GFAP protein expression in ketamine-induced
mice. In addition, Qu et al clearly demonstrated that
tetrahydropalmatine may protect against D-galactose-induced memory
impairment through the inhibition of GFAP expression in rats
(23).
PLCγ1 is a member of the PLC serine/threonine
family; the phosphorylation of tyrosine 783 results in its
activation, signal transmission, and finally its corresponding
cellular effect (36). In the
nervous system epileptic seizures in mice may be significantly
inhibited following induction of the tropomyosin receptor kinase
B/PLCγ1 signaling pathway; similarly, PLCγ1 affects the structural
plasticity of sensory neurons in the vestibular system and neuronal
dendrite formation in the middle of the olfactory bulb (37). In cerebellar neurons and cortical
neurons, PLCγ1 activity is associated with the release of
brain-derived neurotrophic factor-induced glutamate (38). In the present study, the results
indicated that tetrahydropalmatine significantly suppressed PLC-γ1
protein expression in ketamine-induced mice, thus suggesting that
PLC-γ1 may serve an important role in the effects of
tetrahydropalmatine on ketamine-induced toxicity.
In conclusion, the present study clearly
demonstrated that tetrahydropalmatine protects against
ketamine-induced learning and memory impairment in mice. In
addition, it was indicated that the protective effects of
tetrahydropalmatine on learning and memory impairment were
associated with antioxidative, anti-inflammatory and anti-apoptotic
mechanisms. However, further studies are required to clarify the
neurobiological mechanisms.
References
|
1
|
Li J, Yu Y, Wang B, Wu H, Xue G and Hou Y:
Selective regulation of neurosteroid biosynthesis under
ketamine-induced apoptosis of cortical neurons in vitro. Mol Med
Rep. 13:1586–1592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JR, Baek C, Han XH, Shoureshi P and
Soriano SG: Role of glycogen synthase kinase-3β in ketamine-induced
developmental neuroapoptosis in rats. Br J Anaesth. 110 Suppl
1:Si3–Si9. 2013. View Article : Google Scholar
|
|
3
|
Cetin N, Suleyman B, Altuner D,
Kuyrukluyildiz U, Ozcicek F, Coskun R, Kurt N and Suleyman H:
Effect of disulfiram on ketamine-induced cardiotoxicity in rats.
Int J Clin Exp Med. 8:13540–13547. 2015.PubMed/NCBI
|
|
4
|
Liu JR, Liu Q, Li J, Baek C, Han XH,
Athiraman U and Soriano SG: Noxious stimulation attenuates
ketamine-induced neuroapoptosis in the developing rat brain.
Anesthesiology. 117:64–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Souza DC, Ahn K, Bhakta S, Elander J,
Singh N, Nadim H, Jatlow P, Suckow RF, Pittman B and Ranganathan M:
Nicotine fails to attenuate ketamine-induced cognitive deficits and
negative and positive symptoms in humans: Implications for
schizophrenia. Biol Psychiatry. 72:785–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang S, Dai Y, Zhang Z, Hao W and Chen H:
Docosahexaenoic acid intake ameliorates ketamine-induced impairment
of spatial cognition and learning ability in ICR mice. Neurosci
Lett. 580:125–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu J, Piao BK, Pei YX, Qi X and Hua BJ:
Protective effects of tetrahydropalmatine against gamma-radiation
induced damage to human endothelial cells. Life Sci. 87:55–63.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu J, Che J, Liu L, Yang F, Zhu X and Cao
B: Tetrahydropalmatine attenuates irradiation induced lung injuries
in rats. Life Sci. 153:74–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Liang A, Zhang Y, Li C, Yi Y and
Nilsen OG: Impact of Tetrahydropalmatine on the pharmacokinetics of
probe drugs for CYP1A2, 2D6 and 3A isoenzymes in beagle dogs.
Phytother Res. 30:906–914. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koffler SP, Hampstead BM, Irani F, Tinker
J, Kiefer RT, Rohr P and Schwartzman RJ: The neurocognitive effects
of 5 day anesthetic ketamine for the treatment of refractory
complex regional pain syndrome. Arch Clin Neuropsychol. 22:719–729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dahan A, Olofsen E, Sigtermans M, Noppers
I, Niesters M, Aarts L, Bauer M and Sarton E: Population
pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain
relief of chronic pain. Eur J Pain. 15:258–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto K: A BDNF Val66Met Polymorphism
and ketamine-induced rapid antidepressant action. Clin
Psychopharmacol Neurosci. 10:59–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W, Wang N, Yang C, Li XM, Zhou ZQ and
Yang JJ: Ketamine-induced antidepressant effects are associated
with AMPA receptors-mediated upregulation of mTOR and BDNF in rat
hippocampus and prefrontal cortex. Eur Psychiatry. 29:419–423.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Wang B, Wu H, Yu Y, Xue G and Hou Y:
17β-estradiol attenuates ketamine-induced neuroapoptosis and
persistent cognitive deficits in the developing brain. Brain Res.
1593:30–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ben-Azu B, Aderibigbe AO, Ajayi AM and
Iwalewa EO: Neuroprotective effects of the ethanol stem bark
extracts of Terminalia ivorensis in ketamine-induced
schizophrenia-like behaviors and oxidative damage in mice. Pharm
Biol. 54:2871–2879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Liu CN, Wei N, Li XD, Liu YY, Yang R
and Jia YJ: Protective effects of BAY 73–6691, a selective
inhibitor of phosphodiesterase 9, on amyloid-beta peptides-induced
oxidative stress in in-vivo and in-vitro models of Alzheimer's
disease. Brain Res. 1642:327–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu KM, Chuang SM, Long CY, Lee YL, Wang
CC, Lu MC, Lin RJ, Lu JH, Jang MY, Wu WJ, et al: Ketamine-induced
ulcerative cystitis and bladder apoptosis involve oxidative stress
mediated by mitochondria and the endoplasmic reticulum. Am J
Physiol Renal Physiol. 309:F318–F331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Araujo FY, de Oliveira GV, Gomes PX,
Soares MA, Silva MI, Carvalho AF, de Moraes MO, de Moraes ME,
Vasconcelos SM, Viana GS, et al: Inhibition of ketamine-induced
hyperlocomotion in mice by the essential oil of Alpinia zerumbet:
Possible involvement of an antioxidant effect. J Pharm Pharmacol.
63:1103–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Sun LH, Jia W, Liu XM, Dang HX,
Mai WL, Wang N, Steinmetz A, Wang YQ and Xu CJ: Comparison of
ginsenosides Rg1 and Rb1 for their effects on improving
scopolamine-induced learning and memory impairment in mice.
Phytother Res. 24:1748–1754. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hall JM and Savage LM: Exercise leads to
the re-emergence of the cholinergic/nestin neuronal phenotype
within the medial septum/diagonal band and subsequent rescue of
both hippocampal ACh efflux and spatial behavior. Exp Neurol.
278:62–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HW, He XH, Yuan R, Wei BJ, Chen Z,
Dong JX and Wang J: Sesquiterpenes and a monoterpenoid with
acetylcholinesterase (AchE) inhibitory activity from Valeriana
officinalis var. Latiofolia in vitro and in vivo. Fitoterapia.
110:142–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu NG, Xiao ZJ, Zou T and Huang ZL:
Ameliorative effects of physcion 8-O-β-glucopyranoside isolated
from Polygonum cuspidatum on learning and memory in dementia rats
induced by Abeta1-40. Pharm Biol. 53:1632–1638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu Z, Zhang J, Yang H, Huo L, Gao J, Chen
H and Gao W: Protective effect of tetrahydropalmatine against
d-galactose induced memory impairment in rat. Physiol Behav.
154:114–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan D, Xue L, Zhu H and Luo Y: Catalpol
induces neuroprotection and prevents memory dysfunction through the
cholinergic system and BDNF. Evid Based Complement Alternat Med.
2013:1348522013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XY, Xu L, Liu CL, Huang LS and Zhu XY:
Electroacupuncture intervention inhibits the decline of
learning-memory ability and overex-pression of cleaved caspase-3
and bax in hippocampus induced by isoflurane in APPswe/PS 1. Zhen
Ci Yan Jiu. 41:24–30. 2016.PubMed/NCBI
|
|
26
|
Li M, Peng J, Wang MD, Song YL, Mei YW and
Fang Y: Passive movement improves the learning and memory function
of rats with cerebral infarction by inhibiting neuron cell
apoptosis. Mol Neurobiol. 49:216–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xian YF, Mao QQ, Wu JC, Su ZR, Chen JN,
Lai XP, Ip SP and Lin ZX: Isorhynchophylline treatment improves the
amyloid-β-induced cognitive impairment in rats via inhibition of
neuronal apoptosis and tau protein hyperphosphorylation. J
Alzheimers Dis. 39:331–346. 2014.PubMed/NCBI
|
|
28
|
Doniselli N, Monzeglio E, Dal Palù A,
Merli A and Percudani R: The identification of an integral
membrane, cytochrome c urate oxidase completes the catalytic
repertoire of a therapeutic enzyme. Sci Rep. 5:137982015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J, Zhao L, Liu L, Yang F, Zhu X and Cao
B: Tetrahydropalmatine protects rat pulmonary endothelial cells
from irradiation-induced apoptosis by inhibiting oxidative stress
and the calcium sensing receptor/phospholipase C-γ1 pathway. Free
Radic Res. 50:611–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyazaki H, Okuma Y, Nomura J, Nagashima K
and Nomura Y: Age-related alterations in the expression of glial
cell line-derived neurotrophic factor in the senescence-accelerated
mouse brain. J Pharmacol Sci. 92:28–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Tan H, Jiang W and Zuo Z:
Amantadine alleviates postoperative cognitive dysfunction possibly
by increasing glial cell line-derived neurotrophic factor in rats.
Anesthesiology. 121:773–785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pertusa M, Garcia-Matas S, Mammeri H,
Adell A, Rodrigo T, Mallet J, Cristòfol R, Sarkis C and Sanfeliu C:
Expression of GDNF transgene in astrocytes improves cognitive
deficits in aged rats. Neurobiol Aging. 29:1366–1379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sudo G, Kagawa T, Kokubu Y, Inazawa J and
Taga T: Increase in GFAP-positive astrocytes in histone demethylase
GASC1/KDM4C/JMJD2C hypomorphic mutant mice. Genes Cells.
21:218–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silva AF, Aguiar MS, Carvalho OS, Santana
Lde N, Franco EC, Lima RR, Siqueira NV, Feio RA, Faro LR and
Gomes-Leal W: Hippocampal neuronal loss, decreased GFAP
immunoreactivity and cognitive impairment following experimental
intoxication of rats with aluminum citrate. Brain Res. 1491:23–33.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chuang CM, Hsieh CL, Lin HY and Lin JG:
Panax Notoginseng Burk attenuates impairment of learning and memory
functions and increases ED1, BDNF and beta-secretase immunoreactive
cells in chronic stage ischemia-reperfusion injured rats. Am J Chin
Med. 36:685–693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu B, Huang YZ, He XP, Joshi RB, Jang W
and McNamara JO: A peptide uncoupling BDNF receptor TrkB from
phospholipase Cγ1 prevents epilepsy induced by status epilepticus.
Neuron. 88:484–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou L, Martinez SJ, Haber M, Jones EV,
Bouvier D, Doucet G, Corera AT, Fon EA, Zisch AH and Murai KK:
EphA4 signaling regulates phospholipase Cgamma1 activation, cofilin
membrane association and dendritic spine morphology. J Neurosci.
27:5127–5138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cortese GP, Barrientos RM, Maier SF and
Patterson SL: Aging and a peripheral immune challenge interact to
reduce mature brain-derived neurotrophic factor and activation of
TrkB, PLCgamma1 and ERK in hippocampal synaptoneurosomes. J
Neurosci. 31:4274–4279. 2011. View Article : Google Scholar : PubMed/NCBI
|