Introduction
Proteoglycan (PG) is a natural biopolymer consisting
of a core protein covalently attached to one or more
glycosaminoglycan chains. PG forms a complex with collagen,
fibronectin, laminin, hyaluronic acid and other glycoproteins in
the extracellular matrix and exists in the connective tissues, such
as the skin, bone, cartilage and vascular wall of humans and
animals (1). By incorporation with
collagen, fibronectin and laminin, PG has been shown to be involved
in cellular proliferation and adhesion (2). Regarding its complex structure and
biological activity, PG has a high potential for medical and
industrial applications. Nasal cartilage, a by-product accumulated
from salmon (Oncorhynchus keta) consumption, is an
attractive source of PG. PG from salmon nasal cartilage has been
purified and characterized (3,4). It
has been identified as an aggrecan with molecular weight of
approximately 1,400,000 daltons (4). Its core protein comprises 1,324 amino
acids with a molecular weight of 143,276 daltons. Based on its
amino acid sequence, 46 serine residues are estimated to attach to
sugar chains and the disaccharide composition in these sugar chains
contains 60% of chondroitin-6 sulfate (3). Our previous studies have demonstrated
that salmon PG has an immunomodulatory effect (5–9).
Salmon PG suppresses inflammatory responses from mouse macrophages
induced by heat-killed Escherichia coli (5). In mouse experimental models, daily
oral administration of salmon PG attenuates the severity of
inflammatory colitis (6),
autoimmune encephalomyelitis (EAE) (7), collagen-induced arthritis (8) and obesity-induced inflammation
(9). The attenuation of systemic
inflammation in colitis and EAE models by daily oral administration
of PG is associated with a reduction in T helper (Th)17 lineage
differentiation and enhancement of Foxp3+ regulatory T
cells (6,7).
The systemic immunomodulatory effect of PG has
prompted us to investigate the effect of PG on immune responses in
mouse models of allergic asthma. In the present study, the cysteine
protease papain was used as a sensitizing agent for inducing
allergic airway inflammation. In allergic asthma, dendritic cells
present allergens to CD4+ T cells, inducing Th2 cells to
produce interleukin (IL)-4, IL-5 and IL-13, which then lead to
immunoglobulin E (IgE) switching in B cells, airway eosinophilia
and mucous hypersecretion (10).
In addition, certain air pollutants, microbes and glycolipids are
able to induce the release of epithelium-derived cytokines,
including thymic stromal lymphopoietin (TSLP). This cytokine
activates type 2 innate lymphoid cells (ILC2s) to produce high
amounts of IL-5 and IL-13, leading to eosinophilia, mucous
hypersecretion and airway hyperreactivity (11).
In the present study, serum IgE, Th2-related
cytokines, TSLP and eosinophils in bronchoalveolar lavage fluid
(BALF) were evaluated following papain administration. The effect
of salmon PG was investigated, as was whether daily oral
administration of PG attenuated these allergic airway inflammatory
responses.
Materials and methods
Preparation of mice
C57BL/6 mice were purchased from Clea Japan, Tokyo,
Japan. They were maintained under specific pathogen-free conditions
with a temperature-controlled room (22°C) on a 12-h light-dark
cycle at the Institute for Animal Experimentation, Hirosaki
University Graduate School of Medicine. All animal experiments were
carried out in accordance with the Institutional Animal Care and
Use Committees/ethics Committee of Hirosaki University. The study
was approved by the Ethics Committee of the Institute for Animal
Experimentation, Hirosaki University Graduate School of Medicine
(approval no. M08028).
Sensitization and challenge of mice
with papain
Mice were sensitized and challenged with papain as
described previously (12,13), with minor modifications. Briefly,
papain (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
dissolved in phosphate-buffered saline (PBS). Mice (6 to 12-week
old) were sedated by intraperitoneal injection with a mixture of
anesthetic agents [0.075 mg/ml medetomidine (Zenoaq, Tokyo, Japan),
0.4 mg/ml midazolam (Sandoz, Tokyo, Japan) and 0.5 mg/ml
butorphanol (Meiji Seika Pharma Co., Ltd., Tokyo, Japan)] at 100
µl/10 g body weight and allowed to inhale papain (1 µg in 40 µl)
into both nostrils (20 µl each). Inhalation of PBS (40 µl) was
performed in the control group. The sedated mice were reversed by
intraperitoneal injection with 0.075 mg/ml of atipamezol
hydrochloride (Antisedan; Zenoaq) at 100 µl/10 g body weight. The
intranasal administration of papain was performed daily for 5
consecutive days. After 2 weeks, mice were boosted using the same
regimen. A total of 2 weeks post-boost, mice were challenged by
daily intranasal delivery of papain (100 µg in 40 µl) for 3
consecutive days. At 24 h after the final challenge, mice were
euthanized. Serum and BALF were collected for further analysis. To
accelerate acute allergic responses, mice were administered
intranasally as described above with 10 µg papain (in 40 µl) on
days 0 and 7. At 6, 12, 24 and 72 h after the second sensitization
with papain, all mice were euthanized. Then, the serum and BALF
were collected.
PG preparation and administration
PG extracted from salmon nasal cartilage was
purchased from Kakuhiro Co., Ltd., (Aomori, Japan). It was added
into drinking distilled water (DW) at a concentration of 0.2 mg/ml
and the mice drank ad libitum by starting on the first
papain-sensitized day. DW only was used as a negative control. A
mouse drank approximately 5 ml/day. Thus, the consumption of PG was
estimated at 1 mg/day.
Analysis of serum IgE
The titer of total IgE in the serum was determined
by sandwich enzyme-linked immunosorbent assay (ELISA) using
purified rat anti-mouse IgE monoclonal antibody (catalog number
1130-01; Southern Biotechnology Associates, Inc., Birmingham, AL,
USA) as a capture reagent, whereas heat-inactivated papain was used
to determine papain-specific IgE. For detection, goat anti-mouse
IgE antibody conjugated with horseradish peroxidase (cat. no.
1110-05; Southern Biotechnology Associates, Inc.) was used. Mouse
IgE isotype control (cat. no. 0114-01; Southern Biotechnology
Associates, Inc.) was used to establish a standard curve for total
IgE. On the other hand, a pool of sera with a high titer of
papain-specific IgE was used as a standard for the specific IgE.
The optical density obtained from 100 times-diluted standard serum
was assigned the arbitrary unit of 1.0. The titer of
papain-specific IgE in the samples was determined as a relative
value to the standard IgE-positive serum.
Collection of BALF and analysis of
BALF cells
After anesthetization of the mice, BALF was
collected via an incision to the trachea (12). PBS (0.5 ml) was flushed into the
lungs and recovered using 1 mm diameter polyethylene tubing and a 1
ml syringe. BALF cells were collected by centrifugation at 100 × g
at 4°C for 10 min and suspended in 40 µl of PBS. BALF cells were
spread on a glass slide and Giemsa staining was performed according
to the standard protocol. The granulocytes were then counted under
a light microscope (Olympus CX31; Olympus Corporation, Tokyo,
Japan) and the proportion of eosinophils was evaluated from the
number of granulocytes. The number of granulocytes per observed
area was in the range of 100-148. BALF was collected and stored at
−20°C until use.
Histological analysis of lung
tissue
A total of 72 h following the second sensitization
of papain in the acute allergic model, mice were euthanized. Lung
tissue was collected and fixed in 4% (w/v) paraformaldehyde buffer
at 4°C overnight. Tissues were then embedded in paraffin and cut
into 5-µm thick sections. Deparaffinized sections were stained with
hematoxylin and eosin. The tissues were observed under a BZ-X700
microscope (Keyence, Tokyo, Japan). Infiltrated cells were randomly
counted from four histological sections.
Detection of cytokines in BALF and
serum
The titer of TSLP, IL-4, IL-13 and IL-5 in the BALF
and serum was determined using ELISA kits, according to the
manufacturer's protocol. Determination of TSLP and IL-13 production
was achieved using a Mouse TSLP ELISA Ready-SET-Go!™ kit (cat. no.
5017406; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
Novex™ IL13 Mouse Antibody Pair (cat. no. 10180373; Thermo Fisher
Scientific, Inc.), respectively. The production of IL-4 and IL-5
was determined using Mouse IL-4 Antibody Pair and Mouse IL-5
CytoSet™, respectively (cat. nos. CMC0043 and CMC0053; Invitrogen;
Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using Ystat 2018 software
(Igaku Tosho Shuppan Co., Ltd., Tokyo, Japan) together with
Microsoft Excel software (Microsoft Corporation, Redmond, WA, USA).
The statistical significance between two samples was performed by a
Mann-Whitney U-test, whereas ANOVA analysis with a post-hoc Tukey's
test was used for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
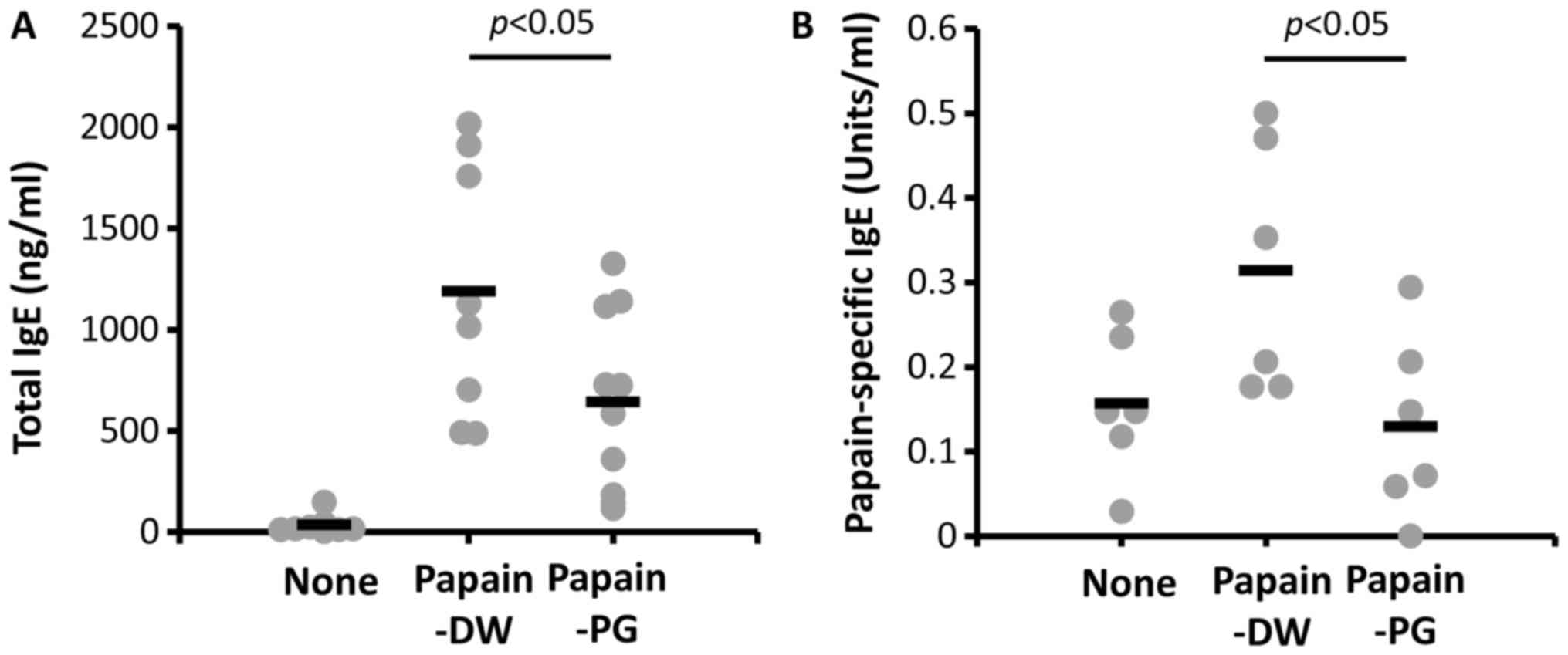
Salmon PG suppresses serum IgE levels
in papain-sensitized mice
To investigate the effect of PG on allergic
responses in papain-sensitized mice, serum IgE levels were
determined. As presented in Fig.
1, the IgE level of papain-sensitized mice with DW-drinking
increased after challenging with papain. The serum IgE of
PG-administered mice was significantly decreased compared with mice
with DW-drinking. This result is promising for the further
examination of the suppressive effect of PG in allergy mouse
models.
Salmon PG suppresses eosinophil
infiltration in lung
To observe the effect of PG on eosinophil
infiltration in the lung, the cells in the BALF were collected and
counted. In the naive mice, the infiltration of eosinophils could
not be detected. Conversely, there was increased infiltration of
eosinophils into the BALF following papain sensitization and
challenge for 3 consecutive days. This infiltration significantly
decreased when mice were orally administered with PG (Fig. 2A and B). To accelerate the acute
allergic response, mice were administered intranasally with 10 µg
papain on days 0 and 7. In this acute model, the effect of PG on
eosinophil infiltration was also confirmed. PG reduced eosinophil
infiltration in the BALF from 12 h after the second sensitization,
and the effect was retained for up to 3 days (Fig. 2C).
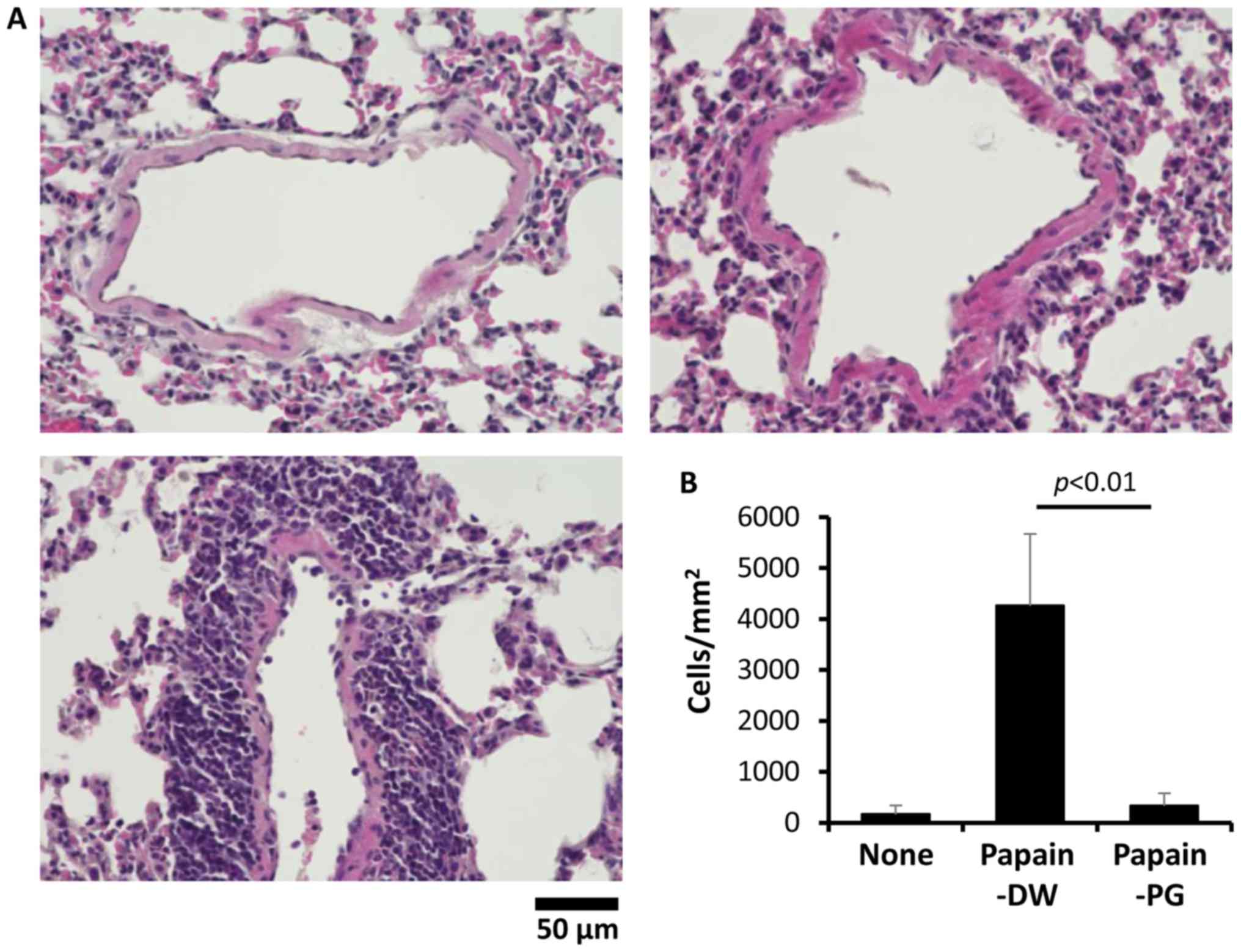
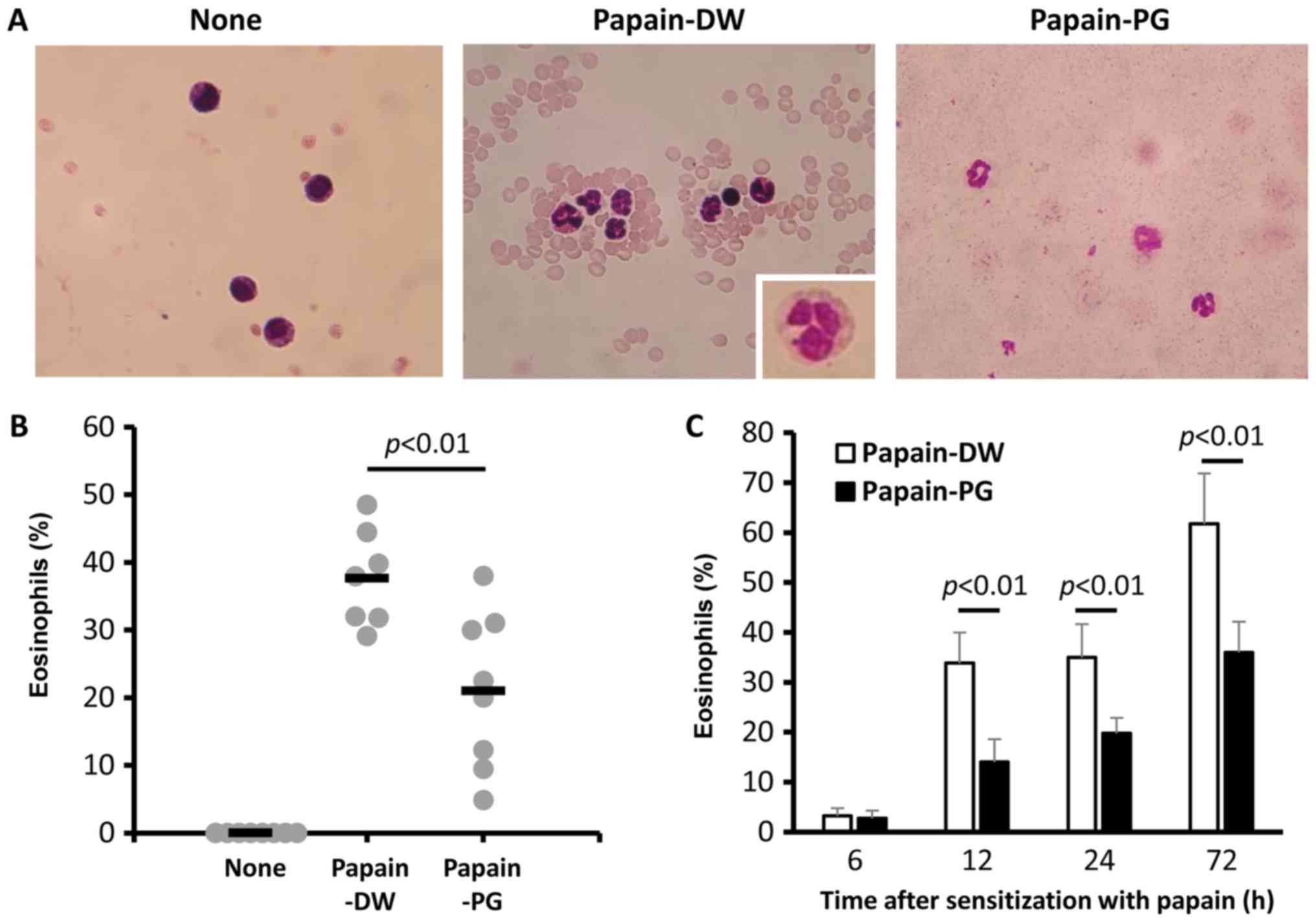 | Figure 2.Effect of PG on eosinophil
infiltration in papain-sensitized mice. Mice were sensitized daily
with 1 µg papain for 5 days. After two weeks the mice were boosted
using the same regimen. A total of 2 weeks post-boost the mice were
challenged by daily intranasal delivery of 100 µg papain for 3
consecutive days. A total of 24 h later, the BALF was collected and
(A) BALF cells were stained with Giemsa. Magnification, ×40. Images
are representative of 3–6 mice from each group. Inset, enlargement
of an eosinophil. (B) BALF cells were counted and the percentage of
eosinophils was calculated from the number of granulocytes. Each
dot on the graph represents the value of an individual mouse. The
horizontal bar indicates the mean value for each group. (C) Mice
were administered 10 µg papain intranasally on days 0 and 7. At 6,
12, 24 and 72 h after the second sensitization with papain, the
BALF was collected. BALF cells were stained with Giemsa and
counted. The percentage of eosinophils was calculated from the
number of granulocytes. BALF, bronchoalveolar lavage fluid; None,
naïve mice; PG, proteoglycan; DW, distilled water. |
PG reduces perivascular cell
infiltration
The histology of the lung tissue was then analyzed
to investigate the effect of PG on lung tissue damage. Lung tissue
damage was not observed in papain-sensitized mice. However, the
papain-sensitized mice with DW-drinking had an increased number of
inflammatory cells infiltrating around the alveolar septa, compared
with the control mice. Conversely, lung inflammation was reduced by
salmon PG. Almost no inflammatory cell infiltration was observed in
the papain-sensitized mice with PG-administration (Fig. 3A). Fig. 3B shows the quantitative analysis of
cell infiltration in the lung tissue.
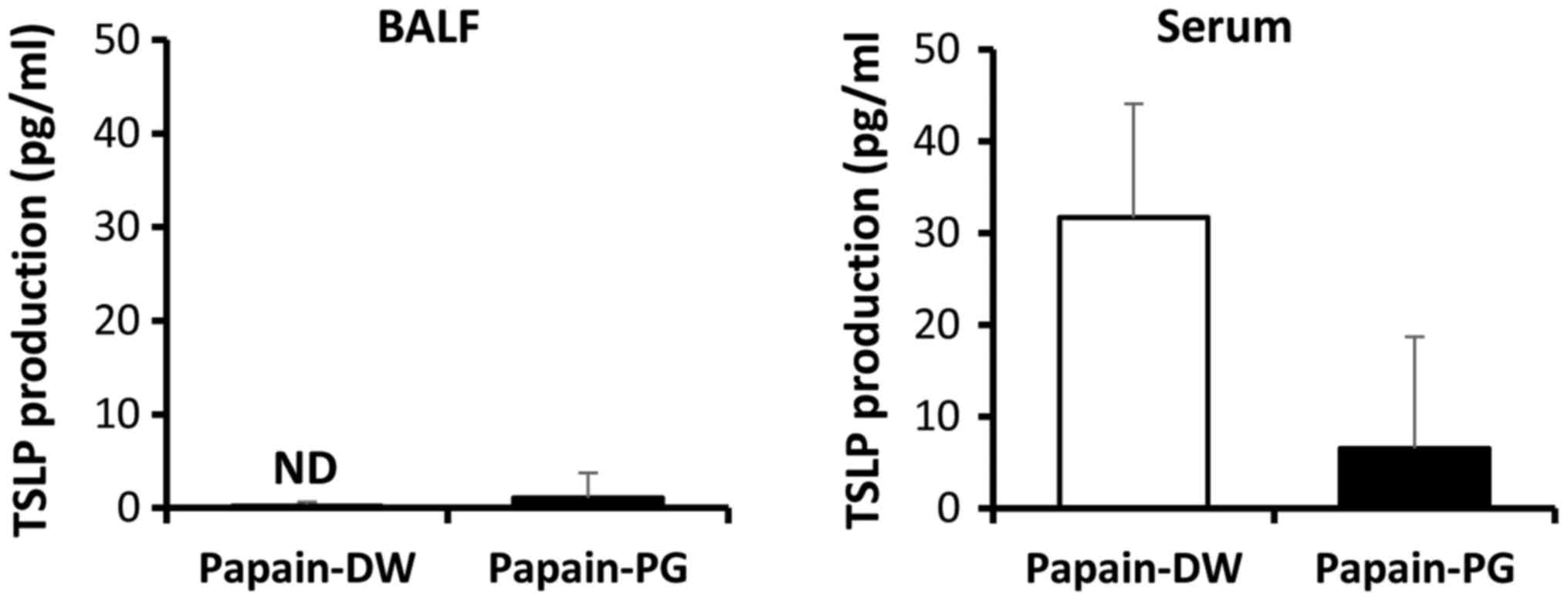
PG suppresses the production of an
epithelium-derived cytokine
Epithelium-derived cytokines have been known to
activate T cells in an antigen-dependent manner and ILC2s in an
antigen-independent manner. Thus, production of the
epithelium-derived cytokine TSLP was examined in acute
allergic-induced mice. At 12 h after the second sensitization, the
titer of TSLP in the BALF and serum was determined by ELISA.
Although the titer of TSLP of papain-sensitized mice in the BALF
was low and PG had no effect, PG suppressed TSLP production in the
serum (Fig. 4).
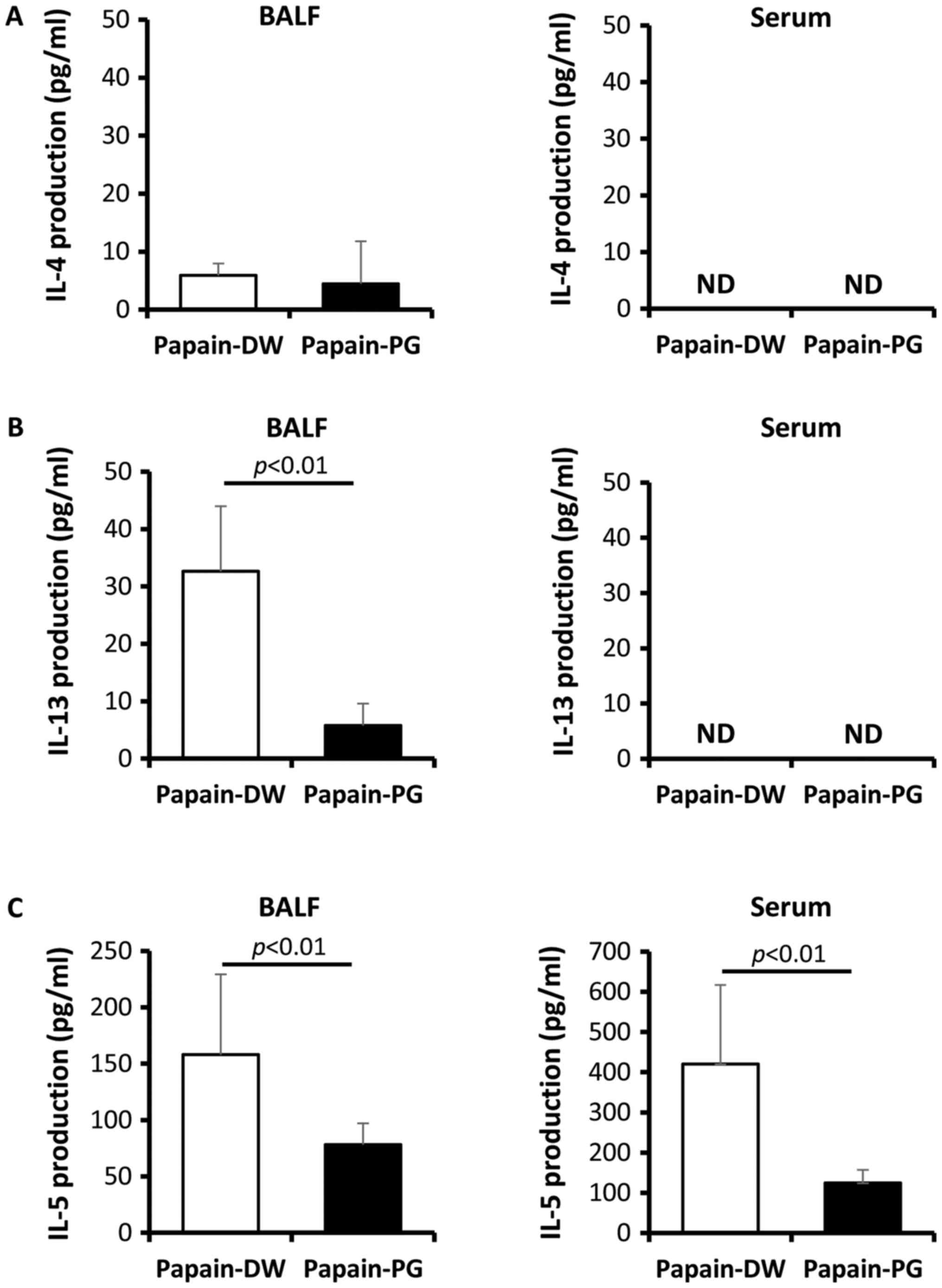
PG suppresses Th2-related cytokines in
the BALF and serum
Th2 cells are known to drive asthma pathobiology,
thus titers of Th2-related cytokines were evaluated. At 12 h after
the second sensitization of acute allergic-induced mice, BALF and
serum were collected and the titers of IL-4, IL-5 and IL-13 were
determined by ELISA. The results showed that both IL-4 and IL-13
could not be detected in the serum (Fig. 5A and B). Although IL-4 production
was detected at low levels in the BALF, PG had no significant
effect (Fig. 5A). In contrast, the
production of IL-13 in the BALF could be detected in
papain-sensitized mice and PG suppressed this IL-13 production
(Fig. 5B). The high titer of IL-5
was detected in both the serum and BALF of papain-sensitized mice.
The production of IL-5 in PG-administered mice was significantly
lower compared with DW-drinking control mice (Fig. 5C).
Discussion
At present, foods are intended not only to provide
necessary nutrients, however also to prevent nutrition-related
diseases and improve physical and mental wellbeing (14,15).
In this regard, the development of functional foods and dietary
supplements is a focus, in order to improve healthcare and disease
prevention (16–19). Salmon nasal cartilage is a major
by-product of salmon consumption (20). PG extracted from salmon nasal
cartilage is a complex biopolymer which has the potential to be a
high-value biomaterial. Several properties of salmon PG have been
characterized. Salmon PG has been shown to exhibit immunomodulatory
effects in both in vitro and in vivo experiments
(5–9). However, daily oral administration of
salmon PG (2 mg/day) for up to 3 months did not promote anti-PG
antibody production or infection susceptibility (data not shown).
This property makes PG to become an attractive prophylactic agent
for use as a supplement. In the present study, the effect of PG on
allergic responses in a mouse model of papain-induced respiratory
inflammation was investigated. The amount of PG and the starting
time of administration were designed based on our previous studies
(6–9).
Allergies are defined as an overreaction of the
immune system to allergens such as pollen or certain foods
(21). This disease is widespread
and affects a large number of people worldwide. Allergens stimulate
plasma cells to produce IgE and bind IgE that has attached to mast
cells. This binding triggers the release of histamine from mast
cells. In the lung, histamine causes bronchiole constriction and
airflow reduction, called asthma (22). Although allergies in specific
tissues are not life-threatening, the allergic response in the
bloodstream can be fatal. To attenuate allergies, the use of
prophylactic agents is an alternative method to allergen avoidance.
To demonstrate whether salmon PG has an immunomodulatory effect on
allergies, a mouse model of papain-induced allergic asthma was used
in the present study. Papain, a cysteine protease, is a homolog of
the major house dust mite allergen Dermatophagoides
pteronyssinus Der p 1 (12,13).
It can induce respiratory inflammation by intranasal administration
with 1 µg several times (12) or
with 10 µg twice (13). From both
models of papain administration, we demonstrated that eosinophil
infiltration in the lung significantly increased in comparison to
naïve mice (Fig. 2).
Th2 cytokines play important roles in allergic
asthma. These cytokines, such as IL-4, IL-5 and IL-13, are thought
to be related to Th2 immunity (10). Conversely, epithelium
derived-cytokines such as IL-33 and TSLP play an important role in
non-allergic airway inflammation (11). These cytokines activate ILC2s to
produce IL-5 and IL-13, however not IL-4. Of the Th2 cytokines,
IL-4 and IL-13 drive naïve Th cells toward the Th2 phenotype and
induce B cells to switch their isotype to IgE, whereas IL-5
produced by Th2 cells is responsible for eosinophil growth,
differentiation, mobilization, recruitment, activation and
survival. Excessive production of IL-4, IL-5 and IL-13 has been
implicated in the development of asthma (22). In the present study, intranasal
papain administration increased TSLP production in the serum
(Fig. 4) and T-cell responsive Th2
cytokines, IL-5 and IL-13 in the BALF (Fig. 5). For an unknown reason, the
production of IL-4 and TSLP was low. This may have been due to the
lower susceptibility of C57BL/6 mice to papain sensitization, in
comparison with that of BALB/c mice. However, PG significantly
suppressed the production of TSLP in the serum, and IL-5 and IL-13
in the BALF (Figs. 4 and 5) which are expected to suppress
eosinophil infiltration and IgE-switching in B cells. In this
regard, salmon PG attenuated perivascular cell infiltration
(Fig. 3). To further confirm the
effect of PG on allergic response, the airway hyperresponsiveness
which is a common finding in allergic bronchial inflammation is
required.
The molecular mechanism of PG and its
immunomodulatory effect remains to be determined. The effect of PG
on intestinal microbiota is one of several possibilities. Daily
oral administration of salmon PG has been shown to improve
intestinal microbiota by enhancing probiotics and short chain fatty
acid-producing bacteria (23).
Alternatively, salmon PG may directly affect the intestinal
epithelium or immune cells, which then regulate intestinal and
systemic immune responses.
In conclusion, the present study demonstrated that
salmon cartilage PG attenuates allergic responses in a mouse model
of papain-induced respiratory inflammation. Oral administration of
PG reduced the serum IgE, eosinophil infiltration and the titers of
epithelium-derived and Th2-related cytokines. Although only a
partial ability of PG to relieve acute allergic asthma was
revealed, and not a chronic reaction, the data in the present study
suggested that continual consumption of salmon PG may be able to
attenuate overreactive allergies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the City Area
Program for the Promotion of Science and Technology in Regional
Areas from the Ministry of Education, Culture, Sports, Science and
Technology.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
AN conceived and designed the study. AN, KA and MT
conceived the study and were involved in funding acquisition. HKO,
SY, SH and KN performed the experiments and analyzed the data. KA
wrote and revised the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Institute for Animal Experimentation, Hirosaki University
Graduate School of Medicine (approval no. M08028).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BALF
|
bronchoalveolar lavage fluid
|
|
EAE
|
autoimmune encephalomyelitis
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IgE
|
immunoglobulin E
|
|
IL
|
interleukin
|
|
ILC2s
|
type 2 innate lymphoid cells
|
|
PG
|
proteoglycan
|
|
TSLP
|
thymic stromal lymphopoietin
|
References
|
1
|
Har-el R and Tanzer ML: Extracellular
matrix. 3: Evolution of the extracellular matrix in invertebrates.
FASEB J. 7:1115–1123. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danen EH and Yamada KM: Fibronectin,
integrins, and growth control. J Cell Physiol. 189:1–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kakizaki I, Mineta T, Sasaki M, Tatara Y,
Makino E and Kato Y: Biochemical and atomic force microscopic
characterization of salmon nasal cartilage proteoglycan. Carbohydr
Polym. 103:538–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kakizaki I, Tatara Y, Majima M, Kato Y and
Endo M: Identification of proteoglycan from salmon nasal cartilage.
Arch Biochem Biophys. 506:58–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sashinami H, Takagaki K and Nakane A:
Salmon cartilage proteoglycan modulates cytokine responses to
Escherichia coli in mouse macrophages. Biochem Biophys Res Commun.
351:1005–1010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsui T, Sashinami H, Sato F, Kijima H,
Ishiguro Y, Fukuda S, Yoshihara S, Hakamada K and Nakane A: Salmon
cartilage proteoglycan suppresses mouse experimental colitis
through induction of Foxp3+ regulatory T cells. Biochem
Biophys Res Commun. 402:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sashinami H, Asano K, Yoshimura S, Mori F,
Wakabayashi K and Nakane A: Salmon proteoglycan suppresses
progression of mouse experimental autoimmune encephalomyelitis via
regulation of Th17 and Foxp3(+) regulatory T cells. Life Sci.
91:1263–1269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimura S, Asano K and Nakane A:
Attenuation of collagen-induced arthritis in mice by salmon
proteoglycan. Biomed Res Int. 2014:4064532014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirose S, Asano K and Nakane A:
Attenuation of obesity-induced inflammation in mice orally
administered with salmon cartilage proteoglycan, a prophylactic
agent. Biochem Biophys Res Commun. 484:480–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wenzel SE: Asthma phenotypes: The
evolution from clinical to molecular approaches. Nat Med.
18:716–725. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brusselle GG, Maes T and Bracke KR:
Eosinophils in the spotlight: Eosinophilic airway inflammation in
nonallergic asthma. Nat Med. 19:977–979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGlade JP, Gorman S, Lenzo JC, Tan JW,
Watanabe T, Finlay-Jones JJ, Thomas WR and Hart PH: Effect of both
ultraviolet B irradiation and histamine receptor function on
allergic responses to an inhaled antigen. J Immunol. 178:2794–2802.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halim TY, Steer CA, Mathä L, Gold MJ,
Martinez-Gonzalez I, McNagny KM, McKenzie AN and Takei F: Group 2
innate lymphoid cells are critical for the initiation of adaptive T
helper 2 cell-mediated allergic lung inflammation. Immunity.
40:425–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roberfroid MB: Global view on functional
foods: European perspectives. Br J Nutr. 88 Suppl 2:S133–S138.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menrad K: Market and marketing of
functional food in Europe. J Food Eng. 56:181–188. 2003. View Article : Google Scholar
|
|
16
|
Tang ML: Probiotics and prebiotics:
Immunological and clinical effects in allergic disease. Nestle Nutr
Workshop Ser Pediatr Program. 64:pp. 219–238, 251-257. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Floch MH and Hong-Curtiss J: Probiotics
and functional foods in gastrointestinal disorders. Curr
Gastroenterol Rep. 3:343–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cencic A and Chingwaru W: The role of
functional foods, nutraceuticals, and food supplements in
intestinal health. Nutrients. 2:611–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alissa EM and Ferns GA: Functional foods
and nutraceuticals in the primary prevention of cardiovascular
diseases. J Nutr Metab. 2012:5694862012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olsen RL, Toppe J and Karunasagar I:
Challenges and realistic opportunities in the use of by-products
from processing of fish and shellfish. Trends Food Sci Technol.
36:144–151. 2014. View Article : Google Scholar
|
|
21
|
Palomares O, Akdis M, Martín-Fontecha M
and Akdis CA: Mechanisms of immune regulation in allergic diseases:
The role of regulatory T and B cells. Immunol Rev. 278:219–236.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kubo M: Innate and adaptive type 2
immunity in lung allergic inflammation. Immunol Rev. 278:162–172.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asano K, Yoshimura S and Nakane A:
Alteration of intestinal microbiota in mice orally administered
with salmon cartilage proteoglycan, a prophylactic agent. PLoS One.
8:e750082013. View Article : Google Scholar : PubMed/NCBI
|