Introduction
Numerous infants and young children receive surgical
treatments and anesthesia (1).
Between the embryonic period and the second year following birth,
the human brain is growing and developing. In particular, the rapid
growth of cerebral neurons, and the formation of axons, dendrites
and synapses, may be observed during this period (2). Nerve cells are sensitive to a number
of general anesthetic drugs; therefore, exposing the developing
central nervous system to these general anesthetics may cause brain
damage (3). Sevoflurane (SEV)
serves as a commonly used general anesthetic drug that has been
widely adopted for infant anesthesia (4). Long-term exposure to SEV may cause
neurological disorders and may lead to neurodegeneration in the
developing brain (5).
Additionally, SEV may impair memory and cognitive functions in
infants and young children (3).
Studies have demonstrated that in rats and mice, exposure to
clinically relevant anesthetics, including isoflurane and SEV,
results in neurological disorders (6–8),
synaptic alterations in the hippocampus (9,10), a
reduction in axonal connections (11) and cerebral cortex axon disorders
(12,13). These findings indicated that
exposure to anesthesia may cause damage to the developing
brain.
Mitogen-activated protein kinases (MAPKs) are a type
of protein kinase with dual serine and threonine phosphorylation
capacity, which act as a signal transduction system that mediates
extracellular signals (14). p38
MAPK (p38) and c-Jun N-terminal kinase (JNK) represent crucial
members of the MAPK family. Studies have demonstrated that p38
serves important roles during anti-inflammatory processes (15,16),
whereas the JNK pathway is considered to be critical for embryonic
morphogenesis, cell differentiation and the regulation of apoptosis
(17,18). However, the modulation of the
p38/JNK pathway by pilose antler polypeptide (PAP) remains to be
elucidated.
Pilose antler (PA) is obtained from male Cervus
nippon Temminck or Cervus elaphus (19) and used to isolate PAP. Studies have
demonstrated that PAP possesses multiple biological activities,
including ossification (20),
anti-inflammation (21) and
anti-oxidative stress (22).
Furthermore, it has been revealed that PA contains insulin-like
growth factors and the associated receptors, which may promote
protein synthesis in nerve cells and accelerate the growth of axons
(23). However, the role of PAP in
protecting nerve cells during SEV-induced injury remains
unclear.
In the present study, the role of PAP and the
p38/JNK pathway in SEV-induced neurocyte injury was
investigated.
Materials and methods
Reagents
PAP was obtained from Affiliated hospital of
Changchun University of Chinese medicine (Jilin, China). SEV-mixed
gas (3%) was purchased from Xilong Scientific Co., Ltd. (Shenzhen,
China).
Cell culture
Sprague Dawley rats (8–12 weeks; 3 males, 7 females;
weight, 220–360 g) were obtained from Guangdong Medical Laboratory
Animal Center (Foshan, China). The animals were kept at 21±2°C with
humidity of 60–70% and a 12-h light/dark cycle, and had free access
to food and water. The animals were mated to produce neonatal rats.
A total of five 24 h-old neonatal rats were used to isolate
neuronal cells. Neonatal rats were sacrificed by rapid cervical
dislocation. Subsequently, the animals were disinfected with 75%
ethanol and transferred to Hank's balanced salt solution. The
hippocampus was removed and digested with 0.125% trypsin (Beyotime
Institute of Biotechnology, Haimen, China) in a cell incubator for
10 min at 37°C with 5% CO2. The supernatant was
discarded. Subsequently, Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) was
added to the tissues and gently agitated at room temperature for
3–4 min. The obtained nerve cells were incubated at 37°C with 5%
CO2. The cells were observed under a light microscope
(magnification, ×200). After 7 days of culture in Neurobasal medium
(Thermo Fisher Scientific, Inc.), nerve cells were treated with
SEV. Neurobasal medium was replaced prior to the treatment with
SEV. The protocols for the animal experiments were approved by the
Ethics Committee of Xinjiang Uygur Autonomous Region Hospital of
TCM (Urumchi, China).
Experimental groups
The five treatment groups in the present study were
as follows: Control group (nerve cells with no treatment), SEV
group (nerve cells treated with 3% SEV mixed gas for 12 h in an
anesthesia box) and PAP+SEV groups (nerve cells pretreated with 10,
20 or 30 µM PAP for 6 h, and subsequently treated with 3% SEV mixed
gas for 12 h in an anesthesia box). The dosage of PAP was set
according to two previous studies (24,25).
SB203580 (10 µM; Selleck Chemicals, Houston, TX, USA) was used to
inhibit p38. SB203580 was added 45 min before the PAP
treatment.
Cell viability analysis
A Cell Counting kit-8 assay (CCK-8; Beyotime
Institute of Biotechnology) was performed to assess the cell
viability of nerve cells. Cultured nerve cells in the logarithmic
phase (~6×104 cells/ml) were seeded into 96-well plates,
and maintained at 37°C with 5% CO2 for 12 h.
Subsequently, 10 µl CCK-8 reagent was added to the wells after 12,
24 and 48 h. Nerve cells were maintained for another 3 h, and a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to record the absorbance at 450 nm. Cell viability was
evaluated as the proportion of cell survival compared with the
control.
Flow cytometry (FCM)
FCM was performed to test the proliferation and
apoptosis of nerve cells. Cultured nerve cells were treated with
0.25% trypsin (Beyotime Institute of Biotechnology). The
supernatant was removed and the nerve cells at a density of
1×106 cells/ml were resuspended in PBS. The
proliferation was detected using CFSE (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were stained with CFSE for 10 min at
37°C. Nerve cells were incubated with Annexin V-phycoerythrin (PE)
and 7-ADD (Annexin V-PE Apoptosis Detection kit, BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA) in the dark at room
temperature for 15 min. Cells were acquired on a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and BD
CellQuest software version 5.1 (BD Biosciences) was used for data
analysis.
Western blot analysis
Proteins were isolated using NP-40 lysis buffer
(Beyotime Institute of Biotechnology). The concentration of
proteins was detected using a Bicinchoninic Acid Assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Proteins (25 µg/lane) were
separated by SDS-PAGE on a 12% gel. The separated products were
transferred onto a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked with 5% non-fat milk
at room temperature for 2 h. Blotting was performed with specific
primary antibodies at 4°C overnight: Anti-activated-caspase-3
(1:500; cat. no. ab13847; rabbit anti-rat), anti-pro-caspase-3
(1:10,000; cat. no. ab32499; rabbit anti-rat),
anti-Bcl-2-associated X protein (Bax; 1:1,000; cat. no. ab32503;
rabbit anti-rat), anti-B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no.
ab59348; rabbit anti-rat), anti-phosphorylated (p)-p38 (1:1,000;
cat. no. ab4822; rabbit anti-rat), anti-p38 (1:1,000; cat. no.
ab170099; rabbit anti-rat), anti-p-JNK (1:1,000; cat. no. ab124956;
rabbit anti-rat), anti-JNK (1:1,000; cat. no. ab179461; rabbit
anti-rat) and anti-GAPDH (1:2,500; cat. no. ab9485; rabbit
polyclonal; all Abcam, Cambridge, UK). The membrane was washed with
PBST buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 3 times (5 min/time). Horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
ab205718; goat anti-rabbit; Abcam) were added and incubated at room
temperature for 1 h. Enhanced chemiluminescent reagents (Millipore,
Billerica, MA, USA) in combination with an ECL system (Amersham
Pharmacia, Piscataway, NJ, USA) were used to visualize the protein
bands. The density of the blots was read by Quantity One software
version 4.6.9 (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cultured nerve cells
using TRIzol® reagent (Beyotime Institute of
Biotechnology). RNA was reverse transcribed to cDNA using BeyoRT II
First Strand cDNA Synthesis kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocols.
SYBR-Green PCR Master Mix (Thermo Fisher Scientific, Inc.) was
performed using an Applied Biosystems® 7500 thermocycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR
cycling conditions were as follows: Pretreatment at 94°C for 4 min,
followed by 35 cycles of 95°C for 15 sec and 68°C for 30 sec, a
final extension at 75°C for 10 min and hold at 4°C. The following
primers were designed by Invitrogen (Thermo Fisher Scientific,
Inc.): caspase-3 forward, 5′-TGTCGATGCAGCTAACCTCA-3′ and reverse,
5′-GCAGTAGTCGCCTCTGAAGA-3′ (241 bp); Bax forward,
5′-GAGACACCTGAGCTGACCTT-3′ and reverse, 5′-CGTCTGCAAACATGTCAGCT-3′
(187 bp); Bcl-2 forward, 5′-AACTCTTCAGGGATGGGGTG-3′ and reverse,
5′-GCTGGGGCCATATAGTTCCA-3′ (209 bp); and GAPDH forward,
5′-AGTCTACTGGCGTCTTCACC-3′ and reverse, 5′-CCACGATGCCAAAGTTGTCA-3′
(225 bp). GAPDH was used as the internal control. The relative gene
expression levels were calculated using the 2−ΔΔCq
method (26).
Statistical analysis
The results are presented as the mean ± standard
deviation for at least three independent experiments. The
experimental data were analyzed by one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. GraphPad Prism
version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used
to analyze the data.
Results
Identification of primary rat
hippocampal neurons
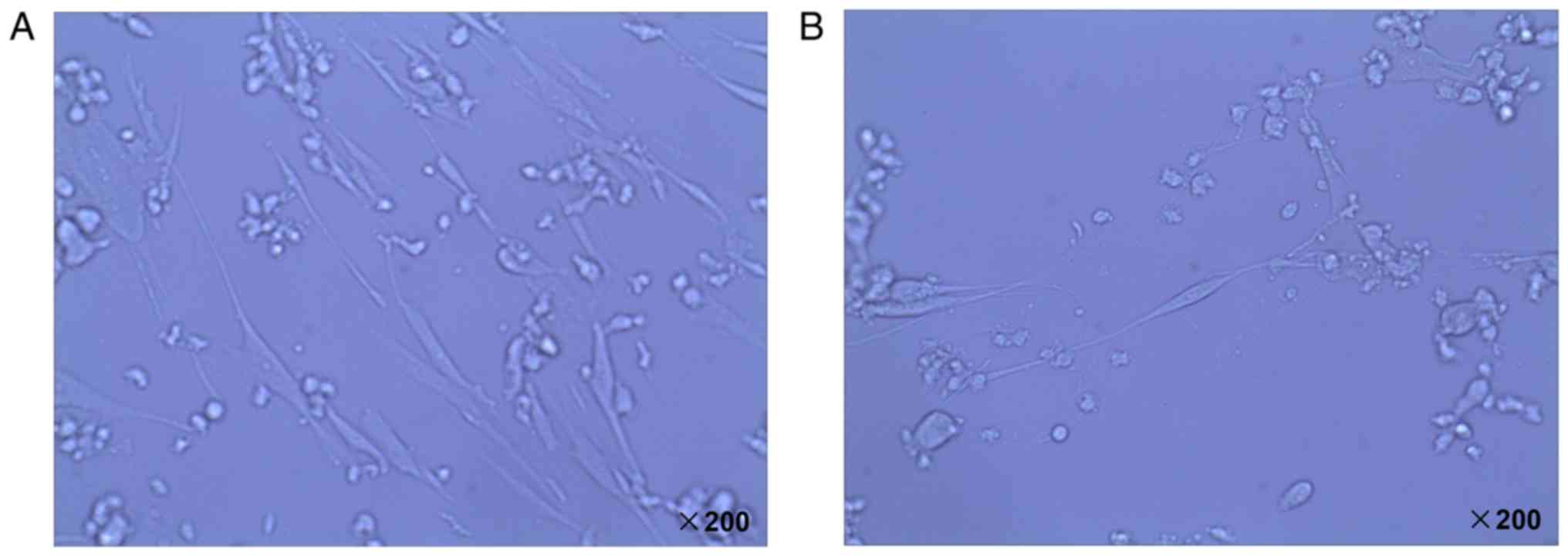
Neurons were isolated from rat hippocampus and
cultured for >2 days before they were visualized under a
microscope. Neuron cells with flat polygonal shape were observed
(Fig. 1A) and a visible halo was
identified around the nerve cells (Fig. 1B). These nerve cells were harvested
and prepared for subsequent experiments.
PAP enhances the cell viability of
SEV-treated nerve cells
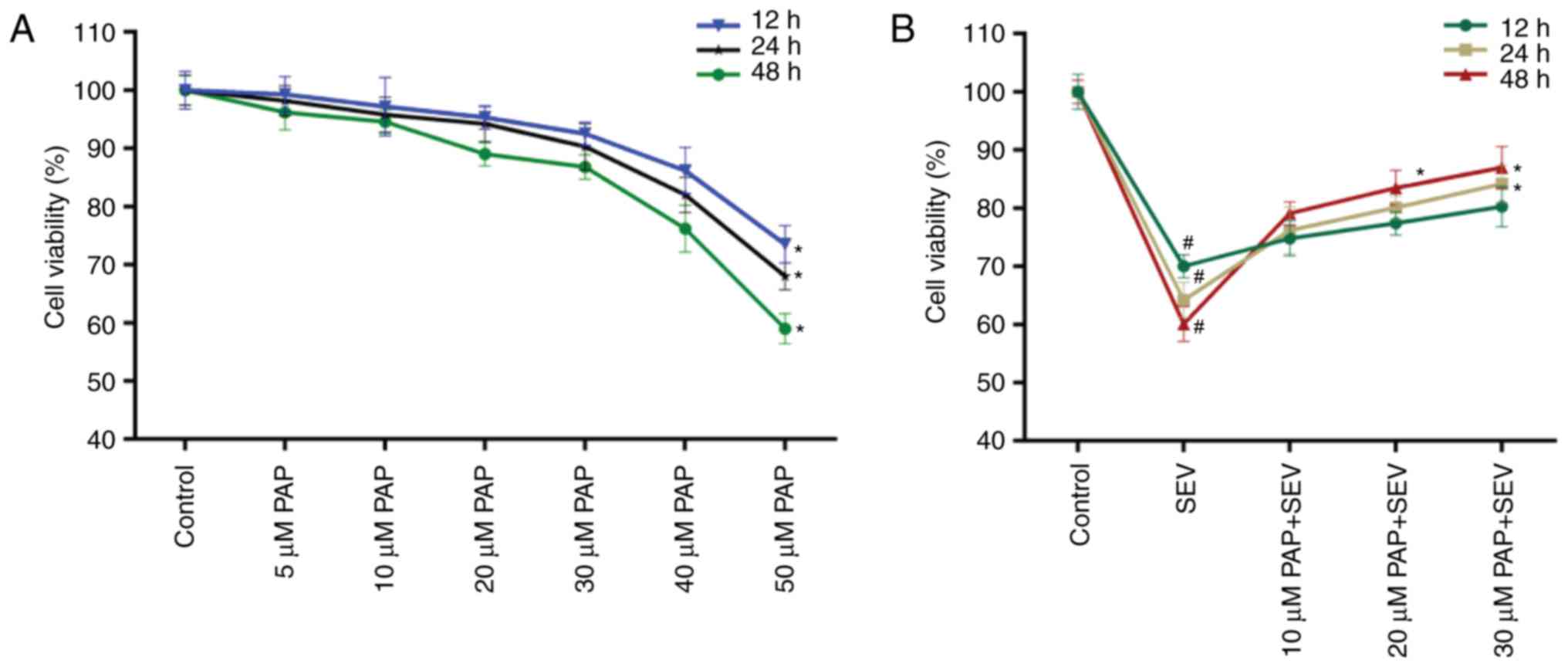
The cell viability of nerve cells treated with
different concentrations of PAP for 12, 24 and 48 h was determined.
A decrease in nerve cell viability was observed when the PAP
concentration was >40 µM (Fig.
2A); therefore, nerve cells were treated with 10, 20 and 30 µM
PAP for all subsequent experiments. The CCK-8 data also
demonstrated that compared with the control, SEV decreased the cell
viability of nerve cells; however, in the presence of PAP, the cell
viability of SEV-treated nerve cells was markedly rescued in a
dose-dependent manner (Fig.
2B).
PAP increases cell proliferation of
SEV-treated nerve cells
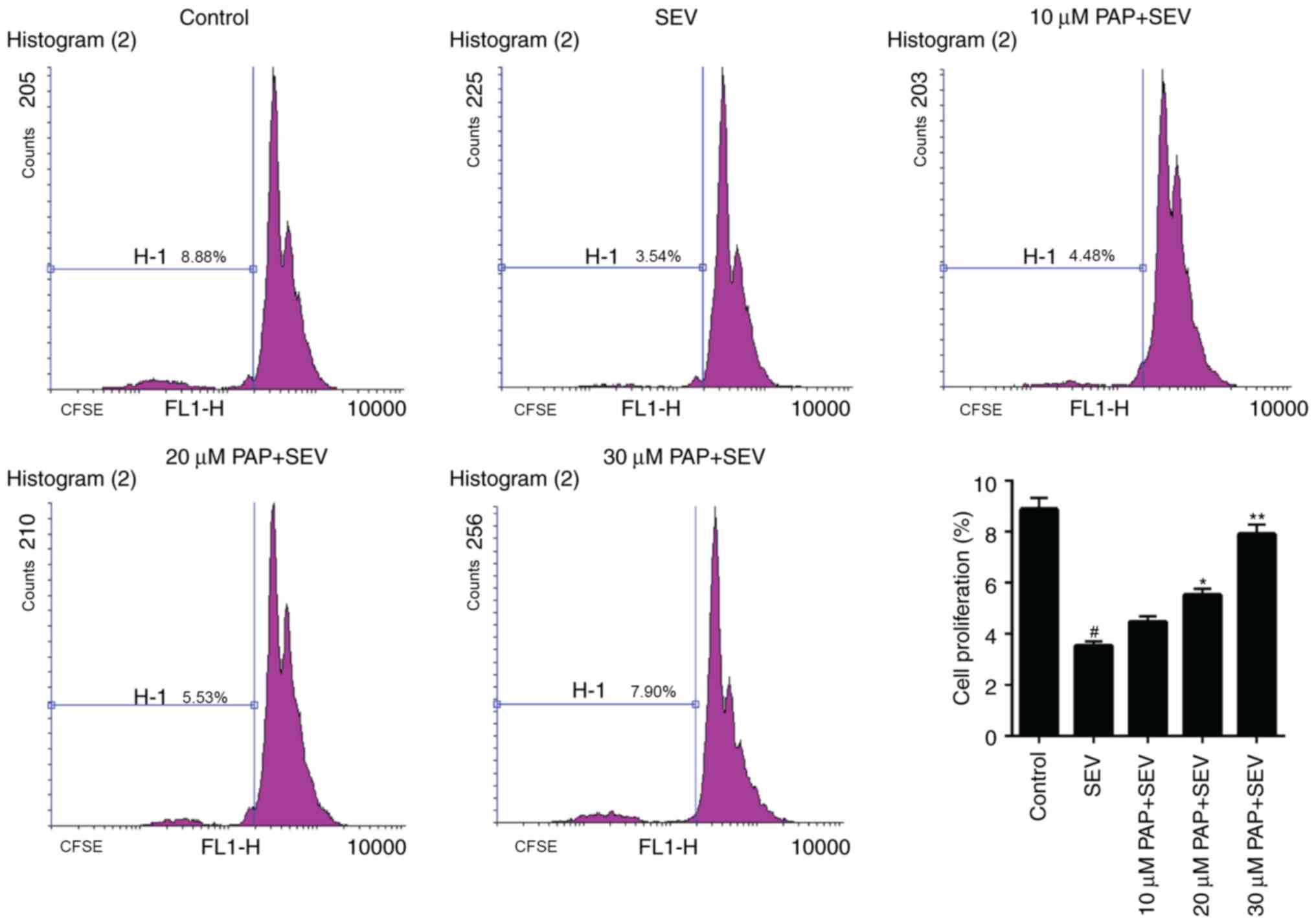
The proliferation of nerve cells treated with SEV
and different concentrations of PAP was evaluated. The FCM data
revealed that treatment with SEV significantly reduced the number
of nerve cells in the M1 phase. However, in the groups that were
pretreated with PAP, the number of nerve cells in M1 phase was
significantly increased (Fig. 3;
P<0.05). These data suggested that SEV reduced the proliferation
capacity of nerve cells, while PAP promoted cell proliferation in
SEV-treated nerve cells.
PAP suppresses the apoptosis of
SEV-treated nerve cells
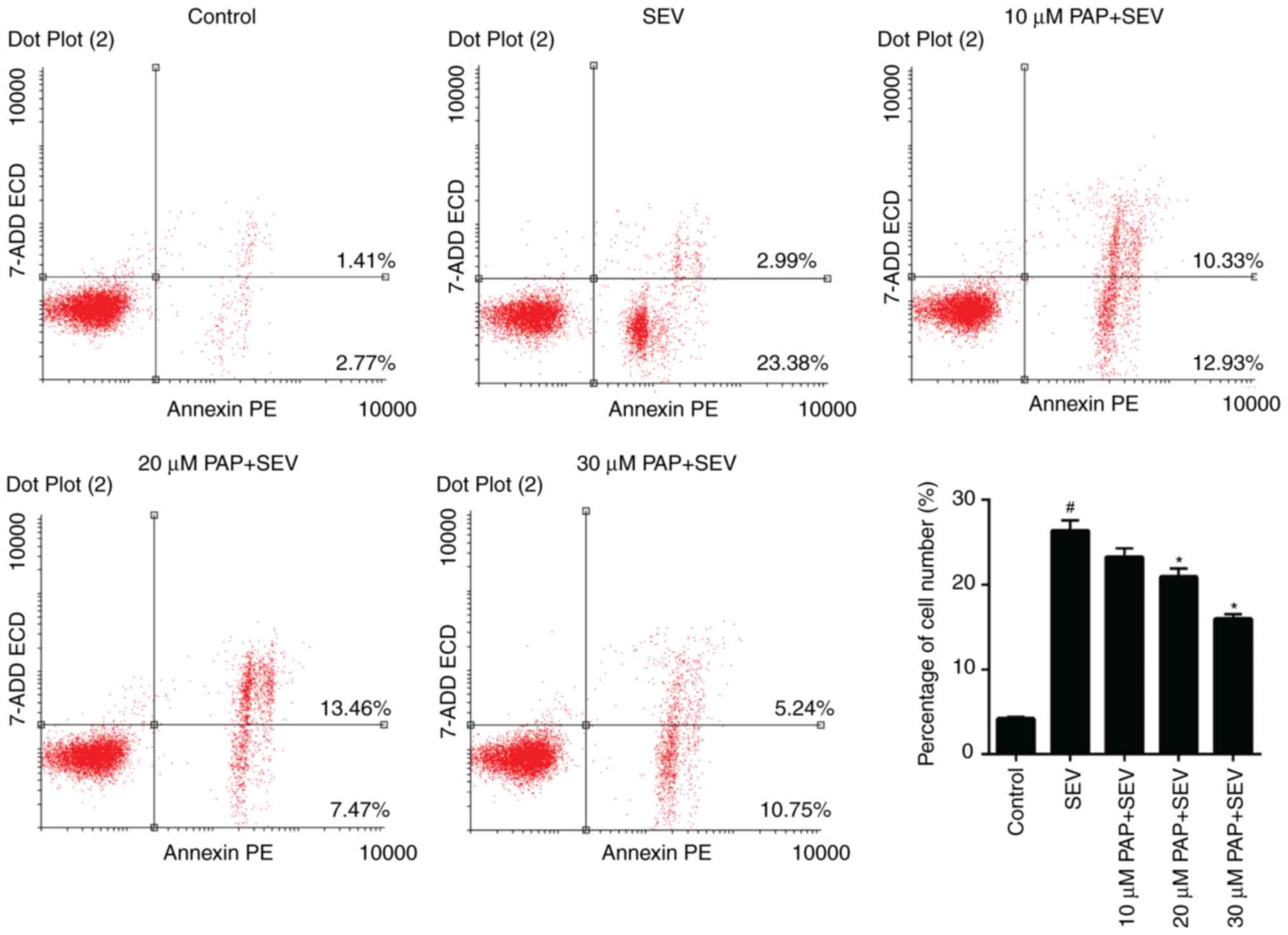
The apoptosis of nerve cells treated with SEV and
different concentrations of PAP was evaluated. The FCM data
revealed that the proportion of apoptotic nerve cells in the SEV
group was 26.37%, which was markedly higher compared with that in
the control group (4.18%). However, in the groups pretreated with
20 and 30 µM PAP, the apoptosis rates of the nerve cells were
decreased from 26.37% to 20.93 and 15.99%, respectively (Fig. 4; P<0.05). According to the FCM
results, the apoptosis of nerve cells was markedly lower in the PAP
groups compared with the SEV group.
PAP modulates expression of
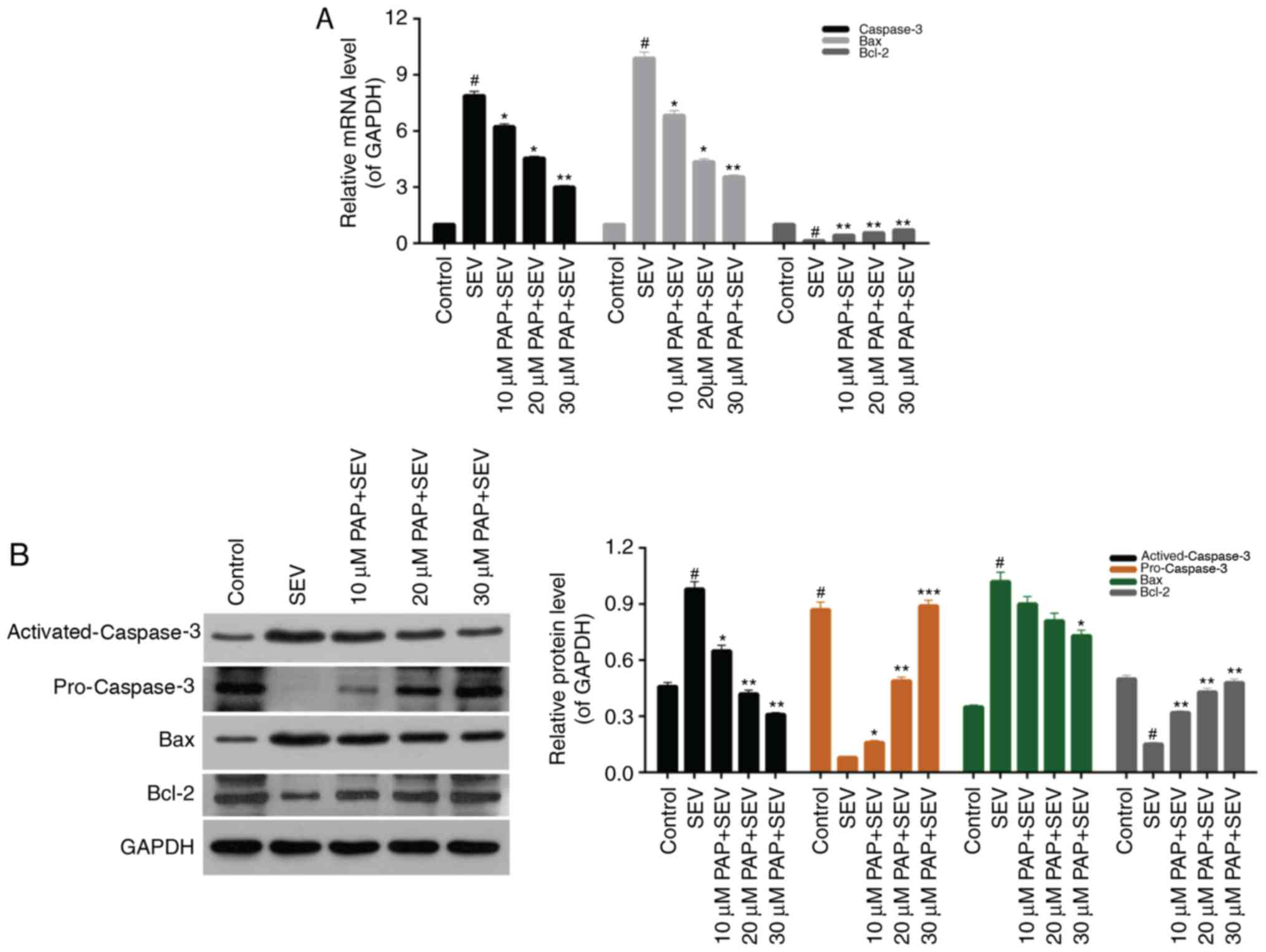
apoptosis-associated proteins
Based on the results of PAP-induced suppression of
apoptosis in SEV-treated nerve cells, the mechanisms were further
investigated in the present study. The expression levels of
apoptosis-associated proteins in nerve cells, including caspase-3,
Bax and Bcl-2, were determined. The RT-qPCR data demonstrated that
the expression levels of caspase-3 and Bax in nerve cells treated
with SEV were significantly upregulated. However, in the groups
pretreated with different concentrations of PAP, significant
decreases in the expression levels of caspase-3 and Bax were
observed. In addition, the results revealed that treatment with SEV
markedly reduced the Bcl-2 expression levels in nerve cells, while
treatment with PAP significantly upregulated Bcl-2 expression
levels in SEV-treated nerve cells (Fig. 5A; P<0.05). The western blot data
displayed similar trends for activated-caspase-3, Bax and Bcl-2
protein expression in nerve cells from the different treatment
groups. Additionally, it was demonstrated that treatment with SEV
significantly downregulated the protein expression levels of
pro-caspase-3 in nerve cells, while pretreatment with PAP
significantly enhanced pro-caspase-3 expression in SEV-induced
nerve cells and decreased the activated-caspase-3 expression in
SEV-induced nerve cells (Fig. 5B;
P<0.05). These results revealed that PAP suppressed the
apoptosis of SEV-treated nerve cells by modulating the expression
levels of caspase-3, Bax and Bcl-2.
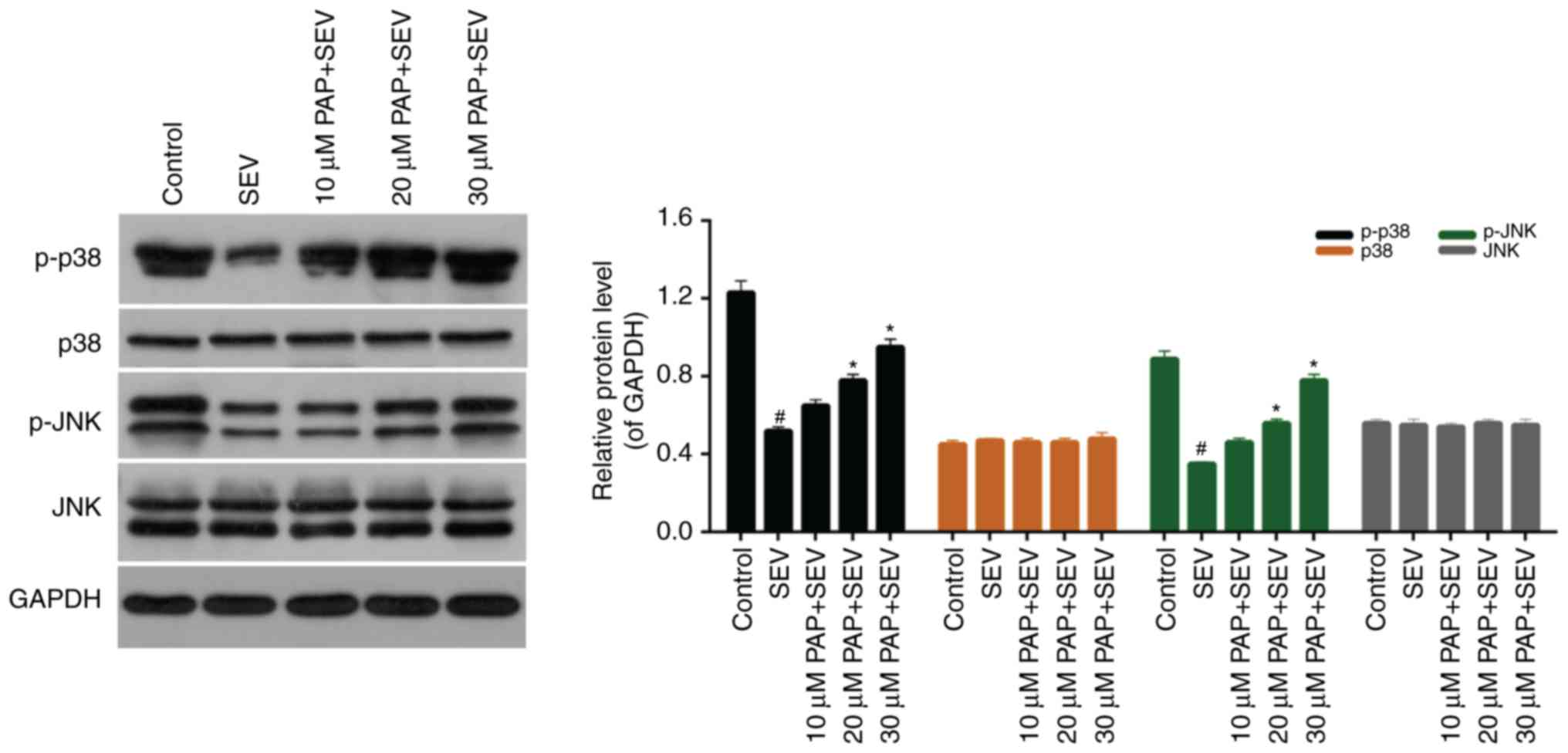
PAP activates the p38/JNK pathway
The western blot results demonstrated that the
expression levels of p-p38 and p-JNK were markedly downregulated by
treatment with SEV. However, increases in p-p38 and p-JNK
expression levels were observed amongst cells that were pretreated
with PAP. Furthermore, there were no significant differences in the
expression levels of p38 and JNK in nerve cells treated with SEV
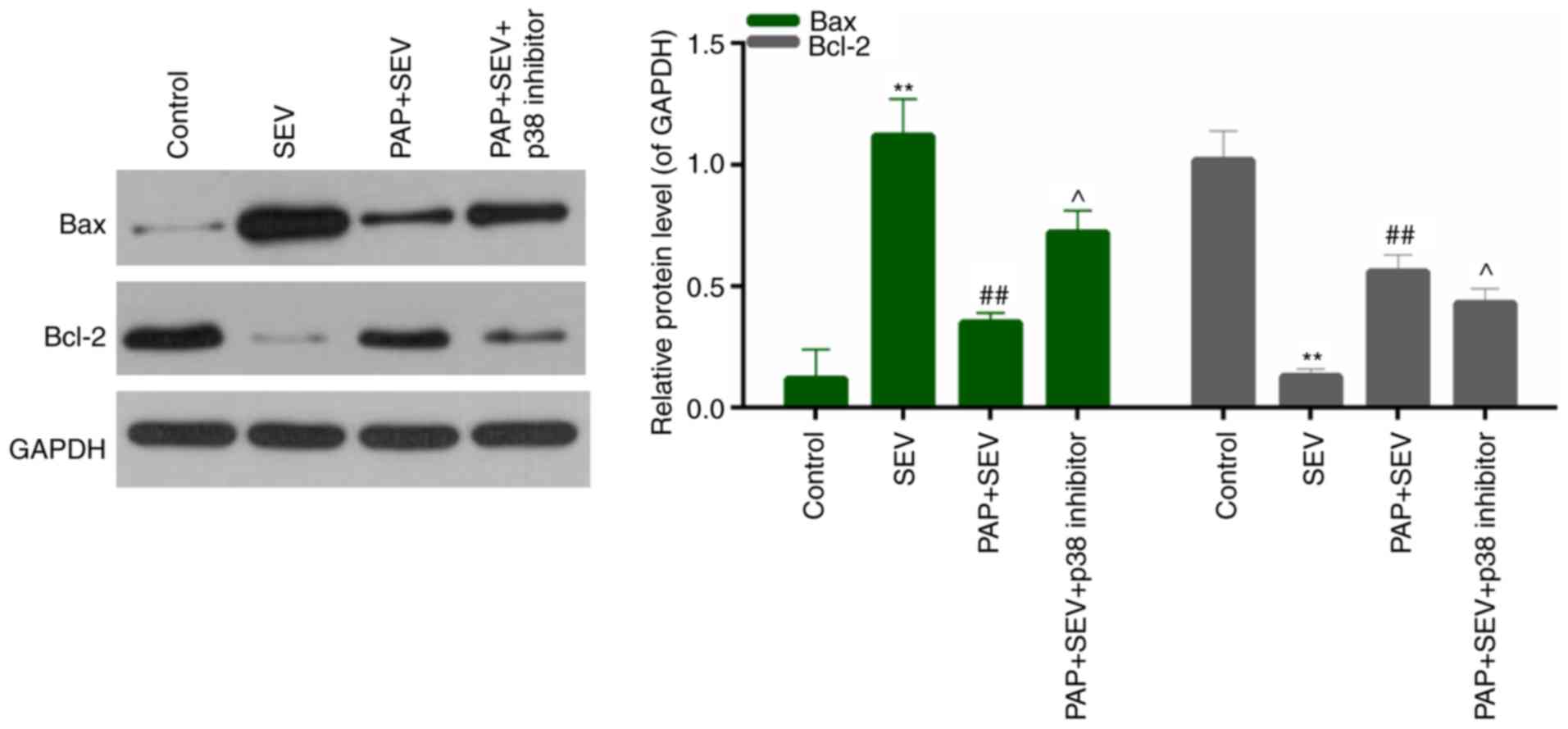
and different concentrations of PAP (Fig. 6; P>0.05). To further investigate
the role of the p38 pathway in mediating the effects of PAP, a p38
pathway inhibitor was employed. The extent of SEV-induced nerve
cell injury was determined by detecting the expression levels of
Bcl-2 and Bax by western blotting. As presented in Fig. 7, in the presence of the p38 pathway
inhibitor, the expression levels of Bcl-2 were decreased, whereas
Bax expression levels were increased compared with the SEV+PAP
group. This suggested that the protective effects conferred by PAP
were largely due to activation of the p38/JNK pathway.
Discussion
The hippocampus is associated with learning, memory
and other brain functions. It consists of a large number of neural
stem cells. The hippocampal tissues of mammals, particularly fetal
rats, may be easily obtained (27); thus, hippocampal neurons cultured
in vitro are considered an ideal experimental model
(28). PAP is the active substance
in PA. Researchers have suggested that PAP may promote the
proliferation of chondrocytes, and serves an important role in the
development and progression of osteogenesis (20,29).
However, little is known about the protective effects of PAP in the
central nervous system. Therefore, in the present study the
potential protective effects of PAP in SEV-induced neurocyte injury
were explored. When the concentration of PAP reached 40 µM, the
cell viability of nerve cells began to decrease. In order to avoid
cytotoxicity caused by PAP, cells were treated with 10, 20 and 30
µM PAP for all subsequent experiments. The results also indicated
that SEV significantly reduced the cell viability of nerve cells,
while PAP markedly increased the cell viability of SEV-treated
nerve cells. Therefore, the results demonstrated that PAP was able
to enhance the cell viability of nerve cells during SEV-induced
injury.
Since PAP enhanced the cell viability of nerve
cells, it was suspected that PAP may affect the proliferation
capacity of SEV-treated nerve cells. Thus, the proliferative
ability of nerve cells treated with SEV and different
concentrations of PAP was tested. The FCM data revealed that
treatment with SEV significantly reduced the proliferation ability
of nerve cells, while PAP increased the proliferation of
SEV-treated nerve cells. These results indicated that PAP may have
protective effects on nerve cells during SEV-induced injury. To
further verify this, the apoptosis rates of nerve cells treated
with SEV and different concentrations of PAP were determined. The
results demonstrated that SEV promoted apoptosis in nerve cells,
while PAP reduced apoptosis in SEV-treated nerve cells.
Furthermore, the mechanisms underlying apoptosis in nerve cells
treated with SEV and different concentrations of PAP were examined.
In the present study, the expression levels of caspase-3, Bax and
Bcl-2 in nerve cells were determined. The expression levels of
caspase-3 and Bax were significantly upregulated by SEV in nerve
cells; however, they were downregulated by PAP in SEV-treated nerve
cells. Conversely, the expression levels of Bcl-2 were markedly
downregulated by SEV and upregulated by PAP in nerve cells. These
results suggested that PAP may suppress the apoptosis of
SEV-treated nerve cells by modulating the expression levels of
caspase-3, Bax and Bcl-2.
It has been previously demonstrated that the p38/JNK
pathway is involved in processes associated with cell proliferation
and apoptosis in various types of cells (30–33).
However, the effect of PAP on the p38/JNK pathway in nerve cells
remains unclear. Therefore, the expression levels of p-p38, p38,
p-JNK and JNK in nerve cells were investigated. SEV markedly
reduced the expression levels of p-p38 and p-JNK in nerve cells,
while PAP increased the phosphorylation of p38 and JNK in
SEV-treated nerve cells. According to these results, PAP modulated
the p38/JNK pathway in SEV-treated nerve cells. Furthermore,
inhibition of the p38/JNK pathway reversed the protective effects
produced by PAP. The expression levels of Bcl-2 and Bax were
decreased and increased, respectively, in the p38 inhibitor+SEV+PAP
group compared with the SEV+PAP group. Therefore, activation of the
p38 pathway may be necessary for mediating the protective effect of
PAP.
In conclusion, the present study demonstrated that
PAP protected against SEV-mediated neurocyte injury, which involved
modulation of the p38/JNK pathway. These findings provided novel
insight on the pathogenesis of neurocyte injury and revealed a
potential agent for treating neurocyte injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL wrote the main manuscript. SL and JH performed
the experiments. SL and JH designed the study. SL and JH performed
data analysis. SL and JH contributed to manuscript revisions and
both authors reviewed the manuscript.
Ethics approval and consent to
participate
The protocols for the animal experiments were
approved by the Ethics Committee of Xinjiang Uygur Autonomous
Region Hospital of TCM.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cornelissen L, Kim SE, Purdon PL, Brown EN
and Berde CB: Age-dependent electroencephalogram (EEG) patterns
during sevoflurane general anesthesia in infants. Elife.
4:e065132015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dehaene-Lambertz G and Spelke ES: The
infancy of the human brain. Neuron. 88:93–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH, Zhang J, Wei L and Yu SP:
Neurodevelopmental implications of the general anesthesia in
neonate and infants. Exp Neurol. 272:50–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lerman J, Sikich N, Kleinman S and Yentis
S: The pharmacology of sevoflurane in infants and children.
Anesthesiology. 80:814–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JR, Lin EP, Hofacer RD, Upton B, Lee
SY, Ewing L, Joseph B and Loepke AW: Alternative technique or
mitigating strategy for sevoflurane-induced neurodegeneration: A
randomized controlled dose-escalation study of dexmedetomidine in
neonatal rats. Br J Anaesth. 119:492–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bignami E, Biondi-Zoccai G, Landoni G,
Fochi O, Testa V, Sheiban I, Giunta F and Zangrillo A: Volatile
anesthetics reduce mortality in cardiac surgery. J Cardiothorac
Vasc Anesth. 23:594–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lunardi N, Ori C, Erisir A and
Jevtovic-Todorovic V: General anesthesia causes long-lasting
disturbances in the ultrastructural properties of developing
synapses in young rats. Neurotox Res. 17:179–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu S, Wang X, Yang F, Chen B, Wu S, He W,
Yuan X, Zhang H, Chen P and Wei G: Propofol induces neuronal
apoptosis in infant rat brain under hypoxic conditions. Brain Res
Bull. 86:29–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Briner A, De Roo M, Dayer A, Muller D,
Habre W and Vutskits L: Volatile anesthetics rapidly increase
dendritic spine density in the rat medial prefrontal cortex during
synaptogenesis. Anesthesiology. 112:546–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briner A, Nikonenko I, De Roo M, Dayer A,
Muller D and Vutskits L: Developmental stage-dependent persistent
impact of propofol anesthesia on dendritic spines in the rat medial
prefrontal cortex. Anesthesiology. 115:282–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Cheng Y, Liu G, Tian X, Tu X and
Wang J: Chronic exposure of gestation rat to sevoflurane impairs
offspring brain development. Neurol Sci. 33:535–544. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mintz CD, Barrett KM, Smith SC, Benson DL
and Harrison NL: Anesthetics interfere with axon guidance in
developing mouse neocortical neurons in vitro via a γ-aminobutyric
acid type A receptor mechanism. Anesthesiology. 118:825–833. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mintz CD, Smith SC, Barrett KM and Benson
DL: Anesthetics interfere with the polarization of developing
cortical neurons. J Neurosurg Anesthesiol. 24:368–375. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arbabi S and Maier RV: Mitogen-activated
protein kinases. Crit Care Med. 30 1 Supp:S74–S79. 2002. View Article : Google Scholar
|
|
15
|
Ryu M, Kim EH, Chun M, Kang S, Shim B, Yu
YB, Jeong G and Lee JS: Astragali Radix elicits anti-inflammation
via activation of MKP-1, concomitant with attenuation of p38 and
Erk. J Ethnopharmacol. 115:184–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang J, Chen X, Tu W, Guo Y, Zhao Z, Xue
Q, Lin C, Xiao J, Sun X, Tao T, et al: Propofol inhibits the
activation of p38 through up-regulating the expression of annexin
A1 to exert its anti-inflammation effect. PLoS One. 6:e278902011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barr RK and Bogoyevitch MA: The c-Jun
N-terminal protein kinase family of mitogen-activated protein
kinases (JNK MAPKs). Int J Biochem Cell Biol. 33:1047–1063. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin A: Activation of the JNK signaling
pathway: Breaking the brake on apoptosis. Bioessays. 25:17–24.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng HY, Qu XB, Li N, Yuan S and Lin Z:
Effects of pilose antler and antler glue on osteoporosis of
ovariectomized rats. Zhong Yao Cai. 32:179–182. 2009.(In Chinese).
PubMed/NCBI
|
|
20
|
Liu G, Ma C, Wang P, Zhang P, Qu X, Liu S,
Zhai Z, Yu D, Gao J, Liang J, et al: Pilose antler peptide
potentiates osteoblast differentiation and inhibits
osteoclastogenesis via manipulating the NF-κB pathway. Biochem
Biophys Res Commun. 491:388–395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma C, Long H, Yang C, Cai W, Zhang T and
Zhao W: Anti-inflammatory role of pilose antler peptide in
LPS-induced lung injury. Inflammation. 40:904–912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chunhui Y, Wenjun C, Hui W, Liquan S,
Changwei Z, Tianzhu Z and Wenhai Z: Pilose antler peptide protects
osteoblasts from inflammatory and oxidative injury through EGF/EGFR
signaling. Int J Biol Macromol. 99:15–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suttie JM, Gluckman PD, Butler JH,
Fennessy PF, Corson ID and Laas FJ: Insulin-like growth factor 1
(IGF-1) antler-stimulating hormone? Endocrinology. 116:846–848.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu T, Yang L, Chen Y, Ni Y, Jiang J, Zhang
W, Zhou Q, Zheng X, Wang Q, Fu Z and Li H: Pilose antler
polypeptides ameliorates hypoxic-ischemic encephalopathy by
activated neurotrophic factors and SDF1/CXCR4 axis in rats. Acta
Biochim Biophys Sin (Shanghai). 50:254–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou QL, Guo YJ, Wang LJ, Wang Y, Liu YQ,
Wang Y and Wang BX: Velvet antler polypeptides promoted
proliferation of chondrocytes and osteoblast precursors and
fracture healing. Zhongguo Yao Li Xue Bao. 20:279–282.
1999.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shitaka Y, Matsuki N, Saito H and Katsuki
H: Basic fibroblast growth factor increases functional L-type Ca2+
channels in fetal rat hippocampal neurons: Implications for neurite
morphogenesis in vitro. J Neurosci. 16:6476–6489. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Facci L and Skaper SD: Culture of rodent
cortical and hippocampal neurons. Methods Mol Biol. 846:49–56.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin JH, Deng LX, Wu ZY, Chen L and Zhang
L: Pilose antler polypeptides promote chondrocyte proliferation via
the tyrosine kinase signaling pathway. J Occup Med Toxicol.
6:272011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han Y, Wu G, Deng J, Tao J, Guo L, Tian X,
Kang J, Zhang X and Yan C: Cellular repressor of E1A-stimulated
genes inhibits human vascular smooth muscle cell apoptosis via
blocking P38/JNK MAP kinase activation. J Mol Cell Cardiol.
48:1225–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hui L, Bakiri L, Mairhorfer A, Schweifer
N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H and
Wagner EF: p38alpha suppresses normal and cancer cell proliferation
by antagonizing the JNK-c-Jun pathway. Nat Genet. 39:741–749. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kostadinova R, Montagner A, Gouranton E,
Fleury S, Guillou H, Dombrowicz D, Desreumaux P and Wahli W:
GW501516-activated PPARβ/δ promotes liver fibrosis via p38-JNK
MAPK-induced hepatic stellate cell proliferation. Cell Biosci.
2:342012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD
and Wang CJ: Induction of apoptosis in the lung tissue from rats
exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol
Interact. 155:31–42. 2005. View Article : Google Scholar : PubMed/NCBI
|