Introduction
Acute pancreatitis is a relatively common disease
and severe acute pancreatitis (SAP) is associated with a high
mortality rate, ranging from 15–40% (1). SAP starts as a local inflammation of
pancreatic tissue and is characterized by the development of
systemic inflammatory response syndrome (SIRS) and multiple organ
dysfunction (2,3). Unfortunately, despite several years
of experimental and clinical research, the precise pathophysiology
of SAP, particularly in the clinical context remains unclear. This
has precluded the development of definitive treatment modalities
for this potentially life-threatening illness.
Experimental and clinical studies over the past
decade have reported the profiles of intrapancreatic and
circulating cytokines, chemokines, adhesion molecules,
transcription factors including nuclear factor (NF)-κB and
high-mobility group box 1 (HMGB1) (4,5), the
nucleotide-binding oligomerization domain receptor, Toll-like
receptor (TLR)9 (6) and protective
pathways, including the heme oxygenase-1 pathway and the peroxisome
proliferator-activated receptor-γ which are involved in SAP
(7).
NF-κB has increasingly gained attention over the
past several years as a factor in human inflammation, immune
regulation and cancer biology. Studies from the last two decades
have suggested that NF-κB is an early, major activator of
pro-inflammatory mediators in the pathogenesis of SAP (8–10).
The HMGB1 protein, as a late mediator of endotoxin lethality, was
demonstrated to exhibit a delayed release by cultured macrophages
of more than 8 h following stimulation with endotoxin, tumor
necrosis factor (TNF), or interleukin (IL)-15 (11,12).
Furthermore, previous studies have demonstrated that HMGB1 may
serve an important role in SAP (9,11)
and it may act as a key inflammatory mediator that participates in
the development of SIRS and multiple organ damage in SAP (10). HMGB1, TLR4 and NFκB are possible
therapeutic targets for SAP treatment; therefore, HMGB1
antagonists, TLR4 antagonists and NFκB inhibitors should be
considered. TLR4 antagonists are the closest to being used in a
clinical setting for SAP treatment. Two TLR4 antagonists, namely
VGX-1027 and eritoran are already in clinical development (13–16).
The inhibition of HMGB1 by sodium butyrate has been
reported to have beneficial effects on SAP development; however,
sodium butyrate is not a specific HMGB1 inhibitor (17).
Dexamethasone (DXM), a type of steroid medication,
can improve microcirculation and inhibit enzymes and inflammatory
mediators (18). It has been used
to treat SAP, but its protective effects and associated mechanisms
on pancreatic injury remain unclear. In the present study, it was
hypothesized that HMGB1 and NF-κBp65 are involved in the
therapeutic mechanism through which DXM acts on SAP. Experiments
were performed to investigate the influence of DXM on the
expression levels of NF-κBp65 and HMGB1, as well as on apoptosis,
in pancreatic cells of SAP rats, to observe the therapeutic
efficacy and investigate the underlying therapeutic mechanisms of
DXM treatment.
Materials and methods
Materials and reagents
Sodium taurocholate solution (TCA) was obtained from
Beijing Solarbio Science & Technology Co., Ltd., (Beijing,
China) and DXM injections were obtained from Hubei Tianyao
Pharmaceutical Co., Ltd., (Fancheng, China) chloral hydrate was
purchased from Sangon Biotech Co., Ltd., (Shanghai, China). The
HMGB1 antibody was from Abcam (Cambridge, UK) and the NF-κB p65
antibody was from Cell Signaling Technology, Inc., (Danvers, MA,
USA).
Animals
In total, 35 male Sprague Dawley rats, 6–8 weeks old
and weighing between 200–250 g, were purchased from Hunan Slake
Jingda Experimental Animal Co. Ltd., (Changsa, China). The study
was approved by the Ethics Committee of Central South University
(Changsa, China).
Experimental model and groups
Rats were raised in rooms with a 12-h light/dark
cycle at 25°C for 1 week prior to the experiment. Rats were fasted
for 12 h and given food and fresh tap water ad libitum up to
the experiment. Anesthesia was administered by intraperitoneal
injection of 10% chloralic hydras (0.3 ml/100 g). The SAP model was
induced by the standard retrograde infusion of a freshly prepared
5% TCA into the biliopancreatic duct following laparotomy. The 35
rats were randomly divided into three groups: (i) Sham-operation
control group (sham group, n=5), where rats received an equivalent
volume of normal saline following a successful sham operation; (ii)
SAP model group (SAP group, n=15), where rats received an
equivalent volume of 5% TCA; and (iii) DXM treatment group (DXM
group, n=15), where rats were administered one dose of DXM (0.5
mg/100 g) intravenously in the tail following successful SAP
induction. The rats were sacrificed by dislocation of the neck in
the state of anesthesia at designated time-points following the
induction of pancreatitis.
Histological examination
For histological analysis, pancreas tissue specimens
were fixed in 4% formaldehyde at room temperature for 3 days,
embedded in paraffin, sectioned at 4 µm and treated with
hematoxylin-eosin (HE) staining for 10 min at room temperature. The
terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling
(TUNEL) assay was used to examine the apoptotic cells in the
pancreas. Following staining with 0.5% hematoxylin for 15 min at
room temperature, the nuclei of healthy cells were stained blue,
while those in apoptotic cells presented brown/yellow staining.
Integrated optical density (IOD) analysis was used to indirect
reaction the apoptosis. The expression levels of HMGB1 and NF-κBp65
in the pancreas were examined by immunohistochemistry (IHC).
Specimens were mounted in Permount and examined using routine light
microscopy.
Cell culture
AR42J pancreatoma cells were obtained from American
Type Culture Collection, (Manassas, VA, USA) and cultured in a
humidified atmosphere with 5% CO2 at 37°C. The cells
were treated with 10−8 mol/L caerulein (CAE) and/or DXM.
Experiments were assigned to three groups: CK group, CAE group and
DXM group. In the DXM group, cells were co-treated with CAE and
10−6, 10−7 or 10−8 mol/L DXM for
24 h. In the CK group, which acted as the control group, the AR42J
cells were only treated with PBS. Cells in the CAE group were
treated with CAE alone.
Detection of cellular apoptosis
assay
For this experiment, 2×106 cells were
plated into 60-mm dishes and then treated with or without CAE/DXM
for 24 h. Cellular apoptosis was detected using the
AnnexinV-fluorescein isothiocyanate Apoptosis kit (BD Biosciences,
Franklin Lakes, NJ, USA) and a FACSCanto II flow cytometer (BD
Biosciences), following the manufacturers protocol.
Cell survival assay
AR42J cells (5×103 cells/well) were
seeded into a 96-well plate and a MTT-based assay was performed
after 24 h. DMSO (150 µl) was used to dissolve the purple formazan
and the absorbance at 570 nm was measured to determine the cell
survival rates.
Immunofluorescence assay
Immunofluorescence was performed on AR42J cells
following blocking with 1X PBS/5% Normal Goat Serum (005-000-121;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA)/0.3% Triton X-100 at room temperature for 60 min using
anti-HMGB1 (1:500; cat. no. ab79823; Abcam), and anti-NF-κBp65
(1:500; cat. no. 8242; Cell Signaling Technology, Inc.) primary
antibodies and cy3-conjugated secondary antibodies (1:500; Abcam).
Nuclei were counterstained with DAPI (1:1,000; Sigma Aldrich; Merck
KGaA, Darmstadt, Germany) for 1 h at room temperature and cover
slips were mounted with Fluorescence Mounting Medium (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) and examined using a
fluorescence microscope.
Western blotting
AR42J cells were harvested using RIPA buffer (P002A;
Auragene; Hunan Aijia Biotechnology Co., Ltd., Hunan, China) and a
BCA Protein Quantitation kit used for protein determination.
Proteins were subjected to SDS-PAGE and immunoblotting, as
previously described (19). A
total of 30 µg protein per lane was separated by 10% SDS-PAGE,
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA), blocked with 5% skimmed milk for 1 h at room
temperature and incubated with primary antibodies at 4°C overnight:
Anti-HMGB1 (1:1,0000; cat. no. ab79823; Abcam), anti-NF-κBp65
(1:1,1000; cat. no. 8242; Cell Signaling Technology, Inc.) and
anti-β-actin (1:1,1000; cat. no. 3700; Cell Signaling Technology,
Inc.). Then the blots were incubated with the corresponding
secondary antibodies (1:5,000; sheep anti-rat; SA001 and sheep anti
rabbit; SA009 Auragene; Hunan Aijia Biotechnology Co., Ltd.) at
room temperature for 2 h. An AuraECL Chemiluminescence detection
kit (Auragene; Hunan Aijia Biotechnology Co., Ltd.) was used for
visualization.
Statistical analysis
All results are expressed as the mean ± standard
deviation from 3 independent experiments. One-way analysis of
variance was used for statistical analysis and Tukey post hoc test
was used for verification. P<0.05 was considered to indicate a
statistically significant difference and SPSS version 17 (SPSS,
Inc., Chicago, IL, USA) was used for analysis.
Results
Histological alterations in the
pancreas
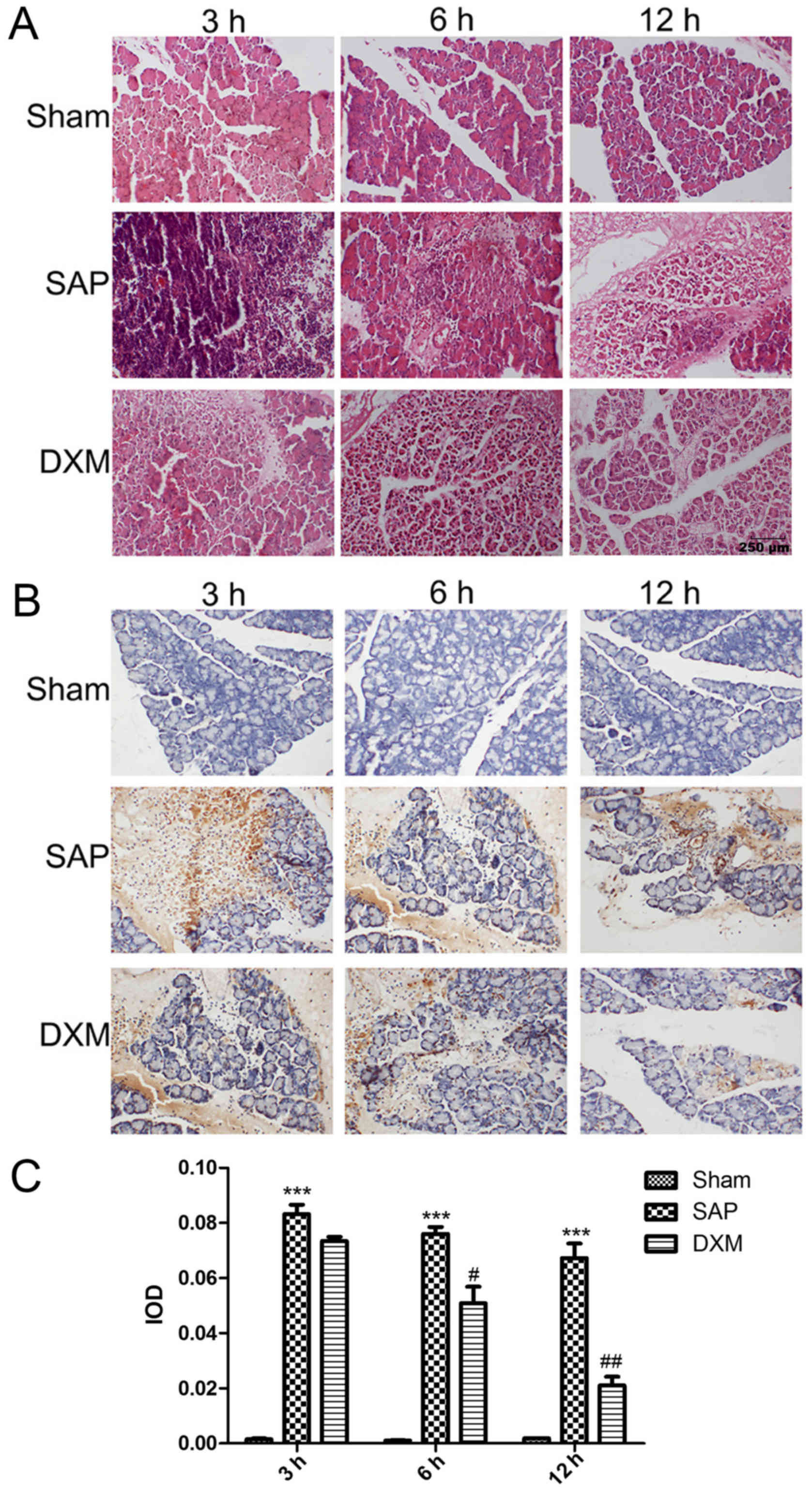
Histological alterations in pancreatic tissues were
observed under a light microscope by HE staining, as presented in
Fig. 1A. In the sham group,
pancreatic interstitial cells demonstrated slight hemorrhage and
mild edema. Pancreatic tissue in the SAP group demonstrated
increased levels of hemorrhage, edema and structural distortion
with necrosis compared with the sham group (data not shown).
Notably, when DXM was infused into the SAP rats, edema formation
and structural alterations with necrosis were reduced. In addition,
the therapeutic influence of DXM was time-dependent and differences
between the DXM group, and the SAP group were especially noticeable
after 12 h. (data not shown). The number of apoptotic acinar cells
increased in the SAP group compared with the sham group. Similarly,
following DXM infusion, the number of apoptotic cells was markedly
reduced compared with the SAP group and this reduction was
time-dependent, with a significant reduction observed after 6 h
(P<0.05; Fig. 1B and C).
IHC analysis of NF-κBp65 and HMGB1
expression in the pancreas
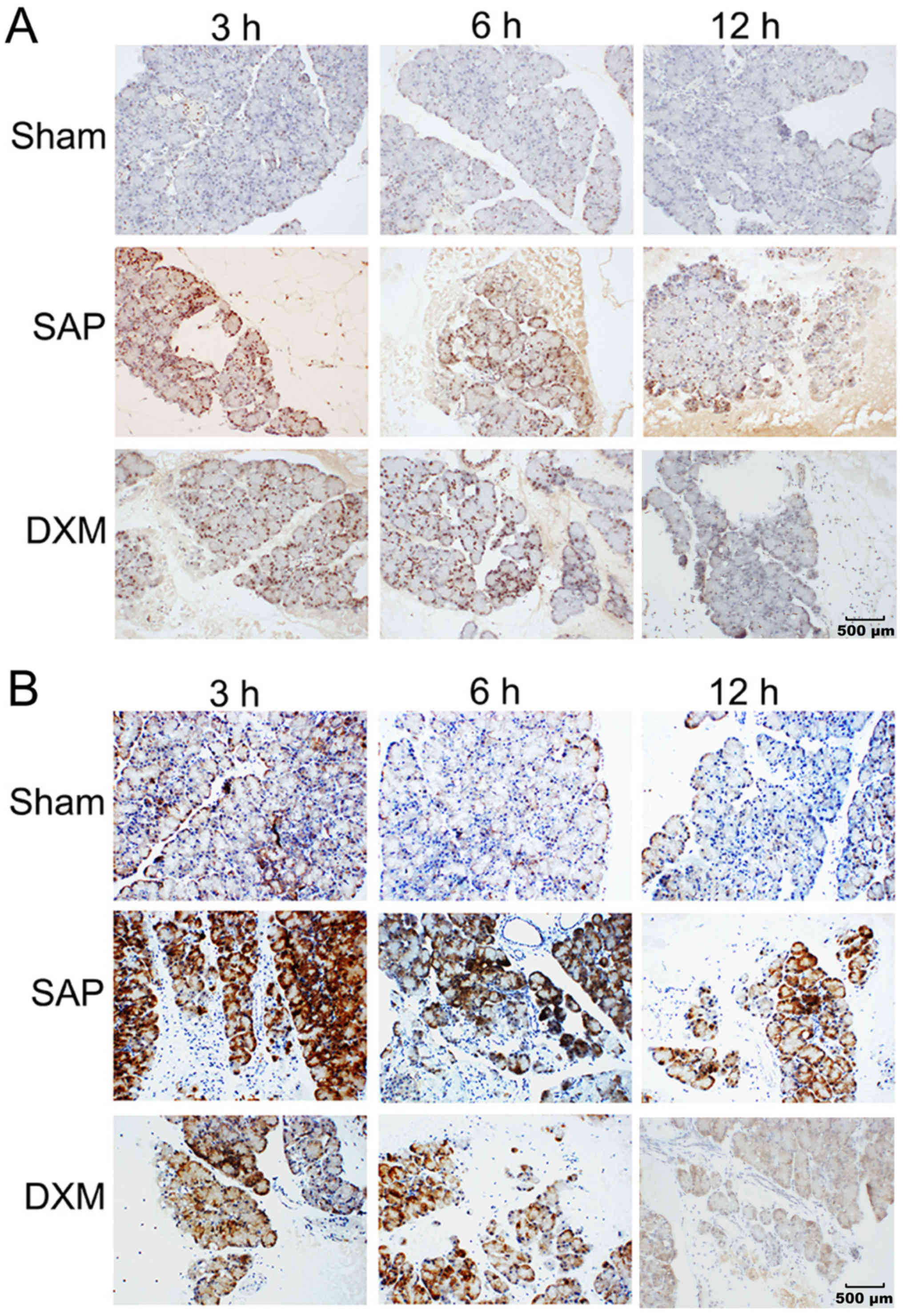
Inflammatory mediators serve key roles in the
pathogenesis of SAP. In the present study, alterations in NF-κBp65
and HMGB1 expression were examined in the pancreas. In the sham
group, the detection of HMGB1 was very weak in the nucleus
(Fig. 2A) and a low level of
NF-κBp65 was concentrated in the cytoplasm (Fig. 2B). In the SAP group, intense
immunoreactivity with HMGB1 and NF-κBp65 was detected in the
nucleus and cytoplasm (Fig. 2). In
the DXM group, the expression levels of HMGB1 and NF-κBp65 were
decreased in the nucleus and the cytoplasm, respectively (Fig. 2), compared with the SAP group.
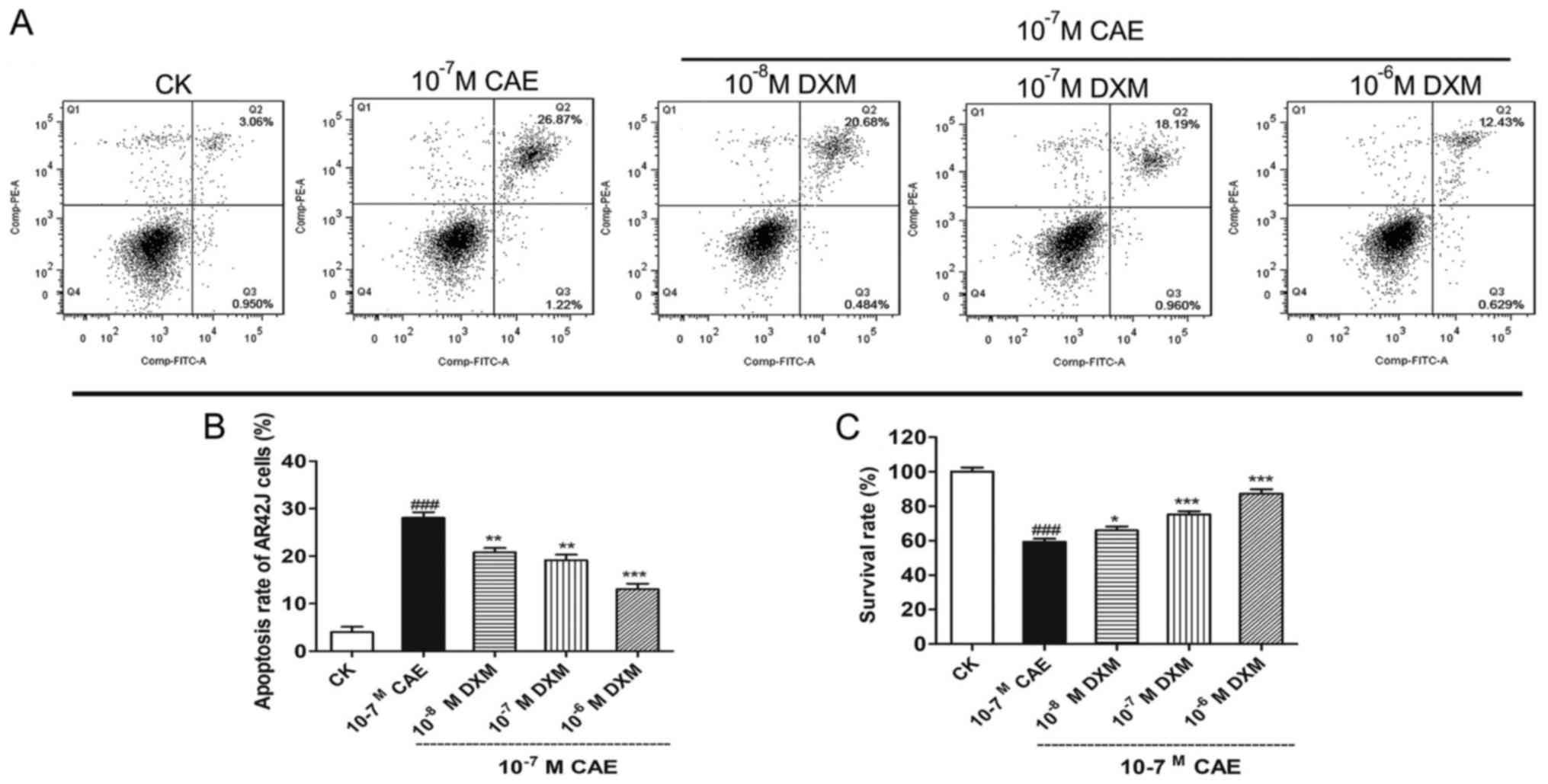
DXM regulates cell apoptosis in AR42J
cells treated with CAE
To confirm that DXM is involved in the regulation of
cellular apoptosis of AR42J cells treated with CAE, flow cytometry
was performed. As presented in Fig.
3, compared with the control group, apoptosis was significantly
increased in AR42J cells following treatment with CAE (P<0.001;
Fig. 3A and B). DXM could
significantly suppress apoptosis induced by CAE (P<0.01) and the
reduction in apoptosis was associated with the DXM concentration
(Fig. 3B).
Cell survival assay in AR42J cells
treated with CAE and DXM
Compared with the CK group, cell survival was
significantly inhibited in the CAE group (P<0.001) and could be
significantly improved by DXM treatment (P<0.05). Additionally,
a positive association was demonstrated between cell viability and
DXM concentration (Fig. 3C).
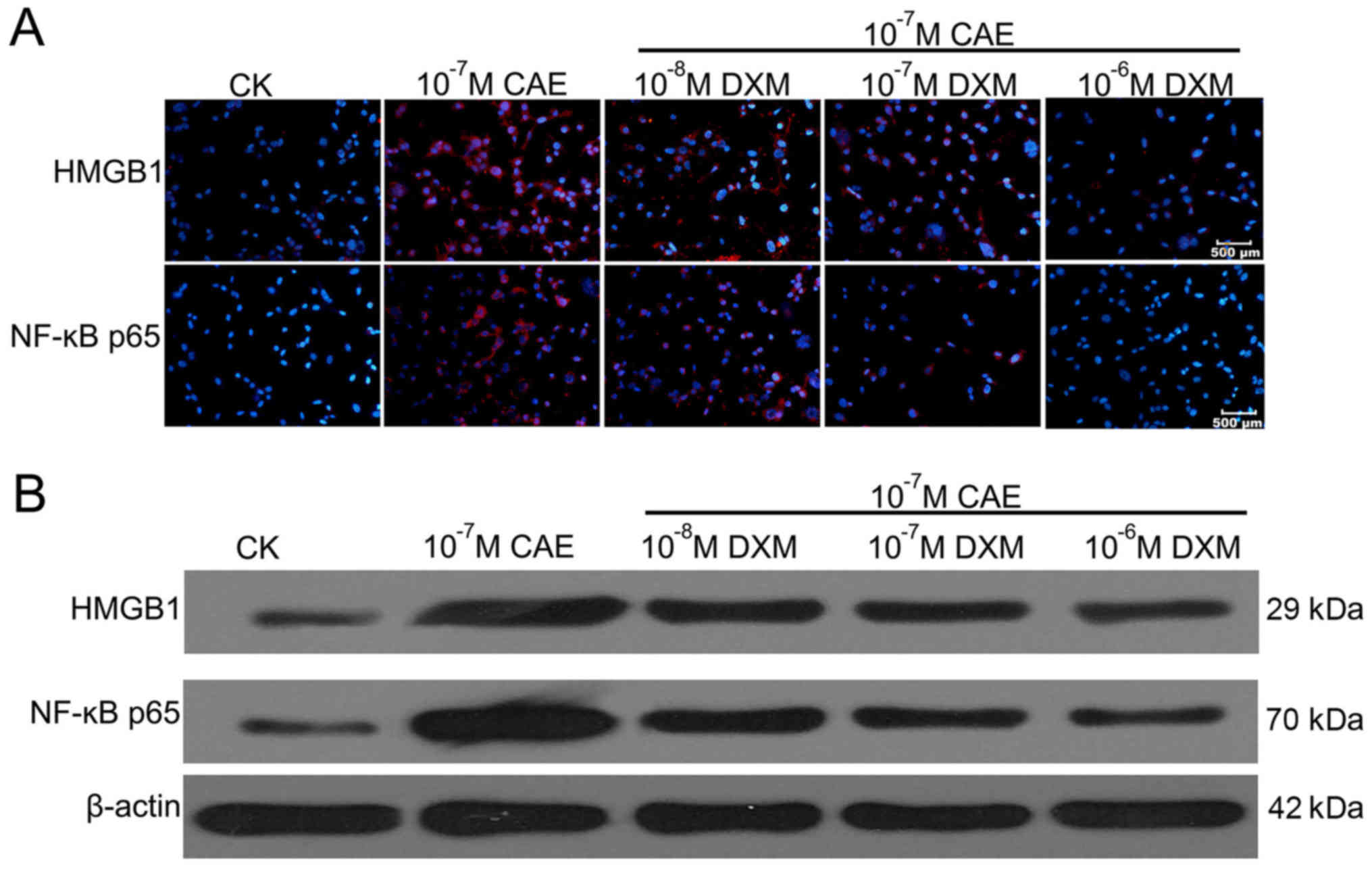
Effect of DXM on HMGB1 and NF-κBp65
expression in AR42J cells
Immunofluorescence (Fig. 4A) and western blotting (Fig. 4B) analyses demonstrated that HMGB1
and NF-κBp65 were expressed at low levels in AR42J cells treated
with PBS (CK group), were expressed at a high level in the CAE
group, and were expressed at an intermediate level in the DXM and
CAE co-treated group. The expression of HMGB1 and NF-κBp65
decreased in a dose-dependent manner with DXM treatment and reached
the lowest levels when treated with 10−6 M DXM, compared
with the CAE group (Fig. 4).
Discussion
SAP is an acute, critical illness with rapid onset,
long duration and rapid progress that results in massive necrosis
of pancreatic tissue, extrapancreatic multiple organ failure, and a
high mortality rate. Despite advances in treatment techniques, the
mortality of SAP has improved slowly over the past several decades
and the pathogenesis of SAP has not been completely clarified
(20,21). However, experts have focused on
inflammatory mediators and their corresponding antagonists as
potential therapeutic targets, due to their contribution to the
injury of the pancreas and other organs, frequently resulting in
patients succumbing to the condition.
DXM is a long-acting corticosteroid. Its therapeutic
effects on SAP are primarily associated with suppressing the
production and/or actions of inflammatory mediators, enhancing the
anti-stress capacity of the body, reducing endotoxemia, and
scavenging oxygen free radicals (22,23).
In the present study, to investigate the protective effects of DXM
on the pancreas of SAP rats the effect of DXM treatment on the
expression levels of NF-κBp65 and HMGB1, as well as on the
histopathology of the pancreas in rats with SAP, was
investigated.
NF-κBp65 is a transcription factor that regulates
various genes involved in inflammatory and immune responses,
including cytokine and adhesion molecules (24). TNF-α expression is directly
regulated by NF-κBp65, as there are NF-κBp65 binding sites on the
TNF-α promoter. Research has demonstrated that the inhibition of
NF-κBp65 can result in decreased expression levels of cytokines,
including TNF-α, reducing the inflammatory response in organisms.
The role of NF-κBp65 activation in the pathogenesis of SAP was
previously reviewed (25) and in a
later investigation, this laboratory demonstrated evidence that the
upregulation of NF-κBp65 could aggravate SAP-induced pancreatic
injury (26). NF-κBp65 inhibitors
in in vitro and in vivo models of SAP have already
been studied (27,28). In the present study, DXM was also
demonstrated as an inhibitor of NF-κBp65 and that DXM can
downregulate the expression of NF-κBp65 protein in the pancreatic
tissue of SAP rats and in AR42J cells treated with CAE. Previous
studies have demonstrated that HMGB1 acts through multiple
mechanisms, including through the NF-κBp65 pathway (29). However, it is not certain whether
there are binding sites for NF-κBp65 on the HMGB1 promoter.
Therefore, it remains unclear whether HMGB1 expression is directly
regulated by NF-κBp65.
HMGB1, a DNA-binding intranuclear protein, is a late
activator of the inflammatory cascade when released into the
extracellular space. HMGB1 is released from necrotic cells or
secreted by activated monocytes or macrophages. HMGB1 induces
pro-inflammatory cytokines in human monocytes via TLR4 and NF-κB
activation (30). HMGB1 can
mediate cell-to-cell signaling by binding to the receptor for
advanced glycation end products and toll-like receptors (TLR),
especially TLR-2 and TLR-4, to enhance the inflammatory response
(29,31). As a late-phase mediator, HMGB1 was
previously discovered to be upregulated in a number of acute and
chronic inflammatory conditions, including sepsis, acute lung
injury and rheumatoid arthritis (32–34).
In contrast to other known pro-inflammatory cytokines, the delayed
kinetics of HMGB1 provide a wide window of opportunity for
therapeutic approaches (35). The
present results also highlight the possible beneficial role of
anti-inflammatory cytokines, including IL-10 and IL-13, in the
pathogenesis of SAP. These cytokines may counteract the type-1
pro-inflammatory cytokines secreted in response to the activation
of the TLR4-NF-κB pathway induced by HMGB1.
As a result, HMGB1 offers the hope of developing an
anti-inflammatory therapy that is practical and effective. In a
previous study serum HMGB1 levels, were demonstrated to be
significantly elevated in patients with SAP and were correlated
with disease severity (36).
Therefore, it has been speculated that HMGB1 may be a target for
anti-inflammatory treatment in SAP. In the present study, it was
demonstrated that HMGB1 expression levels increased in the pancreas
with SAP and decreased following treatment with DXM, suggesting
that HMGB1 may be pivotal for the inflammatory response and organ
injury observed in SAP and may be a therapeutic target for SAP. It
was hypothesized that elevated HMGB1 levels may represent an
aggravating factor in SAP and the associated multiple organ damage.
However, HMGB1 affects a number of angiogenesis-associated
conditions, including cancer, proliferative diabetic retinopathy
and wound healing through the p53 pathway, and it is a promising
therapeutic target in a number of tumors, including epidermal
tumors, prostate cancer and colon cancer (37–39).
Therefore, the exact function of HMGB1 and its mechanism still need
to be elucidated. Notably, while investigating the relevance of
HMGB1-TLR4-NF-κB-induced pro-inflammatory cytokines in SAP, it was
demonstrated that the antibiotic and immunomodulatory agent fusidic
acid may prevent pancreatitis through the reduction of TNF-α and
IL-8. This is of interest because sodium fusidate is clinically
available and could be immediately considered for the treatment of
SAP (40,41).
In conclusion, DXM can reduce the levels of NF-κBp65
and HMGB1 and mitigate the pathological alterations in the pancreas
of rats with SAP and in AR42J cells treated with CAE. In in
vivo and vitro experiments, DXM was identified to have a
therapeutic effect on SAP. Therefore, NF-κBp65 and HMGB1 may serve
auxiliary roles in the treatment of SAP. Overall, each of these
inflammatory mediators has benefits and should be used
appropriately in future clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by Key project of
Hunan science and technology plan (grant no: 2013FJ4093).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors contributions
SZ, LM and KX conceived and designed the study. SZ,
TL, JZ and JY performed the experiments. SZ and JY wrote the
manuscript. KX reviewed and edited the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work were appropriately investigated and
resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Central South University (Changsa, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SAP
|
severe acute pancreatitis
|
|
DXM
|
dexamethasone
|
|
HMGB1
|
high-mobility group box-1
|
|
NF-κB
|
nuclear factor-κB
|
|
CAE
|
caerulein
|
|
SIRS
|
systemic inflammatory response
syndrome
|
|
TNF
|
tumor necrosis factor
|
|
TCA
|
sodium taurocholate solution
|
|
HE
|
hematoxylin-eosin
|
|
TUNEL
|
terminal deoxynucleotidyl transferase,
dUTP nick-end labeling
|
References
|
1
|
Uneno Y, Taneishi K, Kanai M, Okamoto K,
Yamamoto Y, Yoshioka A, Hiramoto S, Nozaki A, Nishikawa Y,
Yamaguchi D, et al: Development and validation of a set of six
adaptable prognosis prediction (SAP) models based on time-series
real-world big data analysis for patients with cancer receiving
chemotherapy: A multicenter case crossover study. PloS One.
12:e01832912017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen Q, Li Z, Huang S, Li L, Gan H and Du
XG: Intestinal mucosal barrier dysfunction in SAP patients with
MODS ameliorated by continuous blood purification. Int J Artif
Organs. 14:02017.
|
|
3
|
Lipinski M and Rydzewska G: Immature
granulocytes predict severe acute pancreatitis independently of
systemic inflammatory response syndrome. Prz Gastroenterol.
12:140–144. 2017.PubMed/NCBI
|
|
4
|
Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen
TK, Zhu YF and Wu L: Antioxidant inhibits HMGB1 expression and
reduces pancreas injury in rats with severe acute pancreatitis. Dig
Dis Sci. 55:2529–2536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang B, Xu XB, Jin XX, Wu XW, Li ML, Guo
MX and Zhang XH: Effects of ω-3 fatty acids on Toll-like receptor 4
and nuclear factor κB p56 in the pancreas of rats with severe acute
pancreatitis. Pancreas. 46:1267–1274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan Y, Lu B, Li P and Wang J: NOD receptor
and TLR9 modulation in severe acute pancreatitisinduced intestinal
injury. Mol Med Rep. 16:8471–8476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong J, Wang K, Yuan C, Xing R, Ni J, Hu
G, Chen F and Wang X: Luteolin protects mice from severe acute
pancreatitis by exerting HO-1-mediated anti-inflammatory and
antioxidant effects. Int J Mol Med. 39:113–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng C, Li B, Wang LL, Chen LI, Zhou X, Lv
FQ and Li TS: Effect of peritoneal lavage with ulinastatin on the
expression of NF-κB and TNF-α in multiple organs of rats with
severe acute pancreatitis. Exp Ther Med. 10:2029–2034. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li G, Wu X, Yang L, He Y, Liu Y, Jin X and
Yuan H: TLR4-mediated NF-κB signaling pathway mediates
HMGB1-induced pancreatic injury in mice with severe acute
pancreatitis. Int J Mol Med. 37:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YJ, Ding Y, Wu J and Ning BT:
Pterostilbene as treatment for severe acute pancreatitis. Genet Mol
Res. 15:2016. View Article : Google Scholar :
|
|
11
|
Yang R, Tenhunen J and Tonnessen TI: HMGB1
and histones play a significant role in inducing systemic
inflammation and multiple organ dysfunctions in severe acute
pancreatitis. Int J Inflam. 2017:18175642017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwak MS, Lim M, Lee YJ, Lee HS, Kim YH,
Youn JH, Choi JE and Shin JS: HMGB1 binds to lipoteichoic acid and
enhances TNF-α and IL-6 production through HMGB1-mediated transfer
of lipoteichoic acid to CD14 and TLR2. J Innate Immun. 7:405–416.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stojanovic I, Cuzzocrea S, Mangano K,
Mazzon E, Miljkovic D, Wang M, Donia M, Al Abed Y, Kim J, Nicoletti
F, et al: In vitro, ex vivo and in vivo immunopharmacological
activities of the isoxazoline compound VGX-1027: Modulation of
cytokine synthesis and prevention of both organ-specific and
systemic autoimmune diseases in murine models. Clin Immunol.
123:311–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fagone P, Muthumani K, Mangano K, Magro G,
Meroni PL, Kim JJ, Sardesai NY, Weiner DB and Nicoletti F: VGX-1027
modulates genes involved in lipopolysaccharide-induced Toll-like
receptor 4 activation and in a murine model of systemic lupus
erythematosus. Immunology. 142:594–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JC, Menacherry S, Diehl MC, Giffear
MD, White CJ, Juba R, Bagarazzi ML, Muthumani K, Boyer J, Agarwal
V, et al: Safety, bioavailability, and pharmacokinetics of
VGX-1027-A novel oral anti-inflammatory drug in healthy human
subjects. Clin Pharmacol Drug Dev. 5:91–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lucas K and Maes M: Role of the Toll Like
receptor (TLR) radical cycle in chronic inflammation: Possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Xia M, Zhan Q, Zhou Q, Lu G and
An F: Sodium butyrate reduces organ injuries in mice with severe
acute pancreatitis through inhibiting HMGB1 expression. Dig Dis
Sci. 60:1991–1999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sellner S, Kocabey S, Zhang T, Nekolla K,
Hutten S, Krombach F, Liedl T and Rehberg M:
Dexamethasone-conjugated DNA nanotubes as anti-inflammatory agents
in vivo. Biomaterials. 134:78–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cen C, Li J, Liu J, Yang M, Zhang T, Zuo
Y, Lin C and Li X: Long noncoding RNA LINC01510 promotes the growth
of colorectal cancer cells by modulating MET expression. Cancer
Cell Int. 18:452018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mofidi R, Madhavan KK, Garden OJ and Parks
RW: An audit of the management of patients with acute pancreatitis
against national standards of practice. Br J Surg. 94:844–848.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buter A, Imrie CW, Carter CR, Evans S and
McKay CJ: Dynamic nature of early organ dysfunction determines
outcome in acute pancreatitis. Br J Surg. 89:298–302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yubero S, Ramudo L, Manso MA and De Dios
I: Mechanisms of dexamethasone-mediated chemokine down-regulation
in mild and severe acute pancreatitis. Biochim Biophys Acta.
1792:1205–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jingmin O, Xiping Z, Chun W, Ping Y and
Qian Y: Study of dexamethasone, baicalin and octreotide on brain
injury of rats with severe acute pancreatitis. Inflamm Res.
61:265–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang
JM, Cai W, Shen HP and Cai J: Influence of dexamethasone on
inflammatory mediators and NF-kappaB expression in multiple organs
of rats with severe acute pancreatitis. World J Gastroenterol.
13:548–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XP, Zhang L, Xu HM, Xu YP, Cheng QH,
Wang JM and Shen HP: Application of tissue microarrays to study the
influence of dexamethasone on NF-kappaB expression of pancreas in
rat with severe acute pancreatitis. Dig Dis Sci. 53:571–580. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ou JM, Zhang XP, Wu CJ, Wu DJ and Yan P:
Effects of dexamethasone and Salvia miltiorrhiza on multiple organs
in rats with severe acute pancreatitis. J Zhejiang Univ Sci B.
13:919–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Zhao Q, Chen Q, Zhang Y, Shao B,
Jin Y and Wu J: Melatonin attenuated inflammatory reaction by
inhibiting the activation of p38 and NFκB in taurocholateinduced
acute pancreatitis. Mol Med Rep. 17:5934–5939. 2018.PubMed/NCBI
|
|
28
|
Qian D, Wei G, Xu C, He Z, Hua J, Li J, Hu
Q, Lin S, Gong J, Meng H, et al: Bone marrow-derived mesenchymal
stem cells (BMSCs) repair acute necrotized pancreatitis by
secreting microRNA-9 to target the NF-κB1/p50 gene in rats. Sci
Rep. 7:5812017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bi Y, Zhu Y, Zhang M, Zhang K, Hua X, Fang
Z, Zhou J, Dai W, Cui Y, Li J and You T: Effect of shikonin on
spinal cord injury in rats via regulation of HMGB1/TLR4/NF-κB
signaling pathway. Cell Physiol Biochem. 43:481–491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M,
Yang H and Tracey KJ: High mobility group 1 protein (HMG-1)
stimulates proinflammatory cytokine synthesis in human monocytes. J
Exp Med. 192:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anton M, Alen F, de Heras Gomez R, Serrano
A, Pavón FJ, Leza JC, García-Bueno B, de Fonseca Rodríguez F and
Orio L: Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-κB
danger signaling in rat frontal cortex and depressive-like behavior
induced by ethanol binge administration. Addict Biol. 22:724–741.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lan KC, Chao SC, Wu HY, Chiang CL, Wang
CC, Liu SH and Weng TI: Salidroside ameliorates sepsis-induced
acute lung injury and mortality via downregulating NF-κB and HMGB1
pathways through the upregulation of SIRT1. Sci Rep. 7:120262017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang W, Tang Y and Li L: HMGB1, a potent
proinflammatory cytokine in sepsis. Cytokine. 51:119–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YB, Xu P, Xu K, Cai YS, Sun MY, Yang L,
Sun J and Lu SM: Methotrexate affects HMGB1 expression in
rheumatoid arthritis, and the downregulation of HMGB1 prevents
rheumatoid arthritis progression. Mol Cell Biochem. 420:161–170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abe A, Kuwata T, Yamauchi C, Higuchi Y and
Ochiai A: High mobility group box1 (HMGB1) released from cancer
cells induces the expression of pro-inflammatory cytokines in
peritoneal fibroblasts. Pathol Int. 64:267–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu GF, Guo M, Tian ZQ, Wu GZ, Zou XP and
Zhang WJ: Increased of serum high-mobility group box chromosomal
protein 1 correlated with intestinal mucosal barrier injury in
patients with severe acute pancreatitis. World J Emerg Surg.
9:612014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weng H, Deng Y, Xie Y, Liu H and Gong F:
Expression and significance of HMGB1, TLR4 and NF-kappaB p65 in
human epidermal tumors. BMC Cancer. 13:3112013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gnanasekar M, Kalyanasundaram R, Zheng G,
Chen A, Bosland MC and Kajdacsy-Balla A: HMGB1: A promising
therapeutic target for prostate cancer. Prostate Cancer.
2013:1571032013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li W, Wu K, Zhao E, Shi L, Li R, Zhang P,
Yin Y, Shuai X, Wang G and Tao K: HMGB1 recruits myeloid derived
suppressor cells to promote peritoneal dissemination of colon
cancer after resection. Biochem Biophys Res Commun. 436:156–161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Widdison AL: Sodium fusidate and the
cytokine response in an experimental model of acute pancreatitis.
Br J Surg. 86:7151999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nicoletti F, Zaccone P, Di Marco R, Magro
G, Grasso S, Morrone S, Santoni A, Tempera G, Meroni PL and
Bendtzen K: Effects of sodium fusidate in animal models of
insulin-dependent diabetes mellitus and septic shock. Immunology.
85:645–650. 1995.PubMed/NCBI
|