Introduction
Osteoarthritis (OA) is characterized by the
degradation and loss of articular cartilage, inflammation of the
synovial membrane, osteophyte formation and changes in the
subchondral bone (1–4). Previous studies focused on cartilage,
the subchondral bone was recently found to play a crucial role in
OA (5,6). Subchondral bone loss occurs during
the early stage of OA while subchondral sclerosis occurs in the
late stage of OA, along with the presence of osteophytes (7). In addition, a vicious cycle between
structural changes in the subchondral bone and cartilage damage
occurs, which contributes to the progression of OA. In a word, the
subchondral bone is another interesting intervention point for the
treatment of OA.
Therefore, to understand the microstructure of the
subchondral bone is very important in OA. Micro-computed tomography
(micro-CT) can detect microstructural changes and measure
parameters of subchondral bone (8). The most important change in the
subchondral bone is the trabecular bone. Therefore, it is necessary
to evaluate the structural changes of trabecular bone and its
related parameters by micro-CT. Moreover, the parameters and
structural changes of subchondral bone are related to bone
remodeling (9,10).
Bone remodeling plays an essential role in the
balance of formation and resorption in bone tissue (11). In this regard, alkaline phosphatase
(ALP) can enhance osteoblast differentiation and promote bone
formation (12,13). Tartrate-resistant acid phosphatase
(TRAP) is used as a marker for osteoclasts and bone resorption
(14). The osteoprotegerin
(OPG)/receptor activator of nuclear factor-κB ligand (RANKL) system
is one of the most essential molecular mechanisms to regulate bone
remodeling. Imbalances in the OPG/RANKL system can cause abnormal
bone metabolism and subchondral bone damage (15–17).
The OPG/RANKL ratio is vital to the bone mass because it maintains
normal bone turnover (18,19). Previous findings have shown that
regulation of bone metabolism occurs through the classical
OPG/RANK/RANKL pathway, the direct and indirect functions of which
are influenced by numerous factors, including IL-1β and TNF-α
(20,21).
Tougu Xiaotong capsule (TGXTC) is an effective
prescription in OA treatment which consists of Ligusticum
chuanxiong, Morinda officinalis, Sarcandra glabra and Paeonia
lactiflora (22,23). There are many effective molecules
in TGXTC, which is multi-drug, multi-path and multi-target of
molecular mechanism for the treatment of OA, and the non-linear
regulation pattern exists in TGXTC ligand-target interaction
network (24). Previous studies
have proved that TGXTC has therapeutic effects on knee OA (25) through multiple targets, such as
inhibiting chondrocyte apoptosis (26), improving the structure and function
of cartilage (27) and slowing
down cartilage degradation (23),
promoting osteoblast proliferation and calcium secretion (22). However, evaluation of the effect of
TGXTC on subchondral bone remodeling using micro-CT combined with
markers of bone formation and bone resorption has not been
reported.
To further elucidate the precise mechanism of the
potential treatment of OA, in the present study, we investigated
the effects of TGXTC in the bone remodeling of subchondral bone on
the basis of micro-CT assessment of trabecular bone. We found could
TGXTC delay the pathological development of OA through regulating
formation/resorption balance and it's relating inflammatory factor
to improve the remodeling of subchondral bone.
Materials and methods
Animals
A total of 18 female New Zealand rabbits (age, 6
months; weight, 1.7–2.3 kg) were purchased from the Shanghai City
Songjiang District Songlian Experimental Animal Farm (Shanghai,
China). These animals were bred in the Animal Center of Fujian
University of Traditional Chinese Medicine (Fujian, China). The
present study was approved by the Animal Care and Use Committee of
the Fujian University of Traditional Chinese Medicine (Fujian,
China).
Drugs and reagents
TGXTC was prepared by the Second People's Hospital
of Fujian University of Traditional Chinese Medicine (Fujian,
China) (approval no. MIN ZIZHI Z20100006). Sodium pentobarbital was
obtained from ShanghaiXitang Biotechnology Co., Ltd., (Shanghai,
China), sodium penicillin was purchased from GE Healthcare Life
Sciences (Logan, UT, USA), paraformaldehyde was from Beijing
Solarbio Science & Technology Co., Ltd., (Beijing, China), and
EDTA was provided by Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China). The ALP and tartrate resistant acid phosphatase
assay kits were obtained from Beyotime Institute of Biotechnology
(Nantong, China). The rabbit OPG ELISA kit and rabbit RANKL ELISA
kit were purchased from Shanghai Westang Bio-Tech Co., Ltd.
(Shanghai, China). Other reagents included TRIzol, which was
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
PrimeScript™ RT reagent kit with gDNA Eraser and SYBR®
Premix Ex Taq TM II were purchased from Takara Bio., Inc. (Otsu,
Japan), mouse anti-β-actin antibody (monoclonal; 1:8,000; cat. no.
HC201), goat anti-mouse horseradish peroxidase-conjugated IgG
(monoclonal; 1:4,000; cat. no. HS201) and goat anti-rabbit
horseradish peroxidase-conjugated IgG (monoclonal, 1:4,000; cat.
no. HS101) were purchased from TransGen Biotech Co., Ltd.,
(Beijing, China), rabbit anti-IL-1β antibody (polyclonal; 1:500;
cat. no. 16806-1-AP) was provided by Wuhan Sanying Biotechnology
(Hubei, China), and rabbit anti-TNF-α (monoclonal; 1:500; cat. no.
MAB2103) antibody was obtained from R&D Systems China
(Shanghai, China). All other chemicals, unless otherwise stated,
were obtained from Sigma-Aldrich (Merck KGaA, St. Louis, MO,
USA).
Grouping, model preparation and
treatments
The 18 rabbits were randomly divided into three
groups, including the normal, model and TGXTC group, with 6 rabbits
in each group.
The rabbit OA model was established by using
modified Hulth's method in all groups except for the normal group
(28). Rabbits were anesthetized
by ear vein injection with sodium pentobarbital (30 mg/kg). After a
routine disinfection, the medial collateral and anterior cruciate
ligaments were transected via the medial approach, and the medial
meniscus was excised. Then the joint capsule was sutured layer by
layer. Prophylactic antibiotic with sodium penicillin (400,000
units) was given for three days after surgery. After a week, the
animals were forced to movement for 30 min daily for one month.
After five weeks, a clinical oral dose of TGXTC (140
mg/kg/day) was given to rabbits in the TGXTC group for four
consecutive weeks. An equivalent volume of saline was administered
to the normal and model group rabbits.
Specimen collection
After intragastric drug administration, the rabbits
were sacrificed by air embolism after anesthesia with 3% sodium
pentobarbital (30 mg/kg ear marginal vein injection). The femur,
tibia and serum were harvested. The medial femoral condyle was
prepared for Safranin O-fast green staining and the tibia for
micro-CT, respectively. The serum was collected for biochemical
parameters assay. The subchondral bone isolated from the lateral
femoral condyle was collected for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Histology
The medial femoral condyle tissues were fixed in 4%
paraformaldehyde for 3 days and decalcified in 10% EDTA at room
temperature for about 4 months. The medial femoral condyle was
longitudinally cut and embedded in paraffin. Sagittal sections (4
μm) were prepared for Safranin O-fast green staining and
observed under an optical microscope (DM4000 B; Leica Microsystems
GmbH, Wetzlar, Germany) and images were captured at a
magnification, ×100. Following that a modified Mankin scoring
principles (29) was used to
evaluate the degeneration of each femoral condyle.
Micro-CT evaluation
A micro-CT system (ZKKS-MCT; Zhongke Kaisheng
Medical Technology Co., Ltd., Guangzhou, China), with a source
voltage of 60 kV, a power of 40 W, a current of 666 μA, and
resolution of 50 µm, was used to obtain X-ray radiographs. The
specimens were attached to a stage that rotated 360° with images
captured every 0.72°. A 3D analysis was used to calculate
parameters of the tibial in the region at 4.2×1.2×1.8 mm from the
subchondral bone. The parameters measured were: Tabecular number
(Tb.N), trabecular thickness (Tb.Th), bone volume fraction (BV/TV)
and trabecular separation (Tb.Sp).
Serum biochemistry assay
After treatment, blood samples were collected and
centrifuged at 3,000 × g for 10 min at 4°C. The supernatants were
stored at −20°C until analysis. ALP and TRAP activity was measured
with autobiochemical analyzer by standard colorimetric methods
using detection kits, according to the manufacturer's protocol.
Serum OPG and RANKL levels were observed by ELISA kit, according to
the manufacturer's protocol.
RT-qPCR
Total RNA was extracted from the subchondral bone of
the lateral femoral condyle using TRIzol and quantified using a
BioPhotometer Plus UV spectrophotometer (Eppendorf AG, Hamburg,
Germany). cDNA was synthesized using a Takara PrimeScript™ RT
reagent kit with gDNA Eraser. Primers were designed and produced by
Takara Bio., Inc. (Table I). The
PCR system was prepared according to the manufacturer's
instructions, with 10 µl of SYBR® Premix Ex Taq II, 0.4
µl of ROX Reference Dye II, 0.8 µl of upstream primer, 0.8 µl of
downstream primer, 2 µl of cDNA, and 6 µl of dH2O, 20 µl in total.
The PCR amplification protocol was: Pre-denaturation at 95°C for 30
sec, denaturation at 95°C for 3 sec, and annealing at 60°C for 30
sec, for a total of 40 cycles. The fluorescence signal of GAPDH
acted as an internal reference for calculating the relative gene
expression levels.
 | Table I.Primers. |
Table I.
Primers.
| Genes | Sequence | Product length
(bp) |
|---|
| IL-1β | F:
5′-ACAAGTGGTGTTCTCCATGAGTTT-3′ | 125 |
|
| R:
5′-GGGTAGGTTTATCGTCTTTCATCAC-3′ |
|
| TNF-α | F:
5′-ACGTAGTAGCAAACCCGCAAG-3′ | 150 |
|
| R:
5′-TGAAGAGAACCTGGGAGTAGATGAG-3′ |
|
| GAPDH | F:
CCACTTTGTGAAGCTCATTTCT −3′ | 140 |
|
|
F:5′-TCGTCCTCCTCTGGTGCTCT-3′ |
|
Western blot analysis
The subchondral bone isolated from the lateral
femoral condyle was immersed 1:10 in lysis buffer containing 50mM
Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% SDS, 0.1% sodium orthovanadate and 2 mM EDTA
(Beijing BLKW Biotechnology Co., Ltd., Shanghai, China),
homogenized using a Tissuelyser-192 (Shanghai Jingxin Industrial
Development Co., Ltd., Shanghai, China) on ice, then centrifuged at
4°C at 12,000 × g for 30 min. A bicinchoninic acid kit (cat. no.
P0010; Beyotime Institute of Biotechnology) was applied to
determine protein concentrations. Protein samples (30 µg) were
electrophoresed by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis for 2 h (Beyotime Institute of Biotechnology,
Shanghai, China), transferred onto polyvinylidene difluoride
(Shanghai Jinghong Laboratory Instrument Co., Ltd.) membranes, and
blocked with 5% skimmed milk for 2 h. Next, the samples were
incubated on a shaker at 4°C overnight with primary antibodies
against IL-1β (1:500), TNF-α (1:500) and β-actin (1:8,000). The
samples were then rinsed with TBST, incubated with their
corresponding secondary antibodies: Goat anti-mouse horseradish
peroxidase-conjugated IgG (monoclonal; 1:4,000; cat. no. HS201) or
goat anti-rabbit horseradish peroxidase-conjugated IgG (monoclonal,
1:4,000; cat. no. HS101) both from TransGen Biotech, Inc., on a
shaker at room temperature for 1 h, rinsed with TBST, and developed
using ECL substrate. Image processing was carried out using the
ChemiDoc™ XRS + system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) with BeyoECL Plus (Beyotime Institute of Biotechnology) to
analyze gray values and to determine the relative expression of the
proteins.
Statistical analysis
Data were expressed as mean ± standard deviation and
were analyzed by SPSS version 20.0 software. The Shapiro-Wilk test
was used to determine the normality of all groups of data. If the
data exhibited a normal distribution, they were analyzed with
one-way analysis of variance (ANOVA) followed by least signifcant
difference or Games Howell post hoc tests. If not, the
Kruskal-Wallis test was used and the Mann-Whitney U with
Bonferroni's correction was applied as a post hoc test. P<0.05
was considered to indicate a statistically signifcant
difference.
Results
TGXTC decreased cartilage damage
To determine the effects of TGXTC on cartilage
morphology, the sections were stained with Safranin O-fast green
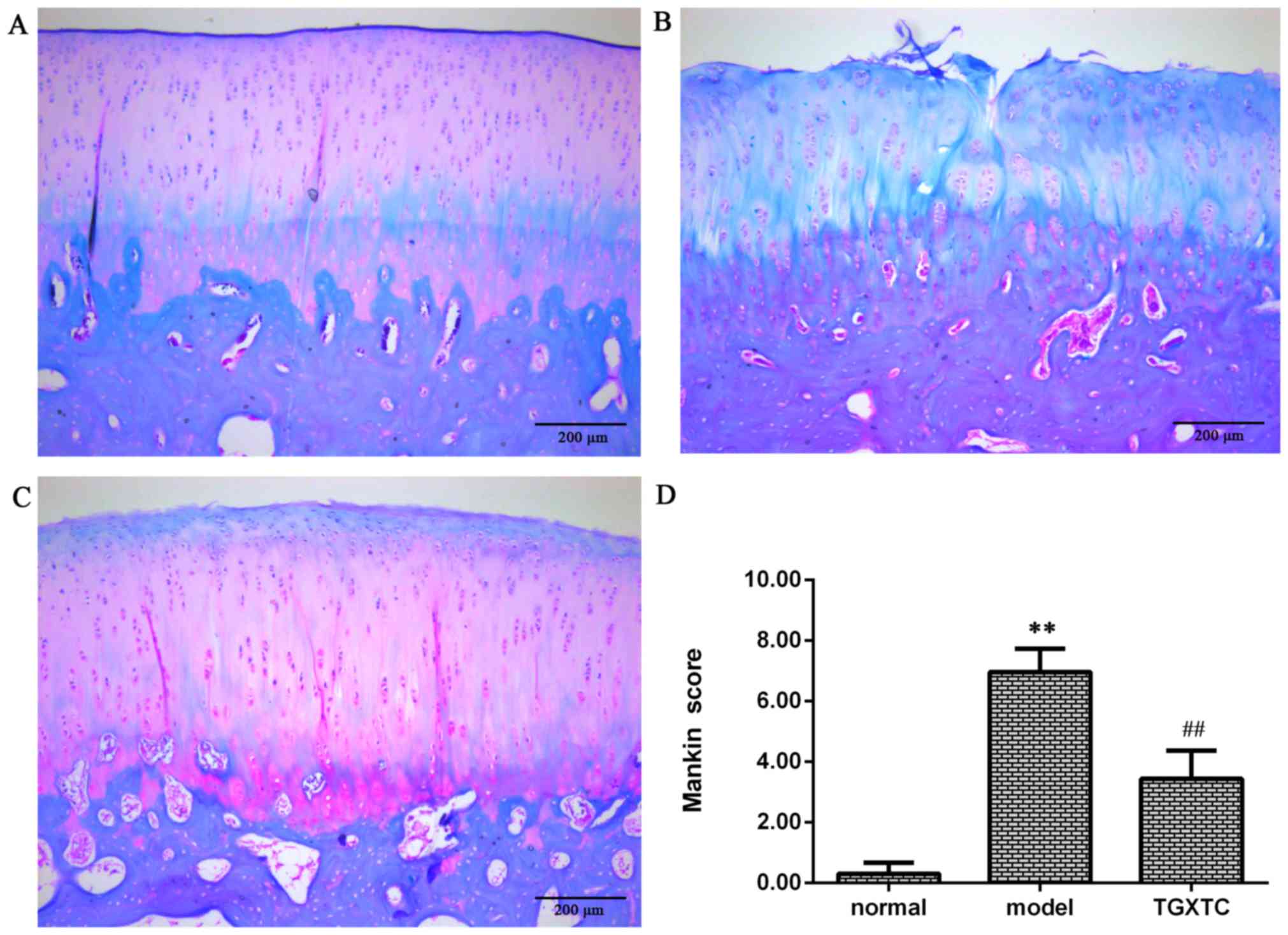
and observed under an optical microscope. As shown in Fig. 1A, the superficial zone, mid zone,
deep zone, and calcified cartilage were visible; the surface of
articular cartilage was smooth and had no cracks, the chondrocytes
were arranged in rows, and the tidemark and cement line were clear.
The proteoglycans were stained by Safranin O, and fast green
staining was evident in the superficial zone and the subchondral
bone in the normal group. In the model group, the cartilage surface
was damaged with cracks and disordered chondrocyte clusters, and
the tidemark replication. In addition, staining of proteoglycans
was severely reduced (Fig. 1B).
However, following treatment with TGXTC, the articular cartilage
had little rough surface without cracks, chondrocytes arrangement
were arranged in order, and the cartilage matrix stained became
deeper and even better than the normal group (Fig. 1C).
We used the scoring principles of Mankin to analyze
the change of cartilage degeneration. The Mankin score in the model
group was significantly higher compared with the normal group
(P<0.01). However, the Mankin score was significantly lower in
the TGXTC group than in the model group (P<0.01). According to
the Mankin score, 1–5 constitutes early stage of OA; 6–9, middle
stage of OA; and 10–14, late stage of OA. The results suggest that
the cartilage damage in the model group was in the middle stages of
OA, while for the TGXTC group cartilage damage occurred in the
early stages of OA (Fig. 1D).
TGXTC reversed microstructural damage
of subchondral bone
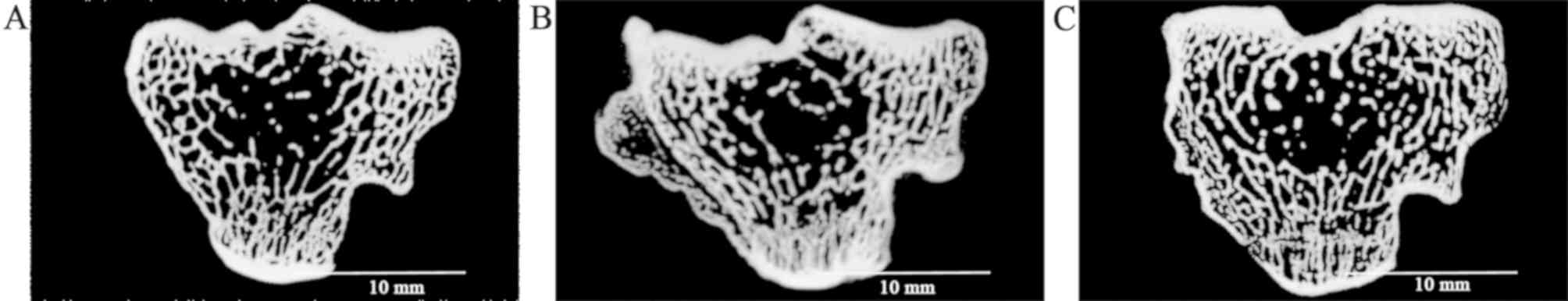
In the sagittal plane of the subchondral bone, the
trabecular bone was arranged in an orderly manner in the normal
group (Fig. 2A). In the model
group, the trabecular bone became broken and disordered (Fig. 2B). However, after treatment with
TGXTC, trabecular bone was relatively regular (Fig. 2C).
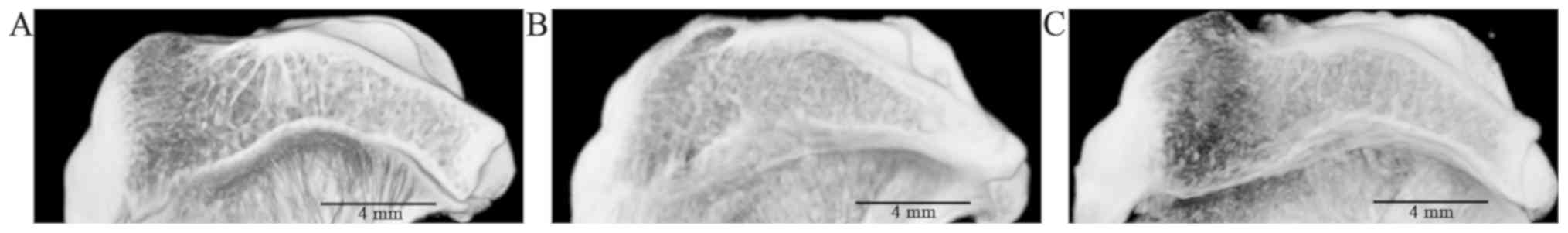
In the cross section of subchondral bone, the
trabecular bone had a uniform network in the normal group (Fig. 3A). In the model group, increased
disorder of the trabecular bone was evident with a large osteophyte
(Fig. 3B). However, after
treatment, the structure of trabecular bone was improved and
arranged neatly (Fig. 3C).
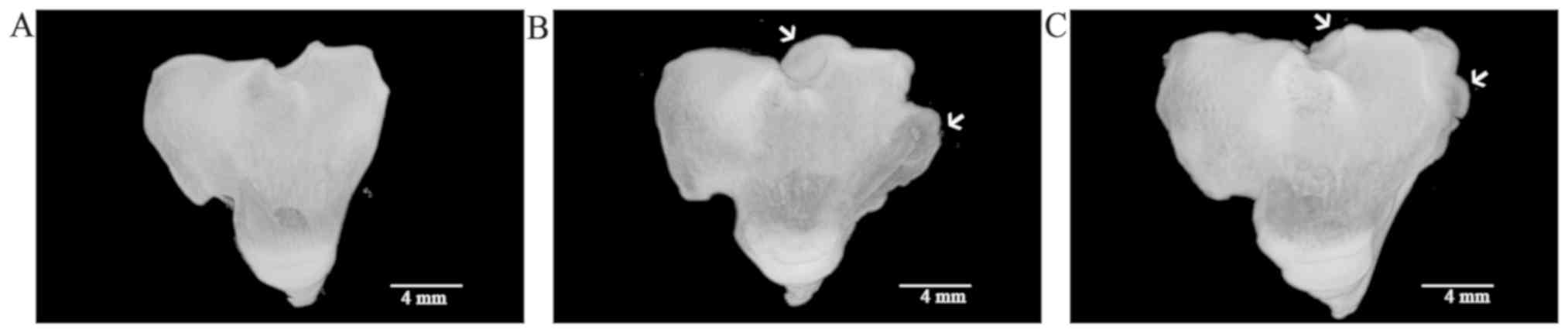
In the 3D models of each experimental group, tibial
plateau became deformed with large osteophyte in the model group
(Fig. 4B), while no osteophyte was
present in the normal group (Fig.
4A). In the TGXTC group, the osteophyte was significantly
decreased compared to the model group (Fig. 4C).
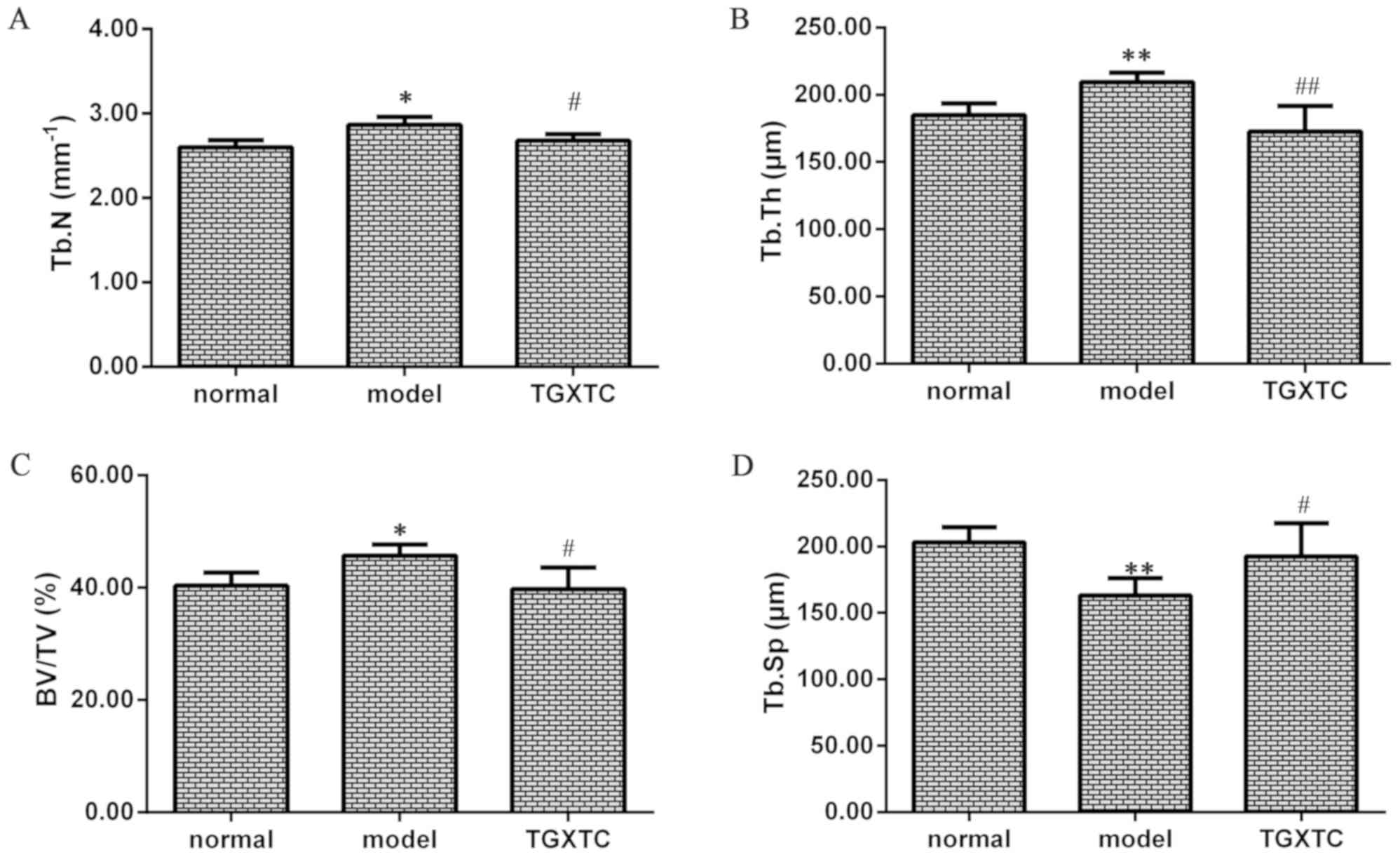
The mean and standard deviation values of the
micro-CT parameters are shown in Fig.
5. The Tb.N, Tb.Th and BV/TV were increased while the Tb.Sp was
decreased in the model group compared with the normal group
(P<0.05 or P<0.01). However, Tb.N, Tb.Th and BV/TV were
reduced in the TGXTC compared with the model group, whereas Tb.Sp
in the TGXTC was increased compared to the model group (P<0.05
or P<0.01). These results suggest that subchondral sclerosis and
microstructure damage in the model group, whereas TGXTC could
improve structural injury of subchondral bone.
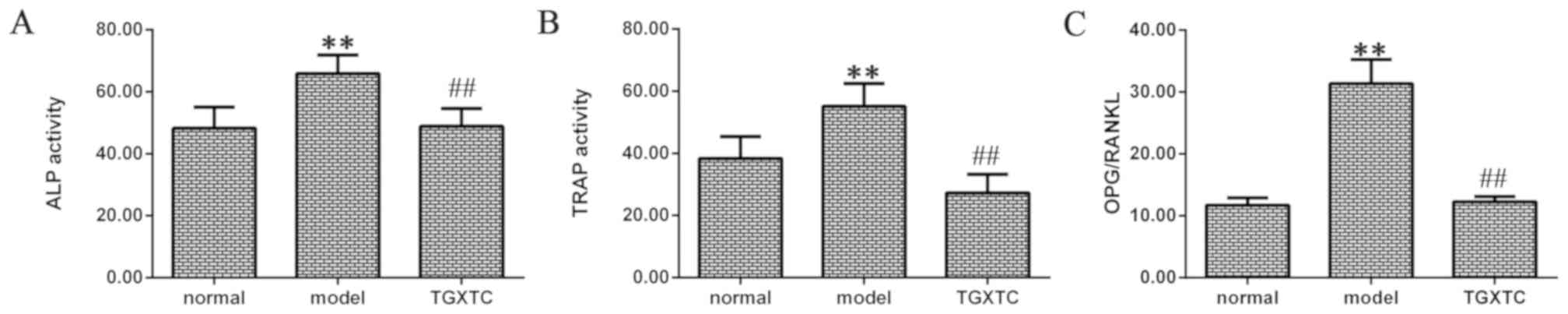
TGXTC decreased ALP and TRAP
ALP activity was significantly higher in the model
than the normal group (P<0.01; Fig.
6A). After treatment, the TGXTC group exhibited a significant
decrease in the ALP activity compared with the model group
(P<0.01; Fig. 6A).
A similar trend was identified for TRAP activity
(Fig. 6B). After inducing OA, TRAP
activity was significantly higher in the model compared to the
normal group (P<0.01). After treatment, the TGXTC group
exhibited a significant decrease in TRAP activity compared to the
model group (P<0.01).
TGXTC decreased the OPG/RANKL
ratio
The ratio of OPG/RANKL was significantly higher in
the model than that in the normal group (P<0.01; Fig. 6C). After treatment, the ratio of
OPG/RANKL was significantly decreased in the TGXTC group compared
to the model group (P<0.01; Fig.
6C).
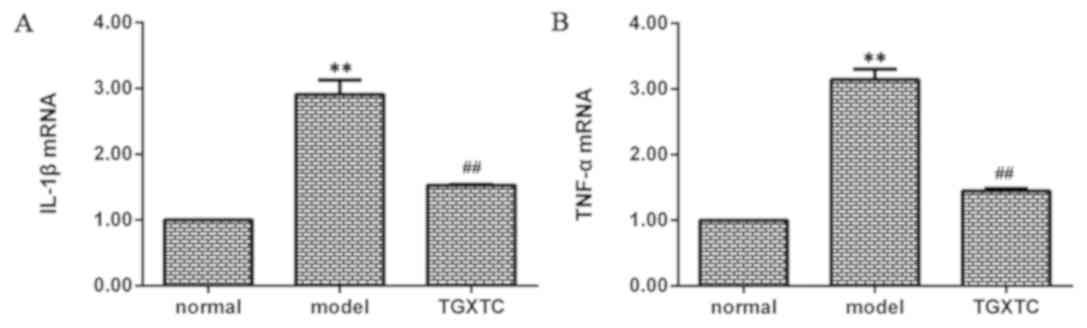
TGXTC inhibits the mRNA expression of
IL-1β and TNF-α
The mRNA expression of IL-1β was significantly
higher in the model than that in the normal group (P<0.01;
Fig. 7A). After treatment, the
IL-1β mRNA expression was significantly decreased in the TGXTC
group compared with the model group (P<0.01; Fig. 7A). mRNA expression of TNF-α was
also significantly higher in the model compared to the normal group
(P<0.01; Fig. 7B). After
treatment, TNF-α mRNA expression was also significantly decreased
in the TGXTC group compared with the model group (P<0.01;
Fig. 7B).
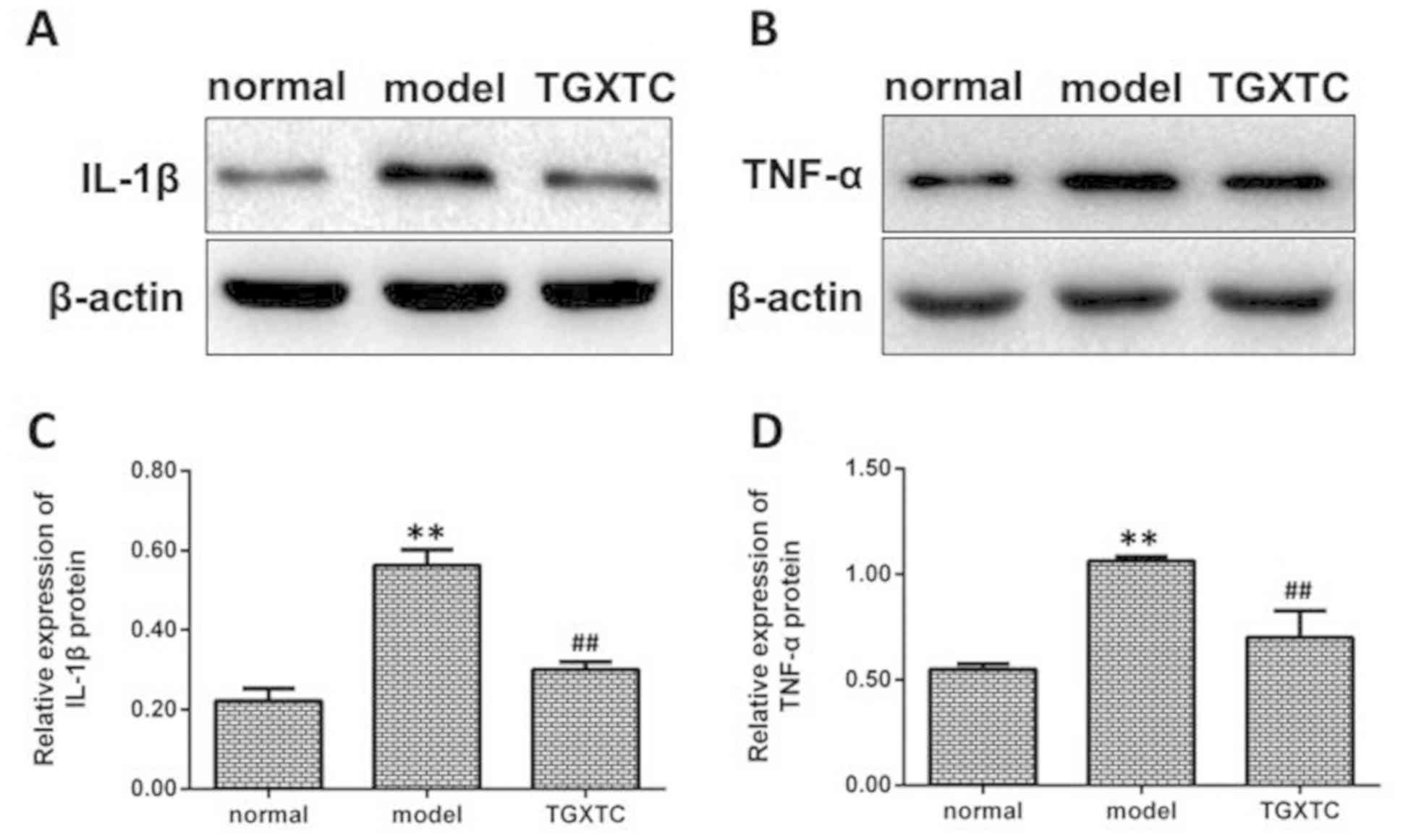
TGXTC inhibits the protein expression
of IL-1β and TNF-α
The protein expression of IL-1β was significantly
higher in the model than the normal group (P<0.01; Fig. 8A and C). After treatment, the IL-1β
protein expression was significantly decreased in the TGXTC
compared with the model group (P<0.01; Fig. 8A and C). The protein expression of
TNF-α was significantly higher in the model than the normal group
(P<0.01; Fig. 8B and D). After
treatment, the TNF-α protein expression was markedly decreased in
the TGXTC compared with the model group (P<0.01; Fig. 8B and D).
Discussion
Although significant progress has been made in
understanding OA pathophysiological pathways, much remains to be
done to develop a specific therapy that could effectively retard or
prevent progression of the disease. To this end, the literature
suggests that in addition to cartilage, other articular tissues,
including the subchondral bone, should be targeted. Previous
findings have shown that TGXTC has therapeutic effects on knee OA
(25) through multiple targets
(22,23,25–27).
In this study, we investigated the effect of TGXTC on subchondral
bone and its relevant mechanisms of inflammatory factors.
To determine the effects of TGXTC on cartilage
structure and the stage of OA, we used the Mankin score. In the
model group, the articular cartilage structure was damaged; the
stained proteoglycans became weaker, showing severe loss of
proteoglycans. However, TGXTC treatment altered loss of
proteoglycans and appearance of the cartilage structure, and the
proteoglycan staining was improved compared to the normal group.
The result shows that TGXTC improves cartilage matrix to promote
increased production of proteoglycans. Additionally, the lower the
modified Mankin score of the animal is, the better the outcome with
respect to delaying the progression of OA (9). Therefore, we used Mankin scoring
principles to analyze alteration of cartilage degeneration after
TGXTC treatment. We found the Mankin score was decreased in the
TGXTC group compared with the model group, which showed the stage
of OA is from the middle stage to the early stage. These findings
suggest that TGXTC has better therapeutic effects on mitigating
cartilage damage and preventing the development of OA.
As the subchondral bone and cartilage are closely
related, structural changes in the subchondral bone can further
aggravate cartilage damage. Thus, we can delay the progression of
OA by regulating bone remodeling to improve the subchondral bone
structure (30,31). In the present study, the effect of
TGXTC on subchondral bone was observed by micro-CT, which enables
non-destructive assessment of the subchondral bone both
quantitatively and qualitatively (10). We found osteophyte formation in the
model group, with osteophytes narrowing the area in TGXTC group. In
the model group, the increase of Tb.N and BV/TV indicate
subchondral bone trabeculae undergo sclerosis. However, after
treatment, the sclerosis was improved. We speculate the treatment
with TGXTC could change these parameters by regulating bone
remodeling, and the structure of subchondral bone was altered by
improving the trabecular bone parameters.
Bone remodeling is a highly complex process by which
old bone is replaced by new bone, in a cycle comprised of three
phases: i) Initiation of bone resorption by osteoclasts, ii) the
transition (or reversal period) from resorption to new bone
formation, and iii) the bone formation by osteoblasts (32,33).
ALP is essential for bone mineralization, which is secreted by
osteoblast (34). Increased ALP
level in serum has been observed in conditions such as rapid bone
loss (35) and fracture risk
(36,37). TRAP is secreted by osteoclasts
during bone resorption, and serum TRAP activity correlates with
resorptive activity in disorders of bone metabolism (14). In order to further explore the
regulatory mechanism of the subchondral bone reconstruction, ALP
and TRAP activity in serum was detected. In the present study a
significant increase in ALP and TRAP levels was observed in the
model group. These results indicated that a high bone formation and
bone resorption of the bone remodeling process were both increased
in OA. Subchondral sclerosis was observed using micro-CT; thus, we
suggest that the rate of bone formation is greater than the rate of
bone resorption. By contrast, treatment of TGXTC showed
significantly decreased ALP and TRAP activity, suggesting that the
potency of TGXTC is due to a decrease in the bone remodeling
process by reducing bone formation and resorption to alleviate
subchondral bone sclerosis in OA rabbits.
During OA, bone remodeling causes an imbalance
between bone resorption and bone formation, leading to structural
changes in the subchondral bone. Recently, the OPG/RANKL signalling
pathway was shown to be involved in the regulation of
osteoblastogenesis and osteoblast activity, which was regarded as a
key factor in inhibiting bone proliferation and directly
participates in the process of bone formation and bone resorption
(38,39). Bone remodeling is also controlled
by the balance of the OPG/RANKL ratio (18,40).
Higher OPG/RANKL ratios mediate bone formation (41), while lower OPG/RANKL ratios mediate
bone resorption (42). The
OPG/RANKL production ratio was significantly increased in the model
group, indicating that the remodeling rate of the subchondral bone
was faster, excessive bone protection developed, and bone
resorption was suppressed. However, following treatment with TGXTC,
the OPG/RANKL ratio was significantly decreased, suggesting that
TGXTC can reduce the remodeling rate by stabilizing bone resorption
and bone formation to delay subchondral bone damage.
Previous studies proved that the regulation of bone
metabolism occurs through the classical OPG/RANK/RANKL pathway, the
direct and indirect functions of which are influenced by
inflammatory factors, such as IL-1β, TNF-α, IL-6 and IL-17
(20,22,43–48).
There are reports that TNF-α and IL-1β and other inflammatory
factors can reduce OPG, stimulate RANKL and have a synergistic
effect, resulting in increased bone resorption (47,49).
In the present study, we found that the mRNA and protein expression
of IL-1 and TNF-α in subchondral bone initially exhibited a
significant increase in the model group and then decreased after
treatment with TGXTC. These results indicated that inflammatory
factors increased in OA, and TGXTC can decrease inflammatory
factors to improve joint damage.
Taken together, results of the present study suggest
that TGXTC could delay the pathological development of OA by
regulating subchondral bone remodeling through regulation of the
formation/resorption balance and its relating inflammatory factors.
This may partly explain its clinical efficacy in the treatment of
knee OA.
Acknowledgements
The authors would like to thank Professor Liu
Xianxiang, from the Academy of Integrative Medicine, Fujian
University of Traditional Chinese Medicine, for his helpful
discussion.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81774345) and the
Developmental Fund of Chen Keji Integrative Medicine (grant no.
CKJ2018003).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
WC and SC conceived and designed the study, and
reviewed and edited the manuscript. YH and MH performed the
histology experiment. RL and ZL performed the micro-CT evaluation
experiment. LZ performed the serum biochemistry assay experiment.
JZ and GW performed the RT-qPCR experiment and the data analysis.
XL and SC performed the western blot analysis experiment. GW
designed the experiment and wrote the manuscript. All authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Fujian University of Traditional Chinese
Medicine (Fujian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mansell JP, Tarlton JF and Bailey AJ:
Biochemical evidence for altered subchondral bone collagen
metabolism in osteoarthritis of the hip. Br J Rheumatol. 36:16–19.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bobinac D, Marinovic M, Bazdulj E,
Cvijanovic O, Celic T, Maric I, Spanjol J and Cicvaric T:
Microstructural alterations of femoral head articular cartilage and
subchondral bone in osteoarthritis and osteoporosis. Osteoarthritis
Cartilage. 21:1724–1730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwan Tat S, Pelletier JP, Lajeunesse D,
Fahmi H, Lavigne M and Martel-Pelletier J: The differential
expression of osteoprotegerin (OPG) and receptor activator of
nuclear factor kappaB ligand (RANKL) in human osteoarthritic
subchondral bone osteoblasts is an indicator of the metabolic state
of these disease cells. Clin Exp Rheumatol. 26:295–304.
2008.PubMed/NCBI
|
|
4
|
Tat SK, Pelletier JP, Lajeunesse D, Fahmi
H, Duval N and Martel-Pelletier J: Differential modulation of RANKL
isoforms by human osteoarthritic subchondral bone osteoblasts:
Influence of osteotropic factors. Bone. 43:284–291. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orth P, Cucchiarini M, Kaul G, Ong MF,
Gräber S, Kohn DM and Madry H: Temporal and spatial migration
pattern of the subchondral bone plate in a rabbit osteochondral
defect model. Osteoarthritis Cartilage. 20:1161–1169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roman-Blas JA and Herrero-Beaumont G:
Targeting subchondral bone in osteoporotic osteoarthritis.
Arthritis Res Ther. 16:4942014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ,
Zhang C and Zheng MH: Subchondral bone in osteoarthritis: Insight
into risk factors and microstructural changes. Arthritis Res Ther.
15:2232013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Effendy NM, Khamis MF and Shuid AN: The
effects of Labisia pumila extracts on bone microarchitecture of
ovariectomized-induced osteoporosis rats: A micro-CT analysis. J
XRay Sci Technol. 25:101–112. 2017.PubMed/NCBI
|
|
9
|
Permuy M, Guede D, López-Peña M, Muñoz F,
Caeiro JR and González-Cantalapiedra A: Effects of diacerein on
cartilage and subchondral bone in early stages of osteoarthritis in
a rabbit model. BMC Vet Res. 11:1432015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohan G, Perilli E, Kuliwaba JS, Humphries
JM, Parkinson IH and Fazzalari NL: Application of in vivo
micro-computed tomography in the temporal characterisation of
subchondral bone architecture in a rat model of low-dose monosodium
iodoacetate-induced osteoarthritis. Arthritis Res Ther.
13:R2102011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellido M, Lugo L, Roman-Blas JA,
Castañeda S, Caeiro JR, Dapia S, Calvo E, Largo R and
Herrero-Beaumont G: Subchondral bone microstructural damage by
increased remodelling aggravates experimental osteoarthritis
preceded by osteoporosis. Arthritis Res Ther. 12:R1522010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Ma XN, Gao YH, Yan JL, Shi WG,
Xian CJ and Chen KM: Sinusoidal electromagnetic fields promote bone
formation and inhibit bone resorption in rat femoral tissues in
vitro. Electromagn Biol Med. 35:75–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang LQ, He HC, He CQ, Chen J and Yang L:
Clinical update of pulsed electromagnetic fields on osteoporosis.
Chin Med J (Engl). 121:2095–2099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janckila AJ and Yam LT: Biology and
clinical significance of tartrate-resistant acid phosphatases: New
perspectives on an old enzyme. Calcif Tissue Int. 85:465–483. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suchecki D and Tufik S: Social stability
attenuates the stress in the modified multiple platform method for
paradoxical sleep deprivation in the rat. Physiol Behav.
68:309–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolon B, Grisanti M, Villasenor K, Morony
S, Feige U and Simonet WS: Generalized Degenerative Joint Disease
in Osteoprotegerin (Opg) Null Mutant Mice. Vet Pathol. 52:873–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen Y, Jiang T, Wang R, He S, Guo M, Zuo
J and He D: (5R)-5-Hydroxytriptolide (LLDT-8) inhibits
osteoclastogenesis via RANKL/RANK/OPG signaling pathway. BMC
Complement Altern Med. 15:772015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trouvin AP and Goëb V: Receptor activator
of nuclear factor-κB ligand and osteoprotegerin: Maintaining the
balance to prevent bone loss. Clin Interv Aging. 5:345–354.
2010.PubMed/NCBI
|
|
19
|
Lange U, Dischereit G, Tarner I, Frommer
K, Neumann E, Müller-Ladner U and Kürten B: The impact of serial
radon and hyperthermia exposure in a therapeutic adit on pivotal
cytokines of bone metabolism in rheumatoid arthritis and
osteoarthritis. Clin Rheumatol. 35:2783–2788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aida Y, Maeno M, Suzuki N, Shiratsuchi H,
Motohashi M and Matsumura H: The effect of IL-1beta on the
expression of matrix metalloproteinases and tissue inhibitors of
matrix metalloproteinases in human chondrocytes. Life Sci.
77:3210–3221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao N, Huang Y, Ye J, Chen W, Li ZF, Lin
R, Li X, Zheng L and Liu X: Protective effects of Tougu Xiaotong
capsule on tumor necrosis factor-α-injured UMR-106 cells. Exp Ther
Med. 10:1908–1914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Huang Y, Chen W, Wu G, Liao N, Li
X, Huang M, Lin R, Yu C, Li X, et al: Protective effects of the
Tougu Xiaotong capsule on morphology and osteoprotegerin/nuclear
factor-κB ligand expression in rabbits with knee osteoarthritis.
Mol Med Rep. 13:419–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng CS, Ye HZ, Xu XJ and Liu XX:
Computational pharmacology study of tougu xiaotong granule in
preventing and treating knee osteoarthritis. Chin J Integr Med.
15:371–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin MN and Liu XX: Observation of Tougu
Xiaotong Recipe for the treatment of 30 patients with knee
osteoarthritis. Fujian J Tradit Chin Med Chin. 36:15–16. 2005.(In
Chinese).
|
|
26
|
Li XH, Wu MX, Ye HZ, Chen WL, Lin JM,
Zheng LP and Liu XX: Experimental study on the suppression of
sodium nitroprussiate-induced chondrocyte apoptosis by Tougu
Xiaotong Capsule -containing serum. Chin J Integr Med. 17:436–443.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Lang W, Ye H, Yu F, Li H, Chen J,
Cai L, Chen W, Lin R, Huang Y, et al: Tougu Xiaotong capsule
inhibits the tidemark replication and cartilage degradation of
papain-induced osteoarthritis by the regulation of chondrocyte
autophagy. Int J Mol Med. 31:1349–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu XX, Li XH and Zhou JT: [Experimental
study on replicating knee osteoarthritis by modified Hulth's
modeling method]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:1104–1108.
2005.(In Chinese). PubMed/NCBI
|
|
29
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cox LG, van Donkelaar CC, van Rietbergen
B, Emans PJ and Ito K: Decreased bone tissue mineralization can
partly explain subchondral sclerosis observed in osteoarthritis.
Bone. 50:1152–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu S, Chen K, Lan Y, Zhang N, Jiang R and
Hu J: Alendronate protects against articular cartilage erosion by
inhibiting subchondral bone loss in ovariectomized rats. Bone.
53:340–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geusens PP and van den Bergh JP:
Osteoporosis and osteoarthritis: Shared mechanisms and
epidemiology. Curr Opin Rheumatol. 28:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Havill LM, Hale LG, Newman DE, Witte SM
and Mahaney MC: Bone ALP and OC reference standards in adult
baboons (Papio hamadryas) by sex and age. J Med Primatol.
35:97–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ni J, Yuan XM, Yao Q and Peng LB: OSM is
overexpressed in knee osteoarthritis and Notch signaling is
involved in the effects of OSM on MC3T3-E1 cell proliferation and
differentiation. Int J Mol Med. 35:1755–1760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bellido M, Lugo L, Roman-Blas JA,
Castañeda S, Calvo E, Largo R and Herrero-Beaumont G: Improving
subchondral bone integrity reduces progression of cartilage damage
in experimental osteoarthritis preceded by osteoporosis.
Osteoarthritis Cartilage. 19:1228–1236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ross PD, Kress BC, Parson RE, Wasnich RD,
Armour KA and Mizrahi IA: Serum bone alkaline phosphatase and
calcaneus bone density predict fractures: A prospective study.
Osteoporos Int. 11:76–82. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hofbauer LC and Schoppet M: Clinical
implications of the osteoprotegerin/RANKL/RANK system for bone and
vascular diseases. JAMA. 292:490–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Whyte MP, Obrecht SE, Finnegan PM, Jones
JL, Podgornik MN, McAlister WH and Mumm S: Osteoprotegerin
deficiency and juvenile Paget's disease. N Engl J Med. 347:175–184.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brzóska MM and Rogalska J: Protective
effect of zinc supplementation against cadmium-induced oxidative
stress and the RANK/RANKL/OPG system imbalance in the bone tissue
of rats. Toxicol Appl Pharmacol. 272:208–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gurban CV and Mederle O: The OPG/RANKL
system and zinc ions are promoters of bone remodeling by osteoblast
proliferation in postmenopausal osteoporosis. Rom J Morphol
Embryol. 52:1113–1119. 2011.PubMed/NCBI
|
|
43
|
Fujii T, Kitaura H, Kimura K, Hakami ZW
and Takano-Yamamoto T: IL-4 inhibits TNF-α-mediated osteoclast
formation by inhibition of RANKL expression in TNF-α-activated
stromal cells and direct inhibition of TNF-α-activated osteoclast
precursors via a T-cell-independent mechanism in vivo. Bone.
51:771–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Onal M, Xiong J, Chen X, Thostenson JD,
Almeida M, Manolagas SC and O'Brien CA: Receptor activator of
nuclear factor κB ligand (RANKL) protein expression by B
lymphocytes contributes to ovariectomy-induced bone loss. J Biol
Chem. 287:29851–29860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Riegel A, Maurer T, Prior B, Stegmaier S,
Heppert V, Wagner C and Hänsch GM: Human polymorphonuclear
neutrophils express RANK and are activated by its ligand, RANKL.
Eur J Immunol. 42:975–981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim KW, Kim HR, Park JY, Park JS, Oh HJ,
Woo YJ, Park MK, Cho ML and Lee SH: Interleukin-22 promotes
osteoclastogenesis in rheumatoid arthritis through induction of
RANKL in human synovial fibroblasts. Arthritis Rheum. 64:1015–1023.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lam J, Takeshita S, Barker JE, Kanagawa O,
Ross FP and Teitelbaum SL: TNF-alpha induces osteoclastogenesis by
direct stimulation of macrophages exposed to permissive levels of
RANK ligand. J Clin Invest. 106:1481–1488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Onal M, Galli C, Fu Q, Xiong J, Weinstein
RS, Manolagas SC and O'Brien CA: The RANKL distal control region is
required for the increase in RANKL expression, but not the bone
loss, associated with hyperparathyroidism or lactation in adult
mice. Mol Endocrinol. 26:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zeng JZ, Ma LF, Meng H, Yu HM, Zhang YK
and Guo A: (5R)-5-hydroxytriptolide (LLDT-8) prevents
collagen-induced arthritis through OPG/RANK/RANKL signaling in a
rat model of rheumatoid arthritis. Exp Ther Med. 12:3101–3106.
2016. View Article : Google Scholar : PubMed/NCBI
|