Introduction
Hepatocellular carcinoma (HCC) is the most commonly
diagnosed primary tumor of the liver, ranking third worldwide in
terms of the most lethal types of cancer (1,2).
While there are some treatment options available for patients, such
as surgical intervention, radiotherapy, locoregional therapy and
chemotherapy, the rates of metastasis and relapse remain high for
patients with HCC (3,4). Therefore, there is currently an
urgent need for improved treatment options in clinical
practice.
Salidroside (SDS) acts as a phenylpropanoid
glycoside and is one of the most potent antioxidant ingredients
that can be isolated from Rhodiola rosea L. It commonly
grows at high altitudes and may be found in parts of Asia, Eastern
Europe and Canada (5,6). SDS functions as an adaptogen that
provides non-specific resistance by suppressing physical, chemical
and biological stressors in the body, and has been used as a
hepato-protective herb in traditional Chinese medicine for decades
(5).
In recent years, SDS has been reported to possess
numerous medicinal properties, including antitumor,
anti-inflammatory, anti-viral, anti-radiation, antioxidative stress
and fatigue-reducing properties (7–16).
Most notably, the anticancer properties of SDS have been
extensively reported by researchers in both in vitro and
in vivo models. SDS has been shown to significantly inhibit
the growth of lung, breast and liver cancer through the promotion
of the activation of cellular apoptotic pathways, and to inhibit
breast tumor growth in vivo (6,17–22).
In addition, SDS has been shown to inhibit metastasis, as Sun et
al demonstrated that SDS inhibited the migration and invasion
of HT1080 human fibrosarcoma cells (23). However, there is limited
information about the role of SDS in preventing the metastasis in
other forms of cancer, and its underlying mechanisms of action
remain unknown.
Notch signaling is highly conserved and is often
activated in many types of tumors, playing complex roles in tumor
development and metastasis (24–30).
Previously, Zhou et al (31) reported Notch1 as a novel candidate
biomarker for assessing patient prognosis, as well as for the
molecular targeted therapy of HCC. This is due to the high
expression of Notch1 in HCC tumor tissues, which has been
associated with tumor size, tumor grade, metastasis, venous
invasion and TNM stage. Patients with a high Notch1 expression were
shown to have significantly shorter overall survival times
(31).
The downregulation of Notch1 has been previously
found to decrease the invasiveness of HCC cells in vitro
(32), which is partially
attributed to the activation of the Notch1/Snail/E-cadherin pathway
(33–36). E-cadherin acts as a homotypic
epithelial cell-cell adhesion molecule with anti-invasive
properties in certain types of cancer (37–39).
The Notch1/matrix metalloproteinase (MMP) pathway has also been
found to play an important role in HCC development, as MMPs are
proteolytic enzymes in the extracellular matrix (ECM) that
contribute to tumor invasion, angiogenesis and metastasis (40–42).
Previously, the downregulation of Notch1 in pancreatic cancer and
lingual squamous cell carcinoma was shown to inhibit tumor invasion
by suppressing the expression of MMP-2 and MMP-9 (28,29,32).
In addition, an inhibitor of the Notch signaling pathway
effectively inhibited the invasion of HCC cells via MMP-2 and MMP-9
suppression (32), confirming that
the Notch1 signaling is closely associated with the metastasis of
HCC.
In the present study, we demonstrate that SDS
suppresses the metastasis of highly metastatic HCC cells. Genes
involved in EMT and metastasis are consisted regulated. Notably,
Notch1 signaling activity is inhibited by SDS. Taken together, the
findings of this study suggest that SDS suppresses the metastasis
of HCC cells through suppression of the activation of the Notch1
signaling pathway.
Materials and methods
Reagents and antibodies
SDS (purity, >99%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). SDS was dissolved in water and filtered
through a 0.22-µm filter prior to use. Hairy and enhancer of split
1 (Hes1; cat. no. BM4488) polyclonal antibodies were purchased from
Boster Biological Co. (Wuhan, China). Hairy/enhancer-of-split
related with YRPW motif 1 (Hey1; cat. no. 19929-1-AP) and hairy and
enhancer of split 5 (Hes5; cat. no. 22666-1-AP) polyclonal
antibodies were purchased from ProteinTech Biological Co. (Wuhan,
China). Cyclooxygenase (COX)-2 (cat. no. KGYT1073-6), E-cadherin
(cat. no. KGYT1453-6), MMP-2 (cat. no. KGYT2798-6) and MMP-9 (cat.
no. KGYT1892-6) polyclonal antibodies were purchased from KeyGEN
BioTech Corp. (Nanjing, China). Snail (cat. no. bs-21598R) and
Notch1 (cat. no. bs-1335R) polyclonal antibodies were purchased
from Bioss Biological Co. (Beijing, China). DMEM was purchased from
the Gibco; Thermo Fisher Scientific Inc. (Waltham, MA, USA). FBS
was purchased from the ExCell Biology Company (Shanghai, China).
All other chemicals were of analytical grade and were commercially
available.
Cells and cell culture
The MHCC97H cells (cat. no. KG340) were obtained
from KeyGEN BioTech Corp. the HMCC97H cells were cultured in DMEM
supplemented with 10% fetal bovine serum (FBS) in a humidified
incubator at 37°C with 5% CO2. The cells were harvested
following trypsinization (0.25% Trypsin-EDTA) and washed with
phosphate-buffered saline (PBS). The cells were subcultured when
the cell density reached 80–90% confluency.
Scratch wound closure assay
For the detection of cell migration, the HMCC97H
cells were seeded onto a 6-well plate and cultured at 37°C for 24
h. When the cell density reached 60% confluency, the monolayer was
scraped away with a sterile tip, as previously described (43). The remaining cells were washed with
PBS, and cultured with new medium with 1, 2, or 4 µg/ml of SDS at
37°C for 24 h. Cell migration into the denuded area was quantified
using a computer-assisted inverted microscope (IX71; Olympus
Corporation, Tokyo, Japan; magnification, ×200).
Transwell assay for in vitro
migration
HMCC97H cell migration was measured using
Matrigel-coated Transwell inserts. The cells were cultured in
serum-free DMEM at 37°C for 24 h before being trypsinized and
seeded at 5×104 cells per upper chamber in 100 µl
serum-free DMEM with 1, 2 or 4 µg/ml SDS. The 24-well plate
containing the Transwell was cultured in a humidified incubator at
37°C and 5% CO2 for 24 h. Cells in the upper side of the
insert membrane were rubbed using a cotton swab. Cells that had
migrated to the underside of the insert membrane were stained with
0.1% crystal violet solution for 30 min at 37°C, rinsed in PBS,
air-dried, and observed under an inverted microscope (IX71; Olympus
Corporation) equipped with a camera to count the number of migrated
cells (magnification, ×200). Three fields per insert were scored
and averaged.
In vitro invasion assay
The invasion of the HMCC97H cells in vitro
was determined using Matrigel-coated Transwell inserts, as
previously described (44).
Serum-free DMEM with 2-fold diluted Matrigel (30 µl/well) was
placed into the upper chamber of the Transwell filter and incubated
for 2 h at 37°C for gelling. Subsequently, 5×104 cells
were seeded in the upper chamber and cultured at 37°C for 24 h. The
invasion of the cells was assessed using the same techniques as
those used for cell migration described above.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to determine the expression levels
of Notch1, Hes1, Hes5, Hey1, COX-2, Snail and
E-cadherin in the SDS-treated HMCC97H cells. The cells were
incubated with serum-free DMEM containing 1, 2 or 4 µg/ml SDS for
24 h. Subsequently, the cells were trypsinized, washed in PBS, and
total RNA was extracted using TRIzol reagent (Thermo Fisher
Scientific Inc.) according to the manufacturer's instructions.
First-strand cDNA was synthesized with a reverse transcription kit
(Thermo Fisher Scientific Inc.) according to the manufacturer's
instructions and used as the template for RT-qPCR. GAPDH was
selected as the reference for internal standardization. The primers
P1 and P2 specific of GAPDH, P3 and P4 specific of
Hes1, P5 and P6 specific of Hes5, P7 and P8 specific
of Hey1, P9 and P10 specific of COX-2, P11 and P12
specific of Snail, P13 and P14 specific of MMP-2, P15
and P16 specific of MMP-9, and P17 and P18 specific of
E-cadherin (Table I) were
designed to amplify the specific fragments of all the genes. qPCR
was performed on an ABI 7500 real-time PCR system (Applied
Biosystems, Foster City, CA, USA) using the 2X SYBR Premix Ex Taq™
kit (Takara, Shiga, Japan). PCR was carried out in a total volume
of 20 µl, containing 10 µl 2X SYBR Premix Ex Taq™, 0.4 µl ROX
Reference Dye II (50X), 1 µl diluted cDNA, 0.2 µl primers (20
mmol/l) and 8.4 µl of DEPC-treated water. The thermal profile for
RT-qPCR was 94°C for 15 sec, followed by 40 cycles of 94°C for 5
sec, 60°C for 15 sec and 72°C for 35 sec. All reactions were run in
triplicate. Dissociation curve analysis of the amplicons was
performed at the end of each PCR to confirm that only one PCR
product was amplified and detected. Data were analyzed with the
comparative Cq method (2−∆∆Cq) based on Cq values for
each gene and GAPDH to calculate relative mRNA expression
levels (45).
 | Table I.Table
I. Sequences of the primers used in this study. |
Table I.
Table
I. Sequences of the primers used in this study.
| Gene | Sequence
(5′-3′) | Amplicon size
(bp) |
|---|
| Notch1 |
F-TCAGCGGGATCCACTGTGAG |
|
|
|
R-ACACAGGCAGGTGAACGAGTTC | 104 |
| Hes1 |
F-CTGAGCACAGACCCAAGTGT |
|
|
|
R-GAGTGCGCACCTCGGTATTA | 115 |
| Hes5 |
F-GAAAAACCGACTGCGGAAGC |
|
|
|
R-GACGAAGGCTTTGCTGTGCT | 184 |
| Hey1 |
F-CGGCTCTAGGTTCCATGTCC |
|
|
|
R-GCTTAGCAGATCCCTGCTTCT | 162 |
| COX-2 |
F-ATAACCCCGCCAAAAGGGG |
|
|
|
R-CTGAGTACCAGGTCTGCAGTG | 145 |
| Snail |
F-CGAGTGGTTCTTCTGCGCTA |
|
|
|
R-GGGCTGCTGGAAGGTAAACT | 160 |
| MMP-2 |
F-GATGACATCAAGGGCATTCAGGAGC |
|
|
|
R-ATCTTTTCCGGGAGCTCAGGCC | 254 |
| MMP-9 |
F-CCAAGGATACAGTTTGTTCCTCGTG |
|
|
|
R-GGTTCAGGGCGAGGACCATAGA | 177 |
|
E-cadherin |
F-GTCAGTTCAGACTCCAGCCC |
|
|
|
R-TGTAGCTCTCGGCGTCAAAG | 196 |
| GAPDH |
F-CATCTTCTTTTGCGTCGCCA |
|
|
|
R-TTAAAAGCAGCCCTGGTGACC | 202 |
Western blot analysis
The HMCC97H cells were cultured with serum-free DMEM
with 1, 2, or 4 µg/ml SDS for 24 h. The cells were washed 3 times
with ice-cold PBS and harvested in lysis buffer. The lysate was
centrifuged at 12,000 × g for 15 min to collect the supernatant.
Bicinchoninic acid (BCA) was used to determine the protein
concentrations. Subsequently, 40 µg of each sample was loaded onto
12% sodium dodecyl sulfate (SDS)-polyacrylamide gels for
electrophoresis and transferred onto nitrocellulose membranes. The
membranes were blocked with 4% BSA in PBS at room temperature for 2
h prior to incubation with primary antibodies, which included
anti-Notch1 (1:1,000), anti-Hey1 (1:1,000), anti-Hes1 (1:1,000),
anti-Hes5 (1:1,000), anti-Snail (1:1,000), anti-COX2 (1:1,000),
anti-E-cadherin (1:1,000), anti-MMP-2 (1:1,000), anti-MMP-9
(1:1,000), and anti-GAPDH (1:2,000). The membranes were incubated
with the primary antibodies overnight at 4°C and washed 4 times
with PBS containing 0.1% Tween-20 (PBST). The membranes were then
incubated with HRP-conjugated anti-mouse/rabbit IgG (cat. no.
bs-0368R-HRP/bs-0369M-HRP; BIOSS, Beijing, China) diluted at
1:5,000 in PBS for 1 h at room temperature before being washed with
PBS-T and detected using the ECL reagent (KeyGEN BioTech Corp.).
The signal intensity of each band was quantified with ImageJ
software (version 1.6.0; National Institutes of Health, Bethesda,
MD, USA), and the results were normalized to those of GAPDH.
Statistical analysis
Data are presented as the means ± standard errors of
the mean (SEM) from 3 or more independent experiments and were
evaluated with analysis of variance (ANOVA) followed by Dunnett's
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
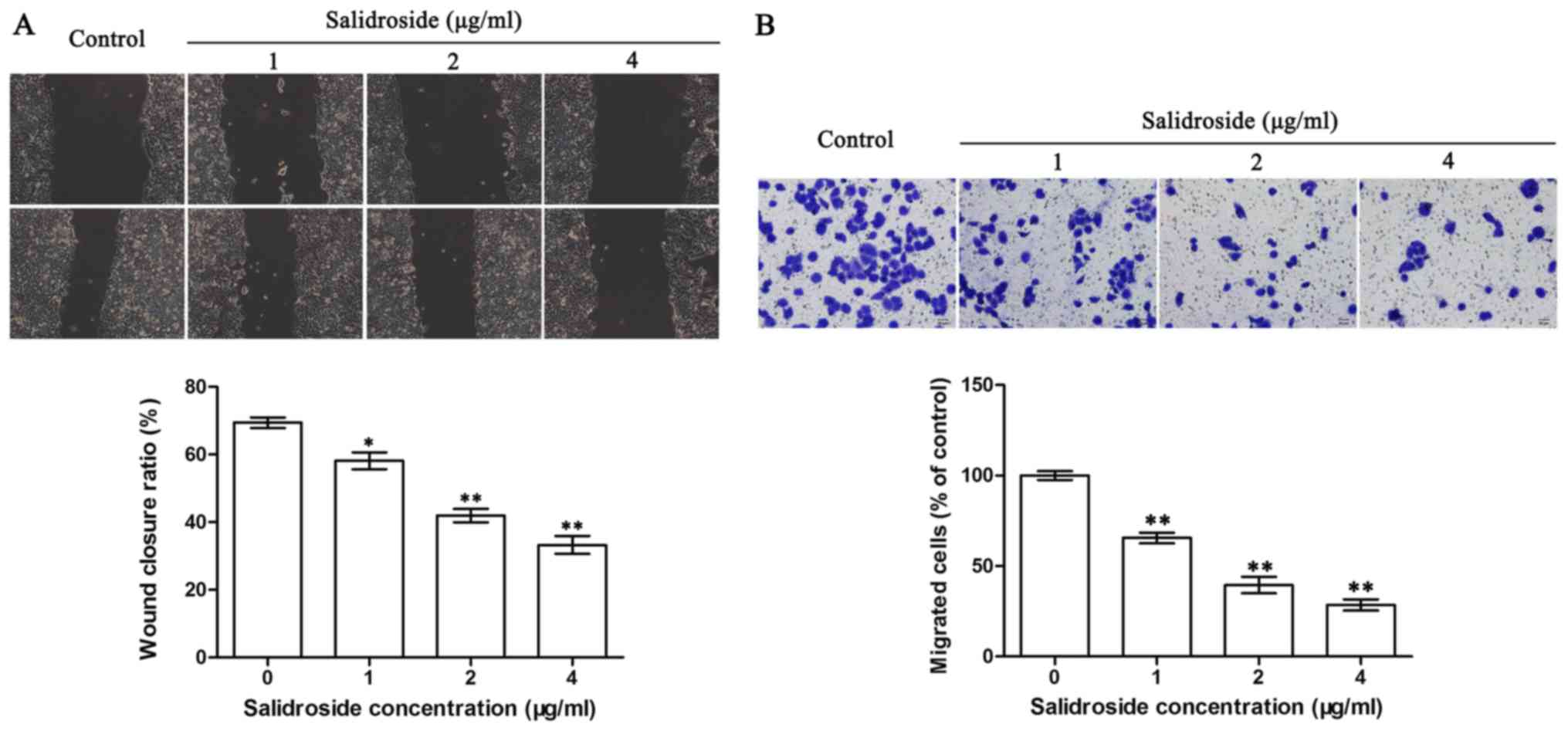
SDS suppresses the migration of
HMCC97H cells in vitro
The scratch test and Transwell assay were used to
determine the effects of SDS on the migration of the HMCC97H cells.
As shown in Fig. 1A, the wound
closure was 69.2% complete in the absence of SDS. Following
treatment with SDS, the wound closure levels were significantly
lower at 58.2, 42.5 and 33.4% in the cells treated with 1, 2 and 4
µg/ml SDS, respectively. The migration of the HMCC97H cells was
significantly suppressed by SDS, as shown by Transwell assay. As
shown in Fig. 1B, cell migration
was reduced to 65.31, 39.68 and 28.12% (relative to the control)
following 24 h of treatment with 1, 2 and 4 µg/ml SDS,
respectively. These results demonstrated that SDS can effectively
suppress HCC cell migration in a concentration-dependent
manner.
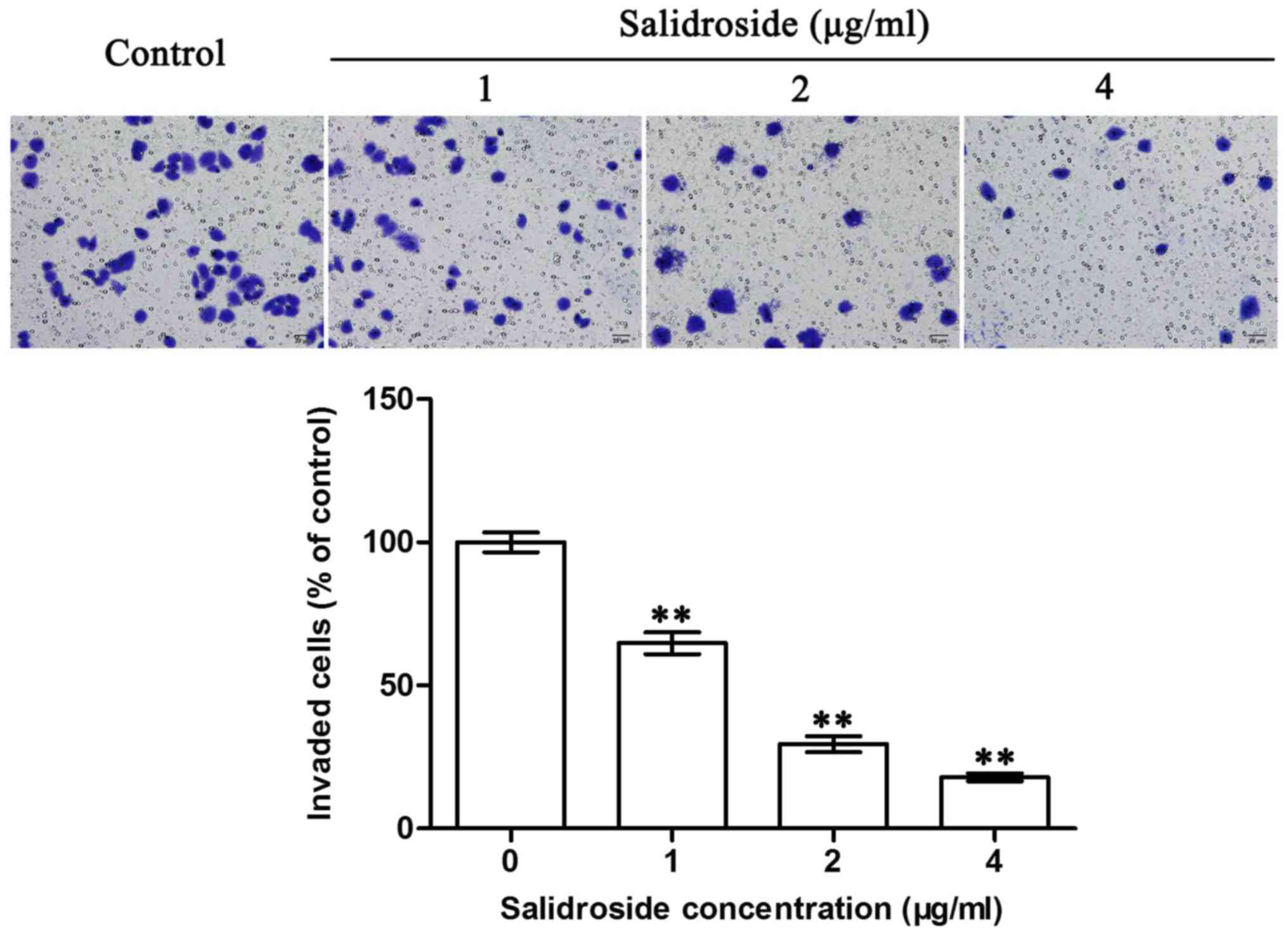
SDS suppresses the invasion of HMCC97H
cells in vitro
The Matrigel-coated Transwell assay was used to
examine the effects of SDS on the invasion of the HMCC97H cells. As
shown in Fig. 2, SDS treatment
significantly suppressed the invasion of the HMCC97H cells in
vitro. Following 24 h of treatment with SDS, the number of
invaded cells had decreased to 64.5, 29.0 and 17.5% when treated
with 1, 2 and 4 µg/ml SDS, respectively. These results indicated
that SDS effectively suppressed the invasion of the HMCC97H cells
in a concentration-dependent manner.
SDS decreases the expression of
Notch1, Snail, COX-2, MMP-2 and MMP-9, whereas it upregulates the
expression of E-cadherin in the HMCC97H cells
Western blot analysis was performed to investigate
protein expression in the HMCC97H cells following treatment with
SDS. Since Snail, COX-2, MMP-2, MMP-9 and E-cadherin are closely
related to the metastasis of tumor cells (43,46–48),
we also investigated the effects of SDS on these genes in the
HMCC97H cells. As shown in the Figs.
3 and 4, expression levels of
Notch1, Snail, COX-2, MMP-2 and MMP-9 were
significantly lower following treatment with SDS (P<0.05).
Moreover, E-cadherin expression was markedly increased
following treatment with SDS (P<0.05).
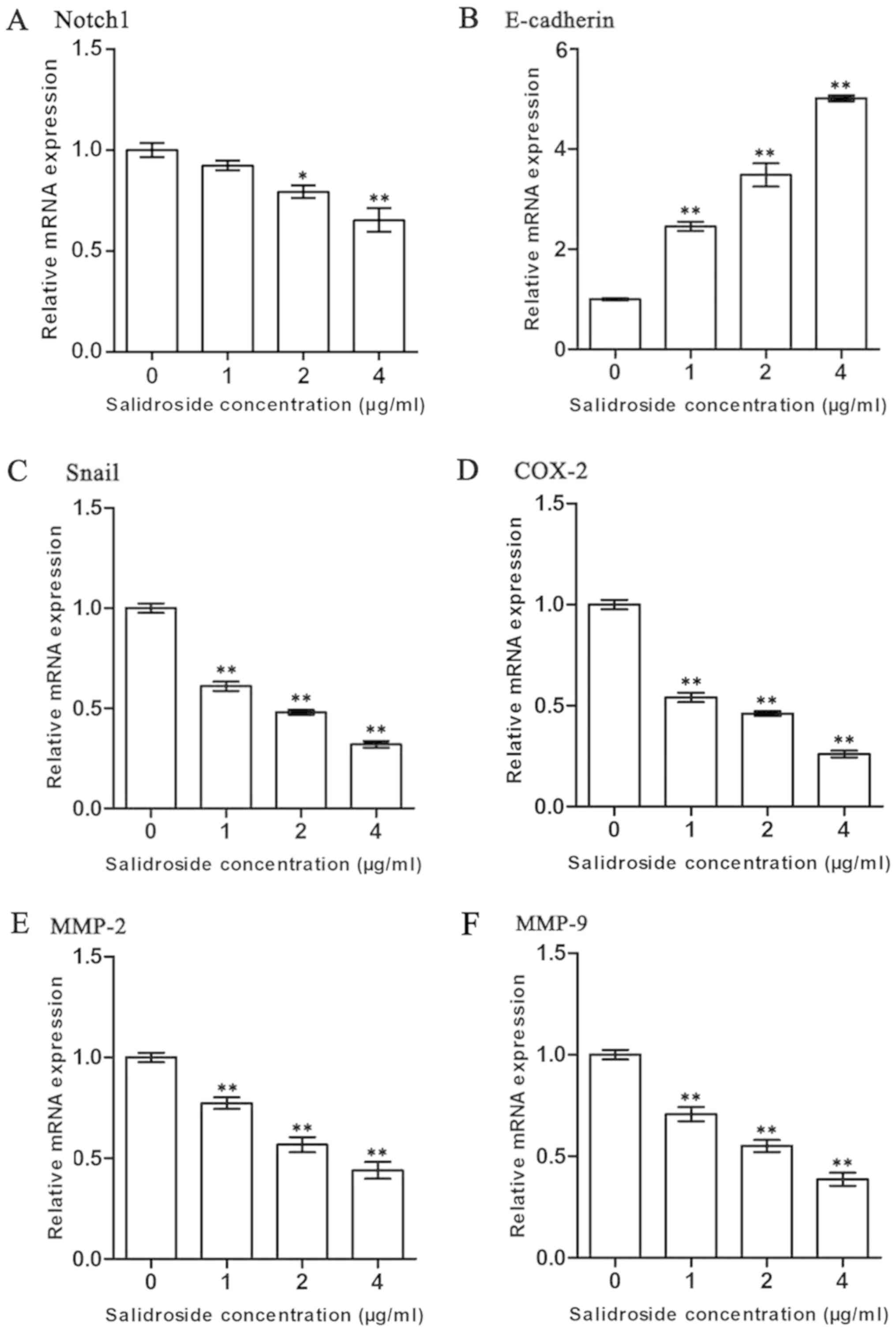 | Figure 3.Effects of SDS on the expression of
Notch1, Snail, COX-2, MMP-2, MMP-9 and E-cadherin in
the HMCC97H cells. The cells were treated with 1, 2, and 4 µg/ml
SDS. (A-F) The expression levels of Notch1, Snail, COX-2, MMP-2,
MMP-9 and E-cadherin were measured by RT-qPCR, and the
data were normalized to the GAPDH gene as an internal
control. Data are presented as the means ± SEM from 3 independent
experiments. Asterisks indicate statistically significant
differences (*P<0.05 and **P<0.01) compared to the control (0
µg/ml). SDS, salidroside; COX-2, cyclooxygenase-2; MMP, matrix
metalloproteinase; SEM, standard error of the mean. |
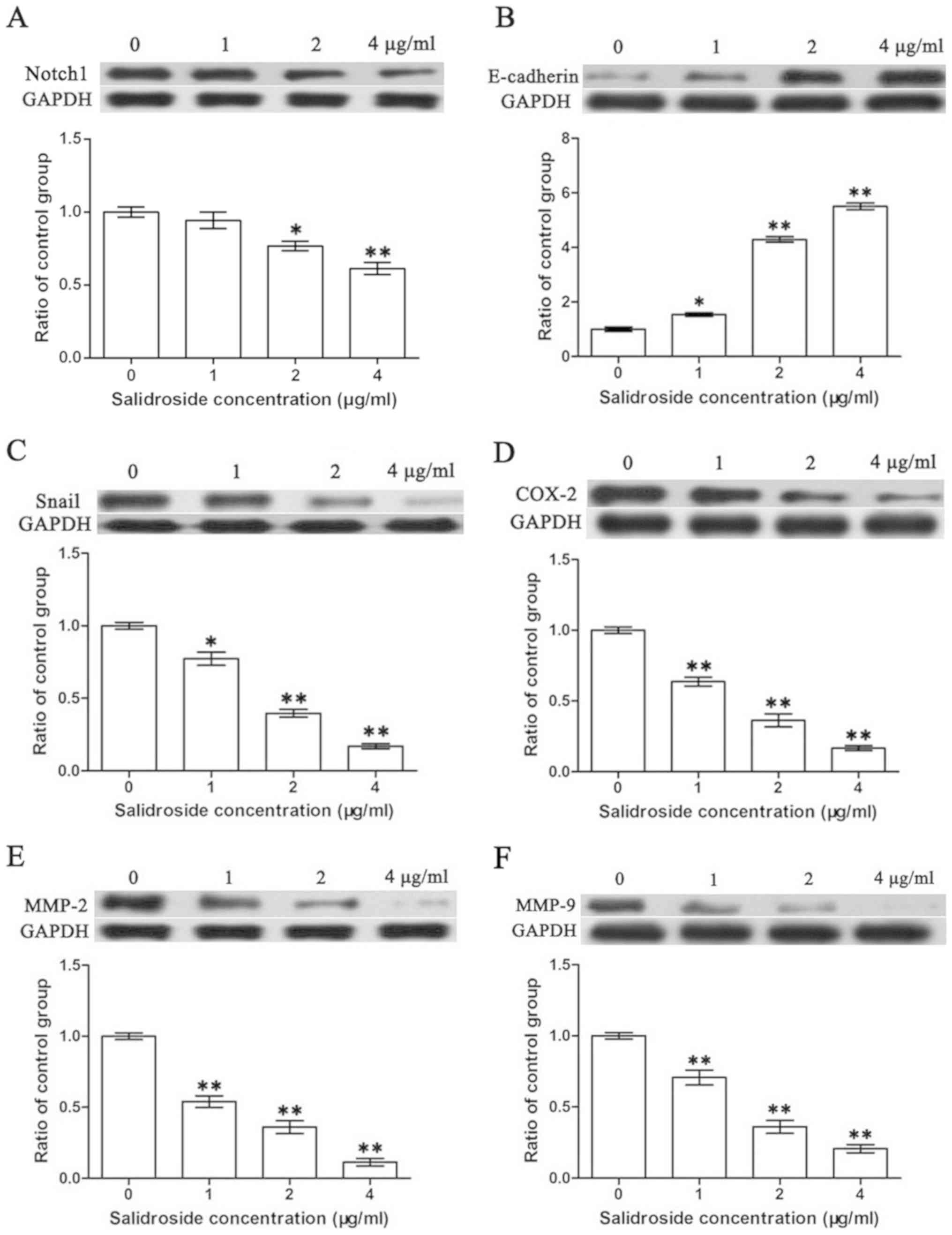 | Figure 4.Effects of SDS on the expression of
Notch1, Snail, COX-2, MMP-2, MMP-9 and E-cadherin in HMCC97H cells.
The cells were treated with 1, 2 and 4 µg/ml SDS. (A-F) The protein
expression levels of Notch1, Snail, COX-2, MMP-2, MMP-9 and
E-cadherin were measured by western blot analysis. Bands were
scanned and quantified and the results were normalized to GAPDH.
Data are presented as the means ± SEM from 3 independent
experiments. Asterisks indicate statistically significant
differences (*P<0.05 and **P<0.01) compared to the control (0
µg/ml). SDS, salidroside; COX-2, cyclooxygenase-2; MMP, matrix
metalloproteinase; SEM, standard errors of the mean. |
SDS inhibits the activation of the
Notch1 signaling pathway
Since the Notch1 signaling pathway is closely
related to tumor metastasis and SDS can inhibit metastasis
(49), we hypothesized that SDS
may function through the Notch1 signaling pathway. The activity of
the Notch signaling pathway was thus assessed by the expression of
its downstream genes, including Hey1, Hes1 and Hes5.
As shown in Fig. 5, treatment with
SDS significantly reduced the expression levels of these genes
(P<0.05). This indicates that SDS likely inhibits the metastasis
of cancer cells by halting the activation of the Notch1 signaling
pathway.
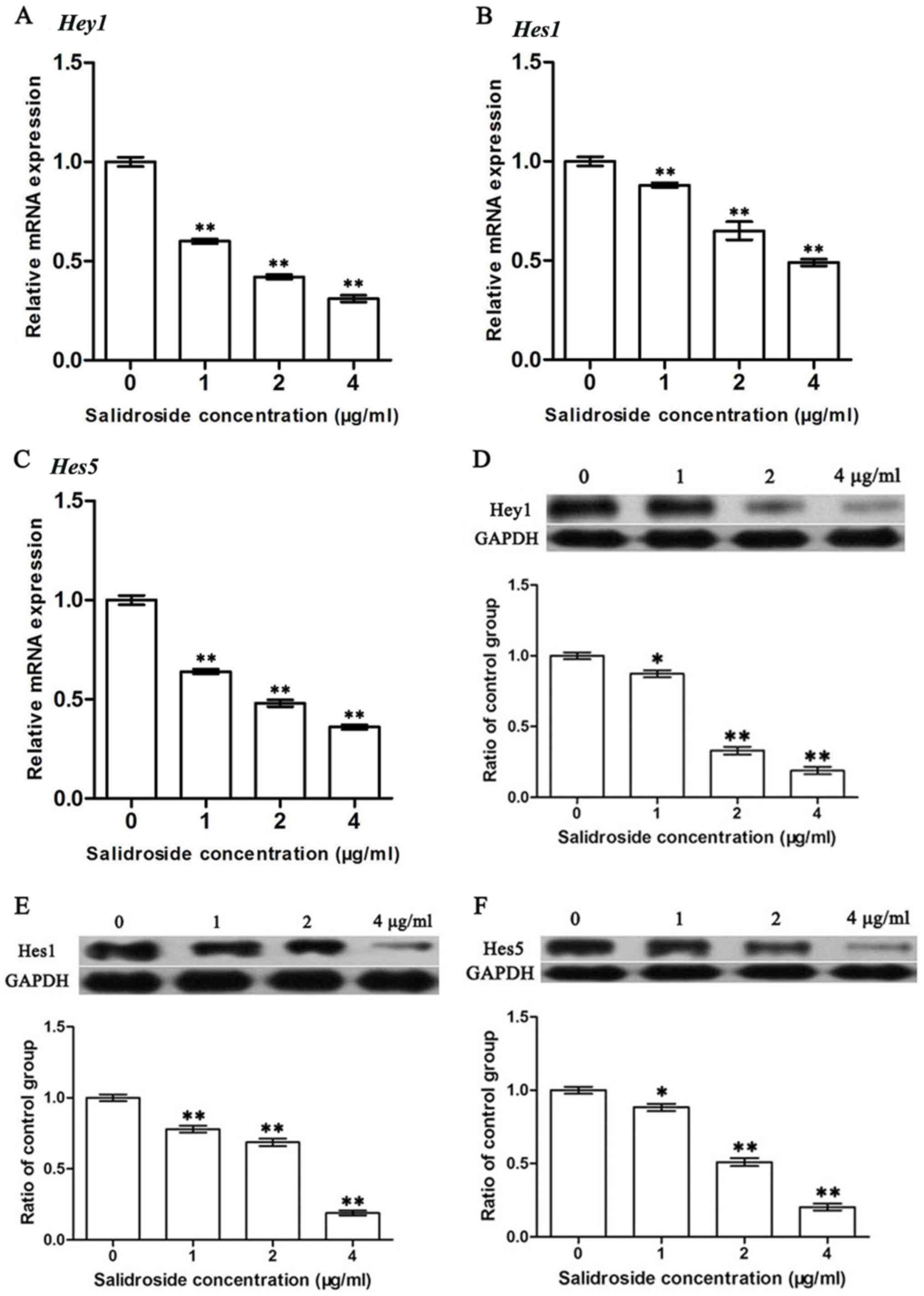 | Figure 5.Effects of SDS on the expression of
Hey1, Hes1 and Hes5 in HMCC97H cells. The cells were
treated with 1, 2 and 4 µg/ml SDS. (A-C) The mRNA expression levels
of Hey1, Hes1 and Hes5 were measured by RT-qPCR and
the data were normalized to the GAPDH gene as an internal
control. (D-F) Hey1, Hes1, and Hes5 protein expression levels were
measured by western blot analysis. Bands were scanned and
quantified and the results were normalized to GAPDH. Data are
presented as the means ± SEM from 3 independent experiments.
Asterisks indicate statistically significant differences
(*P<0.05 and **P<0.01) compared to the control (0 µg/ml).
SDS, salidroside; Hes1, hairy and enhancer of split 1; Hes5, hairy
and enhancer of split 5; Hey1, hairy/enhancer-of-split related with
YRPW motif 1; RT-qPCR, reverse transcription-quantitative PCR; SEM,
standard errors of the mean. |
Discussion
The Notch1 signaling pathway plays an important role
in the differentiation, proliferation and apoptosis of cells during
early development, and may eventually contribute to tumorigenesis
(50,51). Notch1 plays a paradoxical role in
different types of cancer as it is upregulated in several types of
tumors and plays complex roles in tumor development and metastasis
(24–30). Previously, Notch1 expression has
been shown to be associated with the decreased overall survival of
patients with breast, colorectal and liver cancers (31,52,53).
SDS has been reported to display anticancer properties both in
vivo and in vitro. A previous study demonstrated that
SDS inhibited the migration and invasion of HT1080 cells (23). Considering the hepatoprotective
role of Rhodiola rosea L. in traditional Chinese medicine,
in the present study, we aimed to determine whether SDS can inhibit
the metastasis of HCC via the Notch1 signaling pathway.
Tumor metastasis leads to the expression of two gene
sets, including invasion promotors and invasion suppressors
(54,55). E-cadherin acts as a homotypic
homophilic epithelial cell-cell adhesion molecule that can inhibit
tumor cell invasion (37–39). During the invasion and metastasis
of epithelial tumor cells mediated by Notch1, Snail expression is
upregulated, while E-cadherin is downregulated. This indicates that
Notch1 may mediate metastasis by disrupting the normal expression
profiles of Snail and E-cadherin (33).
In the present study, we investigated the effects of
SDS on the migration properties of highly invasive HCC cells via
the scratch wound closure test and Transwell assay. The results
from scratch wound closure assay revealed that wound closure was
suppressed by SDS in a concentration-dependent manner (Fig. 1A). Similarly, the results of
Transwell assay revealed that cell migration was suppressed by SDS
in a concentration-dependent manner (Fig. 1B). This indicates that SDS can
inhibit the migration of cancer cells in a concentration-dependent
manner. In order to determine the possible underlying mechanisms,
we detected the activity of the Notch1 signaling pathway. RT-qPCR
was conducted to investigate the transcription of Notch1, the
Notch1 signaling pathway relevant genes, Hey1, Hes1 and
Hes5, and the migration-associated genes, Snail,
COX-2 and E-cadherin, in HMCC97H cells. The expression
levels of Notch1, Hey1, Hes1, Hes5, Snail and COX-2
in the HMCC97H cells were significantly decreased following
treatment with SDS (Figs. 3 and
5). In addition, the expression of
the migration-related gene, E-cadherin, in the HMCC97H cells
was markedly increased following treatment with SDS (Fig. 3). Western blot analysis was also
conducted to examine the protein expression levels of Notch1, Hey1,
Hes1, Hes5, Snail, COX-2 and E-cadherin in the HMCC97H cells
following treatment with SDS. SDS was found to significantly
inhibit the activation of Notch1, Hey1, Hes1, Hes5, Snail and
COX-2, while it markedly promoted the activation of E-cadherin
(Figs. 4 and 5). These results indicate that SDS may
suppress the migration of HMCC97H cells by inhibiting the
activation of the Notch1 signaling pathway.
MMPs are a family of proteolytic enzymes that
contribute to tumor cell invasion, migration and tumor angiogenesis
by degrading the basement membrane and other ECM components
(23,56–58).
The endothelial cell activities, which are essential for new vessel
development, can be blocked with MMP inhibitors. In return, MMP
inhibitors likely halt the proliferation and invasion of tumor
cells (59). Previously, the
overexpression of tumor suppressor tissue inhibitors of
metalloproteinases (TIMPs) was shown to downregulate the expression
of MMP-2 and inhibit the invasion and metastasis of cancer cells
(58). Previous studies have
indicated that the Notch1 signaling pathway can regulate the
activities of MMP-2 and MMP-9 in pancreatic cancer, lingual
squamous cell carcinoma and breast cancer (28–30).
In HCC cells, the downregulation of Notch1 decreases the expression
and proteolytic activities of MMP-2 and MMP-9 (31). This suggests that the
Notch1/MMP-2/MMP-9 axis may participate in tumor cell invasion.
Previously, Sun et al demonstrated that the SDS decreased
MMP-2 and MMP-9 activities, and inhibited the invasion of HT1080
cells (23); however, it remains
unknown as to whether the SDS-attributed inhibition of MMP-2 and
MMP-9 activities occurs via the Notch1 signaling pathway.
In this study, we examined the effects of SDS on the
invasion of HMCC97H cells, and also aimed to elucidate the
underlying mechanisms. The results of Transwell assay revealed that
SDS suppressed the invasion of MHCC97H cells in a
concentration-dependent manner, and the expression levels of
MMP-2 and MMP-9 in the MHCC97H cells were
significantly reduced following treatment with SDS. In addition,
the expression levels of MMP-2 and MMP-9 in the MHCC97H cells were
also reduced after SDS treatment. Previous studies have shown that
Notch1 mediates the migration and invasion of tumor cells through
regulation of MMP-2 and MMP-9 expression and that Notch1 signaling
pathway activity can be suppressed with SDS treatment (32,60).
Therefore, SDS likely inhibits the migration and invasion of tumor
cells by down-regulating the Notch1 signaling pathway.
In conclusion, the findings of this study suggest
that SDS suppresses the migration and invasion of HMCC97H cells in
a concentration-dependent manner by inhibiting the activation of
the Notch1 signaling pathway. The results suggest that SDS may be
an effective anticancer agent with minimal adverse effects.
Considering the importance of the Notch1 signaling pathway, this
may be an excellent biomarker for the development of novel
therapies for HCC; however, more profound research into the Notch1
signaling pathway and its role in cancer metastasis is
required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Young
Scientist Research Foundation of Medjaden Bioscience Limited (grant
no. MJR20160051) and the National Natural Science Foundation of
China (grant nos. 31770837 and 31800660).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL and SL conducted the experiment, LL, SL, and QD
analyzed the data. LL and SL wrote the manuscript, YX designed the
present study and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cabibbo G, Latteri F, Antonucci M and
Craxì A: Multimodal approaches to the treatment of hepatocellular
carcinoma. Nat Clin Pract Gastroenterol Hepatol. 6:159–169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh S, Singh PP, Roberts LR and Sanchez
W: Chemopreventive strategies in hepatocellular carcinoma. Nat Rev
Gastroenterol Hepatol. 11:45–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutta R and Mahato RI: Recent advances in
hepatocellular carcinoma therapy. Pharmacol Ther. 173:106–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Q, Liu ZY, Chen Q and Lin JS: Mcl-1 as
a potential therapeutic target for human hepatocelluar carcinoma. J
Huazhong Univ Sci Technolog Med Sci. 36:494–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khanna K, Mishra KP, Ganju L and Singh SB:
Golden root: A wholesome treat of immunity. Biomed Pharmacother.
87:496–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: The anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schriner SE, Abrahamyan A, Avanessian A,
Bussel I, Maler S, Gazarian M, Holmbeck MA and Jafari M: Decreased
mitochondrial superoxide levels and enhanced protection against
paraquat in Drosophila melanogaster supplemented with Rhodiola
rosea. Free Radic Res. 43:836–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan
C, Cao G and Wang Z: Protective effects of salidroside on hydrogen
peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
Eur J Pharmacol. 564:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goel HC, Bala M, Prasad J, Singh S,
Agrawala PK and Swahney RC: Radioprotection by Rhodiola
imbricata in mice against whole-body lethal irradiation. J Med
Food. 9:154–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perfumi M and Mattioli L: Adaptogenic and
central nervous system effects of single doses of 3% rosavin and 1%
salidroside Rhodiola rosea L. extract in mice. Phytother
Res. 21:37–43. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spasov AA, Wikman GK, Mandrikov VB,
Mironova IA and Neumoin VV: A double-blind, placebo-controlled
pilot study of the stimulating and adaptogenic effect of
Rhodiola rosea SHR-5 extract on the fatigue of students
caused by stress during an examination period with a repeated
low-dose regimen. Phytomedicine. 7:85–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mishra KP, Padwad YS, Dutta A, Ganju L,
Sairam M, Banerjee PK and Sawhney RC: Aqueous extract of
Rhodiola imbricata rhizome inhibits proliferation of an
erythroleukemic cell line K-562 by inducing apoptosis and cell
cycle arrest at G2/M phase. Immunobiology. 213:125–131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang DZ, Hong HD, Kim KI and Choi SY:
Anti-fatigue effects of fermented Rhodiola rosea extract in
mice. Prev Nutr Food Sci. 20:38–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao K, Wang B, Feng D, Zhang W, Lu F, Lai
J, Huang L, Nie T and Yang Q: Salidroside protects against
6-hydroxydopamine-induced cytotoxicity by attenuating ER stress.
Neurosci Bull. 32:61–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Yao Y, Wang H, Guo Y, Zhang H and
Chen L: Effects of salidroside on glioma formation and growth
inhibition together with improvement of tumor microenvironment.
Chin J Cancer Res. 25:520–526. 2013.PubMed/NCBI
|
|
16
|
Wang H, Ding Y, Zhou J, Sun X and Wang S:
The in vitro and in vivo antiviral effects of
salidroside from Rhodiola rosea L. against coxsackievirus
B3. Phytomedicine. 16:146–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ming DS, Hillhouse BJ, Guns ES, Eberding
A, Xie S, Vimalanathan S and Towers GH: Bioactive compounds from
Rhodiola rosea (Crassulaceae). Phytother Res. 19:740–743.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu X, Zhang X, Qiu S, Yu D and Lin S:
Salidroside induces cell-cycle arrest and apoptosis in human breast
cancer cells. Biochem Biophys Res Commun. 398:62–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Senthilkumar R, Parimelazhagan T,
Chaurasia OP and Srivastava RB: Free radical scavenging property
and antiproliferative activity of Rhodiola imbricata Edgew
extracts in HT-29 human colon cancer cells. Asian Pac J Trop Med.
6:11–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao G, Shi A, Fan Z and Du Y: Salidroside
inhibits the growth of human breast cancer in vitro and
in vivo. Oncol Rep. 33:2553–2560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun C, Wang Z, Zheng Q and Zhang H:
Salidroside inhibits migration and invasion of human fibrosarcoma
HT1080 cells. Phytomedicine. 19:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Song Z, Chen Y, Xia L, Wang J, Fan
R, Du R, Zhang F, Hong L, Song J, et al: Deregulated expression of
Notch receptors in human hepatocellular carcinoma. Dig Liver Dis.
40:114–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicolas M, Wolfer A, Raj K, Kummer JA,
Mill P, van Noort M, Hui CC, Clevers H, Dotto GP and Radtke F:
Notch1 functions as a tumor suppressor in mouse skin. Nat Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sriuranpong V, Borges MW, Ravi RK, Arnold
DR, Nelkin BD, Baylin SB and Ball DW: Notch signaling induces cell
cycle arrest in small cell lung cancer cells. Cancer Res.
61:3200–3205. 2001.PubMed/NCBI
|
|
27
|
Jang MS, Zlobin A, Kast WM and Miele L:
Notch signaling as a target in multimodality cancer therapy. Curr
Opin Mol Ther. 2:55–65. 2000.PubMed/NCBI
|
|
28
|
Ma YC, Shi C, Zhang YN, Wang LG, Liu H,
Jia HT, Zhang YX, Sarkar FH and Wang ZS: The tyrosine kinase c-Src
directly mediates growth factor-induced Notch-1 and Furin
interaction and Notch-1 activation in pancreatic cancer cells. PloS
One. 7:e334142012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu B, Wei J, Qian X, Lei D, Ma Q and Liu
Y: Notch1 signaling pathway participates in cancer invasion by
regulating MMPs in lingual squamous cell carcinoma. Oncol Rep.
27:547–552. 2012.PubMed/NCBI
|
|
30
|
Wang J, Fu L, Gu F and Ma Y: Notch1 is
involved in migration and invasion of human breast cancer cells.
Oncol Rep. 26:1295–1303. 2011.PubMed/NCBI
|
|
31
|
Zhou L, Zhang N, Li QJ, Sun W, Zhang Y,
Wang DS and Dou KF: Associations between high levels of Notch1
expression and high invasion and poor overall survival in
hepatocellular carcinoma. Tumour Biol. 34:543–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and −9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu
X, Sun J, Yang S, Poon RT and Fan ST: Notch1-Snail1-E-cadherin
pathway in metastatic hepatocellular carcinoma. Int J Cancer.
131:E163–E172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim SO, Kim HS, Quan X, Ahn SM, Kim H,
Hsieh D, Seong JK and Jung G: Notch1 binds and induces degradation
of Snail in hepatocellular carcinoma. BMC Biol. 9:832011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lim SO, Park YM, Kim HS, Quan X, Yoo JE,
Park YN, Choi GH and Jung G: Notch1 differentially regulates
oncogenesis by wildtype p53 overexpression and p53 mutation in
grade III hepatocellular carcinoma. Hepatology. 53:1352–1362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khamis ZI, Iczkowski KA and Sang QXA:
Metastasis suppressors in human benign prostate, intraepithelial
neoplasia, and invasive cancer: Their prospects as therapeutic
agents. Med Res Rev. 32:1026–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Debruyne P, Vermeulen S and Mareel M: The
role of the E-cadherin/catenin complex in gastrointestinal cancer.
Acta Gastroenterol Belg. 62:393–402. 1999.PubMed/NCBI
|
|
39
|
Mareel M, Vleminckx K, Vermeulen S, Yan G,
Bracke M and van Roy F: Downregulation in vivo of the
invasion-suppressor molecule E-cadherin in experimental and
clinical cancer. Princess Takamatsu Symp. 24:63–80. 1994.PubMed/NCBI
|
|
40
|
Vihinen P and Kähäri VM: Matrix
metalloproteinases in cancer: Prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Curran S and Murray GI: Matrix
metalloproteinases: Molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis, and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weis M, Heeschen C, Glassford AJ and Cooke
JP: Statins have biphasic effects on angiogenesis. Circulation.
105:739–745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu HM, Zhu SL, He LJ, Liu YH and Xie D:
Clinical significance of macrophage migration inhibitory factor in
invasion of ovarian cancer. Chin J Cancer. 28:1054–1060.
2009.[Clinical significance of macrophage migration inhibitory
factor in invasion of ovarian cancer]. View Article : Google Scholar
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qu L and Liu B: Cyclooxygeanse-2 promotes
metastasis in osteosarcoma. Cancer Cell Int. 15:692015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wieland E, Rodriguez-Vita J, Liebler SS,
Mogler C, Moll I, Herberich SE, Espinet E, Herpel E, Menuchin A,
Chang-Claude J, et al: Endothelial Notch1 activity facilitates
metastasis. Cancer Cell. 31:355–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Egan SE, St-Pierre B and Leow CC: Notch
receptors, partners and regulators: From conserved domains to
powerful functions. Curr Top Microbiol Immunol. 228:273–324.
1998.PubMed/NCBI
|
|
51
|
Callahan R and Egan SE: Notch signaling in
mammary development and oncogenesis. J Mammary Gland Biol
Neoplasia. 9:145–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chu D, Li Y, Wang W, Zhao Q, Li J, Lu Y,
Li M, Dong G, Zhang H, Xie H and Ji G: High level of Notch1 protein
is associated with poor overall survival in colorectal cancer. Ann
Surg Oncol. 17:1337–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Makrilia N, Kollias A, Manolopoulos L and
Syrigos K: Cell adhesion molecules: Role and clinical significance
in cancer. Cancer Invest. 27:1023–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mareel M, Vleminckx K, Vermeulen S, Bracke
M and Van Roy F: E-cadherin expression: A counterbalance for cancer
cell invasion. Bull Cancer. 79:347–355. 1992.PubMed/NCBI
|
|
56
|
Shimada T, Nakamura H, Yamashita K, Kawata
R, Murakami Y, Fujimoto N, Sato H, Seiki M and Okada Y: Enhanced
production and activation of progelatinase A mediated by
membrane-type 1 matrix metalloproteinase in human oral squamous
cell carcinomas: Implications for lymph node metastasis. Clin Exp
Metastasis. 18:179–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rajoria S, Suriano R, George A, Shanmugam
A, Schantz SP, Geliebter J and Tiwari RK: Estrogen induced
metastatic modulators MMP-2 and MMP-9 are targets of
3,3′-diindolylmethane in thyroid cancer. PLoS One. 6:e158792011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yan L, Lin B, Gao L, Gao S, Liu C, Wang C,
Wang Y, Zhang S and Iwamori M: Lewis (y) antigen overexpression
increases the expression of MMP-2 and MMP-9 and invasion of human
ovarian cancer cells. Int J Mol Sci. 11:4441–4452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Murphy AN, Unsworth EJ and
Stetler-Stevenson WG: Tissue inhibitor of metalloproteinases-2
inhibits bFGF-induced human microvascular endothelial cell
proliferation. J Cell Physiol. 157:351–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao HB, Qi SN, Dong JZ, Ha XQ, Li XY,
Zhang QW, Yang YS, Bai J and Zhao L: Salidroside induces neuronal
differentiation of mouse mesenchymal stem cells through Notch and
BMP signaling pathways. Food Chem Toxicol. 71:60–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|