Introduction
Non-small cell lung cancer (NSCLC) accounts for
85–90% lung cancer, and the most common subtypes are lung squamous
cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) (1–4).
LUSC typically occurs in men and is associated with smoking,
accounting for ~25–30% total lung cancer cases (5–7).
Therapies for LUAD are frequently ineffective for LUSC, reflecting
differences between LUAD and LUSC; therefore, distinguishing LUSC
from other types of lung cancer and investigating the molecular
mechanism of LUSC are crucial.
MicroRNAs (miRNAs) are a class of small non-coding
RNAs with lengths of 19–25 nucleotides (8–13).
As post-transcriptional regulators of gene expression, these
molecules are involved in the regulation of a number of biological
processes, including differentiation, proliferation, migration and
apoptosis (14–20). miRNAs may be used as diagnostic
biomarkers and treatment targets for human cancer (21–25).
The miR-1 family is comprised of miR-1-1, miR-1-2 and miR-206. In
the family, miR-1-1 and miR-1-2 locate on chromosomes 20 and 18,
respectively (26,27). The upregulation of miR-1 inhibits
proliferation, promotes apoptosis and reverses the drug resistance
of various types of cancer in vivo and in vitro,
serving a role as a cancer suppressor gene (27). Previous studies demonstrated that
miR-1 is downregulated in approximately all human cancer types and
miR-1 serves a crucial role in the progression of cancer. Han et
al (28) demonstrated that
miR-1 is downregulated in gastric cancer, and inhibits gastric
cancer cell proliferation and migration by targeting MET
proto-oncogene, receptor tyrosine kinase. Wang et al
(29) demonstrated that miR-1-3p,
the mature miRNA of miR-1, suppresses the proliferation and
invasion of bladder cancer cells by inhibiting C-C motif chemokine
ligand 2 expression. Wang et al (30) observed that microRNA-1-3p is
downregulated in oral squamous cell carcinoma (OSCC) tissues and
cells, and serves as a suppressor of OSCC progression. There are a
few previous studies that address the roles of miRNA in lung cancer
progression (31–33). Meta-analyses have additionally been
conducted to confirm the association between miRNA expression and
prognosis in NSCLC (34).
Nevertheless, no consistent conclusion has been reached and direct
evidence of the association between miR-1 and LUSC is limited.
Therefore, the regulatory mechanism of miR-1 in LUSC requires
further investigation.
The present study conducted a meta-analysis to
evaluate the clinical significance of miR-1 in LUSC. Potential
target genes of miR-1 in LUSC were obtained from the gene chip
analysis of LUSC cells transfected with miR-1-3p, combined with
target gene prediction and differential gene expression in The
Cancer Genome Atlas (TCGA). Subsequently, a signaling pathway
analysis was conducted to determine the potential molecular
mechanism of miR-1 in LUSC.
Materials and methods
Collection of microarray datasets from
Gene Expression Omnibus (GEO) and ArrayExpress
To examine the level of miR-1 expression in LUSC and
adjacent non-cancer tissues, retrieval in GEO (http://www.ncbi.nlm.nih.gov/geo/) and
ArrayExpress (http://www.ebi.ac.uk/arrayexpress/) was performed
using the following key words: [‘lung’ OR ‘pulmonary’ OR
‘respiratory’ OR ‘bronchioles’ OR ‘bronchi’ OR ‘alveoli’ OR
‘pneumocytes’ OR ‘air way’ (MeSH)] AND (‘cancer’ OR ‘carcinoma’ OR
‘tumor’ OR ‘neoplas* OR malignan* squamous cell carcinoma’).
‘Series’ and ‘Homo sapiens’ were filtered. Studies with sample
sizes ≥3 and miR-1 expression measured in LUSC and control groups
were included. To identify promising miR-1 target genes, GEO and
ArrayExpress were searched again using the following terms: [‘lung’
OR ‘pulmonary’ OR ‘respiratory’ OR ‘bronchioles’ OR ‘bronchi’ OR
‘alveoli’ OR ‘pneumocytes’ OR ‘air way’ (MeSH)] AND (‘cancer’ OR
‘carcinoma’ OR ‘tumor’ OR ‘neoplas* OR malignan* squamous cell
carcinoma’) AND (‘miR-1’ OR ‘miRNA-1’ OR ‘microRNA-1’ OR ‘miR-1’ OR
‘miRNA-1’ OR ‘microRNA1’ OR ‘miR-1’ OR ‘miRNA-1’ OR ‘microRNA-1’ OR
‘miR-1-3p’ OR ‘miRNA-1-3p’ OR ‘microRNA-1-3p’ OR ‘miR-1-1’ OR
‘miR-1-2’ OR ‘miR1-1’ OR ‘miR1-2’). Gene chips intervened with
miR-1 in LUSC cell lines, knockdown or transfection, were included
in the present analysis. Datasets are presented in Table I.
 | Table I.Forest plot of studies evaluating the
SMD of microRNA-1 expression between patients with lung squamous
cell carcinoma and the control group (a random-effects model). |
Table I.
Forest plot of studies evaluating the
SMD of microRNA-1 expression between patients with lung squamous
cell carcinoma and the control group (a random-effects model).
|
|
| Experimental | Control |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Author, year | Study | Mean | SD | Total | Mean | SD | Total | SMD (95% CI) | (Refs.) |
|---|
| Seike et al,
2009 | GSE14936 | 7.06532 | 0.248859 | 3 | 7.384533 | 0.365138 | 21 | −0.90 (−2.13,
0.34) | (48) |
| Raponi et
al, 2009 | GSE16025 | 4.811725 | 0.183384 | 61 | 4.9321 | 0.158178 | 10 | −0.67 (−1.35,
0.01) | (49) |
| Ohba et al,
2013 | GSE19945 | −1.06547 | 2.263498 | 5 | 2.027512 | 0.642366 | 8 | −2.12 (−3.55,
−0.70) | (50) |
| Nymark et
al, 2011 | GSE25508 | 6.154964 | 0.23209 | 8 | 6.334855 | 0.624779 | 34 | −0.31 (−1.09,
−0.46) | (51) |
| Patnaik et
al, 2017 | GSE40738 | −4.65092 | 0.333811 | 33 | −4.45357 | 0.468942 | 56 | −0.47 (−0.90,
−0.03) | (52) |
| van Jaarsveld et
al, 2014 | GSE47525 | 5.378686 | 2.603978 | 5 | 3.84297 | 1.091469 | 14 | 0.97 (−0.10,
2.04) | (53) |
| Arima et al,
2014 | GSE51853 | −5.83195 | 1.588947 | 29 | −2.78567 | 2.077722 | 5 | −1.84 (−2.89,
−0.79) | (54) |
| Jin et al,
2015 | GSE74190 | 0.321576 | 0.861687 | 30 | 3.414288 | 1.172088 | 44 | −2.92 (−3.59,
−2.26) | (55) |
| Seike et al,
2009 |
PMID:18818206-1 | 0.00442 | 0.00416 | 7 | 0.031476 | 0.021179 | 7 | −1.77 (−3.04,
−0.51) | (48) |
|
|
PMID:18818206-2 | 0.015752 | 0.007522 | 7 | 0.172871 | 0.074251 | 7 | −2.98 (−4.56,
−1.39) |
|
|
| TCGA-1 | 2.861394 | 1.672886 | 444 | 6.550327 | 1.177112 | 45 | −2.26 (−2.60,
−1.92) |
|
|
| TCGA-2 | 2.876746 | 1.652493 | 458 | 6.590287 | 1.154916 | 45 | −2.30 (−2.64,
−1.96) |
|
|
| Total (95% CI) |
|
| 1,090 |
|
| 296 | −1.70 (−1.87,
−1.52) |
|
Acquisition of TCGA miRNA data
The LUSC miRNA matrix was downloaded from TCGA
(http://cancergenome.nih.gov/). Data were
normalized to a log2 scale. The values of miR-1-1 and
miR-1-2 were included in the present study. Samples missing values
for miR-1-1 or miR-1-2 were removed from the analysis. The data
from TCGA were additionally included in the continuous variable
meta-analysis and diagnostic meta-analysis.
Literature reviewing and
selecting
PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Science Direct
(https://www.sciencedirect.com/), Google
Scholar (https://scholar.google.com/), Ovid
(https://ovidsp.ovid.com/), Wiley Online Library
(https://onlinelibrary.wiley.com/),
Embase (https://www.embase.com/), Web of Science
(http://www.webofknowledge.com), Chong
Qing VIP (http://www.cqvip.com), CNKI (http://www.cnki.net/), Wan Fang (http://www.wanfangdata.com.cn/) and China Biology
Medicine Disc (http://http://www.sinomed.ac.cn/) were searched to
identify all studies associated with miR-1-3p in LUSC using the
following keywords: [‘lung’ OR ‘pulmonary’ OR ‘respiratory’ OR
‘bronchioles’ OR ‘bronchi’ OR ‘alveoli’ OR ‘pneumocytes’ OR ‘air
way’ (MeSH)] AND (‘cancer’ OR ‘carcinoma’ OR ‘tumor’ OR ‘neoplas*
OR malignan* squamous cell carcinoma’) AND (‘miR-1’ OR ‘miRNA-1’ OR
‘microRNA-1’ OR ‘miR-1’ OR ‘miRNA-1’ OR ‘microRNA-1’ OR ‘miR-1’ OR
‘miRNA-1’ OR ‘microRNA-1’ OR ‘miR-1-3p’ OR ‘miRNA-1-3p’ OR
‘microRNA-1-3p’ OR ‘miR-1-1’ OR ‘miR-1-2’ OR ‘miR1-1’ OR ‘miR1-2’).
The cut-off point for the search was July 20, 2017, so no studies
later than this date were included. Subsequently, the eligible
literature was independently evaluated in the present study using
the same multi-step process. Eligible studies had to meet the
following criteria: i) They detected miR-1 expression in human
tissue or serum in LUSC and control groups; ii) the study offered
sufficient data to calculate the diagnostic accuracy; and iii) the
study was published in Chinese or English. The following exclusion
criteria were used: i) Duplicated studies, case reports, letters,
reviews and conference articles; ii) studies with unavailable data;
and iii) animal studies.
Data extraction
Information was independently extracted, including
the mean, standard deviation, sample size, true positive, false
positive, true negative and false negative, from the included
studies.
Statistical analysis
All data from the included microarray datasets were
converted to a log2 scale, and the mean, standard
deviation and case numbers of LUSC and control groups were
calculated. Subsequently, Stata 14 (StataCorp LLC, College Station,
TX, USA) was used to combine the standard mean difference (SMD)
value and its 95% confidence interval (CI). The pooled sensitivity,
specificity, positive likelihood ratio (PLR), negative likelihood
ratio (NLR), diagnostic odds ratio (DOR) and 95% CI values from the
accuracy data of each study were calculated using MetaDiSc 1.4
software (http://www.hrc.es/investigacion/metadisc_en.htm). The
DOR value ranged between 0 and ∞, with higher values suggesting
improved discriminatory performance (35–39).
To assess the clinical significance of miR-1 in LUSC, the
summarized receiver operating characteristic (SROC) curves were
obtained using SPSS version 23 (IBM Corp., Armonk, NY, USA). An
area under the curve (AUC) value near 1.0 indicates that the test
has perfect discrimination, whereas, a value close to 0.5 implies
poor discrimination. Potential heterogeneity among the included
studies was estimated using Cochran's Q test and the I2
index, and heterogeneity significantly existed if P<0.05 or
I2>50%. If there was no distinct heterogeneity in the
analysis, a fixed-effect model was used; otherwise, a
random-effects model was employed. Heterogeneity was explained
using the threshold effect analysis and meta-regression analysis. A
sensitivity analysis was conducted to identify sources of
heterogeneity. Finally, a funnel plot was applied to assess the
publication bias among the included studies. P<0.05 was
considered to indicate a statistically significant difference.
Acquisition of potential target genes
of miR-1 in LUSC
The downregulated genes recorded in Genomic Spatial
Event (GSE)56243 were cross-referenced based on two cell lines. The
mRNA data were additionally downloaded at the entry of LUSC in
TCGA. DESeq data R package was applied to obtain the differentially
expressed genes, log2 fold change >2 was considered a
criterion of the differentially expressed genes (40). miRWalk version 2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/custom.html)
was used to predict the target genes of miR-1 based on 12 different
online miRNA prediction tools: miRWalk; Microt4; miRanda;
miRBridge; miRDB; miRMap; miRNAMap; PicTar; PITA; RNA22; RNAhybrid;
and TargetScan (https://bioconductor.org/biocLite/). Subsequently, the
downregulated genes in GSE56243, TCGA differentially expressed
genes and predicted target genes of miR-1 were cross-referenced.
The overlapping genes selected by VENNY diagram (https://omictools.com/venny-tool) were considered
promising target genes of miR-1 in LUSC.
Bioinformatics based on the promising
target genes of miR-1 in LUSC
Gene Ontology (GO) (41,42)
annotation, comprising three parts (biological process, cellular
component and molecular function) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) (43,44) pathway analysis were performed in
Database for the Annotation, Visualization and Integrated Discovery
version 6.8 (https://david-d.ncifcrf.gov/) based on the promising
target genes of miR-1 in LUSC. The BINGO and Enrichment Map
plug-ins of Cytoscape (version 3.6.1) were used to visualize the GO
annotation and KEGG pathways (45–47).
Search Tool for the Retrieval of Interacting Genes/Proteins
(http://www.string-db.org), an online tool, was
used to construct the protein-protein interaction (PPI) network
with the disconnected nodes hidden.
Results
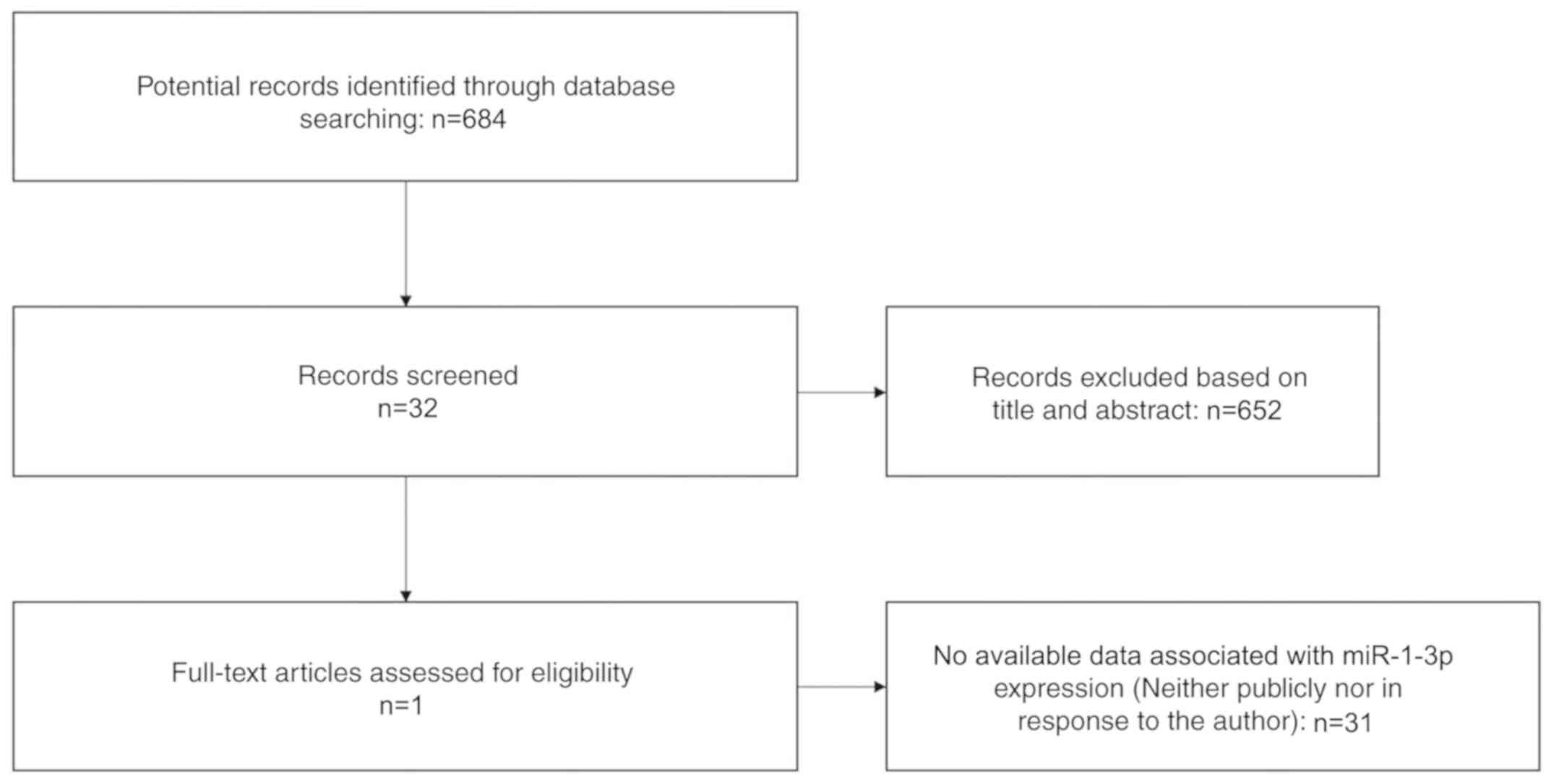
Data selection
A search for literature, and GEO, ArrayExpress and
TCGA datasets was performed to investigate the level of miRNA
expression in LUSC. A total of 684 potential studies were
identified following preliminary literature searches and finally a
study containing two studies was deemed eligible for the present
analysis following abstract screening and examination of the full
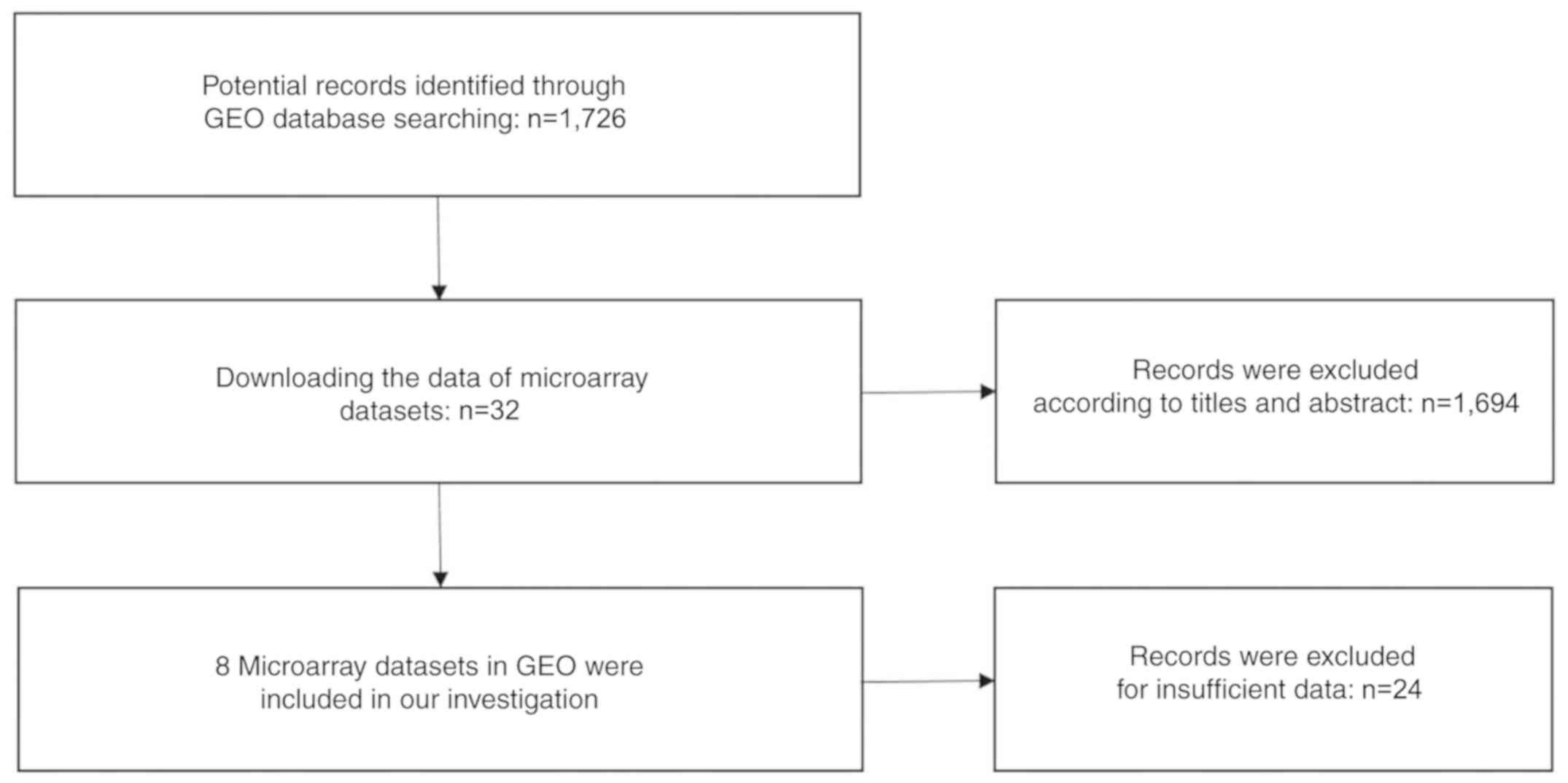
text (41) (Fig. 1). A total of eight GEO datasets
(GSE14936, GSE16025, GSE19945, GSE25508, GSE40738, GSE47525,
GSE51853 and GSE74190) were selected (48–55)
(Fig. 2), and two studies from
TCGA were eligible for the present analysis. Eventually 12 studies
with 1,386 cases were included. The GSE40738 samples were derived
from serum, while the remaining samples were derived from
tissue.
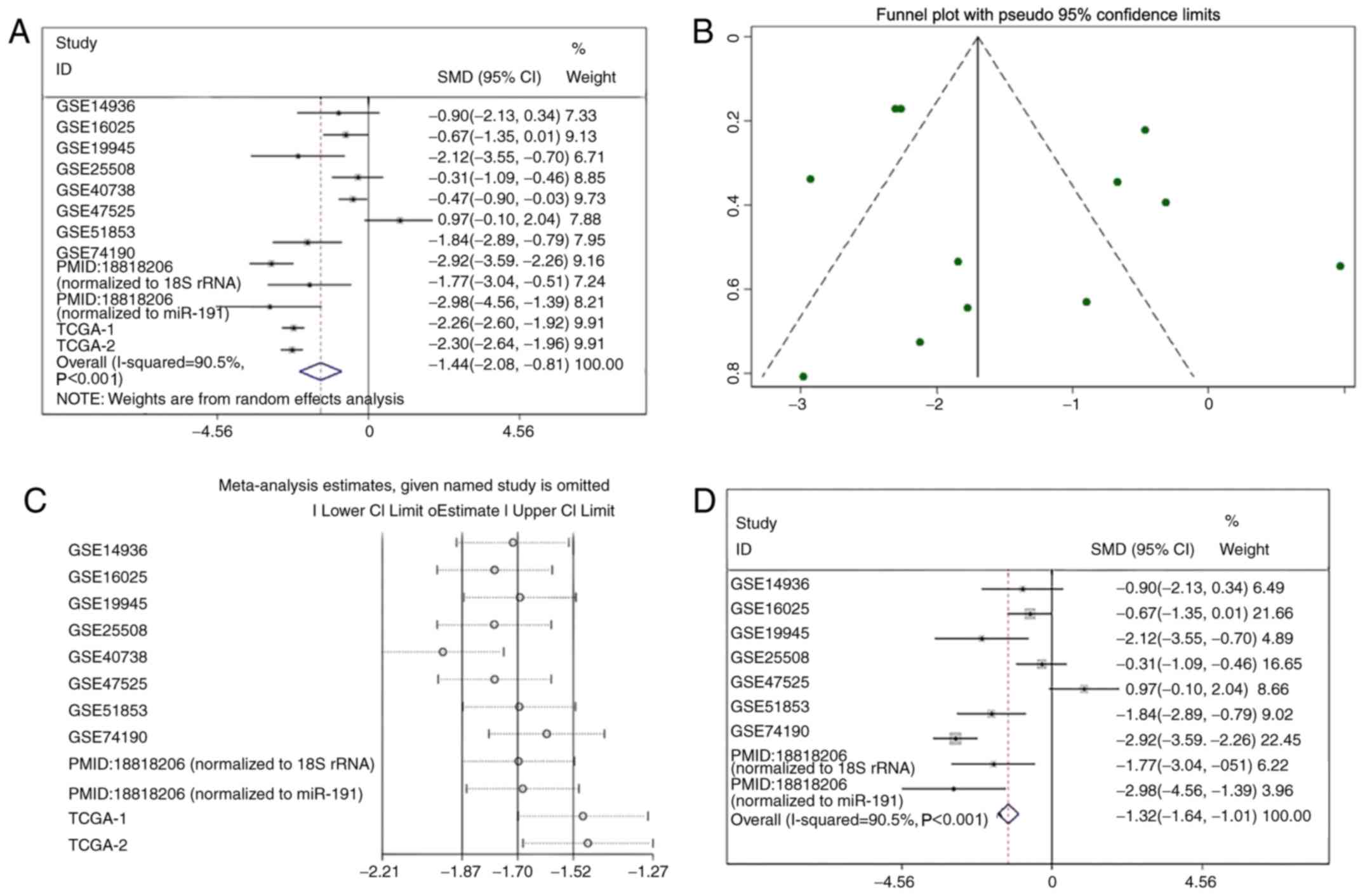
miR-1 expression in LUSC
miR-1 was upregulated in the GSE47525 study and
downregulated in other previous studies (Table I; Fig.
3). A meta-analysis was performed to examine the level of miR-1
expression in LUSC. A random effects model was implied due to the
high heterogeneity (I2=90.5%), and the combined SMD was
−1.44 (95% CI: −2.08, −0.81; Fig.
4A), demonstrating that miR-1 was significantly downregulated
in LUSC. Publication bias was not observed in the present study
(P>0.05; Fig. 4B). Sensitivity
analysis was additionally performed to detect the source of
heterogeneity (Fig. 4C), and the
combined SMD was −1.32 (95% CI: −1.64, −1.01; I2=95.5%)
when the GSE40738 studies and TCGA data were removed (Fig. 4D). The consistent conclusions
obtained confirmed that these results were statistically
stable.
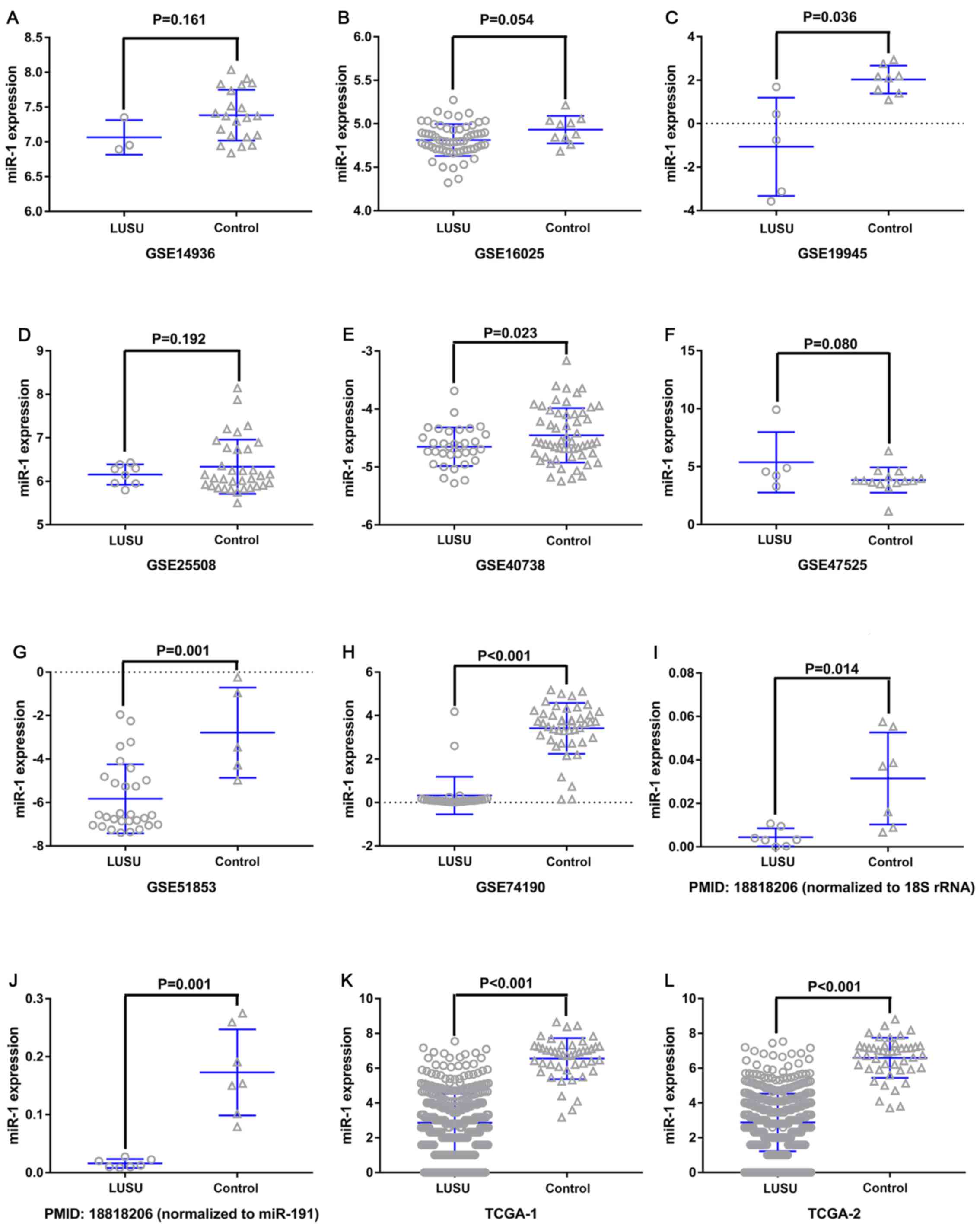 | Figure 3.miR-1 expression in the LUSC and
control groups. (A) GSE14936, (B) GSE16025, (C) GSE19945, (D)
GSE25508, (E) GSE40738, (F) GSE47525, (G) GSE51853, (H) GSE74190,
(I) PMID18818206-1, (J) PMID18818206-2, (K) TCGA-1 and (L) TCGA-2.
The GSE40738 samples were derived from serum, and the remaining
samples were derived from tissue. miR, microRNA; LUSC, lung
squamous cell carcinoma; GSE, Genomic Spatial Event. |
Clinical significance of miR-1 in
LUSC
The ROC curves of miR-1 in LUSC for each study are
presented in Fig. 5. It is evident
that there was significant heterogeneity in these datasets, with
sensitivity ranging from 0.38–1 and specificity ranging from 0.2–1.
To circumvent this heterogeneity, a random effects model was
adopted in the combined analyses (Fig.
6A-E). The combined sensitivity, specificity, PLR, NLR and DOR
were 0.71 (95% CI: 0.66, 0.76; P<0.01), 0.88 (95% CI: 0.86,
0.90; P<0.01), 4.93 (95% CI: 2.54, 9.55; P<0.01), 0.24 (95%
CI: 0.10, 0.54; P<0.01) and 28.24 (95% CI: 10.56, 75.53;
P=0.0022), respectively. The SROC curve is presented in Fig. 6F, with an AUC of 0.9096
(Q*=0.8416), suggesting a good accuracy of miR-1 to distinguish
patients with LUSC from control subjects.
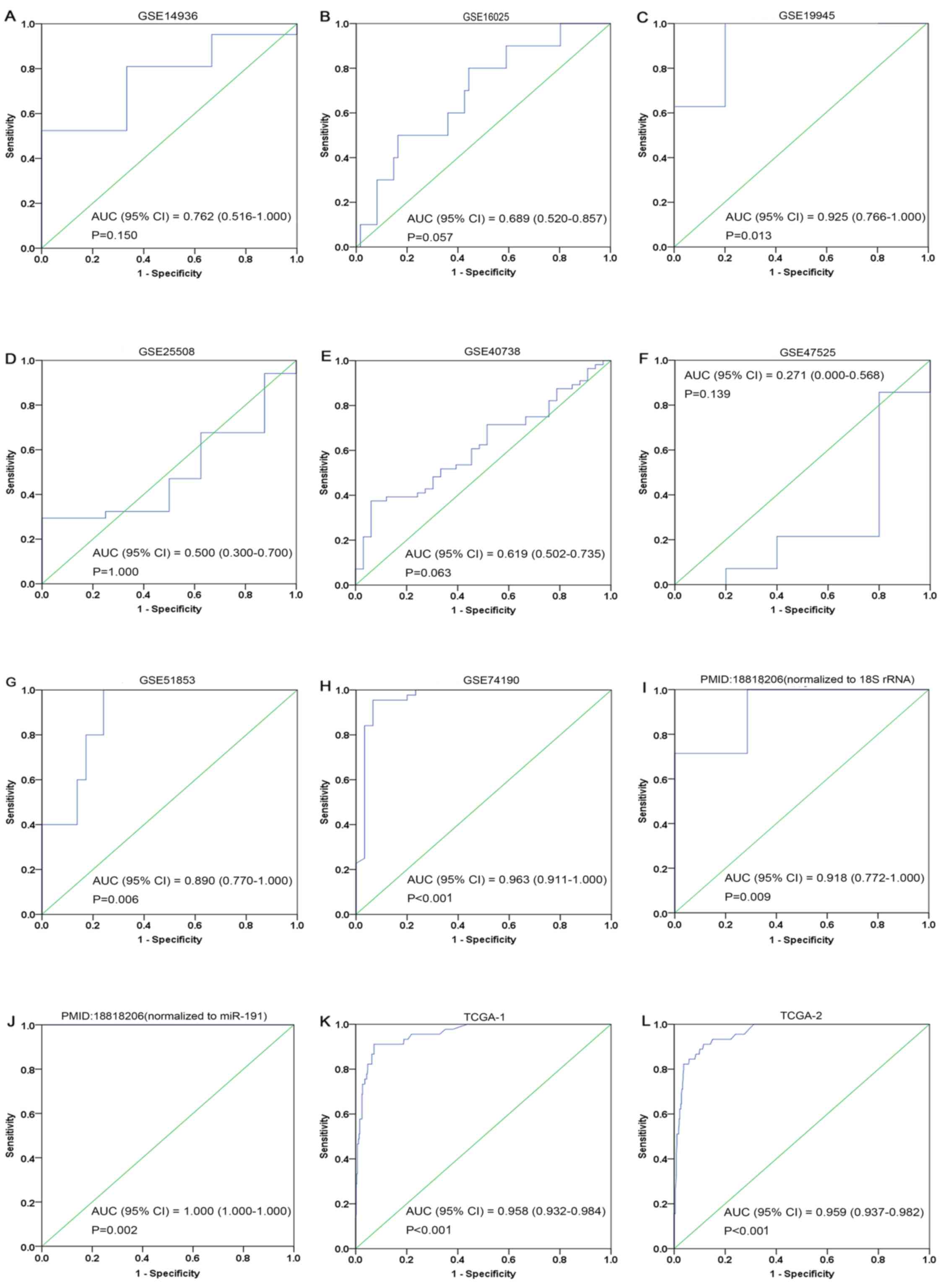 | Figure 5.ROC curves of microRNA-1 in lung
squamous cell carcinoma. (A) GSE14936, (B) GSE16025, (C) GSE19945,
(D) GSE25508, (E) GSE40738, (F) GSE47525, (G) GSE51853, (H)
GSE74190, (I) 18818206-1, (J) 18818206-2, (K) TCGA-1 and (L)
TCGA-2. Blue represents a sensitive curve and green indicates the
identifying line. The X-axis, presented as ‘1-Specificity’,
indicates the false positive rate. The Y-axis, presented as
‘Sensitivity’, indicates the true positive rate. ROC, receiver
operating characteristic. |
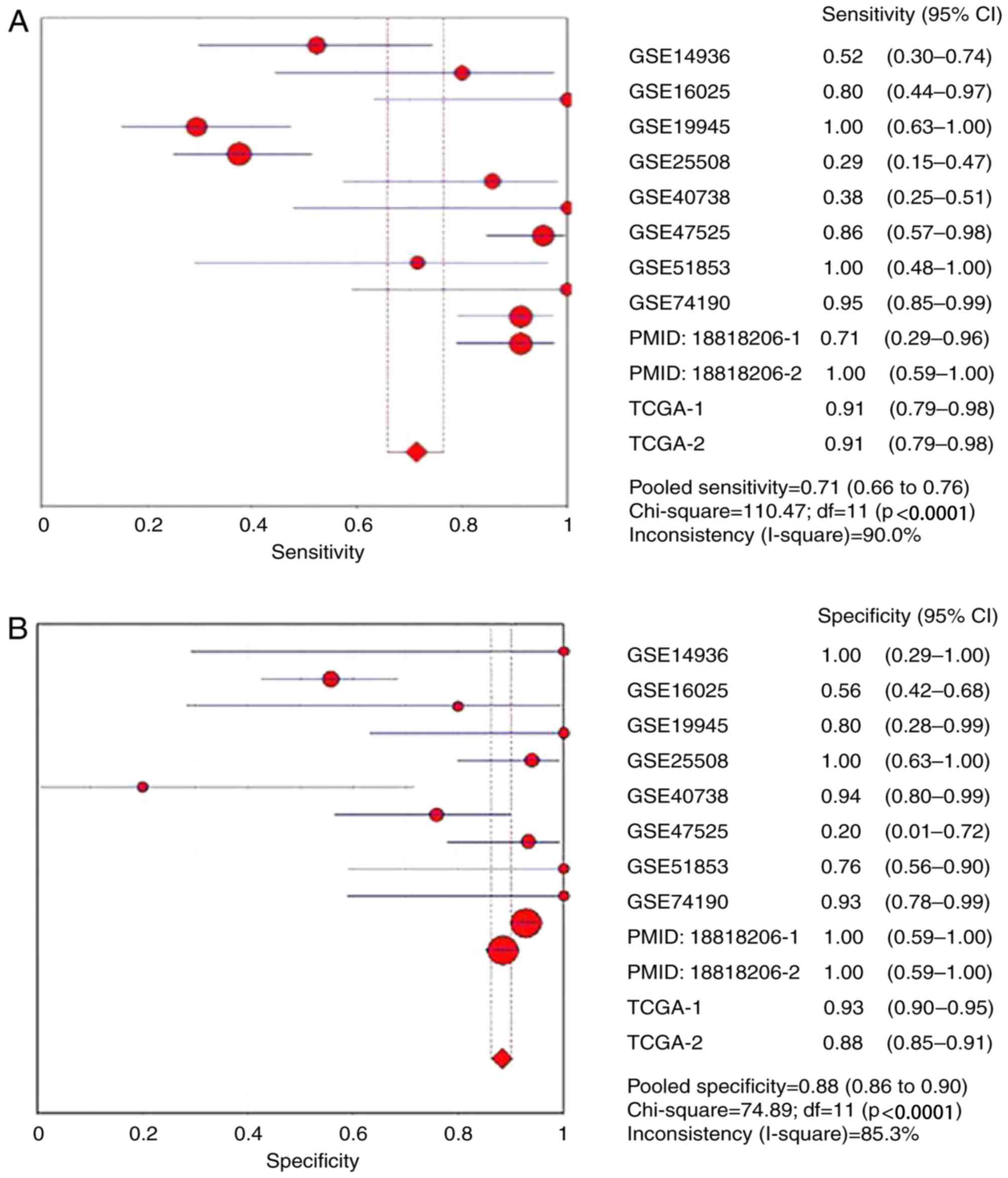 | Figure 6.Forest plots of pooled miR-1 in the
diagnosis of lung squamous cell carcinoma. (A) Sensitivity, (B)
specificity, (C) positive LR, (D) negative LR, indicating the
sensitivity and specificity of all included studies. miR, microRNA;
SROC, summarized receiver operating characteristic; GSE, Genomic
Spatial Event; PMID, PubMed ID; TCGA, The Cancer Genome Atlas;
TCGA, The Cancer Genome Atlas; CI, confidence interval; AUC, area
under the curve; LR, likelihood ratio; OR, odds ratio. Forest plots
of pooled miR-1 in the diagnosis of lung squamous cell carcinoma.
(E) Diagnostic OR and (F) SROC graphs indicating the sensitivity
and specificity of all included studies. |
Function analysis of miR-1-associated
genes in LUSC
A total of 222 overlapping potential target genes of
miR-1 in LUSC were obtained. Subsequently, GO and KEGG analyses
were performed to examine the mechanism of action of miRNA in LUSC.
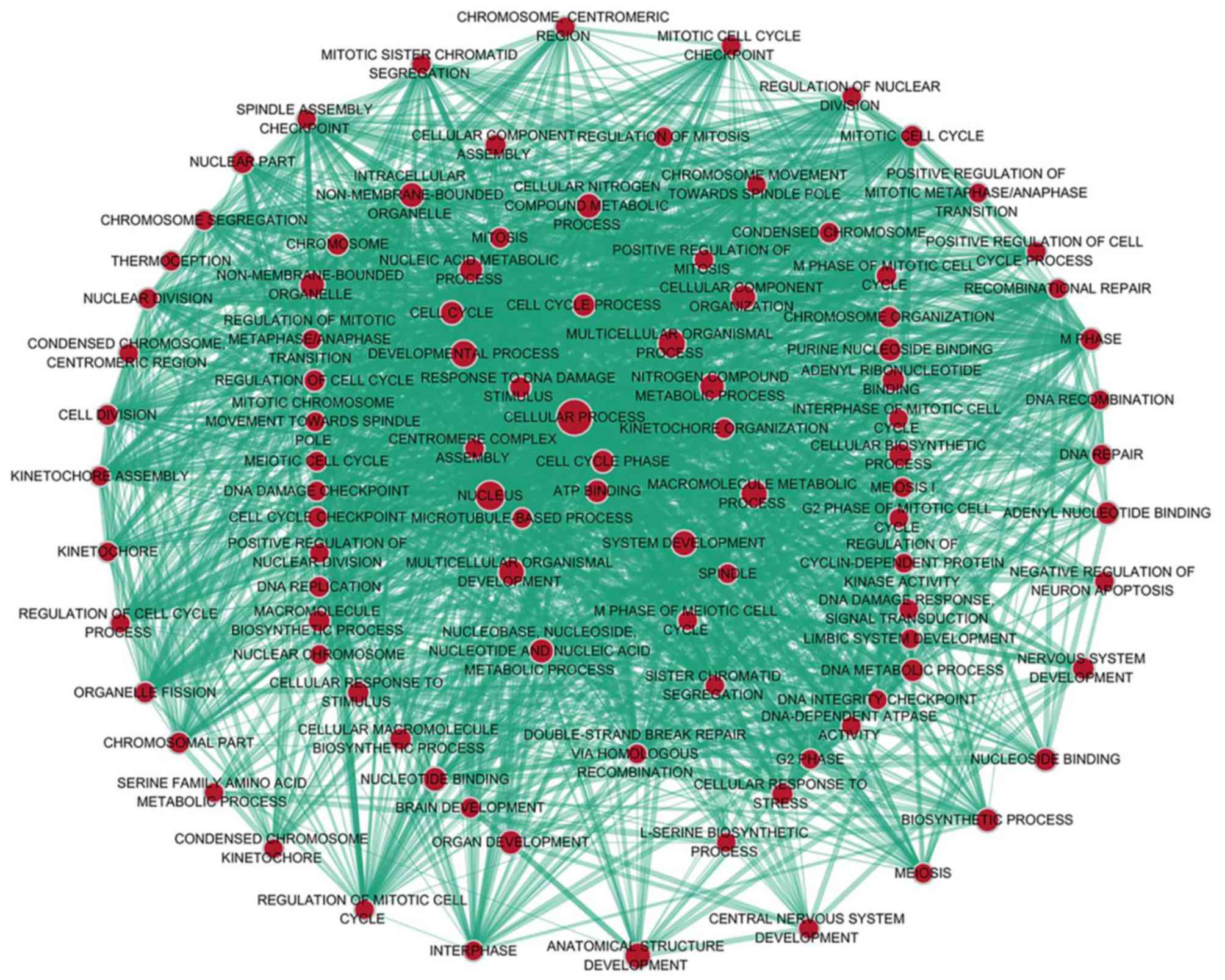
The GO biological process annotation demonstrated that the
promising target genes were primarily enriched in ‘DNA
replication’, ‘cell division’, ‘DNA repair’, ‘G1/S
transition of mitotic cell cycle’ and ‘sister chromatid cohesion’
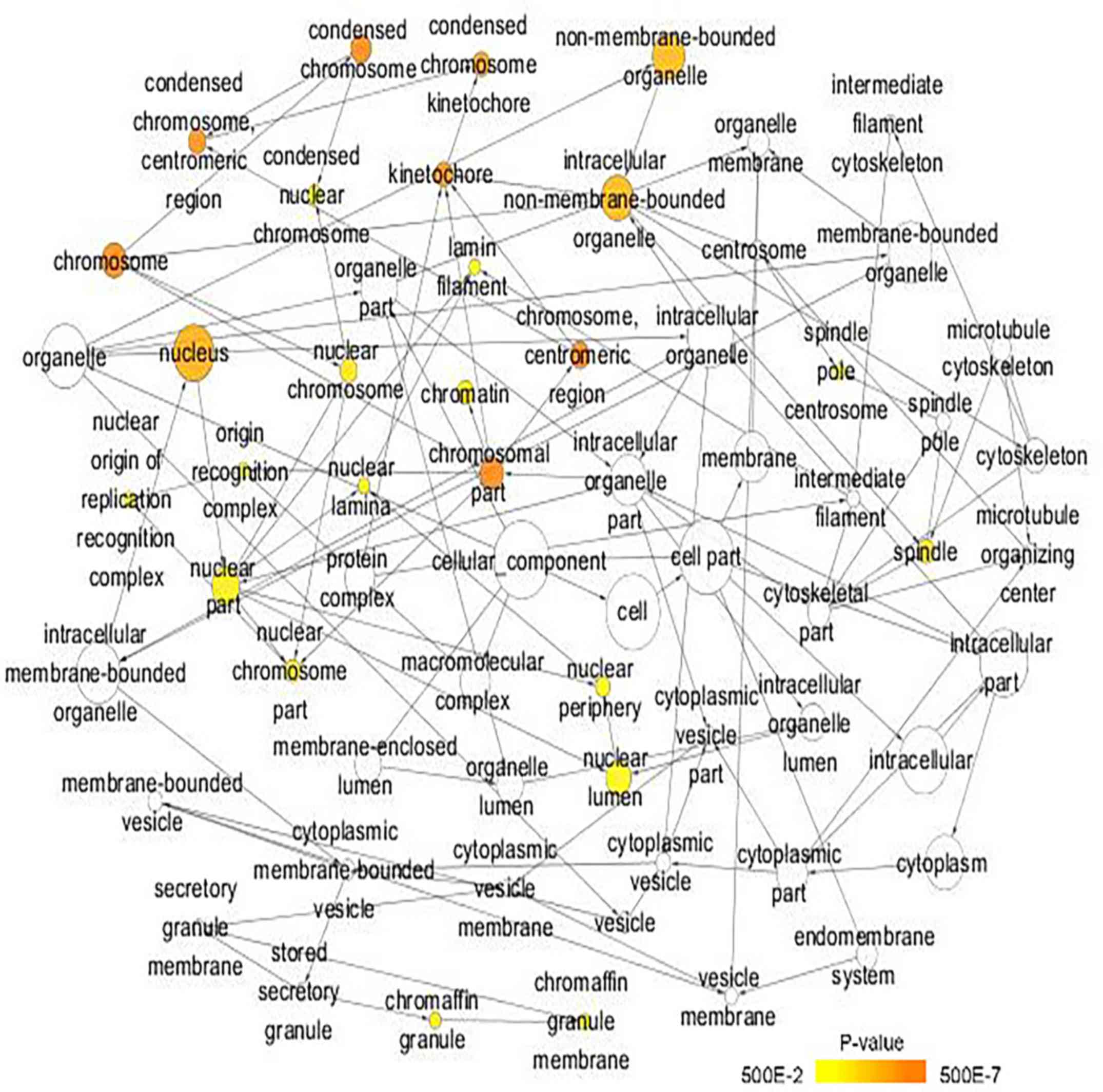
(Table II; Fig. 7). In cellular component, the
promising target genes were closely associated with ‘nucleoplasm’,
‘chromatin’, ‘kinetochore’, ‘GINS complex’ and ‘chromosome,
centromeric region’ (Table II;
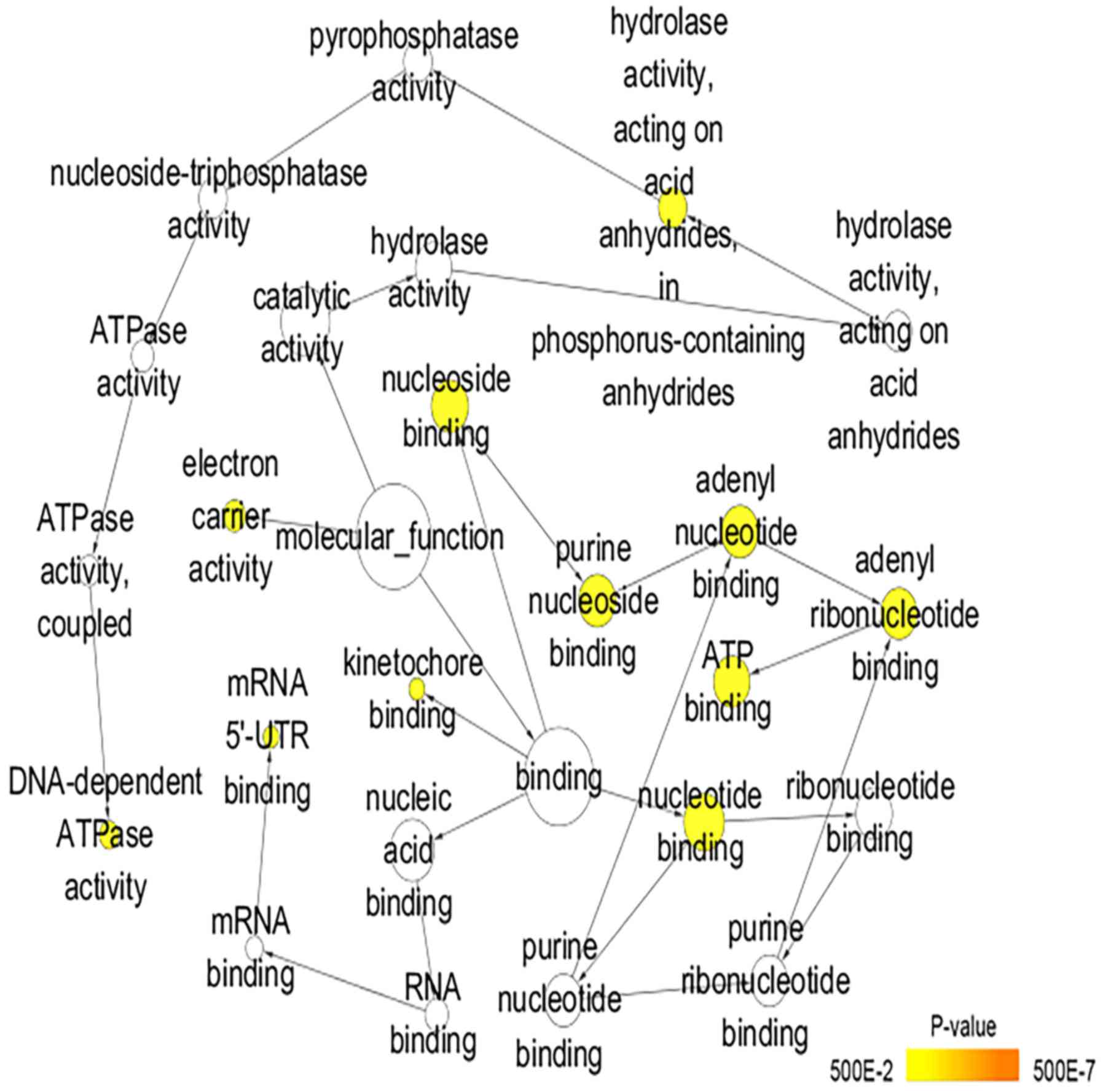
Fig. 8). For molecular function,
five of the most enriched items were ‘chromatin binding’, ‘ATP
binding’, ‘3’-5’ DNA helicase activity’, ‘DNA replication origin
binding’ and ‘microtubule motor activity’ (Table II; Fig. 9). In addition, the significant KEGG
pathways included ‘cell cycle’, ‘p53 signaling pathway’, ‘Fanconi
anemia pathway’, ‘homologous recombination’, ‘glycine, serine and
threonine metabolism’ and ‘oocyte meiosis’ (Table III; Fig. 10). These results suggested that
miR-1 may serve an important role in LUSC via multiple pathways.
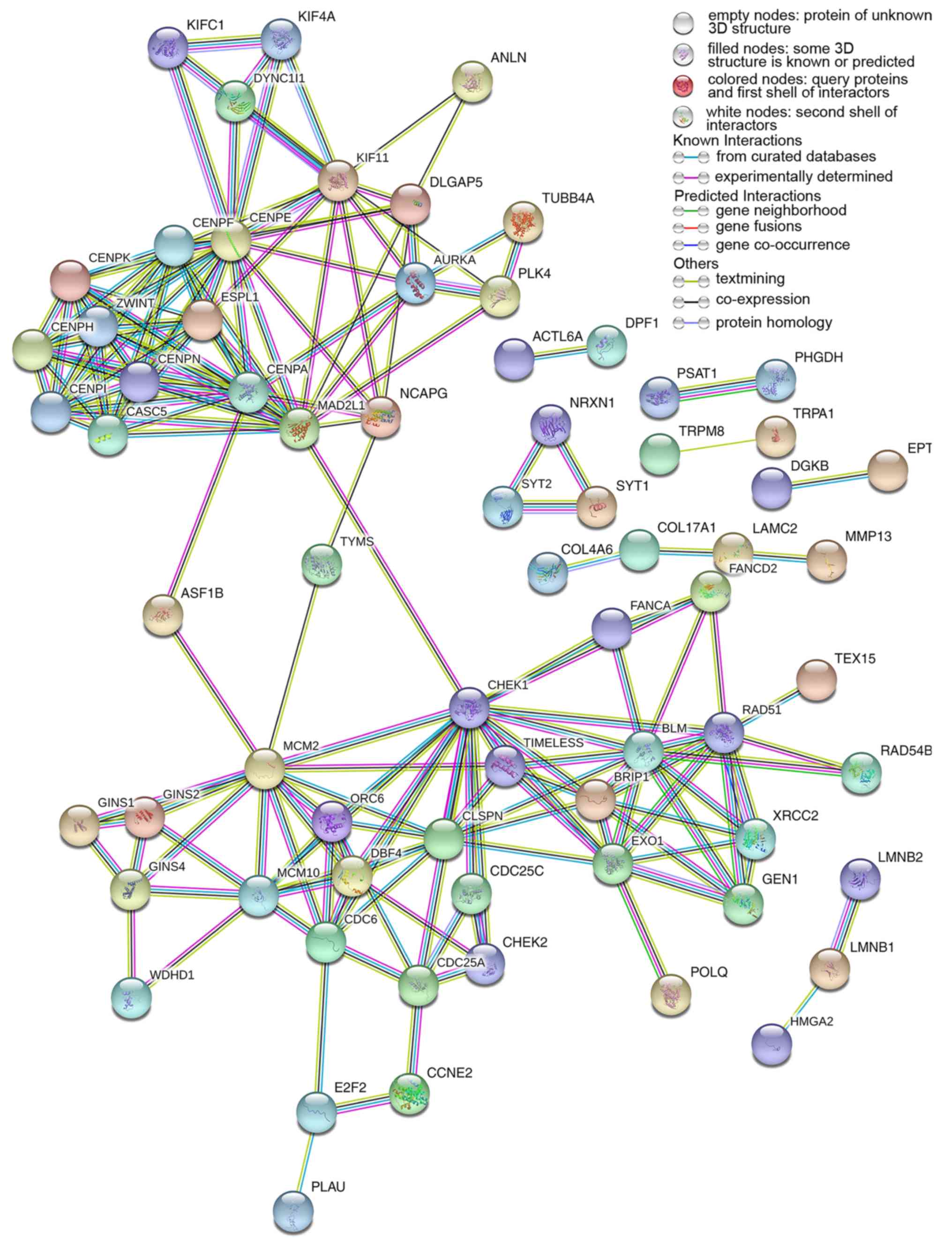
There were 222 nodes and 1,004 edges in the PPI network. In terms
of the PPI network, in the current study, GINS complex subunit 4,
DBF4 zinc finger, GINS complex subunit 1, phosphoserine
aminotransferase 1, minichromosome maintenance complex component 2,
minichromosome maintenance complex component 10, checkpoint kinase
1, GINS complex subunit 2, 3-phosphoglycerate dehydrogenase, cell
division cycle 6 and cell division cycle 25C exhibited the highest
degrees (Table IV; Fig. 11).
 | Table II.GO analysis for the predicted target
genes of microRNA-1 (only the top 10 pathways are presented). |
Table II.
GO analysis for the predicted target
genes of microRNA-1 (only the top 10 pathways are presented).
| Category | Count | P-value | Target genes |
|---|
| A, Biological
process |
|
GO:0051301~cell division | 17 |
4.73×10−6 | CDC6, KIFC1, KIF11,
CENPF, AURKA, CENPE, CHEK2, etc. |
|
GO:0006281~DNA repair | 14 |
4.97×10−6 | EXO1, CLSPN, XRCC2,
BLM, GEN1, CHEK1, RAD51, etc. |
|
GO:0000082~G1/S transition of
mitotic cell cycle | 9 |
2.96×10−5 | CCNE2, CDC6, TYMS,
DBF4, ORC6, MCM2, MCM10, etc. |
|
GO:0007062~sister chromatid
cohesion | 9 |
3.18×10−5 | CENPN, MAD2L1,
CENPA, ZWINT, CENPF, CENPE, etc. |
|
GO:0006270~DNA replication
initiation | 6 |
3.53×10−5 | CNE2, CDC6, GINS4,
ORC6, MCM2, MCM10 |
|
GO:0032508~DNA duplex
unwinding | 6 |
1.70×10−4 | GINS1, GINS2, BLM,
GINS4, BRIP1, RAD54B |
|
GO:0007067~mitotic nuclear
division | 12 |
1.97×10−4 | CENPN, CDC6,
FAM64A, KIF11, TIMELESS, CENPF, etc. |
|
GO:0000070~mitotic sister
chromatid segregation | 5 |
2.03×10−4 | KIFC1, MAD2L1,
CENPA, ZWINT, ESPL1 |
|
GO:0000732~strand
displacement | 5 |
2.38×10−4 | EXO1, XRCC2, BLM,
BRIP1, RAD51 |
| B, Cellular
component |
|
GO:0005654~nucleoplasm | 59 |
3.45×10−6 | E2F2, CLSPN, XRCC2,
DBF4, AURKA, CBX2, MCM10, etc. |
|
GO:0000785~chromatin | 8 |
7.79×10−5 | CENPF, CHEK1, MCM2,
ASF1B, HMGA2, ESCO2, etc. |
|
GO:0000776~kinetochore | 7 |
3.54×10−4 | DYNC1I1, MAD2L1,
ZWINT, CENPF, CENPE, CENPI, CENPH |
|
GO:0000811~GINS complex | 3 |
3.97×10−4 | GINS1, GINS2,
GINS4 |
|
GO:0000775~chromosome,
centromeric region | 6 |
5.09×10−4 | CENPN, MKI67,
CENPA, CENPF, CENPE, HELLS |
|
GO:0000777~condensed
chromosome kinetochore | 7 |
5.20×10−4 | DYNC1I1, CENPN,
MAD2L1, ZWINT, CENPE, CENPK, CENPH |
|
GO:0031298~replication fork
protection complex | 3 |
2.70×10−3 | GINS2, GINS4,
MCM10 |
|
GO:0000922~spindle pole | 6 |
8.77×10−3 | DYNC1I1, CENPN,
MAD2L1, ZWINT, CENPE, CENPK, CENPH |
|
GO:0005634~nucleus | 80 |
9.28×10−3 | KIFC1, FIGNL1,
AURKA, CBX2, MCM10, ZIC1, GLDC, etc. |
|
GO:0005829~cytosol | 53 |
1.12×10−2 | TFERMT1, AURKA,
GTSE1, GLDC, CCNE2, PCP4, etc. |
| C, Molecular
function |
|
GO:0003682~chromatin
binding | 14 |
8.89×10−4 | EXO1, CENPF, ATAD2,
CBX2, VSX1, TP73, RAD51, DLX1, etc. |
|
GO:0005524~ATP binding | 32 |
1.78×10−3 | KIFC1, KIF4A,
XRCC2, GCLC, ATP10B, BLM, FIGNL1,etc. |
|
GO:0043138~3′-5′; DNA helicase
activity | 3 |
2.88×10−3 | GINS1, GINS2,
GINS4 |
|
GO:0003688~DNA replication
origin binding | 3 |
7.30×10−3 | ORC6, MCM2,
MCM10 |
|
GO:0003777~microtubule motor
activity | 5 |
1.55×10−2 | DYNC1I1, KIFC1,
KIF4A, KIF11, CENPE |
|
GO:0030276~clathrin
binding | 4 |
2.43×10−2 | SYT1, SYT2, SYT13,
SYT16 |
|
GO:0003697~single-stranded DNA
binding | 5 |
2.55×10−2 | XRCC2, BLM, NEIL3,
MCM10, RAD51 |
|
GO:0004520~endodeoxyribonuclease
activity | 3 |
2.81×10−2 | XRCC2, GEN1,
RAD51 |
|
GO:0005544~calcium-dependent
phospholipid binding | 4 |
3.22×10−2 | SYT1, SYT2, SYT13,
SYT16 |
|
GO:0008395~steroid hydroxylase
activity | 3 |
3.84×10−2 | CYP2B6, CYP2S1,
CYP2W1 |
 | Table III.KEGG pathway of validated target
genes of microRNA-1. |
Table III.
KEGG pathway of validated target
genes of microRNA-1.
| KEGG Pathway | Count | P-value | Target genes |
|---|
| hsa04110: Cell
cycle | 12 |
3.52×10−7 | CCNE2, E2F2, CDC6,
MAD2L1, DBF4, etc. |
| hsa04115: p53
signaling pathway | 6 |
1.47×10−3 | CCNE2, SERPINB5,
CHEK1, CHEK2, GTSE1, etc. |
| hsa03460: Fanconi
anemia pathway | 5 |
4.29×10−3 | BLM, FANCD2, BRIP1,
FANCA, RAD51 |
| hsa03440:
Homologous recombination | 4 |
5.56×10−3 | XRCC2, BLM, RAD54B,
RAD51 |
| hsa00260: Glycine,
serine and threonine metabolism | 4 |
1.27×10−2 | BHMT, PHGDH, PSAT1,
GLDC |
| hsa04114: Oocyte
meiosis | 5 |
4.81×10−2 | CCNE2, MAD2L1,
AURKA, ESPL1, CDC25C |
| hsa05206: MicroRNAs
in cancer | 8 |
6.69×10−2 | CCNE2, E2F2,
SERPINB5, HMGA2, CDC25C, etc. |
 | Table IV.Top 10 genes with combined scores in
the protein-protein interaction network of potential target genes
of microRNA-1. |
Table IV.
Top 10 genes with combined scores in
the protein-protein interaction network of potential target genes
of microRNA-1.
| Node1 | Node2 | Node1 string
internal ID | Co-expression | Experimentally
determined interaction | Database
annotated | Automated text
mining | Combined score |
|---|
| GINS4 | GINS2 | 1846499 | 0.367 | 0.997 | 0.9 | 0.953 | 0.999 |
| DBF4 | MCM2 | 1845796 | 0.164 | 0.922 | 0.9 | 0.875 | 0.999 |
| GINS1 | GINS2 | 1845151 | 0.764 | 0.997 | 0.9 | 0.953 | 0.999 |
| PSAT1 | PHGDH | 1856523 | 0.971 | 0.417 | 0.941 | 0.743 | 0.999 |
| MCM2 | GINS2 | 1845676 | 0.859 | 0.898 | 0.9 | 0.484 | 0.999 |
| MCM2 | CDC6 | 1845676 | 0.582 | 0.957 | 0.9 | 0.923 | 0.999 |
| MCM10 | MCM2 | 1854261 | 0.769 | 0.934 | 0.9 | 0.842 | 0.999 |
| GINS4 | GINS1 | 1846499 | 0.371 | 0.996 | 0.9 | 0.953 | 0.999 |
| CHEK1 | CDC25C | 1859362 | 0.448 | 0.528 | 0.9 | 0.956 | 0.998 |
Discussion
Recently, numerous studies demonstrated that miR-1
is predominantly downregulated in multiple human tumors, including
lung cancer, prostate cancer, breast cancer and sarcomas (28,56),
and serves as a tumor suppressor gene involved in cancer
progression. However, studies on the expression of miR-1 and its
clinical significance in LUSC are rare, and the underlying
mechanism of miR-1 in LUSC remains to be elucidated. The present
study investigated miR-1 expression, clinical significance and the
potential molecular mechanisms of miR-1 in LUSC.
To the best of the authors' knowledge, the present
study is the first meta-analysis to examine the expression of miR-1
and its clinical significance in LUSC. The present meta-analysis
involved 1,386 cases from 12 eligible studies. The combined SMD was
−1.70 (95% CI: −1.857, 1.52) with high heterogeneity
(I2=90.5%; P=0.001), demonstrating that miR-1 was
significantly downregulated in LUSC. The AUC was 0.9096 (Q*=0.8416)
with a sensitivity of 0.71 (95% CI: 0.66, 0.76, P<0.01) and a
specificity of 0.88 (95% CI: 0.86, 0.90, P<0.01). The pooled PLR
and NLR were 4.93 (95% CI: 2.54, 9.55) and 0.24 (95% CI: 0.10,
0.54), respectively. A PLR value of 4.00 suggested that patients
with LUSC possessed an ~4-fold higher probability of exhibiting
downregulated miR-1 compared with control groups. An NLR value of
0.34 suggested that the probability of having LUSC was 34% when
miR-1 is abnormal. In addition, the pooled DOR was 28.24 (95% CI:
10.56, 75.53). According to these results, miR-1 may better
differentiate between LUSC and patients without cancer. Sensitivity
analysis demonstrated that high heterogeneity may reflect the
GSE40738 studies and TCGA data. To obtain more convincing
conclusions, additional studies using a higher quality, larger
sample size and a consistent standard procedure are required.
Accumulating evidence demonstrated that miR-1 serves
an important role in the development of multiple tumors (57,58).
Mataki et al (59)
demonstrated that miR-1/133a was significantly downregulated in
LUSC tissues and enhanced cancer cell invasion and migration via
the regulation of Coronin1C. However, little is known of the
potential molecular mechanisms of miR-1 in LUSC. Therefore, 222
validated targeting genes of miR-1 were collected and a
comprehensive target genes network analysis performed. GO analysis
demonstrated that miR-1 may be involved in multiple biological
processes, including ‘DNA replication’, ‘cell division’, ‘DNA
repair’ and the ‘G1/S transition of the mitotic cell
cycle’. KEGG pathway analysis identified that miR-1 may serve a
pivotal role in LUSC via different pathways, including ‘cell
cycle’, ‘p53 signaling pathway’, ‘Fanconi anemia pathway’,
‘homologous recombination’, ‘glycine, serine and threonine
metabolism’ and ‘oocyte meiosis’. The p53 gene, a key tumor
suppressor located on chromosome 17p13, is the most frequently
mutated gene in cancer and is involved in in a variety of
biological processes to prevent tumorigenesis through the
transcriptional regulation of downstream target genes (60). A previous study demonstrated that
p53 is associated with cell cycle arrest, apoptosis and drug
resistance in lung cancer cells (61). Zhang et al (62) demonstrated that GluA2 induces
apoptosis in non-small cell lung cancer A549 cells through the p53
signaling pathway; the p53 signaling pathway was a significant
pathway (Table III; P=0.001)
that ranked second in the biological process of LUSC. The p53
pathway may serve an important role in radio sensitivity in
non-small cell lung cancer H460 cells via the upregulation of
phosphatase and tensin homolog expression level (63). A number of previous studies
(64–66) demonstrated that miRNAs may be a key
effector of p53 tumor-suppressor function; mediating the biological
effects of p53 and inactivating this molecule may contribute to
specific cancer types (67),
suggesting that miRNAs may serve a vital role in the p53 gene
signaling pathway. Therefore, it is hypothesized that miR-1 may be
involved in the progression of LUSC through the p53 signaling
pathway.
There are a number of limitations to the present
study. The study size included in this meta-analysis was relatively
small. The prognostic value of miR-1 was not discussed in this
analysis, reflecting that there are no studies regarding the
association between miR-1 and prognosis and clinic pathological
parameters in LUSC. Only studies reported in Chinese and English
were included in this meta-analysis, which may result in the
omission of eligible studies due to language criteria. Furthermore,
among the 12 studies included in the present study, only one was
derived from serum, and the remaining 11 studies used samples
derived from tissue. For a better understanding of the role of
miR-1, large cohort and independent studies are required to examine
the clinical significance of miRNA and its potential mechanism in
LUSC.
In conclusion, the present study demonstrated that
miR-1 is significantly downregulated in LUSC and may be involved in
cancer progression via multiple, crucial pathways. Therefore, miR-1
may be used as a screening tool for LUSC in the future. Large-scale
studies are required to further investigate the clinical
significance of miR-1.
Acknowledgements
The authors would like to acknowledge the public
data provided by The Cancer Genome Atlas and Gene Expression
Omnibus datasets.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, MQ and JH contributed to the design of the
study, data collection, analysis and drafting of the manuscript. JM
and XH contributed to the design of the study, interpretation of
the data and drafting of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen M, Liu X, Du J, Wang XJ and Xia L:
Differentiated regulation of immune-response related genes between
LUAD and LUSC subtypes of lung cancers. Oncotarget. 8:133–144.
2017.PubMed/NCBI
|
|
2
|
Freeman JR, Chu S, Hsu T and Huang YT:
Epigenome-wide association study of smoking and DNA methylation in
non-small cell lung neoplasms. Oncotarget. 7:69579–69591. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng L, Bian XW, Li DK, Xu C, Wang GM, Xia
QY and Xiong Q: Large-scale RNA-Seq transcriptome analysis of 4043
cancers and 548 normal tissue controls across 12 TCGA cancer types.
Sci Rep. 5:134132015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Czarnecka KH, Migdalska-Sęk M, Domańska D,
Pastusza-Lewandoska D, Dutkowska A, Kordiak J, Nawrot E,
Kiszałkiewicz J, Antczak A and Brzeziańska-Lasota E: FHIT promoter
methylation status, low protein and high mRNA levels in patients
with non-small cell lung cancer. Int J Oncol. 49:1175–1184. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang JT, Lee YM and Huang RS: The impact
of the cancer genome atlas on lung cancer. Transl Res. 166:568–585.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Chen Z, An L, Wang Y, Zhang Z, Guo
Y and Liu C: Analysis of long non-coding RNA expression profiles in
non-small cell lung cancer. Cell Physiol Biochem. 38:2389–2400.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bittoni MA, Focht BC, Clinton SK,
Buckworth J and Harris RE: Prospective evaluation of C-reactive
protein, smoking and lung cancer death in the Third National Health
and Nutrition Examination Survey. Int J Oncol. 47:1537–1544. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eldem V, Çelikkol Akçay U, Ozhuner E,
Bakır Y, Uranbey S and Unver T: Genome-wide identification of
miRNAs responsive to drought in peach (Prunus persica) by
high-throughput deep sequencing. PLoS One. 7:e502982012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma N, Zhang W, Qiao C, Luo H, Zhang X, Liu
D, Zang S, Zhang L and Bai J: The tumor suppressive role of
MiRNA-509-5p by targeting FOXM1 in non-small cell lung cancer. Cell
Physiol Biochem. 38:1435–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li P, Liu H, Wang Z, He F, Wang H, Shi Z,
Yang A and Ye J: MicroRNAs in laryngeal cancer: Implications for
diagnosis, prognosis and therapy. Am J Transl Res. 8:1935–1944.
2016.PubMed/NCBI
|
|
11
|
Wang J, Li Y, Ding M, Zhang H, Xu X and
Tang J: Molecular mechanisms and clinical applications of miR-22 in
regulating malignant progression in human cancer (Review). Int J
Oncol. 50:345–355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: Νew trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Subramani R, Gangwani L, Nandy SB,
Arumugam A, Chattopadhyay M and Lakshmanaswamy R: Emerging roles of
microRNAs in pancreatic cancer diagnosis, therapy and prognosis
(Review). Int J Oncol. 47:1203–1210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang P, Yang D, Zhang H, Wei X, Ma T,
Cheng Z, Hong Q, Hu J, Zhuo H, Song Y, et al: Early detection of
lung cancer in serum by a panel of MicroRNA biomarkers. Clin Lung
Cancer. 16:313–319.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma T, Zhao Y, Wei K, Yao G, Pan C, Liu B,
Xia Y, He Z, Qi X, Li Z, et al: MicroRNA-124 functions as a tumor
suppressor by regulating CDH2 and epithelial-mesenchymal transition
in non-small cell lung cancer. Cell Physiol Biochem. 38:1563–1574.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng X, Jiang J, Shi S, Xie H, Zhou L and
Zheng S: Knockdown of miR-25 increases the sensitivity of liver
cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad
signaling pathway. Int J Oncol. 49:2600–2610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meerson A and Yehuda H: Leptin and insulin
up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the
invasiveness of human colon cancer cells. BMC Cancer. 16:8822016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumamoto T, Seki N, Mataki H, Mizuno K,
Kamikawaji K, Samukawa T, Koshizuka K, Goto Y and Inoue H:
Regulation of TPD52 by antitumor microRNA-218 suppresses cancer
cell migration and invasion in lung squamous cell carcinoma. Int J
Oncol. 49:1870–1880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Chen L, Wu Z, Wang M, Jin F, Wang
N, Hu X, Liu Z, Zhang CY, Zen K, et al: miR-124-3p functions as a
tumor suppressor in breast cancer by targeting CBL. BMC Cancer.
16:8262016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu X and Li Z: New insights into MicroRNAs
involves in drug resistance in diffuse large B cell lymphoma. Am J
Transl Res. 7:2536–2542. 2015.PubMed/NCBI
|
|
21
|
Li Z, Yu X, Shen J, Law PT, Chan MT and Wu
WK: MicroRNA expression and its implications for diagnosis and
therapy of gallbladder cancer. Oncotarget. 6:13914–13921.
2015.PubMed/NCBI
|
|
22
|
Cui L, Li Y, Lv X, Li J, Wang X, Lei Z and
Li X: Expression of MicroRNA-301a and its functional roles in
malignant melanoma. Cell Physiol Biochem. 40:230–244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Zhang Y, Liu X, Fang A, Wang J,
Yang Y, Wang L, Du L and Wang C: Direct quantitative detection for
cell-free miR-155 in urine: A potential role in diagnosis and
prognosis for non-muscle invasive bladder cancer. Oncotarget.
7:3255–3266. 2016.PubMed/NCBI
|
|
24
|
Gao Y, Feng B, Han S, Lu L, Chen Y, Chu X,
Wang R and Chen L: MicroRNA-129 in human cancers: From
tumorigenesis to clinical treatment. Cell Physiol Biochem.
39:2186–2202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng T, Yuan Y, Zhang C, Zhang C, Yao W,
Wang C, Liu R and Ba Y: Identification of circulating MiR-25 as a
potential biomarker for pancreatic cancer diagnosis. Cell Physiol
Biochem. 39:1716–1722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu L, Zhou L, Chen EZ, Sun K, Jiang P,
Wang L, Su X, Sun H and Wang H: A Novel YY1-miR-1 regulatory
circuit in skeletal myogenesis revealed by genome-wide prediction
of YY1-miRNA network. PLoS One. 7:e275962012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han C, Shen JK, Hornicek FJ, Kan Q and
Duan Z: Regulation of microRNA-1 (miR-1) expression in human
cancer. Biochim Biophys Acta. 1860:227–232. 2017. View Article : Google Scholar
|
|
28
|
Han C, Zhou Y, An Q, Li F, Li D, Zhang X,
Yu Z, Zheng L, Duan Z and Kan Q: MicroRNA-1 (miR-1) inhibits
gastric cancer cell proliferation and migration by targeting MET.
Tumour Biol. 36:6715–6723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Shen F and Wang C, Lu W, Wei J,
Shang A and Wang C: MiR-1-3p inhibits the proliferation and
invasion of bladder cancer cells by suppressing CCL2 expression.
Tumour Biol. 39:10104283176983832017.PubMed/NCBI
|
|
30
|
Wang Z, Wang J, Chen Z, Wang K and Shi L:
MicroRNA-1-3p inhibits proliferation and migration of oral squamous
cell carcinoma cells by targeting DKK1. Biochem Cell Biol.
96:355–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui R, Meng W, Sun HL, Kim T, Ye Z, Fassan
M, Jeon YJ, Li B, Vicentini C, Peng Y, et al: MicroRNA-224 promotes
tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci
USA. 112:E4288–E4297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang WC, Chin TM, Yang H, Nga ME, Lunny
DP, Lim EK, Sun LL, Pang YH, Leow YN, Malusay SR, et al:
Tumour-initiating cell-specific miR-1246 and miR-1290 expression
converge to promote non-small cell lung cancer progression. Nat
Commun. 7:117022016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Wang C, Shan S, Liu X, Jiang Z
and Ren T: TLR4/ROS/miRNA-21 pathway underlies lipopolysaccharide
instructed primary tumor outgrowth in lung cancer patients.
Oncotarget. 7:42172–42182. 2016.PubMed/NCBI
|
|
34
|
Yu N, Zhang Q, Liu Q, Yang J and Zhang S:
A meta-analysis: microRNAs' prognostic function in patients with
nonsmall cell lung cancer. Cancer Med. 6:2098–2105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao L, Li SH, Tian YX, Zhu QQ, Chen G,
Pang YY and Hu XH: Role of downregulated miR-133a-3p expression in
bladder cancer: A bioinformatics study. Onco Targets Ther.
10:3667–3683. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Xing Z, Zhan P, Liu H, Ye W, Lv T
and Song Y: Is it feasible to detect epidermal growth factor
receptor mutations in circulating tumor cells in nonsmall cell lung
cancer?: A meta-analysis. Medicine (Baltimore). 95:e51152016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Yu Y, Li Y and Wei L: Diagnostic
value of contrast-enhanced ultrasound in hepatocellular carcinoma:
A meta-analysis with evidence from 1998 to 2016. Oncotarget.
8:75418–75426. 2017.PubMed/NCBI
|
|
38
|
Chen WS, Li JJ, Hong L, Xing ZB, Wang F
and Li CQ: Comparison of MRI, CT and 18F-FDG PET/CT in the
diagnosis of local and metastatic of nasopharyngeal carcinomas: An
updated meta analysis of clinical studies. Am J Transl Res.
8:4532–4547. 2016.PubMed/NCBI
|
|
39
|
Ma X, Wang L, Wu H, Feng Y, Han X, Bu H
and Zhu Q: Spleen stiffness is superior to liver stiffness for
predicting esophageal varices in chronic liver disease: A
meta-analysis. PLoS One. 11:e01657862016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Dang YW, Wang X, Yang X, Zhang R,
Lv ZL and Chen G: Comprehensive analysis of long non-coding RNA
PVT1 gene interaction regulatory network in hepatocellular
carcinoma using gene microarray and bioinformatics. Am J Transl
Res. 9:3904–3917. 2017.PubMed/NCBI
|
|
42
|
Zeng JH, Xiong DD, Pang YY, Zhang Y, Tang
RX, Luo DZ and Chen G: Identification of molecular targets for
esophageal carcinoma diagnosis using miRNA-seq and RNA-seq data
from The Cancer Genome Atlas: A study of 187 cases. Oncotarget.
8:35681–35699. 2017.PubMed/NCBI
|
|
43
|
Zhang Y, He RQ, Dang YW, Zhang XL, Wang X,
Huang SN, Huang WT, Jiang MT, Gan XN, Xie Y, et al: Comprehensive
analysis of the long noncoding RNA HOXA11-AS gene interaction
regulatory network in NSCLC cells. Cancer Cell Int. 16:892016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Huang JC, Cai KT, Yu XB, Chen YR,
Pan WY, He ZL, Lv J, Feng ZB and Chen G: Long non-coding RNA HOTTIP
promotes hepatocellular carcinoma tumorigenesis and development: A
comprehensive investigation based on bioinformatics, qRT-PCR and
meta-analysis of 393 cases. Int J Oncol. 51:1705–1721. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of Gene Ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Merico D, Isserlin R, Stueker O, Emili A
and Bader GD: Enrichment map: A network-based method for gene-set
enrichment visualization and interpretation. PLoS One.
5:e139842010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et
al: MiR-21 is an EGFR-regulated anti-apoptotic factor in lung
cancer in never-smokers. Proc Natl Acad Sci USA. 106:12085–12090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Raponi M, Dossey L, Jatkoe T, Wu X, Chen
G, Fan H and Beer DG: MicroRNA classifiers for predicting prognosis
of squamous cell lung cancer. Cancer Res. 69:5776–5783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ohba T and Nagano H: A small-cell lung
cancer subtype with good prognosis found by a three miRNA
signature. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19945Oct
1st–2017
|
|
51
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosomes Cancer. 50:585–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Patnaik SK, Kannisto ED, Mallick R,
Vachani A and Yendamuri S: Whole blood microRNA expression may not
be useful for screening non-small cell lung cancer. PLoS One.
12:e01819262017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
van Jaarsveld MT, Wouters MD, Boersma AW,
Smid M, van Ijcken WF, Mathijssen RH, Hoeijmakers JH, Martens JW,
van Laere S, Wiemer EA and Pothof J: DNA damage responsive
microRNAs misexpressed in human cancer modulate therapy
sensitivity. Mol Oncol. 8:458–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Arima C, Kajino T, Tamada Y, Imoto S,
Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T,
et al: Lung adenocarcinoma subtypes definable by lung
development-related miRNA expression profiles in association with
clinicopathologic features. Carcinogenesis. 35:2224–2231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jin Y, Liu YL and Lu SH: The miRNA
expression profiles in three subtypes of lung carcinomas.
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74190Oct
12th–2017
|
|
56
|
Liu T, Hu K, Zhao Z, Chen G, Ou X, Zhang
H, Zhang X, Wei X, Wang D, Cui M and Liu C: MicroRNA-1
down-regulates proliferation and migration of breast cancer stem
cells by inhibiting the Wnt/β-catenin pathway. Oncotarget.
6:41638–41649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie M, Dart DA, Guo T, Xing XF, Cheng XJ,
Du H, Jiang WG, Wen XZ and Ji JF: MicroRNA-1 acts as a tumor
suppressor microRNA by inhibiting angiogenesis-related growth
factors in human gastric cancer. Gastric Cancer. 21:41–54. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu X, Wu X, Jiang Q, Sun Y, Liu H, Chen R
and Wu S: Downregulation of microRNA-1 and microRNA-145 contributes
synergistically to the development of colon cancer. Int J Mol Med.
36:1630–1638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mataki H, Enokida H, Chiyomaru T, Mizuno
K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T,
Nakagawa M, et al: Downregulation of the microRNA-1/133a cluster
enhances cancer cell migration and invasion in lung-squamous cell
carcinoma via regulation of Coronin1C. J Hum Genet. 60:53–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu J, Zhang C and Feng Z: Tumor
suppressor p53 and its gain-of-function mutants in cancer. Acta
Biochim Biophys Sin (Shanghai). 46:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhong G, Chen X, Fang X, Wang D, Xie M and
Chen Q: Fra-1 is upregulated in lung cancer tissues and inhibits
the apoptosis of lung cancer cells by the P53 signaling pathway.
Oncol Rep. 35:447–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang HY, Yang W and Lu JB: Knockdown of
GluA2 induces apoptosis in non-small-cell lung cancer A549 cells
through the p53 signaling pathway. Oncol Lett. 14:1005–1010. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jung IL, Kang HJ, Kim KC and Kim IG:
PTEN/pAkt/p53 signaling pathway correlates with the radioresponse
of non-small cell lung cancer. Int J Mol Med. 25:517–523.
2010.PubMed/NCBI
|
|
64
|
Zhang C, Liu J, Tan C, Yue X, Zhao Y, Peng
J, Wang X, Laddha SV, Chan CS, Zheng S, et al: microRNA-1827
represses MDM2 to positively regulate tumor suppressor p53 and
suppress tumorigenesis. Oncotarget. 7:8783–8796. 2016.PubMed/NCBI
|
|
65
|
Perdas E, Stawski R, Nowak D and Zubrzycka
M: Potential of liquid biopsy in papillary thyroid carcinoma in
context of miRNA, BRAF and p53 mutation. Curr Drug Targets.
19:1721–1729. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xiao S, Wang R, Wu X, Liu W and Ma S: The
long noncoding RNA TP73-AS1 interacted with miR-124 to modulate
glioma growth by targeting inhibitor of apoptosis-stimulating
protein of p53. DNA Cell Biol. 37:117–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|