Introduction
Acute kidney injury (AKI), characterized by a sharp
decline in renal function, is a severe complication with high
morbidity and mortality rates and is commonly encountered in the
intensive care unit (ICU) (1,2). The
Finnish Acute Kidney Injury study showed that AKI affects 40% of
critically ill patients (3). AKI
can be caused by various events, such as sepsis, cardiac surgery,
liver or kidney transplantation, rheumatic fever, urinary tract
obstruction, pharmacological toxins (4–6) and
acute severe pancreatitis (7).
Among these, endotoxic shock caused by lipopolysaccharide (LPS),
which is the outer membrane component of gram-negative bacteria, is
a common cause of AKI (8–10). Sepsis-associated AKI carries a
particularly high mortality rate. One multicenter, international
study involving an observational cohort of ICU patients
demonstrated that the mortality rate of sepsis-related AKI patients
was as high as 50% (2).
The mechanisms of sepsis-associated AKI are complex
and not well understood. Although the mechanisms of
sepsis-associated AKI are poorly understood, release of
inflammatory factors, oxidative stress, apoptosis and
microcirculatory dysfunction are believed to play an important role
(11–13). So far, there are no effective drugs
for the treatment of AKI. Therefore, novel and effective therapies
to reduce the mortality of AKI are urgently needed.
3,3′-Diindolylmethane (DIM), a natural compound
derived from the acid-catalyzed self-condensation of
indole-3-carbinol, is abundant in cruciferous vegetables including
kale and broccoli (14,15). Studies have found that DIM can
inhibit LPS-induced acute liver injury by regulating the expression
of miRNAs (14,16). Other studies have shown that DIM
has a protective effect on the LPS-induced damage of cardiomyocytes
and brain inflammation by reducing the release of pro-inflammatory
mediators and negative regulation of the NF-κB signaling pathway
(17,18). In addition, DIM may also exert its
organ protective function by mitigating oxidative stress and
apoptosis (19).
However, the potentially protective characteristics
of DIM have not yet been tested in LPS-triggered AKI. To address
this, an experiment was designed to evaluate how DIM modifies
disease progression in LPS-induced AKI.
Materials and methods
Animal protocols
Research protocols were reviewed and approved by the
Experimental Animal Ethics Committee of Chongqing Medical
University, while all the handling and care of animals were
performed in strict compliance with the U.S. National Institute of
Health Guide for the Care and Use of Laboratory Animals (1996
revision). Mice (male, aged 8 weeks old, body weight 22–25 g) were
bred in a specific pathogen-free laboratory and allowed free access
to food and tap water. The animal room was kept on a 12 h
light/dark cycle at a constant temperature (25°C) and relative
humidity of 55±5% throughout the experimental period. Mice were
divided into 4 groups of 10 mice (40 mice in total). The four
groups comprised the control, LPS (10 mg/kg; Sigma Aldrich; Merck
KGaA), DIM (40 mg/kg; MedChemExpress) and LPS + DIM (10 mg/kg LPS +
40 mg/kg DIM) groups. The choice of DIM concentration was based on
a previous study (19) and
incorporated similar DIM doses as administered in previous
experiments (14,19). An AKI murine model was produced by
intraperitoneally injecting LPS (10 mg/kg in 200 µl saline
solution) and allowing 24 h for renal damage to develop. Mice were
administered two intraperitoneal injections of DIM (40 mg/kg). The
first and second doses were administered 24 and 2 h prior to the
LPS inoculation, respectively. The animals were sacrificed after 24
h exposure to LPS and blood and kidney samples were harvested.
Histological examination
Tissues from the right kidney were first fixed in 4%
paraformaldehyde (4°C, 24 h) and embedded in paraffin. Sections of
4 µm thickness were cut and processed and then stained with
hematoxylin (0.2%) and eosin (1%) (H&E) at room temperature for
30 sec and 1 min respectively. Histological alterations in renal
tissues and the degree of kidney injury were scored on a scale of
0–4, as previously described (20), upon visualization by light
microscopy (magnification, ×400; Olympus Corporation).
Evaluation of renal function
Blood samples were extracted via the retro-orbital
venous plexus and processed to extract the serum. In this present
study, serum creatinine (SCr) and blood urea nitrogen (BUN) levels
were employed as markers of renal function and were analyzed with
an AutoAnalyzer (Roche Diagnostics GmbH). SCr and BUN were
quantified in accordance with the manufacturer's instructions.
TUNEL staining
Tissues of the right kidney were fixed in 4%
paraformaldehyde, paraffin-embedded, and resected into 4 µm-thick
sections according to the aforementioned procedure. TUNEL staining
was conducted with a commercially available kit (Roche Diagnostics
GmbH) according to the manufacturer's instructions. Briefly, the
dehydrated sections were treated with DNase-free proteinase K
(37°C, 30 mins), followed by 3% H2O2 to
quench endogenous peroxidase activity. Free 3′-OH termini were
labeled with digoxigenin-dUTP for 1 h at 37°C using the TUNEL
reaction mixture. Then, the sections were incubated with
converter-POD at 37°C for 30 min. DAB chromogenic reagent was
employed to develop the stain and hematoxylin was used to stain the
nucleus. Finally, the samples were cleared in xylene, mounted with
neutral balsam and coverslipped. Under optical microscopy
(magnification, ×400; Olympus Corporation), the number of
TUNEL-positive cells in 400 histological fields were counted per
kidney section.
Fluorescence microscopy analysis for
kidney reactive oxygen species (ROS)
ROS generated in the kidney were detected using
dihydroethidium (DHE; Beyotime Institute of Biotechnology). The
left kidney tissues were stored in liquid nitrogen before
preparation into frozen sections. The kidney sections were washed
three times with ice-cold PBS, treated with DHE (10−5 M,
final concentration) and incubated for 30 min at 37°C. After
treatment, sections were again washed three times with ice-cold
PBS. The excitation (480 nm) and emission (590 nm) wavelengths for
these experiments were set by fluorescent microscopy. All mean
fluorescence values were analyzed by comparing each group using the
ZEN 2012 software (Carl Zeiss AG, Germany).
Measurement of kidney lipid
peroxidation and glutathione (GSH) content
The malondialdehyde (MDA) level in kidney
homogenates was analyzed by measuring the level of trimethine, a
reaction product of MDA and thiobarbituric acid,
spectrophotometrically at a wavelength of 535 nm. The reduced GSH
level in the kidney homogenates was determined using
5,5′-dithiobis-2-nitrobenoicacid (DTNB) at a wavelength of 412 nm.
Thereafter, oxidized GSH (GSSG) is reduced to GSH by GSH reductase,
total GSH (T-GSH) is measured by the same method. The level of GSSG
can be calculated by subtracting GSH from T-GSH.
Western blot analysis
A protein extraction kit (RIPA Lysis Buffer,
Beyotime Institute of Biotechnology) was used to isolate proteins
from the left renal tissue samples. The bicinchoninic acid method
was employed to quantify protein concentrations. Each protein
sample of 20 µg was separated via 10% SDS-PAGE gel electrophoresis
and transferred to a PVDF membrane (EMD Millipore) and successively
incubated with 5% non-fat milk at room temperature for 1.5 h. PVDF
membranes were allowed to incubate overnight at 4°C with the
indicated primary antibodies, including caspase-3 (1:500; cat. no.
wl01927; Wanleibio Co., Ltd.), cleaved caspase-3 (1:500; cat. no.
wl01992; Wanleibio Co., Ltd.), poly (ADP-ribose) polymerase (PARP;
1:500; cat. no. wl0326; Wanleibio Co., Ltd.), cleaved PARP (1:500;
cat. no. wl01932; Wanleibio co., Ltd.), NADPH oxidase-1, −2, −3 and
−4 (NOX1, NOX2, NOX3 and NOX4, respectively; 1:500; sc-518023,
sc-17844, sc-7662 and sc-20781, respectively; Santa Cruz
Biotechnology, Inc.,USA), and β-actin (1:1,000; cat. no. A0208;
Beyotime Institute of Biotechnology). The following day, HRP
labeled goat anti-rabbit IgG (H+L) (1:3,000; Beyotime Institute of
Biotechnology) was added and further incubated at room temperature
for 1 h. Immunoreactive bands were visualized using an ECL (cat.
no. WLA003a, Wanleibio co., Ltd.) system and quantified with using
Image J software (version 1.8.0).
Statistical analysis
All experiments were conducted at least three times.
All data are presented as the mean ± standard error of the mean.
All statistical analyses were performed using SPSS 17.0 statistical
software (SPSS, Inc.). Statistical significance of multiple groups
was determined by one-way ANOVA, followed by the least significant
difference for multiple comparisons test. P<0.05 was considered
to indicate a statistically significant difference.
Results
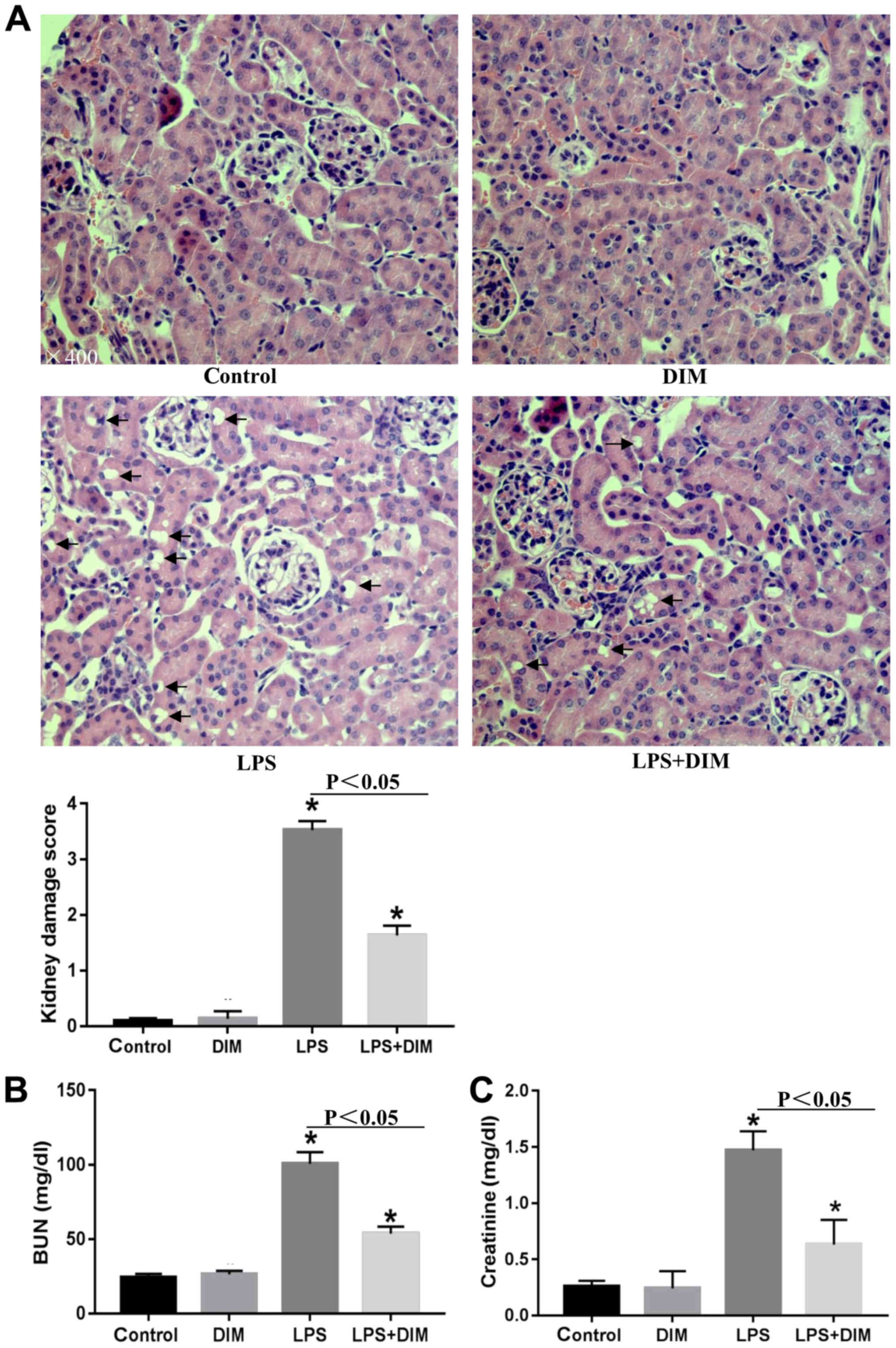
DIM mitigates histopathological
changes and restores renal function in LPS-induced AKI mouse
models
To determine how DIM affected the progression of
LPS-mediated AKI, the kidneys were examined for histopathological
changes. Mice that were exposed to LPS exhibited several indicators
of AKI, including destroyed renal tubules, aberrant renal tubular
epithelial cells, interstitial edema in renal epithelial cells and
renal tubule dilation (Fig. 1A).
H&E staining showed that the epithelial cell structure and the
glomerular membrane were normal in the control and DIM groups.
AKI-related kidney lesions appeared to be attenuated in AKI mouse
models treated with DIM.
To further confirm the results from the H&E
staining, changes in SCr and BUN levels were also examined. As
shown in Fig. 1B and C, DIM
significantly improved the renal function of AKI mice.
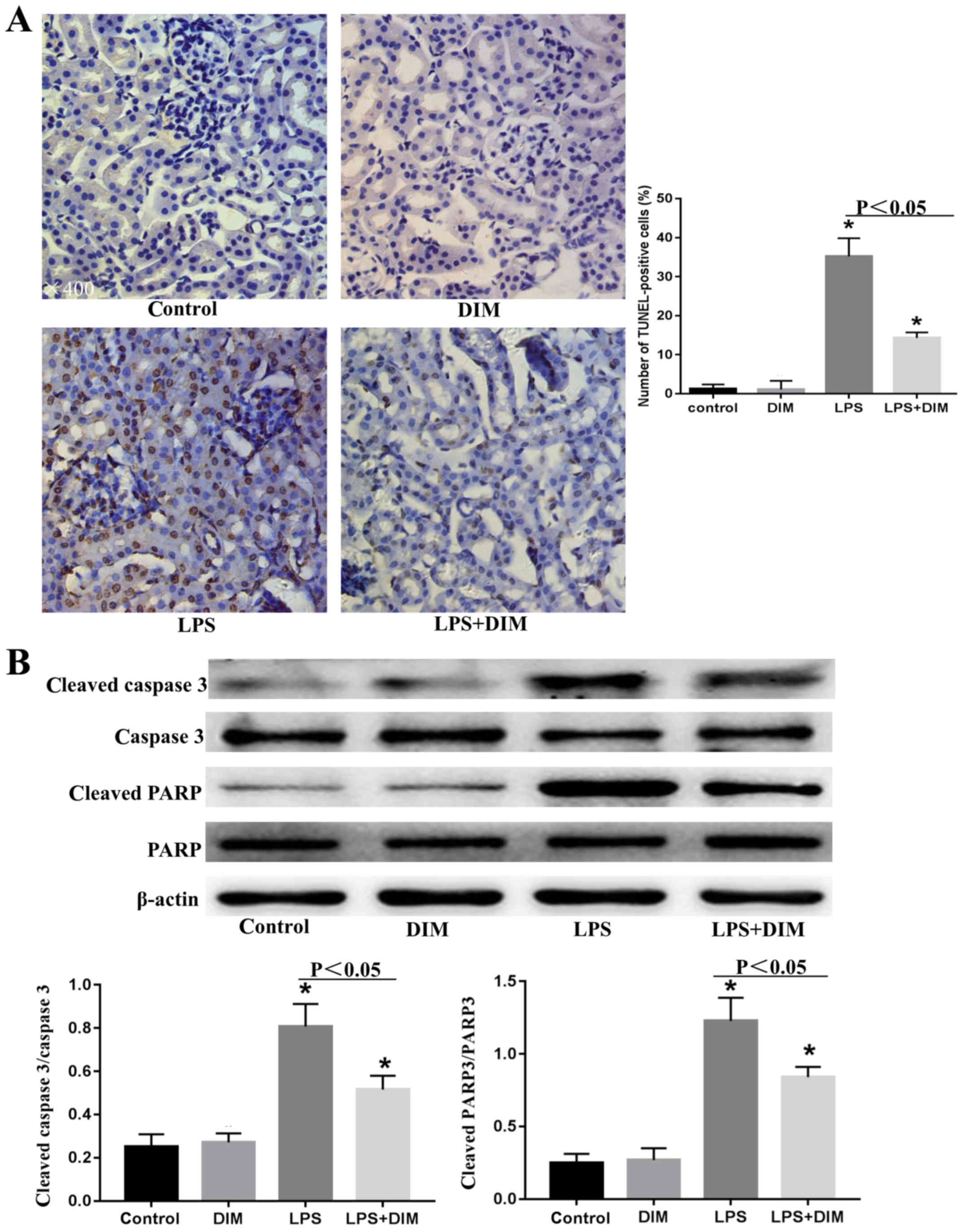
DIM treatment inhibits the apoptosis
of renal tubular epithelial cells in mice with LPS-triggered
AKI
As the apoptosis of renal tubular epithelial cells
is an important mechanism leading to AKI, the effects of DIM on
LPS-induced tubular epithelial cell apoptosis were examined.
As shown in Fig.
2A, numerous TUNEL-positive renal tubular epithelial cells were
observed in the kidney tissues of the LPS-induced AKI mouse models.
However, fewer TUNEL-positive tubular epithelial cells were
observed in the kidney tissues of the DIM-treated AKI mice.
Consistent with this, western blot analysis revealed that the
expression levels of apoptotic markers, including cleaved-caspase-3
and cleaved-PARP, were significantly increased in the LPS-induced
AKI mouse model and were attenuated by DIM pre-treatment (Fig. 2B).
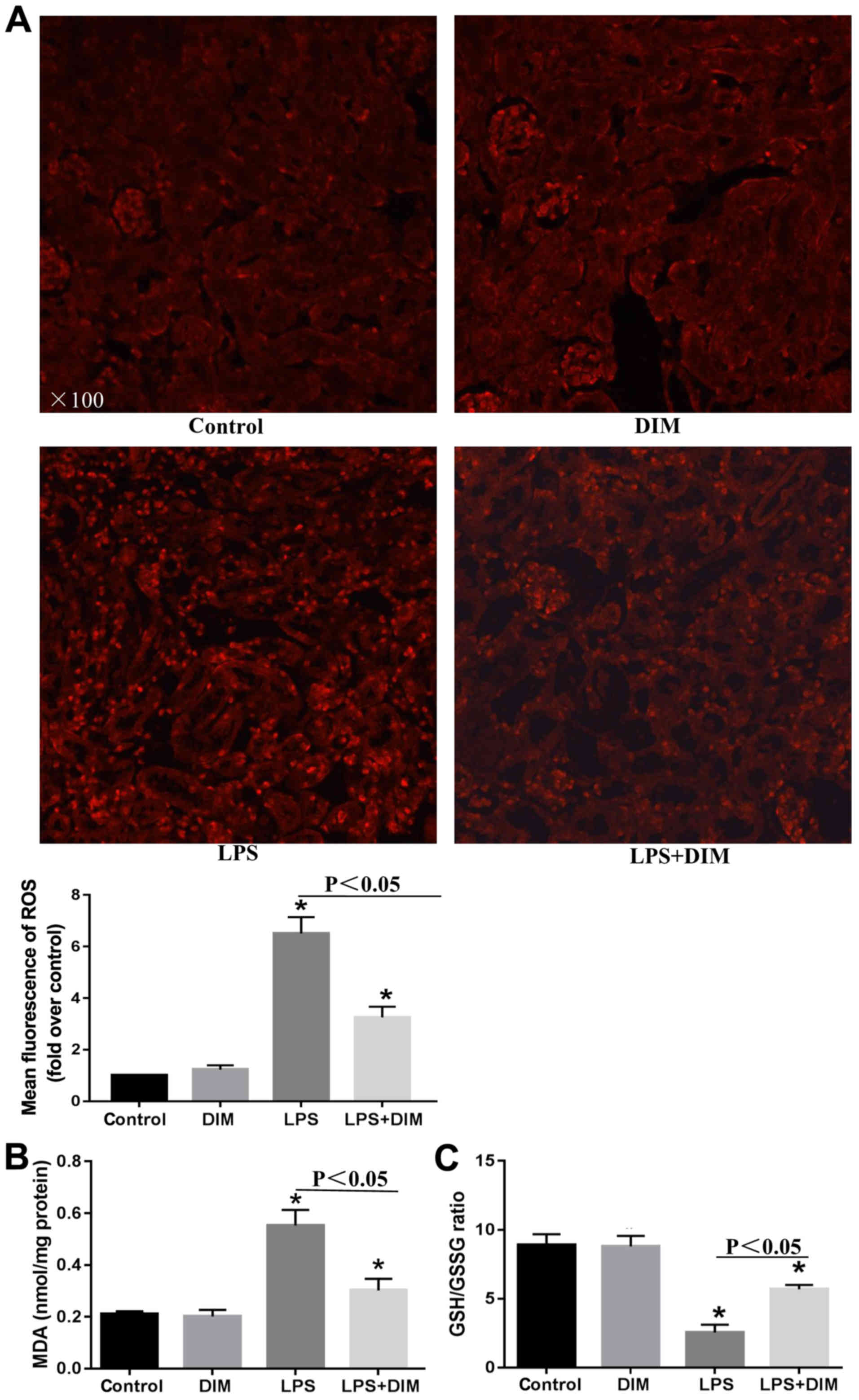
DIM treatment alleviates oxidative
stress in mice with LPS-triggered AKI
To further elucidate the mechanisms allowing DIM to
mediate its anti-apoptotic effects on LPS-induced cell apoptosis,
alterations in oxidative stress were investigated. Mice that
received LPS had elevated levels of ROS and MDA and a decreased
GSH/GSSG ratio in comparison with the control and DIM groups
(Fig. 3). The alterations in
oxidative stress indexes significantly improved when treated with
DIM.
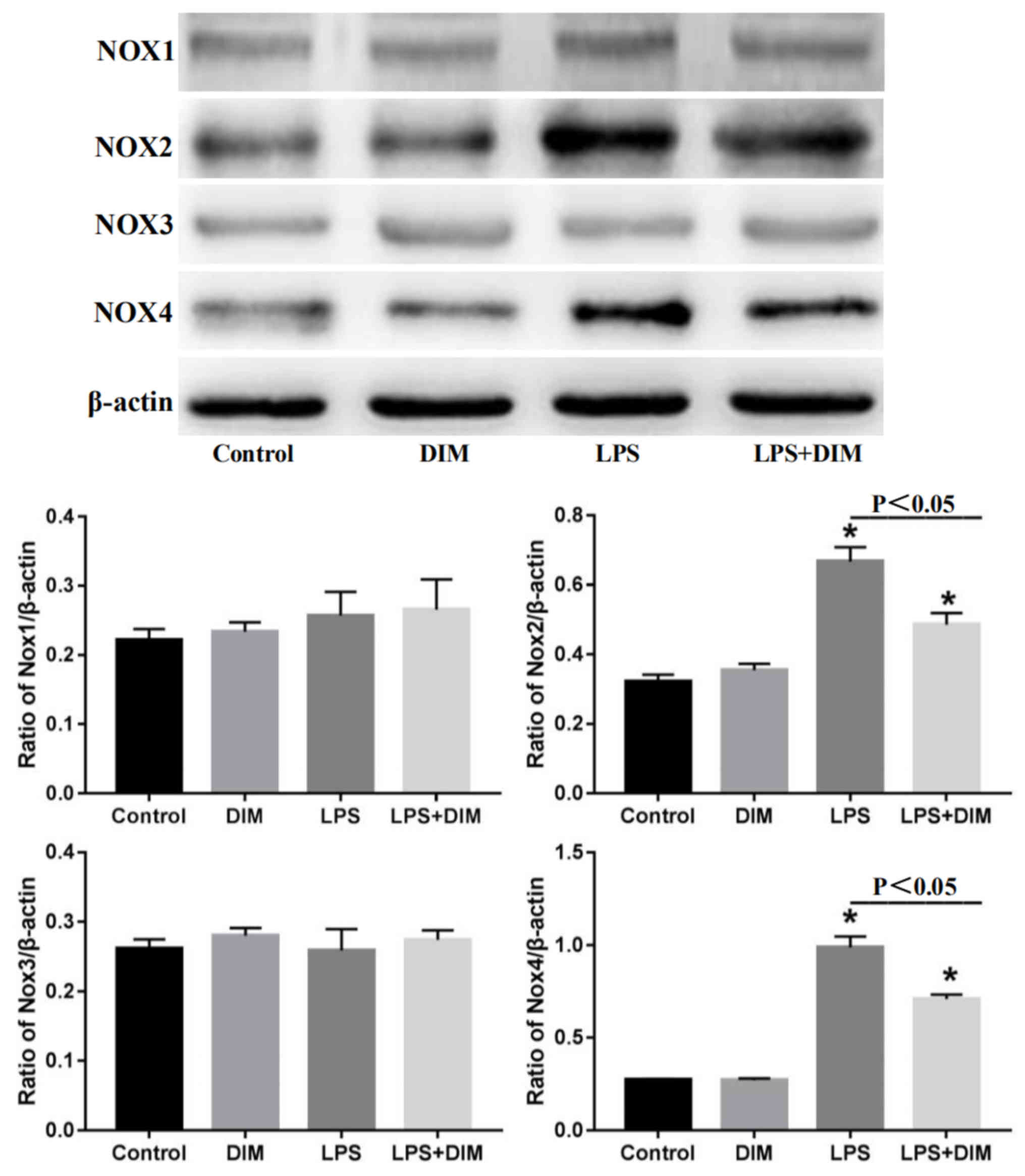
DIM treatment suppresses the
expression of NOX2 and NOX4 in mice with LPS-triggered AKI
As the NOX enzymes are major source of ROS in the
kidneys, the effect of DIM on the expression of NOX family members
was tested. As shown in Fig. 4,
LPS had no effects on the expression of NOX1 and NOX3, but
significantly promoted the expression of NOX2 and NOX4.
Nevertheless, DIM effectively blocked the LPS-induced expression of
NOX2 and NOX4.
Discussion
AKI, which often arises in sepsis cases, is a common
problem in critical patients and carries high morbidity and
mortality rates (2,21). However, effective medical
treatments for AKI are not commonly available. In the present
study, the effects of DIM on LPS-induced AKI in mice was tested.
DIM conferred protection against AKI induced by LPS, as
demonstrated by the improvements in kidney function and the
attenuation of the kidney damage score. These effects may be
achieved by inhibiting the NOX-mediated oxidative stress and
programmed cell death in renal tubular epithelial cells.
Apoptosis is an important pathological mechanism
leading to AKI (13,22–25).
As expected, the LPS-induced AKI mouse model showed increased
apoptosis in renal tubular epithelial cells, as determined by the
elevated expression levels of cleaved caspase-3 and cleaved PARP
measured by western blot analysis. Consistent with the effects of
DIM on renal function in the AKI mouse model, DIM could decrease
the levels of both cleaved caspase-3 and cleaved PARP in kidneys
stimulated with LPS. TUNEL staining also showed that DIM could
alleviate the level of apoptosis in LPS-induced tubular epithelial
cells. Thus, DIM may mitigate LPS-induced AKI by reducing the level
of apoptosis in renal tubular epithelial cells.
ROS play an important role in apoptosis induction
under both physiological and pathological conditions (26). LPS is a strong inducer of ROS
production, and the excessive production of ROS is closely related
to the apoptosis of renal tubular cells by causing the release of
apoptotic factors, including cytochrome C, and by destroying the
permeability of the mitochondrial membrane. Aside from the actions
on mitochondria, ROS may also activate sphingomyelinase-generating
ceramide, an intracellular mediator of apoptosis in granulocytes
(25–28). In the present study, mice
challenged with LPS showed a significant increase in ROS and MDA
(lipid peroxidation) levels coinciding with a rapid decrease in the
GSH/GSSG ratio. Reduced GSH plays an important role as a free
radical scavenger to counteract the deleterious effects of ROS
(29). These changes were all
attenuated by DIM. Thus, DIM may protect against LPS-induced
apoptosis by reducing oxidative stress.
ROS are primarily generated in the mitochondria by
NOX enzymes, cyclooxygenase and xanthine oxidase. The NOX family is
the main source of ROS in the kidneys (30–32).
To determine the mechanism by which DIM reduces ROS, the effect of
DIM on the expression of NOX enzymes (NOX1-NOX4) was investigated.
The data revealed that LPS promoted ROS production primarily
through NOX2 and NOX4. The expression of NOX2 and NOX4 was
decreased in the presence of DIM. Taken together, the data
presented in this present study support the hypothesis that DIM
suppresses LPS-triggered oxidative stress by inhibiting the NOX2
and NOX4 pathways.
A total of three major findings have been revealed
in this present study. First, DIM significantly improved renal
function as demonstrated by the lowering of SCr and BUN levels, as
well as the attenuation of pathological kidney damage in the
LPS-stimulated AKI mouse model. Secondly, DIM suppressed the
pro-apoptotic effects of LPS. Finally, DIM may confer its
protective benefits by inhibiting NOX/ROS signaling.
In conclusion, DIM is a potential therapeutic agent
for use in AKI triggered by LPS. The possible mechanism through
which DIM attenuates renal damage in the LPS-induced AKI mouse
model is via the inhibition of NOX-mediated oxidative stress and
programmed cell death of renal tubular epithelial cells. This
present study focused on an animal model of AKI and was not
sufficient to describe how DIM acts on AKI through the regulation
of NOX-mediated oxidative stress and programmed cell death of renal
tubular epithelial cells. Therefore, further studies using cells
are required to elucidate the specific mechanism of DIM
regulation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JH and TH performed the experiments, data analysis
and manuscript writing. LZ designed the experiments, participated
in the generation of ideas and data interpretation and was
responsible for the overall direction of this work. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Animal Care and Use Committee of Chongqing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoste EA and Kellum JA: Acute kidney
injury: Epidemiology and diagnostic criteria. Curr Opin Crit Care.
12:531–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchino S, Kellum JA, Bellomo R, Doig GS,
Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al:
Acute renal failure in critically ill patients: A multinational,
multicenter study. JAMA. 294:813–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nisula S, Kaukonen KM, Vaara ST, Korhonen
AM, Poukkanen M, Karlsson S, Haapio M, Inkinen O, Parviainen I,
Suojaranta-Ylinen R, et al: Incidence, risk factors and 90-day
mortality of patients with acute kidney injury in Finnish intensive
care units: The FINNAKI study. Intensive Care Med. 39:420–428.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kellum JA, Lameire N, Aspelin P, Barsoum
RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A,
Levey AS, et al: Kidney disease: Improving global outcomes (KDIGO)
acute kidney injury work group. KDIGO clinical practice guideline
for acute kidney injury. Kidney Int Suppl. 2:1–138. 2012.
|
|
5
|
Liang R, Zhao Q, Jian G, Cheng D, Wang N,
Zhang G and Wang F: Tanshinone IIA attenuates contrast-induced
nephropathy via Nrf2 activation in rats. Cell Physiol Biochem.
46:2616–2623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu R, Kong Y, Yin J, Liang R, Lu Z, Wang
N, Zhao Q, Zhou Y, Yan C, Wang F and Liang M: Antithrombin III is a
novel predictor for contrast induced nephropathy after coronary
angiography. Kidney Blood Press Res. 43:170–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong Y, Yin J, Cheng D, Lu Z, Wang N, Wang
F and Liang M: Antithrombin III attenuates AKI following acute
severe pancreatitis. Shock. 49:572–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mårtensson J and Bellomo R:
Pathophysiology of septic acute kidney injury. Contrib Nephrol.
187:36–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skube SJ, Katz SA, Chipman JG and
Tignanelli CJ: Acute kidney injury and sepsis. Surg Infect
(Larchmt). 19:216–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zarbock A, Gomez H and Kellum JA:
Sepsis-induced acute kidney injury revisited: Pathophysiology,
prevention and future therapies. Curr Opin Crit Care. 20:588–595.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomez H, Ince C, De Backer D, Pickkers P,
Payen D, Hotchkiss J and Kellum JA: A unified theory of
sepsis-induced acute kidney injury: Inflammation, microcirculatory
dysfunction, bioenergetics, and the tubular cell adaptation to
injury. Shock. 41:3–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacobs R, Honore PM, Joannes-Boyau O, Boer
W, De Regt J, De Waele E, Collin V and Spapen HD: Septic acute
kidney injury: The culprit is inflammatory apoptosis rather than
ischemic necrosis. Blood Purif. 32:262–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan L, Bagshaw SM, Langenberg C, Saotome
T, May C and Bellomo R: Pathophysiology of septic acute kidney
injury: What do we really know? Crit Care Med 36 (4 Suppl).
S198–S203. 2008. View Article : Google Scholar
|
|
14
|
Tomar S, Nagarkatti M and Nagarkatti PS:
3,3′-Diindolylmethane attenuates LPS-mediated acute liver failure
by regulating miRNAs to target IRAK4 and suppress Toll-like
receptor signalling. Br J Pharmacol. 172:2133–2147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho HJ, Seon MR, Lee YM, Kim J, Kim JK,
Kim SG and Park JH: 3,3′-Diindolylmethane suppresses the
inflammatory response to lipopolysaccharide in murine macrophages.
J Nutr. 138:17–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Busbee PB, Nagarkatti M and Nagarkatti PS:
Natural indoles, indole-3-carbinol (I3C) and 3,3′-diindolylmethane
(DIM), attenuate staphylococcal enterotoxin B-mediated liver injury
by downregulating miR-31 expression and promoting
caspase-2-mediated apoptosis. PLoS One. 10:e01185062015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Wu Q, Deng W and Tang Q: GW26-e4573
Anti-inflammatory effect of 3,3′-Diindolylmethane on LPS-induced
inflammatory injury in neonatal rat cardiac myocytes via
suppressing TLR-4/MAPKs signaling pathways. J Am College Cardiol.
66:C592015. View Article : Google Scholar
|
|
18
|
Kim HW, Kim J, Kim J, Lee S, Choi BR, Han
JS, Lee KW and Lee HJ: 3,3′-Diindolylmethane inhibits
lipopolysaccharide-induced microglial hyperactivation and
attenuates brain inflammation. Toxicol Sci. 137:158–167. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hajra S, Basu A, Singha Roy S, Patra AR
and Bhattacharya S: Attenuation of doxorubicin-induced
cardiotoxicity and genotoxicity by an indole-based natural compound
3,3′-diindolylmethane (DIM) through activation of Nrf2/ARE
signaling pathways and inhibiting apoptosis. Free Radic Res.
51:812–827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tilyek A, Chai C, Hou X, Zhou B, Zhang C,
Cao Z and Yu B: The protective effects of Ribes diacanthum Pall on
cisplatin-induced nephrotoxicity in mice. J Ethnopharmacol.
178:297–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zarjou A and Agarwal A: Sepsis and acute
kidney injury. J Am Soc Nephrol. 22:999–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cantaluppi V, Quercia AD, Dellepiane S,
Figliolini F, Medica D and De Lena M: New mechanisms and recent
insights in the pathogenesis of acute kidney injury (AKI). G Ital
Nefrol. 29:535–547. 2012.(In Italian). PubMed/NCBI
|
|
23
|
Langenberg C, Bagshaw SM, May CN and
Bellomo R: The histopathology of septic acute kidney injury: A
systematic review. Crit Care. 12:R382008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lerolle N, Nochy D, Guérot E, Bruneval P,
Fagon JY, Diehl JL and Hill G: Histopathology of septic shock
induced acute kidney injury: Apoptosis and leukocytic infiltration.
Intensive Care Med. 36:471–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bae EH, Kim IJ, Choi HS, Kim HY, Kim CS,
Ma SK, Kim IS and Kim SW: Tumor necrosis factor α-converting enzyme
inhibitor attenuates lipopolysaccharide-induced reactive oxygen
species and mitogen-activated protein kinase expression in human
renal proximal tubule epithelial cells. Korean J Physiol Pharmacol.
22:135–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants, and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J, Kim HY and Lee SM: Protective
effects of geniposide and genipin against hepatic
ischemia/reperfusion injury in mice. Biomol Ther (Seoul).
21:132–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiose A, Kuroda J, Tsuruya K, Hirai M,
Hirakata H, Naito S, Hattori M, Sakaki Y and Sumimoto H: A novel
superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem.
276:1417–1423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao L, Wu WF, Dong L, Ren GL, Li HD, Yang
Q, Li XF, Xu T, Li Z, Wu BM, et al: Protocatechuic aldehyde
attenuates cisplatin-induced acute kidney injury by suppressing
nox-mediated oxidative stress and renal inflammation. Front
Pharmacol. 7:4792016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|