Introduction
Podocytes are terminally differentiated glomerular
cells with elaborate extensions of the cell body and play an
important role in maintaining the structural and functional
integrity of the filtration barrier (1). Studies have indicated that podocytes
damage and the reduction in podocyte density are the main
contributors to the development of proteinuria and
glomerulosclerosis (2). Thus,
podocytes serve as vital role in preventing proteinuria and
glomerulosclerosis from getting worse.
Long non-coding RNAs (lncRNAs), a heterogeneous
class of long (>200 nucleotides) 5 transcripts, is characterized
by an apparent lack of protein-coding potential (3). lncRNAs have attracted much attention
of scientists for its diagnostic, prognostic, and predictive
potential (4). lncRNA
taurine-upregulated gene 1 (TUG1) was firstly identified in a
genomic screen as a part of photoreceptors and overexpressed in
response to retinal development in mouse retinal cells (5). Researchers have indicated that TUG1
could be a predictive biomarkers of kidney neoplasms. Besides,
accumulated studies have demonstrated that TUG1 contributed to the
development of chronic kidney diseases (6). However, the underlying molecular
mechanisms are not fully understood.
Researches have shown that TUG1 has moderation
effects on various diseases via interacting with microRNAs (miRNAs
or miRs), including miR-204-5p (7), miR-144-3p (8), miR-382 (9) and so on. Studies over the last
decades have suggested that miRNAs served as pathogenic or
therapeutic factor to be involved in the development of a variety
of renal diseases, such as chronic kidney disease (10), kidney fibrosis (11), type 2 diabetic kidney disease
(12) and glucose-induced podocyte
apoptosis (13). According to
miRDB, at least two binding sites were identified between miR-197
and TUG1. Besides, TargetScan and miRwalk have predicted the high
binding possibility between MAPK1 and miR-197. As we all know, MAPK
pathway could induce cell autophagy in various types of cells
(14), and autophagy played a
positive role in defending podocyte against lipopolysaccharide
(LPS)-induced damage (15). Thus,
miR-197 may be a potential biomarker and a regulation factor in the
treatment of podocyte injury.
In this study, we investigated the underlying
mechanisms of TUG1 in treating LPS-induced podocytes injury. Our
results strongly demonstrated that TUG1 could protect podocyte
against LPS-induced injury by targeting miR-197 through activating
MAPK pathway.
Materials and methods
Cell culture
The mouse immortalized podocyte cell line MPC5 was
purchased from National Infrastructure of Cell Line Resource. Cells
were cultivated at 33°C with RPMI-1640 containing 10% FBS and
recombinant mouse interferon-γ (50 U/ml, Toyobo Co., Osaka, Japan)
to propagate cells. After reaching confluence, cells were incubated
without interferon-γ to induce cell differentiation into the
podocyte lineage at 37°C for more than 6 days. The culture medium
was changed every three days. Then, cells were divided into
different groups.
Cell transfection
SB203580 was purchased from Calbiochem (San Diego,
CA, USA) and dissolved in dimethyl sulfoxide (DMSO). PcDNA-TUG1,
pcDNA vector, miR-197 mimic and miR-197 inhibitor were purchased
from Addgene (Cambridge, MA, USA). MPC5 cells were transfected with
pcDNA-TUG1 (40 µg/ml), pcDNA vector (40 µg/ml), miR-197 mimic (80
nM) or miR-197 inhibitor (160 nM) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to manufacturers instruction. 48 h after transfection,
transfected MPC5 podocytes and normal MPC5 podocytes were treated
with lipopolysaccharide (LPS; 1 µg/ml) or the combination of LPS (1
µg/ml) and SB203580 (10 µM) for 24 h for further experiments.
Western blot assay
The total protein was isolated from MPC5 cells in
different groups, and separated by SDS-PAGE and transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). After blocking with 5% BSA and washing with 0.05%
Tween-20, membranes were incubated with primary antibodies
[Podocin, Nephrin, CCAAT/enhancer-binding protein (CHOP), p-MAPK,
MAPK, Beclin1, p62, light chain (LC)3 and β-actin] (Abcam,
Cambridge, UK) at 4°C overnight. Then, all membranes were
incubation with horseradish peroxidase (HRP)-conjugated secondary
antibodies for 1.5 h at room temperature. The bands were analyzed
using ImageJ [National Institutes of Health (NIH), Sacaton, AZ,
USA].
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNAs were extracted from MPC5 podocytes in
different groups using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
RT-qPCR was conducted using Applied Biosystems StepOnePlus™ system.
The RT-PCR primers for Podocin, Nephrin, CHOP, TUG1 and miR-197
purchased from GeneCopoeia Inc. (Rockville, MD, USA). The specific
primers were as follows: Podocin (forward:
5′-GCATCAAGCCCTCTGGATTAG-3′ and reverse:
3′-AGACGGAGATCAACCTTGTGATA-5′); Nephrin (forward:
5′-ATGGGAGCTAAGGAAGCCACA-3′ and reverse:
3′-CCACACCACAGCTTAACTGTC-5′), CHOP (forward:
5′-TTGCCCTCTTATTGGTCCAGC-3′ and reverse:
3′-TAGCGACTGTTCTGTTCCCAC-5′). GAPDH was used as the internal
control. The relative levels were measured using the
2−ΔΔCq analysis method (16).
Albumin influx assay
Filtration barrier function of podocyte monolayers
was assessed using albumin influx assay. Briefly, 5×103
podocytes were seeded into a 3 µm pores trans-well filter coated
with collagen (Corning Incorporated, Corning, NY, USA), then
incubated for 10 days. After that, MPC5 podocytes were
serum-starved overnight and washed with PBS containing 1 mmol/l
MgCl2 and 1 mmol/l CaCl2. Then, top chamber
was filled with 0.15 ml RPMI-1640 and the bottom chamber with 1 ml
RPMI-1640 containing 40 mg/ml of bovine serum albumin at 37°C with
5% CO2. Cells were cultured for 24 h. Albumin
concentration was measured using an ELISA kit (Thermo Fisher)
according to the manufacturer's instructions.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze all the data. The data are presented as the mean ±
standard deviation. Analysis was performed using one-way analysis
of variance followed by a Bonferroni post hoc test. Statistical
significance was assigned at P<0.05.
Results
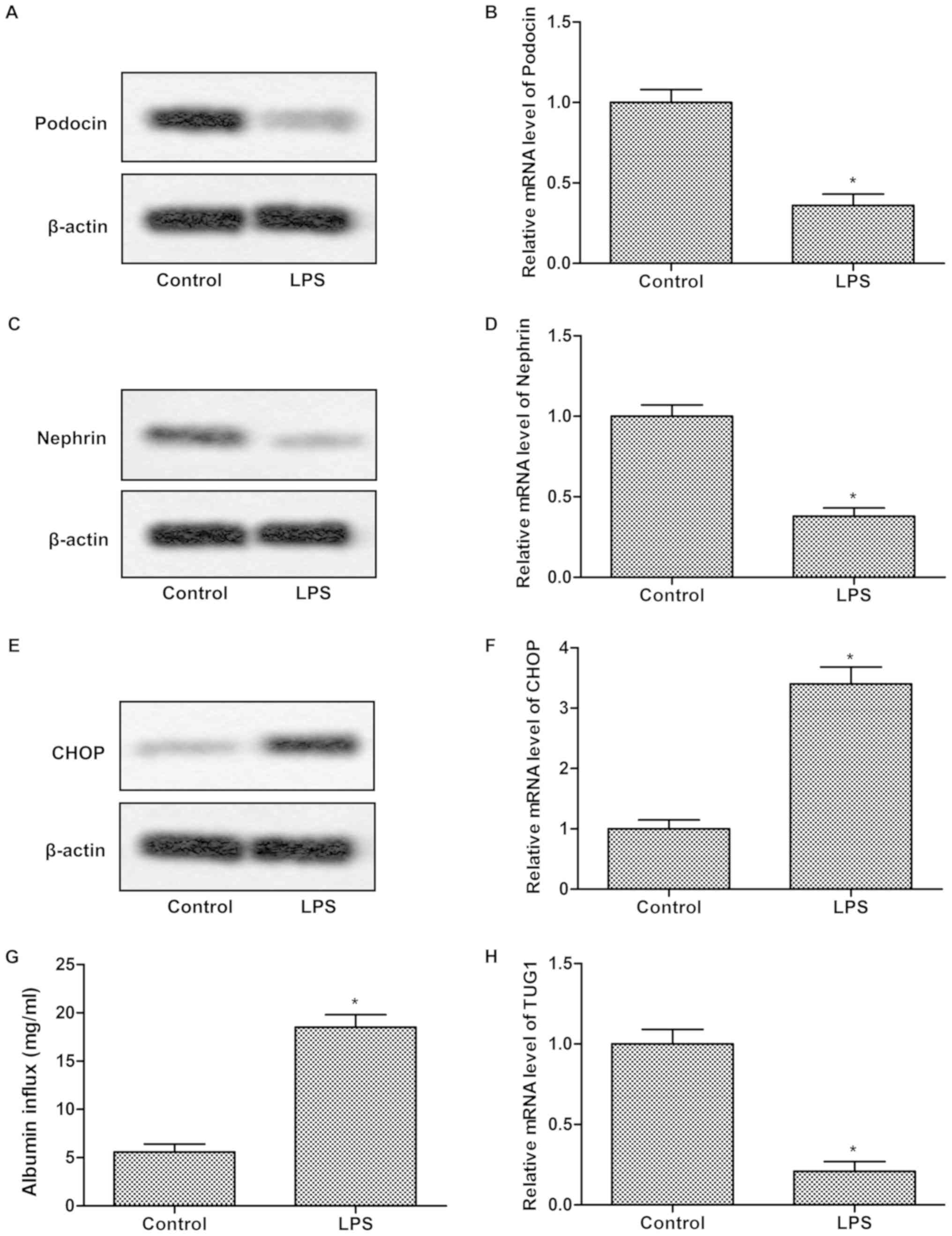
TUG1 was downregulated in LPS impaired
podocyte
To investigate whether TUG1 expression was related
to LPS-induced damage, MPC5 podocytes were treated with LPS (1
µg/ml) for 24 h. Results indicated that the protein expressions of
Podocin and Nephrin were remarkably suppressed while CHOP
significantly elevated in LPS group compared with control group
(Fig. 1A, C and E). The mRNA
expression was consistent with protein expression (Fig. 1B, D and F). LPS treatment result in
a greater albumin influx across the podocyte monolayer compared
with control (Fig. 1G). These
results suggested that the function of MPC5 podocytes was impaired
by LPS. Besides that, the relative mRNA expression of TUG1 was
dramatically inhibited in LPS group in comparison with control
group (Fig. 1H), indicating that
TUG1 was downregulated in LPS damaged podocyte.
Overexpression of TUG1 alleviates
LPS-induced podocyte injury
To explore whether TUG1 could attenuate the cell
damage induced by LPS, MPC5 podocytes were transfected with
pcDNA-TUG1. As shown in Fig. 2A,
the relative expression of TUG1 was largely enhanced in TUG1 group,
indicating the high transfection efficiency. The reduced Podocin
and Nephrin expression along with the increased CHOP expression
induced by LPS compared with control were significantly inhibited
by TUG1 transfection (Fig. 2B-E).
Moreover, the elevated albumin influx caused by LPS compared with
control was remarkably repressed by TUG1 overexpression (Fig. 2F). These results suggested that
LPS-induced podocyte injury could be alleviated by TUG1
overexpression.
 | Figure 2.Overexpression of TUG1 alleviates
LPS-induced podocyte injury. (A) Podocytes were randomly divided
into 3 groups: Control group, cells without treatment; the mock
group, cells transfected with pcDNA vector; and cells transfected
with pcDNA-TUG1. The relative mRNA expression of TUG1 was assessed
using reverse transcription-quantitative polymerase chain reaction.
Podocytes were then randomly divided into 4 groups: Control group,
cells without treatment; LPS group, cells induced by LPS; LPS+mock
group, cells transfected with pcDNA vector that were induced by
LPS; and LPS+TUG1 group, cells transfected with pcDNA-TUG1 that
were induced by LPS. (B) The protein expressions of Nephrin,
Podocin and CHOP were measured using western blot analysis. Data
summary and analysis of the expression of (C) Nephrin, (D) Podocin
and (E) CHOP in podocyte cells according to the results of western
blotting. (F) Albumin influx assay was used to evaluate the
glomerular filtration function. The experiments were repeated at
least 3 times, and data is presented as the mean ± standard
deviation. *P<0.05 vs. control group; #P<0.05 vs.
TUG1, taurine-upregulated gene 1; LPS group. LPS,
lipopolysaccharide; CHOP, CCAAT/enhancer-binding protein. |
miR-197 is a direct target of
TUG1
The targeting relationship between miR-197 and MAPK1
was predicted by bioinformatics (Fig.
3A). To further evaluate the potential relationship between
miR-197 and TUG1, RT-qPCR and western blotting were conducted in
podocytes transfected with pcDNA-TUG1/pcDNA-vector/miR-197
mimic/miR-197 inhibitor. As illustrated in Fig. 3B, the expression of miR-197 was
significantly increased in LPS group compared with control group.
The elevated level of miR-197 in cells treated with LPS was
inhibited by transfection with TUG1 (Fig. 3C). Besides that, the reduced
Nephrin and Podocin expression along with the enhanced CHOP
expression were slightly enhanced by TUG1 mimic transfection while
remarkably suppressed by TUG1 inhibitor transfection (Fig. 3D-G). Moreover, the increased
albumin influx induced by LPS was slightly enhanced by TUG1 mimic
transfection while remarkably inhibited by TUG1 inhibitor
transfection (Fig. 3H). These
results indicated that miR-197 is a potential target of TUG1.
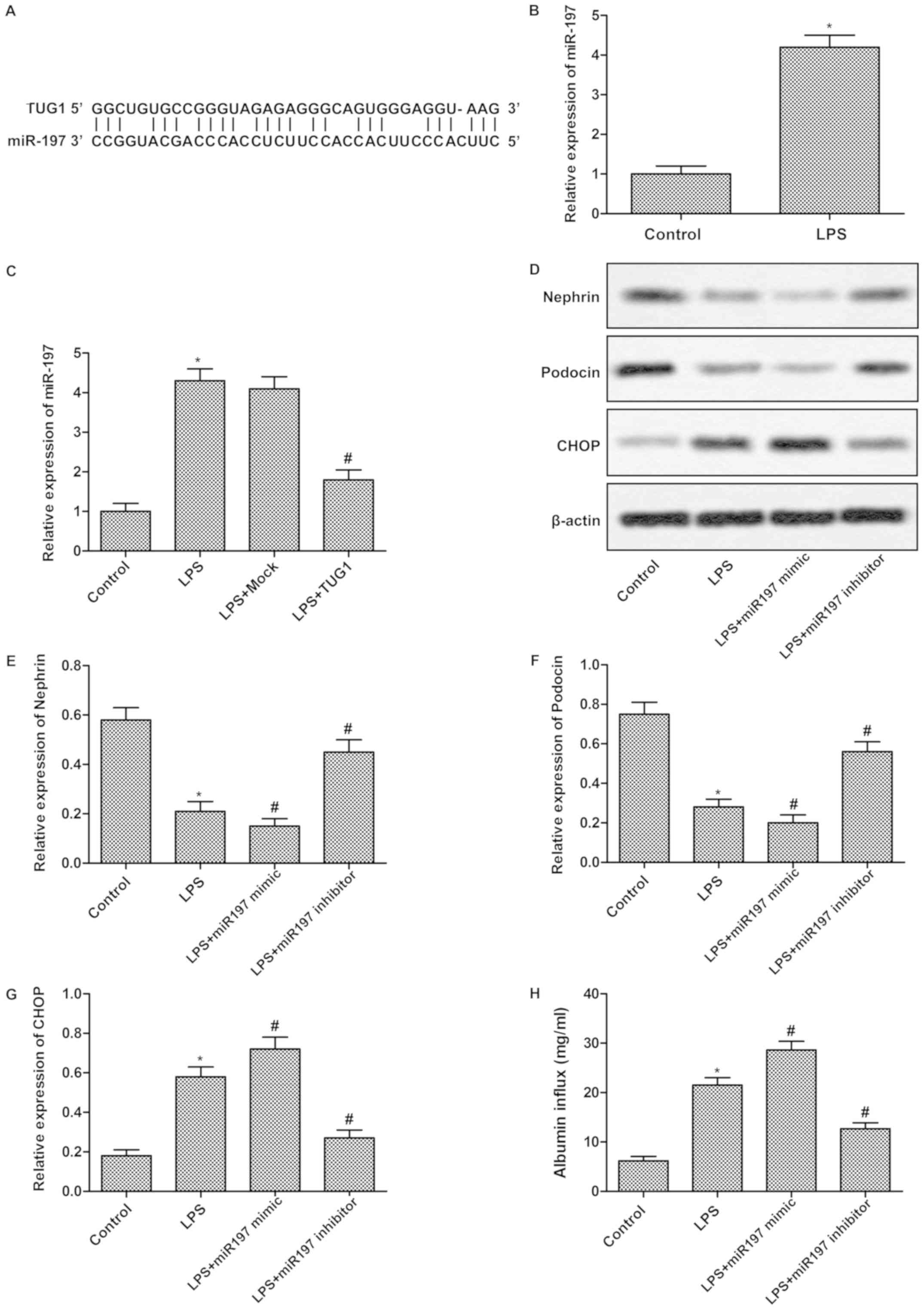 | Figure 3.miR-197 is a direct target of TUG1.
(A) The targeting association between miR-197 and TUG1 was
predicted through bioinformatics. (B) The expression of miR-197 in
podocytes treated with or without LPS was measured using RT-qPCR.
(C) Podocytes were randomly divided into 4 groups: Control group,
cells without treatment; LPS group, cells induced by LPS; LPS+mock
group, cells transfected with pcDNA vector that were induced by
LPS; and LPS+TUG1 group, cells transfected with pcDNA-TUG1 that
were induced by LPS. The relative miR-197 expression was detected
using RT-qPCR. (D) Podocytes were also randomly divided into 4
groups: Control group, cells without treatment; LPS group, cells
induced by LPS; LPS+mimic group, cells transfected with miR-197
mimic that were induced by LPS; and LPS+inhibitor group, cells
transfected with miR-197 inhibitor that were induced by LPS. The
protein expressions of Nephrin, Podocin and CHOP were detected by
western blot analysis. Data summary and analysis of the expression
of (E) Nephrin, (F) Podocin and (G) CHOP in podocytes cells
according to the results of western blotting. (H) Albumin influx
assay was applied to assess the glomerular filtration function. The
experiments were repeated at least 3 times, and data is presented
as the mean ± standard deviation. *P<0.05 vs. control group;
#P<0.05 vs. LPS group. TUG1, taurine-upregulated gene
1; LPS, lipopolysaccharide; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CHOP,
CCAAT/enhancer-binding protein; miR, microRNA. |
lncRNA TUG1 aggravates podocytes
damage via regulating MAPK pathway
The target sequences of miR-197 in the 3′-UTR region
of MAPK1 was predicted through bioinformatics analysis (Fig. 4A). To detect the regulatory
relationship between TUG1 and MAPK1, MPC5 podocytes were
transfected with pcDNA-TUG1/pcDNA-vector. As illustrated in
Fig. 4B, the decreased expression
of p-MAPK/MAPK induced by LPS compared with control was strongly
inhibited by TUG1 transfection. Besides, the decreased Beclin1, LC3
II/LC3 I along with upregulated p62 levels were vastly suppressed
by TUG1 transfection, indicating TUG1 could regulate cell autophagy
via regulating MAPK pathway (Fig.
4C). To further validate this result, SB203580 was added into
cells to inactivate MAPK pathway. Results indicated that SB203580
could reverse all the changes in the expressions of Beclin1, LC3
II/LC3 I and p62 caused by TUG1 (Fig.
4D). These results suggested that lncRNA TUG1 could aggravate
podocytes damage via regulating MAPK pathway indirectly.
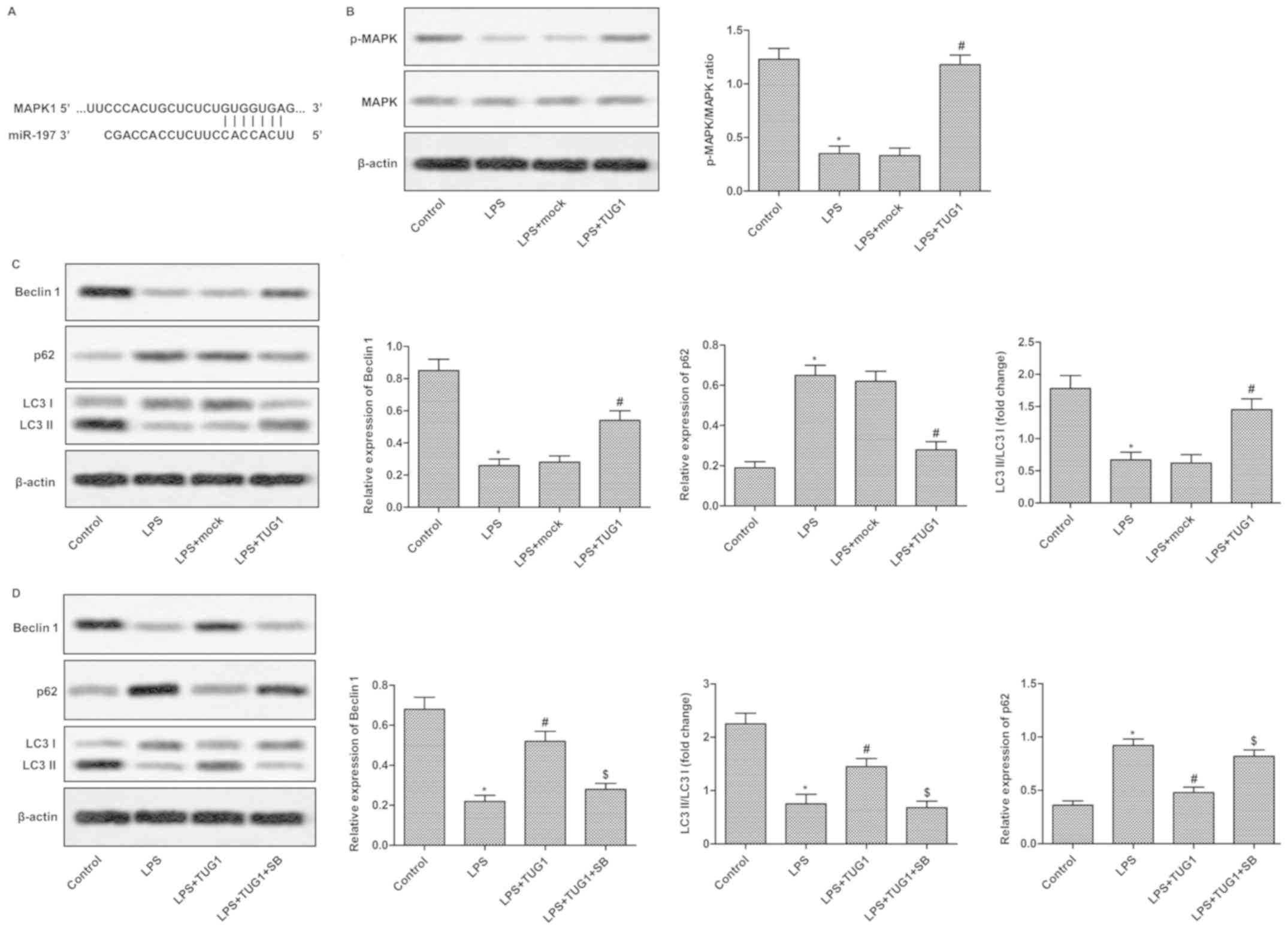 | Figure 4.miR-197 aggravates podocyte damage by
regulating the MAPK signaling pathway. (A) The targeting
association between miR-197 and MAPK1 was predicted by
bioinformatics. (B and C) Podocytes were randomly divided into 4
groups: Control group, cells without treatment; LPS group, cells
induced by LPS; LPS+mock group, cells transfected with pcDNA vector
that were induced by LPS; and LPS+TUG1 group, cells transfected
with pcDNA-TUG1 that were induced by LPS. (B) The expression of
p-MAPK/MAPK was measured using western blotting. (C) The levels of
Beclin1, p62 and LC3 I/LC3 II were evaluated using western
blotting. (D) Podocytes were randomly divided into 4 groups:
Control group, cells without treatment; LPS group, cells induced by
LPS; LPS+TUG1 group, cells transfected with pcDNA-TUG1 that were
induced by LPS; and LPS+TUG1+SB group, cells transfected with
pcDNA-TUG1 were treated with LPS and SB. The expression level of
Beclin1, p62 and LC3 I/LC3 II were detected using western blot
analysis. The experiments were repeated at least 3 times, and data
is presented as the mean ± standard deviation. *P<0.05 vs.
control group; #P<0.05 vs. LPS group;
$P<0.05 vs. LPS+TUG1 group. MAPK, mitogen-activated
protein kinase; LPS, lipopolysaccharide; TUG1, taurine-upregulated
gene 1; LC3, light chain 3; p-, phosphorylated; SB, SB203580
(inhibitor). |
Discussion
The podocyte is a kind of special glomerular
epithelial cell and key constituent of the layer of the filtration
barrier in the kidney (17).
Intact kidney filtration barrier maintains the balance of proteins
in the blood (18). If the
podocytes are impaired, plasma albumin can leak into the urinary
space, leading to the deterioration of kidney diseases and
hypoalbuminemia. If the albuminuria persist and increase in
patients, it will develop into systemic diseases, such as anemia,
postural hypotension, bradyarrhythmia. Thus, treatment for podocyte
damage is extremely important to the prevention of various renal
diseases and related systemic diseases.
It has been suggested and supported by researchers
that lncRNAs have extensive biological functions. Abnormal
expression of lncRNAs is linked to a variety of diseases, such as
cancer as well as neurological, cardiac and renal diseases
(19,20). Among these lncRNAs, Long and
colleagues observed that the expression of TUG1 was remarkably
inhibited in the podocytes of diabetic mice. Podocyte-specific
overexpression of TUG1 could elevate PGC-1α expression, resulting
in the improvement of mitochondrial bioenergetics (21). Similarly, in our research,
overexpression of TUG1 could protect podocyte from LPS-induced
damage, evidences were the reduced Nephrin, Podocin expression,
elevated CHOP expressions along with the enhanced albumin influx
were vastly inhibited by TUG1 transfection. However, how did TUG1
worked need further exploration.
Previous studies have shown that TUG1 acted as a
regulation role in a variety of diseases through interacting with
miRNAs. miRNAs, existed in eukaryotic cells, could regulate various
physiological biological metabolism through combining with 3′-UTR
of target genes mRNA, thereby promoting mRNA cleavage or
suppressing the translation of mRNA (22). More and more researchers have
demonstrated that many miRNAs were involved in the development of
multiple kidney diseases. For example, miR-21 acts as an oncogene
in renal cancer via inducing cell proliferation and invasion
(23). miR-214, −132, −21 and −15b
have been testified to participate in the renal fibrosis process
(10). It is predicted by
TargetScan and miRwalk that miR-197 has a close relationship to
TUG1, at least two binding sites were found between them. Our
research further provided evidences that miR-197 may be a target of
TUG1 through detecting the miR-197 content in podocytes transfected
with TUG1. In addition to this, the decreased Nephrin, Podocin
expression, upregulated CHOP expressions along with the increased
albumin influx induced by LPS were slightly enhanced by miR-197
mimic transfection while significantly suppressed by miR-197
inhibitor transfection in podocytes.
Apoptotic cell death is a genetically programmed
mechanism that allows the cell to commit suicide (24), and plays a vital role in the
development of multiple organ dysfunction in LPS-induced sepsis
(25). miRNAs serve as the useful
laboratory markers of organ failure during sepsis and have various
effects on the processes of pro-apoptosis or anti-apoptosis
(26,27). According to the previous report,
miR-197 induced apoptosis and suppressed tumorigenicity in multiple
myeloma xenograft models (28).
Report also indicate that the level of miR-197 was elevated in
severe acute viral hepatitis with coagulopathy (29). Thus, cell apoptosis regulated by
miR-197 may be involved in the pathological process of LPS-induced
podocytes injury. Autophagy serves a vital role in keeping the
balance between anabolism and catabolism, maintaining protein
quality control and removing impaired proteins, organelles and
lipids (30). The results obtained
from the current studies have demonstrated that the dysfunction of
podocyte autophagy taken responsibility for the development of
various kidney diseases, including diabetic nephropathy (31), immunoglobulin A Nephropathy
(32) and proteinuric kidney
disease (33). Besides, Tan et
al (15) pointed out that the
podocytes autophagy was remarkably suppressed by LPS, which result
in LPS-induced injury of podocytes. The inducing autophagy ability
of MAPK pathway was demonstrated by many researchers in multiple
diseases (34,35). Thus, MAPK pathway may be a major
target in treating LPS-induced podocytes injury. According to the
prediction of TargetScan and miRwalk, miR-197 may inactivate MAPK
pathway via binding to MAPK1, and then restrain the autophagy of
podocyte. Considering that miR-197 is a target of TUG1, TUG1 might
induce the autophagy of podocytes via motivating MAPK pathway
indirectly. Our study demonstrated that TUG1 transfection could
remarkably inhibit the increased p-MAPK/MAPK level in LPS-induced
podocytes. Besides that, the reduced Beclin1, p62 and LC3 II/I and
increased p62 expression caused by LPS were remarkably inhibited by
TUG1 transfection. Moreover, the p38 MAPK inhibitor SB203580 could
reverse all the changes caused by TUG1 in LPS-induced
podocytes.
All in all, our study indicated that TUG1 level was
downregulated in podocytes impaired by LPS. Elevated TUG1 by
transfection suppressed the LPS-induced alteration of podocyte
injury related proteins (Nephrin, Podocin and CHOP) expression and
elevated albumin influx. Further research demonstrated that miR-197
was a direct target of TUG1. TUG1 was confirmed to restore the
function of podocytes via sponging miR-197 to enhance the level of
p-MAPK/MAPK, thereby inducing autophagy. In summary, the
TUG1-miR-197-MAPK pathway may act as a potential new target for
LPS-induced podocytes injury therapy.
Acknowledgements
The authors would like to thank Dr Dong Zhao (Jining
No. 1 People's Hospital, Jining, China) and Dr Heng Zhang (The
Third Military Medical university, Chongqing, China), for providing
helpful discussions and technical support concerning the present
study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ analyzed and interpreted the data regarding cell
culture, transfection and western blot analysis, and conducted the
statistical analysis. ZL was involved in RT-qPCR analysis. HZ was
responsible for the Albumin influx assay, as well as the study
design and drafting of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MAPK
|
mitogen-activated protein kinase
|
|
LPS
|
lipopolysaccharide
|
|
lncRNAs
|
long non-coding RNAs
|
|
TUG1
|
taurine-upregulated gene 1
|
|
miRNA/miR
|
microRNA
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Kriz W, Gretz N and Lemley KV: Progression
of glomerular diseases: Is the podocyte the culprit? Kidney Int.
54:687–697. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ijpelaar DH, Schulz A, Koop K, Schlesener
M, Bruijn JA, Kerjaschki D, Kreutz R and de Heer E: Glomerular
hypertrophy precedes albuminuria and segmental loss of podoplanin
in podocytes in Munich-Wistar-Fromter rats. Am J Physiol Renal
Physiol. 294:F758–F767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young TL, Matsuda T and Cepko CL: The
noncoding RNA taurine upregulated gene 1 is required for
differentiation of the murine retina. Curr Biol. 15:501–512. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li SY and Susztak K: The long noncoding
RNA Tug1 connects metabolic changes with kidney disease in
podocytes. J Clin Invest. 126:4072–4075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu C, Li L, Xie F, Guo S, Liu F, Dong N
and Wang Y: lncRNA TUG1 sponges miR-204-5p to promote osteoblast
differentiation through upregulating Runx2 in aortic valve
calcification. Cardiovasc Res. 114:168–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao J, Han X, Qi X, Jin X and Li X: TUG1
promotes osteosarcoma tumorigenesis by upregulating EZH2 expression
via miR-144-3p. Int J Oncol. 51:1115–1123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao L, Sun H, Kong H, Chen Z, Chen B and
Zhou M: The Lncrna-TUG1/EZH2 axis promotes pancreatic cancer cell
proliferation, migration and EMT phenotype formation through
sponging Mir-382. Cell Physiol Biochem. 42:2145–2158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv W, Fan F, Wang Y, Gonzalez-Fernandez E,
Wang C, Yang L, Booz GW and Roman RJ: Therapeutic potential of
microRNAs for the treatment of renal fibrosis and CKD. Physiol
Genomics. 50:20–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park EJ, Jung HJ, Choi HJ, Cho JI, Park HJ
and Kwon TH: miR-34c-5p and CaMKII are involved in
aldosterone-induced fibrosis in kidney collecting duct cells. Am J
Physiol Renal Physiol. 314:F329–F342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie Y, Jia Y, Cuihua X, Hu F, Xue M and
Xue Y: Urinary exosomal MicroRNA profiling in incipient type 2
diabetic kidney disease. J Diabetes Res. 2017:69789842017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Lin B, Nie L and Li P:
microRNA-20b contributes to high glucose-induced podocyte apoptosis
by targeting SIRT7. Mol Med Rep. 16:5667–5674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie H, Li J, Gao H, Wang J, Li C, Xu Y and
Liu C: Total flavone of desmodium styracifolium relieved apoptosis
and autophagy of COM-induced HK-2 cells by regulating KIM-1 via
p38/MAPK pathway. Mol Cell Biochem. 442:169–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan X, Chen Y, Liang X, Yu C, Lai Y, Zhang
L, Zhao X, Zhang H, Lin T, Li R and Shi W:
Lipopolysaccharide-induced podocyte injury is mediated by
suppression of autophagy. Mol Med Rep. 14:811–818. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo B, Lyu Q, Slivano OJ, Dirkx R,
Christie CK, Czyzyk J, Hezel AF, Gharavi AG, Small EM and Miano JM:
Serum response factor is essential for maintenance of podocyte
structure and function. J Am Soc Nephrol. 29:416–422. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan Y, Nakano D, Zhang Y, Li L, Liu W,
Nishida M, Kuwabara T, Morishita A, Hitomi H, Mori K, et al: A
protease-activated receptor-1 antagonist protects against podocyte
injury in a mouse model of nephropathy. J Pharmacol Sci. (pii):
S1347 8613. 30128–30137. 2017.
|
|
19
|
Feng M, Tang PM, Huang XR, Sun SF, You YK,
Xiao J, Lv LL, Xu AP and Lan HY: TGF-β mediates renal fibrosis via
the Smad3-Erbb4-IR long noncoding RNA axis. Mol Ther. 26:148–161.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang HW, Xie H, Ma X, Zhao F and Gao Y:
Upregulation of lncRNA PANDAR predicts poor prognosis and promotes
cell proliferation in cervical cancer. Eur Rev Med Pharmacol Sci.
21:4529–4535. 2017.PubMed/NCBI
|
|
21
|
Long J, Badal SS, Ye Z, Wang Y, Ayanga BA,
Galvan DL, Green NH, Chang BH, Overbeek PA and Danesh FR: Long
noncoding RNA Tug1 regulates mitochondrial bioenergetics in
diabetic nephropathy. J Clin Invest. 126:4205–4218. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tana C, Giamberardino MA and Cipollone F:
microRNA profiling in atherosclerosis, diabetes, and migraine. Ann
Med. 49:93–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Gu Y and Shen W: MicroRNA-21
functions as an oncogene and promotes cell proliferation and
invasion via TIMP3 in renal cancer. Eur Rev Med Pharmacol Sci.
21:4566–4576. 2017.PubMed/NCBI
|
|
24
|
Fulda S, Gorman AM, Hori O and Samali A:
Cellular stress responses: Cell survival and cell death. Int J Cell
Biol. 2010:2140742010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei WY, Ma ZG, Zhang N, Xu SC, Yuan YP,
Zeng XF and Tang QZ: Overexpression of CTRP3 protects against
sepsis-induced myocardial dysfunction in mice. Mol Cell Endocrinol.
476:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Liu C, Wang Z, Huang J and Zeng Q:
microRNA-23a-5p acts as a potential biomarker for sepsis-induced
acute respiratory distress syndrome in early stage. Cell Mol Biol
(Noisy-le-grand). 62:31–37. 2016.PubMed/NCBI
|
|
27
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Li F, Saha MN, Abdi J, Qiu L and
Chang H: miR-137 and miR-197 induce apoptosis and suppress
tumorigenicity by targeting MCL-1 in multiple myeloma. Clin Cancer
Res. 21:2399–2411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weseslindtner L, Macheleidt I, Eischeid H,
Strassl R, Hofer H, Popow-Kraupp T, Dienes HP, Holzmann H and
Odenthal M: Micro RNAs mir-106a, mir-122 and mir-197 are increased
in severe acute viral hepatitis with coagulopathy. Liver Int.
36:353–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Li QX, Wang XJ, Zhang C, Duan YQ,
Wang ZY, Zhang Y, Yu X, Li NJ, Sun JP and Yi F: β-Arrestins promote
podocyte injury by inhibition of autophagy in diabetic nephropathy.
Cell Death Dis. 7:e21832016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang S, Jin J, Lin B, Gong J, Li Y and He
Q: Rapamycin induces autophagy and reduces the apoptosis of
podocytes under a stimulated condition of immunoglobulin a
nephropathy. Kidney Blood Press Res. 42:177–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L and Liu Y: Wnt/β-catenin signalling
and podocyte dysfunction in proteinuric kidney disease. Nat Rev
Nephrol. 11:535–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu S, Niu P, Chen K, Xia Y, Yu Q, Liu N,
Li J, Li S, Wu L, Feng J, et al: The liver protection of propylene
glycol alginate sodium sulfate preconditioning against ischemia
reperfusion injury: Focusing MAPK pathway activity. Sci Rep.
7:151752017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Niu W, He Z, Zhang Q, Wu Y, Jiang
C, Tang C, Hu Y and Jia J: Autophagy suppression by exercise
pretreatment and p38 inhibition is neuroprotective in cerebral
ischemia. Brain Res. 1587:127–132. 2014. View Article : Google Scholar : PubMed/NCBI
|