Fibrosis is defined by the overgrowth, hardening
and/or scarring of various tissues, and is responsible for the
excessive deposition of extracellular matrix (ECM) proteins,
including collagen (Col), fibronectin (FN) and hyaluronan, in the
surrounding tissues (1,2). Fibrosis affects a number of different
organ systems and is the hallmark of fibroproliferative diseases
(FPDs) such as systemic sclerosis (SSc), liver cirrhosis, kidney
fibrosis and interstitial lung disease (2). Given that various diseases are
associated with the same fibrotic changes in different organ
systems, the involvement of common pathogenic pathways may be
speculated (3). Furthermore, it is
well documented that fibrosis may cause progressive organ
dysfunction and lead to significant morbidity and mortality
(2–6), highlighting the requirement for new
therapeutic targets. However in the past decade, the results of
several promising early-phase studies of anti-fibrotic therapies
have not been confirmed in large randomised clinical trials, in
terms of both expected efficacy and unexpected side-effects; this
has revealed a knowledge gap, particularly in the field of
immune-associated tissue fibrosis (3).
CD248 (also known as endosialin and TEM-1) is
considered to be a specific marker of fibrosis. It is localized to
fibroblasts and pericytes in FPDs and cancer, serving a pivotal
role in tissue remodelling and repair (20,21).
Given its high expression level in different types of tumours,
numerous studies have evaluated its role in angiogenesis despite
the hypothesis that CD248, by interaction with the ECM, may enhance
the invasiveness of cancer cells (22–24).
The role of CD248 has also been investigated in FPDs; a number of
in vitro studies have illustrated the interplay between
CD248 and several profibrotic molecules, including TGFβ,
platelet-derived growth factor subunit B (PDGF-BB) and ECM
proteins, which were the basis for further in vivo
investigations of fibrosis (25–28).
Additionally, CD248 was over-expressed in the lungs of patients
with pulmonary (29) and hepatic
fibrosis (30,31); in this context, the genetic loss of
CD248 significantly reduced both microvascular rarefaction and
fibrosis via the modulation of pericyte and stromal cell function
in experimental models of kidney (32) and hepatic fibrosis (30,31,33).
Therefore, it has been suggested that CD248 over-expression may
serve an important role in fibrosis, and that it may be considered
as a possible therapeutic target (21,32–34).
In the present review, current understanding of the
structure and function of CD248 has been described, and the role of
CD248-associated pathological angiogenesis assessed. Additionally,
the role of CD248 in the pathogenesis of FPDs has been highlighted,
indicating that CD248 inhibition may potentially modulate both
microvascular alterations and myofibroblast generation during
tissue fibrosis.
CD248 is a heavily glycosylated, single-pass
transmembrane protein that was initially identified as an
overexpressed cell surface marker in the cancer vasculature
(35,36). It has subsequently been revealed
that CD248 is expressed by pericytes and not by the underlying ECs
(37). CD248 is regarded as a
mesenchymal marker involved in the regulation of cellular
proliferation (33,37), and is expressed on mesenchymal stem
cells (MSCs), fibroblasts, pericytes, smooth muscle cells and
osteoblasts (21,26,38).
The 2,274 bp human CD248 gene does not contain introns and is
localized to the long arm of chromosome 11 (11q13); it has a 2,274
bp-long open reading frame which encodes a 757 amino acid type I
transmembrane protein (39). CD248
is also a C-type lectin-like protein with a signal leader peptide,
5 globular extracellular domains consisting of a C-lectin domain,
one domain similar to the Sushi/ccp/scr pattern and three EGF
repeats, followed by a mucin-like region, a transmembrane segment
and a short cytoplasmic tail (40). Its extracellular N-terminal domain
(360 amino acids in length) shares structural and sequence homology
with thrombomodulin (CD141) and C1qRp (CD93) proteins (39,41,42).
The cytoplasmic tail of CD248 contains a putative
PSD-95/Discs-large/ZO-1 (PDZ) binding domain (42,43),
which is involved in protein-protein interactions and acts as an
adaptor molecule, holding receptor and signalling molecule in large
protein complexes (40). Notably,
the cytoplasmatic domain of CD248 is highly conserved among
different vertebrate species (Fig.
1). Structural homologies to receptor proteins suggest that
CD248 may be a cell surface receptor (44), and previous molecular and cellular
studies have demonstrated that CD248 selectively binds the ECM
proteins FN, ColI and ColIV; in fact, engineered cells expressing
CD248 exhibit enhanced adhesion to FN and migration through an
FN-enriched cancer matrix (40),
which are suppressed by anti-CD248 antibodies (44). FN, ColI and IV bind to the
ectodomain of CD248, promoting cell attachment and migration during
cancer invasion by stimulating the release of active matrix
metalloproteinase (MMP)-9 (40,42).
Furthermore, CD248 may bind the endothelial-specific ECM protein
multimerin-2 (MMRN2) (45), which
is typically deposited along blood vessels and serves
anti-(46–48) and pro-(49) angiogenic roles, depending on the
specific step of vessel development involved. Moreover, CD248-MMRN2
complexes may bind to CD93 expressed on the surface of ECs, where
MMRN2 acts as an ‘extracellular glue’ between ECs and
pericytes/fibroblasts.
A primary feature of CD248 is its temporal pattern
of expression during development, which is high in the embryo and
progressively diminished postnatally (40). In line with this, CD248 may be
functionally involved not only pathologically, but also in
physiological angiogenesis (50).
In the embryonic mouse, CD248 is predominantly expressed on stromal
fibroblasts and cells of the developing vasculature (51–53).
It is also expressed to greater degree on fibroblasts closely
associated with epithelial structures, such as those adjacent to
immature alveoli in the embryonic lung and the dermal condensate of
developing skin (51).
Furthermore, it has been shown that gene knockout (KO) mice may
develop with a lack of CD248 expression altogether (23), thus CD248 may be redundant in
certain physiological mechanisms. Rupp et al (52) showed that, at embryonic stage
E10.0, CD248 was expressed in the ECs of the dorsal aorta. During
stages E10.5 and E12.0, a prominent CD248+ perineural vascular
plexus may develop in the head region, and angiogenic sprouts may
be generated from the perineural plexus to invade the proliferating
neuroectoderm. At stage E12.0, the vascular network is
significantly developed throughout entire embryo; during stages
E13.5-E14.5, CD248 may be expressed in clusters of mesenchymal
cells in the head region and in the developing genitourinary
system. Furthermore, CD248 expression is observed in the lung and
salivary glands, where CD248+ fibroblast-like cells have been
reported at the epithelial-mesenchymal interface. By
late-gestation, clusters of CD248+ mesenchymal cells are present in
the mucosa of the gastric cavity, in the dermis and in the area
separating the skeletal muscle fibres (41,52,53).
In healthy adult mice, CD248 is undetectable in all blood vessel
types of the organs and tissues examined, with the exception of
scattered stromal fibroblasts of the uterus and ovary, specialized
cells of the kidney glomeruli and bone marrow fibroblasts (41,52)
(Table I). This may reflect the
dynamic remodelling of the uterus during this period, which closely
resembles that in embryonic tissues (52–54).
As aforementioned, CD248 is involved in the
fibro-proliferative process by modulating the PDGF-BB (24) and TGFβ pathways, and promoting
alpha smooth muscle actin (αSMA) expression (25,27).
On this basis, CD248 may induce the proliferation of both pericytes
and fibroblasts following tissue injury, resulting in myofibroblast
accumulation. CD248 could potentially alter PDGF-BB signalling,
acting downstream of its receptor (PDGFR) to induce the
proliferation of pericytes following fibrotic tissue damage.
Furthermore, a high expression level of CD248 may induce TGFβ
signalling. Recent studies have highlighted the importance of the
TGFβ-CD248 signalling pathway as a potential therapeutic target for
cancer and FPDs such as SSc. These studies revealed that the
expression of CD248 by non-cancerous cells of mesenchymal origin
was downregulated at both the transcriptional and protein level. On
the contrary, in a pathological setting characterized by higher
CD248 expression levels, TGFβ failed to downregulate the expression
level of CD248 (25,28). Suresh Babu et al (28) speculated that CD248 may be one of
the TGFβ-effector molecules that undergoes context-dependent
switching (55–58). Moreover, it has been reported that
the increased expression of CD248 may upregulate the expression of
various other genes, including IL6, CCL2, TGFβ1 and TGFβR1
(22,59), which may stimulate αSMA and
ColI-associated myofibroblast generation. In addition, TGFβ was
unable to induce αSMA expression in CD248-silenced pericytes
(25,27).
Conflicting results have been reported concerning
the expression of different smooth muscle-associated genes during
fibroblast proliferation and activation. Transcript expression
levels of transgelin were shown to be elevated in fibroblasts
derived from mice lacking the cytoplasmatic domain of CD248
(40). In addition, CD248
knock-out did not affect the in vitro expression of αSMA in
normal human lung fibroblasts (29). Furthermore, in a model of renal
fibrosis, no increase in αSMA expression level was observed in
CD248-deficient mice (32), and in
an experimental model of liver fibrosis, the total hepatic mRNA
levels of Col and αSMA were reduced in CD248-deficient, compared
with wild-type (WT) mice (33).
These discrepancies may be partially explained by the use of
different experimental models, and the reported differences in cell
manipulation.
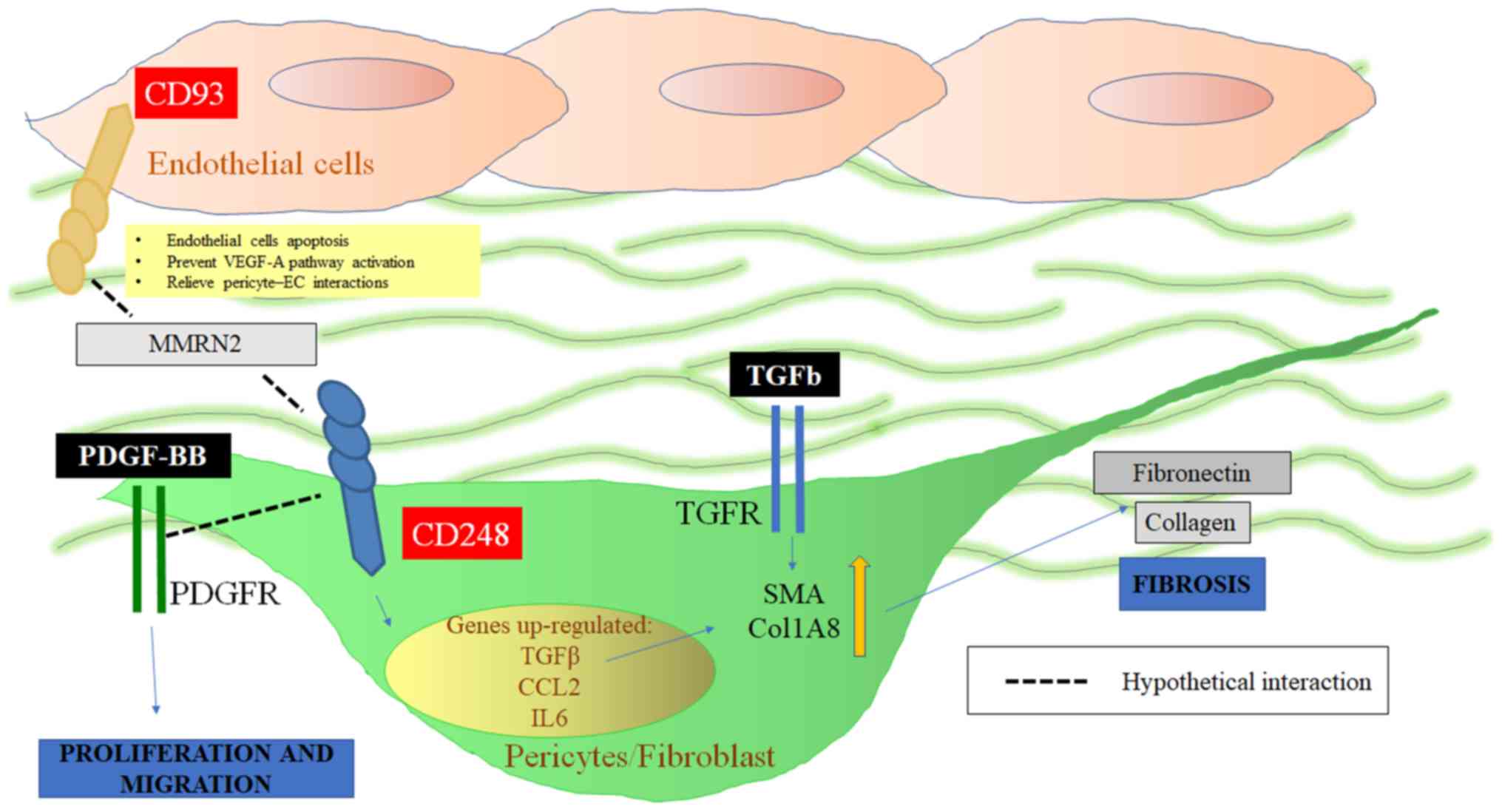
As far as its angiogenic role is concerned, CD248
may promote the interaction between ECs, pericytes and fibroblasts
via MMRN2. In fact, EC-expressed CD93 may bind to MMRN2 present in
the ECM, which in turn binds CD248 expressed by fibroblasts or
vasculature-associated pericytes. CD93 also serves a pivotal role
in endothelial migration and tube formation; CD93-deficient mice
displayed defects in angiogenesis (60) and the CD93-binding fragment of
MMRN2 exhibited anti-angiogenic effects, presumably by disrupting
its normal function (47). In this
context, pericyte-expressed CD248 may promote EC apoptosis via
MMRN2. Simonavicius et al (61) speculated that CD248 was able to
bind to the vascular basement membrane, directly disrupting EC
adhesion to the matrix. Alternatively, matrix-bound CD248 may
impair cross-talk between endothelial integrins and vascular
endothelial growth factor receptor 2 (VEGFR2), resulting in the
attenuation of vascular endothelial growth factor (VEGF) signalling
and subsequent EC apoptosis. These findings are summarized in
Fig. 2.
The Analysis of CD248 expression in 250 clinical
specimens (158 carcinomas and 92 sarcomas), revealed that 19 out of
the 20 cancer subtypes in question had CD248-positive specimens
(62). Regarding its expression
pattern in cancer tissues, where the formation and reorganisation
of blood vessels occurs at a high rate, CD248 has been proposed to
be a potential target of antiangiogenic cancer therapy (21,22,61).
In vitro, Brett et al (63) demonstrated that the CD248+
subpopulation within the lipoaspirate stromal vascular fraction
(SVF) showed an increased expression of angiogenic genes. The
authors speculated that CD248+ pro-angiogenic cells obtained from
SVF could represent a suitable strategy in wound healing by
promoting increased vessel growth in the wound. In line with this,
CD248-deficient mice displayed a specific defect in the early stage
of angiogenesis during muscle remodelling (64). Facciponte et al (65) developed a DNA vaccine that
expressed full-length mouse Tem1 cDNA fused to a TT adjuvant;
immunization with the Tem1-TT vaccine reduced tumour vasculature
compared with the control vaccine, as determined by
microvasculature density, functional imaging (ultrasound imaging of
blood perfusion and blood flux) and haematocrit levels. However,
conflicting results have been reported; Nanda et al
(23) illustrated that
abdominally-implanted cancers proliferated significantly more
slowly in CD248-KO mice than in their WT counterparts, but that the
number of small blood vessels was increased in the KO mice.
Similarly, murine brain tumours from the CD248-KO animals possessed
~40% more vessels than tumours extracted from WT mice (41). The authors speculated that CD248
served a critical role in determining cancer progression, and that
the increase in small blood vessels observed in the stroma of
CD248-KO mice may represent the pro-angiogenic response to an
abnormal microenvironment (23,28,66,67).
Furthermore, CD248 is expressed predominantly on cells which line
the blood vessels, but is also detectable in fibroblast-like
stromal cells (23); the authors
did not elucidate whether the effects of CD248 disruption were due
its absence in blood vessels or fibroblast-like stromal cells. It
is possible to speculate that the effects of CD248 expression on
cancer progression may also be associated with its absence in the
fibroblastic stroma. The interaction between the stroma and cancer
cells may induce the expression of ECM proteins, MMPs and growth
factors (68), thus facilitating
invasiveness by stimulating the growth of irregular and tortuous
new vessels (69–71). Viski et al (24) revealed that in 3 different
preclinical models, upregulated CD248 expression levels may have
promoted cancer cell intravasation into the circulation,
facilitating interactions with perivascular cells and promoting
transmigration across the endothelium. In this study, impaired
cancer cell intravasation in CD248-KO mice was not associated with
vascular alterations, suggesting that CD248 inhibition in cancer
has minimal impact on vascular integrity. Concurrently, treatment
of syngeneic cancer-bearing human-CD248 knock-in mice with the
anti-human endosialin antibody MORAb-004 did not lead to a
reduction in vessel number or destabilization of the vasculature,
but significantly impaired cancer cell proliferation following
subcutaneous or intravenous inoculation (24,27).
Another consideration is to clarify the phase of
angiogenesis that CD248 is associated with. Physiological
angiogenesis is initiated in response to the local production of
pro-angiogenic factors (particularly VEGF-A), which promotes
vascular sprout formation by the induction and migration of leading
tip cells, and by stimulating the proliferation of neighbouring
stalk cells. After sprouting, the initial vascular plexus is
extensively remodelled. A pivotal feature of this remodelling is
the pruning of unwanted capillaries through selective branch
regression (61). CD248 may serve
a primary role in vascular pruning, promoting vessel regression and
apoptosis of redundant ECs (61).
The capillary regression resulting from the apoptosis of ECs marks
the end of vessel plasticity and reflects the quiescent, mature
state of the new vascular network (61,72).
In pathological conditions in which CD248 is overexpressed, it is
possible that the remodelling and pruning of new vessels may be
increased, promoting the formation of irregular, tortuous and leaky
blood vessels. Of note, retarding CD248 function may prevent
vasculature remodelling during cancer (72), resulting in more stable vessels.
This would be advantageous in the treatment of cancer, allowing
efficient delivery of therapeutics and increasing the
responsiveness to VEGF inhibition (61,71).
Furthermore, during FPDs characterized by microvascular alteration,
blocking CD248 may prevent endothelial damage, inhibiting vessel
remodelling towards myofibroblasts generation (32). In addition, hypoxia (a primary
promoter of angiogenesis) regulates CD248 gene transcription via
the HIF-2 transcription factor (73). It is possible to speculate that in
fibrotic tissues with impaired angiogenesis, hypoxia may further
stimulate CD248 over-expression, resulting in the exacerbation of
microvascular damage from excessive CD248-mediated pruning
(61,74,75).
Although CD248 has been proposed as a potential
target of antiangiogenic cancer therapy, blocking CD248 did not
lead to a reduction in vessel number, though did prevent cancer
stromal cell interaction with vessels (23,24).
Notably, in pathological fibrosis, CD248 inhibition may even
reverse microvascular damage (30–33).
In fact, it has been shown that CD248+ pericytes may promote EC
apoptosis, resulting in the attenuation of VEGF signalling
(61). In a previous study
(61), the authors investigated
the potential mechanism by which CD248+ pericytes promote vessel
regression, via the generation of a soluble CD248-Fc construct
cultured with ECs; ECs exhibited attenuated VEGF-mediated
signalling with reduced VEGFR2 Tyr1175 and ERK1/2 phosphorylation.
Furthermore, flow cytometric analysis highlighted an increase in
apoptotic ECs compared with cells cultured with a control Fc
construct, suggesting a potential role for CD248. In another
experimental model, HeLa cells were transfected with the
phCMV1-CD248 plasmid; as a consequence, the genes involved in
cell-cell communication, adhesion and motility (Ang-2,
Angiopoietin-like 3 and 4, IL-1β, EGF, TGF-β receptor, Ephrin-A3,
Neuropilin 1) were upregulated, while VEGF-A expression levels were
significantly decreased (22).
Using an animal model of renal fibrosis, following
unilateral ureteral obstruction (UUO)-associated kidney injury,
Smith et al (32) showed
that the genetic deletion of CD248 protected mice from
microvascular rarefaction. Prior to UUO injury, there was no
difference in the size of the vessel area between WT and KO mice.
However, following kidney injury in WT mice, there was an initial
increase in vessel density 3 days post-injury, followed by
progressive vascular regression up to 14 days post-injury. These
results suggested an early phase of reparative angiogenesis
followed by vascular regression (microvascular rarefaction).
Conversely, CD248-KO mice did not exhibit an early phase of
reparative angiogenesis, and the kidneys of these animals were
protected from vessels loss in response to UUO injury (32).
Despite the fact that the exact mechanism by which
CD248-expressing cells contribute to microvascular rarefaction is
not fully elucidated, CD248 may bind the ECM proteins FN, ColI,
ColIV, and MMRN2 (26,46) in fibrotic tissues. Furthermore, the
absence of CD248 may reduce the interaction between pericytes, ECM
proteins and stromal fibroblasts, preventing migration from the
vasculature into the tissue stroma, and subsequent myofibroblast
generation. In turn, this would result in reduced stromal fibrosis
and microvascular rarefaction (32).
FPDs are able to cause significant morbidity and
mortality in affected patients (1,2,78),
thus the development of novel therapies are still required
(79–81). For this reason, gaining a greater
understanding of the origin and differentiation pathways of
myofibroblasts in vivo is a priority. In this context,
recent studies (82–87) have suggested a role for mesenchymal
perivascular cells (88); these
cells have held various names including mural cells and pericytes,
and several of their functions remain largely unknown. Dulauroy
et al (89) used genetic
studies in mice to map the fate of neural crest cell-derived
embryonic mesenchymal cells that expressed A disintegrin and
metalloprotease isoform 12 (ADAM12). It was observed that fetal
ADAM12+ cells contributed to the generation of perivascular cells
in adult skeletal muscle. In fact, a subset of these cells derived
from the ADAM12+ lineage expressed pericyte markers and lined the
capillaries. Following fibrotic injury, the reactivation of ADAM12+
cells stimulated ontogenetic signalling to restore vascular
integrity. ADAM12+ cells were shown to be significantly increased
in the perivascular skin cells of SSc, a model of chronic FPDs,
suggesting that myofibroblasts may originate from perivascular
cells (83). Therefore, the
investigation of novel targets involved in
pericyte-to-myofibroblast transition may reveal therapeutic
possibilities for FDPs. It has also been shown that CD248+
perivascular cells may serve a role in myofibroblast generation. In
fact, CD248 expression is required for TGFβ-induced αSMA expression
on normal human pericytes, in addition to that on the perivascular
MSCs of patients with SSc (25,27).
Furthermore, in injured fibrotic kidney tissue exhibits increased
CD248 expression within a CD248+/αSMA+ subpopulation of
myofibroblasts, in addition to a population of CD248+/αSMA-stromal
fibroblasts. Notably, a subset of CD248-/αSMA+ myofibroblasts was
also identified, emphasizing the heterogeneity of these cells in
fibrotic kidney tissues (32). Of
note, Smith et al (32)
demonstrated that CD248-deficient mice were protected from renal
fibrosis, illustrating a pathogenic role for CD248 in the
development of renal fibrosis. In this context the loss of CD248
may modulate the response of renal pericytes and stromal
fibroblasts to UUO injury. Although the precise mechanism has not
been fully elucidated, it has been revealed that the genetic loss
of CD248 significantly reduced the formation of matrix-depositing
myofibroblasts in response to renal injury by UUO, with a
subsequent decrease in tissue fibrosis. However, it should be
clarified whether the reduction in stromal myofibroblasts and
pericytes in CD248-deficient mice was the result of impaired
migration or a specific proliferative defect in vivo.
PDGF-BB/PDGFR signalling between ECs and pericytes has been shown
to be important for vascular stabilization following kidney injury
(26,90–92),
indicating that CD248 may exert its effects by modulating the
PDGF-BB pathway. Furthermore, PDGF-induced autophosphorylation of
extracellular signal-regulated kinase (ERK; but not PDGFR itself)
was markedly diminished in CD248-deficient pericytes (26). These findings suggest that CD248
may perturb PDGF signalling downstream of PDGFR and upstream of
ERK1/2, by an as yet unidentified mechanism. CD248 may therefore
promote the proliferation of pericytes after injury, as they
differentiate into Col-secreting myofibroblasts (26,93).
Studies of hepatic fibrosis revealed that CD248
promoted fibrosis by enhancing a PDGF pro-proliferative signal to
hepatic stellate cells (33).
Combined with the established roles of the PDGF signalling axis in
renal fibrosis (93), the
CD248-associated enhancement of PDGF signalling may be speculated
in pericytes during fibrosis.
In order to identify the source(s) of αSMA-positive
myofibroblasts in different pathological conditions, fibrosis and
cancer development, a number of cell tracing studies were performed
(94). In response to both injury
and dysregulated tissue homeostasis, local fibroblasts were able to
undergo trans-differentiation to myofibroblasts, and activation in
the skin, liver, lung, heart and kidney, and in the stromal
reaction to epithelial tumours (95,96).
Epithelial mesenchymal transition is another mechanism of
myofibroblast generation from local epithelial layers during cancer
development (97,98), mirroring what observed in kidney
(99) and lung fibrosis (100,101). In addition, the
de-differentiation of perivascular cells into ECM-producing cells
may contribute to myofibroblast development and function (102). Furthermore, a number of studies
have reported that bone marrow MSCs and hematopoietic stem cells
may be the precursors of myofibroblasts (103,104). Independent of this speculation,
few essential factors are required for the activation of
myofibroblasts. TGFβ is the most potent myofibrogenic growth
factor, and the inhibition of cancer cell responsiveness to TGFβ
has metastasis-suppressing effects (105). Another important factor in
myofibroblast activation is the mechanical stress resulting from
remodelling activities in the stroma, and the mechanical properties
of the ECM (106,107). The activation and differentiation
of stromal cells into cancer-associated myofibroblasts has
consequences for tumour development, progression and metastasis
(108). Fibrosis and cancer are
known to be intimately linked and myofibroblast activation may
serve a pivotal role in cancer chemoresistance (109,110). Also, a dense fibrotic stroma
correlated with a poor response to neoadjuvant treatments,
including 5-fluorouracil epirubicin and cyclophosphamide (FEC) in
breast cancer, and gemcitabine in pancreatic ductal adenocarcinoma
(PDAC) (111,112). Furthermore, cancer-associated
fibroblasts may secrete hyaluronan, which is responsible for the
regulation of interstitial pressure within the tumour, and results
in blood vessel collapse and impaired drug delivery (113,114). It has also been shown that CD248
was expressed by these cancer-associated fibroblasts and mural
cells, but not ECs (114), and
that its expression correlated with poor prognosis in patients with
gastric cancer (115). These
findings suggest that the inhibition of CD248 expression may
represent a novel strategy for perturbing the differentiation of
stromal cells into cancer-associated fibroblasts.
The results of a first-in-human, open-label, phase I
study have recently been published, enrolling patients with
refractory solid cancers treated with a humanized monoclonal
antibody against CD248 (MORAb-004). Following the administration of
MORAb-004, a prolonged, stable disease state of >106 days was
observed in patients with cancer subtypes believed to be of
epithelial origin, including colorectal carcinoma (116). Although the mechanism of action
of MORAb-004 is not completely understood, preclinical models have
suggested that CD248 is removed from the cell surface upon
MORAb-004-mediated internalization, while MORAb-004 exhibits no
antibody-dependent cellular cytotoxicity (45). Furthermore, it is possible that
MORAb-004 may disrupt protein-protein interactions that serve to
signal between tumour and stromal cells within the cancer
microenvironment (116). Another
phase I study enrolling children with relapsed or refractory solid
cancer, demonstrated that MORAb-004 was well tolerated and that its
pharmacokinetics did not significantly differ in children compared
with adults (117). Concurrently,
a multicentre, open-label, phase II study did not produce the same
encouraging results; this study evaluated the 24-week
progression-free survival, pharmacokinetics and tolerability of 2
doses of MORAb-004 in patients with metastatic melanoma. However,
the efficacy of single-agent MORAb-004 treatment in melanoma was
low. The principal limitations of this study were the small sample
size and population heterogeneity of previously treated melanoma
patients (118). Furthermore, a
randomized, double-blind, placebo-controlled phase II study of
patients with chemo-refractory metastatic colorectal cancer
confirmed that MORAb-004 was well tolerated, despite the fact that
no improvement in overall survival and/or response rate was
demonstrated (119). However, it
should be noted that the results of this trial may be skewed by the
enrolment of patients at advanced cancer stages; indeed, it was
speculated that MORAb-004 may be more effective at treating
patients affected by early-onset cancer and with a short duration
of disease.
Future studies with more stringent inclusion
criteria are necessary to fully evaluate the efficacy of MORAb-004,
and an alternative approach may be considered to improve efficacy,
including antibody-drug conjugates to selectively deliver cytotoxic
agents to tumour sites. Anti-CD248 drug conjugates delivering
monomethyl auristatin E, a synthetic antineoplastic agent, were
tested for their activity in 4 human cancer cell lines. The study
demonstrated that CD248-positive tumours may be specifically and
effectively targeted by a monoclonal anti-CD248 antibody conjugated
to an anti-neoplastic agent, and the response was complete and
sustained (120). Collectively,
the results of these studies suggest that additional pre-clinical
investigations are required to better understand the mechanism of
action of anti-CD248 monoclonal antibodies, and to determine their
most suitable clinical application (116). However, despite the CD248
expression pattern and encouraging in vivo results
suggesting a potential anti-cancer target, the exact role of CD248
in cancer is not fully understood. Current research is hindered by
inadequate knowledge of the factors and pathways that control CD248
expression and distribution. It is also unclear whether the effects
of CD248 on tumour growth are due to its expression in fibroblastic
stromal cells or vascular cells (23).
Although the pathogenic role of CD248 in fibrosis
has been strongly indicated, there are currently no published
studies assessing its potential as a therapeutic target in patients
affected by FPDs. Future studies are required to completely
elucidate the possible therapeutic applications of targeting
CD248.
Fibrosis is the hallmark of pathologic remodelling
in a number of tissues, a contributor to clinical disease and one
of the leading causes of mortality in the developed world (2). Therefore, there is a great deal of
interest in identifying a means of inhibiting, or even reversing
the progression of tissue fibrosis. Notably, FPDs are characterized
by common pathogenic pathways preserved between different organs,
thus an understanding of the mediators and pathways activated in
tissue fibrosis may help to establish potential therapeutic targets
across different diseases and organs systems (108). Another unifying characteristic of
FPDs is microvascular alteration and its common pathogenic
pathways, the constituents of which may also be an attractive
therapeutic target. At present, an improved understanding of the
mechanisms involved in vascular remodelling are essential for the
implementation of therapies stimulating vascular network
stabilization during FPDs. Interestingly, this could also be
required for cancers vascularised by immature, disorganised and
leaky blood vessels, with structural abnormalities which contribute
to blood flow disturbances and cancer cell extravasation. Indeed,
for more effective anti-angiogenic therapies, it has been
speculated that in addition to targeting excessive angiogenesis,
the cancer vasculature should also be normalized (121).
CD248 is a glycosylated transmembrane protein that
is overexpressed in the perivascular cells and fibroblasts of
several diseases characterized by abnormal vascular remodelling,
including cancer and FPDs. The exact role of CD248 is not fully
understood; however, in vitro and in vivo studies
have revealed that inhibiting CD248 signalling may prevent
myofibroblast accumulation and promote vessel stabilisation. In
line with these findings, anti-CD248 therapy may be considered a
promising therapeutic strategy in both cancer and FPDs. Indeed,
interfering with CD248 may prevent the myofibroblast proliferation
responsible for ECM stiffening, which in turn contributes to the
persistence and progression of both cancer and fibrosis.
Furthermore, anti-CD248 therapy may also prevent pericyte
trans-differentiation into mature αSMA+ cells, which may
subsequently limit myofibroblast generation (25,82,87,121). In future, further studies are
required to clarify the role of CD248 in FPDs, securing this
molecule as a potential therapeutic target in a clinical setting,
in which an effective therapeutic approach to prevent fibrosis has
yet to be developed.
The authors would like to thank Mrs. Federica
Sensini (Department of Biotechnological and Applied Clinical
Sciences, Rheumatology Unit, School of Medicine, University of
L'Aquila, L'Aquila, Italy) for her assistance.
No funding was received.
Not applicable.
PDB, PR, FDG, RG and PC conceived and designed the
review, performed the literature search, and wrote and revised the
manuscript. VL performed the literature search and revised the
manuscript. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu KM, Sava P and Gonzalez AL:
Microvascular targets for anti-fibrotic therapeutics. Yale J Biol
Med. 86:537–554. 2013.PubMed/NCBI
|
|
3
|
Wynn TA: Common and unique mechanisms
regulate fibrosis in various fibroproliferative diseases. J Clin
Invest. 117:524–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sziksz E, Pap D, Lippai R, Béres NJ,
Fekete A, Szabó AJ and Vannay A: Fibrosis related inflammatory
mediators: Role of the IL-10 cytokine family. Mediators Inflamm.
2015:7646412015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giacomelli R, Afeltra A, Alunno A, Baldini
C, Bartoloni-Bocci E, Berardicurti O, Carubbi F, Cauli A, Cervera
R, Ciccia F, et al: International consensus: What else can we do to
improve diagnosis and therapeutic strategies in patients affected
by autoimmune rheumatic diseases (rheumatoid arthritis,
spondyloarthritides, systemic sclerosis, systemic lupus
erythematosus, antiphospholipid syndrome and Sjogren's syndrome)?
The unmet needs and the clinical grey zone in autoimmune disease
management. Autoimmun Rev. 16:911–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cox TR and Erler JT: Remodeling and
homeostasis of the extracellular matrix: Implications for fibrotic
diseases and cance. Dis Models Mech. 4:165–178. 2011. View Article : Google Scholar
|
|
7
|
Gabbiani G: The myofibroblast in wound
healing and fibrocontractive diseases. J Pathol. 200:500–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hinz B, Phan SH, Thannickal VJ, Galli A,
Bochaton-Piallat ML and Gabbiani G: The myofibroblast: One
function, multiple origins. Am J Pathol. 170:1807–1816. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cipriani P, Marrelli A, Liakouli V, Di
Benedetto P and Giacomelli R: Cellular players in angiogenesis
during the course of systemic sclerosis. Autoimmun Rev. 10:641–646.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Desmouliere A, Darby IA and Gabbiani G:
Normal and pathologic soft tissue remodeling: Role of the
myofibroblast, with special emphasis on liver and kidney fibrosis.
Lab Invest. 83:1689–1707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virag JI and Murry CE: Myofibroblast and
endothelial cell proliferation during murine myocardial infarct
repair. Am J Pathol. 163:2433–2440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phan SH: The myofibroblast in pulmonary
fibrosis. Chest. 122:286S–289S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cipriani P, Di Benedetto P, Ruscitti P,
Capece D, Zazzeroni F, Liakouli V, Pantano I, Berardicurti O,
Carubbi F, Pecetti G, et al: The Endothelial-mesenchymal transition
in systemic sclerosis is induced by endothelin-1 and transforming
growth factor-β and May Be blocked by macitentan, a dual
endothelin-1 receptor antagonist. J Rheumatol. 42:1808–1816. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liakouli V, Cipriani P, Di Benedetto P,
Ruscitti P, Carubbi F, Berardicurti O, Panzera N and Giacomelli R:
The role of extracellular matrix components in angiogenesis and
fibrosis: Possible implication for systemic sclerosis. Mod
Rheumatol. 15:1–11. 2018.
|
|
15
|
Sacchetti C, Bai Y, Stanford SM, Di
Benedetto P, Cipriani P, Santelli E, Piera-Velazquez S, Chernitskiy
V, Kiosses WB, Ceponis A, et al: PTP4A1 promotes TGFβ signaling and
fibrosis in systemic sclerosis. Nat Commun. 8:10602017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abe R, Donnelly SC, Peng T, Bucala R and
Metz CN: Peripheral blood fibrocytes: differentiation pathway and
migration to wound sites. J Immunol. 166:7556–7562. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leaf IA and Duffield JS: What can target
kidney fibrosis? Nephrol Dial Transplant. 32 (Suppl 1):i89–i97.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magro CM, Ross P, Marsh CB, Allen JN, Liff
D, Knight DA, Waldman WJ and Cowden DJ: The role of
anti-endothelial cell antibody-mediated microvascular injury in the
evolution of pulmonary fibrosis in the setting of collagen vascular
disease. Am J Clin Pathol. 127:237–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruart M, Chavarria L, Campreciós G,
Suárez-Herrera N, Montironi C, Guixé-Muntet S, Bosch J, Friedman
SL, Garcia-Pagán JC and Hernández-Gea V: Impaired endothelial
autophagy promotes liver fibrosis by aggravating the oxidative
stress response during acute liver injury. J Hepatol. 70:458–469.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lax S, Hardie D, Wilson A, Douglas M,
Anderson G, Huso D, Isacke CM and Buckley CD: The pericyte and
stromal marker CD248 (endosialin) is required for efficient lymph
node expansion. Eur J Immunol. 40:1884–1889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bagley RG, Honma N, Weber W, Boutin P,
Rouleau C, Shankara S, Kataoka S, Ishida I, Roberts BL and Teicher
BA: Endosialin/TEM1/CD248 is a pericyte marker of embryonic and
tumour neovascularisation. Microvasc Res. 76:180–188. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kontsekova S, Polcicova K, Takacova M and
Pastorekova S: Endosialin: Molecular and functional links to tumour
angiogenesis. Neoplasma. 63:183–192. 2016.PubMed/NCBI
|
|
23
|
Nanda A, Karim B, Peng Z, Liu G, Qiu W,
Gan C, Vogelstein B, St Croix B, Kinzler KW and Huso DL: Tumor
endothelial marker 1 (TEM1) functions in the growth and progression
of abdominal tumours. Proc Natl Acad Sci USA. 103:3351–3356. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Viski C, König C, Kijewska M, Mogler C,
Isacke CM and Augustin HG: Endosialin-expressing pericytes promote
metastatic dissemination. Cancer Res. 76:5313–5325. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di Benedetto P, Liakouli V, Ruscitti P,
Berardicurti O, Carubbi F, Panzera N, Di Bartolomeo S, Guggino G,
Ciccia F, Triolo G, et al: Blocking CD248 molecules in perivascular
stromal cells of patients with systemic sclerosis strongly inhibits
their differentiation toward myofibroblasts and proliferation: A
new potential target for antifibrotic therapy. Arthritis Res Ther.
20:2232018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomkowicz B, Rybinski K, Nicolaides NC,
Grasso L and Zhou Y: Endosialin/TEM-1/CD248 regulates pericyte
proliferation through PDGF receptor signaling. Cancer Biol Ther.
9:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rybinski K, Imtiyaz HZ, Mittica B,
Drozdowski B, Fulmer J, Furuuchi K, Fernando S, Henry M, Chao Q,
Kline B, et al: Targeting endosialin/CD248 through
antibody-mediated internalization results in impaired pericyte
maturation and dysfunctional tumour microvasculature. Oncotarget.
22:25429–25440. 2015.
|
|
28
|
Suresh Babu S, Valdez Y, Xu A, O'Byrne AM,
Calvo F, Lei V and Conway EM: TGFβ-mediated suppression of CD248 in
non-cancer cells via canonical Smad-dependent signaling pathways is
uncoupled in cancer cells. BMC Cancer. 14:1132014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartis D, Crowley LE, D'Souza VK,
Borthwick L, Fisher AJ, Croft AP, Pongrácz JE, Thompson R, Langman
G, Buckley CD and Thickett DR: Role of CD248 as a potential
severity marker in idiopathic pulmonary fibrosis. BMC Pulm Med.
16:512016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mogler C, Wieland M, König C, Hu J, Runge
A, Korn C, Besemfelder E, Breitkopf-Heinlein K, Komljenovic D,
Dooley S, et al: Hepatic stellate cell-expressed endosialin
balances fibrogenesis and hepatocyte proliferation during liver
damage. EMBO Mol Med. 7:332–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mogler C, König C, Wieland M, Runge A,
Besemfelder E, Komljenovic D, Longerich T, Schirmacher P and
Augustin HG: Hepatic stellate cells limit hepatocellular carcinoma
progression through the orphan receptor endosialin. EMBO Mol Med.
9:741–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith SW, Croft AP, Morris HL, Naylor AJ,
Huso DL, Isacke CM, Savage CO and Buckley CD: Genetic deletion of
the stromal cell marker CD248 (Endosialin) protects against the
development of renal fibrosis. Nephron. 131:265–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilhelm A, Aldridge V, Haldar D, Naylor
AJ, Weston CJ, Hedegaard D, Garg A, Fear J, Reynolds GM, Croft AP,
et al: CD248/endosialin critically regulates hepatic stellate cell
proliferation during chronic liver injury via a PDGF-regulated
mechanism. Gut. 65:1175–1185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith SW, Eardley KS, Croft AP, Nwosu J,
Howie AJ, Cockwell P, Isacke CM, Buckley CD and Savage CO: CD248+
stromal cells are associated with progressive chronic kidney
disease. Kidney Int. 80:199–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rettig WJ, Garin-Chesa P, Healey JH, Su
SL, Jaffe EA and Old LJ: Identification of endosialin, a cell
surface glycoprotein of vascular endothelial cells in human cancer.
Proc Natl Acad Sci USA. 89:10832–10836. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brady J, Neal J, Sadakar N and Gasque P:
Human endosialin (tumor endothelial marker 1) is abundantly
expressed in highly malignant and invasive brain tumors. J
Neuropathol Exp Neurol. 63:1274–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B and Kinzler KW: Genes expressed in human tumor
endothelium. Science. 289:1197–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
MacFadyen JR, Haworth O, Roberston D,
Hardie D, Webster MT, Morris HR, Panico M, Sutton-Smith M, Dell A,
van der Geer P, et al: Endosialin (TEM1, CD248) is a marker of
stromal fibroblasts and is not selectively expressed on tumour
endothelium. FEBS Lett. 579:2569–2575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naylor AJ, Azzam E, Smith S, Croft A,
Poyser C, Duffield JS, Huso DL, Gay S, Ospelt C, Cooper MS, et al:
The mesenchymal stem cell marker CD248 (Endosialin) is a negative
regulator of bone formation in mice. Arthritis Rheum. 64:3334–3343.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Christian S, Ahorn H, Koehler A,
Eisenhaber F, Rodi HP, Garin-Chesa P, Park JE, Rettig WJ and Lenter
MC: Molecular cloning and characterization of endosialin, a C-type
lectin-like cell surface receptor of tumour endothelium. J Biol
Chem. 276:7408–7414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valdez Y, Maia M and Conway EM: CD248:
Reviewing its role in health and disease. Curr Drug Targets.
13:432–439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carson-Walter EB, Watkins DN, Nanda A,
Vogelstein B, Kinzler KW and St Croix B: Cell surface tumour
endothelial markers are conserved in mice and humans. Cancer Res.
61:6649–6655. 2001.PubMed/NCBI
|
|
43
|
Maia M, de Vriese A, Janssens T, Moons M,
van Landuyt K, Tavernier J, Lories RJ and Conway EM: CD248 and its
cytoplasmic domain: A therapeutic target for arthritis. Arthritis
Rheum. 62:3595–3606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gardiol D: PDZ-containing proteins as
targets in human pathologies. FEBS J. 279:35292012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
O'Shannessy DJ, Smith MF, Somers EB,
Jackson SM, Albone E, Tomkowicz B, Cheng X, Park Y, Fernando D,
Milinichik A, et al: Novel antibody probes for the characterization
of endosialin/TEM-1. Oncotarget. 7:69420–69435. 2016.PubMed/NCBI
|
|
46
|
Khan KA, Naylor AJ, Khan A, Noy PJ,
Mambretti M, Lodhia P, Athwal J, Korzystka A, Buckley CD and
Willcox BE: Multimerin-2 is a ligand for group 14 family C-type
lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte
interface. Oncogene. 36:6097–7008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Andreuzzi E, Colladel R, Pellicani R,
Tarticchio G, Cannizzaro R, Spessotto P, Bussolati B, Brossa A, De
Paoli P, Canzonieri V, et al: The angiostatic molecule Multimerin 2
is processed by MMP-9 to allow sprouting angiogenesis. Matrix Biol.
64:40–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Colladel R, Pellicani R, Andreuzzi E,
Paulitti A, Tarticchio G, Todaro F, Colombatti A and Mongiat M:
MULTIMERIN2 binds VEGF-A primarily via the carbohydrate chains
exerting an angiostatic function and impairing tumor growth.
Oncotarget. 7:2022–2037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lorenzon E, Colladel R, Andreuzzi E,
Marastoni S, Todaro F, Schiappacassi M, Ligresti G, Colombatti A
and Mongiat M: MULTIMERIN2 impairs tumor angiogenesis and growth by
interfering with VEGF-A/VEGFR2 pathway. Oncogene. 31:3136–3147.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Galvagni F, Nardi F, Spiga O, Trezza A,
Tarticchio G, Pellicani R, Andreuzzi E, Caldi E, Toti P and Tosi
GM: Dissecting the CD93-Multimerin 2 interaction involved in cell
adhesion and migration of the activated endothelium. Matrix Biol.
64:112–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Opavsky R, Haviernik P, Jurkovicova D,
Garin MT, Copeland NG, Gilbert DJ, Jenkins NA, Bies J, Garfield S
and Pastorekova S: Molecular characterization of the mouse
Tem1/endosialin gene regulated by cell density in vitro and
expressed in normal tissues in vivo. Biol Chem. 276:38795–38807.
2001. View Article : Google Scholar
|
|
52
|
Rupp C, Dolznig H, Puri C, Sommergruber W,
Kerjaschki D, Rettig WJ and Garin-Chesa P: Mouse endosialin, a
C-type lectin-like cell surface receptor: Expression during
embryonic development and induction in experimental cancer
neoangiogenesis. Cancer Immun. 6:102006.PubMed/NCBI
|
|
53
|
MacFadyen J, Savage K, Wienke D and Isacke
CM: Endosialin is expressed on stromal fibroblasts and CNS
pericytes in mouse embryos and is downregulated during development.
Gene Expr Patterns. 7:363–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lax S, Hou TZ, Jenkinson E, Salmon M,
MacFadyen JR, Isacke CM, Anderson G, Cunningham AF and Buckley CD:
CD248/Endosialin is dynamically expressed on a subset of stromal
cells during lymphoid tissue development, splenic remodeling and
repair. FEBS Lett. 581:3550–3556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Croft AP, Naylor AJ, Marshall JL, Hardie
DL, Zimmermann B, Turner J, Desanti G, Adams H, Yemm AI,
Müller-Ladner U, et al: Rheumatoid synovial fibroblasts
differentiate into distinct subsets in the presence of cytokines
and cartilage. Arthritis Res Ther. 18:2702016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rahimi RA and Leof EB: TGF-beta signaling:
A tale of two responses. J Cell Biochem. 102:593–608. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schiemann WP: Targeted TGF-beta
chemotherapies: Friend or foe in treating human malignancies? Exp
Rev Anticancer Ther. 7:609–611. 2007. View Article : Google Scholar
|
|
58
|
Tian M and Schiemann WP: The TGF-beta
paradox in human cancer: An update. Future Oncol. 5:259–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Murray LA, Argentieri RL, Farrell FX,
Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff
HL, et al: Hyper-responsiveness of IPF/UIP fibroblasts: Interplay
between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol.
40:2174–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Langenkamp E, Zhang L, Lugano R, Huang H,
Elhassan TE, Georganaki M, Bazzar W, Lööf J, Trendelenburg G,
Essand M, et al: Elevated expression of the C-type lectin CD93 in
the glioblastoma vasculature regulates cytoskeletal rearrangements
that enhance vessel function and reduce host survival. Cancer Res.
75:4504–4516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Simonavicius N, Ashenden M, van Weverwijk
A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H and Isacke
CM: Pericytes promote selective vessel regression to regulate
vascular patterning. Blood. 120:1516–1527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rouleau C, Curiel M, Weber W, Smale R,
Kurtzberg L, Mascarello J, Berger C, Wallar G, Bagley R, Honma N,
et al: Endosialin protein expression and therapeutic target
potential in human solid tumors: Sarcoma versus carcinoma. Clin
Cancer Res. 14:7223–7236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brett E, Zielins ER, Chin M, Januszyk M,
Blackshear CP, Findlay M, Momeni A, Gurtner GC, Longaker MT and Wan
DC: Isolation of CD248-expressing stromal vascular fraction for
targeted improvement of wound healing. Wound Repair Regen.
25:414–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Naylor AJ, McGettrick HM, Maynard WD, May
P, Barone F, Croft AP, Egginton S and Buckley CD: A differential
role for CD248 (Endosialin) in PDGF-mediated skeletal muscle
angiogenesis. PLoS One. 22:e1071462014. View Article : Google Scholar
|
|
65
|
Facciponte JG, Ugel S, De Sanctis F, Li C,
Wang L, Nair G, Sehgal S, Raj A, Matthaiou E, Coukos G and
Facciabene A: Tumor endothelial marker 1-specific DNA vaccination
targets tumor vasculature. J Clin Invest. 124:1497–1511. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tlsty TD and Hein PW: Know thy neighbor:
Stromal cells can contribute oncogenic signals. Curr Opin Genet
Dev. 11:54–59. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bissell MJ, Kenny PA and Radisky DC:
Microenvironmental regulators of tissue structure and function also
regulate tumor induction and progression: The role of extracellular
matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol.
70:343–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang F, Tuxhorn JA, Ressler SJ, McAlhany
SJ, Dang TD and Rowley DR: Stromal expression of connective tissue
growth factor promotes angiogenesis and prostate cancer
tumorigenesis. Cancer Res. 65:8887–8895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kucerova L, Zmajkovic J, Toro L, Skolekova
S, Demkova L and Matuskova M: Tumor-driven molecular changes in
human mesenchymal stromal cells. Cancer Microenviron. 8:1–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nagy JA, Chang SH, Dvorak AM and Dvorak
HF: Why are tumour blood vessels abnormal and why is it important
to know? Br J Cancer. 100:865–869. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kiyohara E, Donovan N, Takeshima L, Huang
S, Wilmott JS, Scolyer RA, Jones P, Somers EB, O'Shannessy DJ and
Hoon DS: Endosialin expression in metastatic melanoma tumor
microenvironment vasculature: Potential therapeutic implications.
Cancer Microenviron. 8:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ohradanova A, Gradin K, Barathova M,
Zatovicova M, Holotnakova T, Kopacek J, Parkkila S, Poellinger L,
Pastorekova S and Pastorek J: Hypoxia upregulates expression of
human endosialin gene via hypoxia-inducible factor 2. Br J Cancer.
99:1348–1356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tian Y, Deng H, Han L, Hu S and Qi X:
Hypoxia-inducible factor may induce the development of liver
fibrosis in budd-chiari syndrome by regulating CD248/endosialin
Expression: A Hypothesis. J Transl Int Med. 6:66–69. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lin SL, Chang FC, Schrimpf C, Chen YT, Wu
CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, et al: Targeting
endothelium-pericyte cross talk by inhibiting VEGF receptor
signaling attenuates kidney microvascular rarefaction and fibrosis.
Am J Pathol. 178:911–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zanivan S, Maione F, Hein MY,
Hernandez-Fernaud JR, Ostasiewicz P, Giraudo E and Mann M:
SILAC-based proteomics of human primary endothelial cell
morphogenesis unveils tumor angiogenic markers. Mol Cell
Proteomics. 12:3599–3611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wynn TA: Fibrotic disease and the
T(H)1/T(H)2 paradigm. Nat Rev Immunol. 4:583–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fraticelli P, Gabrielli B, Pomponio G,
Valentini G, Bosello S, Riboldi P, Gerosa M, Faggioli P, Giacomelli
R, Del Papa N, et al: Imatinib in Scleroderma Italian Study Group.
Low-dose oral imatinib in the treatment of systemic sclerosis
interstitial lung disease unresponsive to cyclophosphamide: A phase
II pilot study. Arthritis Res Ther. 16:R1442014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rosenbloom J, Macarak E, Piera-Velazquez S
and Jimenez SA: Human fibrotic diseases: Current challenges in
fibrosis research. Methods Mol Biol. 1627:1–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Giacomelli R, Afeltra A, Alunno A,
Bartoloni-Bocci E, Berardicurti O, Bombardieri M, Bortoluzzi A,
Caporali R, Caso F, Cervera R, et al: Guidelines for biomarkers in
autoimmune rheumatic diseases-evidence based analysis. Autoimmun
Rev. 18:93–106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Krieg T, Abraham D and Lafyatis R:
Fibrosis in connective tissue disease: The role of the
myofibroblast and fibroblast-epithelial cell interactions.
Arthritis Res Ther. 9 (Suppl 2):S42007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cipriani P, Di Benedetto P, Ruscitti P,
Liakouli V, Berardicurti O, Carubbi F, Ciccia F, Guggino G,
Zazzeroni F, Alesse E, et al: Perivascular cells in diffuse
cutaneous systemic sclerosis overexpress activated ADAM12 and are
involved in myofibroblast transdifferentiation and development of
fibrosis. J Rheumatol. 43:1340–1349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cipriani P, Di Benedetto P, Ruscitti P,
Verzella D, Fischietti M, Zazzeroni F, Liakouli V, Carubbi F,
Berardicurti O, Alesse E and Giacomelli R: Macitentan inhibits the
transforming growth factor-β profibrotic action, blocking the
signaling mediated by the ETR/TβRI complex in systemic sclerosis
dermal fibroblasts. Arthritis Res Ther. 17:2472015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sato M, Suzuki S and Senoo H: Hepatic
stellate cells: Unique characteristics in cell biology and
phenotype. Cell Struct Funct. 28:105–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lin SL, Kisseleva T, Brenner DA and
Duffield JS: Pericytes and perivascular fibroblasts are the primary
source of collagen-producing cells in obstructive fibrosis of the
kidney. Am J Pathol. 173:1617–1627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cipriani P, Di Benedetto P, Ruscitti P,
Campese AF, Liakouli V, Carubbi F, Pantano I, Berardicurt O,
Screpanti I and Giacomelli R: Impaired endothelium-mesenchymal stem
cells cross-talk in systemic sclerosis: A link between vascular and
fibrotic features. Arthritis Res Ther. 16:4422014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cipriani P, Marrelli A, Di Benedetto P,
Liakouli V, Carubbi F, Ruscitti P, Alvaro S, Pantano I, Campese AF,
Grazioli P, et al: Scleroderma mesenchymal stem cells display a
different phenotype from healthy controls; implications for
regenerative medicine. Angiogenesis. 16:595–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dulauroy S, Di Carlo SE, Langa F, Eberl G
and Peduto L: Lineage tracing and genetic ablation of ADAM12(+)
perivascular cells identify a major source of profibrotic cells
during acute tissue injury. Nat Med. 18:1262–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chang-Panesso M and Humphreys BD:
CD248/Endosialin: A novel pericyte target in renal fibrosis.
Nephron. 131:262–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL,
Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, et al: Platelet-derived
growth factor receptor signalling activates pericyte-myofibroblast
transition in obstructive and post-ischemic kidney fibrosis. Kidney
Int. 80:1170–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cipriani P, Di Benedetto P, Dietrich H,
Ruscitti P, Liakouli V, Carubbi F, Pantano I, Berardicurti O, Sgonc
R and Giacomelli R: Searching for a good model for systemic
sclerosis: The molecular profile and vascular changes occurring in
UCD-200 chickens strongly resemble the early phase of human
systemic sclerosis. Arch Med Sci. 12:828–843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Boor P, Ostendorf T and Floege J: PDGF and
the progression of renal disease. Nephrol Dial Transplant. 29
(Suppl 1):i45–i54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hinz B, Phan SH, Thannickal VJ, Prunotto
M, Desmoulière A, Varga J, De Wever O, Mareel M and Gabbiani G:
Recent developments in myofibroblast biology: Paradigms for
connective tissue remodeling. Am J Pathol. 180:1340–1355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cruz-Solbes AS and Youker K: Epithelial to
mesenchymal transition (EMT) and endothelial to mesenchymal
transition (EndMT): Role and implications in kidney fibrosis.
Results Probl Cell Differ. 60:345–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chapman HA: Epithelial-mesenchymal
interactions in pulmonary fibrosis. Annu Rev Physiol. 73:413–435.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Piera-Velazquez S, Li Z and Jimenez SA:
Role of endothelial-mesenchymal transition (EndoMT) in the
pathogenesis of fibrotic disorders. Am J Pathol. 179:1074–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Coen M, Gabbiani G and Bochaton-Piallat
ML: Myofibroblast-mediated adventitial remodeling: An
underestimated player in arterial pathology. Arterioscler Thromb
Vasc Biol. 31:2391–2396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lim H and Moon A: Inflammatory fibroblasts
in cancer. Arch Pharm Res. 39:1021–1031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Padua D, Zhang XH, Wang Q, Nadal C, Gerald
WL, Gomis RR and Massagué J: TGFbeta primes breast tumors for lung
metastasis seeding through angiopoietin-like 4. Cell. 133:66–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hinz B: The myofibroblast: Paradigm for a
mechanically active cell. J Biomech. 43:146–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Otranto M, Sarrazy V, Bonté F, Hinz B,
Gabbiani G and Desmoulière A: The role of the myofibroblast in
tumor stroma remodeling. Cell Adh Migr. 6:203–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liao Z, Tan ZW, Zhu P and Tan NS:
Cancer-associated fibroblasts in tumor microenvironment-Accomplices
in tumor malignancy. Cell Immunol. 2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ireland LV and Mielgo A: Macrophages and
fibroblasts, key players in cancer chemoresistance. Front Cell Dev
Biol. 6:1312018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Farmer P, Bonnefoi H, Anderle P, Cameron
D, Wirapati P, Becette V, André S, Piccart M, Campone M, Brain E,
et al: A stroma-related gene signature predicts resistance to
neoadjuvant chemotherapy in breast cancer. Nat Med. 15:68–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pandol S, Edderkaoui M, Gukovsky I, Lugea
A and Gukovskaya A: Desmoplasia of pancreatic ductal
adenocarcinoma. Clin Gastroenterol Hepatol 7 (11 Suppl). S44–S47.
2009. View Article : Google Scholar
|
|
113
|
DuFort CC, Delgiorno KE and Hingorani SR:
Mounting pressure in the microenvironment: Fluids, solids, and
cells in pancreatic ductal adenocarcinoma. Gastroenterology.
150:1545–1557.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Christian S, Winkler R, Helfrich I, Boos
AM, Besemfelder E, Schadendorf D and Augustin HG: Endosialin (Tem1)
is a marker of tumor-associated myofibroblasts and tumor
vessel-associated mural cells. Am J Pathol. 172:486–494. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fujii S, Fujihara A, Natori K, Abe A,
Kuboki Y, Higuchi Y, Aizawa M, Kuwata T, Kinoshita T, Yasui W and
Ochiai A: TEM1 expression in cancer-associated fibroblasts is
correlated with a poor prognosis in patients with gastric cancer.
Cancer Med. 4:1667–1678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Diaz LA Jr, Coughlin CM, Weil SC, Fishel
J, Gounder MM, Lawrence S, Azad N, O'Shannessy DJ, Grasso L,
Wustner J, et al: A first-in-human phase I study of MORAb-004, a
monoclonal antibody to endosialin in patients with advanced solid
tumors. Clin Cancer Res. 21:1281–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Norris RE, Fox E, Reid JM, Ralya A, Liu
XW, Minard C and Weigel BJ: Phase 1 trial of ontuxizumab
(MORAb-004) in children with relapsed or refractory solid tumors: A
report from the Children's Oncology Group Phase 1 Pilot Consortium
(ADVL1213). Pediatr Blood Cancer. 65:e269442018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
D'Angelo SP, Hamid OA, Tarhini A,
Schadendorf D, Chmielowski B, Collichio FA, Pavlick AC, Lewis KD,
Weil SC, Heyburn J, et al: A phase 2 study of ontuxizumab, a
monoclonal antibody targeting endosialin, in metastatic melanoma.
Invest New Drugs. 36:103–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Grothey A, Strosberg JR, Renfro LA,
Hurwitz HI, Marshall JL, Safran H, Guarino MJ, Kim GP, Hecht JR,
Weil SC, et al: A randomized, double-blind, placebo-controlled
phase II study of the efficacy and safety of monotherapy
ontuxizumab (MORAb-004) plus best supportive care in patients with
chemorefractory metastatic colorectal cancer. Clin Cancer Res.
24:316–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Rouleau C, Gianolio DA, Smale R, Roth SD,
Krumbholz R, Harper J, Munroe KJ, Green TL, Horten BC, Schmid SM
and Teicher BA: Anti-endosialin antibody-drug conjugate: Potential
in sarcoma and other malignancies. Mol Cancer Ther. 14:2081–2089.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lee S: What tumor vessels can tell us.
Pigment Cell Melanoma Res. 23:309–311. 2010. View Article : Google Scholar : PubMed/NCBI
|