Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection. With its high morbidity and mortality, sepsis
poses a serious threat to human health, and has become a global
public health challenge (1). Organ
damage is caused by organ ischemia and infection in the progression
of sepsis, and mortality rises sharply to 56–100% when multiple
organ dysfunctions (MODS) occur (2,3).
Sepsis can further develop into septic shock and multiple organ
failure, and patients with severe sepsis often suffer from cardiac
dysfunction (4). Previous studies
have found that the heart is one of the most significantly damaged
organs in MODS. Approximately 50% of sepsis patients may have
cardiac dysfunctions, and the mortality rate can even rise to 70%
in the case that myocardial inhibition occurs in sepsis (5,6).
Therefore, in the treatment of sepsis, effective prevention and
treatment of myocardial damage is of great significance in
controlling the disease and preventing the deterioration of the
disease.
In essence, sepsis is caused by infection-induced
host immunity (7). Many factors
may be involved in sepsis-induced heart dysfunction, such as the
production of nitric oxide, release of inflammatory factors,
adhesion and infiltration of monocytes, activation of the
coagulation system, oxidative metabolic disorders and so forth
(8–10). However, the specific mechanism of
myocardial injury in sepsis is still not fully elucidated (11). When the system is infected by
bacteria, factors such as lipopolysaccharide (LPS) in bacteria
participate in the process of initial organism damage (12). LPS is the outermost
lipopolysaccharide of the Gram-negative bacterial cell wall, which
can bind to various protein molecules on the cell surface, initiate
intracellular signaling molecules, activate different tissues or
cells to produce cytokines, release oxygen free radicals and lipid
mediators, induce microvascular obstruction and changes in vascular
homeostasis, leading to coagulopathy, fever, vasodilatation,
capillary leakage, and ultimately resulting in multiple organ
failure of sepsis (13). Among
them, LPS can activate macrophages through the TLR4 receptor on
macrophages, and initiate gene expression of inflammatory factors,
such as tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6).
These inflammatory cytokines can directly lead to myocardial
dysfunction (14,15). The process of blocking these
pathways may be a potential strategy for treating myocardial damage
in sepsis.
Sestrin2, an important member of the Sestrin family,
is a highly conserved stress response protein that is induced in
response to stresses, such as DNA damage, oxidative stress and
hypoxia (16,17). Sestrin2 has been reported to
prevent oxidative liver damage (18) and age-related pathologies (19). Recent research has discovered that
Sestrin2 protects the myocardium from radiation-induced dysfunction
(20). There is further evidence
that Sestrin2 is involved in ischemic AMP-activated protein kinase
(AMPK) signaling and the toxicological actions of ischemic stress
to the heart (21,22). The mechanism of Sestrin2 in
sepsis-induced cardiac dysfunction remains unclear.
Hence, a model of LPS sepsis-induced myocardial
injury was constructed, and the mechanism of Sestrin2
overexpression was investigated using this model. As a result, it
was found that Sestrin2 overexpression could relieve cardiac
oxidative stress induced by LPS, which is valuable for the
treatment of myocardial damage in sepsis.
Materials and methods
Animal models
Female healthy Sprague-Dawley (SD) rats weighing
220–250 g, which were purchased from Beijing Vital River Company
(Beijing, China), were used for this study. The animals were
maintained in a 25°C atmosphere, with a cycle of 12 h light/12 h
dark and ad libitum access to food and water. The animal
model of endotoxin shock was established by injecting LPS (10
mg/kg) into the peritoneal cavity of the rats and the control group
was injected with normal saline. Blood was collected at 6, 12, and
24 h after LPS injection, rats were anesthetized by injection with
3% (v/v) pentobarbital sodium solution (40 mg/kg) into the
abdominal cavity, and blood was collected from the inferior vena
cava. The serum was separated by centrifugation at 1,000 × g for 10
min at 4°C and preserved at −80°C for subsequent experimentation.
The cardiac troponin T (CTnT) levels in the serum were assessed by
ELISA. In addition, heart specimens were taken 6, 12 and 24 h after
LPS injection. After anesthesia, rats were sacrificed using a
continuous flow of CO2 using a flow meter unit for 3–5
min at the flow rate of 2 l/min (displacement rate, 20% chamber
volume/min). The right region of the heart was homogenized for
ELISA detection of TNF-α and IL-6 levels, as well as colorimetric
analysis for malondialdehyde (MDA). The left region of the heart
was placed into paraformaldehyde fixative for 24 h, and used for
paraffin sections (4-µm thick) for hematoxylin and eosin (H&E)
staining. All procedures for animal care were approved by the
Animal Management Committee of The First Affiliated Hospital of
Soochow University.
Cell treatment
H9c2 rat embryo cardiomyocytes were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
used for cell transfection and LPS injury. Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) were used for cell
culture. Sestrin2 siRNA was purchased from MISSION predesigned
siRNA (Sigma-Aldrich; Merck KGaA). The sequences of si-Sestrin2
were as follows: Sense, 5′-CAGAGUAUUGUAACAU-3′ and antisense,
5′-AUAGUGUUACAAUACUCUG-3′. Sestrin2 siRNA (50 nM, siSestrin2 group)
and Sestrin 2 overexpression (Sestrin2 group) recombinant plasmids
(Sigma-Aldrich; Merck KGaA), as well as the empty vector (NC group)
were respectively transfected into H9c2 cells using Lipofectamine
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Cells without
any treatment were used for control. Then, 50 µg/ml LPS was applied
in all the above groups for 48 h for most experiments of cell
functions, namely, LPS, LPS+NC, LPS+Sestrin2, LPS+siSestrin2
groups. After a 48-h incubation, the cells were collected and used
for subsequent experiments.
Hematoxylin and eosin (H&E)
staining
The paraffin sections of the hearts excised at 6, 12
and 24 h after LPS injection were first dewaxed with xylene I and
II for 15 min, and then dewaxed in gradient ethanol at different
concentrations (100, 95, 85 and 70%, respectively) for 3 min each,
and subsequently rinsed with steaming water for 5 min. Next, the
sections were stained with hematoxylin for 10 min and then stained
with eosin for 5 min. The sections were again placed into gradient
concentrations of ethanol (70, 85, 95 and 100%, respectively) for 3
min each for dehydration. After that, the slices were clarified
with xylene I, II for 5 min each time and finally sealed with
neutral gum.
Cell viability assay
The cell viability of H9c2 cells treated with LPS
(0, 5, 10, 50 µg/ml) for 0, 24 and 48 h was measured using a Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology,
Nantong, China) first. In addition, cell viability of the cells in
the different groups (control, NC, Sestrin2, siSestrin2, LPS,
LPS+NC, LPS+Sestrin2, LPS+siSestrin2 groups) was also determined.
The processes were mainly performed as follows: cells were cultured
in 96-well plates (5×103 cells/well) for 0, 24 and 48 h
and stained with 20 µl CCK-8 stain for 1 h. The optical density
values (OD) at 450 nm as detected by a microplate reader (BioTek,
Winooski, VT, USA) were used for cell viability analysis.
Enzyme linked immunosorbent assay
(ELISA)
The quantities of CTnT in rat blood, and TNF-α and
IL-6 in rat hearts collected at 6, 12, and 24 h after LPS
injection, as well as CTnT, TNF-α and IL-6 in the different cell
groups (control, NC, Sestrin2, siSestrin2, LPS, LPS+NC,
LPS+Sestrin2, LPS+siSestrin2 groups) were determined by ELISA kits
(Cusabio, Wuhan, China), following the manufacturer's instructions.
The samples and standard substances were added into 96-well plate
and incubated for 90 min at 37°C, cultured with biotinylated
antibodies for 60 min, and later seeded with avidin peroxidase
complex for 30 min before tetramethylbenzidine (TMB) coloration.
Finally, OD values at 450 nm as read by a microplate reader
(BioTek) were used for quantity calculation.
Colorimetric detection
The levels of lipid peroxidation product MDA in rat
heart collected at 6, 12, and 24 h after LPS injection, as well as
the levels in the different cell groups (control, NC, Sestrin2,
siSestrin2, LPS, LPS+NC, LPS+Sestrin2, LPS+siSestrin2 groups) were
determined with the Lipid Peroxidation MDA Assay kit (Beyotime
Institute of Biotechnology). According to the manufacturer's
instructions, MDA reacts with thiobarbituric acid (TBA) and the red
MDA-TBA products were observed at 535 nm using a colorimetric
method.
Western blot analysis
The protein levels of ribosomal protein S6 kinase
(p-S6K), phosphorylated AMP-activated protein kinase (p-AMPK) and
Sestrin2 in the heart tissues of rats treated with LPS (10 mg/kg)
for 6, 12 and 24 h, as well as these proteins in the different cell
groups (control, NC, Sestrin2, siSestrin2, LPS, LPS+NC,
LPS+Sestrin2, LPS+siSestrin2 groups) were detected by western blot
analysis. First, the total proteins were extracted using RIPA lysis
buffer (Boster Biological Technology, China), and quantified by BCA
protein assay reagent kit (Beyotime Institute of Biotechnology).
Then, 10 µg protein was loaded in each lane and were separated by
12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF)
membranes (Amersham; GE Healthcare, UK). The PVDF membranes were
blocked with Tris-buffered saline (TBS) containing 5% skim milk
with 0.1% Tween 20 at 37°C for 1 h, and then the membranes were
incubated overnight at 4°C with the following primary antibodies:
p-AMPK antibody (cat. no. 4186; dilution, 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), AMPK antibody (cat. no. 12063;
dilution, 1:1,000; Cell Signaling Technology, Inc.), p-S6K antibody
(cat. no. 9209; dilution, 1:1,000; Cell Signaling Technology,
Inc.), S6K antibody (cat. no. 9202; dilution, 1:1,000; Cell
Signaling Technology, Inc.), anti-Sestrin2 (ab178518; dilution,
1:1,000; Abcam, Cambridge, UK) and anti-GAPDH (cat. no. ab8245;
dilution, 1:1,000; Abcam). GAPDH was used as internal control.
Subsequently, the proteins were probed with goat anti-rabbit
secondary antibody (HRP) (cat. no. Ab205718, 1:5,000; Abcam) for 1
h at 37°C. The proteins were detected by enhanced chemiluminescence
(ECL) reagents (Amersham; GE Healthcare) and X-ray exposure.
Finally, the proteins were quantified using Bio-Rad ChemiDoc system
with Image Lab software (version 6.0; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Data are presented as mean ± standard deviations,
and were statistical analyzed using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA), one-way analysis of variance (ANOVA), and
Dunnett's test. P<0.05 was considered as indicative of
statistical significance.
Results
LPS induces changes in myocardial
morphology and inflammatory responses in the rats
The morphological structures of the myocardial
tissues of rats injected with LPS (10 mg/kg) for 6, 12 and 24 h
were determined by H&E staining. The images showed that the
fiber bundles in the control group were normal, neatly arranged,
without fractures and dissolution, and no edema was observed in the
interstitial. The sepsis rat tissues at 6 h after injection were
observed as normal and neatly arranged myocardial fiber bundles,
with moderate inflammatory cell infiltration. At 12 h, the
myocardial fiber bundles were loosely arranged; some myocardial
fibers were broken and dissolved, accompanied by mild interstitial
edema and moderate inflammatory cell infiltration. At 24 h,
myocardial fiber bundles were loosely arranged, some myocardial
fibers were broken and dissolved, partial myocardial cells were
degenerated and dissolved, accompanied by necrosis, interstitial
edema, and moderate inflammatory cell infiltration (Fig. 1A). Then, the levels of inflammatory
factors including CTnT, TNF-α and IL-6 in rat blood collected at 6,
12 and 24 h after LPS injection were measured by ELISA. The results
showed that serum CTnT levels were increased time-dependently.
Levels of TNF-α and IL-6 in the heart were increased by LPS,
reached a peak at 6 h after LPS injection and slightly decreased
subsequently (P<0.05; Fig.
1B-D). In addition, the lipid peroxidation product MDA levels
in the heart were found to be increased time-dependently, as
determined by the colorimetric method (P<0.05; Fig. 1E).
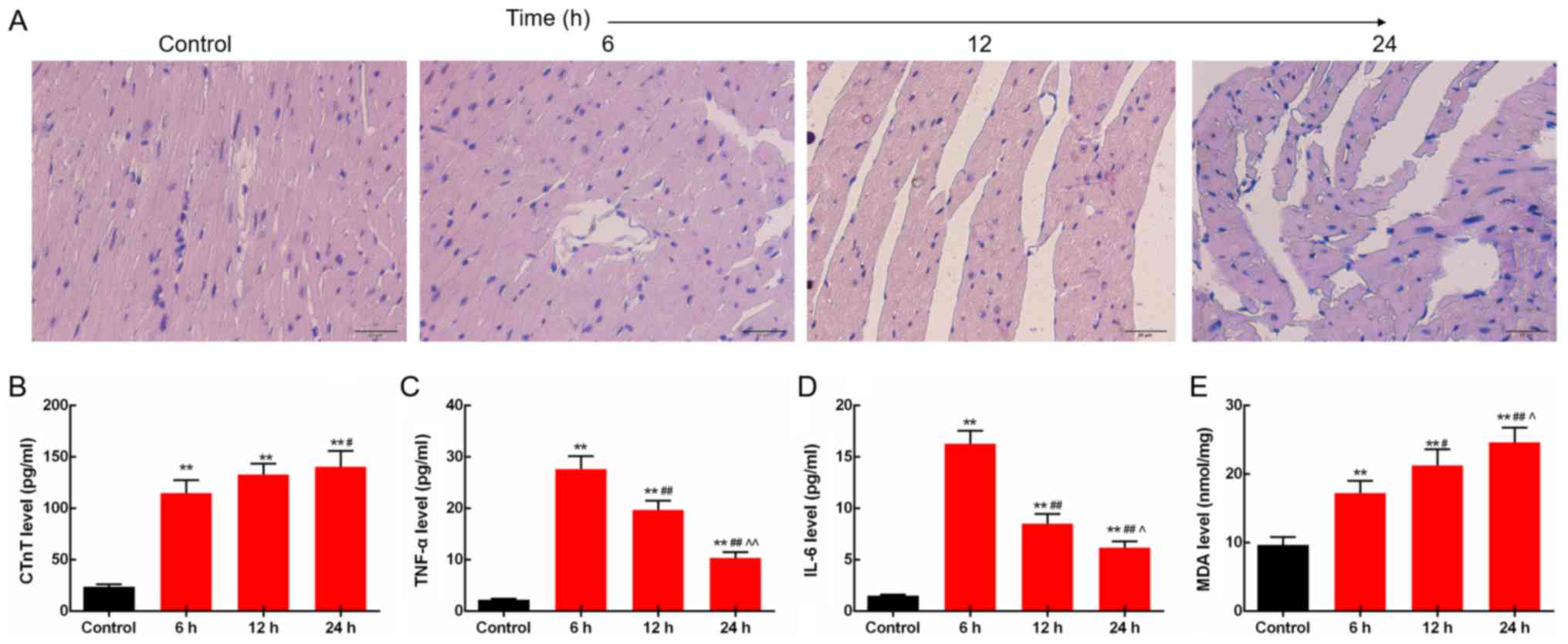 | Figure 1.LPS induces changes in myocardial
morphology and inflammatory responses in rats. (A) In the septic
rats, myocardial fiber rupture, interstitial edema, and
inflammatory cell infiltration were observed under light
microscope. Scale bar, 20 µm. (B) Serum CTnT levels were increased
time-dependently. (C) TNF-α levels in the heart were increased by
LPS. (D) Protein expression level of IL-6 in the heart was
increased by LPS. (E) Levels of lipid peroxidation product MDA in
the heart were increased time-dependently. **P<0.01 vs. the
control group. #P<0.05 and ##P<0.01 vs.
the 6 h group, ^P<0.05 and ^^P<0.01 vs.
the 12 h group. LPS, lipopolysaccharide; CTnT, cardiac troponin T;
TNF-α, tumor necrosis factor-α; IL-6, levels of interleukin 6; MDA,
malondialdehyde; AMPK, AMP-activated protein kinase. |
LPS regulates the expression levels of
p-S6K, p-AMPK and Sestrin2 in the rats
The protein levels of S6K, p-S6K, p-AMPK, AMPK and
Sestrin2 were determined by western blot analysis in the rats
injected with LPS (10 mg/kg) for 6, 12 and 24 h. The results
demonstrated that the protein levels of p-S6K were adaptively
decreased, and those of p-AMPK and Sestrin2 were adaptively
increased by LPS treatment. Moreover, changes at 6 h were the most
significant (P<0.01; Fig. 2).
Notably, the protein expression levels of total S6K and AMPK were
not significantly changed.
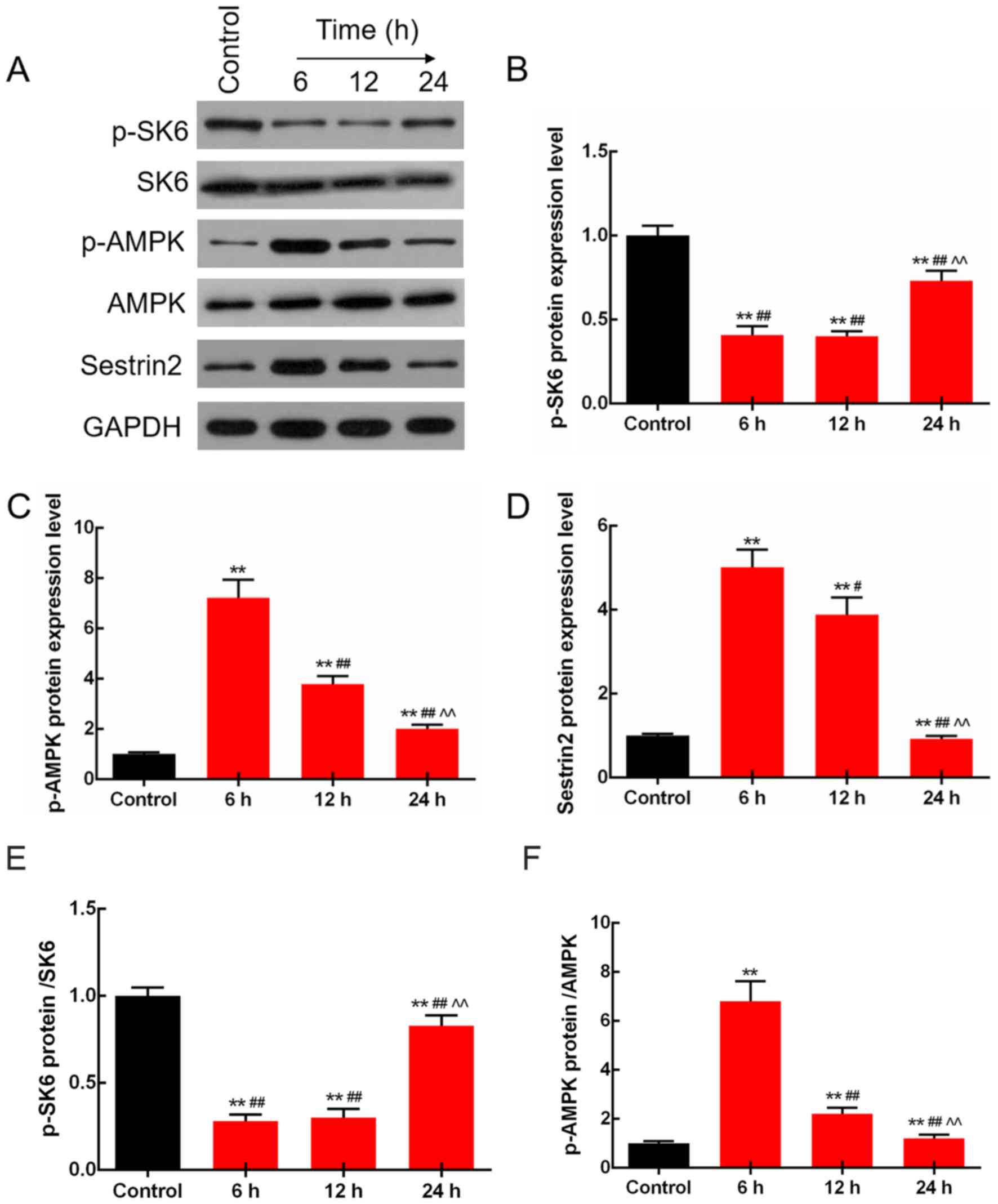 | Figure 2.LPS regulates the expression levels of
p-S6K, p-AMPK and Sestrin2 in rats. (A) p-S6K, S6K, p-AMPK, AMPK
and Sestrin2 protein levels were detected by western blot analysis.
(B) Protein levels of p-S6K were adaptively decreased, and those of
(C) p-AMPK (D) and Sestrin2 were adaptively increased by LPS
treatment, and the change at 6 h was the most significant. (E) The
ratio of p-S6K/S6K protein was significantly decreased. (F) The
ratio of p-AMPK/AMPK was adaptively increased. **P<0.01 vs. the
control group. #P<0.05 and ##P<0.01 vs.
the 6 h group, ^^P<0.01 vs. the 12 h group. LPS,
lipopolysaccharide; p-, phosphorylated; AMPK, AMP-activated protein
kinase. |
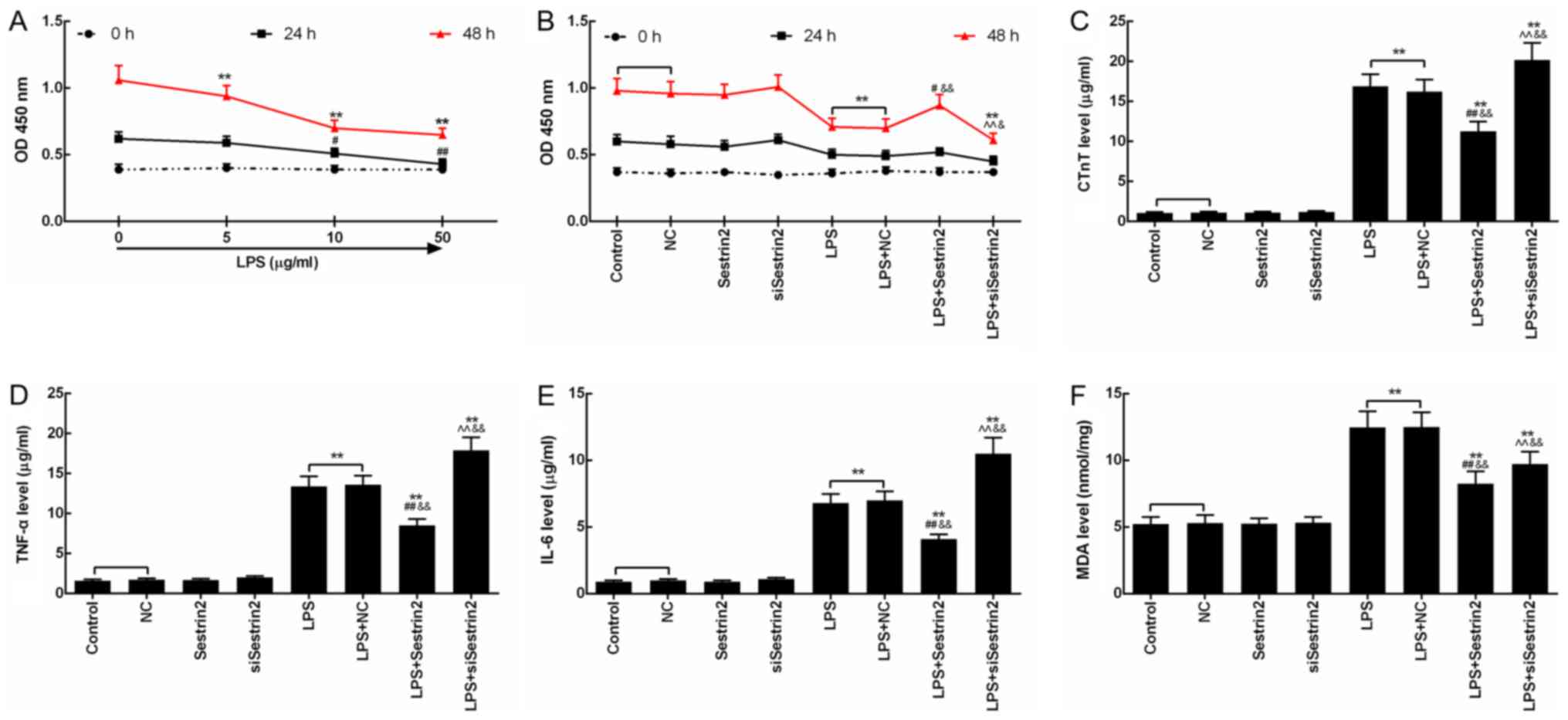
Sestrin2 alleviates LPS-mediated
injured cell viability and LPS-enhanced inflammatory response in
cardiomyocytes
The injury effect of LPS on cardiomyocytes was
evaluated by CCK-8 assay. Cell viability of the cardiomyocytes
treated with LPS (5, 10 and 50 µg/ml) for 24 and 48 h was inhibited
time-dependently, and the decrease in cell viability of the
cardiomyocytes treated with 50 µg/ml LPS at 48 h was most
significant (P<0.05; Fig. 3A).
Then, the function of Sestrin2 on cell viability of the
cardiomyocytes was detected at 24 and 48 h after LPS treatment. The
results showed that the cell viability changed significantly after
48 h of LPS injury. Compared with the control group, cell viability
in the LPS and LPS+NC groups was significantly suppressed following
treatment with LPS. Cell viability in the LPS+Sestrin2 group was
significantly increased, and cell viability in the LPS+siSestrin2
group was significantly decreased, compared with the LPS group.
Cell viability in the LPS+Sestrin2 group was lower than that in the
Sestrin2 group, and a similar tendency between that in the
LPS+siSestrin2 group and in the siSestrin2 group was observed
(P<0.05; Fig. 3B). Then, levels
of inflammatory factors such as CTnT, TNF-α and IL-6 were detected,
showing a significant increase in the LPS and LPS+NC groups, a
decrease in the LPS+Sestrin2 group by Sestrin2, and an increase in
the LPS+siSestrin2 group by siSestrin2 (Fig. 3C-E). In addition, levels of lipid
peroxidation product MDA as determined by colorimetric method,
indicated that the MDA levels were increased in the LPS group, and
decreased in the LPS+Sestrin2 group (Fig. 3F).
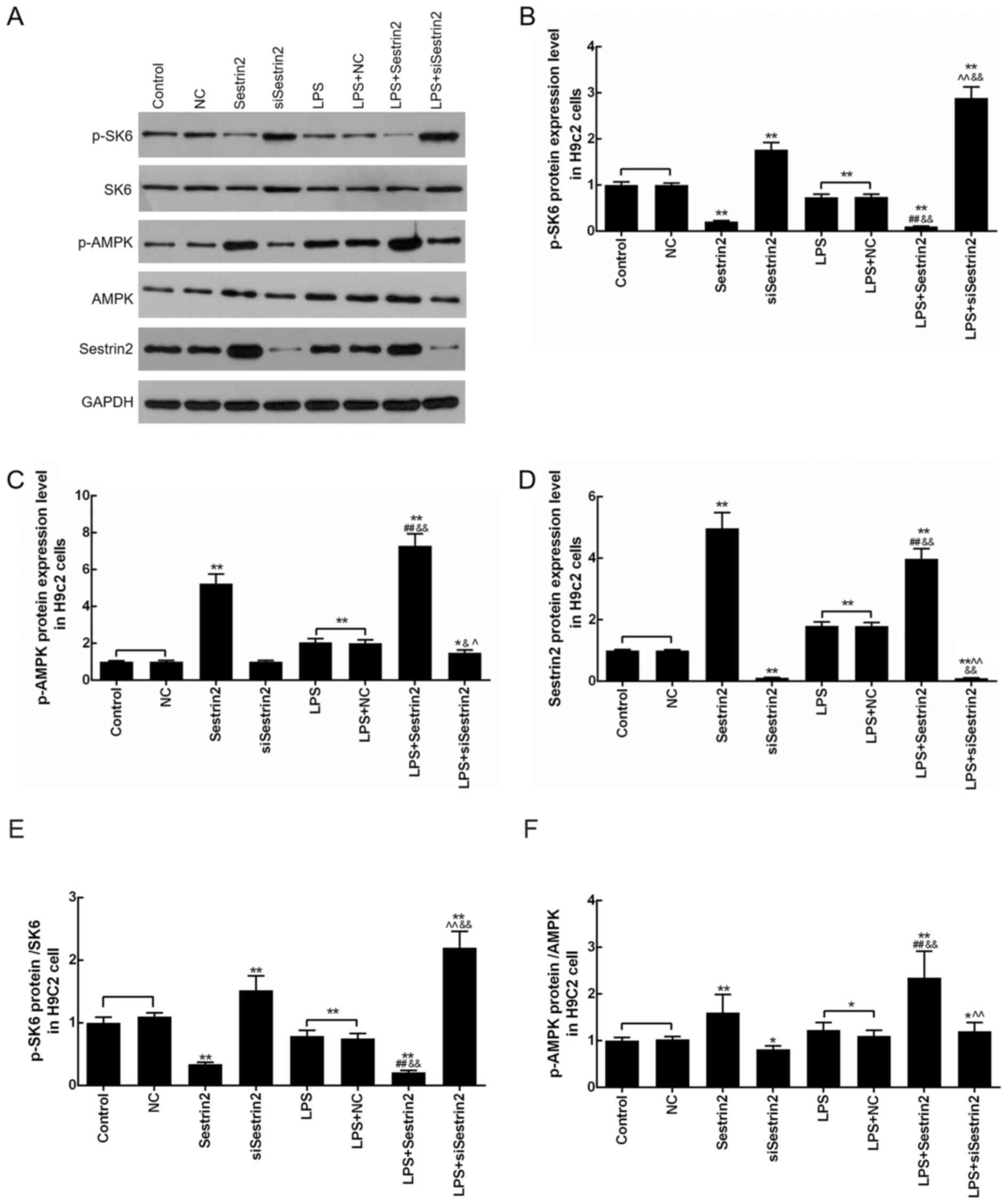
Sestrin2 alleviation of LPS-mediated
injured cell viability is related to regulated levels of p-S6K and
p-AMPK in cardiomyocytes
Levels of p-S6K, p-AMPK and Sestrin2 in
cardiomyocytes were evaluated by western blot analysis after
Sestrin2 overexpression or siSestrin2 transfection or LPS
treatment. The results demonstrated that p-S6K levels were
significantly decreased in the LPS, LPS+NC and Sestrin2 groups, and
markedly increased in the siSestrin2 group, compared with the
control group. Meanwhile, p-S6K levels were decreased in the
LPS+Sestrin2 group, and increased in the LPS+siSestrin2 group,
compared with the LPS group (Fig. 4A
and B). In addition, levels of p-AMPK and Sestrin2 were
increased in the LPS, LPS+NC and Sestrin2 groups, and decreased in
the siSestrin2 group, compared with the control group; while p-AMPK
and Sestrin2 levels were increased in the LPS+Sestrin2 group, and
decreased in LPS+siSestrin2 group, compared with the LPS group. The
present results suggested that there was no significant difference
in S6K and AMPK levels between the LPS+siSestrin2 group and the LPS
group (Fig. 4A, C-F).
Discussion
Lipopolysaccharide (LPS), an important component of
the outer membrane of the cell wall of Gram-negative bacteria,
plays an important role in the infection and disease evolution of
Gram-negative bacteria, and is considered to be the main cause of
systemic inflammatory syndrome (23). Sepsis-induced myocardial injury is
one of the manifestations of multiple organ dysfunctions in sepsis,
which has been confirmed in clinical and sepsis animal experiments
(24). LPS is an important
substance that causes myocardial damage, by triggering the
inflammatory response and seriously affecting cardiac function
(25). The sepsis model by
injection of LPS can mimic the complex pathophysiological changes
of the body caused by bacterial release of LPS during sepsis
(26). In the present study, an
animal model of sepsis was prepared by intraperitoneal injection of
LPS (10 mg/kg) into SD rats. In the septic rats, myocardial fiber
rupture, interstitial edema, and inflammatory cell infiltration
were observed under light microscope. The above processes were
gradually increased as time prolonged, and myocardial tissue
necrosis was observed at 24 h after LPS injection. Thus, it is
feasible to prepare an animal model of myocardial injury in sepsis
by intraperitoneal injection of LPS (10 mg/kg) into SD rats. Serum
CTnT is a sensitive indicator of myocardial injury, and elevated
serum CTnT is observed in 31–85% of patients with severe sepsis
(27). In patients with septic
shock, an increase in serum CTnT predicts a higher mortality rate
and a poor prognosis (28). In the
present study, it was observed that CTnT was increased in serum 6 h
after LPS injection, and gradually increased time-dependently.
LPS, by initiating transcription and translation of
various cytokine genes, causes inflammatory cells to release large
amounts of inflammatory factors (29). TNF-α is recognized as a main
mediator of septic shock, and is the first inflammatory factor
produced in the early stage of sepsis (30). As a triggering factor of
inflammation, TNF-α is involved in the induction of IL-1
production, and together with IL-1 induces the formation of
secondary inflammatory factors such as IL-6, resulting in an
inflammatory cascade (31,32). In the serum of patients with
sepsis, inflammatory factors, such as TNF-α and IL-6 are elevated,
and these inflammatory factors have a direct inhibitory effect on
myocardial cell contraction (33).
IL-6 causes myocardial damage by activating nitric oxide synthase,
and elimination of IL-6 relieves myocardial damage in sepsis
(34). Injecting TNF-α into
animals can replicate the complex metabolic, hemodynamic, and
pathological changes in septic shock syndrome, whereas rats with
TNF-α-encoding gene loss do not develop septic shock after LPS
injection (35). Therefore, TNF-α
and IL-6 are the main inflammatory factors in sepsis-induced
multiple organ dysfunctions (MODS). In the present study, TNF-α and
IL-6 were significantly increased in the myocardial homogenate,
indicating that TNF-α and IL-6 were involved in the inflammatory
reaction after LPS injection. In addition, the concentrations of
TNF-α and IL-6 at 6 h were higher than those at 12 and 24 h after
LPS injection, indicating that concentrations of TNF-α and IL-6
peaked in the myocardial homogenate 6 h after LPS injection, and
gradually decreased time-dependently. The cause of its occurrence
is unknown and may be related to the metabolism and the adaptive
upregulation response. In addition, MDA is a degradation product of
lipid peroxide, and its content is positively correlated with the
degree of cell damage (36). The
changes in MDA content can indirectly reflect the changes in oxygen
free radical content in tissues (37). In the present study, after the
establishment of the sepsis model, MDA content in the myocardial
homogenate increased significantly, and the concentration gradually
increased with time, indicating that the oxidative damage gradually
increased time-dependently after LPS injection.
The signaling pathways related to inflammatory
reactions should play important roles in sepsis-induced cardiac
dysfunctions. mTOR is a type of conserved kinase, which stimulates
cell anabolism and growth by increasing the synthesis of proteins
and lipids through p-S6K (19).
p-S6K was found to be increased in tubulointerstitial inflammation
and fibrosis (38). In addition,
the important regulatory role of the AMPK pathway in the
inflammatory response has also drawn much attention. In
vitro studies have found that AMPK activation can also
significantly reduce the expression of inflammatory mediators and
tissue inflammatory damage, and it is an important new target for
inflammation regulation (39–41).
In this study, after the establishment of the sepsis model, the
expression levels of p-S6K were decreased at 6 h after LPS
injection, and increased again at 12 and 24 h. The expression
levels of p-AMPK and Sestrin2 were increased at 6 h after LPS
injection, and decreased at 12 and 24 h. The cause of its
occurrence is unknown and may be related to the adaptive
upregulation response.
Sestrin2, as an important oxidative stress response
protein, was reported to protect the myocardium from
radiation-induced dysfunction (20). We overexpressed Sestrin2 and
silenced Sestrin2 in LPS-injured H9c2 cells. The results indicated
that the cell viability of the LPS-injured H9c2 cells was enhanced
by Sestrin2 over-expression, whereas the cell viability was
inhibited by Sestrin2 interference. Decreased levels of CTnT,
TNF-α, IL-6 and MDA in the LPS-injured H9c2 cells by Sestrin2
overexpression were observed. The results suggested that Sestrin2
overexpression inhibited the inflammatory response and the lipid
peroxidation chain reaction of the cell membrane, leading to a
reduction in myocardial tissue damage. In regards to the
inflammation related signaling, Sestrin2 overexpression promoted
levels of Sestrin2 and p-AMPK, and inhibited p-S6K levels, while
Sestrin2 interference inhibited levels of Sestrin2 and p-AMPK, and
promoted p-S6K levels in H9c2 cells with or without LPS injury.
Although LPS showed the same tendency as Sestrin2 in H9c2 cells,
this may depend on the adaptive upregulation response.
In conclusion, Sestrin2 promoted cell viability and
inhibited inflammatory responses in LPS-injured myocardial cells.
The phenomena may be associated with inhibition of p-S6K and
activation of the p-AMPK pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Suzhou
Municipal Science and Technology Project (grant no. SYS201616), the
Natural Science Foundation of Jiangsu Province of China (grant no.
BK20170369) and the National Natural Science Foundation of China
(grant no. 81701213).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW wrote the manuscript. LB and PY performed the
experiments. ZW designed the study. SF and FX analyzed the data. ZW
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures for animal care were approved by the
Animal Management Committee of The First Affiliated Hospital of
Soochow University, Suzhou, Jiangsu.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McNevin C, McDowell R, Fitzpatrick F,
O'Sullivan R and Wakai A: The prevalence of severe sepsis or septic
shock in an irish emergency department. Ir Med J.
111:6922018.PubMed/NCBI
|
|
2
|
Koivikko P, Arola O, Inkinen O and
Tallgren M: One-year survival after inhospital cardiac arrest-does
prearrest sepsis matter? Shock. 50:38–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruangchan S, Chusri S, Saengsanga P,
Kiamkan N, Phunpairoth P and Chayakul P: Clinical outcomes of
community-acquired severe sepsis after implementation of a simple
severe sepsis fast track. J Med Assoc Thai. 99:877–885.
2016.PubMed/NCBI
|
|
4
|
Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L,
Kalbfleisch JH, Gao X, Kao RL, Williams DL and Li C: Attenuation of
cardiac dysfunction in polymicrobial sepsis by MicroRNA-146a is
mediated via targeting of IRAK1 and TRAF6 expression. J Immunol.
195:672–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walley KR: Sepsis-induced myocardial
dysfunction. Curr Opin Crit Care. 24:292–299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fattahi F, Frydrych LM, Bian G, Kalbitz M,
Herron TJ, Malan EA, Delano MJ and Ward PA: Role of complement C5a
and histones in septic cardiomyopathy. Mol Immunol. 102:32–41.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sagy M, Al-Qaqaa Y and Kim P: Definitions
and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health
Care. 43:260–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abraham E and Singer M: Mechanisms of
sepsis-induced organ dysfunction. Crit Care Med. 35:2408–2416.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu YC, Yu MM, Shou ST and Chai YF:
Sepsis-induced cardiomyopathy: Mechanisms and treatments. Front
Immunol. 8:10212017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cimolai MC, Alvarez S, Bode C and Bugger
H: Mitochondrial mechanisms in septic cardiomyopathy. Int J Mol
Sci. 16:17763–17778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C, Wu K, Li SH and You Q: Protective
effect of curcumin against cardiac dysfunction in sepsis rats.
Pharm Biol. 51:482–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cavaillon JM and Adib-Conquy M:
Bench-to-bedside review: Endotoxin tolerance as a model of
leukocyte reprogramming in sepsis. Crit Care. 10:2332006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong W, Kang K, Gao Y, Liu H, Meng X, Yang
S, Yu K and Zhao M: Dexmedetomidine alleviates LPS-induced septic
cardiomyopathy via the cholinergic anti-inflammatory pathway in
mice. Am J Transl Res. 9:5040–5047. 2017.PubMed/NCBI
|
|
14
|
Zhao P, Kuai J, Gao J, Sun L, Wang Y and
Yao L: Delta opioid receptor agonist attenuates
lipopolysaccharide-induced myocardial injury by regulating
autophagy. Biochem Biophys Res Commun. 492:140–146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silwal P, Lim K, Heo JY, Park JI, Namgung
U and Park SK: Adenine attenuates lipopolysaccharide-induced
inflammatory reactions. Korean J Physiol Pharmacol. 22:379–389.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Budanov AV and Karin M: Sestrins
orchestrate cellular metabolism to attenuate aging. Cell Metab.
18:792–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JH, Budanov AV, Talukdar S, Park EJ,
Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, et al:
Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell
Metab. 16:311–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bae SH, Sung SH, Oh SY, Lim JM, Lee SK,
Park YN, Lee HE, Kang D and Rhee SG: Sestrins activate Nrf2 by
promoting p62-dependent autophagic degradation of Keap1 and prevent
oxidative liver damage. Cell Metab. 17:73–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Budanov AV, Park EJ, Birse R, Kim
TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E and Karin M:
Sestrin as a feedback inhibitor of TOR that prevents age-related
pathologies. Science. 327:1223–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng YC, Chi F, Xing R, Zeng J, Gao S,
Chen JJ, Wang HM, Duan QY, Sun YN, Niu N, et al: Sestrin2 protects
the myocardium against radiation-induced damage. Radiat Environ
Biophys. 55:195–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun W, Quan N, Wang L, Yang H, Chu D, Liu
Q, Zhao X, Leng J and Li J: Cardiac-specific deletion of the Pdha1
gene sensitizes heart to toxicological actions of ischemic stress.
Toxicol Sci. 151:193–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morrison A, Chen L, Wang J, Zhang M, Yang
H, Ma Y, Budanov A, Lee JH, Karin M and Li J: Sestrin2 promotes
LKB1-mediated AMPK activation in the ischemic heart. FASEB J.
29:408–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mendes SJF, Sousa FIAB, Pereira DMS, Ferro
TAF, Pereira ICP, Silva BLR, Pinheiro AJMCR, Mouchrek AQS,
Monteiro-Neto V, Costa SKP, et al: Cinnamaldehyde modulates
LPS-induced systemic inflammatory response syndrome through
TRPA1-dependent and independent mechanisms. Int Immunopharmacol.
34:60–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abulizi P, Loganathan N, Zhao D, Mele T,
Zhang Y, Zwiep T, Liu K and Zheng X: Growth differentiation
factor-15 deficiency augments inflammatory response and exacerbates
septic heart and renal injury induced by lipopolysaccharide. Sci
Rep. 7:10372017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huxtable AG, Smith SM, Vinit S, Watters JJ
and Mitchell GS: Systemic LPS induces spinal inflammatory gene
expression and impairs phrenic long-term facilitation following
acute intermittent hypoxia. J Appl Physiol (1985). 114:879–887.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong W, Kang K, Gao Y, Liu H, Meng X, Cao
Y, Yang S, Liu W, Zhang J, Yu K and Zhao M: GTS-21 protected
against LPS-induced sepsis myocardial injury in mice through
α7nAChR. Inflammation. 41:1073–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahman A and Broadley SA: Review article:
Elevated troponin: Diagnostic gold or fool's gold? Emerg Med
Australas. 26:125–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choon-ngarm T and Partpisanu P: Serum
cardiac troponin-T as a prognostic marker in septic shock. J Med
Assoc Thai. 91:1818–1821. 2008.PubMed/NCBI
|
|
29
|
Yuan Y, Ding D, Zhang N, Xia Z, Wang J,
Yang H, Guo F and Li B: TNF-α induces autophagy through ERK1/2
pathway to regulate apoptosis in neonatal necrotizing enterocolitis
model cells IEC-6. Cell Cycle. 17:1390–1402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lv S, Han M, Yi R, Kwon S, Dai C and Wang
R: Anti-TNF-α therapy for patients with sepsis: A systematic
meta-analysis. Int J Clin Pract. 68:520–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tripsianis G, Papadopoulou E,
Anagnostopoulos K, Botaitis S, Katotomichelakis M, Romanidis K,
Kontomanolis E, Tentes I and Kortsaris A: Coexpression of IL-6 and
TNF-α: Prognostic significance on breast cancer outcome. Neoplasma.
61:205–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hua F, Li CH, Wang H and Xu HG:
Relationship between expression of COX-2, TNF-α, IL-6 and
autoimmune-type recurrent miscarriage. Asian Pac J Trop Med.
6:990–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hwang TL and Yeh CC: Hemodynamic and
hepatic microcirculational changes in endotoxemic rats treated with
different NOS inhibitors. Hepatogastroenterology. 50:188–191.
2003.PubMed/NCBI
|
|
34
|
Ward JR, Wilson HL, Francis SE, Crossman
DC and Sabroe I: Translational mini-review series on immunology of
vascular disease: Inflammation, infections and Toll-like receptors
in cardiovascular disease. Clin Exp Immunol. 156:386–394. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aosasa S, Ono S, Mochizuki H, Tsujimoto H,
Ueno C and Matsumoto A: Mechanism of the inhibitory effect of
protease inhibitor on tumor necrosis factor alpha production of
monocytes. Shock. 15:101–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsikas D, Rothmann S, Schneider JY, Suchy
MT, Trettin A, Modun D, Stuke N, Maassen N and Frölich JC:
Development, validation and biomedical applications of
stable-isotope dilution GC-MS and GC-MS/MS techniques for
circulating malondialdehyde (MDA) after pentafluorobenzyl bromide
derivatization: MDA as a biomarker of oxidative stress and its
relation to 15(S)-8-iso-prostaglandin F2α and nitric oxide (NO). J
Chromatogr B Analyt Technol Biomed Life Sci. 1019:95–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou F, Sun W and Zhao M: Controlled
formation of emulsion gels stabilized by salted myofibrillar
protein under malondialdehyde (MDA)-induced oxidative stress. J
Agric Food Chem. 63:3766–3777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang B, Ding W, Zhang M, Li H and Gu Y:
Rapamycin attenuates aldosterone-induced tubulointerstitial
inflammation and fibrosis. Cell Physiol Biochem. 35:116–125. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xue B, Yang Z, Wang X and Shi H: Omega-3
polyunsaturated fatty acids antagonize macrophage inflammation via
activation of AMPK/SIRT1 pathway. PLoS One. 7:e459902012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Calvert JW, Gundewar S, Jha S, Greer JJ,
Bestermann WH, Tian R and Lefer DJ: Acute metformin therapy confers
cardioprotection against myocardial infarction via
AMPK-eNOS-mediated signaling. Diabetes. 57:696–705. 2008.
View Article : Google Scholar : PubMed/NCBI
|