Introduction
Teeth develop as a result of sequential and
reciprocal interactions between the oral ectoderm and neural
crest-derived mesenchyme (1). In
the absence of the dental epithelium or dental mesenchyme, tooth
morphogenesis would be abolished (2). Bone morphogenetic protein (3), fibroblast growth factor (4), Hedgehog (5) and wingless/integrated (Wnt) families
(6) are involved in this
epithelial-mesenchymal interaction.
Osteoprotegerin is a member of the tumor necrosis
factor receptor superfamily (7).
Lacking transmembrane and cytoplasmic domains, it is presented
mainly in soluble form in extracellular fluids. Functioning as a
decoy receptor for receptor activator of nuclear factor kappa B
ligand (RANKL), it is an integral part of the
RANKL/RANK/osteoprotegerin system because it ‘tunes’ the balance
between the formation and resorption of bone (8). Recent studies have demonstrated that
osteoprotegerin is involved in orthodontic tooth movement (9), periodontitis (10), tooth eruption (11) and various other physiologic or
pathologic processes (12).
However, whether osteoprotegerin is involved in tooth development
has not been studied.
In the present study, osteoprotegerin expression was
dynamically assessed during teeth development. In vitro and
in vivo experiments were carried out to ascertain if removal
of the dental epithelium could lift suppression of osteoprotegerin
expression and generate an effect similar to that elicited by
osteoprotegerin treatment. Finally, it was investigated whether
osteoprotegerin could influence mesenchymal Wnt/β-catenin activity
(13).
Materials and methods
Ethical approval of the study
protocol
The study protocol for animal studies was approved
by the Ethics Committee of the Hospital of Stomatology within Sun
Yat-sen University (ERC-2013-15; Guangzhou, China).
Animals
The ‘tooth germ’ is an aggregation of cells that
eventually forms a tooth. Our aim was to isolate specimens of tooth
germs at different stages of tooth development for further study,
and to carry out experiments in vitro and in
vivo.
Female Chinese Kunming mice (n=9, 4 weeks old),
Chinese Kunming mice (n=20, 7 days old) and 4 male nude mice (n=4,
4 weeks old) were purchased from San Yet-sun University. For all
experiments involving isolation of tooth germs, three mice were
used for each group. In each experiment, the mice from all groups
originated from same pregnant mouse to minimize genetic
differences. In total, 6 mice were sacrifice at days E14.5 (25±1.5
g), 3 mice were sacrifice at days E16.5 (25±1.5 g), 20 mice were
sacrificed at P7 (6.4 g) and 4 nude mice (20 g) were used for
Subrenal capsule assays. All mice were kept at 26–28°C, 40%
humidity, and 10-h light and 14-h dark cycle. All mice were fed
ad libitum with drinking water filtered and autoclaved
before use.
Organ cultures
In order to study the influence of various factors
on tooth-germ development in a controlled environment, tooth germs
of first molars were dissected from the mandibles of embryonic day
14.5 (E14.5) mice and cultured in vitro. The tissue
surrounding tooth germs was removed carefully under microscope
guidance. The effect of the removal of the dental epithelium on
tooth development was analyzed. First, isolated tooth germs were
incubated in 1.2 U/ml of dispase II (cat. no. 17105041; Gibco;
Thermo Fisher Scientific, Inc.) for 5 min at room temperature, and
then the dental mesenchyme was isolated with a fine needle. Tooth
germs and the isolated dental mesenchyme were cultured for 7 days
on 6-well Transwell™ plates with 1,000 µl/well of Dulbecco's
modified Eagle's medium (cat. no. 11885092; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum, 100
µg/ml of ascorbic acid (cat. no. PHR1008; Sigma-Aldrich; Merck
KGaA) and 2 mM of L-glutamine (cat. no. 25030081, Gibco; Thermo
Fisher Scientific, Inc.). Recombinant mouse osteoprotegerin protein
(100 or 200 ng/ml; cat. no. 375-TL; R&D Systems) was added to
the culture medium to study its effect on tooth development.
Cultures were incubated at 37°C in a humidified atmosphere of 5%
CO2. The culture medium was changed every 3 days.
Tissue preparation and histology. To assess
osteoprotegerin expression dynamically during tooth development,
mice were sacrificed at E14.5, E16.5, post-partum day 1 (P1), P3,
P5 and P7. Embryos at E14.5 and E16.5 and mandibles from P1, P3,
P5, and P7 were fixed with 4% paraformaldehyde for 72 h at room
temperature. Then, the tooth germs from first molars were
dissected. Following decalcification with 0.5 M of ethylenediamine
tetraacetic acid solution for 2 weeks, samples were dehydrated with
graded solutions of alcohol and embedded. Tooth germs from E14.5
mice were dehydrated with graded solutions of alcohol and embedded
without decalcification.
Expression of osteoprotegerin, the osteogenic marker
osteocalcin, and the odontogenic marker dentin sialoprotein (DSP)
was assessed by immunohistochemical (IHC) staining.
Anti-osteoprotegerin polyclonal antibody (5 µg/ml; cat. no. AF459;
R&D Systems), anti-osteocalcin polyclonal antibody (50 µl/ml;
cat. no. ab93876; Abcam) and anti-DSP monoclonal antibody (2 µg/ml;
cat. no. MABT37; EMD Millipore) were used as primary antibodies.
Terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL; cat. no. ab206386; Abcam) staining was carried out
according to manufacturer's instructions to evaluate apoptosis
activity in tooth germs with/without osteoprotegerin treatment.
Immunofluorescence analyses were carried out to
evaluate the nuclear translocation of β-catenin. Tissue sections
were incubated with anti-β-catenin polyclonal antibody (50 µl/ml;
cat. no. ab2365; Abcam) overnight at 4°C. Then, the sections were
incubated with secondary antibody Alexa Fluor 488 (1:1,000
dilution; cat. no. A-21206; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h in a dark chamber. Finally, tissue sections were
counterstained with 4′6-diamidino-2-phenylindole (DAPI; 0.5 µg/ml)
for 15 min for nuclear labeling.
Quantitative reverse
transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tooth germs or samples
of isolated dental mesenchyme using PureLink® RNA Mini
Kit (cat. no. 12183018A; Invitrogen; Thermo Fisher Scientific,
Inc.) according to manufacturer's instructions. Complimentary-DNA
synthesis was performed with random 6-mer primers using a
PrimeScript™ 1st Strand cDNA Synthesis kit (cat. no. 6110A; Takara
Bio, Inc.). Messenger-RNA expression was assessed by RT-qPCR using
the SYBR®-Green method. The thermocycling conditions
were: Initial denaturation for 30 sec at 95°C, followed by 40
cycles of 5 sec at 95°C and 30 sec at 60°C. The relative fold
change for expression of the target gene was calculated following
the method proposed by of Livak and Schmittgen (14). Glyceraldehyde-3-phosphate
dehydrogenase was used as endogenous reference for normalization,
and the expression level in tooth germ with no osteoprotegerin
treatment as a calibrator. The primers employed are listed in
Table SI.
Subrenal capsule assays
Subrenal capsule assays were carried out to confirm
the findings from in vitro experiments and compare tooth
development between whole tooth germs and dental-mesenchyme tissue
over longer periods of time. Using gel sponges as scaffolds, tooth
germs or dental-mesenchyme tissue were transplanted under the
subrenal capsule of 4-week-old nude mice according to the protocol
of Fingert et al (15).
After 3 weeks, the mice were sacrificed to obtain the transplanted
tissue for further study.
Protein microarrays
In order to compare the expression of various
extracellular cytokines between tooth germs and those in isolated
dental-mesenchyme tissue (Fig.
S1), the changes introduced by removal of the dental epithelium
using protein microarray assays were identified.
Quantibody® glass-based antibody arrays (product code:
QAM-CAA-4000; RayBiotech) were used to assess cytokine levels.
First, the glass chips were blocked with 100 µl of diluted samples
and incubated on shakers for 1 h. After decanting buffer from each
well, 100 µl of samples were added, and incubated overnight at 4°C.
Microarray assays and data analyses were carried out according to
manufacturer's protocols. A stock culture medium without growth
factors was used as a negative control. In each chip, there were
two positive controls providing a reference for normalization among
different chips. The fold change in protein level was calculated
using the following equation:
Foldchange=log2Vtooth
germVmesenchyme
where Vtooth germ denotes the
microarray reading for expression of a certain protein from
tooth-germ culture fluid, and Vmesenchyme is the
reading for that protein from isolated dental-mesenchyme culture
fluid.
Statistical analysis
Upon confirmation of a normal distribution of data,
all quantitative datasets were subjected to Student's t-tests or
one-way analysis of variance (ANOVA), with P<0.05 considered to
indicate a statistically significant difference. Dunnett's test was
used for post hoc test following ANOVA. Statistical analyses were
carried out using R 3.4.2 (R Foundation). Heatmaps for differential
expression of proteins were created with pheatmap (R Foundation).
All experiments were performed in triplicate.
Results
Influence of the dental epithelium on
extracellular levels of cytokines secreted from tooth germs
Results from protein microarray analysis revealed
that removal of the dental epithelium altered expression of various
secreted proteins, among which changes in osteoprotegerin
expression was one of the most significant (P<0.001; Fig. 1A). Heatmaps of microarray data
further demonstrated a consistent difference in osteoprotegerin
expression between culture fluid from the dental mesenchyme and
whole tooth germs (Fig. 1B).
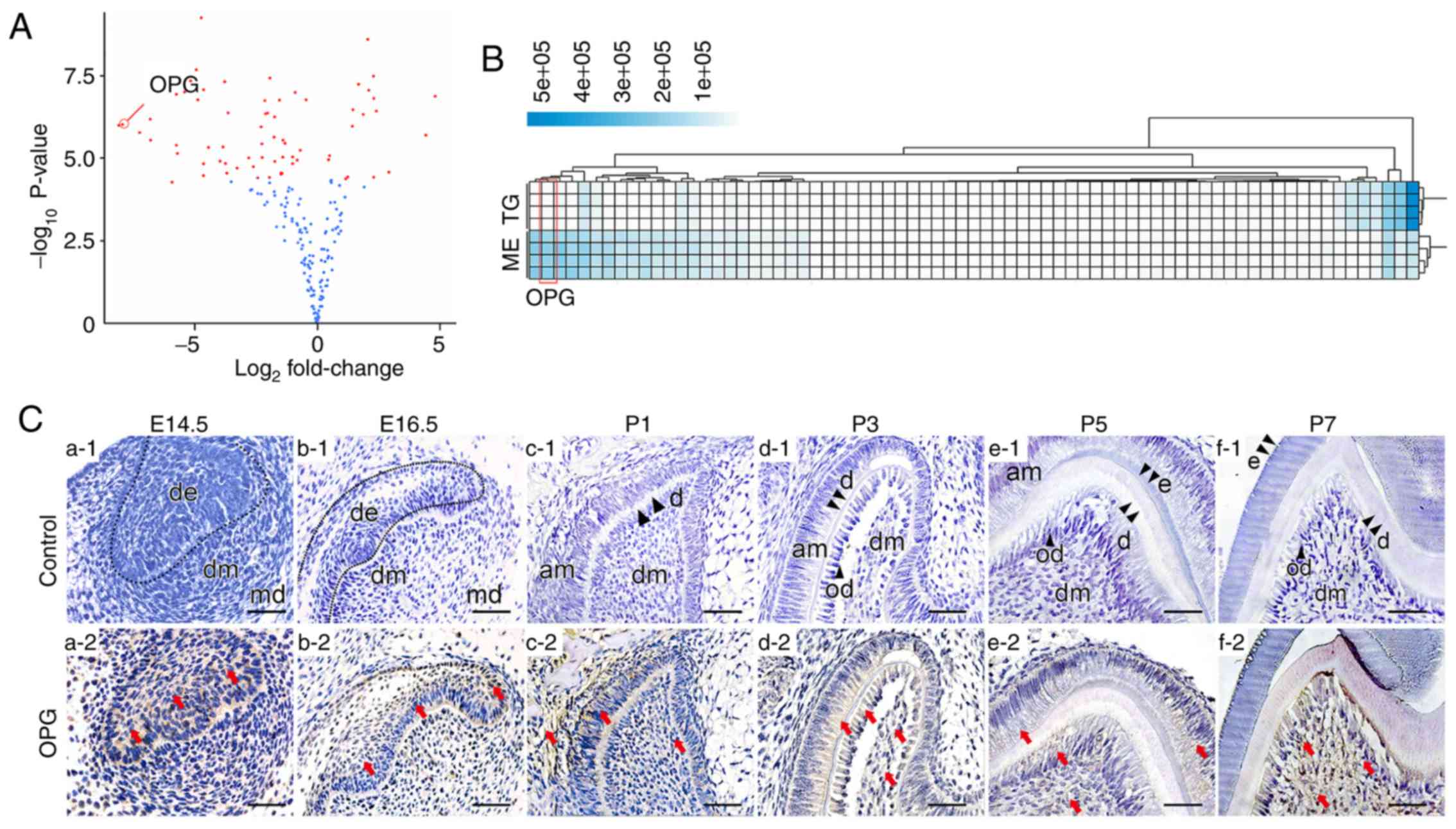 | Figure 1.Expression pattern of OPG. (A and B)
Volcano plot and heatmap of differential protein expression
comparing extracellular protein levels between tooth germs and
samples of isolated dental mesenchyme. OPG is indicated in the
figures. In the volcano plot, the log2 fold change was
calculated by comparing tooth germs vs. the dental mesenchyme.
Microarray analysis for tooth germs and the dental mesenchyme was
performed in triplicate. (C) Spatial-temporal pattern of OPG
expression during tooth development. At first, OPG expression was
confined to the dental epithelium (a1 and a2). Then, OPG expression
in the dental mesenchyme gradually increased as development
proceeded (b-f). (C) The red arrows indicate parts of the tissue
that were positive for immunohistochemical staining. Scale bar, 50
µm. OPG, osteoprotegerin; TG, tooth germ; ME, dental mesenchyme;
am, ameloblasts; d, dentin; de, dental epithelium; dm, dental
mesenchyme; md, mandible; od, odontoblast. |
Osteoprotegerin expression at different stages of
tooth development was evaluated further by IHC staining. At E14.5,
osteoprotegerin expression was found only in the epithelium of
internal and external enamel (Fig.
1C-a1 and a2), and a small amount of osteoprotegerin was found
in the dental mesenchyme at E16.5 (Fig. 1C-b1 and b2). As development
proceeded, osteoprotegerin expression in the dental mesenchyme
became more prominent, but its level in the epithelium did not
change much until P3. As the dental epithelium differentiated into
ameloblasts, osteoprotegerin expression in the dental epithelium
also decreased (Fig. 1C-c-f).
Influence of osteoprotegerin on
development of tooth germs
To study the influence of osteoprotegerin on the
development of tooth germs, extraneous osteoprotegerin protein (100
or 200 ng/ml) was added to the culture medium of the first-molar
tooth germs from E14.5. Tooth-germ structures in the control group
(Fig. 2A-b1) and the
osteoprotegerin (100 ng/ml) group (Fig. 2A-b2) were normal. However,
considerable structural disruption was revealed in the
osteoprotegerin (200 ng/ml) group (Fig. 2A-b3), whereby the polarity and
continuity of tooth structures were lost. Staining with Masson's
trichrome also revealed disrupted dentin formation in the
osteoprotegerin (200 ng/ml) group (Fig. 2A-c). TUNEL staining revealed no
significant difference in number of positive staining cells between
the osteoprotegerin-treated groups and the control group (Fig. 2B). These results demonstrated that,
during the early stages of tooth development, a high concentration
of osteoprotegerin disrupted the developmental process through
mechanisms other than cytotoxicity.
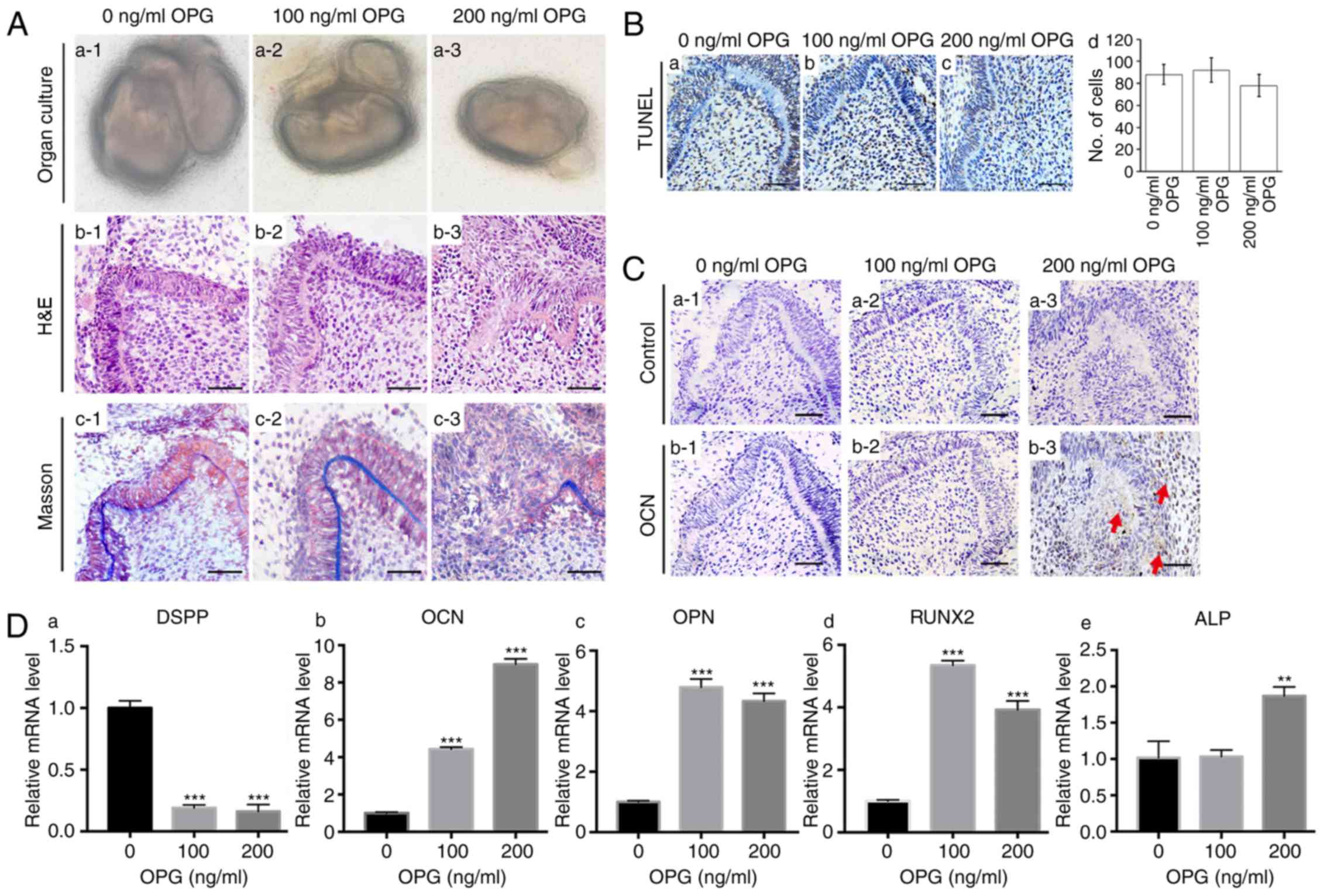 | Figure 2.Effects of OPG on tooth development.
(A) (a1-a3) Tooth germs of first molars were isolated from E14.5
mandibles. (b1-b3) H&E staining of tissue sections demonstrated
tooth-structure disruption after stimulation with 200 ng/ml of OPG.
(c1-c3) Staining (Masson's trichrome) of collagen structures
demonstrated disruption of dentin structure after stimulation with
200 ng/ml of OPG. (B) (a-c) TUNEL staining of tissue sections
revealed no significant cytotoxic effect after OPG stimulation. (d)
Quantification of positive cells for TUNEL staining. (C)
Immunohistochemical staining of OPG in tooth germs treated with
different concentrations of OPG. OCN expression was not high enough
to be detected in the control group (b1) or 100 ng/ml OPG-treated
group (b2), however, 200 ng/ml of OPG significantly increased OCN
levels in the dental mesenchyme (b3). Three tooth germs of E14.5
mice first molars were used in each group. (D) RT-qPCR revealed
that expression of the odontogenic marker DSPP (a) was suppressed
after OPG stimulation. OPG increased levels of the osteogenic
markers OCN (b), OPN (c), RUNX2 (d) and ALP (e). Each experiment
was performed in triplicate. Scale bar, 50 µm (A-b1-b3, A-c1-c3, B
and C). Data are presented as the mean ± SD. **P<0.01 and
***P<0.001. The red arrows indicate parts of the tissue that
were positive for immunohistochemical staining. OPG,
osteoprotegerin; OCN, osteocalcin; DSPP, dentin
sialophosphoprotein; OPN, osteopontin; RUNX2, runt-related
transcription factor 2; ALP, alkaline phosphatase. |
The influence of osteoprotegerin on various
osteogenic markers was evaluated by RT-qPCR. Compared with the
control group, expression of osteocalcin (P<0.001; Fig. 2D-b), osteopontin (P<0.001;
Fig. 2D-c) and runt-related
transcription factor 2 (RUNX2) (P<0.001; Fig. 2D-d) was higher in the
osteoprotegerin-treated groups, whereas an increase in alkaline
phosphatase (ALP) was revealed only in the osteoprotegerin (200
ng/ml) group (P<0.01; Fig.
2D-e). IHC staining further confirmed that treatment with
osteoprotegerin (200 ng/ml) not only disrupted tooth structures,
but also promoted expression of osteocalcin (Fig. 2C). Conversely, expression of the
important odontogenic marker dentin sialophosphoprotein (DSPP) was
suppressed significantly after osteoprotegerin treatment
(P<0.001; Fig. 2D-a).
Alteration in osteoprotegerin
expression and tooth development after removal of the dental
epithelium
The dental mesenchyme was isolated from E14.5 tooth
germs and cultured in vitro for 7 days to study whether
removal of the dental epithelium increased osteoprotegerin
expression and generated a similar effect on tooth development to
that observed with extraneous osteoprotegerin. Staining with
hematoxylin and eosin revealed that removal of the dental
epithelium disrupted tooth structures (Fig. 3A-a and b). RT-qPCR results revealed
a significant decrease in expression of the odontogenic marker DSPP
in samples of isolated dental mesenchyme (P<0.001, Fig. 3A-c). Compared with that in tooth
germs, expression of osteoprotegerin was significantly upregulated
in samples of isolated dental mesenchyme (P<0.001; Fig. 3A-d), as was that of osteocalcin
(P<0.001; Fig. 3A-e), RUNX2
(P<0.001; Fig. 3A-f) and ALP
(P<0.001; Fig. 3A-g). These
results were consistent with data from protein microarrays and the
effects of external osteoprotegerin supplementation.
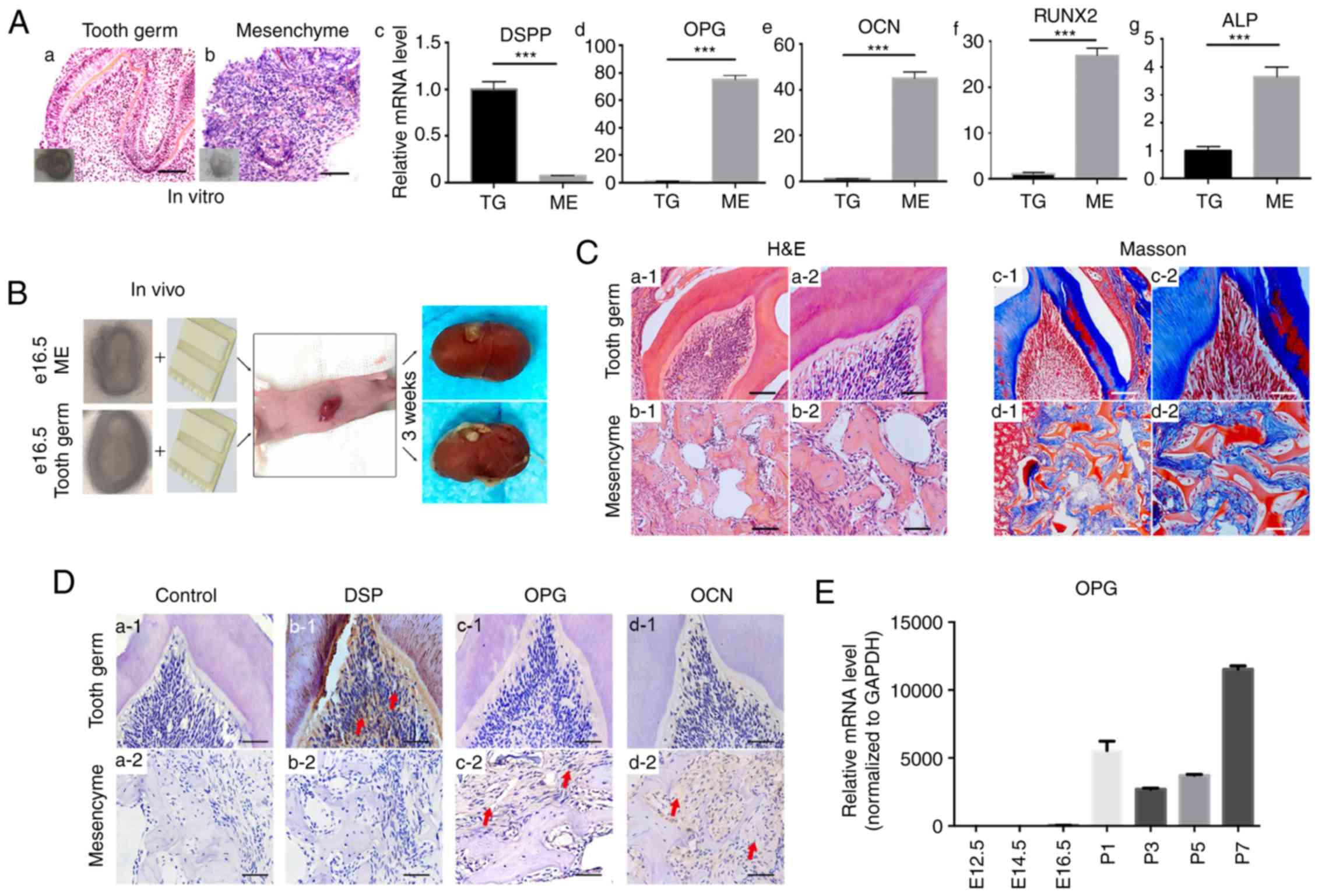 | Figure 3.Effects of the removal of the dental
epithelium on dental mesenchyme evaluated by in vitro and
in vivo cultures. (A) H&E staining of tooth germs (a)
and dental the mesenchyme (b) cultured for 7 days. RT-qPCR revealed
that the level of DSPP (c) decreased in the dental mesenchyme,
whereas that of OPG (d), OCN (e), RUNX2 (f) and ALP (g) in the
dental mesenchyme was significantly higher than that in tooth
germs. Each experiment was performed in triplicate. (B) In
vivo subrenal capsule culture with gel sponges (schematic).
Three E16.5 tooth germs and three samples of the dental mesenchyme
were cultured for further study. (C) H&E staining (a and b) and
Masson's trichrome staining (c and d) revealed that, while tooth
germs could form normal tooth structures after 3 weeks of subrenal
capsule culture, without the dental epithelium, the dental
mesenchyme could only form bone-like tissue. (D) (a) Samples
without primary antibody were used as negative control.
Immunohistochemical staining for DSP (b), OPG (c) and OCN (d)
revealed that, after 3 weeks in vivo culture, the DSP level
was higher in tooth germ-derived tissue (b1), whereas levels for
OPG and OCN were higher in dental mesenchyme-derived tissue (c2 and
d2). (E) RT-qPCR revealed that, compared with the prenatal period,
OPG expression in postnatal first-molar tooth germs significantly
increased. Scale bars, 100 µm (A-a and b, C-a1-d1) and 50 µm
(C-a2-d2 and D). Data are presented as the mean ± SD.
***P<0.001. The red arrows indicate parts of the tissue that
were positive for immunohistochemical staining. DSPP, dentin
sialophosphoprotein; OPG, osteoprotegerin; OCN, osteocalcin; RUNX2,
runt-related transcription factor 2; ALP, alkaline phosphatase;
DSP, dentin sialoprotein. |
To further confirm these in vitro findings,
and to study the effect of removal of the dental epithelium over a
long period of time, subrenal capsule cultures were carried out
(Fig. 3B). After culture for 3
weeks, implanted tooth germs formed normal tooth structures
(Fig. 3C-a1 and a2), whereas
implanted mesenchymal tissue formed only irregular structures
(Fig. 3C-b1 and b2). Staining with
Masson's trichrome revealed that implanted tooth germs formed bone
structures and dentin (Fig. 3C-c1 and
c2), whereas implanted mesenchyme formed only bone-like
structures (Fig. 3C-d1 and d2).
Comparison of IHC staining between these two types of implanted
tissue revealed that DSP expression was higher in tooth
germ-derived tissue (Fig. 3D-b1 and
b2). With regard to osteoprotegerin (Fig. 3D-c1 and c2) and osteocalcin
(Fig. 3D-d1 and d2) expression,
however, no prominent differences were revealed between the two
groups.
As revealed by IHC staining (Fig. 1C), osteoprotegerin expression in
the dental mesenchyme increased during postnatal tooth development.
RT-qPCR revealed that osteoprotegerin expression in tooth germs
increased >1,000-fold in the postnatal period (Fig. 3E). These results indicated that
suppression by the dental epithelium of osteoprotegerin expression
in the dental mesenchyme was relieved in the postnatal period.
Effect of osteoprotegerin on the
Wnt/β-catenin signaling pathway
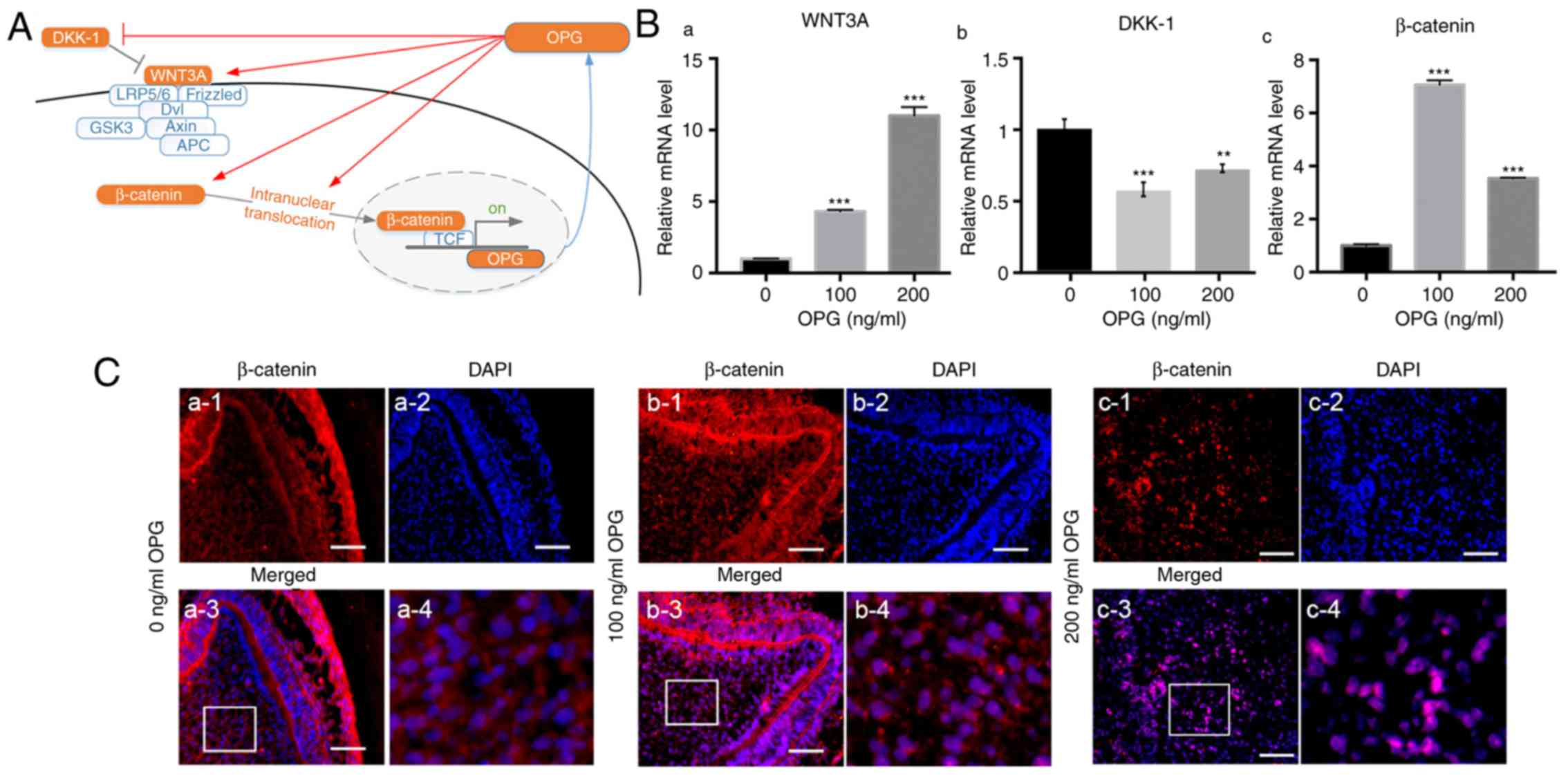
The canonical Wnt/β-catenin signaling pathway plays
an important part in regulation of osteogenic differentiation
(16). Hence, the influence of
osteoprotegerin on several key molecules in the canonical
Wnt/β-catenin signaling pathway was investigated (Fig. 4A). mRNA expression of WNT3A was
upregulated in whole tooth germs treated with osteoprotegerin
(P<0.001; Fig. 4B-a), whereas
that of Dickkopf-related protein-1 (DKK-1, an antagonist of this
pathway) was downregulated (P<0.01; Fig. 4B-b). β-catenin expression was also
significantly increased in osteoprotegerin-treated groups
(P<0.001; Fig. 4B-c). Then,
nuclear translocation of β-catenin was evaluated with
immunofluorescence assays (Fig.
4C). Compared with the control group, more overlaps between
β-catenin and DAPI were observed in osteoprotegerin-treated tooth
germs (especially 200 ng/ml) (Fig.
4C-b3 and b4; C-c3 and c4). These results indicated that
osteoprotegerin stimulation could enhance canonical Wnt/β-catenin
signaling.
Discussion
Removal of the dental epithelium abolishes the
normal development of teeth (17,18).
In the present study, protein microarray analysis revealed that,
after removal of the dental epithelium, osteoprotegerin expression
in the dental mesenchyme significantly increased. Järvinen et
al (13) revealed that, during
prenatal tooth development, the Wnt/β-catenin signaling pathway in
the epithelium inhibits Wnt/β-catenin signaling in the mesenchyme.
Osteoprotegerin expression is promoted mainly by the Wnt/β-catenin
pathway, which recruits hepatocyte nuclear factor 1 homeobox A
(HNF1A, also known as TCF1) to its promoter (19). Hence, removal of the dental
epithelium eases suppression of Wnt/β-catenin signaling in the
dental mesenchyme, leading to upregulation of osteoprotegerin
expression. These results indicated that the dental epithelium
plays an important role in regulating osteoprotegerin expression in
the dental mesenchyme.
In the present study, IHC staining and RT-qPCR
revealed that osteoprotegerin expression in the dental mesenchyme
was quite low during the prenatal period, but increased
significantly after birth. E14.5 tooth germs treated with
osteoprotegerin revealed disruption in tooth structures and failure
of dentin formation. Further evaluation revealed that
osteoprotegerin stimulation promoted expression of the osteogenic
biomarkers RUNX2 and osteocalcin, but suppressed DSPP expression,
thereby tilting the balance away from odontogenesis. In accordance
with our results, Ohazama et al revealed local injection of
osteoprotegerin protein or upregulation of osteoprotegerin
expression resulted in temporal retardation of tooth development,
which may cause defective mineralization in teeth (20). Conversely, a micro-computed
tomography study indicated that knockout of osteoprotegerin genes
would increase the thickness of enamel and dentin (21). Those studies and our findings
indicate that suppression of osteoprotegerin expression in the
dental mesenchyme is important for appropriate development of
teeth, and that extraneous osteoprotegerin in the early stages of
tooth development will disrupt this process.
Results from in vitro cultures revealed that,
compared with intact tooth germs, removal of the dental epithelium
not only increased osteoprotegerin expression in the isolated
dental mesenchyme, but also increased levels of ALP, osteocalcin
and RUNX2, and suppressed DSPP expression. Conversely, in
vivo cultures revealed no difference in osteoprotegerin
expression between tissue derived from tooth germs and isolated
dental mesenchyme. Such seemingly inconsistent results could be
reconciled by the difference in culture times. For in vitro
experiments, tooth germs were cultured for 7 days and, according to
a study by Ahmad and Ruch (22),
cultured tooth germs develop only to the stage between E16.5 and
P1. For in vivo culture, tooth germs or samples of isolated
dental mesenchyme were implanted in the subrenal capsule space for
3 weeks. Lungová et al demonstrated that interactions
between the dental epithelium and mesenchyme decline gradually
after birth (23). Hence, halfway
through the 3-week in vivo culture, suppression of
osteoprotegerin expression from the dental epithelium had already
been removed. Thus, no significant difference in osteoprotegerin
expression was revealed between tissue derived from tooth germs and
that from samples of the isolated dental mesenchyme.
The Wnt/β-catenin signaling pathway plays a critical
part in regulation of tooth development (24,25).
In the present study, it was revealed that osteoprotegerin was not
only a downstream target of the Wnt/β-catenin signaling pathway
(19), but could also influence
Wnt/β-catenin signaling. Osteoprotegerin increased WNT3A expression
in E14.5 tooth germs, and suppressed DKK-1 expression. In addition,
extraneous osteoprotegerin increased expression and intra-nuclear
translocation of β-catenin. Collectively, these results revealed
that osteoprotegerin treatment promoted the Wnt/β-catenin pathway
in E14.5 tooth germs. The Wnt/β-catenin signaling pathway has been
revealed to promote osteoprotegerin expression (19), thus these results indicated a
reciprocal activating relationship between osteoprotegerin and the
Wnt/β-catenin pathway. Choi et al demonstrated reciprocal
interactions between β-catenin and osterix in cementogenesis
(26), and a complex interplay
between Wnt/β-catenin and nuclear factor-κB signaling pathways in
the initiation and maintenance of organ development (27). In the present study, for the first
time, evidence was provided for a reciprocal activating
relationship between osteoprotegerin and the Wnt/β-catenin
signaling pathway during the early stages of tooth development.
Such interplay could lead to uncontrolled over-activation of
Wnt/β-catenin signaling in the dental mesenchyme and, as pointed
out by Aurrekoetxea et al (28), lead to delays/deficiencies in tooth
development.
During the early stages of tooth development,
osteoprotegerin expression in the dental mesenchyme was suppressed
by the dental epithelium. Addition of osteoprotegerin to prenatal
tooth germs promoted the expression of osteogenic markers,
inhibited the expression of odontogenic markers, and disrupted
tooth structures and dentin formation. In vitro experiments
confirmed that removal of the dental epithelium promoted
osteoprotegerin expression, and generated a similar effect to that
observed upon osteoprotegerin treatment. In vivo experiments
revealed similar results. The present data also indicated that the
underlying mechanism for these effects is related to enhancement of
mesenchymal Wnt/β-catenin signaling. Such promotion could further
increase osteoprotegerin expression, forming a positive feedback
loop, and lead to dysregulation of the early stages of tooth
development.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81800961),
Science and Technology Program of Guangdong Province (grant no.
2016B030229003) and Natural Science Foundation of Guangdong
Province (grant no. 2018A030313098).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, RZ and LX contributed to conception and design
of the study, XG, JZ and LX contributed to data analysis, ST and BC
contributed to data acquisition, and BC, RZ and LX critically
revised the manuscript.
Ethics approval and consent to
participate
The protocol for animal studies was approved by the
Ethics Committee of the Hospital of Stomatology within Sun Yat-sen
University (ERC-2013-15; Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RUNX2
|
runt-related transcription factor
2
|
|
DSPP
|
dentin sialophosphoprotein
|
|
DSP
|
dentin sialoprotein
|
|
RANKL
|
receptor activator of nuclear factor
kappa B ligand
|
|
IHC
|
immunohistochemical
|
|
RT-qPCR
|
quantitative reverse
transcription-polymerase chain reaction
|
References
|
1
|
Tucker A and Sharpe P: The cutting-edge of
mammalian development; how the embryo makes teeth. Nat Rev Genet.
5:499–508. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arakaki M, Ishikawa M, Nakamura T, Iwamoto
T, Yamada A, Fukumoto E, Saito M, Otsu K, Harada H, Yamada Y and
Fukumoto S: Role of epithelial-stem cell interactions during dental
cell differentiation. J Biol Chem. 287:10590–10601. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie X, Liu C, Zhang H, Jani PH, Lu Y, Wang
X, Zhang B and Qin C: Abrogation of epithelial BMP2 and BMP4 causes
amelogenesis imperfecta by reducing MMP20 and KLK4 expression. Sci
Rep. 6:253642016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanyama M, Shimo T, Sugito H, Nagayama M,
Kuboki T, Pacifici M and Koyama E: Regulation of CCN2 gene
expression and possible roles in developing tooth germs. Arch Oral
Biol. 58:1659–1666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gritli-Linde A, Bei M, Maas R, Zhang XM,
Linde A and McMahon AP: Shh signaling within the dental epithelium
is necessary for cell proliferation, growth and polarization.
Development. 129:5323–5337. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu X, Li Y, Wang F, Hu L, Li Y, Wang J,
Zhang C and Wang S: Spatiotemporal expression of wnt/β-catenin
signaling during morphogenesis and odontogenesis of deciduous molar
in miniature pig. Int J Biol Sci. 13:1082–1091. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki T, Suda N and Ohyama K:
Osteoclastogenesis during mouse tooth germ development is mediated
by receptor activator of NFKappa-B ligand (RANKL). J Bone Miner
Metab. 22:185–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kearns AE, Khosla S and Kostenuik PJ:
Receptor activator of nuclear factor kappaB ligand and
osteoprotegerin regulation of bone remodeling in health and
disease. Endocr Rev. 29:155–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi M: RANK/RANKL/OPG during
orthodontic tooth movement. Orthod Craniofac Res. 12:113–119. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Babur C, Ozcan G, Cebi DU, Pervane B,
Ozdemir B, Yücel A, Biri AA and Babür C: Gingival crevicular fluid
levels of osteoprotegerin (OPG) in premenopausal and postmenopausal
women with or without chronic periodontitis. J Dent. 40:364–371.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dorotheou D, Gkantidis N, Karamolegkou M,
Kalyvas D, Kiliaridis S and Kitraki E: Tooth eruption: Altered gene
expression in the dental follicle of patients with cleidocranial
dysplasia. Orthod Craniofac Res. 16:20–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walsh MC and Choi Y: Biology of the
RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol.
5:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Järvinen E, Shimomura-Kuroki J, Balic A,
Jussila M and Thesleff I: Mesenchymal Wnt/β-catenin signaling
limits tooth number. Development. 145:1580482018. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fingert HJ, Treiman A and Pardee AB:
Transplantation of human or rodent tumors into cyclosporine-treated
mice: A feasible model for studies of tumor biology and
chemotherapy. Proc Natl Acad Sci USA. 81:7927–7931. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Shao J, Zhou Y, Friis T, Yao J, Shi
B and Xiao Y: The impact of Wnt signalling and hypoxia on
osteogenic and cementogenic differentiation in human periodontal
ligament cells. Mol Med Rep. 14:4975–4982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saxen L and Thesleff I:
Epithelial-mesenchymal interactions in murine organogenesis. Ciba
Found Symp. 165:183–193; discussion 193–188. 1992.PubMed/NCBI
|
|
18
|
Puthiyaveetil JS, Kota K, Chakkarayan R,
Chakkarayan J and Thodiyil AK: Epithelial-mesenchymal interactions
in tooth development and the significant role of growth factors and
genes with emphasis on mesenchyme - a review. J Clin Diagn Res.
10:ZE05–ZE09. 2016.PubMed/NCBI
|
|
19
|
Boyce BF, Xing L and Chen D:
Osteoprotegerin, the bone protector, is a surprising target for
beta-catenin signaling. Cell Metab. 2:344–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohazama A, Courtney JM and Sharpe PT: Opg,
Rank, and Rankl in tooth development: Co-ordination of
odontogenesis and osteogenesis. J Dent Res. 83:241–244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng ZF, Ye W, Wang J, Li CH, Liu JH,
Liang QC, Li S, Xu K and Liao EY: OPG knockout mouse teeth display
reduced alveolar bone mass and hypermineralization in enamel and
dentin. Arch Oral Biol. 55:288–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmad N and Ruch J: Comparison of growth
and cell proliferation kinetics during mouse molar odontogenesis in
vivo and in vitro. Cell Tissue Kinet. 20:319–329. 1987.PubMed/NCBI
|
|
23
|
Lungová V, Radlanski RJ, Tucker AS, Renz
H, Míšek I and Matalová E: Tooth-bone morphogenesis during
postnatal stages of mouse first molar development. J Anat.
218:699–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu F and Millar SE: Wnt/beta-catenin
signaling in oral tissue development and disease. J Dent Res.
89:318–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu F, Chu EY, Watt B, Zhang Y, Gallant
NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al:
Wnt/beta-catenin signaling directs multiple stages of tooth
morphogenesis. Dev Biol. 313:210–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi H, Kim TH, Yang S, Lee JC, You HK and
Cho ES: A reciprocal interaction between β-catenin and osterix in
cementogenesis. Sci Rep. 7:81602017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Tomann P, Andl T, Gallant NM,
Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML,
et al: Reciprocal requirements for EDA/EDAR/NF-kappaB and
Wnt/beta-catenin signaling pathways in hair follicle induction. Dev
Cell. 17:49–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aurrekoetxea M, Lopez J, Garcia P,
Ibarretxe G and Unda F: Enhanced Wnt/β-catenin signalling during
tooth morphogenesis impedes cell differentiation and leads to
alterations in the structure and mineralisation of the adult tooth.
Biol Cell. 104:603–617. 2012. View Article : Google Scholar : PubMed/NCBI
|