Introduction
Neuropathic pain is defined as ‘pain caused by a
lesion or disease of the somatosensory system’ by the International
Association for the Study of Pain (1). Nociceptive pain involves peripheral
noxious stimulation, for instance, by inflammatory factors
(2). In 174 clinical trials, the
efficacies of multiple drugs with analgesic effects, such as, local
anesthetic drugs, opioids, antidepressants, NMDA receptor
antagonists, botulinum toxin and topical capsaicin have been
compared with placebo agents in patients with neuropathic pain
(3). Despite the high prevalence
of neuropathic pain, it is rarely diagnosed, and adequately and
effectively treated. Thus, is important to develop novel potential
therapies for treating patients with neuropathic pain.
Tumor necrosis factor (TNF)-α, which is a major
proinflammatory cytokine and is produced during the transmission of
pain (4), serves a role in the
pathogenesis of neuropathic pain (5). Spinal nerve injury in rats induces
mechanical allodynia and thermal hyperalgesia, which are
accompanied with concomitant increases of interleukin (IL)-1β and
TNF-α in the brain (6).
Increasing evidence has suggested that oxyntomodulin
is peripherally and centrally distributed (7,8).
Oxyntomodulin exhibits numerous functions. For instance, in mice
administered a high-fat diet, intraperitoneal and intravenous
treatment of oxyntomodulin ameliorates glucose intolerance
(9), and increases insulin/glucose
density in the plasma (10);
additionally, oxyntomodulin regulates the intrinsic heart rate of
mice (11). Furthermore,
oxyntomodulin exhibits antinociceptive effects in mice (12). However, the potential molecules
responsible for the attenuation of nociception by oxyntomodulin
require further investigation of which we aimed to determine in the
present study.
Materials and methods
Ethics statement
Each experimental procedure in the present study was
approved by the Ethical Committee for the Use of Laboratory Animals
of Ningbo No. 6 Hospital, and was conducted strictly according to
the ‘Guide for Care and Use of Laboratory Animals published by the
National Institutes of Health (13) and the ethical guidelines of the
International Association for the Study of Pain (14)’.
Reagents
For in vivo experiments, 5 µl oxyntomodulin
(0.2 µg/µl; Phoenix Pharmaceuticals) was dissolved in saline and
administered to mice via an intrathecal (i.t.) injection according
to a previous study (12). A total
of 5 µl TNF-α (20 pg/µl; Sigma-Aldrich; Merck KGaA) was i.t.
injected into mice (12).
For in vitro experiments, BV2 cells were
purchased from American Type Culture Collection and were treated
with 0.01, 0.1 and 1 µM of oxyntomodulin (15). Then, 1 pg/µl TNF-α was added into
the culture medium (16).
Animals
A total of 24 adult (8-week old, weight 20 g) male
C57BL/6 mice were obtained from and housed in Ningbo No.6 Hospital.
Mice were housed under controlled conditions with a temperature of
23±1°C, humidity of 70±10% and a light-dark cycle of 12 h, with
ad libitum access to water and food. All of the experiments
in the current study were performed in the light phase of the
light/dark cycle.
Mice were injected intrathecally with oxyntomodulin
to determine the potential antinociceptive effects of
oxyntomodulin. Thereafter, 24 mice were randomly divided into three
different groups as described in a previously reported study
(12): Control group, i.t.
injection of 5 µl saline and 5 µl saline (n=8); TNF-α group, i.t.
injection with 5 µl saline and 5 µl TNF-α (20 pg/µl) (n=8);
oxyntomodulin + TNF-α group, i.t. injection with 5 µl oxyntomodulin
(0.2 µg/µl) prior to 5 µl TNF-α (20 pg/µl) (n=8). Each mouse was
euthanized by thoracic dislocation directly after the behavior
tests (described below).
Nociceptive behavioral testing
Mice were acclimatized to the new condition for
nociceptive behavioral testing for ~1 h before the experiments.
After i.t. injection with TNF-α, mice were placed in a chamber (20
cm high, 20 cm diameter). Within half an hour, the cumulative
response time of nociceptive behavioral responses of each mouse,
including licking, scratching and biting episodes to the lumbar and
caudal spinal cord were recorded with a stop-watch timer (17).
Western blotting
Lumbar 4–6 spinal cords were obtained mixed with
radioimmunoprecipitation assay lysis (Roche Diagnostics) buffer
containing phosphatase and proteinase inhibitors (Roche
Diagnostics). The concentration of each protein sample was
determined with a BCA kit (Beyotime Institute of Biotechnology).
Protein samples (15 µg) were separated by 8% SDS-PAGE followed by
transferring to a polyvinylidene difluoride (PVDF) membrane (EMD
Millipore). The membranes were incubated with corresponding primary
antibodies against IL-6 (cat. no. 12912; dilution 1:1,000), IL-1β
(cat. no. 31202; dilution 1:1,000), phosphorylated-NF-κB (Ser536,
cat. no. 3033; dilution 1:1,000), p65 (cat. no. 59674; dilution
1:1,000) and GAPDH (cat. no. 5174; dilution 1:1,000), which acted
as a loading control. All the above primary antibodies were
obtained from Cell Signaling Technology, Inc. and applied at 4°C
overnight. The antibody for IBA1 (cat. no. ab178846; dilution
1:1,000) was obtained from Abcam and applied at 4°C overnight.
Prior, the PVDF membrane was blocked with 5% skim milk at room
temperature for ~1 h. The next day, the PVDF membrane was incubated
with an anti-rabbit horseradish peroxidase-conjugated secondary
antibody (cat. no. 7074; dilution 1:200, Cell Signaling Technology,
Inc.) at room temperature for 1 h. Then, the protein bands were
visualized via enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.). The quantity of each target protein band was
analyzed by Image Quant LAS 500 (GE Healthcare Life Sciences).
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the lumbar 4–6 spinal
cords and BV2 cells using TRIzol® (Thermo Fisher
Scientific, Inc.). cDNA was synthesized from RNA with a
PrimerScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocols. RT-qPCR was performed by SYBR Premix Ex
Taq (Takara Bio, Inc.) and conducted by a CFX96™ real-time PCR
system (Bio-Rad Laboratories, Inc.). The thermocycling conditions
were: Denaturation (95°C, 5 min), followed by 36 cycles of
amplification (95°C, 25 sec) and quantification (62°C, 40 sec), the
melting curve was them obtained (95°C, 25 sec and 60°C, 1 min). The
2−ΔΔCq method was used to quantify the expression level
of each gene (18). GAPDH acted as
a control. The primer sequences were as listed: IL-6, forward,
5′-GGCTACTGCTTTCCCTACCC-3′ and reverse, 5′-TTTTCTGCCAGTGCCTCTT-3′;
IL-1β, forward, 5′-TCCAGGATGAGGACATGAGCAC-3′ and reverse,
5′-GAACGTCACCCAGCAGGTTA-3′; GAPDH, forward,
5′-GTGATGCTGGTGCTGAGTATC-3′ and reverse,
5′-GTGATGGCATGGACKGTGG-3′.
Cell culture
BV2 microglial cells were seeded into 6-well plates,
cultured with Dulbecco's Modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were maintained
at 37°C in an incubator with 5% CO2 for 24 h. Cells
(3×105) were divided into three groups and maintained at
37°C in an incubator with 5% CO2 with the treatments as
followed: Control group, cultured with only culture medium; TNF-α
group, treated with 1 pg/µl TNF-α at 37°C for 24 h (16); oxyntomodulin + TNF-α group, treated
with 0.01, 0.1 and 1 µM of oxyntomodulin at 37°C at 24 h (15) prior to the treatment of 1 pg/µl
TNF-α.
MTT assay
The effects of oxyntomodulin on BV2 cell viability
were analyzed via an MTT assay (Sigma-Aldrich; Merck KGaA). Cells
(4×106 cells/well) were first incubated for 8 h at 37°C
in an incubator with 5% CO2. Thereafter, 10 µl MTT was
added into each well and cells were incubated for further 4 h at
37°C. The optical density (OD) at 570 nm was measured with a
microplate reader (Bio-Rad 550; Bio-Rad Laboratories, Inc.).
ELISA
Supernatants of the culture medium were cleared of
cells by spinning the 96-well plates at 400 × g at room temperature
for 5 min. The supernatants were transferred to the round bottom,
non-treated 96-well plates followed by spinning at 400 × g at room
temperature for 5 min. Thereafter, the supernatants were
transferred to a new 96-well plate for the subsequent experiments.
The protein expression levels of IL-1β (SMLB00C, mouse IL-1
β/IL-1F2 Quantikine ELISA Kit) and IL-6 (SM6000B, mouse IL-6
Quantikine ELISA Kit) in the supernatants of the culture medium
were tested by ELISA (R&D Systems, Inc.) according to the
manufacturer's protocols. The OD at 490 nm was measured with a
microplate reader (Spectra MAX 340PC Molecular Devices LLC).
Statistical analysis
Each experiment was repeated three times.
Statistical analyses were conducted using GraphPad Prism 6 software
(GraphPad Software, Inc.). Data were presented as the mean ±
standard error of the mean. One-way ANOVA followed by a
Student-Newman-Keuls test were applied for the analyses of
differences among 3 or more groups. P<0.05 indicated a
statistically significant difference.
Results
Oxyntomodulin attenuates TNF-α-induced
pain hypersensitivity
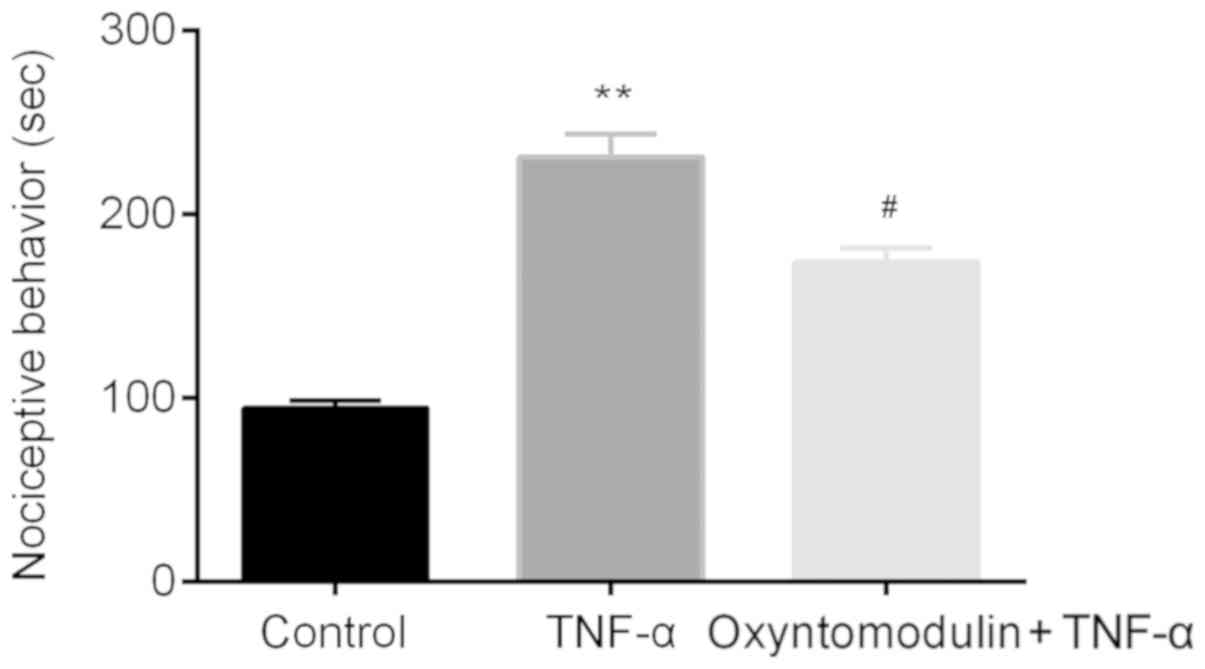
The effects of oxyntomodulin on TNF-α-induced
nociceptive behaviors in mice were assessed by nociceptive
behavioral testing with a stop-watch timer. Compared with mice in
the control group, mice in the TNF-α group presented acute licking,
scratching, and biting the lumbar or caudal region which lasted for
about half an hour; this was significantly longer than the control.
On the contrary, a significant decrease in the duration of
nociceptive behavior was reported following oxyntomodulin
administration compared with TNF-α treatment alone (Fig. 1).
Oxyntomodulin decreases TNF-α-induced
expression of IBA-1 and NF-κB p-p65 in the spinal cord
Over the past 15 years, neuropathic conditions have
been proved to be related with the activation of glia in the
central nervous system (CNS), which are important contributors to
central sensitization (19).
Molecular changes caused by glial activation leads to pain
hypersensitivity, including upregulation of chemokine receptors and
the release of glial cytokines (20).
Nuclear factor-κB is activated by IκB kinases, which
then migrates to the nucleus (21). Spinal nerve injury in rats
increases mechanical allodynia and thermal hyperalgesia which are
accompanied with increases in NF-κB expression in the brain
(6).
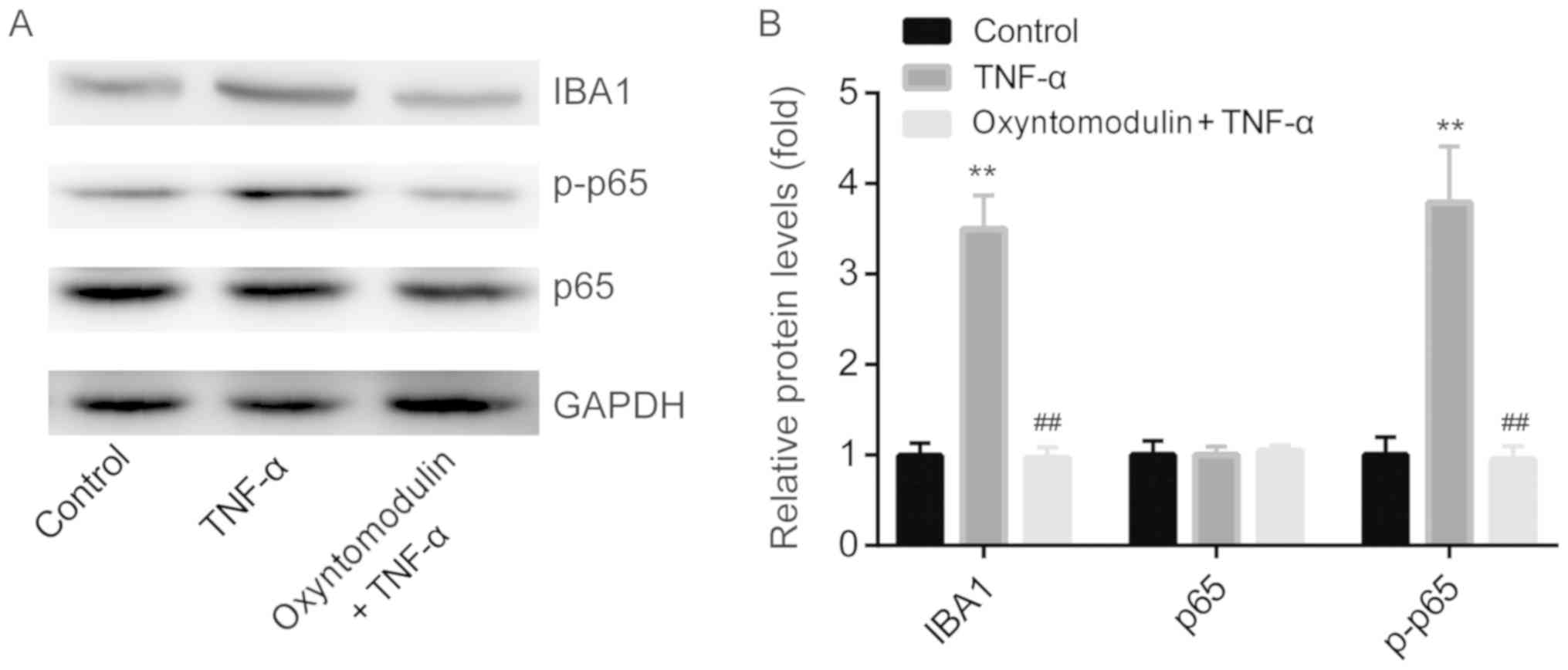
Therefore, we explored the expression levels of
ionized calcium binding adaptor molecule-1 (IBA1, microglia marker)
and NF-κB in the spinal cord of mice by western blotting. The
results showed that, compared with the control group, TNF-α
significantly induced the protein expression of IBA1 and NF-κB
p-p65, but was attenuated by pretreatment with oxyntomodulin
(Fig. 2A and B); no significant
changes in NF-κB p65 expression were observed among the three
groups.
Oxyntomodulin attenuates TNF-α-induced
IL-6 and IL-1β production in the spinal cord
NF-κB activates the transcription of proinflammatory
genes (18), while, NF-κB responds
to ~150 different stimuli (22).
Spinal nerve injury in rats induces mechanical allodynia and
thermal hyperalgesia accompanied with concomitant increases of
IL-1β and TNF-α in the brain (6).
Several studies indicate that various cytokines can directly
stimulate nociceptors (23,24).
Cytokines (such as IL-1β and TNF-α) which are released after stress
induce the activation of NF-κB (25).
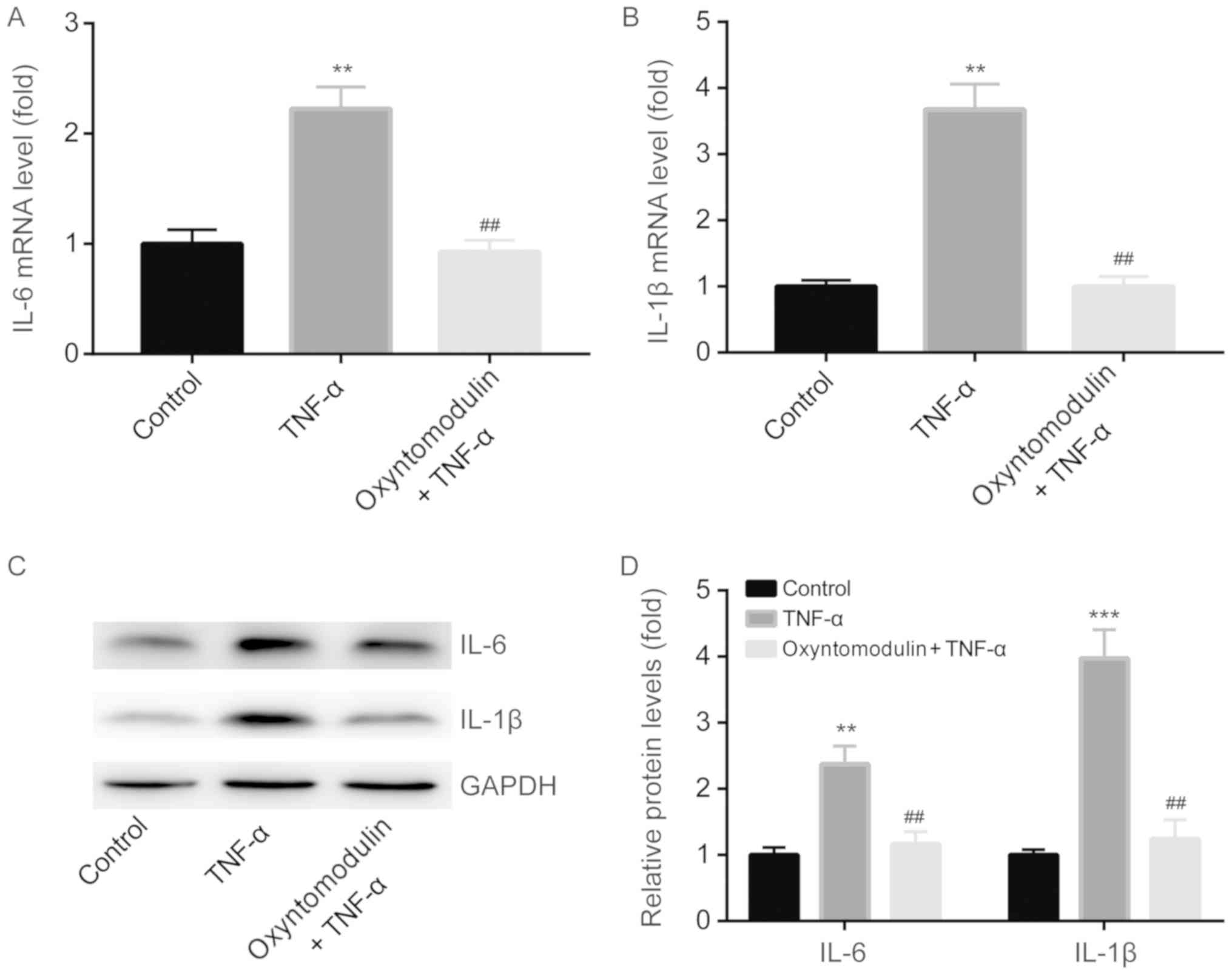
Correspondingly, we detected the expression levels
of IL-1β and TNF-α in the spinal cord of mice by RT-qPCR and
western blotting. RT-qPCR demonstrated that compared with the
control group, TNF-α significantly induced IL-6 and IL-1β mRNA
expression in spinal cord, but was attenuated by oxyntomodulin
administration (Fig. 3A and B).
Western blotting demonstrated similar results to the findings of
RT-qPCR (Fig. 3C and D).
Oxyntomodulin protects BV2 cells from
TNF-α-induced toxicity
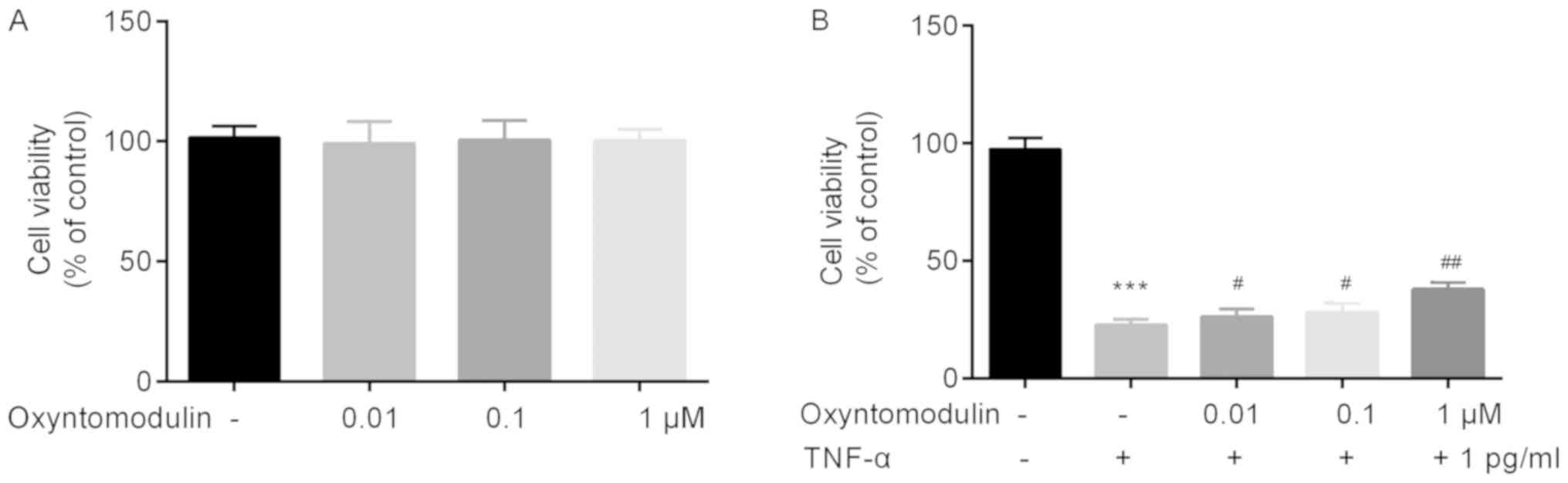
BV2 cell viability in different groups was
investigated by an MTT assay. At 12 h after treatment,
oxyntomodulin (0.01, 0.1 and 1 µM) did not affect BV2 cell
viability as presented in Fig. 4A,
indicating that oxyntomodulin exerted no toxicity on BV2 cells.
Compared with the control group, TNF-α significantly
inhibited the cell viability of BV2 cells, which was rescued by
oxyntomodulin treatment (0.01, 0.1 and 1 µM) (Fig. 4B).
Oxyntomodulin attenuates TNF-α-induced
IL-6 and IL-1β production and release in BV2 cells and culture
medium
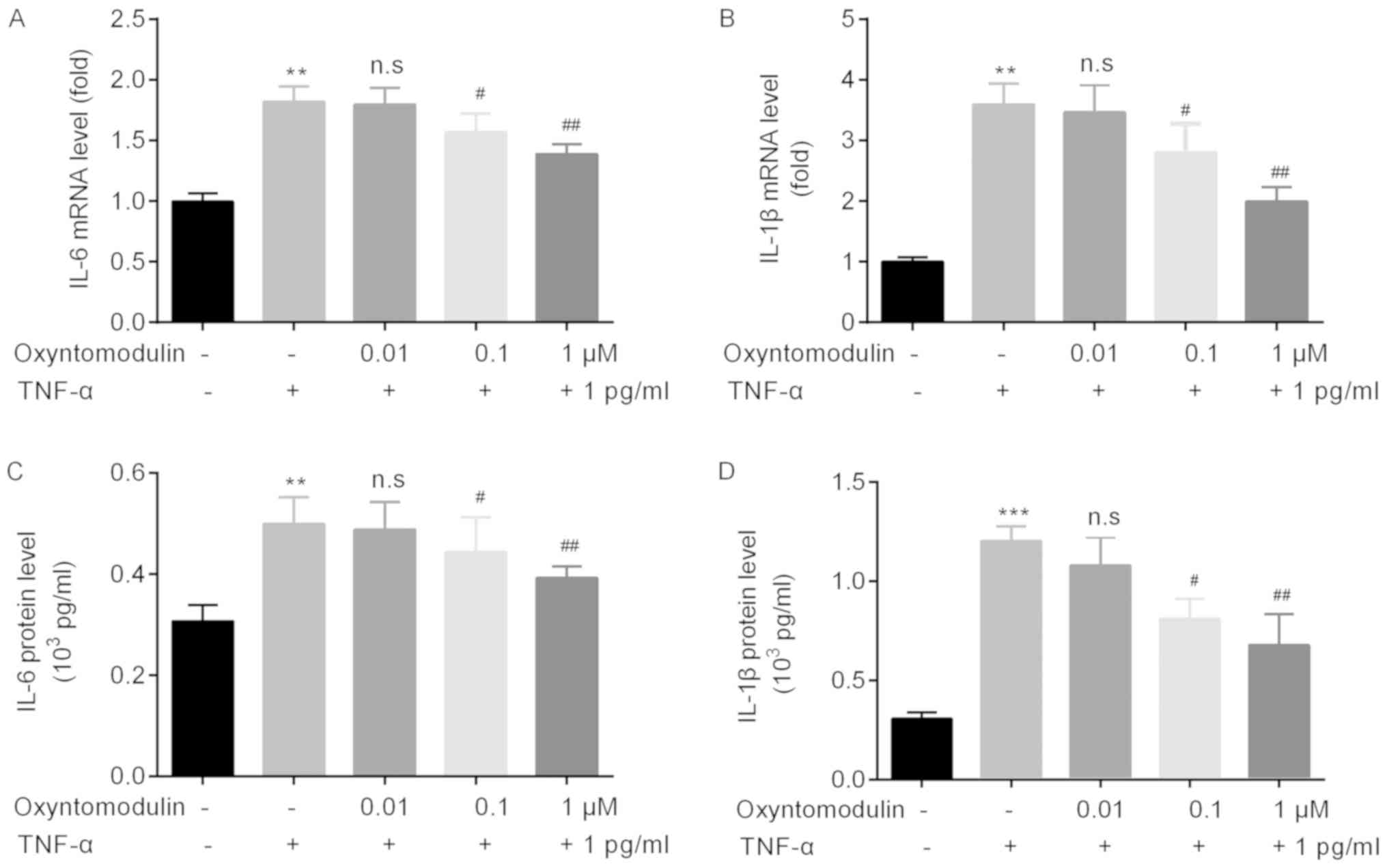
Additionally, we detected the effects of
oxyntomodulin on the release of glia cytokine. RT-qPCR was
performed to determine the mRNA levels of IL-6 and IL-1β in BV2
cells. Compared with the control group, TNF-α significantly
increased the mRNA levels of IL-6 and IL-1β in BV2 cells, which
were significantly attenuated by oxyntomodulin treatment (0.1 and 1
µM) (Fig. 5A and B).
Furthermore, ELISA was used for the analyze the
protein levels of IL-6 and IL-1β in the supernatants of the culture
medium. The results demonstrated that compared with control group,
TNF-α significantly increased protein levels of IL-6 and IL-1β,
which were attenuated by oxyntomodulin treatment (0.1 and 1 µM) in
the supernatants of culture medium (Fig. 5C and D).
Discussion
The effects of oxyntomodulin in the CNS have not
been well elucidated (26). One
previous study indicated that D-Ser2-oxytomodulin (a
protease-resistant oxyntomodulin analogue) functions in the CNS.
For instance, it improves locomotor activities and protects
dopaminergic neurons in a mouse model of Parkinson's Disease (PD)
(27). Although oxyntomodulin is
expressed in the brain and the spinal cord (7,8), the
function and potential molecules responsible for the function of
oxyntomodulin in nociception remain to be further explored.
TNF-α which is a major proinflammatory cytokine that
is produced during the transmission of pain (4), serves a role in the pathogenesis of
neuropathic pain (5). In the
present study, we reported that oxyntomodulin attenuated
TNF-α-induced neuropathic pain in mice in nociceptive behavior
tests; however, the molecules that are responsible for the
aforementioned changes remain to be further investigated.
As an important upstream molecule of NF-κB, TNF-α
activates NF-κB, which further promotes the expression of IL-6
(28); TNF-α also leads to an
increase of IL-1β expression (29). NF-κB plays a crucial role in the
transcriptional regulation of various pro-inflammatory genes,
including IL-1β and IL-6 (30).
Our results showed that, compared with the control group, TNF-α
induced the expression of NF-κB, as well as IL-6 and IL-1β, which
were in agreement with the aforementioned findings, indicating that
TNF-α induced the expression of IL-6 and IL-1β via activation of
NF-κB (28–30).
Furthermore, the effects of TNF-α on nociceptive
behaviors, and NF-κB, IL-6 and IL-1β levels were found to be
attenuated by pretreatment of oxyntomodulin in the present study.
These findings were in agreement with a recent study (12) and a report that investigated the
potential treatment of oxyntomodulin on PD (27).
In conclusion, we revealed for the first time, to
the best of our knowledge, that oxyntomodulin attenuates
TNF-α-induced neuropathic pain (release of glial cytokines IL-6 and
IL-1β) via inhibiting the activation of NF-κB pathway, suggesting a
potential role of oxyntomodulin the clinical management of pain in
the future.
Unfortunately, there are several limitations in the
present study: i) The time-dependent effects of oxyntomodulin on
BV2 were not investigated, which could provide insight into the
effects of intracellular cytokines; and ii) little evidence
regarding the effects of oxyntomodulin on TNF-α-induced neuropathic
pain has been reported; thus; further analysis should be conducted
in the future.
Acknowledgements
Not applicable.
Funding
The present study was funded by Natural Science
Foundation of Ningbo (grant no. 2009A610140).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, LY, YC, CL and GY performed the experiments and
analyzed the data. LY prepared the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee for the Use of Laboratory Animals of Ningbo No. 6
Hospital, and was conducted strictly according to the ‘Guide for
Care and Use of Laboratory Animals’ published by the National
Institutes of Health (13) and the
ethical guidelines of the International Association for the Study
of Pain (14).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jensen TS, Baron R, Haanpää M, Kalso E,
Loeser JD, Rice AS and Treede RD: A new definition of neuropathic
pain. Pain. 152:2204–2205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woolf CJ: American College of Physicians;
American Physiological Society Pain: Moving from symptom control
toward mechanism-specific pharmacologic management. Ann Intern Med.
140:441–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finnerup NB, Sindrup SH and Jensen TS: The
evidence for pharmacological treatment of neuropathic pain. Pain.
150:573–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vicario N, Parenti R, Arico G, Turnaturi
R, Scoto GM, Chiechio S and Parenti C: Repeated activation of delta
opiod receptors counteracts nerve injury-induced TNF-α
up-regulation in the sciatic nerve of rats with neuropathic pain: A
possible correlation with delta opiod receptors-mediated
antiallodinic effect. Mol Pain. 12:17448069166679492016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leung L and Cahill CM: TNF-alpha and
neuropathic pain-a review. J Neuroinflammation. 7:272010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie W, Luo S, Xuan H, Chou C, Song G, Lv
R, Jin Y, Li W and Xu J: Betamethasone affects cerebral expressions
of NF-kappaB and cytokines that correlate with pain behavior in a
rat model of neuropathy. Ann Clin Lab Sci. 36:39–46.
2006.PubMed/NCBI
|
|
7
|
Blache P, Kervran A and Bataille D:
Oxyntomodulin and glicentin: Brain-gut peptides in the rat.
Endocrinology. 123:2782–2787. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vrang N and Larsen PJ: Preproglucagon
derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: Role of
peripherally secreted and centrally produced peptides. Prog
Neurobiol. 92:442–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parlevliet ET, Heijboer AC, Schroder-van
der Elst JP, Havekes LM, Romijn JA, Pijl H and Corssmit EP:
Oxyntomodulin ameliorates glucose intolerance in mice fed a
high-fat diet. Am J Physiol Endocrinol Metab. 294:E142–E147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
ThanThan S, Zhao H, Yannaing S, Ishikawa T
and Kuwayama H: Oxyntomodulin increases the concentrations of
insulin and glucose in plasma but does not affect ghrelin secretion
in Holstein cattle under normal physiological conditions. Domest
Anim Endocrinol. 39:163–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sowden GL, Drucker DJ, Weinshenker D and
Swoap SJ: Oxyntomodulin increases intrinsic heart rate in mice
independent of the glucagon-like peptide-1 receptor. Am J Physiol
Regul Integr Comp Physiol. 292:R962–R970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SH, Lee JR, Jang SP, Park SH, Lee HJ,
Hong JW and Suh HW: Antinociceptive profiles and mechanisms of
centrally administered oxyntomodulin in various mouse pain models.
Neuropeptides. 68:7–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guide for the Care and Use of Laboratory
Animals, . 8th. The National Academies Collection: Reports funded
by National Institutes of HealthNational Academies Press;
Washington, DC: 2011
|
|
14
|
Ethical standards for investigations of
experimental pain in animals. The Committee for Research and
Ethical issues of the international association for the study of
pain. Pain. 9:141–143. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Wu KJ, Yu SJ, Tamargo IA, Wang Y and
Greig NH: Neurotrophic and neuroprotective effects of oxyntomodulin
in neuronal cells and a rat model of stroke. Exp Neurol.
288:104–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El Karim I, McCrudden MT, Linden GJ,
Abdullah H, Curtis TM, McGahon M, About I, Irwin C and Lundy FT:
TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity
in odontoblast-like cells. Am J Pathol. 185:2994–3002. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hylden JL and Wilcox GL: Intrathecal
substance P elicits a caudally-directed biting and scratching
behavior in mice. Brain Res. 217:212–215. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuda M, Inoue K and Salter MW:
Neuropathic pain and spinal microglia: A big problem from molecules
in ‘small’ glia. Trends Neurosci. 28:101–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji RR, Berta T and Nedergaard M: Glia and
pain: Is chronic pain a gliopathy? Pain. 154 (Suppl 1):S10–S28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109 (Suppl 1):S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Obreja O, Rathee PK, Lips KS, Distler C
and Kress M: IL-1 beta potentiates heat-activated currents in rat
sensory neurons: Involvement of IL-1RI, tyrosine kinase, and
protein kinase C. FASEB J. 16:1497–1503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parada CA, Yeh JJ, Joseph EK and Levine
JD: Tumor necrosis factor receptor type-1 in sensory neurons
contributes to induction of chronic enhancement of inflammatory
hyperalgesia in rat. Eur J Neurosci. 17:1847–1852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allan SM and Rothwell NJ: Inflammation in
central nervous system injury. Philos Trans R Soc Lond B Biol Sci.
358:1669–1677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bataille D and Dalle S: The forgotten
members of the glucagon family. Diabetes Res Clin Pract. 106:1–10.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Li Y, Jalewa J, Saunders-Wood T, Li
L and Hölscher C: Neuroprotective effects of an oxyntomodulin
analogue in the MPTP mouse model of Parkinson's disease. Eur J
Pharmacol. 765:284–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee KM, Jeon SM and Cho HJ: Tumor necrosis
factor receptor 1 induces interleukin-6 upregulation through
NF-kappaB in a rat neuropathic pain model. Eur J Pain. 13:794–806.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Segond von Banchet G, König C, Patzer J,
Eitner A, Leuchtweis J, Ebbinghaus M, Boettger MK and Schaible HG:
Long-lasting activation of the transcription factor CREB in sensory
neurons by interleukin-1β during Antigen-induced arthritis in rats:
A mechanism of persistent arthritis pain? Arthritis Rheumatol.
68:532–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsing CH, Lin MC, Choi PC, Huang WC, Kai
JI, Tsai CC, Cheng YL, Hsieh CY, Wang CY, Chang YP, et al:
Anesthetic propofol reduces endotoxic inflammation by inhibiting
reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS
One. 6:e175982011. View Article : Google Scholar : PubMed/NCBI
|