Introduction
In the current medical field, the lumbar laminectomy
is deemed the most valid treatment for lumbar illnesses; these
include lumbar disc herniation and other associated diseases, which
may result in spinal canal stenosis. However, due to inaccurate
recognition and inadequate treatment, following surgery the
formation of fibrosis on local dura and lumbosacral adhesive
arachnoiditis occurs (1). With
epidural fibrosis patients often experience chronic lower back and
leg pain, as well disability (2).
There is also a study that indicated that the function of local
inflammatory factors and hematoma organization could be primary
contributors of epidural fibrosis (3). Additionally, further studies have
illustrated that the excessive proliferation of fibroblasts in the
operative region is the most significant element of local fibrosis
formation (4,5). Therefore, the methods of inhibiting
fibroblast proliferation to reduce fibrosis have become an
attractive area of study and previous studies have demonstrated
positive results (6–8). However, there are numerous
limitations for clinical application, hence further research is
still required to solve the problem completely.
Laminins are a type of biofunctional glycoprotein in
the extracellular matrix, which consist of three different
polypeptide α, β and γ chains with disulfide bonds; there are five
kinds of α chains (α1-α5), three β chains (β1-β3) and three γ
chains (γ1-γ3) (9–11). Furthermore, some studies have shown
that all fifteen different laminin trimer-formations are associated
with multiple cell biological behaviors, such as adhesion,
differentiation, migration and proliferation in various cell lines
(12–16). For further research, as the major
constituent protein of the extracellular matrix, most laminins
extensively express the α5 chain (referred as laminin α5), which
has a greater influence on the aforementioned cell behaviors than
the other chains (17,18). Studies have also indicated that
laminin α5 is a crucial constituent of the basement membrane in
some tissues and organs, such as the skin, hair, lung, intestines
and kidney, and plays a significant role in the organism (19). For instance, in mice experiments
laminin α5 was found to accelerate the morphogenesis of embryonic
skin and hair (20). According to
previous studies, laminin α5 was also demonstrated to be associated
with human myasthenia, ligament lesions, dermopathy, visual
impairment, scar formation malabsorption and vitreous detachment
(21,22). Thus, laminin α5 is of great
significance, however there are no studies to the best of our
knowledge, involving laminin α5, fibroblast and epidural fibrosis
until now.
The PI3K/AKT/mTOR signaling pathway is a classical
pathway, which has been demonstrated to be associated with various
biological cell behaviors including proliferation (23,24).
There was also research indicated that AKT pathway is probably
involved with epidural fibrosis (25). However, there are few further
studies on the signaling pathway involved in fibroblast
proliferation and epidural fibrosis. Therefore, the present study
explored whether laminin α5 is associated with epidural fibrosis
and modulates fibroblast proliferation through the activation of
the PI3K/AKT/mTOR signaling pathway. The results of the present
study may aid the development of a novel treatment for the
prevention of epidural fibrosis.
Materials and methods
Animals
A total of 40 Sprague-Dawley male rats (mean weight,
250 g; age, 8 weeks) were provided by the Medical College of
Yangzhou University (Yangzhou, China). The rats were acclimatized
for a week to adapt to the laboratory environment of 23±2°C and
50–60% humidity, with a 12-h light/dark cycle and free access to
food and water. Rats were then randomly divided into two groups:
2-week group and 4-week group (the number represents the
postoperative euthanasia-time; 20 rats per group). During the
preparation process, all rats were appropriately treated according
to the standards of International Laboratory Animal Care.
Animal laminectomy model
To simulate the situation of clinical patients,
lumbar laminectomy was carefully performed in all rats according to
a laminectomy model and the procedure was conducted as previously
reported (26). Following the
application of 1% pentobarbital sodium for anesthesia (40 mg/kg),
the rats were shaved on the back among the first and second lumbars
(L1 and L2) to expose the operative area clearly and the local skin
was sterilized three times with iodine. The local connective tissue
and muscles were separated layer by layer until the vertebral
plate, and the plates of L1 and L2 were carefully removed with a
rongeur to expose the spinal cord distinctly, avoiding causing any
other injuries. Following thorough hemostasis, the wound was
sutured in mattress-style with Coated Vicryl Plus Antibacterial
Suture (Johnson Co., Ltd). All procedures aforementioned were
performed in sterile conditions by professional veterinarians with
the certification from Jiangsu Experimental Animal Association
(Jiangsu, China).
Histological analysis of hematoxylin
and eosin (H&E) and Masson trichrome stains
After lumbar laminectomy operation, the two groups
of rats were individually euthanized at 2 and 4 weeks to perform
histological analysis to detect the degree of local fibrosis. The
rats were anesthetized with 1% pentobarbital sodium and perfused
with 4% paraformaldehyde intracardially for euthanasia.
Subsequently, the L1 and L2 lumbar column with local muscles and
epidural fibrosis were excised and fixed with 10% buffered formalin
for one week at room temperature, then immersed in Ethylene Diamine
Tetraacetic Acid (EDTA) for 40 days for decalcification. Finally,
the columns were embedded in paraffin and then sliced into
successive 4-µm transverse sections.
For H&E staining, the sections were subsequently
stained with hematoxylin for 5 min and then eosin for 5 min, both
at room temperature. For Masson trichrome staining, the sections
were subsequently immersed in 50% potassium dichromate overnight at
room temperature, stained with hematoxylin for 3 min at room
temperature and incubated in Ponceau S dye for 5 min at room
temperature. Then, the sections were washed and incubated with 1%
phosphomolybdic acid for 2 min at room temperature prior to being
stained with aniline blue for 5 min at room temperature. The degree
of fibrosis, local fibroblast counting, and the content of epidural
collagen were observed by optical photographic light microscopy at
×40 and ×200 magnification. Stained cells were counted in three
random views of fibrotic area per section by Image Pro Plus 6.0
software (Media Cybernetics, Inc.).
Immunohistochemistry
Following the initial fixing steps described above
and obtaining paraffin-embedded sections (4 µm),
immunohistochemistry analysis of laminin α5 protein expression was
performed with a Ready-to-use HP IHC detection kit (Absin
Bioscience, Inc.), according to the manufacturer's protocol.
Briefly, sections of each group underwent antigen retrieval in
sodium citrate at 100°C for 20 min. Sections were subsequently
deparaffinized in xylene at room temperature and rehydrated in a
descending alcohol series (100, 85 and 75%), and blocked in 100%
FBS (Gibco; Thermo Fisher Scientific, Inc.) for 15 min at room
temperature. Sections were subsequently incubated with the laminin
α5 primary antibody (1:200; cat. no. NBP2-42391; Novus Biologicals,
Ltd.) at 4°C overnight, then incubated with the secondary antibody
included in the kit at room temperature for 2 h. Finally, the
sections were stained with DAB reagent for 2 min at room
temperature and then, hematoxylin for 2 min at room temperature.
Stained cells were observed under an optical photographic light
microscope at ×200 magnification and analyzed by Image Pro Plus 6.0
software (Media Cybernetics, Inc.).
Culture and treatment of
fibroblasts
The human fibroblasts were obtained from Shanghai
Cell Repository of the Chinese Academy of Sciences. Cells were
cultured at 37°C in 5% CO2 with DMEM (Gibco; Thermo
Fisher Scientific, Inc.), supplemented with 15% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin & streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). A total of
1×106 fibroblasts were seeded in petri dishes with a
variety of specifications overnight until a confluence of 70% was
attained; then the dishes were washed twice with PBS. One third of
the fibroblasts were set as the LY group and were treated with the
PI3K inhibitor LY294002 (MedChemExpress) diluted in
dimethylsulfoxide to 50 µM for 24 h. Another third of the
fibroblasts were treated with siRNA for knockdown or lentiviral
vectors for overexpression. All fibroblasts were maintained in the
growth phase between 3 and 6 passages.
Small interfering (si)RNA
siRNA of laminin α5 (5′-GCATCAGCTTCGACAGTCA-3′) and
the negative control (cat. no. siN0000001-1-5; with same sequence
length as siRNA-laminin α5 but non-targeting) were purchased from
Guangzhou RiboBio, Co., Ltd. The fibroblasts were transfected with
50 nM siRNA at a confluence of 70% for 48 h with Opti-MEM (Gibco;
Thermo Fisher Scientific, Inc.) and Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. The efficiency of transfection was
detected by reverse transcription-quantitative PCR (RT-qPCR) and
immunofluorescence. Transfected cells were maintained in culture
for 48 h prior to subsequent experiments.
Gene overexpression by lentiviral
infection
The GV418 and GV419 lentiviral vectors for
overexpressing the laminin α5 target gene and the scramble control
(empty vector) were obtained from Shanghai Genechem Co., Ltd.
Lentiviral infection was performed to overexpress laminin α5
following the manufacturer's protocol. Fibroblasts in the
overexpression and scramble control group were cultured until they
reached 70% confluence. Subsequently, 1×106
fibroblasts/well were transfected with 2×107 TU
(multiplicity of infection of 20) laminin α5 GV418 lentiviral
vector or GV418 scramble control empty vector overnight in the
presence of 2 mg/ml polybrene (Gibco; Thermo Fisher Scientific,
Inc.) and then replaced with fresh complete medium. After 48
h-transfection at 37°C, cells were cultured in puromycin
(Sigma-Aldrich; Merck KGaA) at a concentration of 2 µg/ml for 72 h
for the preliminary screening to eliminate non-transfected
cells.
Subsequently, the laminin α5 overexpression GV419
lentiviral and the GV419 scramble control empty vector were
transfected into 1×106 fibroblasts as described
previously. Then, fibroblasts were screened with 400 µg/ml G418
Sulfate (Thermo Fisher Scientific, Inc.) for 72 h. The transfection
efficiency was verified as aforementioned, with an untreated group
used as the control group, and cells were kept in culture for
subsequent experiments.
RNA preparation and RT-qPCR
Total RNA was extracted from fibroblasts with
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Reverse transcription to
cDNA was performed using the FastKing DNA Dispelling RT SuperMix
(Tiangen Biotech Co., Ltd.), according to the manufacturer's
protocol. The RT conditions were 42°C for 15 min and 95°C for 3
min. All qPCR reactions were run on the StepOnePlus Real-Time PCR
System (Thermo Fisher Scientific, Inc.) with a SYBR®
Green Master Mix kit (Vazyme Biotech Co. Ltd), according to the
manufacturer's protocol. The primers used are displaying in
Table I. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 5 min; 40 cycles at 95°C for 10 sec and
60°C for 30 sec; and 95°C for 15 sec, 60°C for 60 sec and 95°C for
15 sec. Expression levels were quantified using the
2−ΔΔCq method (27) and
normalized to the loading control GAPDH.
 | Table I.Primers for reverse
transcription-quantitative PCR. |
Table I.
Primers for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| Laminin α5 | F:
TGCACCCGCCCTACTTCAA |
|
| R:
GGGTGACGTTGACCTCGTTGTA |
| GAPDH | F:
GAAGCTTGTCATCAATGGAAAT |
|
| R:
TGATGACCCTTTTGGCTCCC |
Western blot analysis
A total of 3×106 fibroblasts were lysed
on ice with RIPA lysis buffer (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Total
protein concentration was determined with a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). Western blot
analysis was performed as previously reported (28). Briefly, 30 µg protein/lane was
separated via 10% SDS-PAGE and transferred onto a PVDF membrane.
The membranes were blocked with 5% skim milk in TBS and 0.05%
Tween-20 for 2 h at room temperature and subsequently incubated
with primary antibodies at 4°C overnight. The primary antibodies
were against proliferating cell nuclear antigen (PCNA; 1:1,000;
cat. no. 13110; Cell Signaling Technology, Inc.), cyclin D1
(1:1,000; cat. no. 55506; Cell Signaling Technology, Inc.),
phosphorylated (p)-focal adhesion kinase 1 (FAK1; 1:1,000; cat. no.
3281; Cell Signaling Technology, Inc.), FAKT1 (1:1,000; cat. no.
71433; Cell Signaling Technology, Inc.), AKT (1:1,000; cat. no.
4685; Cell Signaling Technology, Inc.), p-AKT (1:1,000; cat. no.
4060; Cell Signaling Technology, Inc.), mTOR (1:1,000; cat. no.
2983; Cell Signaling Technology, Inc.), p-mTOR (1:1,000; cat. no.
5536; Cell Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no.
5174; Cell Signaling Technology, Inc.). Subsequently, the membranes
were incubated with a horseradish peroxidase-conjugated anti-rabbit
IgG secondary antibody (1:2,000; cat. no. 7074; Cell Signaling
Technology, Inc.) for 2 h at room temperature. The target protein
expression was detected with ECL reagents (Beyotime Institute of
Biotechnology) using a ChemiDoc XRSþ system (Bio-Rad Laboratories,
Inc.). The results were analyzed using ImageJ version 1.46r
software (National Institutes of Health).
Immunofluorescence staining
A total of 2.5×105 fibroblasts from each
group were simultaneously cultured in 24-well plates overnight
until 70% confluent. Then cells were fixed with 4% polyoxymethylene
in PBS at room temperature for 15 min, then immersed in 0.1% Triton
X-100. Subsequently, the sections were blocked in 3% BSA (Gibco;
Thermo Fisher Scientific, Inc.) for 30 min at room temperature and
incubated with anti-laminin α5 primary antibody (1:100; cat. no.
220399; Abcam) overnight at 4°C and probed with a FITC-conjugated
goat anti-rabbit IgG secondary antibody (1:200; cat. no. 33112ES60;
Yeasen Biotechnology (Shanghai) Co., Ltd.) for 2 h at room
temperature. Finally, the cell nuclei were stained with Hoechst for
5 min at room temperature, then observed with a Zeiss inverted
fluorescence microscope (magnification, ×200) to determine the
expression levels of the target protein. The data were analyzed
using Image Pro Plus 6.0 (Media Cybernetics, Inc.).
Cell viability
Cell viability was analyzed using the Cell Counting
Kit-8 assay (CCK-8; cat. no. CK04; Dojindo Molecular Technologies,
Inc.), according to the manufacturer's protocol. Fibroblasts were
cultured in triplicate in 96-well plates for 24 h at 37°C, then
treated with 10 µl CCK-8 reagent for 2 h at 37°C. The optical
density value at 450 nm was determined with a microplate absorbance
reader (Bio-Tek; Elx800). The cell survival rate was calculated
according to the manufacturer's specification.
EdU incorporation assay
The EdU incorporation assay was conducted to
evaluate fibroblast proliferation. The kFlour555 Click-iT EdU kit
was obtained from KeyGen Biotech Co., Ltd. A total of
2.5×105 fibroblasts were cultured in 24-well plates for
24 h until 70% confluent. Then cells were subsequently incubated in
10 µmol/l EdU working solution for 2 h at 37°C, fixed in 4%
polyoxymethylene for 30 min at room temperature and incubated with
0.5% Triton X-100 for 20 min in the dark at room temperature. After
immerged in Click-iT mixture system, cell nuclei were stained with
Hoechst 33342 for 5 min at room temperature. Finally, the cells
were observed under a Zeiss inverted fluorescence microscope
(magnification, ×200). Orange was deemed as a positive signal of
proliferation and the cell nucleus was royal blue. The positive EdU
rate was calculated using ImageJ software.
Statistical analysis
The data of the present study are presented as the
mean ± SD and statistical analysis was performed using SPSS 19.0
statistical software (IBM Corp.). Each experiment was performed in
triplicate. The significance of the differences among groups was
evaluated by Student's t-test or one-way ANOVA followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Laminin α5 is positively associated
with epidural fibrosis
To detect epidural fibroblast density and fibrosis,
histological analysis by H&E staining, fibroblast counting, and
Masson trichrome stains were performed. As shown in Fig. 1A and C, from H&E staining and
fibroblast counting, extensive fibrosis and significantly high
densities of fibroblasts were found in the postoperative area in
the 4-week group compared with the 2-week group (P<0.05).
Similarly, the Masson trichrome stains (Fig. 1B) indicated that the presence of
collagen in local tissue on the dura mater of the 4-week group was
markedly increased compared with the 2-week group. The results also
demonstrated that the epidural fibrosis level and fibroblast
density in the laminectomy area were increased in a time-dependent
manner. This supports the previous conclusion that the increased
presence of fibroblasts in the operative region is a significant
cause of epidural fibrosis. To determine whether laminin α5 is
involved in epidural fibrosis, its expression in the two groups was
further assessed, as shown in Fig.
1D. The laminin α5 content was significantly increased in the
4-week group compared with the 2-week group (P<0.05). These
results supported the assumption that laminin α5 may be positively
associated with epidural fibrosis.
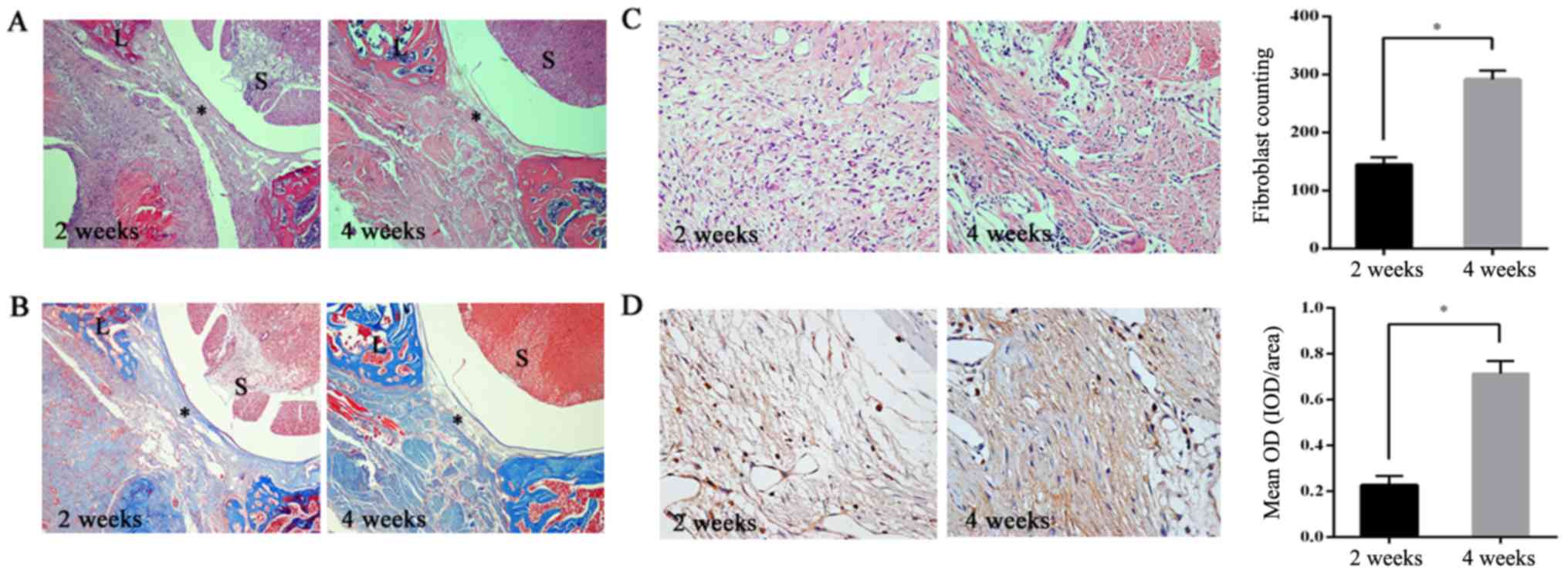 | Figure 1.Laminin α5 expression is related to
epidural fibrosis. (A) In H&E staining, excessive fibrosis ‘*’
with thick adherence to spinal dura was observed in the 4 weeks
group. In the 2 weeks group, there were notably fewer areas of
fibrosis, which was observed in a time-dependent manner. In the
images, the surgical area is marked by ‘L’ and the spinal cord by
‘S’. Magnification, ×40. (B) In Masson's trichrome staining,
collagen is indicated in royal blue. Images indicated that epidural
collagen was gradually synthesized with increasing time.
Magnification, ×40. (C) H&E staining images showed that the
number of fibroblasts within the surgical area around the spinal
dura increased with time, as shown in the histogram. Magnification,
×200. The data are presented as the mean ± SD of the two groups.
*P<0.05. (D) Immunohistochemical staining of laminin α5 in
epidural fibrosis tissues. The results of laminin α5 expression are
shown as the mean integral OD in the histogram. Magnification,
×200. Analysis was conducted using Image Pro Plus 6.0 (Media
Cybernetics, Inc.). The data are presented as the mean ± SD of two
groups, *P<0.05. SD, standard deviation; H&E, hematoxylin
and eosin; OD, optical density. |
Laminin α5 modulates fibroblast
proliferation
Based on the results of the animal model, the effect
of laminin α5 on fibroblast proliferation was further studied. The
fibroblasts were transfected with laminin α5 siRNA, which was
followed with RT-qPCR and immunofluorescence to detect the
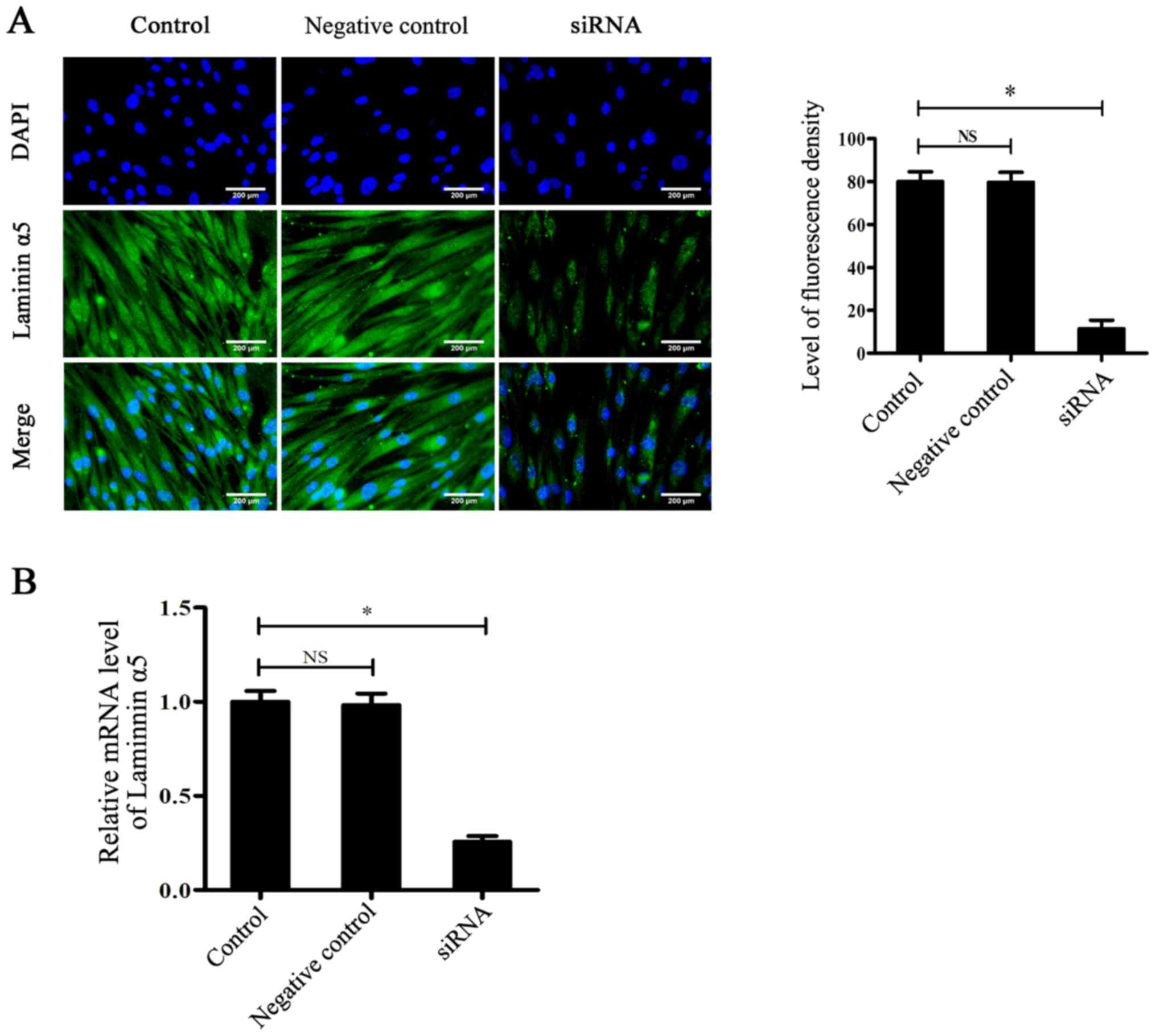
transfection efficiency. As shown in Fig. 2A, immunofluorescence staining
indicated that the expression of laminin α5 in the siRNA group was
significantly reduced after siRNA-knockdown compared with the
control group (P<0.05), which was further demonstrated by
RT-qPCR (Fig. 2B). After
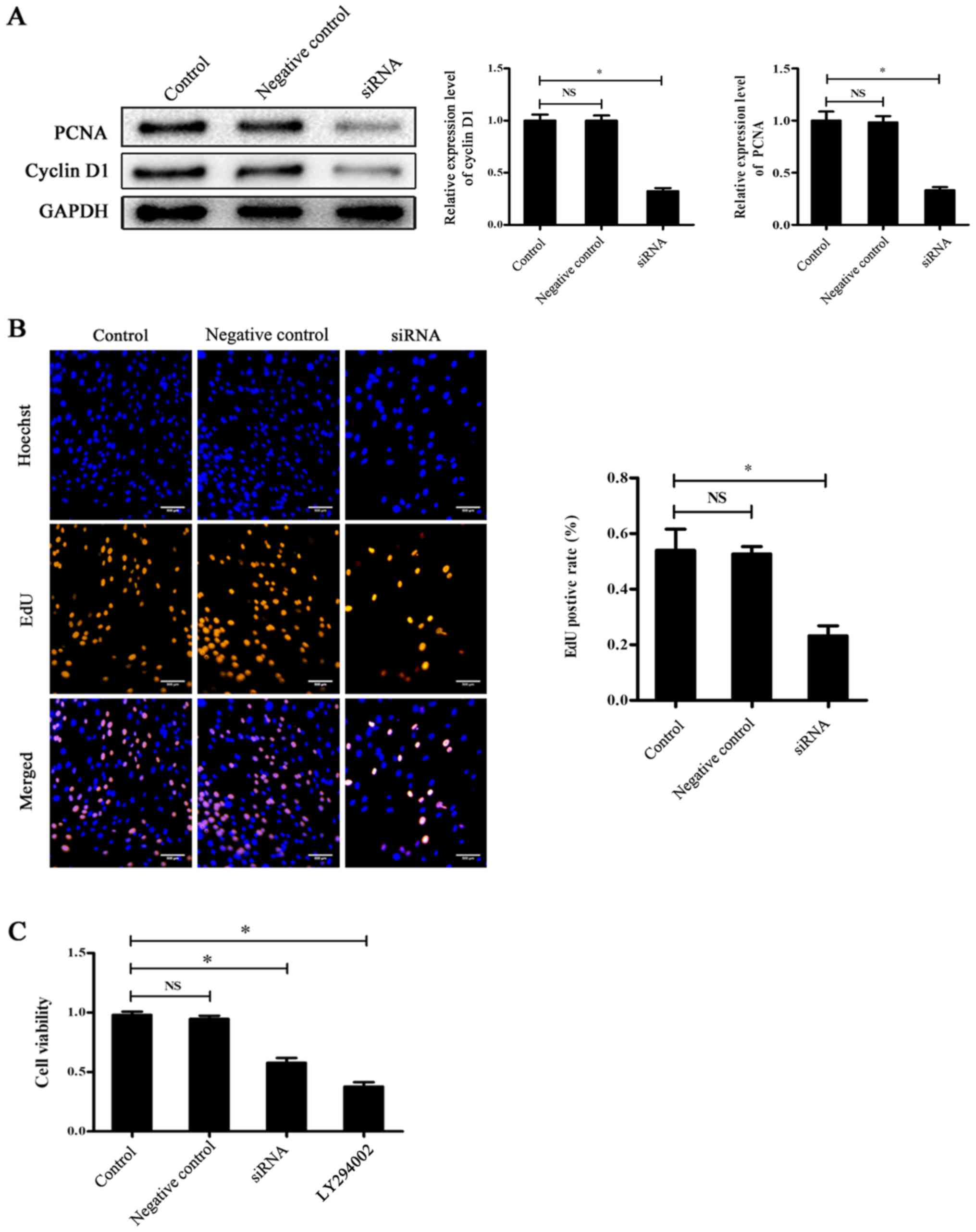
transfection, the PCNA and cyclin D1 (marker of cell proliferation)
levels were determined by western blotting. The results
demonstrated that the expression levels of PCNA and cyclin D1 were
significantly reduced in the siRNA group compared with the control
group (P<0.05; Fig. 3A). An EdU
incorporation assay was subsequently performed to further study the
proliferative level, which demonstrated that the positive rate of
proliferation was also significantly decreased in the siRNA group
compared with the control group (P<0.05; Fig. 3B). The results of the CCK-8 assay
also demonstrated that the cell viability of the siRNA group was
significantly reduced compared with the control group (P<0.05;
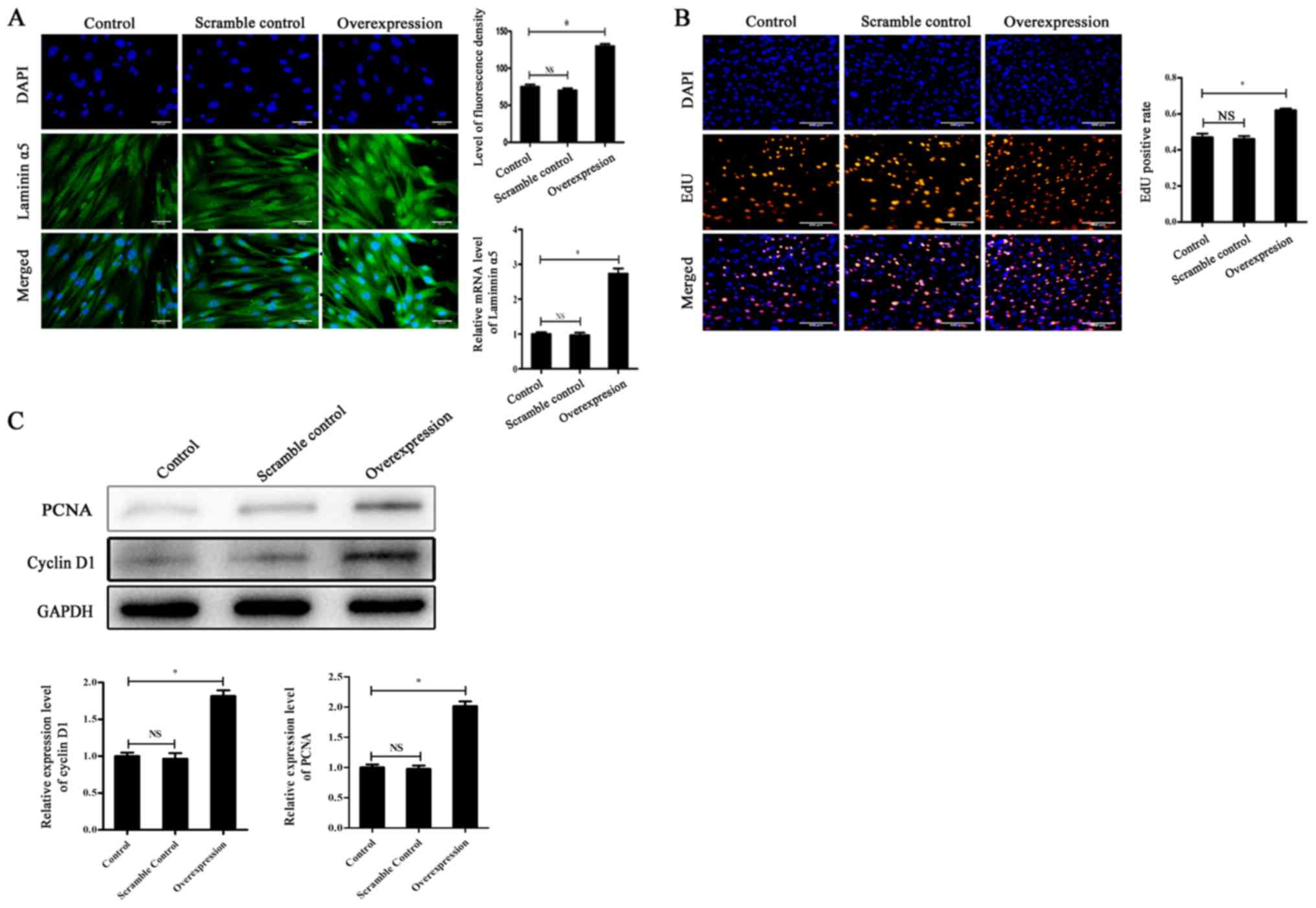
Fig. 3C). Subsequently, laminin α5
was overexpressed by lentiviral vectors to perform the same
experiments. The transfection efficiency was confirmed by RT-qPCR
and immunofluorescence (Fig. 4A).
The EdU positive rate and the expression levels of PCNA and cyclin
D1 were significantly higher in the overexpression group compared
to the control group (Fig. 4B and
C). This indicated that the proliferative rate of cells
following laminin α5 overexpression was increased compared with the
control group, which was opposite to the results obtained with
siRNA (Fig. 4B and C).
Collectively, these results suggested that laminin α5 may be
associated with fibroblast viability and it could modulate cell
proliferation.
Laminin α5 interferes with the
activation of the PI3K/AKT/mTOR signaling pathway
After transfection with laminin α5 siRNA, western
blotting was performed to determine the influence of target
proteins of the PI3K/AKT/mTOR signaling pathway. The results
indicated that after gene knockdown, the expression ratio of
p-AKT/AKT and p-mTOR/mTOR significantly decreased compared with the
control group (P<0.05; Fig.
5A). Thus, downregulation of laminin α5 prevented the
activation of PI3K/AKT/mTOR signaling. The expression level of
p-FAK, an upstream protein of the PI3K/AKT/mTOR signaling pathway,
was also detected and there was also a significant decrease in the
siRNA group compared with the control group (P<0.05; Fig. 5A). Subsequently, the expression
levels of proteins in the PI3K/AKT/mTOR signaling pathway in
laminin α5-overexpressing fibroblasts were also investigated and
the results in Fig. 5B
demonstrated a reversed tendency of signaling: The expression ratio
of p-AKT/AKT and p-mTOR/mTOR in the overexpression group was
significantly increased compared with the control group, as well as
the expression ratio of p-FAK/FAK (P<0.05). All of the results
indicated that laminin α5 may interfere with PI3K/AKT/mTOR
signaling activation and the expression of p-FAK.
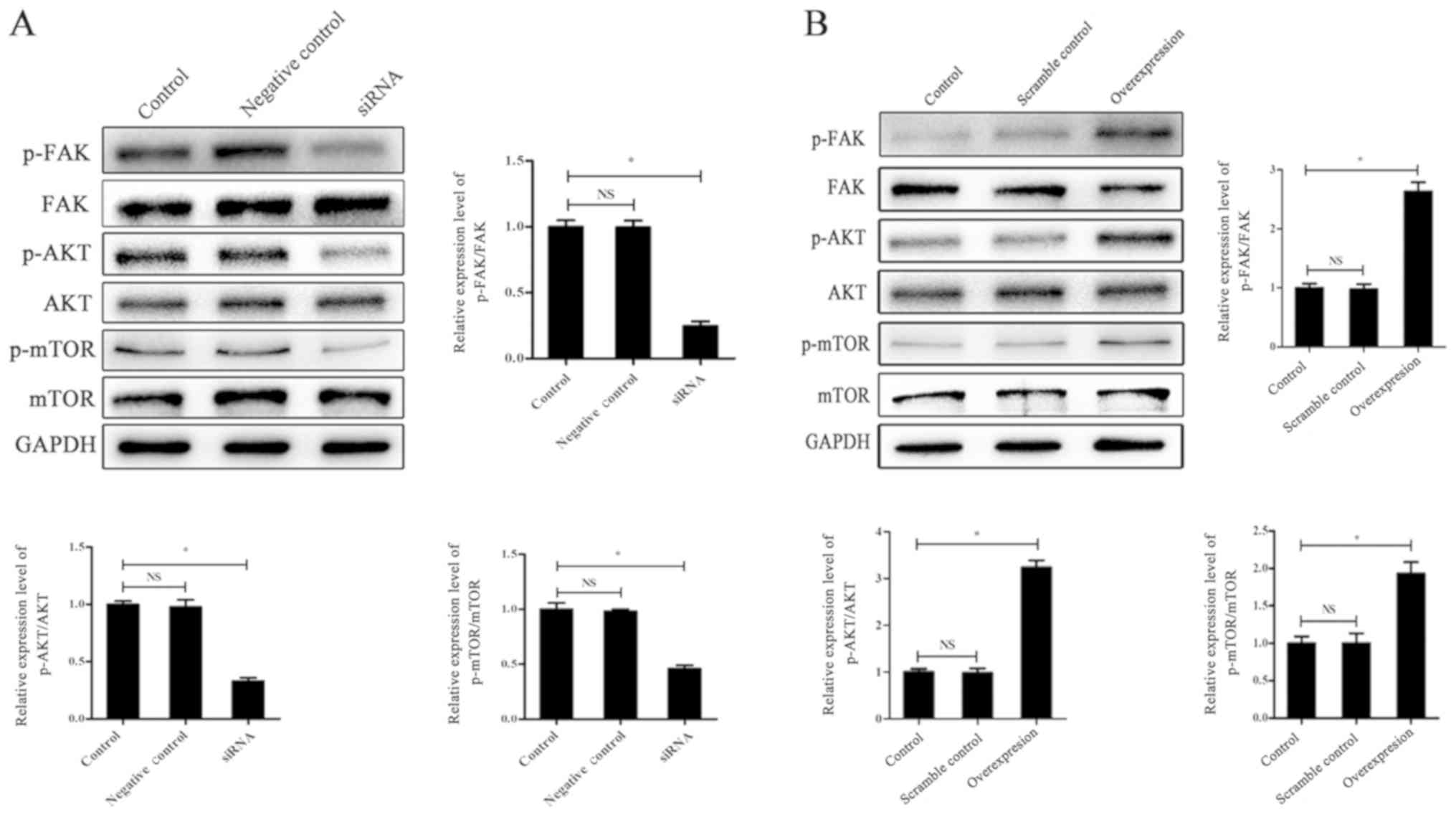 | Figure 5.Laminin α5 interferes with the
activation of the PI3K/AKT/mTOR signaling pathway. (A) Knockdown of
laminin α5 reduces the activation of the PI3K/AKT/mTOR signaling
pathway. Western blotting of p-FAK, FAK, p-AKT, AKT, p-mTOR and
mTOR expression levels in fibroblasts within the control, siRNA and
negative control groups. GAPDH was set as the control. The data are
presented as the mean ± SD of three independent experiments.
*P<0.05. (B) Overexpression of laminin α5 promotes the
activation of the PI3K/AKT/mTOR signaling pathway. Western blot
assay of p-FKA, FAK, p-AKT, AKT, p-mTOR and mTOR expression levels
in fibroblasts within the control, scramble control and
overexpression group. GAPDH was set as the control. The data are
presented as the mean ± SD of three independent groups. *P<0.05.
SD, standard deviation; si, small interfering RNA; p,
phosphorylated; FAK, focal adhesion kinase; AKT, protein kinase B;
mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3
kinase; NS, not significant. |
The PI3K/AKT/mTOR signal pathway
regulates fibroblast proliferation
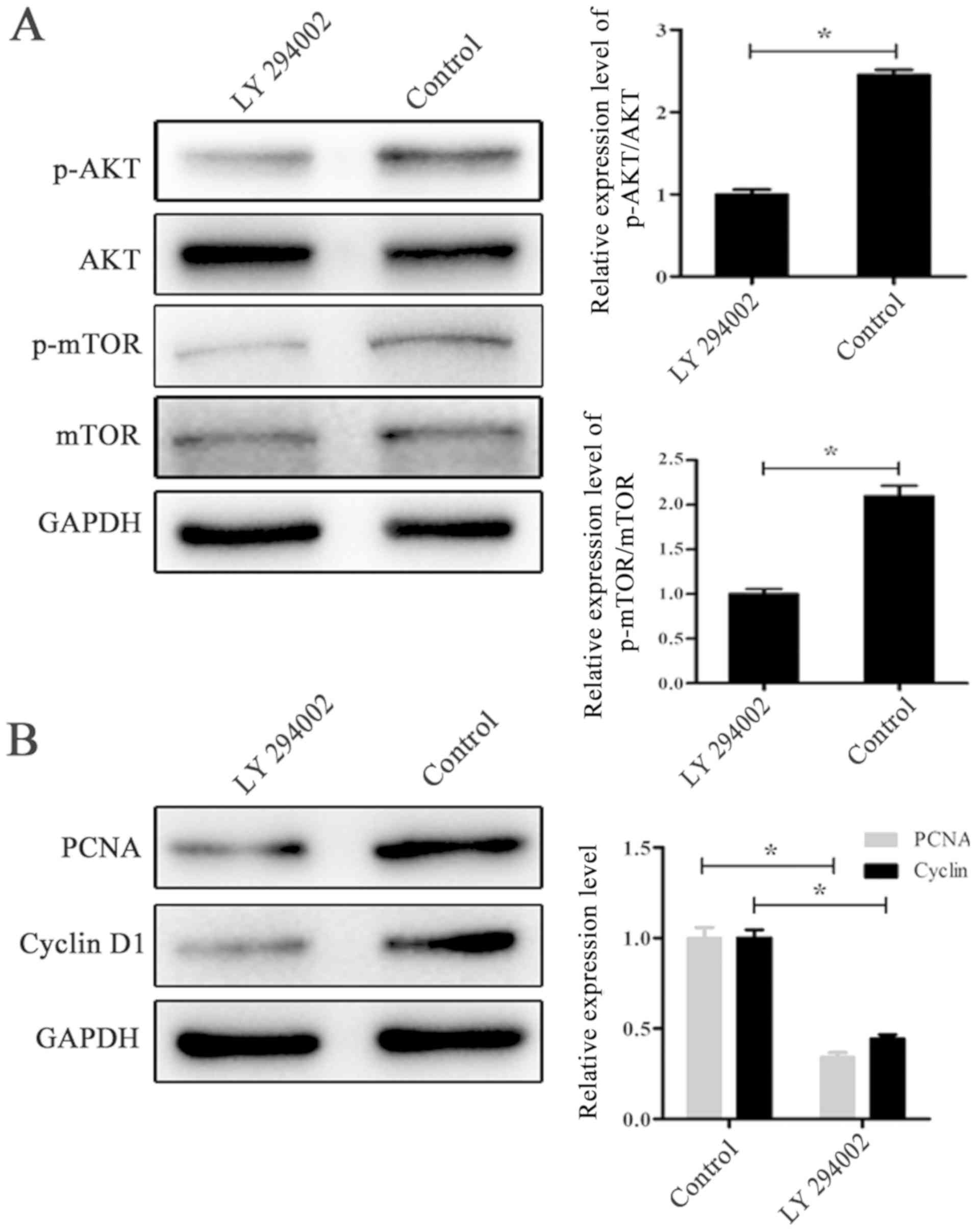
To confirm whether PI3K/AKT/mTOR signaling could
regulate fibroblast proliferation, cells were treated with the
signaling pathway inhibitor LY294002. Following treatment with
LY294002 for 24 h, western blotting was performed to detect the
expression of PCNA, cyclin D1, p-AKT and p-mTOR. As shown in
Fig. 6A, the expression ratio of
p-AKT/AKT and p-mTOR/mTOR were significantly decreased in the
LY294002 group compared with the control group (P<0.05), which
indicated successful inhibition. In Fig. 6B, the downregulation of PCNA and
cyclin D1 in the LY294002 group suggested that the PI3K/AKT/mTOR
signaling pathway could regulate fibroblast proliferation, which
was further proved in the CCK-8 assay (Fig. 3C).
Discussion
Previous studies have indicated the epidural
fibrosis on dura mater after laminectomy operation, which
ultimately results in a negative outcome for patients (8,29).
Various studies have attempted to solve this problem by local drug
applications (30,31) and biomaterials (32). However, there are numerous
disadvantages that limit clinical popularization. Therefore, the
prevention of epidural fibrosis through the reduction of fibroblast
proliferation has been a continuously popular research topic.
The extracellular matrix is a structure with an
important role to support cell construction and promote various
functions, such as adhesion, differentiation, migration and
proliferation, which are also associated with the development of
numerous diseases (33,34). The laminins are an important part
of the extracellular matrix and serve a primary role in multiple
biological behaviors (12).
Additionally, there are several studies indicate that the α5 chain
(laminin α5) is widely expressed in the laminin glycoprotein
family, which suggests that laminins may serve important roles in
most cell functions (17,18). Further studies also illustrated the
involvement of laminin α5 in maintaining the stability of the
basement membrane and organ formation, including the placenta
during the embryonic phase and dental epithelium growth (35,36).
Therefore, laminin α5 is a crucial factor in an organism, so the
present study assumed it might also be involved in the formation of
epidural fibrosis.
Several studies have illustrated that the
PI3K/AKT/mTOR signaling pathway modulates cellular proliferation
and various biological behaviors (23,24).
PI3K is a bridge factor between extracellular signaling and
cellular response effects, where activated PI3K could promote the
transformation of AKT, which accelerates the phosphorylation of
downstream factor mTOR to inhibit cell apoptosis (37,38).
Thus, it is reasonable to assume that laminin α5 could be a pivotal
point in epidural fibrosis and modulate fibroblast proliferation
through the activation of the PI3K/AKT/mTOR signalling pathway.
In the initiation of the present study, the
association between laminin α5 and epidural fibrosis was
investigated. There are a series of methods to detect the epidural
fibrosis formation, such as H&E staining, Masson trichrome
stains, local fibroblast number counting (7), MR imaging assessment (39) and high-resolution CT scan (1). In the present study histological
evaluation was used to assess the epidural fibrosis level. The
result from H&E staining, fibroblast counting and Masson
trichrome stains indicated that epidural fibrosis got thicker and
local fibroblast number increased over time. On that basis, further
immunohistochemistry of laminin α5 showed that the expression was
similar to epidural fibrosis formation and presented in a
time-dependent manner. It demonstrated that laminin α5 was strongly
associated with epidural fibrosis and local fibroblast
proliferation.
After animal model experiments, analysis was
conducted at the cellular level to study the detail of the
mechanism involved in laminin α5 and fibroblast proliferation.
Laminin α5 was knocked down for further studies including western
blotting, EdU incorporation assay and CCK-8 assay, which showed
that fibroblasts following knockdown presented a lower level of
proliferation and cell vitality. For further confirmation, the
laminin α5 was overexpressed, in which the present study
demonstrated an increase in cell proliferation. The results
indicated that laminin α5 could modulate fibroblast proliferation,
which is similar to previous studies where laminin α5 played a
marked role in cell behaviors, such as proliferation (17,18).
Further detection of p-AKT/AKT and p-mTOR/mTOR in laminin
α5-knockdown fibroblasts implied that the PI3K/AKT/mTOR signal
pathway activation was reduced, which dramatically increased
following overexpression. Thus, laminin α5 could modulate the
activation of PI3K/AKT/mTOR signaling, which corroborates with the
finding of another study, that laminin α5 serves a biological role
through this signaling pathway (40). Furthermore, the expression of p-FAK
decreased after laminin α5 knockdown but increased following its
overexpression. FAK is the hub of multiple signal transduction
pathways; it can be phosphorylated to an active form by the
activation of integrin, which initiates multiple signaling pathways
including PI3K/AKT/mTOR (41). The
change in p-FAK expression in the present study indicated that the
mechanism of laminin α5 modulates the activation of the
PI3K/AKT/mTOR signaling pathway might be through integrin and FAK,
which was similar to the study of Santos et al (42), although this requires further
confirmation. Then after the inhibition of the signaling pathway
with LY294002, cell proliferation was decreased, which revealed
that the PI3K/AKT/mTOR signaling pathway could regulate fibroblast
proliferation. Combined with the results that laminin α5 modulates
fibroblast proliferation and interferes the activation of the
PI3K/AKT/mTOR signaling pathway, it can be concluded that laminin
α5 might modulate fibroblast proliferation in epidural fibrosis
through the PI3K/AKT/mTOR signaling pathway.
Fukumoto et al (36) found laminin α5 is necessary for
oral cavity epithelium generation and plays a significant role in
cell behavior. There is also a study that indicated that laminins
with α5 chain are essential for several biological behaviors among
epidermal cells (43). These
studies all showed that laminin α5 is a crucial factor in
biological functions and participates in several cell behaviors.
The data of the present study suggest that laminin α5 is associated
with epidural fibrosis and might modulate fibroblast proliferation
through the PI3K/AKT/mTOR signaling pathway.
In conclusion, the present study confirmed the
association between laminin α5 and epidural fibrosis. Furthermore,
a possible mechanism was also found that laminin α5 might modulate
fibroblast proliferation through the PI3K/AKT/mTOR signaling
pathway. The results of this study could indicate a potential
treatment to prevent epidural fibrosis. However, due to time
limitations in this study, there are also more complex experiments
have not been performed such as using an inducible laminin α5
knockout mouse which would take 1–2 years. In the future, the
present authors may perform this experiment to aid further
conclusions and find out more regarding the potential
mechanism.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81772331, 81371971
and 81271994), the Jiangsu Provincial Medical Youth Talent (grant
no. QNRC2016344), the Six talent peaks project of Jiangsu Province
(grant no. 2015-WSN-108 and 2015 WSN 110), the Jiangsu Provincial
333 Project Foundation (grant no. BRA2018194), the Social
Development Projects of Yangzhou Science and Technology Bureau
(grant no. YZ2017073), the China Postdoctoral Science Foundation
(grant no. 2016M590431) and the Jiangsu Provincial Medical
Innovation Team (grant no. CXTDB2017004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL designed the research, performed the experiments
and wrote the manuscript. HC contributed to the reagents,
materials, analysis tools and analyzed the data. LY prepared the
figures and tables. YS helped design the experiments, prepared the
animal models and collected the tissue, and reviewed the drafts of
the manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Research Ethics Committee of the Northern Jiangsu People's Hospital
(Yangzhou, China) and written informed consent was obtained from
all the participants for their tissues to be used for the purposes
of this research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burton CV, Kirkaldy-Willis WH, Yong-Hing K
and Heithoff KB: Causes of failure of surgery on the lumbar spine.
Clin Orthop Relat Res. 157:191–199. 1981.
|
|
2
|
Songer MN, Rauschning W, Carson EW and
Pandit SM: Analysis of peridural scar formation and its prevention
after lumbar laminotomy and discectomy in dogs. Spine (Phila Pa
1976). 20:571–580. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sen O, Kizilkilic O, Aydin MV, Yalcin O,
Erdogan B, Cekinmez M, Caner H and Altinors N: The role of
closed-suction drainage in preventing epidural fibrosis and its
correlation with a new grading system of epidural fibrosis based on
MRI. Eur Spine J. 14:409–414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirzai H, Eminoglu M and Orguc S: Are
drains useful for lumbar disc surgery? A prospective, randomized
clinical study. J Spinal Disord Tech. 19:171–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cekinmez M, Erdogan B, Tufan K, Sarica FB,
Ozen O and Caner H: Is topical tissue plasminogen activator
application effective on prevention of post-laminectomy epidural
fibrosis? An experimental study. Neurol Res. 31:322–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Zhao S, Li X, Yan L, Wang J, Wang
D, Chen H, Dai J and He J: Local application of rapamycin reduces
epidural fibrosis after laminectomy via inhibiting fibroblast
proliferation and prompting apoptosis. J Orthop Surg Res.
11:582016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Yan L, Wang J, Sun Y, Li X, Zhao
S, Wang D, Zhu G and Liang Y: Methotrexate prevents epidural
fibrosis through endoplasmic reticulum stress signalling pathway.
Eur J Pharmacol. 796:131–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai J, Li X, Yan L, Chen H, He J, Wang S,
Wang J and Sun Y: The effect of suramin on inhibiting fibroblast
proliferation and preventing epidural fibrosis after laminectomy in
rats. J Orthop Surg Res. 11:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atsuta I, Yamaza T, Yoshinari M, Goto T,
Kido MA, Kagiya T, Mino S, Shimono M and Tanaka T: Ultrastructural
localization of laminin-5 (gamma2 chain) in the rat peri-implant
oral mucosa around a titanium-dental implant by immuno-electron
microscopy. Biomaterials. 26:6280–6287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Durbeej M: Laminins. Cell Tissue Res.
339:259–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burgeson RE, Chiquet M, Deutzmann R,
Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M,
Sanes J, et al: A new nomenclature for the laminins. Matrix Biol.
14:209–211. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domogatskaya A, Rodin S and Tryggvason K:
Functional diversity of laminins. Annu Rev Cell Dev Biol.
28:523–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petz M, Them N, Huber H, Beug H and
Mikulits W: La enhances IRES-mediated translation of laminin B1
during malignant epithelial to mesenchymal transition. Nucleic
Acids Res. 40:290–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aumailley M and Rousselle P: Laminins of
the dermo-epidermal junction. Matrix Biol. 18:19–28. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petz M, Them NC, Huber H and Mikulits W:
PDGF enhances IRES-mediated translation of Laminin B1 by
cytoplasmic accumulation of La during epithelial to mesenchymal
transition. Nucleic Acids Res. 40:9738–9749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Wang TL, Toh WS and Pei M: The role
of laminins in cartilaginous tissues: From development to
regeneration. Eur Cell Mater. 34:40–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savino W, Mendes-da-Cruz DA, Golbert DC,
Riederer I and Cotta-de-Almeida V: Laminin-mediated interactions in
thymocyte migration and development. Front Immunol. 6:5792015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laperle A, Hsiao C, Lampe M, Mortier J,
Saha K, Palecek SP and Masters KS: α-5 Laminin synthesized by human
pluripotent stem cells promotes self-renewal. Stem Cell Rep.
5:195–206. 2015. View Article : Google Scholar
|
|
19
|
Miner JH: Laminins and their roles in
mammals. Microsc Res Tech. 71:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, DeRouen MC, Chen CH, Nguyen M,
Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, et
al: Laminin-511 is an epithelial message promoting dermal papilla
development and function during early hair morphogenesis. Genes
Dev. 22:2111–2124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sampaolo S, Napolitano F, Tirozzi A,
Reccia MG, Lombardi L, Farina O, Barra A, Cirillo F, Melone MAB,
Gianfrancesco F, et al: Identification of the first dominant
mutation of LAMA5 gene causing a complex multisystem syndrome due
to dysfunction of the extracellular matrix. J Med Genet.
54:710–720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Napolitano F, Di Iorio V, Di Iorio G,
Melone MAB, Gianfrancesco F, Simonelli F, Esposito T, Testa F and
Sampaolo S: Early posterior vitreous detachment is associated with
LAMA5 dominant mutation. Ophthalmic Genet. 40:39–42. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lam L, Hu X, Aktary Z, Andrews DW and
Pasdar M: Tamoxifen and ICI 182,780 increase Bcl-2 levels and
inhibit growth of breast carcinoma cells by modulating PI3K/AKT,
ERK and IGF-1R pathways independent of ERalpha. Breast Cancer Res
Treat. 118:605–621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Li X, Yan L, Nie Q, Dai J, Chen H,
Wang J and Sun Y: Tamoxifen inhibits fibroblast proliferation and
prevents epidural fibrosis by regulating the AKT pathway in rats.
Biochem Biophys Res Commun. 497:937–942. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Wang LX, Wang L, Sun SX, Cao XJ,
Wang P and Feng L: A comparison of the effectiveness of mitomycin C
and 5-fluorouracil in the prevention of peridural adhesion after
laminectomy. J Neurosurg Spine. 7:423–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai J, Sun Y, Yan L, Wang J, Li X and He
J: Upregulation of NOXA by 10-Hydroxycamptothecin plays a key role
in inducing fibroblasts apoptosis and reducing epidural fibrosis.
PeerJ. 5:e28582017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiao R, Chen H, Wan Q, Zhang X, Dai J, Li
X, Yan L and Sun Y: Apigenin inhibits fibroblast proliferation and
reduces epidural fibrosis by regulating Wnt3a/β-catenin signaling
pathway. J Orthop Surg Res. 14:2582019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Chen H, Wang S, Dai J, Yan L, Wang J
and Sun Y: Tacrolimus induces fibroblasts apoptosis and reduces
epidural fibrosis by regulating miR-429 and its target of RhoE.
Biochem Biophys Res Commun. 490:1197–1204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Wang S, Dai J, Yan L, Zhao S, Wang J
and Sun Y: Homoharringtonine prevents surgery-induced epidural
fibrosis through endoplasmic reticulum stress signaling pathway.
Eur J Pharmacol. 815:437–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sandoval MA and Hernandez-Vaquero D:
Preventing peridural fibrosis with nonsteroidal anti-inflammatory
drugs. Eur Spine J. 17:451–455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pozzi A, Yurchenco PD and Iozzo RV: The
nature and biology of basement membranes. Matrix Biol. 57-58:1–11.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hynes RO: The evolution of metazoan
extracellular matrix. J Cell Biol. 196:671–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spenle C, Simon-Assmann P, Orend G and
Miner JH: Laminin α5 guides tissue patterning and organogenesis.
Cell Adh Migr. 7:90–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fukumoto S, Miner JH, Ida H, Fukumoto E,
Yuasa K, Miyazaki H, Hoffman MP and Yamada Y: Laminin alpha5 is
required for dental epithelium growth and polarity and the
development of tooth bud and shape. J Biol Chem. 281:5008–5016.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma N, Nanta R, Sharma J, Gunewardena
S, Singh KP, Shankar S and Srivastava RK: PI3K/AKT/mTOR and sonic
hedgehog pathways cooperate together to inhibit human pancreatic
cancer stem cell characteristics and tumor growth. Oncotarget.
6:32039–32060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su JS, Woods SM and Ronen SM: Metabolic
consequences of treatment with AKT inhibitor perifosine in breast
cancer cells. NMR Biomed. 25:379–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bundschuh CV, Modic MT, Ross JS, Masaryk
TJ and Bohlman H: Epidural fibrosis and recurrent disk herniation
in the lumbar spine: MR imaging assessment. AJR Am J Roentgenol.
150:923–932. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ritie L, Spenle C, Lacroute J,
Bolcato-Bellemin AL, Lefebvre O, Bole-Feysot C, Jost B, Klein A,
Arnold C, Kedinger M, et al: Abnormal Wnt and PI3Kinase signaling
in the malformed intestine of lama5 deficient mice. PLoS One.
7:e377102012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia H, Nho RS, Kahm J, Kleidon J and Henke
CA: Focal adhesion kinase is upstream of phosphatidylinositol
3-kinase/Akt in regulating fibroblast survival in response to
contraction of type I collagen matrices via a beta 1 integrin
viability signaling pathway. J Biol Chem. 279:33024–33034. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Santos AR, Corredor RG, Obeso BA,
Trakhtenberg EF, Wang Y, Ponmattam J, Dvoriantchikova G, Ivanov D,
Shestopalov VI, Goldberg JL, et al: β1 integrin-focal adhesion
kinase (FAK) signaling modulates retinal ganglion cell (RGC)
survival. PLoS One. 7:e483322012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wegner J, Loser K, Apsite G, Nischt R,
Eckes B, Krieg T, Werner S and Sorokin L: Laminin α5 in the
keratinocyte basement membrane is required for epidermal-dermal
intercommunication. Matrix Biol. 56:24–41. 2016. View Article : Google Scholar : PubMed/NCBI
|