Introduction
Gastric cancer (GC) is one of the most common
malignancies worldwide, ranking 5th in morbidity and 3rd in
mortality among all malignancies (1,2).
Surgery along with systemic chemotherapy remains the most common
treatment regimen for GC. However, it has recently been observed
that GC is generally refractory to conventional chemotherapy drugs
(3), resulting in the poor
prognosis. Thus, new chemotherapy drugs are urgently required.
Toosendanin (TSN) is a tetracyclic triterpenoid
extracted from Melia toosendan Sieb, et Zucc, which
primarily grows in specific areas of China. Previous studies
indicate that TSN can induce the apoptosis of multiple human cancer
cells (4,5). It has been reported that TSN induces
apoptosis of AGS and HGC-27 human GC cell lines (5). Wang et al (6) have previously reported that TSN can
induce apoptosis of human GC SGC-7901 cells partly through microRNA
(miRNA/miR)-200a-mediated downregulation of the β-catenin pathway.
However, the regulatory mechanisms of the effect of TSN on GC cells
remain to be elucidated.
miRNAs are a class of evolutionarily conserved small
single-stranded non-coding RNAs with a length of 18–25 nt that
exhibit a post-transcriptional level regulatory function primarily
through binding to the 3′-untranslated (3′-UTR) region of mRNAs
(7,8). Several studies have reported that
~60% of human genes are regulated by different miRNAs (9,10).
In addition, miRNAs directly or indirectly affect various
biological processes such as cell proliferation, differentiation,
apoptosis and migration (11–14).
Specifically, miR-23a, has been shown to serve different roles
within tumour cells (15,16). It functions as a tumour suppressor
in osteosarcoma, in which its overexpression leads to reduced
proliferation, migration and invasion of osteosarcoma cells
(16). Additionally, miR-23a
inhibits pancreatic cancer cell progression by directly targeting
polo-like kinase 1 mRNA (17). There were 1,547 reads of
hsa-miR-23a-5p detected from 115 experiments, while 3,017,274 reads
of hsa-miR-23a-3p were detected from 159 experiments data from
miRbase database (http://www.mirbase.org/cgi-bin/mirna_entry.pl?acc=MI0000079/,
version 22.1). This indicates that the expression abundance of
hsa-miR-23a-3p is much higher compared with that of hsa-miR-23a-5p,
thus hsa-miR-23a-3p was selected in the present study. It was known
from the miRbase database that only 1547 reads of hsa-miR-23a-5p
were in the 115 experiments, while 3017274 reads of hsa-miR-23a-3p
were in the 159 experiments. This indicates that the expression
abundance of hsa-miR-23a-3p is many times higher than that of
hsa-miR-23a-5p, so hsa-miR-23a-3p was selected. The present study
found that the expression level of miR-23a-3p increased
significantly in MKN-45 cells following treatment with varying
concentrations of TSN. The role of miR-23a-3p, and its underlying
mechanism have not been previously investigated. Thus, the present
study aimed to elucidate the functions and mechanisms of miR-23a-3p
in TSN-induced apoptosis of GC cells.
Materials and methods
Cell culture
The human GC cell line MKN-45 and 293T cells were
purchased from the Beijing Beina Chuanglian Biotechnology
Institute. MKN-45 cells were cultured in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc.), 293T cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% foetal
bovine serum (Biological Industries), 100 µg/ml streptomycin, and
100 U/ml penicillin, and incubated at 37°C in 5% CO2 in
a humidified chamber. Fluorouracil (5-FU) and 0 nmol/l TSN were
used as positive control and negative control, respectively. The
cells were treated with different concentrations of TSN (0, 60, 80
and 100 nmol/l) and 5-FU (80 nmol/l) for 48 h, or transfected with
pcDNA3.1(+) (2 µg/ml), pcDNA3.1(+)-miR-23a-3p (2 µg/ml), miR-23a-3p
inhibitor (miR-23a-3p-I; 1.0×10−4 mmol/l) or miR-23a-3p
inhibitor-NC (miR-23a-3p-I-NC; 1.0×10−4 mmol/l)
(Guangzhou RiboBio Co., Ltd.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, then cultured for 48 h. The sequences
used were as follows: miR-23a-3p-I 5′-GGAAAAUCCCUGGCAAUGUGAU-3′;
miR-23a-3p-I-negative control (NC) 5′-CAGUACUUUUGUGUAGUACAAA-3′.
miR-23a-3p-I and miR-23a-3p-I-NC were 2′-O-methyl modified.
Cell Counting Kit-8 (CCK-8) assay for
chemosensitivity
Cell survival rate and chemosensitivity were
determined using a CCK-8 assay (Beyotime Institute of
Biotechnology). A total of 1×105 MKN-45 cells were
seeded in a 96-well plate. Three duplicated wells were set for each
group, and the total volume of each well was 100 µl. The plate was
then placed in a humidified incubator at 37°C with 5%
CO2, and the cells were cultured for 24, 48 and 72 h
with different concentrations of TSN (0, 10, 20, 30, 40, 50, 60,
70, 80, 90 and 100 nmol/l). After the indicated incubation times,
10 µl of CCK-8 was added to the plates and incubated at 37°C for an
additional 2 h. The absorbance at 450 nm was measured using a Spark
10 M multi-well plate reader (Tecan Group, Ltd.).
Vector construction
Total DNA was extracted from the MKN-45 cells using
a Genomic DNA Extraction kit (centrifugal column) according to the
manufacturer's instructions (BioTeke Corporation). Pre-miR-23a DNA
fragment (5′-ggc cgg cug ggg uuc cug ggg aug gga uuu gcu ucc ugu
cac aaA UCACAUUGCCAGGGAUUUCCa acc gac c-3′) was amplified by PCR
from the total DNA using Rapid PCR amplification kit according to
the manufacturer's instructions (BioTeke Corporation). The PCR
thermocycling conditions were as follows: Initial denaturation at
94°C for 5 min; followed by 35 cycles of denaturation at 94°C for
30 sec, primer annealing at 61°C for 30 sec, extension at 72°C for
1 min, and a final extension at 72°C for 10 min. Then pre-miR-23a
DNA fragment cloned into pcDNA3.1(+) plasmids (Promega Corporation)
to construct the overexpression vector of miR-23a-3p, which was
termed pcDNA3.1(+)-miR-23a-3p. The 3′-UTR of BCL2 was
PCR-amplified from MKN-45 cell genomic DNA using the same PCR
protocol as the pre-miR-23a-3p and cloned into psiCHECK-2
dual-luciferase reporter plasmids (Promega Corporation) immediately
downstream of the stop codon of the luciferase gene to generate
psiCHECK-2-BCL2-wt. The mutant BCL2 3′-UTR reporter,
designated as psiCHECK-2-BCL2-mu, was created by mutating the seed
region of the predicted miR-23a-3p site (TAT ATG T to TAC ACA A)
using PCR and the T-BCL primers was that used to amplify the
sequence contained the mutant seed region of the predicted
miR-23a-3p site (18). The primers
used in plasmid construction are shown in Table I.
 | Table I.Primers for BCL2 mRNA 3′-UTR
plasmid construction. |
Table I.
Primers for BCL2 mRNA 3′-UTR
plasmid construction.
| Gene | Primer sequences
(5′→3′) |
|---|
| Pre-miR-23a
F |
CGGGGTACCGGGAGGTGTCCCCAAATCTCATTAC |
| Pre-miR-23a
R |
CCGGAATTCGAACTTAGCCACTGTGAACACGACT |
| BCL2-3′UTR
F |
CCGCTCGAGTCAAACAAGACGCCAACA |
| BCL2-3′UTR
R |
AAGGAAAAAAGCGGCCGCTAACAGCCACTGCCTTAAAAGTAC |
| mu-BCL2-3′UTR
F |
AACAAATAGTTTATAATACACTACTTAAACTCTAATTAATTCC |
| mu-BCL23′UTR
R |
TTAGGATAAGTTCAATTACAAATA |
Acridine orange nuclear staining
Cells were cultured on glass cover slides and
treated with either different concentrations of TSN (0, 60, 80 and
100 nmol/l) or transfected with pcDNA3.1(+) (2 µg/ml),
pcDNA3.1(+)-miR-23a-3p (2 µg/ml), miR-23a-3p-I-NC
(1.0×10−4 mmol/l) or miR-23a-3p-I (1.0×10−4
mmol/l) for 48 h. Subsequently, cells were rinsed twice with PBS
and fixed with 4% paraformaldehyde in PBS for 10 min. Next, the
cells were stained with 0.1 mg/ml acridine orange (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) for 1–2 min. The images were
captured using a Leica TCS SP8 confocal laser-scanning microscope
(Leica Microsystems GmbH) at 488 nm excitation and 515 nm emission
wavelengths (magnification, ×63).
Cell cycle analysis
Cells were cultured to the logarithmic phase of
growth in a cell culture flask and treated with different
concentrations of TSN (0, 60, 80 and 100 nmol/l) or transfected
with pcDNA3.1(+), pcDNA3.1(+)-miR-23a-3p (2 µg/ml), miR-23a-3p-I-NC
or miR-23a-3p-I (1.0×10−4 mmol/l) for 48 h. The cells
were then collected and centrifuged at 300 × g at 4°C for 5 min.
The pellet was resuspended in PBS and centrifuged again using the
same conditions. Next, the pellet was resuspended in ice-cold 70%
ethanol and stored at 4°C for 18 h. Fixed cells were then washed
with PBS and incubated in 0.5 ml staining solution [0.1 mg/ml RNase
A and 0.05 mg/ml propidium iodide (PI)] at 37°C in the dark for 30
min. The cell cycles were determined on a Cytomics FC 500 flow
cytometer and CXP software 2.3 (Beckman Coulter, Inc.).
Apoptosis assay
Cells were cultured to the logarithmic phase in a
cell culture flask and treated with different concentrations of TSN
(0, 60, 80 and 100 nM) or transfected with pcDNA3.1(+),
pcDNA3.1(+)-miR-23a-3p (2 µg/ml), miR-23a-3p-I-NC or miR-23a-3p-I
(1.0×10−4 mmol/l) for 48 h. The cells were collected,
washed twice with cold PBS and 500 µl binding buffer was added to
each sample. The cells were then stained with 5 µl annexin V-FITC
and 5 µl PI for 15 min. The cells were immediately analysed by FC
500 flow cytometer and CXP software 2.3 (Beckman Coulter, Inc.) to
determine the rate of apoptosis.
Mitochondrial membrane potential (MMP)
assay
MMP was measured by flow cytometry using JC-1
(Nanjing KGI Biological Technology Development Co., Ltd.,)
staining. Briefly, transfected cells were incubated with 500 µl
diluted JC-1 (10 µg/ml) reagent at 37°C in the dark for 20 min.
After incubation, cells were washed with PBS and analysed within 30
min using FC 500 flow cytometer and CXP software 2.3 (Beckman
Coulter, Inc.). If the mitochondrial membrane potential is high,
JC-1 is gathered in the mitochondrial matrix and the formation of
polymer (J-aggregates) produces red fluorescence; When the
mitochondrial membrane potential is low, JC-1 cannot be
concentrated in the mitochondrial matrix. Then JC-1 is a monomer
and produces green fluorescence. Thus it is convenient to detect
the change of mitochondrial membrane potential by the change of
fluorescent color.
Reverse transcription-quantitative PCR
(RT-qPCR)
MKN-45 cells were seeded in 6-well plates
(1.0×105 cells/well) and treated with different
concentrations of TSN (0, 60, 80 and 100 nmol/l) or transfected
with pcDNA3.1(+), pcDNA3.1(+)-miR-23a-3p (2 µg/ml),
miR-23a-3p-I-NC, or miR-23a-3p-I (1.0×10−4 mmol/l) for
48 h. Total RNA was extracted based on the TRIzol®
method (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentrations of total RNA were determined spectrophotometrically
using a NanoDrop 2000C Spectrophotometer (Thermo Fisher Scientific,
Inc.). A first-strand cDNA was synthesized with 1µg of total RNA
from each sample using a TransScriptROne-Step gDNA Removal and cDNA
Synthesis Super Mix (Beijing TransGen Biotech Co., Ltd) according
to the manufacturer's instructions. 2X Plus SYBR Real-time PCR
mixture (BioTeke Corporation) was used for qPCR. The thermocycling
conditions were as follows: 95°C for 2 min, followed by 40 cycles
of 95°C for 15 sec, and 60°C for 30 sec. miRNAs are short and have
no ploy (A) structure, so stem-loop RT-PCR was used to detect the
miRNA expression (19). The
expression of human βactin or U6 was used as an
internal control for mRNA or miRNA expression levels, respectively,
and the relative expression level was measured by the
2−∆∆Cq method (20).
Primers used are detailed in Table
II.
 | Table II.Primers for RT-qPCR. |
Table II.
Primers for RT-qPCR.
|
|
| qPCR primer
sequences |
|---|
|
|
|
|
|---|
| Gene | RT primer
(5′→3′) | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
| miR-23a-3p |
CTCAACTGGTGTCGTGGAGTCG |
ACACTCCAGCTGGGATCA |
CTCAACTGGTGTCGT |
|
|
GCAATTCAGTTGAGGGAAATCC | CATTGCCAGGGAT | GGA |
| miR-148 |
CTCAACTGGTGTCGTGGAGTCGG |
ACACTTCCAGCTGGGAA |
CTCAACTGGTGTCGT |
|
|
CAATTCAGTTGAGAGTTCGGAG | AGTTGAGACACTC | GGA |
| miR-34a-5p |
CTCAACTGGTGTCGTGGAGTCGG |
ACACTCCAGCTGGGTGG |
CTCAACTGGTGTCGT |
|
|
CAATTCAGTTGAGACAACCAG |
CAGTGTCTTAGCTGG | GGA |
| miR-365a-3p |
CTCAACTGGTGTCGTGGAGTCG |
ACACTCCAGCTGGGTAA |
CTCAACTGGTGTCGT |
|
|
GCAATTCAGTTGAGATAAGGAT |
TGCCCCTAAAAATCC | GGA |
| BCL2 |
|
ATGTGTGTGAGAGCGTC | AGACAGCCAGGAGA |
|
|
| AAC | AATCAAAC |
| Bax |
|
AAGCTGAGCGAGTGTATC | CAAAGTAGAAAAGG |
|
|
| AAG | GCGACAAC |
| Fas |
|
TGATGTGGAACACAGCA |
GGCTGTGGTGACTCT |
|
|
| AGG | TAGTGATAA |
| Cyt c |
|
CTGGGTGACGAGTGAAA | TGAGCACAACAGGA |
|
|
| CTG | ACTGGA |
|
Caspase-3 |
|
GGAACGAACGGACCT | GCCTCCACTGGTAT |
|
|
| GTG | CTTCTG |
|
Caspase-8 |
|
GAGACAAGGGCATCATC |
TGGGTTTACCACGAA |
|
|
| TACGGC | GGGAAGG |
|
Caspase-9 |
|
GGACATCCAGCGGGC |
TCTAAGCAGGAGATG |
|
|
| AGG | AACAAAGG |
| APAF-1 |
|
AAGGTGGAGTACCACA |
TCCATGTATGGTGAC |
|
|
| GAG | CCATCC |
| β-actin |
|
AGCGAGCATCCCCCAAA | GGGCACGAAGGCTC |
|
|
| GTT | ATCATT |
| U6 |
|
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATT |
|
|
|
| TGCGT |
Western blot analysis
MKN-45 cells were seeded in 6-well plates
(1.0×105 cells/well) and treated with different
concentrations of TSN (0, 60, 80 and 100 nmol/l) or transfected
with pcDNA3.1(+) (2 µg/ml), pcDNA3.1(+)-miR-23a-3p (2 µg/ml),
miR-23a-3p-I-NC (1.0×10−4 mmol/l), or miR-23a-3p-I
(1.0×10−4 mmol/l) for 48 h. Cells were harvested and
lysed in RIPA buffer containing a protease inhibitor cocktail
(Beyotime Institute of Biotechnology). The lysate was centrifuged
at 12,000 × g for 10 min at 4°C. The supernatant was then
collected, and the protein concentration was determined using the
BCA Protein Assay Kit (Beyotime Institute of Biotechnology). The
same protein amounts (10 µg in each lane) were loaded and separated
by 10% SDS-PAGE, followed by transfer to polyvinylidene fluoride
membranes (EMD Millipore) using the western blotting apparatus. The
membranes were blocked with 5% (w/v) fat-free milk in Tris-buffered
saline containing 0.05% Tween-20 (TBS-T) and then incubated with
primary antibodies at 4°C overnight. Membranes were probed with the
following primary antibodies (all 1:300): Anti-BCL2 polyclonal
(cat. no. ab196495; Abcam), anti-Bax polyclonal (cat. no. ab53154;
Abcam), anti-active caspase-3 monoclonal (cat. no. bsm-33284M;
BIOSS), anti-caspase-8 polyclonal (cat. no. bs-0052R; BIOSS),
anti-caspase-9 polyclonal (cat. no. bs-0049R; BIOSS),
anti-cytochrome c polyclonal (cat. no. bs-0013R; BIOSS),
anti-Fas polyclonal (cat. no. bs-6477R; BIOSS), anti-apoptotic
protease activating factor 1 polyclonal (cat. no. ab2000; Abcam)
and anti-β actin polyclonal (cat. no. ab8227; Abcam). The membranes
were subsequently incubated with IRDye 800CW® goat
anti-rabbit (926–32211) and IRDye 800CW® goat anti-mouse
(32210) secondary antibody (1:15,000; LI-COR Biosciences) for 90
min at 20–25°C and washed with PBS. The bands were visualized and
quantified using the Li-COR Odyssey infrared imaging system (Li-COR
Biosciences); each band was normalised to its respective β-actin
band.
Dual-luciferase reporter assay
To explore the mechanisms of miR-23a-3p-induced
apoptosis, the target genes of mir-23a-3p were predicted by
TargetScan websites (TargetScan: https://www.targetscan.org, version 7.1) (21) and examined by luciferase reporter
assay. 293T cells (2×104 cells/well) were plated in a
24-well plate (Corning, Inc.) 24 h before transfection. Cells were
co-transfected with 0.5 µg of either the psiCHECK-2-BCL2-wt,
psiCHECK-2-BCL2-mu or empty vector psiCHECK-2, and either
pcDNA3.1(+) (2 µg/ml), pcDNA3.1(+)-miR-23a-3p (2 µg/ml),
miR-23a-3p-I-NC (1×10−4 mmol/l) or miR-23a-3p-I
(1×10−4 mmol/l) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After 48 h of transfection, cells were
lysed in Passive Lysis Buffer (Promega Corporation), and the
activities of the firefly and Renilla luciferase were measured with
a GloMax 20/20 Luminometer (Promega Corporation) using the
Dual-Luciferase Reporter Assay System according to the
manufacturer's protocols. Transfections were performed in duplicate
and were repeated three times.
Statistical analysis
All the results were expressed as mean ± SEM. All
statistical analyses and graphing were performed using SPSS 17.0
software (SPSS, Inc.) and GraphPad Prism 5.0 software (GraphPad
Software, Inc.), respectively. Statistical differences were
determined using one-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TSN inhibits proliferation and induces
apoptosis in MKN-45 cells
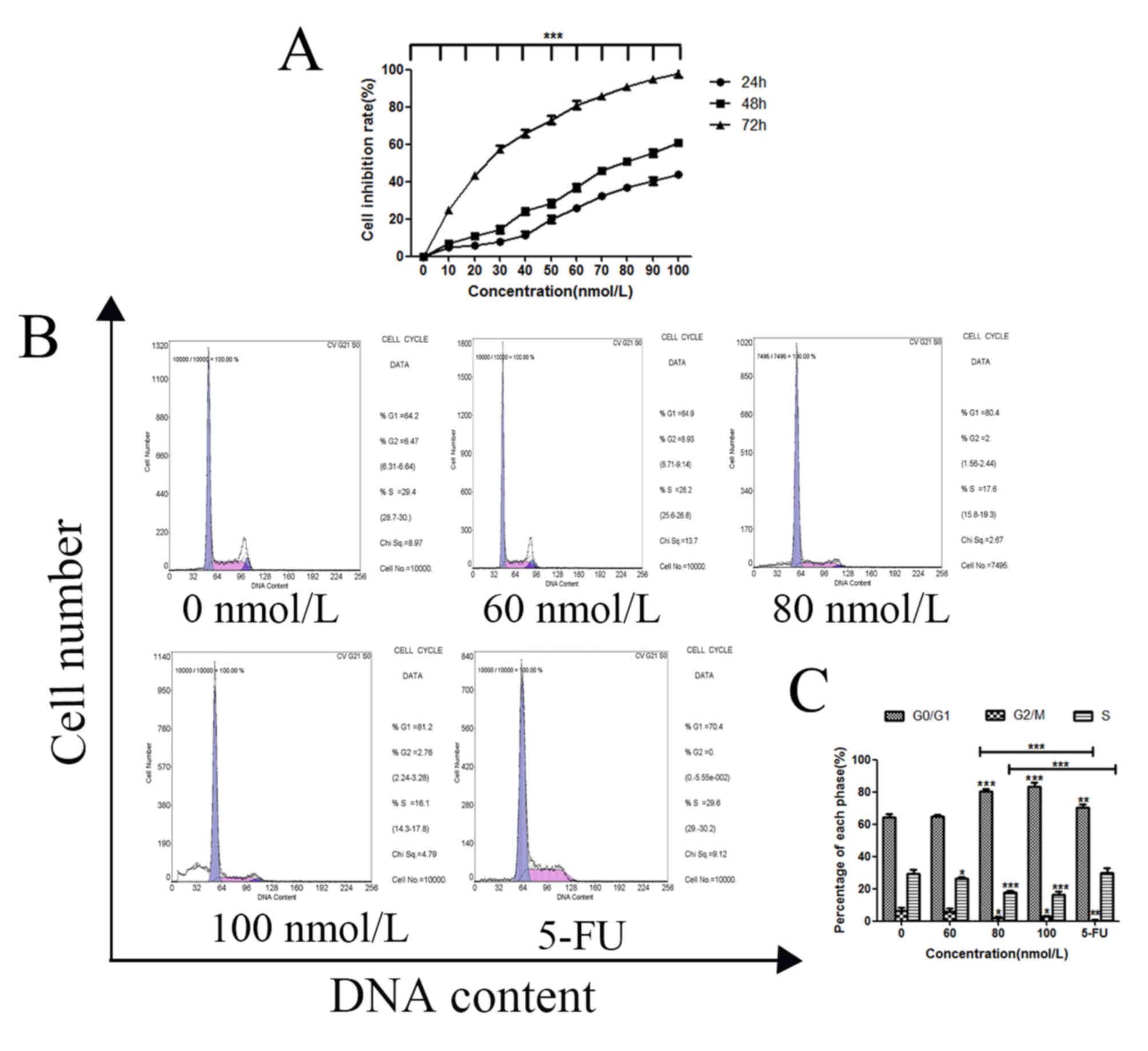
CCK-8 and cell cycle assays were carried out to
evaluate the effect of TSN on MKN-45 cell proliferation. The
results showed that inhibitory rates on MKN-45 cells treated with
TSN significantly increased as the rise of TSN concentration and
prolongation of time, (Fig. 1A),
the IC50 value was 81.06 nmol/l for 48 h, and the cell
cycle was arrested at the G1 phase with increasing concentrations
of TSN compared with the negative control (Fig. 1B and C). Compared with those in the
negative control, the TSN-treated cells showed the cell nucleus
cleavage and chromatin morphological changes. Acridine orange
staining revealed a higher number of apoptotic bodies following the
addition of TSN (Fig. 2A), and
flow cytometry demonstrated that the early apoptotic rates
gradually increased (Fig. 2B and
C). Moreover, compared with the negative control, the mRNA and
protein expression levels of Bax, cytochrome c, Fas,
caspase-3, caspase-8, caspase-9 and APAF-1 were significantly
upregulated, reaching the maximum at 80 nmol/l concentration of
TSN, and decreasing following the addition of 100 nmol/l (Fig. 2D-F). However, the expression of
BCL2 was significantly downregulated at 80 nmol/l TSN, and
increased following the addition of 100 nmol/l, which suggested
that TSN may also activate other mechanisms to inhibit the
proliferation of gastric cancer cells at 100 nmol/l. The effect in
the 80 nmol/l TSN treatment group was more evident compared with
the 5-FU treatment group.
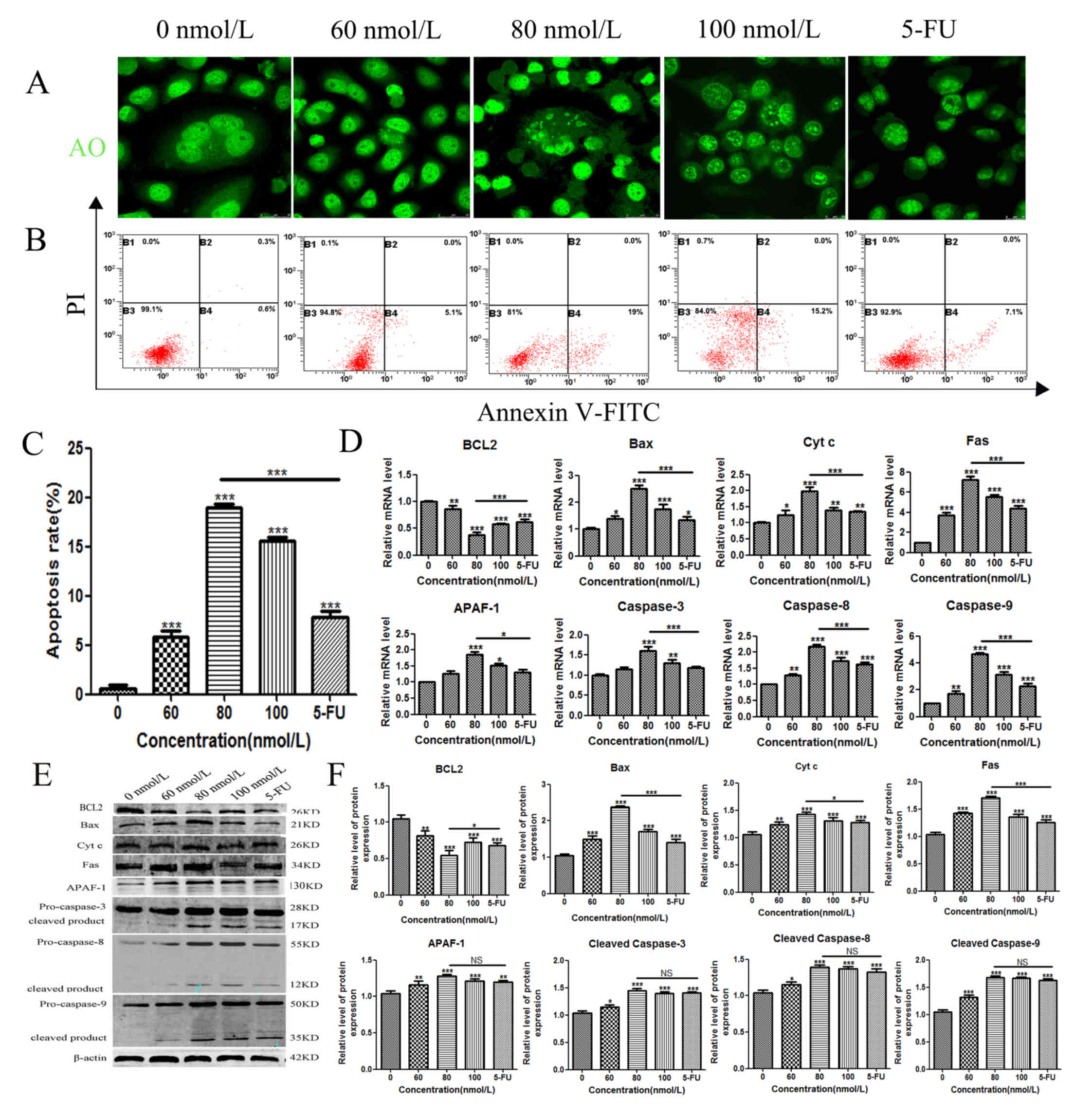 | Figure 2.Apoptotic effects of TSN on MKN-45
cells. (A) MKN-45 cells were seeded into 6-well plates and treated
with varying concentrations of TSN for 48 h, incubated with
acridine orange, and visualised using laser confocal microscope. (B
and C) Early apoptotic effects of TSN were evaluated by flow
cytometry analysis after staining with annexin V-FITC and PI. (D)
The mRNA expression of Caspase-3, 8, 9, BAX, BCL2, FAS, Cyt
c and APAF1 was detected using reverse transcription
quantitative PCR in TSN-treated MKN-45 cells. (E and F) Protein
expression levels of Caspase-3, 8, 9, Bax, BCL2, Fas, Cyt c
and APAF-1 were detected using western blotting in TSN-treated
MKN-45 cells. β-actin was used as an internal control. All results
are expressed as the mean ± SEM of three independent experiments;
n=3; *P<0.05, **P<0.01, ***P<0.001 vs. 0 nM. Cyt c,
cytochrome c; TSN, toosendanin; APAF1, apoptotic protease
activating factor 1; 5-FU, fluorouracil. |
TSN suppresses the expression of BCL2
partly through upregulation of miR-23a-3p
BCL2 is an integral mitochondrial membrane protein
and acts as a switch to control the process of mitochondrial
pathway apoptosis (22,23). As shown in Fig. 2D-F, the expression of BCL2 was
significantly downregulated in MKN-45 cells following treatment
with TSN. To explore the apoptotic mechanism employed by TSN,
miRNAs targeting BCL2 were predicted (Fig. 3A), and the expression of these
miRNAs was evaluated using RT-qPCR in MKN-45 cells following
treatment with different concentrations of TSN for 48 h. After TSN
treatment, the expression levels of miR-23a-3p, miR-34a-5p and
miR-365a-3p were markedly increased compared with the negative
control; however, a similar change was not observed in miR-148a-5p
expression (Fig. 3B). It was found
that the expression of miR-23a-3p increased significantly upon
increasing TSN concentration and reached a maximum when the TSN
concentration was increased to 80 nmol/l. Since the expression
level of miR-23a-3p was the more significant, the study focused on
miR-23a-3p for the subsequent experiments.
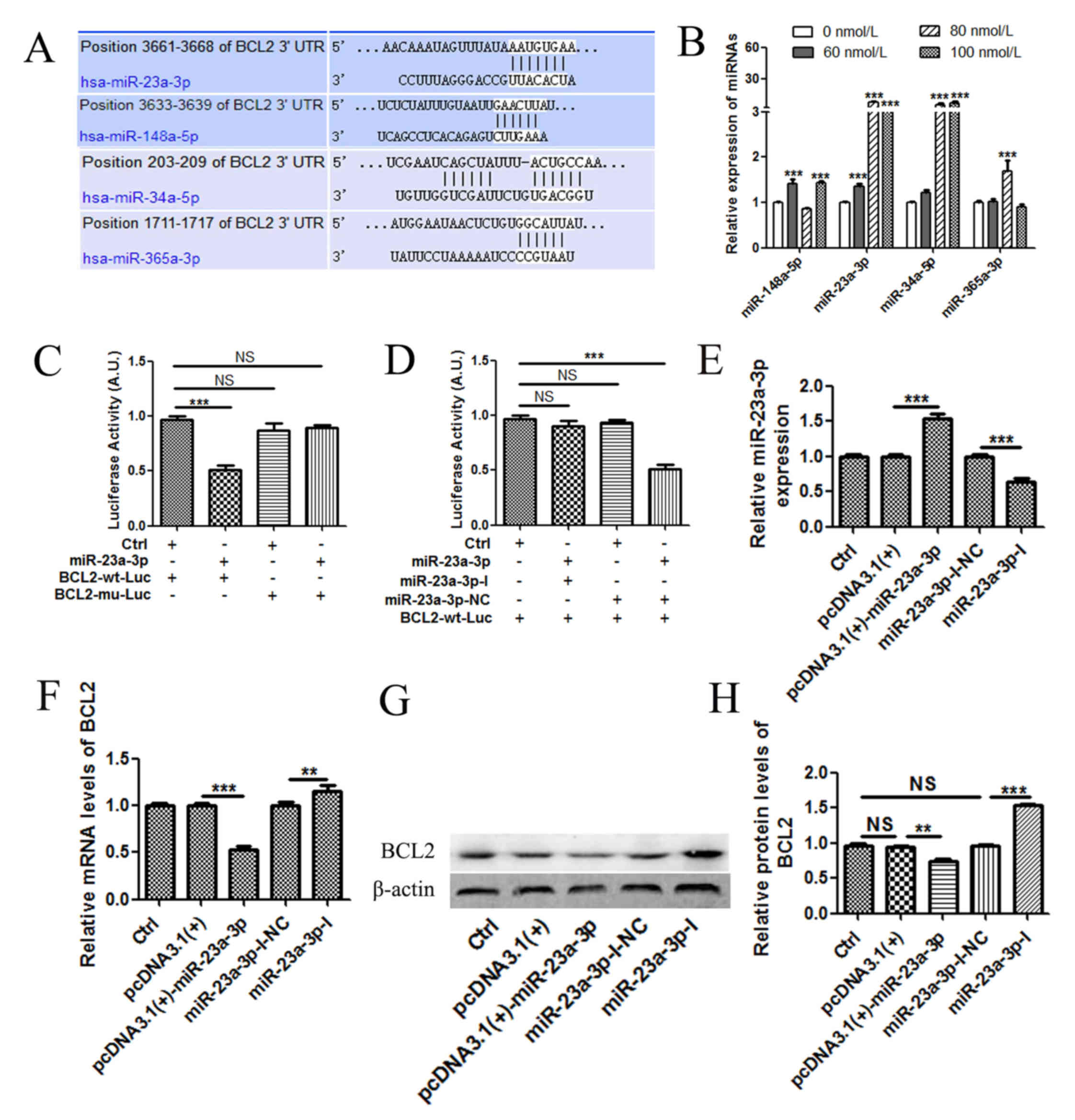 | Figure 3.TSN upregulates the expression of
miR-23a-3p, which subsequently downregulates the expression of
BCL2. (A) As predicted in the TargetScan database, the
BCL2 3′-UTR contained several potential miRNA binding sites.
(B) MKN-45 cells were treated with varying concentrations of TSN
for 48 h, and the changes in miRNA expression levels were detected
by RT-qPCR. (C) miR-23a-3p significantly inhibited the luciferase
activities. (D) miR-23a-3p inhibitors abolished the targeting 3′UTR
of BCL2. (E) The expression of miR-23a-3p in MKN-45 cells
after transfection with pcDNA3.1(+), pcDNA3.1(+)-miR-23a-3p,
miR-23a-3p-I-NC or miR-23a-3p-I. The expression of BCL2 mRNA
was determined by (F) RT-qPCR and (G and H) western blot analysis.
All results are expressed as the mean ± SEM of three independent
experiments; n=3; **P<0.01, ***P<0.001. Ctrl, control; miR,
microRNA; RT-qPCR, reverse transcription-quantitative PCR; UTR,
untranslated region; mu, mutant; wt, wild-type; luc, luciferase;
NC, negative control; NS, not significant; miR, microRNA; I,
inhibitor; TSN, toosendanin. |
To validate whether BCL2 was the direct
target genes of miR-23a-3p, a dual-luciferase reporter system was
employed. 3′UTR sequences containing the predicted target site of
miR-23a-3p or mutated sequences were cloned into the psiCHECK-2,
respectively. Co-transfection of pcDNA3.1 (+)-miR-23a-3p with
psiCHECK-2-BCL2-wt in 293T cells showed that luciferase activity
was significantly lower than those of controls (P<0.001)
(Fig. 3C). Co-transfection
pcDNA3.1 (+)-miR-23a-3p with psiCHECK-2- BCL2-mu did not change the
luciferase activities (P>0.05) (Fig. 3C). Compared with miR-23a-3p-I-NC,
miR-23a-3p-I abolished the targeting 3′UTR of BCL2 (Fig. 3D). This finding suggested that
miR-23a-3p may directly target the 3′-UTR of the BCL2 mRNA.
Based on this evidence, whether miR-23a-3p regulated the expression
of BCL2 in MKN-45 cells was examined. Endogenous mRNA and
protein expression levels of BCL2 were markedly downregulated
following overexpression of miR-23a-3p and upregulated following
the silencing of miR-23a-3p with the inhibitor (Fig. 3E-H).
miR-23a-3p induces MKN-45
apoptosis
To identify the effects of miR-23a-3p on apoptosis
in MKN-45 cells, a series of cell apoptosis experiments were
performed. Compared with those in the pcDNA3.1(+) group, cells
transfected with pcDNA3.1(+)-miR-23a-3p exhibited more pronounced
nuclear morphology changes, which included the appearance of
concentrated, agglomerated and marginalised chromatin as well as a
small number of nuclei becoming ruptured (Fig. 4A). By contrast, compared with cells
in the miR-23a-3p-I-NC group, the nuclei transfected with
miR-23a-3p-I were intact. Subsequently, annexin V-FITC/PI double
staining assay was performed to detect apoptosis in transfected
MKN-45 cells (Fig. 4B and C). The
results indicated that the early apoptotic rate of cells
transfected with pcDNA3.1(+)-miR-23a-3p was significantly higher
compared with that of cells transfected with pcDNA3.1(+) for 48 h.
By contrast, the rate of apoptosis of cells transfected with
miR-23a-3p-I was lower compared with that of cells transfected with
miR-23a-3p-I-NC.
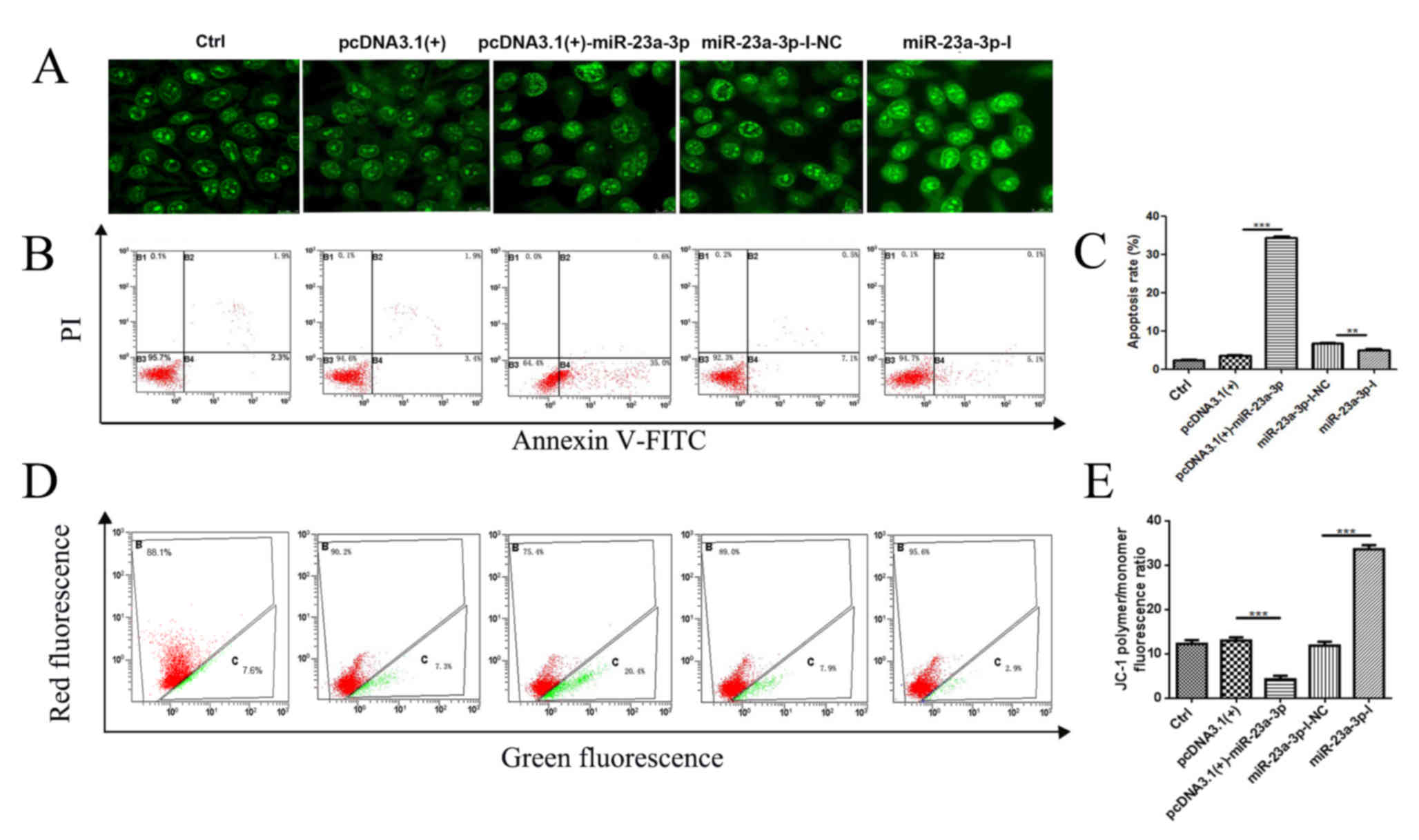 | Figure 4.miR-23a-3p induces apoptosis of
MKN-45 cells. MKN-45 cells were transfected with pcDNA3.1(+),
pcDNA3.1(+)-miR-23a-3p, miR-23a-3p-I-NC or miR-23a-3p-I for 48 h.
(A) Morphological changes in MKN-45 cells nuclei, observed after
acridine orange staining. The images were obtained with a Leica SP8
and analysed under HCX PL APO CS (magnification, 63×). (B and C)
The early apoptotic rates of transfected MKN-45 cells were
investigated by flow cytometry analysis using annexin V-FITC and PI
double labelling. (D and E) Mitochondrial membrane potential
changes in MKN-45 cells were determined by JC-1 staining. Red
channels: JC-1 polymer; Green channels: JC-1 monomer. All results
are expressed as the mean ± SEM of three independent experiments;
n=3; **P<0.01, ***P<0.001. miR, microRNA; I, inhibitor; NC,
negative control; Ctrl, control; MMP; PI, propidium iodide. |
The changes in MMP were determined by flow cytometry
(Fig. 4D and E). Following
transfection with pcDNA3.1(+)-miR-23a-3p, MMP was decreased in
MKN-45 cells compared with its level in cells transfected with
pcDNA3.1(+). However, the MMP was increased in cells transfected
with miR-23a-3p-I compared with the NC cells transfected with
miR-23a-3p-I-NC. These findings indicated that miR-23a-3p may
induce apoptosis in MKN-45 cells.
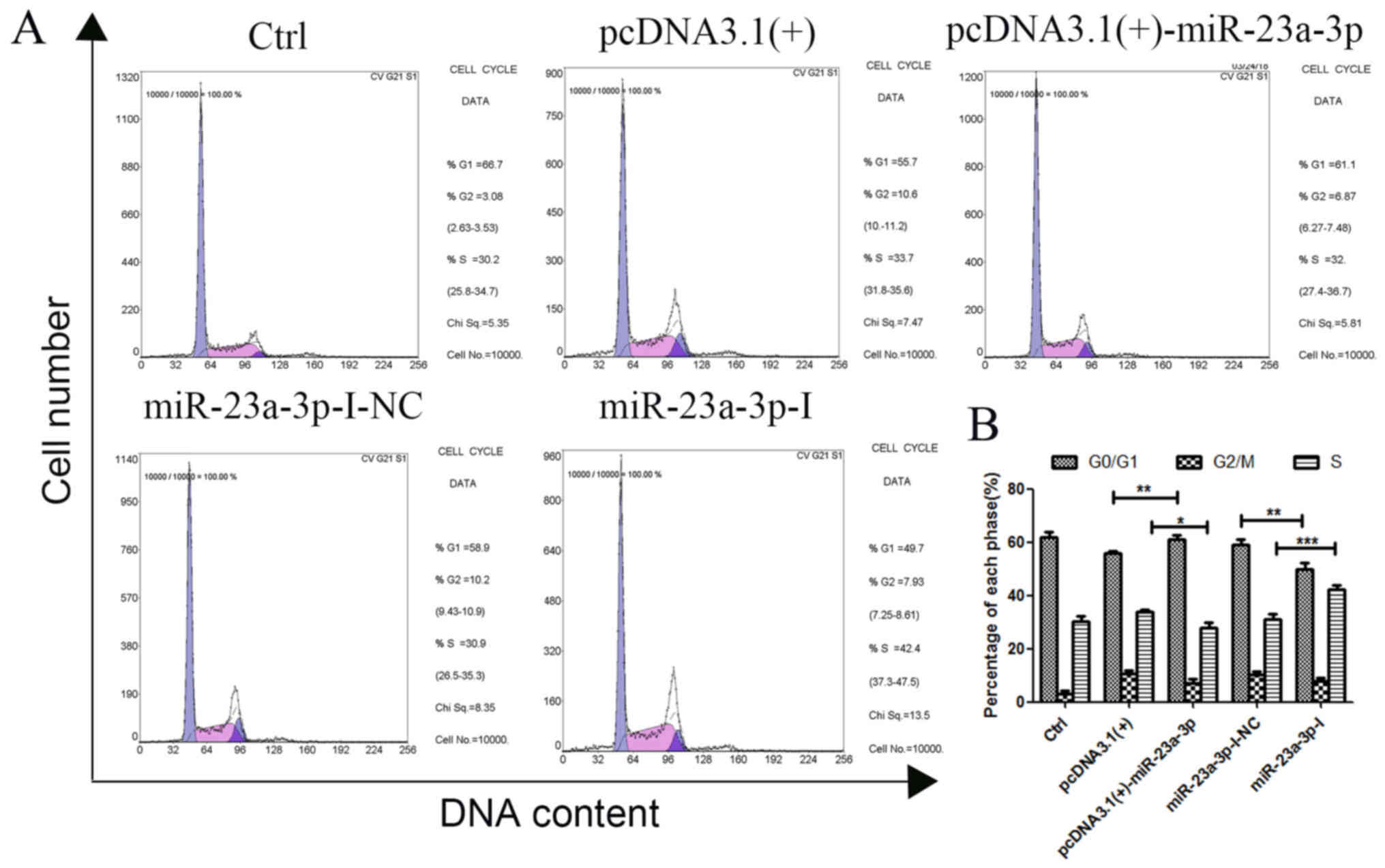
miR-23a-3p arrests cell cycle in
MKN-45 cells
To identify the effects of miR-23a-3p on cell cycle
distribution, a cell cycle study was conducted in MKN-45 cells by
PI single staining (Fig. 5A and
B). Compared with pcDNA3.1(+), the number of cells in the
G1/G0 phase of MKN-45 cells increased
significantly after transfection with pcDNA3.1(+)-miR-23a-3p, and
the number of cells in the S phase decreased significantly,
resulting in inhibition of cell proliferation in the G1
phase after transfection. Conversely, compared with that in the
miR-23a-3p-I-NC group, the number of cells in the
G1/G0 phase of MKN-45 cells decreased,
whereas the number in the S phase increased after transfection with
miR-23a-3p-I. These results indicated that the cycle became
arrested in the G1 phase following overexpression of
miR-23a-3p in MKN-45 cells.
Discussion
TSN has been regarded as an effective drug to induce
apoptosis of tumor cells (24).
TSN has been reported to inhibit proliferation and induce apoptosis
in human colorectal cancer (25)
and hepatocarcinoma cells (26).
However, the exact functions and mechanisms of the apoptotic
effects of TSN on human GC cells remain largely unclear. In the
present study, the results demonstrated that TSN inhibited
proliferation and induced apoptosis in MKN-45 cells. Previous
studies have reported that TSN inhibits a number of pathways and
targets that are crucial to cancer cell survival and proliferation
(4), such as TSN induced apoptosis
of cells through the β-catenin and MAPK pathways (6,5).
Zhang et al (27) has
previously shown that TSN selectively inhibits U87 and C6 glioma
cell proliferation and induces apoptosis through oestrogen
receptor-dependent machinery. In order to study the mechanism of
TSN-induced apoptosis of human gastric cancer MKN-45 cells, the
expression of apoptosis-related genes was examined. The expression
of these genes (cytochrome c, Fas, caspase-3, caspase-8,
caspase-9 and APAF-1) increased with the increase of TSN
concentration. In addition, toosendanin was found to upregulate the
expression of Bax and downregulate the expression of Bcl-2,
altering the Bax/Bcl-2 ratio.
Apoptosis is a complex process involving several
genes and miRNAs (28). However,
which miRNAs are involved in TSN-induced apoptosis of gastric
cancer cells remains to be elucidated. The present study found that
the expression of miR-23a-3p increased significantly with the
increase in TSN concentration. This is similar to the results of
Zhao et al (29), who found
that mir-148a inhibits cell proliferation and promotes
paclitaxel-induced apoptosis of ovarian cancer cells.
miR-23a has been reported to be associated with
human GC (30). However, the role
of miR-23a-3p in GC, along with its underlying mechanisms, remains
to be fully elucidated. The present study demonstrated that
overexpression of miR-23a-3p significantly inhibited proliferation
and induced the apoptosis of GC cells, whereas inhibition of
miR-23a-3p had the opposite effects. Therefore, miR-23a-3p may be a
key target for the induction of apoptosis in GC cells. However, the
effects of miR-23a on cancer cell proliferation remain
controversial. For example, miR-23a is known to suppress the
proliferation of osteosarcoma cells by targeting special AT-rich
sequence-binding protein-1 (31)
but promotes colorectal cancer cell survival by targeting
PDK4 (32). Collectively,
these findings suggest that the function of miR-23a in cancer cells
differs by targeting different genes. However, the genes targeted
by miR-23a-3p to induce apoptosis in GC cells remain to be
clarified. In this study, an inverse correlation was noted between
the expression of miR-23a-3p and BCL2 in GC cells,
suggesting that miR-23a-3p may be involved in the apoptosis of
MKN-45 cells.
BCL2 is an essential mitochondrial membrane protein
and is regulated by a number of miRNAs (33). Liao et al (34) reported that miRNA-448 inhibits cell
growth by targeting BCL2 in hepatocellular carcinoma. Chen
et al (35) reported that
miRNA-449a is downregulated in osteosarcoma and promotes cell
apoptosis by targeting BCL2. In the present study,
BCL2 was identified as a target gene of miR-23a-3p, and
miR-23a-3p was able to downregulate the mRNA and protein expression
levels of BCL2 by binding directly to the 3′-UTR of BCL2
mRNA in MKN-45 GC cells.
In this study, the expression level of miR-34a-5p
also increased significantly after TSN treatment, which suggested
that miR-34a-5p may also be involved in the regulation of
TSN-induced apoptosis of GC cells. However, whether miR-34a-5p is
involved in TSN-induced apoptosis requires further study. In the
future more work to intensify this research in other GC cell lines
is needed, so that we can better improve the mechanism of
TSN-induced apoptosis; meanwhile future studies are also required
to explore whether miR-23a has any effect on the migration and
invasion of GC cells.
In conclusion, the present study demonstrated that
TSN induces apoptosis of MKN-45 cells in part through the
miR-23a-3p/BCL2 axis. These results may provide novel
insights into the growth of GC cells. Further studies identifying
additional mechanisms of TSN will be interesting to pursue.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant number no. 31670843), the
Natural Science Foundation of Heilongjiang Province, China (grant
nos. C2016057 and C201241), the Scientific Research Fund of
Heilongjiang Provincial Education Department (grant nos. 135109104
and 135209260), and the Basic scientific research Fund of
Heilongjiang Provincial institutions of university (grant nos.
STSXK201809 and LTSW201737).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
SL performed data analysis and drafted the
manuscript. CL, SZ, YF and YP performed data analysis and
participated in intellectual discussion. WZ designed flow cytometry
experiments and assisted with critical revisions of the manuscript.
ZZ captured the images using a confocal laser microscope. SS
obtained funding for the study and assisted with the design and
interpretation of the study, as well as critical revisions of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rugge M, Genta RM, Di Mario F, El-Omar EM,
El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano
K and Graham DY: Gastric cancer as preventable disease. Clin
Gastroenterol Hepatol. 15:1833–1843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kosaka T, Endo M, Toya Y, Abiko Y, Kudara
N, Inomata M, Chiba T, Takikawa Y, Suzuki K and Sugai T: Long-term
outcomes of endoscopic submucosal dissection for early gastric
cancer: A single-center retrospective study. Dig Endosc.
26:183–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilson DH: Advances in the treatment of
gastric cancer. Curr Opin Gastroenterol. 34:465–468. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao T, Xie A, Liu X, Zhan H, Zeng J, Dai M
and Zhang B: Toosendanin induces the apoptosis of human Ewing's
sarcoma cells via the mitochondrial apoptotic pathway. Mol Med Rep.
20:135–140. 2019.PubMed/NCBI
|
|
5
|
Zhou Q, Wu X, Wen C, Wang H, Wang H, Liu H
and Peng J: Toosendanin induces caspase-dependent apoptosis through
the p38 MAPK pathway in human gastric cancer cells. Biochem Biophys
Res Commun. 505:261–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang G, Huang Y, Zhang R, Hou L, Liu H,
Chen X, Zhu J and Zhang J: Toosendanin suppresses oncogenic
phenotypes of human gastric carcinoma SGC-7901 cells partly via
miR-200a-mediated downregulation of β-catenin pathway. Int J Oncol.
51:1563–1573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams BD, Parsons C, Walker L, Zhang WC
and Slack FJ: Targeting noncoding RNAs in disease. J Clin Invest.
127:761–771. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Wang Q, Liu H, Hu B, Zhou W and
Cheng Y: MicroRNA expression and its implication for the diagnosis
and therapeutic strategies of gastric cancer. Cancer Lett.
297:137–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han R, Chen X, Li Y, Zhang S, Li R and Lu
L: MicroRNA-34a suppresses aggressiveness of hepatocellular
carcinoma by modulating E2F1, E2F3, and Caspase-3. Cancer Manag
Res. 11:2963–2976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang T, Zeng H, Chen W, Zheng R, Zhang Y,
Li Z, Qi J, Wang M, Chen T, Lou J, et al: Helicobacter pylori
infection, H19 and LINC00152 expression in serum and risk of
gastric cancer in a Chinese population. Cancer Epidemiol.
44:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Melton C, Li Y, Shenoy A, Zhang X,
Subramanyam D and Blelloch R: miR-294/miR-302 promotes
proliferation, suppresses G1-S restriction point, and inhibits ESC
differentiation through separable mechanisms. Cell Rep. 4:99–109.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XH, Lu KH, Wang KM, Sun M, Zhang EB,
Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, et al: MicroRNA-196a
promotes non-small cell lung cancer cell proliferation and invasion
through targeting HOXA5. BMC Cancer. 12:3482012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding F, Lai J, Gao Y, Wang G, Shang J,
Zhang D and Zheng S: NEAT1/miR-23a-3p/KLF3: A novel regulatory axis
in melanoma cancer progression. Cancer Cell Int. 19:2172019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu P, Wang C, Ma C, Wu Q, Zhang W and Lao
G: MicroRNA-23a regulates epithelial-to-mesenchymal transition in
endometrial endometrioid adenocarcinoma by targeting SMAD3. Cancer
Cell Int. 16:672016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He Y, Meng C, Shao Z, Wang H and Yang S:
MiR-23a functions as a tumor suppressor in osteosarcoma. Cell
Physiol Biochem. 34:1485–1496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen B, Zhu A, Tian L, Xin Y, Liu X, Peng
Y, Zhang J, Miao Y and Wei J: miR23a suppresses pancreatic cancer
cell progression by inhibiting PLK1 expression. Mol Med Rep.
18:105–112. 2018.PubMed/NCBI
|
|
18
|
Rigotto C, Sincero TC, Simões CM and
Barardi CR: Detection of adenoviruses in shellfish by means of
conventional-PCR, nested-PCR, and integrated cell culture PCR
(ICC/PCR). Water Res. 39:297–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Ridzon DA, Broomer KJ, Zhou ZH,
Lee DH, Nguyen JT, Barbisin N, Xu LN, Mahuvakar VR, Andersen MR, et
al: Real-time quantification of microRNAs by stem-loop RT-PCR.
Nucleic Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sui CG, Meng FD, Li Y and Jiang YH:
miR-148b reverses cisplatin-resistance in non-small cell cancer
cells via negatively regulating DNA (cytosine-5)-methyltransferase
1(DNMT1) expression. J Transl Med. 13:1322015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schenk RL, Strasser A and Dewson G: BCL-2:
Long and winding path from discovery to therapeutic target. Biochem
Biophys Res Commun. 482:459–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Li J, Yin F, Lin B, Wang Z, Xu J,
Wang H, Zuo D, Wang G, Hua Y and Cai Z: Toosendanin demonstrates
promising antitumor efficacy in osteosarcoma by targeting STAT3.
Oncogene. 36:6627–6639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang G, Feng C, Chu S, Zhang R, Lu Y, Zhu
J and Zhang J: Toosendanin inhibits growth and induces apoptosis in
colorectal cancer cells through suppression of
AKT/GSK-3beta/beta-catenin pathway. Int J Oncol. 47:1767–1774.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu XL, Wang H, Zhang L, Wang YL, Wang J,
Wang P, He X and He YJ: Anticancer effects of crude extract from
Melia toosendan Sieb. et Zucc on hepatocellular carcinoma in vitro
and in vivo. Chin J Integr Med. 22:362–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Cao L, Wang ZR, Li Z and Ma J:
Anti-cancer effect of toosendanin and its underlying mechanisms. J
Asian Nat Prod Res. 21:270–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao S, Wen Z, Liu S, Liu Y, Li X, Ge Y
and Li S: MicroRNA-148a inhibits the proliferation and promotes the
paclitaxel-induced apoptosis of ovarian cancer cells by targeting
PDIA3. Mol Med Rep. 12:3923–3929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hua K, Chen YT, Chen CF, Tang YS, Huang
TT, Lin YC, Yeh TS, Huang KH, Lee HC, Hsu MT, et al:
MicroRNA-23a/27a/24-2 cluster promotes gastric cancer cell
proliferation synergistically. Oncol Lett. 16:2319–2325.
2018.PubMed/NCBI
|
|
31
|
Wang G, Li B, Fu Y, He M, Wang J, Shen P
and Bai L: miR-23a suppresses proliferation of osteosarcoma cells
by targeting SATB1. Tumour Biol. 36:4715–4721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng Y, Deng Z, Hao H, Wu X, Gao H, Tang S
and Tang H: MicroRNA-23a promotes colorectal cancer cell survival
by targeting PDK4. Exp Cell Res. 373:171–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weyhenmeyer B, Murphy AC, Prehn JH and
Murphy BM: Targeting the anti-apoptotic Bcl-2 family members for
the treatment of cancer. Exp Oncol. 34:192–199. 2012.PubMed/NCBI
|
|
34
|
Liao ZB, Tan XL, Dong KS, Zhang HW, Chen
XP, Chu L and Zhang BX: miRNA-448 inhibits cell growth by targeting
BCL-2 in hepatocellular carcinoma. Dig Liver Dis. 51:703–711. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Zhou J, Chen X, Yang B, Wang D,
Yang P, He X and Li H: miRNA-449a is downregulated in osteosarcoma
and promotes cell apoptosis by targeting BCL2. Tumour Biol.
36:8221–8229. 2015. View Article : Google Scholar : PubMed/NCBI
|