Introduction
Ketamine is an intravenous anesthetic with analgesic
effects that is commonly used for pediatric surgery and superficial
operations (1). However,
increasing evidence has suggested that early exposure to commonly
used anesthetic agents can enhance cellular death and negatively
impact neurodevelopment (2,3).
Ketamine affects multiple signaling pathways and proteins
associated with synaptic plasticity and memory formation, including
the PI3K/Akt and CREB/BDNF/Bcl-2 pathways (4) and inflammatory cytokines interleukin
(IL)-6, IL-1β, and tumor necrosis factor α (5). Ketamine can induce neurotoxicity and
cause neurocyte apoptosis in the nervous system during early neural
development, leading to widespread neurodegeneration and long-term
neurocognitive deficits, particularly when administered at high
doses over prolonged periods of time (6,7). The
majority of studies investigating anesthetic neurotoxicity in
neonates have focused on neuronal apoptosis, as neuronal cell
apoptosis and necrosis are critical events during ketamine-induced
neurotoxicity. Ketamine-induced neurodegeneration has emerged as a
public health concern (8).
The Hippo signaling pathway is a protein kinase
cascade composed of a series of protein kinases and various
transcription factors, which is highly evolutionarily conserved
from lower animals to mammals (9).
As an important transcriptional co-activator protein downstream of
the Hippo signaling pathway, yes-associated protein (YAP) is a
major regulator of organ growth via its actions on embryonic
precursor cells (10). Previous
studies have advanced the knowledge of how YAP maintains stem cell
self-renewal and differentiation, regulates organ size and inhibits
apoptosis by interacting with other transcription factors (11–13).
Dysregulation of the Hippo-YAP signaling pathway has significant
impacts on cell proliferation and apoptosis. Overexpression of a
Hippo-resistant YAP mutant leads to progenitor cell expansion in
multiple organs (14), for
example, YAP overexpression increases airway basal stem cell
self-renewal (11,15). Inactivation studies have further
demonstrated that YAP is largely dispensable during homeostasis in
several adult organs (16–18). Moreover, activation of Hippo leads
to phosphorylation of the co-transcription factor Yorkie
(YAP/tafazzin in vertebrates), which favors resistance to apoptosis
and proliferation (19). However,
the expression or exact function of YAP in ketamine-induced neural
apoptosis or proliferation has not been previously reported.
Based on the effects of YAP on cell proliferation
and apoptosis, the present study hypothesized that YAP may serve a
role in regulating ketamine-induced apoptosis. A human
neuroblastoma cell line (SH-SY5Y), which has been widely used to
study neurological disease and therapeutic effects, was used to
investigate the impact of ketamine-induced toxicity (20,21).
An in vitro neurotoxicity model was established to
investigate the effects of different concentrations of ketamine and
to clarify the precise role of YAP. The results of the present
study enhanced the current understanding of YAP modulation during
ketamine-induced neural apoptosis.
Materials and methods
Cell culture
The SHSY-5Y cell line (American Type Culture
Collection) was cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) containing 300 mg/l L-glutamine, 4.5 mg/l D-glucose, 10 mg/l
sodium pyruvate, 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin solution. Cells were seeded
(2×105 cells/ml) and maintained at 37°C in a 5%
CO2 humidified incubator. The culture medium was changed
every 2 days. For subsequent experiments, cells were treated with
various concentrations of ketamine (Fujian Gutian Pharmaceutical
Industry Co., Ltd.) and incubated at 37°C for 12 and 24 h.
Cell proliferation-neurotoxicity
assay
The effects of different concentrations of ketamine
(0, 400, 800, 1,200 and 1,600 µM) on cell
proliferation-neurotoxicity (viability) were assessed by performing
the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc.). It has been reported that high doses of
ketamine are required to induce SH-SY5Y cell apoptosis and the
pre-experiment results indicated that 1,600 µM induced cell death
but not excessive cell death (22). Cells were seeded (1×104
cells/well) into 96-well plates and maintained at 37°C with 5%
CO2 for 24 h. Cells were treated with ketamine for 12 or
24 h at 37°C. Hydrogen peroxide (H2O2; 400
mM) was used as a positive toxicity control for 12 or 24 h at 37°C
(23). Cell viability was assessed
using the CCK-8 assay, according to the manufacturer's protocol.
Briefly, 10 µl CCK-8 solution was added to each well and incubated
at 37°C with 5% CO2 for 1.5 h. The absorbance of each
well was measured at a wavelength of 450 nm using a microplate
reader. At 48 h post-transfection, cells were selected using
puromycin (1 mg/ml), this process took ~2 or 3 days, cells
harvested after selection were used for subsequent experiments.
Lentiviral infection
Lentiviral infection was used to increase the
expression level of YAP in SH-SY5Y cells. LV5 (cat. no. A2831-3;
Shanghai GenePharma Co., Ltd.) was used for YAP overexpression
lentiviral particle production and LV5-negative control (NC) was
used as the control for the YAP overexpression plasmid. LV5-based
lentiviral vectors (1 µg/µl) were transfected into 293T cells at
80–90% confluence, 3.5×105/ml. After purification and
titration, the viral supernatant was harvested at 48–72 h
post-transfection, passed through a 0.45-mm filter and diluted 2:3
with fresh medium containing 8 mg/ml polybrene. At 80% confluence,
the SH-SY5Y target cells were infected with the viral supernatant.
At 48 h post-transfection, cells were selected using puromycin (1
mg/ml). SH-SY5Y cells were seeded (0.5×105 cells/well)
into 24-well plates and incubated at 37°C for 24 h in a 5%
CO2 humidified incubator. Subsequently, the cell culture
medium was aspirated and the virus was diluted in DMEM containing
10% FBS and 5 µg/ml polybrene. Blank and negative control (treated
with the LV5NC plasmid) groups were established. After incubation
for 12–24 h at 37°C, the viral suspension was removed, and DMEM was
added before incubation at 37°C with 5% CO2 for 24–48
h.
Small interfering RNA (siRNA)
transfection
An siRNA was used to reduce the expression level of
YAP in SH-SY5Y cells. The siRNAs (Shanghai GenePharma Co., Ltd.)
used in the present study were as follows: YAP1-homo-1858 siRNA
forward, CUGCCACCAAGCUAGAUAATT and reverse, UUAUCUAGCUUGGUGGCAGTT;
and NC-siRNA forward, UUCUCCGAACGUGUCACGUTT and reverse,
ACGUGACACGUUCGGAGAATT . The siRNA targeting YAP was fluorescently
labeled and si-fluorescein amidite (FAM), which has an excitation
wavelength of 480 nm and a emission wavelength of 520 nm, was used
as the negative control. Cells were transfected with 20 µM
YAP-siRNA or NC-siRNA using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C, according to
the manufacturer's protocol. Briefly, 0.5–2×105 cells
and 500 µl DMEM without penicillin/streptomycin was added to each
well and incubated for 24 h. Lipofectamine® 2000 was
diluted in 50 µl Opti-MEM I Reduced Serum medium (Gibco; Thermo
Fisher Scientific, Inc.), mixed gently and incubated at room
temperature for 5 min. Subsequently, 2 µl si-FAM diluted in 50 µl
Opti-MEM I Reduced Serum medium was added to the
Lipofectamine® 2000 dilution and the cells incubated for
20 min at room temperature; YAP-siRNA prepared in the same way. The
time interval between transfection and subsequent experimentation
was 48 h.
Immunofluorescence assay
After aspirating the culture media, cells were
gently rinsed with PBS and fixed with 4% paraformaldehyde at 25°C
for 15 min. Cells were rinsed twice with warm PBS and permeabilized
using PBS containing 0.2% Triton X-100 and 0.2% BSA [Yeasen
Biotechnology (Shanghai) Co., Ltd.] on ice for 15 min. Cells were
blocked with PBS containing 0.02% Triton X-100 and 5% BSA for
>30 min at room temperature. Subsequently, cells were incubated
at 4°C overnight with an anti-cleaved caspase-3 (cat. no. 9661;
1:400; Cell Signaling Technology, Inc.) primary antibody. Following
primary incubation, cells were incubated for 1 or 2 h at room
temperature with Alexa 488-conjugated anti-rabbit IgG (cat. no.
A11034; 1:300; Invitrogen; Thermo Fisher Scientific, Inc.) and
594-conjugated anti-rabbit IgG (cat. no. A21207; 1:300; Invitrogen;
Thermo Fisher Scientific, Inc.) secondary antibodies, then cleaved
caspase-3 protein labeled with the green fluorophore of the
secondary antibody. Finally, nuclei were stained using DAPI (cat.
no. 62248; 1:1,000; Thermo Fisher Scientific, Inc.) at room
temperature for 10 min and sealed with 90% glycerin. Stained cells
were visualized using a LSM88 confocal microscope (Zeiss GmbH) at
magnifications of ×200, 400 and 630, ZEN 2.3 lite (Zeiss GmbH) was
used for image capture and analysis.
Flow cytometry
SH-SY5Y cell apoptosis following ketamine treatment
was analyzed by flow cytometry. Cells were seeded (3×105
cells/ml) into 6-well plates and incubated at 37°C with 5%
CO2 for 24 h. Cells were treated with ketamine for 12 or
24 h at 37°C, harvested and washed twice with PBS. Subsequently,
5–10×104 cells were re-suspended in 1X binding buffer.
To each 5-ml culture tube, 100 µl cell suspension (1×105
cells), 5 µl APC Annexin V and 5 µl 7-aminoactinomycin D (7-AAD)
were added (cat. no. 561012, BD Pharmingen). Cells were incubated
for 15 min at room temperature in the dark with gentle agitation.
Subsequently, 400 µl 1X binding buffer was added to each tube and
samples were analyzed by flow cytometry within 1 h. The following
controls were used to set up compensation and quadrants: Unstained
cells, cells stained with APC Annexin V and cells stained with
7-AAD, early and late apoptosis was assessed. FlowJo v10 (FlowJo
LLC) was used for analysis and the model and supplier of the
instrument used for flow cytometry was a Sony SA3800-Spectral Cell
Analyzer (Sony Biotechnology).
Western blotting
Western blotting was performed as previously
described (24). Briefly, total
protein was extracted from ketamine-treated cells using RIPA buffer
(Beyotime Institute of Biotechnology) containing
phenylmethylsulfonyl fluoride, phosphatase inhibitors and protease
inhibitors. Subsequently, the samples were centrifuged at 16,363 ×
g at 4°C for 10 min. Total protein was quantified using the
bicinchoninic acid method. Protein (25 mg per lane) was separated
via 12% SDS-PAGE and transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc.) using the Trans-Blot Turbo Transfer
system (Bio-Rad Laboratories, Inc.). After 90 min, the membranes
were blocked with 5% BSA [Yeasen Biotechnology (Shanghai) Co.,
Ltd., cat. no. 36101ES76] in 1X Tris-buffered saline containing
0.1% Tween-20 (TBST; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. Subsequently, the membranes were incubated at 4°C
overnight with primary antibodies (diluted in 5% BSA in 1X TBST)
targeted against: Cleaved caspase-3 (Asp175; cat. no. 9661;
1:1,000; Cell Signaling Technology, Inc.), Bax (cat. no. 2772;
1:1,000; Cell Signaling Technology, Inc.), Bcl-2 (cat. no. ab32124;
1:1,000; Abcam), YAP (cat. no. 4912; 1:1,000; Cell Signaling
Technology, Inc.), β-tubulin (cat. no. 2146; 1:5,000; Cell
Signaling Technology, Inc.) and β-actin (cat. no. 4967; 1:5,000;
Cell Signaling Technology, Inc.). Following primary incubation, the
membranes were washed three times for 10 min with 1X TBST and
incubated with the Goat anti-Rabbit IgG (H+L; cat. no. 31460;
1:5,000, Thermo Fisher Scientific, Inc.) secondary antibodies at
room temperature for 1 h. The membranes were washed three times for
10 min with 1X TBST and protein bands were visualized by
chemiluminescence (cat. no. RPN 2322, Cytiva) and analyzed with
Image Lab v.5.2.1 62311 (Bio-Rad Laboratories, Inc.). β-tubulin and
β-actin were used as the loading controls.
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± SEM. Statistical analyses were
performed using GraphPad Prism software (version 5.0; GraphPad
Software, Inc.). Comparisons between two groups were analyzed using
paired Student's t-test. Comparisons among multiple groups were
analyzed using one-way or two-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
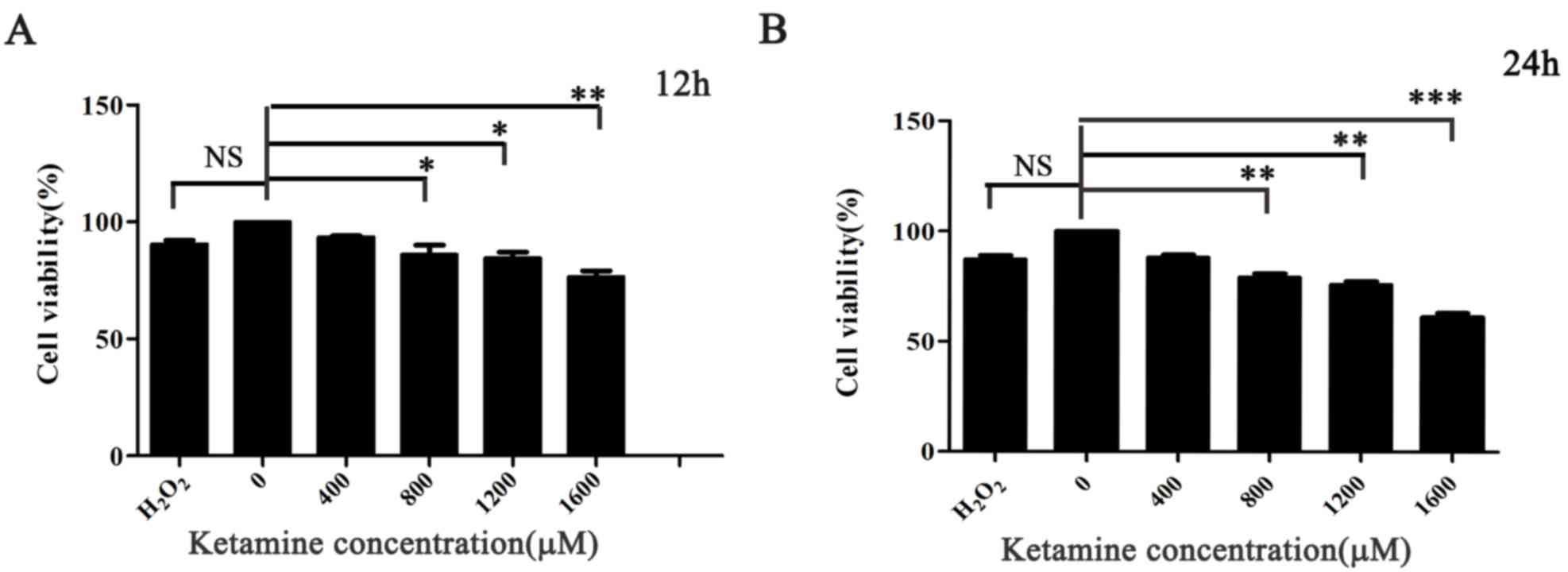
Effects of ketamine on SH-SY5Y cell
viability
The CCK-8 assay was used to investigate the optimum
dose and exposure time of ketamine-induced cytotoxicity in SH-SY5Y
cells. Different concentrations of ketamine induced cell toxicity,
and cell viability was suppressed following treatment with ketamine
for 12 and 24 h compared with the 0 µM group, particularly at a
concentration of 1,600 µM. However, extensive cell death was
observed when cells were treated with 2,000 µM ketamine (data not
shown). Ketamine attenuated SH-SY5Y cell viability in a
dose-dependent manner under the 24 h conditions. Short exposure to
ketamine (12 h) also induced neurotoxicity in SH-SY5Y cells, but
was less compared with 24 h. Nevertheless, cell viability was
markedly decreased in a dose-dependent manner when the exposure
time was extended to 24 h (P<0.01; Fig. 1A and B).
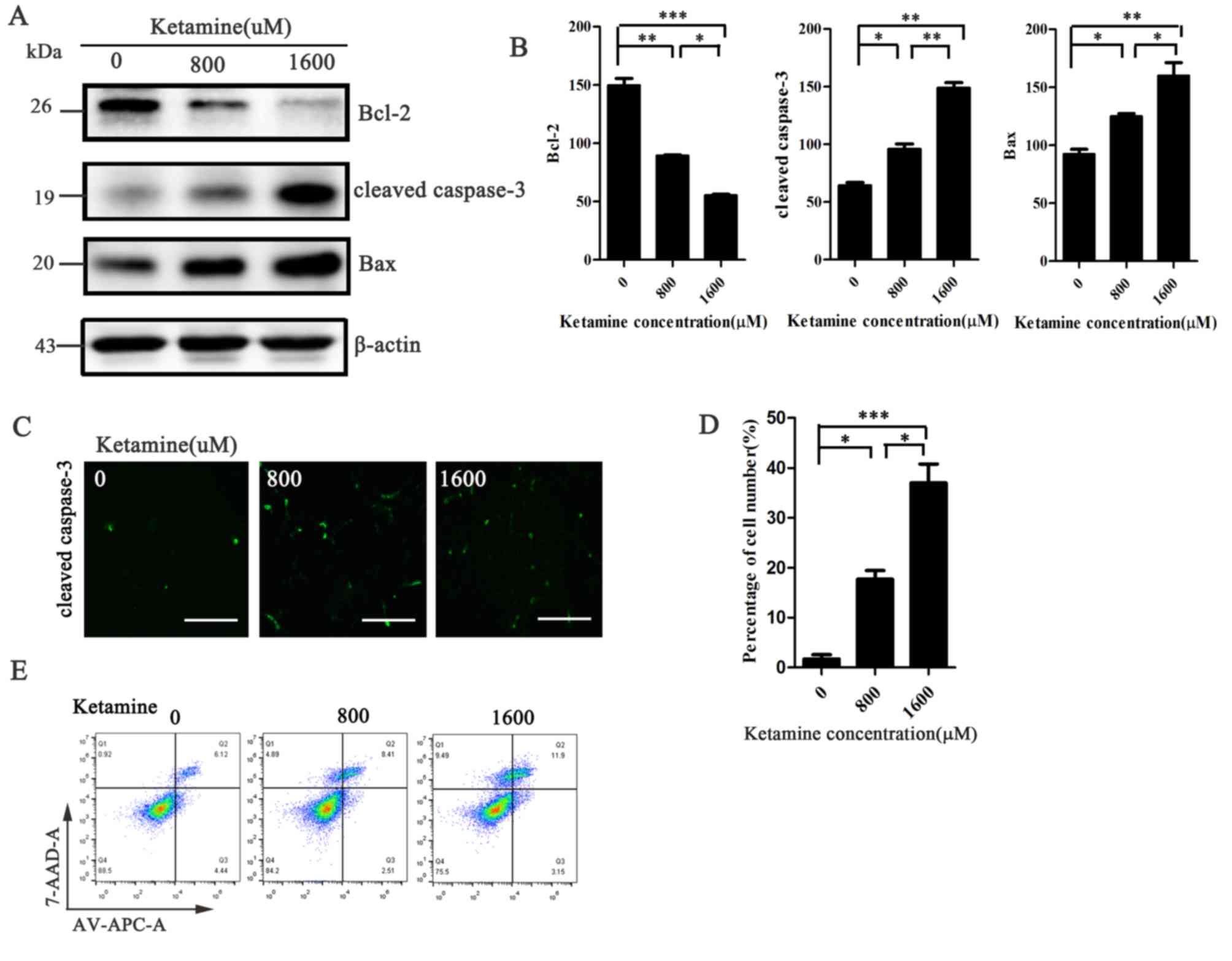
Ketamine induces SH-SY5Y cell
apoptosis in a dose-dependent manner
To further investigate whether ketamine-induced
inhibition of cell viability was associated with apoptosis
induction, western blotting assays were performed to measure the
expression levels of apoptotic markers. SH-SY5Y cells treated with
higher concentrations of ketamine displayed increased expression
levels of cleaved caspase-3 and Bax, and decreased expression
levels of Bcl-2 compared with the 0 µM group (Fig. 2A and B). Similar results were
obtained for the immunofluorescence and flow cytometry assays.
Cleaved caspase-3 protein expression levels (labeled with green
fluorescent protein) and the rate of cell apoptosis increased with
increasing ketamine concentrations (Fig. 2C-E). The results indicated that
ketamine inhibited cell viability in a dose-dependent manner, which
was associated with the activation of apoptosis.
YAP overexpression enhances
ketamine-treated SH-SY5Y cell viability
To investigate the potential role of YAP in
regulating the activity and viability of ketamine-treated SH-SY5Y
cells, the present study induced YAP overexpression by lentiviral
infection. The rate of apoptosis was decreased in the YAP
overexpression group compared with the NC group (P<0.001;
Fig. 3A and B). The CCK-8 assay
indicated that YAP overexpression increased SH-SY5Y cell activity
and viability following treatment with ketamine for 12 or 24 h
compared with the NC group (P<0.05; Fig. 3C and D). Therefore, the results
indicated that YAP overexpression protected SH-SY5Y cells against
ketamine exposure.
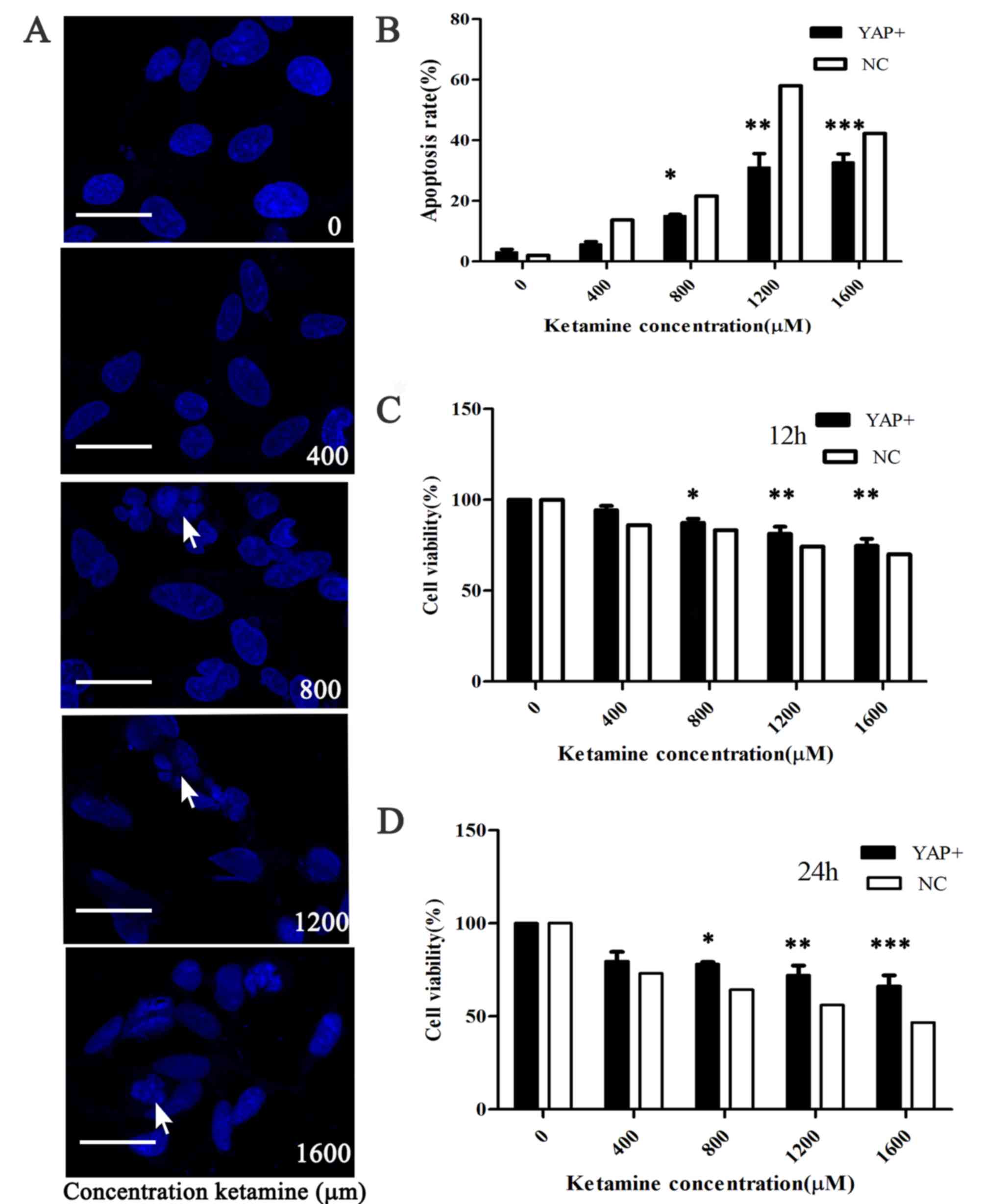 | Figure 3.YAP overexpression reverses
ketamine-induced reductions in SH-SY5Y cell viability. (A) Confocal
microscopy indicated that different concentrations of ketamine (0,
400, 800, 1,200, 1,600 µM) induced apoptosis, as indicated by the
breakdown of the cell nucleus. The arrow represents the broken down
cell nucleus (scale bar, 20 µm). (B) Apoptotic cells were counted
to calculate the rate of apoptosis following YAP overexpression.
The Cell Counting Kit-8 assay was used to investigate the effects
of YAP overexpression on SH-SY5Y cell viability following treatment
with ketamine for (C) 12 or (D) 24 h. *P<0.05, **P<0.01 and
***P<0.001 vs. NC. YAP, yes-associated protein; NC, negative
control. |
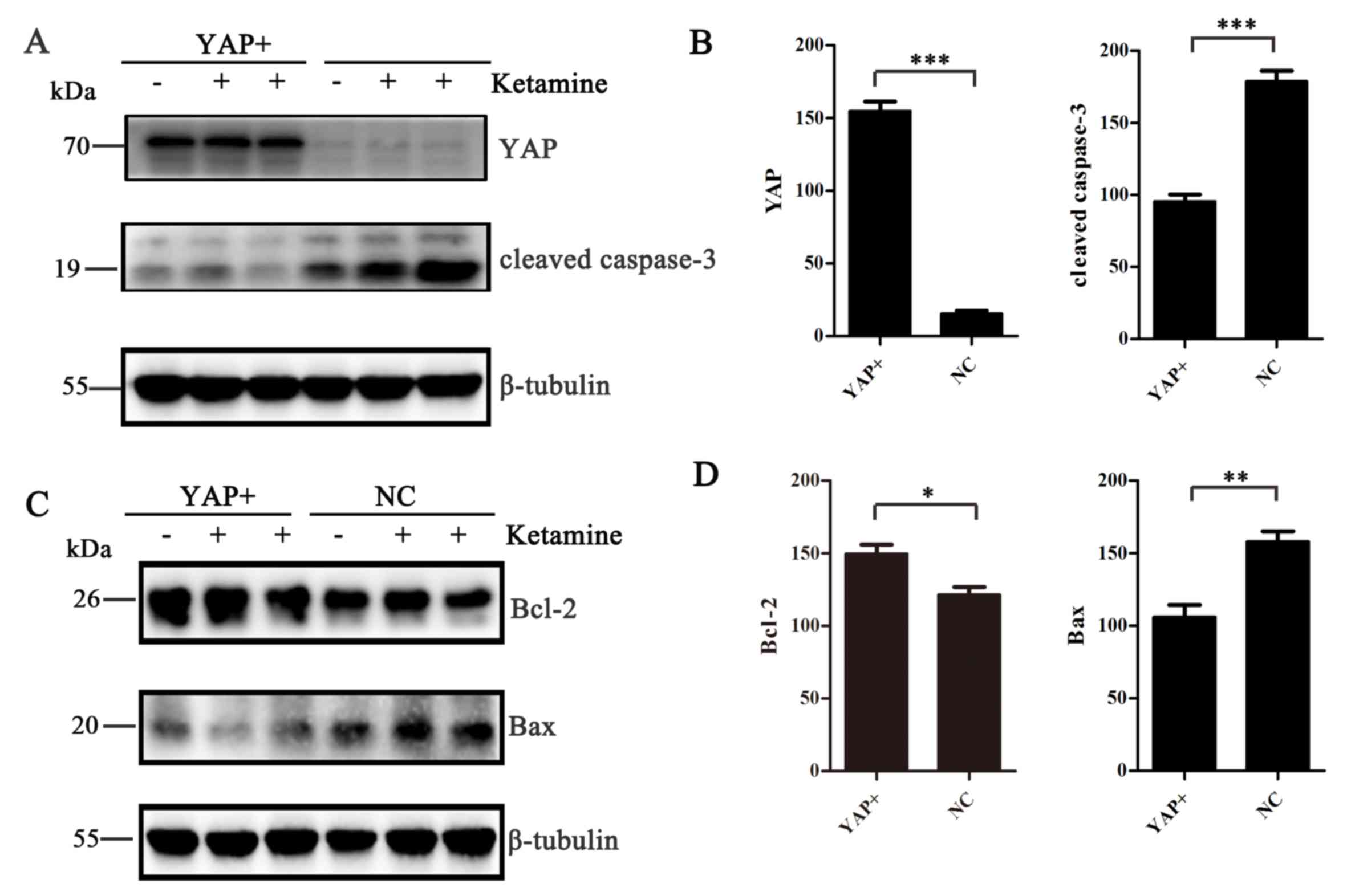
YAP overexpression decreases apoptotic
protein production in SH-SY5Y cells
To improve the current understanding of the role of
YAP in ketamine-induced apoptosis, western blotting was performed
to measure the expression of apoptotic markers, including cleaved
caspase-3, Bcl-2 and Bax. Following YAP overexpression, SH-SY5Y
cells were treated with 800 or 1,600 µM ketamine for 24 h.
Following YAP overexpression, the expression levels of cleaved
caspase-3 and Bax were decreased (P<0.01; Fig. 4A-D), whereas Bcl-2 expression
levels were increased (P<0.05; Fig.
4C and D), compared with the NC group.
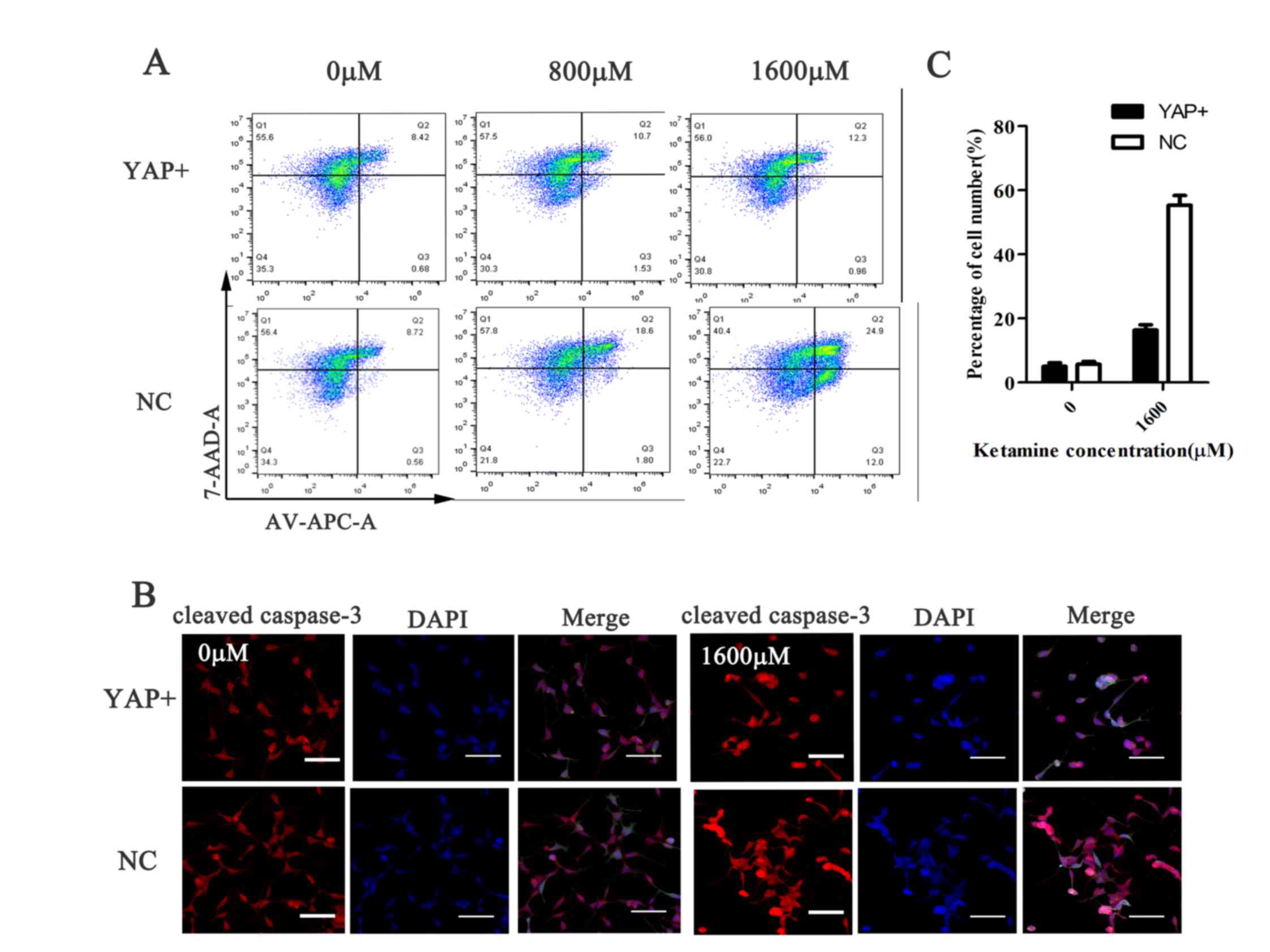
YAP overexpression decreases
ketamine-induced SH-SY5Y cell apoptosis
Flow cytometry was performed to assess
ketamine-induced alterations to SH-SY5Y cell apoptosis. Compared
with the NC group, ketamine-induced apoptosis was reduced following
YAP overexpression. Furthermore, ketamine treatment increased
SH-SY5Y cell apoptosis in a dose-dependent manner; however, YAP
overexpression decreased the rate of ketamine-induced apoptosis at
each concentration compared with the NC group (Fig. 5A). Similarly, the
immunofluorescence results indicated that ketamine-induced cleaved
caspase-3 expression was significantly decreased in the YAP
overexpression group compared with the NC group (P<0.001;
Fig. 5B and C). Therefore, the
results suggested that YAP overexpression decreased
ketamine-induced SH-SY5Y cell apoptosis.
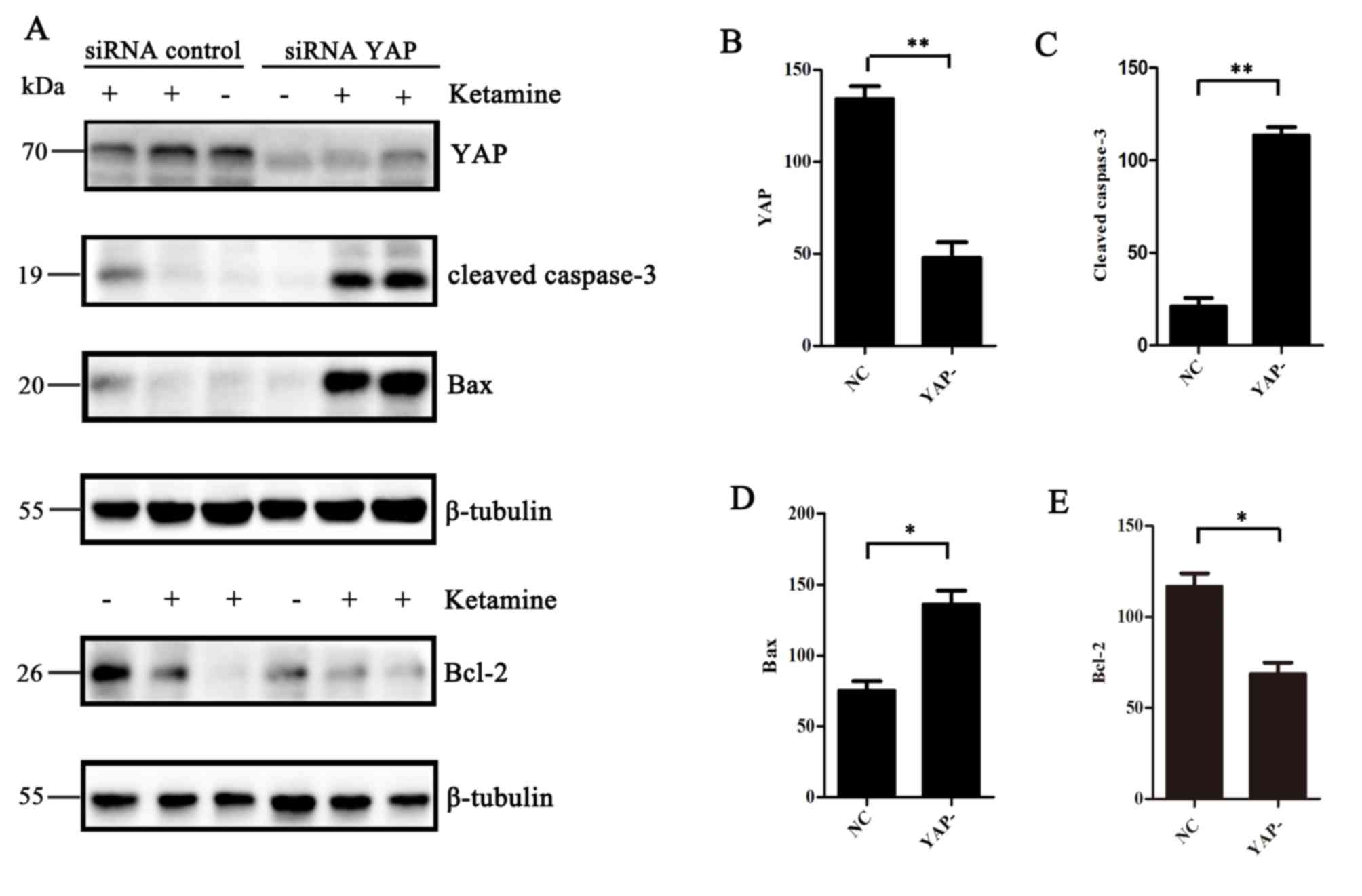
YAP knockdown aggravates
ketamine-induced apoptosis
To further investigate whether YAP knockdown altered
ketamine-induced cell apoptosis, YAP expression was knocked down
using an siRNA. Transfection efficiency of the siRNA was confirmed
by performing western blotting (P<0.01; Fig. 6A and B). In addition, the
expression levels of cleaved caspase-3, Bcl-2 and Bax in SH-SY5Y
cells following treatment with ketamine for 24 h were measured.
Cleaved caspase-3 and Bax expression levels were increased in the
YAP knockdown group compared with the NC group (P<0.05; Fig. 6A, C and D). In addition, the
expression levels of the anti-apoptotic protein Bcl-2 were
decreased in the YAP knockdown group compared with the NC group
(P<0.05; Fig. 6A and E).
Therefore, the results suggested that YAP knockdown aggravated
ketamine-induced apoptosis.
Discussion
The present study used different dose of ketamine to
decrease SH-SY5Y cell viability and proliferation. A number of
previous studies have demonstrated that ketamine can induce
irreversible neurotoxicity in the developing central nervous system
(25,26). On the one hand, it has been
reported that repeated and prolonged exposure to ketamine leads to
malignant consequences, and may be neurotoxic for the developing
nerve (27,28); however, it has also been reported
that ketamine may be neuroprotective in the presence of strong pain
stimuli (29). Furthermore, the
present study indicated that ketamine induced cell apoptosis in a
dose-dependent manner, which was consistent with previous studies
that used similar drug concentrations (23,30).
Ketamine-induced neurotoxicity is associated with a
number of signaling pathways. A previous study reported that the
NLR family pyrin domain containing 3 inflammasome and
apoptosis-associated speck-like protein and caspase-1 proteins
regulate the cleavage and release of proinflammatory interleukin-1β
(31). Moreover, the Akt/Glycogen
synthase kinase-3β/caspase-3-dependent signaling pathway also
serves a key role in protecting erythropoietin against
ketamine-induced apoptosis in cortical neurons (32). Another study using an in
vivo model of ketamine-induced neurotoxicity in the hippocampus
revealed that caspase-1-dependent cell damage was an important
factor that may be relevant to mitochondria-associated apoptosis
(33). The study also reported
that pyroptosis and inflammatory responses were involved in
mitochondria-associated apoptosis. Ketamine also inhibits protein
kinase C (PKC)/ERK to alter early and late apoptosis of hippocampal
neurons, and activation of the excitatory N-methyl-D-aspartate
receptor reverses ketamine-induced effects. The neurotoxic effect
of ketamine is associated with the PKC/ERK signaling pathway in
developing hippocampal neurons (34).
The present study indicated that YAP may serve a
crucial role in mediating ketamine-induced SH-SY5Y cell apoptosis.
As a component of the Hippo pathway, YAP is involved in organ
development via its effects on cell proliferation, apoptosis and
migration (35). The present study
also suggested that the expression levels of typical apoptotic
markers were altered and the rate of apoptosis was decreased. YAP
overexpression decreased apoptotic protein production and decreased
ketamine-induced SH-SY5Y cell apoptosis compared with the control
group. Conversely, YAP knockdown by siRNA-mediated gene
transfection resulted in increased cell apoptosis compared with the
NC group. Based on the results of the present study, YAP
overexpression may attenuate ketamine-induced damage. The results
of the present study were consistent with the results of previous
studies, which indicated that YAP stimulates cell proliferation
(9,36). Ketamine increases early and late
apoptosis in the developing brain by inhibiting the PKC/ERK
signaling pathway (34). The
present study identified a potential mechanism underlying
ketamine-induced apoptosis, highlighting the potential important
role of YAP, which is different from previous studies that have
suggested the involvement of other signaling pathways (37,38).
However, the present study had a number of
limitations. First, the YAP-associated neuroprotection mechanism
was not investigated using an in vivo model. Secondly, the
present study only assessed the effect of YAP on ketamine-induced
neurotoxicity in a single cell line. Therefore, due to the
important differences between in vitro and in vivo
environments, future studies are required to verify the neurotoxic
role of YAP identified in the present study in additional cell
lines or in an in vivo model.
In conclusion, the present study suggested that
ketamine induced neurotoxicity and cell apoptosis in a
dose-dependent manner, YAP regulation may serve as an important
event during ketamine-induced neurotoxicity in vitro. YAP
may facilitate pivotal cross talk between the Hippo signaling
pathway and ketamine-induced neural apoptosis. In addition, the
results suggested that YAP overexpression protected cells against
ketamine-induced apoptosis and neurotoxicity. By contrast, YAP
knockdown enhanced cell apoptosis. Although the precise mechanism
underlying ketamine-induced cell apoptosis via YAP requires further
investigation, the results of the present study highlighted the
role of YAP in mediating ketamine-induced apoptosis and enhanced
the current understanding of ketamine-induced neural damage in the
developing brain and may aid with the identification of novel
therapeutic strategies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shanghai (grant no. 18ZR1443100), the
Shanghai Jiao Tong University School of Medicine, Innovation Center
of Translational Medicine Collaboration (grant no. TM201729) and
the National Natural Science Foundation of China (grant no.
81401279).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YC and YL designed the study, performed the
experiments, analyzed the data and wrote the manuscript. ZY
contributed to the design of the study, provided financial support
and revised the manuscript. LW made substantial contributions to
the conception and design of the work and supervised the
experiments. JW, YW and WX aquired and analyzed the data. Jl and ZK
made substantial contributions to conception and design of the
current study. ZK, JL and YL drafted the manuscript and revised it
critically for important intellectual content, approved the final
version of the manuscript and provided financial support. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang JD, Zheng XC, Huang FY, Gao F, You
MZ and Zheng T: MicroRNA-107 regulates anesthesia-induced neural
injury in embryonic stem cell derived neurons. IUBMB Life.
71:20–27. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Z and Ma D: Anaesthetics-induced
neurotoxicity in developing brain: An update on preclinical
evidence. Brain Sci. 4:136–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan J, Li YR, Zhang Y, Lu Y and Jiang H:
Repeated exposure to anesthetic ketamine can negatively impact
neurodevelopment in infants: A prospective preliminary clinical
study. J Child Neurol. 29:1333–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuo D, Lin L, Liu Y, Wang C, Xu J, Sun F,
Li L, Li Z and Wu Y: Baicalin attenuates ketamine-induced
neurotoxicity in the developing rats: Involvement of PI3K/Akt and
CREB/BDNF/Bcl-2 Pathways. Neurotox Res. 30:159–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowak K, Meyza K, Nikolaev E, Hunt MJ and
Kasicki S: Local blockade of NMDA receptors in the rat prefrontal
cortex increases c-Fos expression in multiple subcortical regions.
Acta Neurobiol Exp (Wars). 72:207–218. 2012.PubMed/NCBI
|
|
6
|
Eustaquio T, Wang C, Dugard CK, George NI,
Liu F, Slikker W Jr, Paule MG, Howard PC and Paredes AM: Electron
microscopy techniques employed to explore mitochondrial defects in
the developing rat brain following ketamine treatment. Exp Cell
Res. 373:164–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang L, Liu Y, Jin W, Ji X and Dong Z:
Ketamine potentiates hippocampal neurodegeneration and persistent
learning and memory impairment through the PKCgamma-ERK signaling
pathway in the developing brain. Brain Res. 1476:164–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Green SM and Cote CJ: Ketamine and
neurotoxicity: Clinical perspectives and implications for emergency
medicine. Ann Emerg Med. 54:181–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Porazinski S, Wang H, Asaoka Y, Behrndt M,
Miyamoto T, Morita H, Hata S, Sasaki T, Krens SFG, Osada Y, et al:
YAP is essential for tissue tension to ensure vertebrate 3D body
shape. Nature. 521:217–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juan WC and Hong W: Targeting the hippo
signaling pathway for tissue regeneration and cancer therapy. Genes
(Basel). 7:552016. View Article : Google Scholar
|
|
13
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu H, Wei L, Fan F, Ji S, Zhang S, Geng J,
Hong L, Fan X, Chen Q, Tian J, et al: Integration of Hippo
signalling and the unfolded protein response to restrain liver
overgrowth and tumorigenesis. Nat Commun. 6:62392015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu M, Zhang Z, Sampson L, Zhou X,
Nalapareddy K, Feng Y, Akunuru S, Melendez J, Davis AK, Bi F, et
al: RHOA GTPase Controls YAP-Mediated EREG signaling in small
intestinal stem cell maintenance. Stem Cell Reports. 9:1961–1975.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Zhang N, Gray RS, Li H, Ewald AJ,
Zahnow CA and Pan D: A temporal requirement for Hippo signaling in
mammary gland differentiation, growth, and tumorigenesis. Genes
Dev. 28:432–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song H, Mak KK, Topol L, Yun K, Hu J,
Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al: Mammalian
Mst1 and Mst2 kinases play essential roles in organ size control
and tumor suppression. Proc Natl Acad Sci USA. 107:1431–1436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The Hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bohere J, Mancheno-Ferris A, Al Hayek S,
Zanet J, Valenti P, Akino K, Yamabe Y, Inagaki S, Chanut-Delalande
H, Plaza S, et al: Shavenbaby and Yorkie mediate Hippo signaling to
protect adult stem cells from apoptosis. Nat Commun. 9:51232018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Biedler JL, Roffler-Tarlov S, Schachner M
and Freedman LS: Multiple neurotransmitter synthesis by human
neuroblastoma cell lines and clones. Cancer Res. 38:3751–3757.
1978.PubMed/NCBI
|
|
21
|
Gilany K, Van Elzen R, Mous K, Coen E, Van
Dongen W, Vandamme S, Gevaert K, Timmerman E, Vandekerckhove J,
Dewilde S, et al: The proteome of the human neuroblastoma cell line
SH-SY5Y: An enlarged proteome. Biochim Biophys Acta. 1784:983–985.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mansouri S, Agartz I, Ogren SO, Patrone C
and Lundberg M: PACAP protects adult neural stem cells from the
neurotoxic effect of ketamine associated with decreased apoptosis,
ER stress and mTOR pathway activation. PLoS One. 12:e01704962017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mak YT, Lam WP, Lu L, Wong YW and Yew DT:
The toxic effect of ketamine on SH-SY5Y neuroblastoma cell line and
human neuron. Microsc Res Tech. 73:195–201. 2010.PubMed/NCBI
|
|
24
|
Mahmood T and Yang PC: Western blot:
Technique, theory, and trouble shooting. N Am J Med Sci. 4:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sampaio TB, de Oliveira LF, Constantino
LC, Costa AP, Poluceno GG, Martins WC, Dal-Cim T, de Oliveira KA,
Ludka FK, Prediger RD, et al: Long-term neurobehavioral
consequences of a single ketamine neonatal exposure in rats:
Effects on cellular viability and glutamate transport in frontal
cortex and hippocampus. Neurotox Res. 34:649–659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davidson AJ: Anesthesia and neurotoxicity
to the developing brain: The clinical relevance. Paediatr Anaesth.
21:716–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi H, Dikkes P and Soriano SG:
Repeated administration of ketamine may lead to neuronal
degeneration in the developing rat brain. Paediatr Anaesth.
12:770–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pohl D, Bittigau P, Ishimaru MJ, Stadthaus
D, Hübner C, Olney JW, Turski L and Ikonomidou C:
N-Methyl-D-aspartate antagonists and apoptotic cell death triggered
by head trauma in developing rat brain. Proc Natl Acad Sci USA.
96:2508–2513. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan J and Jiang H: Dual effects of
ketamine: Neurotoxicity versus neuroprotection in anesthesia for
the developing brain. J Neurosurg Anesthesiol. 26:155–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takadera T, Ishida A and Ohyashiki T:
Ketamine-induced apoptosis in cultured rat cortical neurons.
Toxicol Appl Pharmacol. 210:100–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li JM, Liu LL, Su WJ, Wang B, Zhang T,
Zhang Y and Jiang CL: Ketamine may exert antidepressant effects via
suppressing NLRP3 inflammasome to upregulate AMPA receptors.
Neuropharmacology. 146:149–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shang Y, Wu Y, Yao S, Wang X, Feng D and
Yang W: Protective effect of erythropoietin against
ketamine-induced apoptosis in cultured rat cortical neurons:
Involvement of PI3K/Akt and GSK-3 beta pathway. Apoptosis.
12:2187–2195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye Z, Li Q, Guo Q, Xiong Y, Guo D, Yang H
and Shu Y: Ketamine induces hippocampal apoptosis through a
mechanism associated with the caspase-1 dependent pyroptosis.
Neuropharmacology. 128:63–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang S, Li X, Jin W, Duan X, Bo L, Wu J,
Zhang R, Wang Y, Kang R and Huang L: Ketamine-induced neurotoxicity
blocked by N-Methyl-d-aspartate is mediated through activation of
PKC/ERK pathway in developing hippocampal neurons. Neurosci Lett.
673:122–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, He F, Zhao X, Zhang Y, Chu X, Hua C,
Qu Y, Duan Y and Ming L: YAP inhibits the apoptosis and migration
of human rectal cancer cells via suppression of
JNK-Drp1-mitochondrial fission-HtrA2/Omi pathways. Cell Physiol
Biochem. 44:2073–2089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deng Y, Wu LMN, Bai S, Zhao C, Wang H,
Wang J, Xu L, Sakabe M, Zhou W, Xin M and Lu QR: A reciprocal
regulatory loop between TAZ/YAP and G-protein Galphas regulates
Schwann cell proliferation and myelination. Nat Commun.
8:151612017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai X, Yan Y, Canfield S, Muravyeva MY,
Kikuchi C, Zaja I, Corbett JA and Bosnjak ZJ: Ketamine enhances
human neural stem cell proliferation and induces neuronal apoptosis
via reactive oxygen species-mediated mitochondrial pathway. Anesth
Analg. 116:869–880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Braun S, Gaza N, Werdehausen R, Hermanns
H, Bauer I, Durieux ME, Hollmann MW and Stevens MF: Ketamine
induces apoptosis via the mitochondrial pathway in human
lymphocytes and neuronal cells. Br J Anaesth. 105:347–354. 2010.
View Article : Google Scholar : PubMed/NCBI
|