Introduction
Hypertrophic scars (HSs) comprise a type of
pathological scar induced by surgery, burn injuries or trauma
during the healing process (1).
HSs most commonly occur in the outer layers of the skin and
arthroses, resulting in damage to the individual appearance and
severe dysfunction, including itchiness, susceptibility to
infection, pain and disfigurement (2,3). It
is well established that HSs are a type of tissue fibrosis caused
by the accumulation of the extracellular matrix, exhibiting a
robust inflammatory response and fibroblast proliferation (2,4). The
therapeutic strategies for HSs include surgery, radiotherapy and
combination therapy (1); however,
the therapeutic efficacy of these treatments remains
unsatisfactory. For example, previous studies investigated the
efficacy of laser therapy combined with silicone gel sheeting and
steroid injection, but found that there was no significant effect
for HSs treatment (5,6). Furthermore, HSs have been reported to
be regulated by a number of complicated regulatory mechanisms,
including inflammation (7) and
immune response (8). Nonetheless,
to the best of our knowledge, the mechanisms behind the
pathophysiological processes of HSs remain unknown (9). Therefore, it remains an urgent
requirement to investigate the potential molecular events of HSs to
identify novel therapeutic targets.
Autophagy is an evolutionarily highly conserved
catabolic pathway that maintains the cellular energy balance
through recycling cytoplasmic proteins and controlling the quality
of organelles (10,11); it also provides efficient
protection for cells under various stress conditions (12,13).
Previous studies have reported that autophagy was involved in
numerous types of disease, including cancer, lung disease and
neurodegenerative diseases (14–17).
In addition, the involvement of autophagy in the formation of HSs
has been demonstrated under starvation stress (18). Thus, these findings provide
reasoning for researchers to further investigate the relationship
between HSs and autophagy.
MicroRNAs (miRNA/miR) are endogenous, conserved
non-coding RNA molecules of 19–22 nucleotides in length (19). miRNAs serve as critical regulators
of target genes through multiple mechanisms, including inhibiting
translation, promoting mRNA degradation and repressing protein
synthesis (20–22). In addition, an abundance of
evidence has identified that miRNAs were involved in numerous
metabolic reactions, including cell proliferation, differentiation,
autophagy and apoptosis, by directly binding to the 3′-untranslated
region (3′-UTR) of their target mRNAs (23–26).
To date, a small number of studies have suggested a potential role
between miRNAs and HSs; for example, the expression levels of
miR-21 were reported to be upregulated in HS-derived fibroblasts
(HSFBs) and inhibiting miR-21 expression significantly slowed the
formation of HSs in vivo (27). Conversely, the expression levels of
another miRNA, miR-137, were markedly downregulated in HSs, which
induced the proliferation and metastasis of fibroblasts (28). Thus, as multiple miRNAs have been
reported to be aberrantly expressed during HS formation, it deems
worthy to investigate potential miRNAs candidates for HS
therapy.
Resveratrol was discovered to be highly effective in
the treatment of numerous types of tumor, including colon cancer,
liver cancer and neuroendocrine tumors, as well as inflammatory
reaction (29,30). Several previous studies have
reported that resveratrol was involved in miRNA-induced autophagy
during the treatment of multiple types of disease, such as chronic
diabetic nephropathy (31),
Alzheimer's disease (32) and
cancer (33). For HSs, resveratrol
has been approved as a potential agent to suppress scar formation
(34). Interestingly, Zeng et
al (35) identified that
resveratrol significantly inhibited cell growth by inducing
fibroblast apoptosis, whereas Bai et al (36) discovered that sirtuin 1 was
upregulated by resveratrol, leading to autophagy during HSs
treatment. Therefore, the present study hypothesized that
resveratrol may inhibit the viability of hypertrophic scars by
activating autophagy via the miRNAs. The results revealed that
resveratrol induced autophagy by inhibiting the expression levels
of Rheb. Notably, miR-4654 served as the ‘bridge’ between
resveratrol and the GTP-binding protein Rheb. Taken together, the
findings of the present study confirmed that Rheb was a target gene
of miR-4654 and partially determined the novel mechanism of
miR-4654-induced autophagy, thereby providing further insights into
putative targets for HS therapy.
Materials and methods
Chemicals and cell culture
Resveratrol was purchased from Target Molecule Corp.
Normal skin-derived fibroblasts (NSFBs) and HSFBs were kindly
provided by Dr Li Min at Department of Dermatology, Gulou Hospital
(Nanjing, China). 293T cells were obtained from the American Type
Culture Collection. All cell lines were cultured in high glucose
DMEM (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic (Thermo Fisher Scientific, Inc.), and
maintained at 37°C in an atmosphere of 5% CO2. HSFBs
were treated by resveratrol or transfected with miR-4654 mimic and
inhibitor or Rheb vectors. HSFBs treated with mixed vehicle
controls were used as control.
MTT assay
An MTT assay was used to determine cell viability.
Briefly, HSFBs were seeded in the 96-well plate at a density of
106 cells per well. Then resveratrol was diluted to
various concentrations (0, 1, 10 or 100 µmol/l) using PBS and
incubated with the cells (1×106) for 0, 24, 48 or 72 h
at 37°C. Following the incubation, 200 µl MTT medium was added/well
and incubated with the cells at 37°C for a further 4 h. Following
treatment with dimethyl sulfoxide (100 µl), the optical density was
measured at a wavelength of 570 nm for each experimental group
using a microplate reader (Thermo Fisher Scientific, Inc.).
Cell transfection
The miR-4654 mimics (5′-UGUGGGAUCUGGAGGCAUCUGG-3′),
miR-4654 inhibitors (5′-AGAUGCCUCCAGAUCCCACAAA-3′), mimic-NC
(5′-UUUGUACUACACAAAAGUACUG-3′) and inhibitor-NC
(5′-CAGUCCUUUUGUGUAGUACAA−3′) were obtained from Shanghai
GenePharma Co., Ltd. HSFBs were plated on the 6-well plate at a
density of 106 cells pre well and transfected with
miR-4654 mimics, miR-4654 inhibitors or the respective negative
controls (NCs) (5 nM for all) for 72 h using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
According to the manufacturer's protocols, Rheb
overexpression (OE) was accomplished using a Rheb OE vector
(pcDNA3.1; Synthgene Biotech). The Rheb knockdown (KD) was
accomplished using a short hairpin RNA targeting Rheb contained
within a pcDNA3.1 vector. An empty pcDNA3.1 vector was used as the
NC for the KD and OE vectors. HSFBs were plated in the 6-well plate
(106 cells pre well) and transfected with these vectors
at 5 nM using Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The cells were used for further
study 72 h following transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. A total of 1 µg
RNA was reverse transcribed into cDNA using AMV reverse
transcriptase (RR019A, Takara Biotechnology Co., Ltd.), according
to the manufacturer's protocol, and a miR-4654 RT primer
(5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCAGAT-3′); U6 RT
primer (5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCATGCT-3′).
The RT reaction conditions were as follows: 42°C for 30 min, 95°C
for 5 min and 5°C for 5 min.
qPCR was subsequently performed using a SYBR Green
qPCR mix (Vazyme Biotech Co., Ltd.), according to the
manufacturer's protocol, on an ABI 7300 sequence detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR (performed on a
96-well plates): Initial denaturation at 95°C for 10 min; followed
by 40 cycles at 95°C for 30 sec, 56°C for 30 sec and 72°C for 1
min. The following primer sequences were used for the qPCR:
miR-4654 forward, 5′-CGTGTGGGATCTGGAGGC−3′ and reverse,
5′-AGTGCAGGGTCCGAGGTATT-3′; U6 forward, 5′-CGGTCCAACGATACAGAGAAG−3′
and reverse, 5′-AGTGCAGGGTCCGAGGTATT-3′; Rheb forward,
5′-TGGGAATAAGAAAGACCTG-3′ and reverse, 5′-GAAGACTTGCCTTGTGAA−3′;
and GAPDH forward, 5′-GATATTGTTGACATCAATGAC−3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′. The expression levels were quantified
using the 2−ΔΔCq method (37), and GAPDH expression levels were
used to normalize the relative abundance of Rheb, whereas U6
expression levels were used to normalize the relative abundance of
miR-4654.
Detection of the target site of
miR-4654 on the 3′-UTR of Rheb using a dual-luciferase reporter
assay
The potential target sequence for miR-4654 on the
3′-UTR of Rheb was predicted using TargetScan (www.targetscan.org). Subsequently, 1×106
293T cells were plated into six-well plates and cultured for 12 h
at 37°C and 5% CO2. The pGL3 luciferase reporter vector
was obtained from Promega Corporation and the Rheb wild-type (WT)
or mutant (MUT) 3′-UTR were cloned into the pGL3 plasmid to
synthesize pGL3-Rheb-WT or pGL3-Rheb-MUT. To establish the 3′-UTR
of mutant Rheb (Rheb-MUT), the binding sites were mutated via the
site directed mutagenesis kit (NEB E0554, New England BioLabs,
Inc.). 293T cells were co-transfected with 5 nM of miR-4564 mimics
or mimic-NC and pGL3-Rheb-WT or pGL3-Rheb-MUT using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following incubation for 48 h at 37°C, the transfected cells were
harvested by centrifugation (350 × g; 3 min; 20°C) and firefly
luciferase activity was detected using a Dual-Luciferase Reporter
Assay system (Promega Corporation). The data was normalized to
Renilla luciferase activity.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with protease and phosphatase inhibitors (Thermo Fisher Scientific,
Inc.). Total protein was quantified using a bicinchoninic acid
assay (Thermo Fisher Scientific, Inc.) and 45 µg protein/lane was
separated via 12% SDS-PAGE for 2.5 h at a voltage of 100 V. The
separated proteins were subsequently transferred onto PVDF
membranes for 1.5 h under a current of 320 mA and blocked with 5%
non-fat milk for 2 h at 37°C. The membranes were then incubated
with the following primary antibodies overnight at 4°C: Anti-Rheb
rabbit polyclonal (ab92313, 1:2,000; Abcam),
anti-microtubule-associated protein 1A/1B-light chain 3 (LC3)
rabbit polyclonal (14600-1-ap, 1:1,000; ProteinTech Group, Inc.),
anti-Beclin 1 rabbit monoclonal (ab210498, 1:2,000; Abcam) and
anti-GAPDH mouse monoclonal (40004-1-lg, 1:10,000; ProteinTech
Group, Inc.). GAPDH served as the internal loading control.
Following the primary antibody incubation, the membranes were
washed with PBS-Tween 20 (1%) and incubated with horseradish
peroxidase-conjugated secondary antibodies: Anti-Rat IgG (HRP)
(ab7097, 1:5,000, Abcam) or Anti-Mouse IgG (HRP) (ab97040, 1:5,000,
Abcam) at room temperature for 2 h. Protein bands were visualized
using an ECL reagent (Thermo Fisher Scientific, Inc.) and an ECL
immunoblotting system (Tanon Science and Technology Co., Ltd.) and
the expression levels were semi-quantified using ImageJ software
(V1.8.0.112, National Institutes of Health).
Verification of the role of
autophagy
HSFBs (1×106) were co-treated with 5 mM
3-Methyladenine (3-MA; cat. no. HY-19312; MedChemExpress) and 100
µmol/l resveratrol for 72 h at 37°C. Then, the cells were collected
by centrifugation (350 × g; 3 min; room temperature) for further
experiments.
Fluorescence assay
HSFBs were infected with adenoviruses expressing
GFP-LC3B fusion protein [Umibio (Shanghai) Co., Ltd.] using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) for 72 h to obtain the stable GFP-LC3 cell line.
Briefly, 1×106 cells were seeded upon glass confocal
dishes and allowed to settle for 12 h at 37°C. miR-4654 mimics,
miR-4654 inhibitors and the respective NCs were subsequently
transfected into the stable cell line with or without Rheb OE
plasmid or Rheb KD plasmid transfection. Subsequently, each group
was treated with 100 µmol/l resveratrol or an equal volume of PBS.
Following incubation for 72 h at 37°C, cells in each group were
fixed by 4% paraformaldehyde for 10 min at room temperature. The
cell nuclei were stained with DAPI (1:2,000; Abcam) for 5 min in
the dark at room temperature. The images were observed at 200×
magnification using a confocal microscope.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism v5 software (GraphPad Software, Inc.) and data are presented
as the mean or percentage change ± SD from three independent
experiments. Statistical differences between the two treatment
groups were compared using a paired Student's t-test, whereas
comparisons between >2 groups were performed using a one-way
ANOVA and a Tukey's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Autophagy is triggered by resveratrol
in a dose-dependent manner in HSFBs
To identify the pharmacological effect of
resveratrol, HSFBs were treated with resveratrol at different
concentrations, and the cell viability was subsequently determined
using a MTT assay, which revealed a significant dose-dependent
decrease in cell viability following the treatment with resveratrol
compared with the untreated cells (Fig. 1A). Notably, the most significant
level of inhibition occurred following 100 µmol/l resveratrol
treatment. Subsequently, changes in the expression levels of the
autophagy-related protein marker, LC3, were investigated. The
results revealed that the LC3-II/LC3-I ratio was significantly
increased in a dose-dependent manner (Fig. 1B). Conversely, the expression
levels of the upstream gene, Rheb, were significantly decreased
dose-dependently. These findings suggested that resveratrol may
induce autophagy in HSFBs.
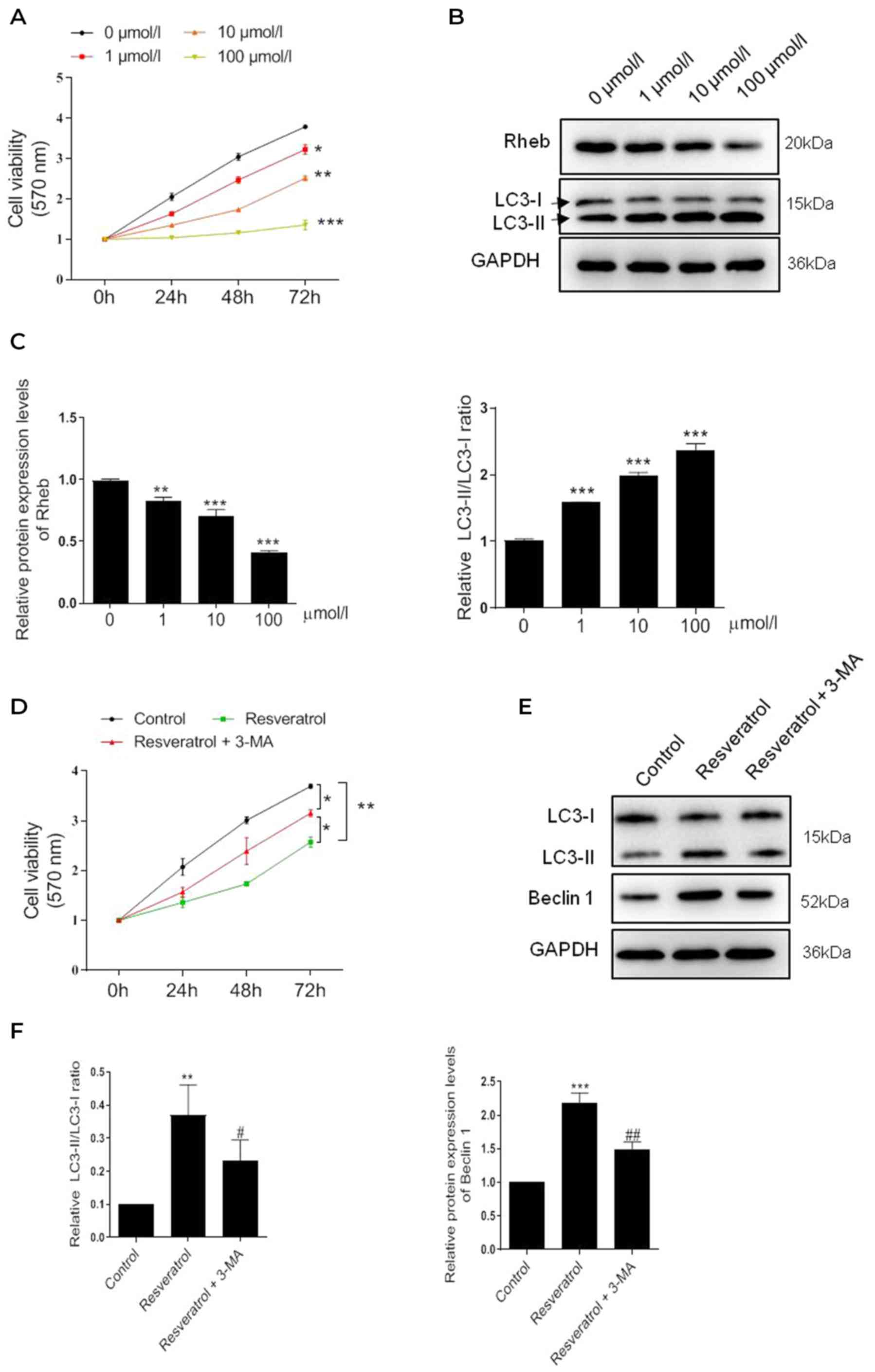 | Figure 1.Resveratrol inhibits the viability of
HSFBs through regulating Rheb and activating autophagy. (A) MTT
assay was performed to determine the cell viability of HSFBs in
each group at 72 h following the treatment with different
concentrations of resveratrol. *P<0.05, **P<0.01,
***P<0.001 vs. 0 µmol/l group. (B) and (C) Western blotting was
used to analyze the protein expression levels of Rheb and the
LC3-II/I ratio in HSFBs following the treatment with different
concentrations of resveratrol. **P<0.01, ***P<0.001 vs. 0
µmol/l group. (D) MTT assay was performed to detect the cell
viability of HSFBs in each group at 72 h following the treatment
with 100 µmol/l resveratrol with or without 5 mM 3-MA treatment.
*P<0.05, **P<0.01. (E) and (F) Western blotting was used to
analyze the protein expression levels of Rheb and LC3 in HSFBs
following the treatment with resveratrol and 3-MA or resveratrol
alone. **P<0.01, ***P<0.001 vs. the control group;
#P<0.05, ##P<0.01 vs. the resveratrol
group. Data are presented as the mean ± SD from three independent
experiments. Control, the cell was treated with PBS; HSFBs,
hypertrophic scar-derived fibroblasts; LC3, microtubule-associated
protein 1A/1B-light chain 3; 3-MA, 3-Methyladenine. |
To further confirm whether resveratrol inhibited the
viability of HSFBs through activating autophagy, HSFBs were
co-treated with the autophagy inhibitor 3-MA (5 mM) and 100 µmol/l
resveratrol. Interestingly, 3-MA treatment partially reversed the
inhibitory effect of resveratrol on cell viability (Fig. 1C). The western blotting results
further revealed that 3-MA treatment markedly regulated the effect
of resveratrol-induced autophagy; the LC3-II/LC3-I ratio and Beclin
1 expression levels were partially but notably downregulated in
HSFBs treated with resveratrol and 3-MA compared with resveratrol
alone (Fig. 1D). These results
indicated that autophagy may be triggered by resveratrol in a
dose-dependent manner to inhibit HSFB cell viability.
miR-4654 downregulates the protein
expression levels of Rheb
The subsequent experiments investigated the effect
of separate controls and a mixed control on the expression levels
of Rheb, cell viability and autophagy. The results revealed that
the mimic-NC or inhibitor-NC transfections did not alter the
protein or mRNA expression levels of Rheb compared with the
untreated group (Fig. S1A and B).
Similarly, following the transfection of the cells with the mixed
control (mimic-NC + inhibitor-NC + PBS), the expression levels of
Rheb were slightly downregulated; however, no statistical
differences were recorded among the different groups (Fig. S1A and B). A similar trend in the
cell viability response was observed in each group (Fig. S2A). Similarly, no significant
differences were identified between the separate controls and mixed
control transfections on the LC-3II/I ratio or Beclin 1 expression
levels (Fig. S2B and C). In
addition, the effect of the control transfections on the expression
levels of LC3 were investigated using GFP-LC3 stable cells.
Compared with the untreated group, each separated control group did
not display significant changes in the LC3 signal intensity
(Fig. S3). In fact, even in the
mimic-NC + inhibitor-NC + vector + PBS group, the LC3 signal was
similar compared to the untreated group. Taken together, the
results identified that the mixed control presented a similar
effect to the single controls, and had no effect on Rheb expression
levels, HFSB viability or autophagy. Thus, the mixed controls were
chosen for use in subsequent experiments.
The expression levels of miR-4654 were subsequently
investigated in NSFBs and HSFBs from 14 independent repeated
experiments. Compared with the NSFBs, the expression levels of
miR-4654 in the HSFBs were significantly downregulated (Fig. 2A). Following resveratrol treatment,
the expression levels of miR-4654 were significantly upregulated in
a dose-dependent manner compared with the untreated HSFBs (Fig. 2B). Changes in miR-4654 expression
in HSFBs transfected with miR-4654 mimic and inhibitor were
assessed by RT-qPCR. The results revealed that the cells
transfected with the miR-4654 mimic had significantly upregulated
miR-4654 expression levels compared with the mimic-NC group. In
addition, a 50% decrease was observed in the expression levels of
miR-4654 following the transfection with the miR-4654 inhibitor
compared with the inhibitor-NC group (Fig. 2C). Subsequently, the mRNA and
protein expression levels of Rheb were analyzed in the presence of
the miR-4654 mimic, miR-4653 inhibitor or resveratrol treatment;
however, no significant differences were observed in the expression
levels of Rheb mRNA between these groups (Fig. 2D). Interestingly, neither miR-4654
nor resveratrol were able to influence Rheb mRNA expression levels;
however, resveratrol treatment significantly downregulated the
protein expression levels of Rheb, while the inhibition of miR-4654
expression levels in HSFBs led to the significant upregulation of
the protein expression levels of Rheb compared with the control
group (Fig. 2E). These findings
indicated that Rheb may be influenced by miR-4654 or resveratrol in
the process of translation.
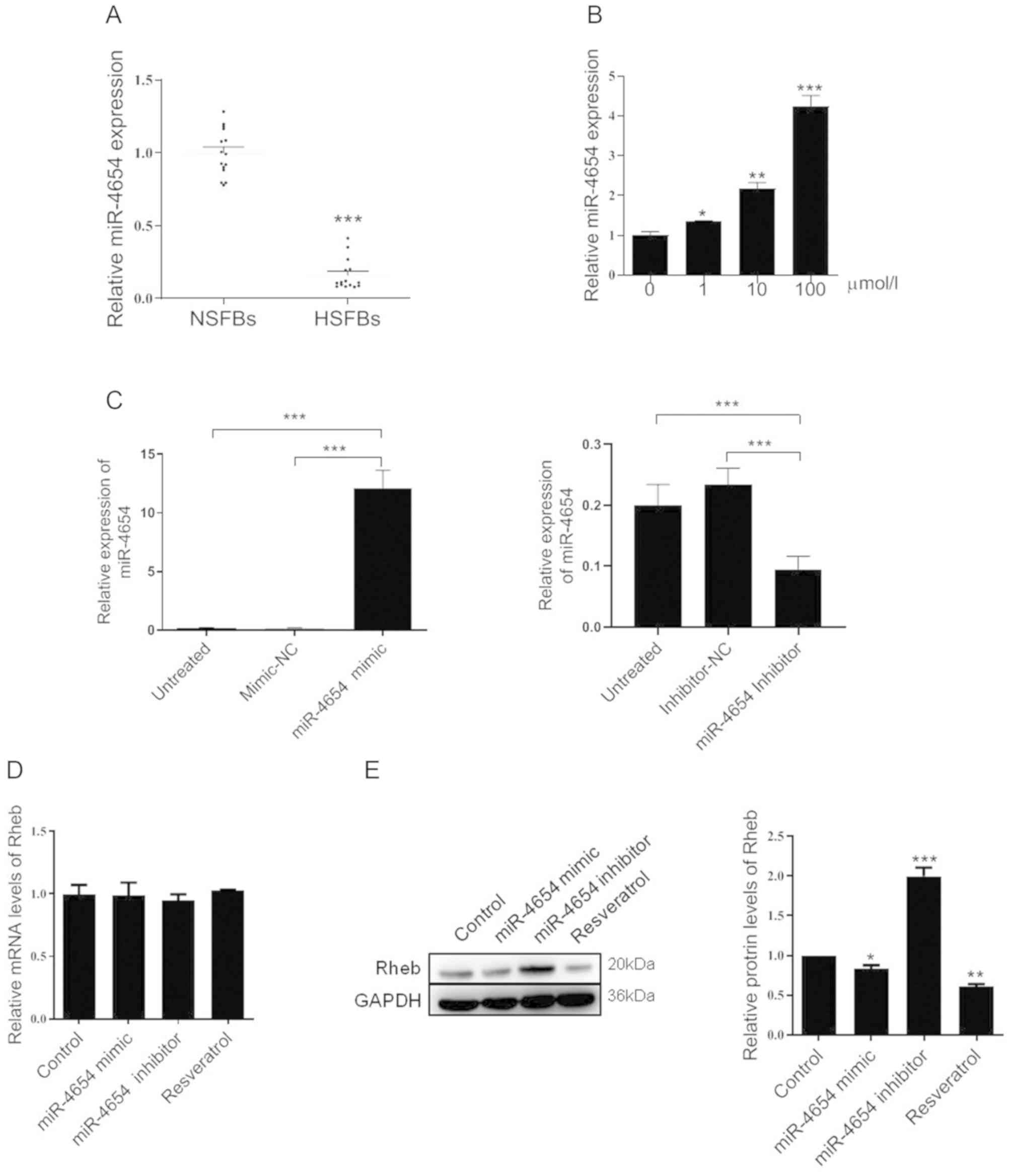 | Figure 2.Effect of miR-4654 on Rheb
expression. (A) Relative expression levels of miR-4654 in NSFBs and
HSFBs. N=14, ***P<0.001 vs. NFSBs. (B) RT-qPCR was performed to
analyze the miR-4654 expression levels in HSFBs following the
treatment with resveratrol at different concentrations. *P<0.05,
**P<0.01, ***P<0.001 vs. 0 µmol/l group. (C) Transfection
efficiency of miR-4654 mimic or miR-4654 inhibitor in HSFBs was
determined using RT-qPCR. ***P<0.001. (D) RT-qPCR was performed
to analyze the mRNA expression levels of Rheb in HSFBs transfected
with miR-4654 mimic or miR-4654 inhibitor or treated with 100
µmol/l resveratrol. (E) Western blotting was used to analyze the
protein expression levels of Rheb in HSFBs transfected with
miR-4654 mimic or miR-4654 inhibitor, or treated with 100 µmol/l
resveratrol. *P<0.05, **P<0.01, ***P<0.001 vs. the control
group. Both the mRNA and protein expression levels from parts (D)
and (E) were normalized to GAPDH. Data are presented as the mean ±
SD; n=3. HSFBs, hypertrophic scar-derived fibroblasts; NSFBs,
normal skin-derived fibroblasts; Control, mimic-NC + inhibitor-NC +
PBS group; RT-qPCR, reverse transcription-quantitative PCR; miR,
microRNA. |
miR-4654 regulates the translation of
Rheb through targeting its 3′-UTR region
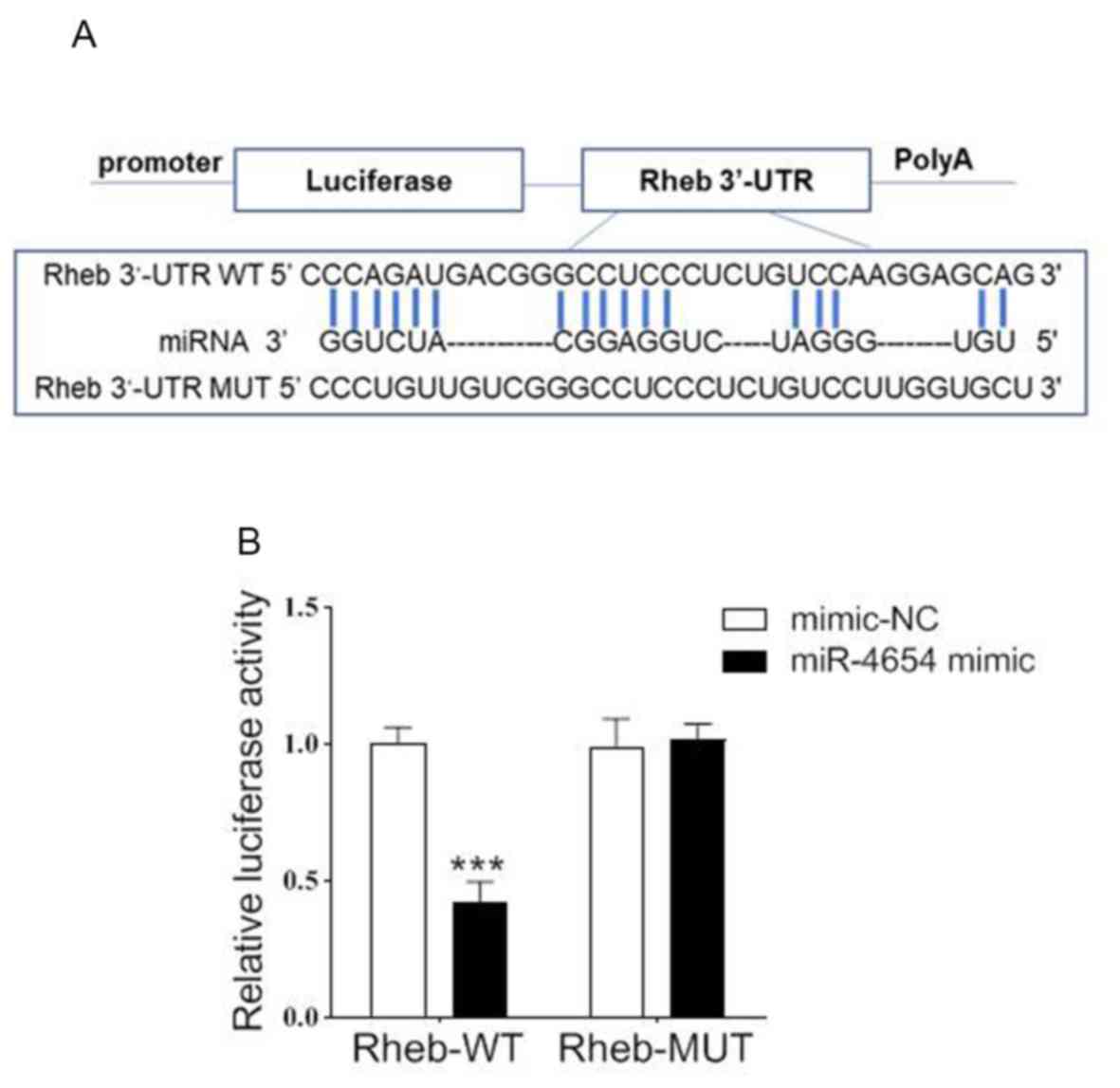
To confirm the relationship between miR-4654 and
Rheb, the potential binding sites of miR-4654 on the 3′-UTR of Rheb
were investigated using bioinformatics analysis (Fig. 3A). To further confirm the
hypothesis of the present study, a dual-luciferase reporter assay
was performed by co-transfecting miR-4654 mimics or mimic-NC with
the luciferase reporter vector containing the WT or MUT 3′-UTR of
Rheb. The transfection of the miR-4654 mimic led to a significant
reduction in the relative luciferase activity of Rheb-WT cells
compared with the mimic-NC group; however, no significant
differences were observed in the Rheb-MUT cells with miR-4654
treatment as compared with the mimic-NC group (Fig. 3B). These findings suggested that
miR-4654 may target the 3′-UTR of WT Rheb and regulate the
expression of Rheb negatively.
miR-4654 enhances Rheb-induced
autophagy in HSFBs
A previous study demonstrated the critical role of
Rheb in the regulation of cell autophagy and proliferation
processes (38). Therefore, the
present study aimed to investigate whether the miRNA-dependent
downregulation of Rheb affected these processes in HSFBs. A 5-fold
upregulation in mRNA expression levels and a 2.4-fold upregulation
in protein expression levels was identified in HSFBs transfected
with the Rheb OE vector compared with the control vector.
Conversely, the mRNA expression levels of Rheb were significantly
downregulated in cells transfected with Rheb KD vector compared
with the control vector and the western blotting results revealed a
60% reduction in Rheb expression levels following Rheb KD in the
cells (Fig. 4A).
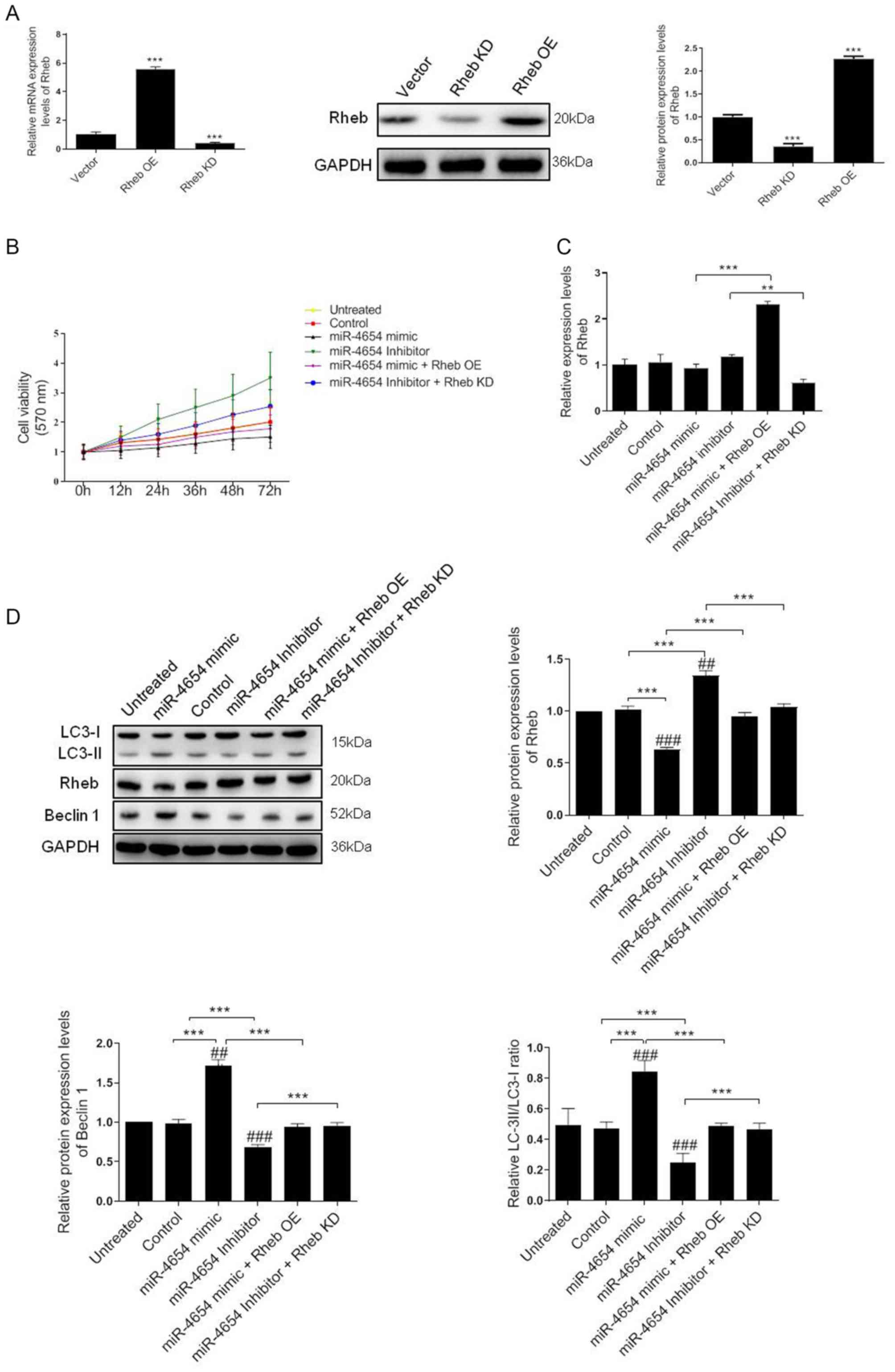 | Figure 4.Effect of miRNA-mediated Rheb
downregulation on the viability and autophagy of HFSBs. (A) HFSBs
were transfected with the Rheb OE plasmid, Rheb KD plasmid or the
empty control vector. The mRNA and protein expression levels of
Rheb were analyzed using RT-qPCR or western blotting, respectively.
***P<0.001 vs. vector group. (B) MTT assay was performed to
determine the cell viability in each group at 72 h. (C) RT-qPCR
analysis of Rheb expression levels in HFSBs in the different
groups. **P<0.01, ***P<0.001. (D) Western blotting was used
to determine the protein expression levels of Beclin 1, Rheb and
LC3 in HFSBs in the different groups. ***P<0.001;
##P<0.01, ###P<0.001 vs. the untreated
group. Data are presented as the mean ± SD from three independent
experiments. HSFBs, hypertrophic scar-derived fibroblast; OE,
overexpression; KD, knockdown; Control, mimic-NC + inhibitor-NC +
vector treated group; LC3, microtubule-associated protein
1A/1B-light chain 3; miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR. |
Based on these results, the current study aimed to
determine whether miR-4654 induced HSFB cell viability through a
Rheb-dependent mechanism The MTT assay results revealed that the
inhibition of miR-4654 expression levels increased the cell
viability of HSFBs compared with the untreated group or control
group. However, the cell viability was reduced following the
transfection with the miR-4654 mimic. In addition, following the
co-transfection with the Rheb KD vector prior to the miR-4654
inhibitor, the cell viability was re-increased compared with the
miR-4654 inhibitor group (Fig.
4B). The mRNA expression levels of Rheb were subsequently
investigated following the different transfections. The regulation
of miR-4654 expression levels using the mimic or inhibitor did not
significantly alter Rheb expression levels in the HSFBs compared
with the control groups; however, a 2.3-fold increase and a 50%
reduction was observed in Rheb expression levels in the Rheb OE +
miR-4654 mimic and miR-4654 inhibitor + Rheb KD groups,
respectively (Fig. 4C). Finally,
the expression levels of autophagy-related protein markers,
including LC3 and Beclin 1, were analyzed. The data revealed that
the overexpression of miR-4654 significantly upregulated the
expression levels of these proteins compared with the control
group. Moreover, following the transfection with miR-4654 mimic,
the expression levels of Rheb were significantly downregulated
compared with the control group. In contrast, following the
transfection of cells with the miR-4654 inhibitor, the expression
levels of Rheb were significantly upregulated. Next, Rheb was
overexpressed prior to the miRNA-mimic/inhibitor transfection; the
results demonstrated that the transfection with Rheb OE partially
reversed the effect of miR-4654 on autophagy. Similarly, the
co-transfection of Rheb KD and the miR-4654 inhibitor into HSFBs
also significantly reversed the effect of the miR-4654-inhibitor
alone (Fig. 4D). These data
suggested that Rheb may be one of the targets of miR-4654
responsible for autophagy and the viability of HSFBs.
Resveratrol induces HSFB autophagy
through miR-4654/Rheb axis
In light of the aforementioned findings, it was
hypothesized that the effect of resveratrol on cell autophagy may
be mediated by miRNA. As previously demonstrated, resveratrol
treatment promoted the upregulation of miR-4654 expression levels
in HSFBs. The subsequent results revealed that the cell viability
was markedly reduced following resveratrol treatment compared with
the control group, and this effect was exacerbated by the addition
of the miR-4654 mimic (resveratrol + miR-4654 mimic group).
Conversely, the inhibition of miR-4654 expression alongside
resveratrol treatment effectively alleviated the effects of
resveratrol treatment alone over cell viability. When Rheb was
overexpressed before resveratrol and miR-4654 mimic treatment, the
cell viability was re-increased significantly compared with the
resveratrol + miR-4654 mimic group. The silencing of Rheb reversed
the effect of resveratrol + miR-4654 inhibitor treatment (Fig. 5A). Moreover, RT-qPCR analysis
revealed that neither resveratrol treatment alone or combined with
miR-4654 mimics downregulated the expression level of Rheb compared
with the control group (Fig. 5B).
However, these effects were significantly reversed following the
co-transfection with the Rheb OE plasmid (resveratrol + miR-4654
mimic + Rheb OE group; Fig. 5B).
Similarly, the results obtained from the western blotting
experiments also suggested that the downregulated expression levels
of Rheb following resveratrol treatment in HSFBs were miR-4654
dependent. Rheb was markedly reduced following resveratrol
treatment alone or combined with miR-4654 mimic compared with the
control group. By contrast, the inhibition of miR-4654 expression
alongside resveratrol treatment effectively increased the effects
of resveratrol treatment alone. However, the expression level of
Beclin 1 and the LC3-II/LC3-I ratio were both increased in the
resveratrol group, and further increased in the resveratrol +
miR-4654 mimic group. While the levels of such molecules were both
downregulated when the cells were treated with miR-4654 inhibitor,
which exhibited no difference compared with the resveratrol alone
group. When the cells were co-transfected with the miR-4654 mimic
and Rheb OE prior to resveratrol treatment, no change was observed
in the expression level of Beclin 1 and the LC3-II/LC3-I ratio
compared with the resveratrol + miR-4654 mimic group, while the
protein level of Rheb was reupregulated significantly. Moreover,
following the treatment of cells with the miR-4654 inhibitor and
downregulating Rheb expression levels with the Rheb KD plasmid,
resveratrol treatment significantly inhibited cell autophagy
(Fig. 5C). These findings
suggested that the increase in autophagy by resveratrol in HSFBs
may be dependent on the miR-4654/Rheb axis.
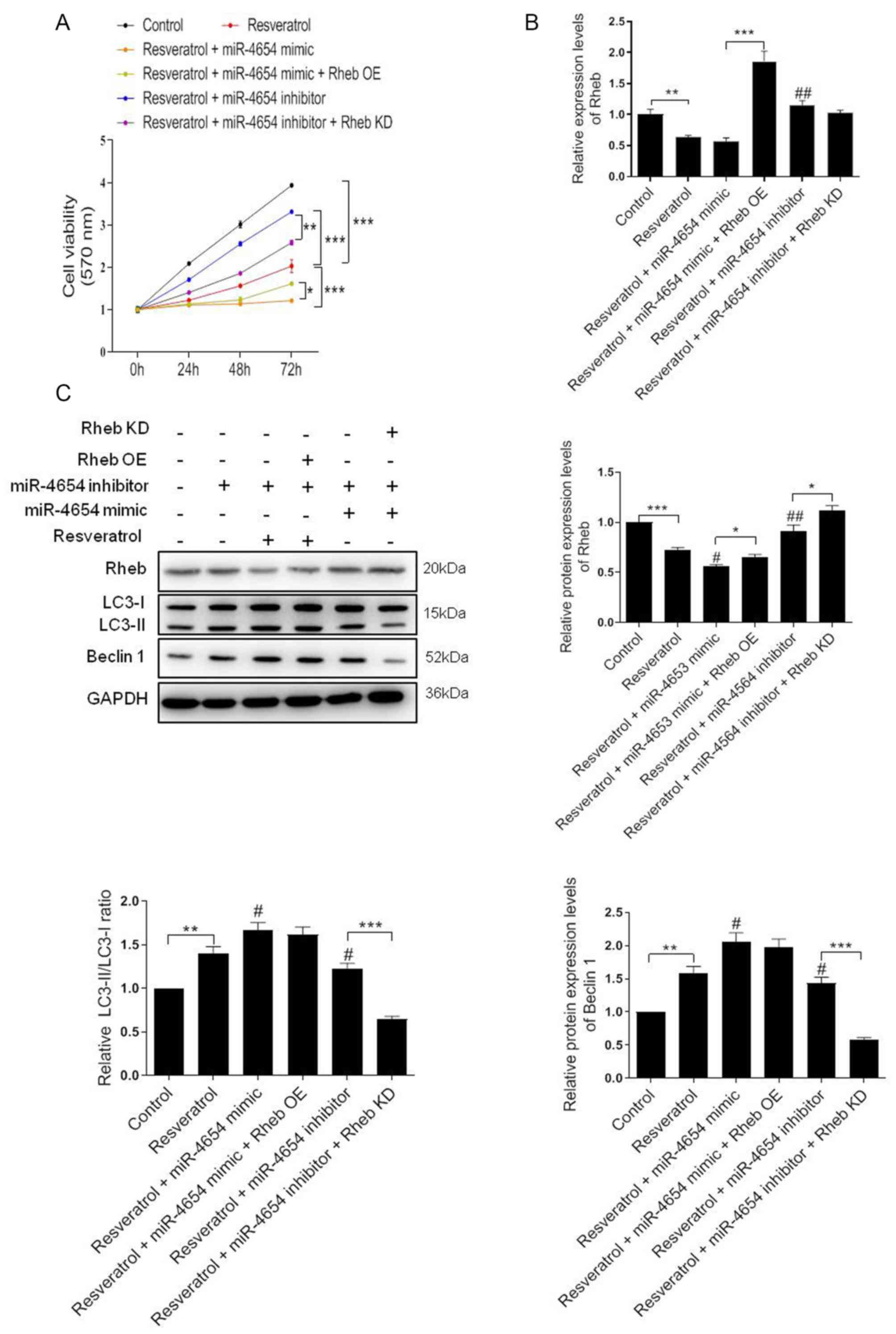 | Figure 5.Resveratrol activates autophagy
through the miR-4654/Rheb axis. (A) Cell viability was analyzed in
HSFBs in the different groups using an MTT assay. *P<0.05,
**P<0.01, ***P<0.001. (B) RT-qPCR analysis of the expression
levels of Rheb in HSFBs in the different groups. **P<0.01,
***P<0.001; ##P<0.01 vs. the resveratrol group.
(C) Western blotting was used to analyze the protein expression
levels of Rheb, LC3 and Beclin 1 in HSFBs in the different groups.
*P<0.05, **P<0.01, ***P<0.001; #P<0.05,
##P<0.01 vs. the resveratrol group. Data are
presented as the mean ± SD. N=3. HSFBs, hypertrophic scar-derived
fibroblasts; LC3, microtubule-associated protein 1A/1B-light chain
3; Control, mimic-NC + inhibitor-NC + PBS + vector group; OE,
overexpression; KD, knockdown; miR, microRNA. |
miR-4654 promotes the formation of
autophagosomes in HSFBs following resveratrol treatment
To further confirm the role of miR-4654 in the
activation of autophagy, the fluorescence intensity in the stable
GFP-LC3 HSFBs cell line was analyzed in each group; miR-4654 mimics
or inhibitors were transfected into stable GFP-LC3 cell lines.
Following 72 h of transfection, DAPI was used to stain the cell
nuclei and the cells were observed under confocal microscopy to
determine the GFP-LC3 fluorescent intensities (Fig. 6). The results revealed that
excessive autophagy was induced following resveratrol treatment,
while the transfection with the miR-4654 inhibitor reversed the
effect of resveratrol treatment. In addition, upon the
co-transfection of the cells with the miR-4654 mimic and Rheb OE
prior to resveratrol treatment, the increased GFP-LC3 fluorescent
intensity in the resveratrol + miR-4654 mimic group was reduced.
Moreover, the co-transfection of cells with the miR-4654 inhibitor
and Rheb KD plasmid prior to resveratrol treatment re-increased the
effect of resveratrol + miR-4654 inhibitor treatment.
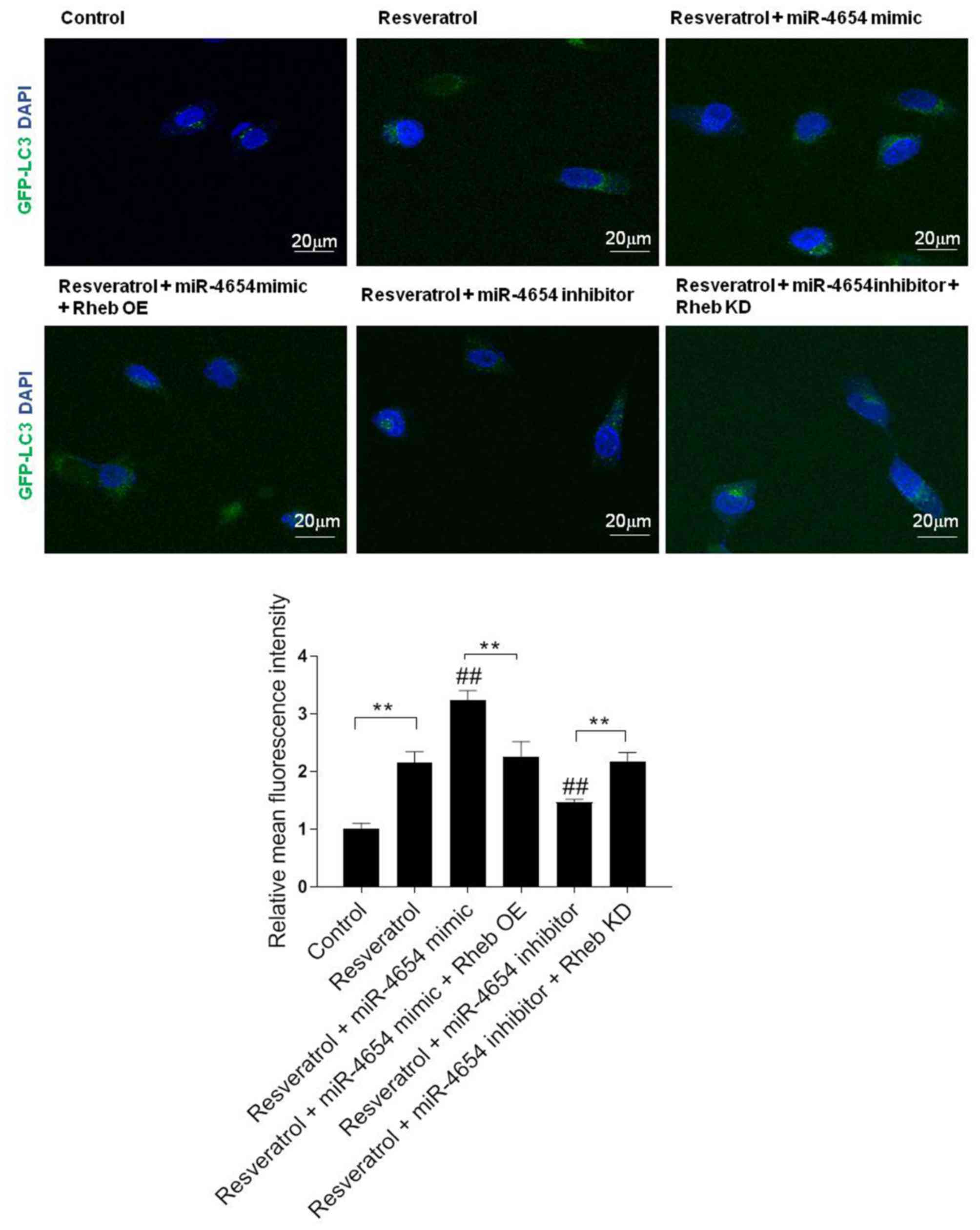 | Figure 6.miR-4654 promotes the formation of
autophagosomes in HSFBs following resveratrol treatment. HSFBs were
infected with adenoviruses expressing GFP-LC3 fusion protein to
obtain a stable GFP-LC3 cell line. Different
treatments/transfections were applied in the stable cell line,
including resveratrol treatment alone, resveratrol + miR-4654 mimic
transfection, resveratrol + miR-4654 mimic + Rheb OE transfection,
resveratrol + miR-4654 inhibitor transfection + Rheb KD,
resveratrol + miR-4654 inhibitor transfection and the control
group. After 72 h, DAPI was used to stain cells and the cells were
observed at a magnification of ×200 using confocal microscopy.
Scale bar=20 µm. The GFP-LC3 fluorescence intensity was normalized
against the control group and determined using Image J software.
Data are presented as the mean ± SD, n=3, **P<0.01;
##P<0.01 vs. the resveratrol group. LC3,
microtubule-associated protein 1A/1B-light chain 3; OE,
overexpression; KD, knockdown; Control, mimic-NC + inhibitor-NC +
PBS + vector group; HSFBs, hypertrophic scar-derived fibroblasts;
miR, microRNA. |
Discussion
Autophagy serves a vital role in sustaining cellular
metabolism; however, in certain cellular settings, autophagy is
also known to induce cell death (39,40).
Autophagy has also been identified to regulate the apoptotic
response (41). It is also
established that autophagy is an essential process for the
maintenance of cellular homeostasis (42). Furthermore, the association between
autophagy and HSs has been widely reported by an increasing number
of studies; for example, Shi et al (43) compared the autophagic capacity of
HSs and normal skin and discovered that the generation of LC3 is
prevented in HSs, which was suggested to benefit HSs formation. In
addition, Shi et al (44)
demonstrated that WT p53-modulated autophagy and autophagic-induced
fibroblast apoptosis suppresses the formation of HSs in a rabbit
ear model. Moreover, Shi et al (45) reported that interleukin-10 inhibits
autophagy in HSFBs under starvation stress to reduce HS formation.
These findings suggested that autophagy may be involved in the
proliferation and survival of HSFBs (46). The present study demonstrated that
resveratrol could efficiently trigger HSFBs autophagy, as evidenced
by the increased LC3-II/LC3-I ratio and upregulated expression
level of Beclin 1, which are the markers of autophagy. The most
marked effects were observed in the cells with 100 µmol/l
resveratrol treatment for 72 h, in which the cell viability was
seriously impaired. In addition, using the common inhibitor of the
autophagic pathway 3-MA, it was found that inhibition of autophagy
could significantly reverse the effect of resveratrol treatment. It
was hypothesized that high level of autophagy in HSFBs might be
essential to inhibit cell viability.
The present study also demonstrated that resveratrol
treatment markedly reduced the expression levels of Rheb in a
dose-dependent manner. Rheb has been identified as a critical
regulator of autophagy during the disease state; a previous study
has reported its ability to activate autophagy, thereby leading to
its own inactivation via the mTOR complex 1 pathway (37). Another study suggested that
reduction of Rheb can initiate autophagy in macrophages with
increased Beclin1 and autophagy-related protein 7 (47). These finding suggest that
inhibition of Rheb may be important to suppress HSFBs proliferation
and induce autophagy, which was consistent with the present
study.
An increasing number of miRNAs have been reported to
serve as essential components of multiple pathophysiological and
biomechanical processes (38,48,49).
HSs have been discovered to be associated with abnormal changes in
the expression level of multiple miRNAs, including the upregulation
of miRNAs such as miR-30A-5p and miR-152, and the downregulation of
miRNAs such as miR-143-5p and miR-4328 (50,51).
In addition, miRNAs have been identified to affect the
proliferation and apoptosis of fibroblasts, as well as
extracellular matrix deposition (52,53).
The roles of numerous miRNAs have been reported in both biological
and clinical processes, although the roles for the majority of
miRNAs have yet to be elucidated. The present study revealed that
the expression levels of miR-4654 were significantly downregulated
in HSFBs as compared to the NSFBs. While its level was
significantly increased in the cells following the treatment with
different concentrations of resveratrol. In addition, the
expression levels of miR-4654 were closely associated with the
degree of autophagy, which is consistent with the findings of a
previous study (54). In the
present study, overexpression of miR-4654 notably inhibited Rheb
expression at the protein level and transfection of the miR-4654
inhibitor markedly increased the level of Rheb when compared with
the control group. Subsequently, the current study further
investigated the effect of miR-4654 and Rheb on the autophagic
process. Results from bioinformatics analysis and a dual-luciferase
reporter assay suggested that miR-4654 may directly inhibit the
translation of Rheb by targeting its 3′-UTR region. Restoring the
Rheb expression reversed the cell viability inhibition and
autophagy initiation of HFSBs induced by miR-4654 overexpression.
Similarly, suppressing the Rheb expression in Rheb-depleted cells
inhibited the cell viability and re-enhanced cell autophagy as
compared with the miR-4654 inhibitor group. Accordingly, these data
indicated that resveratrol induced HFSBs autophagy might be
regulated by upregulation of miR-4654 which in turn suppressed
Rheb.
Finally, co-treatment with miR-4654 mimic further
suppressed cell viability and enhanced cell autophagy in
resveratrol-treated HFSBs. When the cells were treated with
miR-4654 inhibitor prior to resveratrol administration, a higher
cell viability and lower degree of autophagy were observed compared
with the resveratrol treated group. By contrast, dysregulation of
Rheb reversed the function of resveratrol and miR-4654 inhibitor
treatment, and demonstrated no effect on the role of miR-4654 mimic
treatment upon the resveratrol administration. The hypothesis of
the present study was further verified by the fluorescence assay,
which revealed that a higher number of autophagosomes were present
following the transfection with the miR-4654 mimic and resveratrol
treatment, whereas knocking down miR-4654 expression inhibited the
fluorescence intensity of LC3 in HFSBs.
In conclusion, the findings of the present study
suggested that resveratrol treatment may promote autophagy by
upregulating miR-4654 expression levels, and thus downregulating
the expression levels of the downstream gene, Rheb. However, the
findings of the current study are limited due to the fact that the
effect of resveratrol treatment on NSFBs was not investigated,
which may limit the clinical impact of these results. However,
despite this limitation, the findings still shed light on the
molecular mechanism underlying the miR-4654-mediated activation of
autophagy and the results suggested that miR-4654 may be a
potential biomarker and therapeutic target for the treatment and
diagnosis of HSs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Fund (grant no. 81774089), The Jiangsu Province,
The Medical Innovation Team (grant no. CXTDA2017048) and Xuzhou
Clinical Technology Diaphysis Training Program (grant no.
2019GG005).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KP, BL, ZT, WY, LH, ZS, JZ, LC, RL, YL and QL made
substantial contributions to the conception and design of the
study, and acquired, analyzed and interpreted the data. LH, JZ, LC,
RL, YL, and QL also contributed to drafting the manuscript and
revising it critically for intellectual content. JD and CH made
substantial contributions to the conception and design of the
study, gave final approval of the version to be published and
agreed to be accountable for the work in ensuring that questions
related to the integrity of any part of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee HJ and Jang YJ: Recent Understandings
of biology, prophylaxis and treatment strategies for hypertrophic
scars and keloids. Int J Mol Sci. 19:34402018.
|
|
2
|
Zhu Z, Ding J, Shankowsky HA and Tredget
EE: The molecular mechanism of hypertrophic scar. J Cell Commun
Signal. 7:239–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Zhang Y, Jiang BH, Zhang Q, Zhou
RP, Zhang L and Wang C: Study on the role of Hsa-miR-31-5p in
hypertrophic scar formation and the mechanism. Exp Cell Res.
361:201–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alster T: Laser scar revision: Comparison
study of 585-nm pulsed dye laser with and without intralesional
corticosteroids. Dermatol Surg. 29:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wittenberg GP, Fabian BG, Bogomilsky JL,
Schultz LR, Rudner EJ, Chaffins ML, Saed GM, Burns RL and Fivenson
DP: Prospective, single-blind, randomized, controlled study to
assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser
and silicone gel sheeting in hypertrophic scar treatment. Arch
Dermatol. 135:1049–1055. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Hori K, Ding J, Huang Y, Kwan P,
Ladak A and Tredget EE: Toll-like receptors expressed by dermal
fibroblasts contribute to hypertrophic scarring. J Cell Physiol.
226:1265–1273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Armour A, Scott PG and Tredget EE:
Cellular and molecular pathology of HTS: Basis for treatment. Wound
Repair Regen. 15 (Suppl 1):S6–S17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, He QY and Luo CQ: Overexpression of
miR-200b inhibits the cell proliferation and promotes apoptosis of
human hypertrophic scar fibroblasts in vitro. J Dermatol.
41:903–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Wang J and Yang X: Functions of
autophagy in pathological cardiac hypertrophy. Int J Biol Sci.
11:672–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu H, Wang Y, Wang X, Li R and Yin D:
MicroRNA-365 accelerates cardiac hypertrophy by inhibiting
autophagy via the modulation of Skp2 expression. Biochem Biophys
Res Commun. 4:304–310. 2017. View Article : Google Scholar
|
|
12
|
Goutas A, Syrrou C, Papathanasiou I,
Tsezou A and Trachana V: The autophagic response to oxidative
stress in osteoarthritic chondrocytes is deregulated. Free Radical
Biol Med. 126:122–132. 2018. View Article : Google Scholar
|
|
13
|
Xu J, Camfield R and Gorski SM: The
interplay between exosomes and autophagy-partners in crime. J Cell
Sci. 131:jcs2152102018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Huang C, He Y, Sang Z, Liu G and Dai
H: Knockdown of long non-coding RNA GAS5 increases miR-23a by
targeting ATG3 involved in autophagy and cell viability. Cell
Physiol Biochem. 48:1723–1734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun K, Deng W, Zhang S, Cai N, Jiao S,
Song J and Wei L: Paradoxical roles of autophagy in different
stages of tumorigenesis: Protector for normal or cancer cells. Cell
Bio Sci. 3:352013.
|
|
16
|
Pabon MA, Ma KC and Choi AM: Autophagy and
obesity-related lung disease. Am J Respir Cell Mol Biol.
54:636–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo L and Qin ZH: Autophagy, aging, and
longevity. Adv Exp Med Biol. 1206:509–525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv L, Lin K, Gao W, He ZL, Gao ZM, Li ZF
and Li JJ: Starvation induces autophagy of hypertrophic scar
fibroblasts. Chin J Pathophysiology. 29:330–333. 2013.
|
|
19
|
Xu J, Zhao J, Evan G, Xiao C, Cheng Y and
Xiao J: Circulating microRNAs: Novel biomarkers for cardiovascular
diseases. J Mol Med (Berl). 90:865–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alevizos I and Illei GG: MicroRNAs as
biomarkers in rheumatic diseases. Nat Rev Rheumatol. 6:391–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Bei Y, Huang P, Zhou Q, Shi J, Sun
Q, Zhong J, Li X, Kong X and Xiao J: Inhibition of miR-155 protects
against LPS-induced cardiac dysfunction and apoptosis in mice. Mol
Ther Nucleic Acids. 5:e3742016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Behm-Ansmant I, Rehwinkel J and Izaurralde
E: MicroRNAs silence gene expression by repressing protein
expression and/or by promoting mRNA decay. Cold Spring Harb Symp
Quant Biol. 71:523–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi J, Bei Y, Kong X, Liu X, Lei Z, Xu T,
Wang H, Xuan Q, Chen P, Xu J, et al: miR-17-3p contributes to
exercise-induced cardiac growth and protects against myocardial
ischemia-reperfusion injury. Theranostics. 7:664–676. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv J, Yang L, Guo R, Shi Y, Zhang Z and Ye
J: Ox-LDL-induced MicroRNA-155 promotes autophagy in human
endothelial cells via repressing the Rheb/mTOR pathway. Cell
Physiol Biochem. 43:1436–1448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang P, Zhang J, Zhang L, Zhu Z, Fan J,
Chen L, Zhuang L, Luo J, Chen H, Liu L, et al: MicroRNA 23b
regulates autophagy associated with radioresistance of pancreatic
cancer cells. Gastroenterology. 145:1133–1143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Zhou R, Zhang Q, Jiang B, Wu Q and
Wang C: Fibroproliferative effect of microRNA-21 in hypertrophic
scar derived fibroblasts. Exp Cell Res. 339:360–366.
2015.PubMed/NCBI
|
|
27
|
Zhang Q, Guo B, Hui Q, Chang P and Tao K:
miR-137 inhibits proliferation and metastasis of hypertrophic scar
fibroblasts via targeting pleiotrophin. Cell Physiol Biochem.
49:985–995. 2018. View Article : Google Scholar
|
|
28
|
Xu XH, Ding DF, Yong HJ, Dong CL, You N,
Ye XL, Pan ML, Ma JH, You Q and Lu YB: Resveratrol
transcriptionally regulates miRNA-18a-5p expression ameliorating
diabetic nephropathy via increasing autophagy. Eur Rev Med
Pharmacol Sci. 21:4952–4965. 2017.PubMed/NCBI
|
|
29
|
Han Y, Jo H, Cho JH, Dhanasekaran DN and
Song YS: Resveratrol as a tumor-suppressive nutraceutical
modulating tumor microenvironment and malignant behaviors of
cancer. Int J Mol Sci. 20:9252019. View Article : Google Scholar
|
|
30
|
Jimenez-Gomez Y, Mattison JA, Pearson KJ,
Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM,
Lewis K, Allard JS, et al: Resveratrol improves adipose insulin
signaling and reduces the inflammatory response in adipose tissue
of rhesus monkeys on high-fat, high-sugar diet. Cell Metab.
18:533–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kou X and Chen N: Resveratrol as a natural
autophagy regulator for prevention and treatment of Alzheimer's
disease. Nutrients. 9:9272017. View Article : Google Scholar
|
|
32
|
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X,
Liu CG and Yang JM: Regulation of autophagy by a Beclin 1-targeted
microRNA, miR-30a, in cancer cells. Autophagy. 6:816–623. 2009.
View Article : Google Scholar
|
|
33
|
Mehta M, Branford OA and Rolfe KJ: The
evidence for natural therapeutics as potential anti-scarring agents
in burn-related scarring. Burns Trauma. 4:152016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sciarretta S, Zhai P, Shao D, Maejima Y,
Robbins J, Volpe M, Condorelli G and Sadoshima J: Rheb is a
critical regulator of autophagy during myocardial ischemia:
Pathophysiological implications in obesity and metabolic syndrome.
Circulation. 125:1134–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng G, Zhong F, Li J, Luo S and Zhang P:
Resveratrol-mediated reduction of collagen by inhibiting
proliferation and producing apoptosis in human hypertrophic scar
fibroblasts. Biosci Biotechnol Biochem. 77:2389–2396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai XZ, Liu JQ, Yang LL, Fan L, He T, Su
LL, Shi JH, Tang CW, Zheng Z and Hu DH: Identification of sirtuin 1
as a promising therapeutic target for hypertrophic scars. Br J
Pharmacol. 173:1589–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Braunwald E: The war against heart
failure: The Lancet lecture. Lancet. 385:812–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Doherty J and Baehrecke EH: Life, death
and autophagy. Nat Cell Biol. 20:1110–1117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi JH, Hu DH, Zhang ZF, Bai XZ, Wang HT,
Zhu XX, Su YJ and Tang CW: Reduced expression of
microtubule-associated protein 1 light chain 3 in hypertrophic
scars. Arch Dermatol Res. 304:209–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi J, Xiao H, Li J, Zhang J, Li Y, Zhang
J, Wang X, Bai X, Tao K, Hu D and Guan H: Wild-type p53-modulated
autophagy and autophagic fibroblast apoptosis inhibit hypertrophic
scar formation. Lab Invest. 98:1423–1437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi J, Wang H, Guan H, Shi S, Li Y, Wu X,
Li N, Yang C, Bai X, Cai W, et al: IL10 inhibits starvation-induced
autophagy in hypertrophic scar fibroblasts via cross talk between
the IL10-IL10R-STAT3 and IL10-AKT-mTOR pathways. Cell Death Dis.
7:e21332016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Felice B, Garbi C, Santoriello M,
Santillo A and Wilson RR: Differential apoptosis markers in human
keloids and hypertrophic scars fibroblasts. Mol Cell Biochem.
327:191–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Babuta M, Furi I, Bala S, Bukong TN, Lowe
P, Catalano D, Calenda C, Kodys K and Szabo G: Dysregulated
autophagy and lysosome function are linked to exosome production by
Micro-RNA 155 in Alcoholic liver disease. Hepatology. 70:2123–2141.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen LJ, Wei SY and Chiu JJ: Mechanical
regulation of epigenetics in vascular biology and pathobiology. J
Cell Mol Med. 17:437–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Donaldson CJ, Lao KH and Zeng L: The
salient role of microRNAs in atherogenesis. J Mol Cell Cardiol.
122:98–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu ZY, Lu L, Liang J, Guo XR, Zhang PH and
Luo SJ: Keloid microRNA expression analysis and the influence of
miR-199a-5p on the proliferation of keloid fibroblasts. Genet Mol
Res. 13:2727–2738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li C, Bai Y, Liu H, Zuo X, Yao H, Xu Y and
Cao M: Comparative study of microRNA profiling in keloid fibroblast
and annotation of differential expressed microRNAs. Acta Biochim
Biophys Sin (Shanghai). 45:692–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kozlova A, Pachera E, Maurer B, Jüngel A,
Distler JHW, Kania G and Distler O: Regulation of fibroblast
apoptosis and proliferation by MicroRNA-125b in systemic sclerosis.
Arthritis Rheumatol. 71:2068–2080. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang C, Zhang Y, Zhu H, Hu J and Xie Z:
MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat
cardiac fibroblasts and extracellular matrix deposition in vivo and
in vitro. Cell Signal. 46:145–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jing Z, Han W, Sui X, Xie J and Pan H:
Interaction of autophagy with microRNAs and their potential
therapeutic implications in human cancers. Cancer Lett.
356:332–338. 2015. View Article : Google Scholar : PubMed/NCBI
|