Introduction
Breast cancer is by far the most common malignant
tumor in women. In 2018, there were 2,088,849 new breast cases
(11.6% of the total cancer cases) and 626,679 deaths (6.6% of the
total cancer deaths) worldwide (1).
Surgery, radiotherapy, chemotherapy, and molecular targeted
therapies are currently used for breast cancer treatment; however,
effective therapies for patients diagnosed with triple-negative
breast cancer [TNBC; i.e., those that are negative for estrogen
receptor (ER), progesterone receptor (PR), and human epidermal
growth factor receptor 2 (HER2)] remain limited (2–4). TNBC
accounts for ~15% of invasive breast cancers; moreover, it tends to
be aggressive and is associated with a poor prognosis (2,5,6). TNBC
is more common in young women than in older women and is frequently
associated with invasion and metastatic disease (2,5–7). As
such, highly sensitive and specific monoclonal antibodies (mAbs)
are required to facilitate the diagnosis of and treatment decisions
for this breast cancer subtype.
The trophoblast cell-surface antigen (TROP2), also
known as human tumor-associated calcium signal transducer
(TACSTD2), is a type I transmembrane glycoprotein originally
identified in human trophoblast cells (8–10).
Previously, Schon and Orfanos reported that tunicamycin treatment
of living cells and N-glycanase digestion of immunopurified
TROP2 revealed that the molecular heterogeneity of TROP2 is due to
the different N-glycosylation in normal and transformed
keratinocytes (11). In transformed
keratinocytes, two distinct precursor proteins at 38 and 42 kDa
were detected, whereas in normal cells the 38-kDa signal was
dramatically decreased, indicating that quantitative and
qualitative changes of N-glycan of TROP2 are associated with
the transformation process of human keratinocytes. TROP2 is highly
expressed in several cancers and may play a critical role in tumor
progression in association with the pathways involving both the
extracellular signal-related kinase (ERK) and c-Jun N-terminal
kinase (JNK) (12,13). The expression of TROP2 has been
reported in more than 85% of all tumors; as such, TROP2 may be a
useful marker for cancer diagnosis and immunotherapy (2,14,15).
It has also been identified in the stem cells of various tissues,
including basal cells, all of which are capable of self-renewal,
regeneration, and differentiation (2,16,17).
Several mAbs targeting TROP2 are currently evaluated in clinical
trials, including PF-06664178 (12,18),
IMMU-132 (12,19,20),
and DS-1062a (12,21).
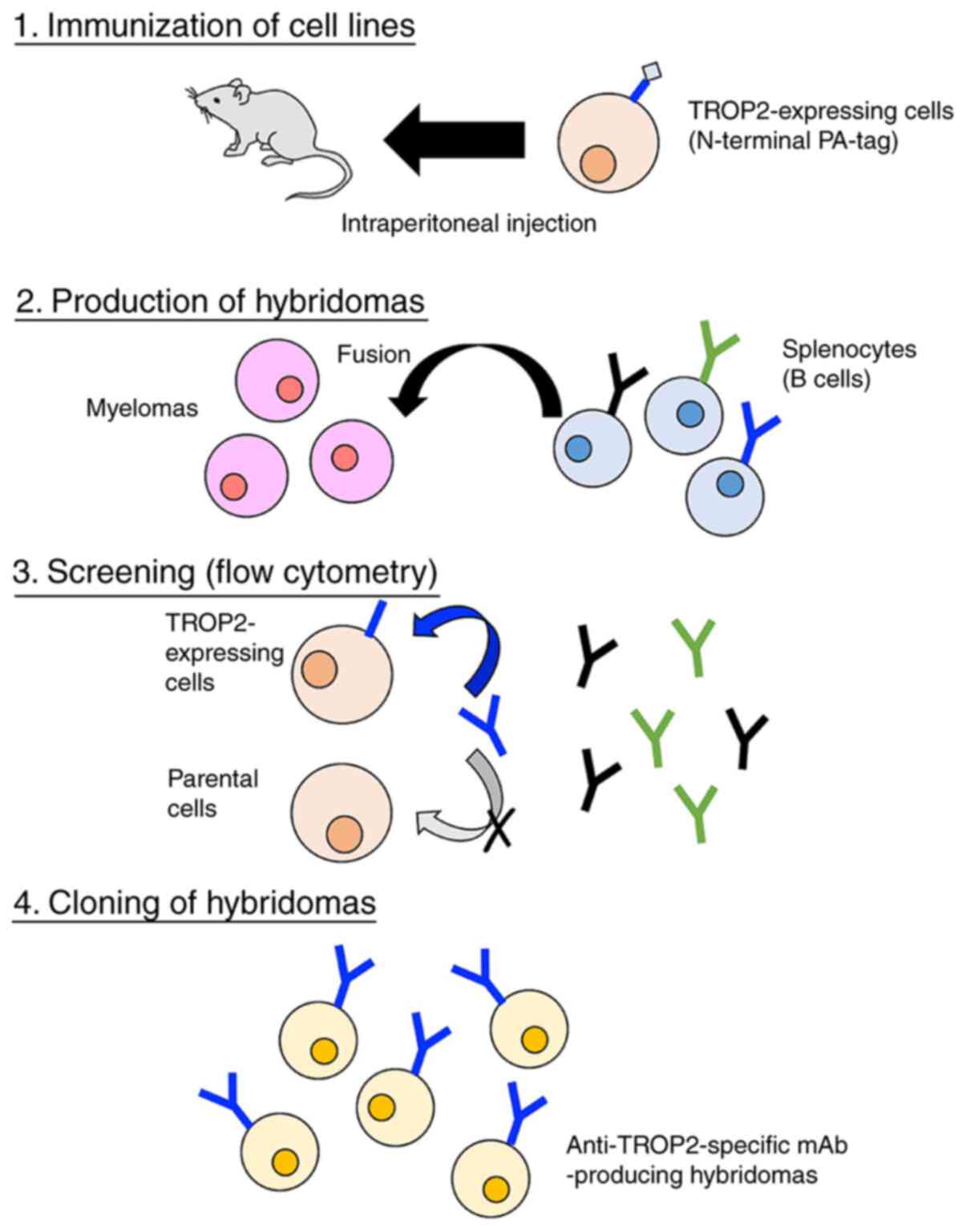
In our previous studies, we developed the Cell-Based
Immunization and Screening (CBIS) method; in this method, cell
lines are used exclusively for both immunization and screening
(22). CBIS has been employed to
develop sensitive and specific mAbs against numerous transmembrane
proteins, including CD19 (23),
CD20 (24), CD44 (25), CD133 (22), and PD-L1 (26). Of note, mAbs developed using this
method have proven to be extremely useful in flow cytometry,
Western blot, and immunohistochemical analyses.
In this study, we developed novel anti-TROP2 mAbs
and evaluated their capacity to target breast cancer cells using
flow cytometry, Western blot, and immunohistochemical analyses.
Materials and methods
Plasmid preparation
Human TROP2 DNA was synthesized commercially by
Thermo Fisher Scientific (Waltham, MA, USA). TROP2 DNA with an
N-terminal PA16 tag (27) and a
C-terminal RAP tag (28)/MAP tag
(29) (PA16-TROP2-RAP-MAP) was
subcloned into the pCAG-Ble expression vector (FUJIFILM Wako Pure
Chemical Corporation) using an In-Fusion HD Cloning Kit (Takara
Bio, Inc.); the recombinant expression vector was named
pCAG/PA16-TROP2-RAP-MAP. TROP2 DNA with a C-terminal PA tag
(27) alone was also subcloned into
the pCAG-Ble vector using an In-Fusion HD Cloning Kit; this
expression vector was named pCAG/TROP2-PA. The amino acid sequences
of each tag are as follows: PA16 tag, 16 amino acids
(GLEGGVAMPGAEDDVV); PA tag, 12 amino acids (GVAMPGAEDDVV); RAP tag,
12 amino acids (DMVNPGLEDRIE); and MAP tag, 12 amino acids
(GDGMVPPGIEDK).
Cell lines
Chinese hamster ovary (CHO)-K1, P3X63Ag8U.1 (P3U1),
BT-474, Lec1, Lec2, and Lec8 cell lines were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). MCF7
was obtained from the Cell Resource Center for Biomedical Research,
Institute of Development, Aging and Cancer, Tohoku University
(Miyagi, Japan).
CHO-K1 cells that overexpress TROP2-PA
(CHO/TROP2-PA) and PA16-TROP2-RAP-MAP (CHO/PA16-TROP2-RAP-MAP) were
generated by transfection of pCAG/TROP2-PA and
pCAG/PA16-TROP2-RAP-MAP to CHO-K1 cells, respectively, using
Lipofectamine LTX Reagent (Thermo Fisher Scientific, Inc.). Cell
lines Lec1/TROP2, Lec2/TROP2, and Lec8/TROP2 were generated by
transfection of pCAG/TROP2-PA to Lec1, Lec2, and Lec8 cells,
respectively, using the Neon Transfection System (Thermo Fisher
Scientific, Inc.). Several days after the transfection, the
transfected cells were confirmed as TROP2-positive by flow
cytometry (EC800, Sony Corp.) using a commercial anti-TROP2
antibody (Cat#LS-C489657, LS Bio). The transfected cells were
selected by limiting dilution culture and cultivation in the medium
containing 0.5 mg/ml of zeocin (InvivoGen). We confirmed the
transfection efficiency using western blotting.
The TROP2 gene-deleted cell line, MCF7/TROP2-KO
(BINDS-29), was generated by transfection of CRISPR/Cas9 plasmids
targeting TROP2 using the Neon Transfection System (Thermo Fisher
Scientific, Inc.). Stable transfectants were established by cell
sorting using SH800 (Sony Corp.).
CHO-K1, CHO/PA16-TROP2-RAP-MAP, CHO/TROP2-PA, P3U1,
MCF7, Lec1/TROP2, Lec2/TROP2, Lec8/TROP2, and BINDS-29 cells were
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(Nacalai Tesque, Inc., Kyoto, Japan); BT-474 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Inc.).
All media were supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin,
100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B (Nacalai
Tesque, Inc.). Cells were grown in an incubator at 37°C with
humidity and 5% CO2 and 95% air atmosphere.
Animals
Female BALB/c mice (6 weeks old) were purchased from
CLEA Japan and kept under specific pathogen-free conditions. All
animal experiments were conducted in accordance with the relevant
guidelines and regulations in order to minimize animal suffering
and distress in the laboratory. The Animal Care and Use Committee
of Tohoku University approved all the animal experiments (permit
no. 2019NiA-001). Mice were euthanized by cervical dislocation
under inhalation anesthesia using 2% of isoflurane, and the death
was verified to be respiratory and cardiac arrest.
Hybridoma production
We employed CBIS to develop new mAbs against TROP2.
Two mice were immunized with CHO/PA16-TROP2-RAP-MAP cells
(1×108) via the intraperitoneal route (i.p.)
together with the Imject Alum (Thermo Fisher Scientific, Inc.).
After several additional immunizations, a booster immunization was
administered via the i.p. route 2 days before spleen cell
collection. Mice were euthanized by cervical dislocation under
inhalation anesthesia using isoflurane, and the death was verified
to be respiratory and cardiac arrest. We chopped spleens, and
collected spleen cells using serum-free RPIM-1640 medium. We
further broke the red blood cells with 3 ml of Red Blood Cell
Lysing Buffer Hybri-Max (Sigma-Aldrich Corp.) at 37°C for 1 min,
and washed the spleen cells using serum-free RPIM-1640 medium. The
collected spleen cells were fused with P3U1 mouse myeloma cells
using polyethylene glycol 1500 (Roche Diagnostics) (30,31);
the resulting hybridomas were selected in RPMI medium, including
hypoxanthine, aminopterin, and thymidine (Thermo Fisher Scientific,
Inc.). The culture supernatants were screened via flow
cytometry using CHO/TROP2-PA and CHO-K1 cells.
Flow cytometry
Cells were collected following a brief exposure to
0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA;
Nacalai Tesque, Inc.). The cells were washed with 0.1% bovine serum
albumin in phosphate-buffered saline (PBS) and treated with
anti-TROP2 mAbs, such as TrMab-6 (1 µg/ml) or EPR20043 (1/60
dilution; Abcam) for 30 min at 4°C. After incubation, the cells
were treated with Alexa Fluor 488-conjugated anti-mouse IgG
(1:1,000; Cell Signaling Technology, Inc.) or Alexa Fluor
488-conjugated anti-rabbit IgG (1:1,000; Cell Signaling Technology,
Inc.). Fluorescence data were collected using SA3800 Spectral Cell
Analyzer (Sony Corp.) and analyzed using FlowJo (BD
Biosciences).
Determination of the binding
affinity
MCF7 or BT-474 cells (2×105) were
suspended in 100 µg of serially diluted TrMab-6 (6 ng/ml-100 µg/ml)
for 30 min at 4°C, followed by the addition of Alexa Flour
488-conjugated anti-mouse IgG (1:200; Cell Signaling Technologies,
Inc.). Fluorescence data were collected using a cell analyzer
(EC800). The dissociation constant (KD) was
calculated by fitting the binding isotherms to built-in, one-site
binding models in GraphPad Prism 8 (GraphPad Software, Inc.).
Western blot analysis
Cell lysates (10 µg) were boiled in sodium dodecyl
sulfate (SDS) sample buffer (Nacalai Tesque, Inc.). Proteins were
separated on 5–20% polyacrylamide gels (FUJIFILM Wako Pure Chemical
Corporation) and transferred onto polyvinylidene difluoride (PVDF)
membranes (Merck KGaA). After blocking with 4% skim milk (Nacalai
Tesque, Inc.) in PBS with 0.05% Tween-20, the membranes were
incubated with 1 or 5 µg/ml of TrMab-6, 1/2000 dilution of EPR20043
(Abcam), 1 µg/ml of NZ-1 (anti-PA tag), or 1 µg/ml of anti-β-actin
(clone AC-15; Sigma-Aldrich Corp.). This was followed by incubation
with peroxidase-conjugated anti-mouse immunoglobulins (Agilent
Technologies Inc.; diluted 1:1,000) to detect TrMab-6 and
anti-β-actin, peroxidase-conjugated anti-rabbit immunoglobulins
(Agilent Technologies Inc.; diluted 1:1,000) to detect EPR20043, or
anti-rat IgG (Sigma-Aldrich Corp; diluted 1:10,000) to detect NZ-1,
respectively. Finally, protein bands were detected with ImmunoStar
LD (FUJIFILM Wako Pure Chemical Corporation) using a Sayaca-Imager
(DRC Co. Ltd.).
Immunohistochemical analysis
Paraffin-embedded tissue sections of the breast
cancer tissue array (Cat#T8235721-5, Lot#B104066; BioChain, San
Francisco, CA, USA) were autoclaved in EnVision FLEX Target
Retrieval Solution High pH (Agilent Technologies, Inc.) for 20 min.
After blocking with SuperBlock T20 (Thermo Fisher Scientific,
Inc.), tissue sections were incubated with TrMab-6 (5 µg/ml) or
EPR20043 (1/500 dilution; Abcam) for 1 h at room temperature and
then treated with the EnVision+ Kit for mouse (Agilent Technologies
Inc.) and EnVision+ Kit for rabbit (Agilent Technologies Inc.) for
30 min, respectively. Color was developed using
3,3′-diaminobenzidine tetrahydrochloride (DAB; Agilent Technologies
Inc.) for 2 min. Counterstaining was performed with hematoxylin
(FUJIFILM Wako Pure Chemical Corporation).
Results
Development of novel anti-TROP2 mAbs
using the CBIS method
We immunized two mice with CHO/PA16-TROP2- RAP-MAP
cells and anti-TROP2 mAbs were screened via flow cytometry
(Fig. 1). The first screening
approach identified strong signals from CHO/TROP2-PA cells and weak
to no signals from CHO-K1 cells using hybridoma supernatants from
90 of the 956 wells (9.4%). The second screening approach
identified strong signals from MCF7 cells from 84 of the 90
hybridoma supernatants identified in the earlier step (93.3%).
After limiting dilution, we established 30 positive clones. Further
screening via Western blot and immunohistochemistry led to
the establishment of TrMab-6. The subclass of TrMab-6 was
determined to be mouse IgG2b as shown in Fig. S1A.
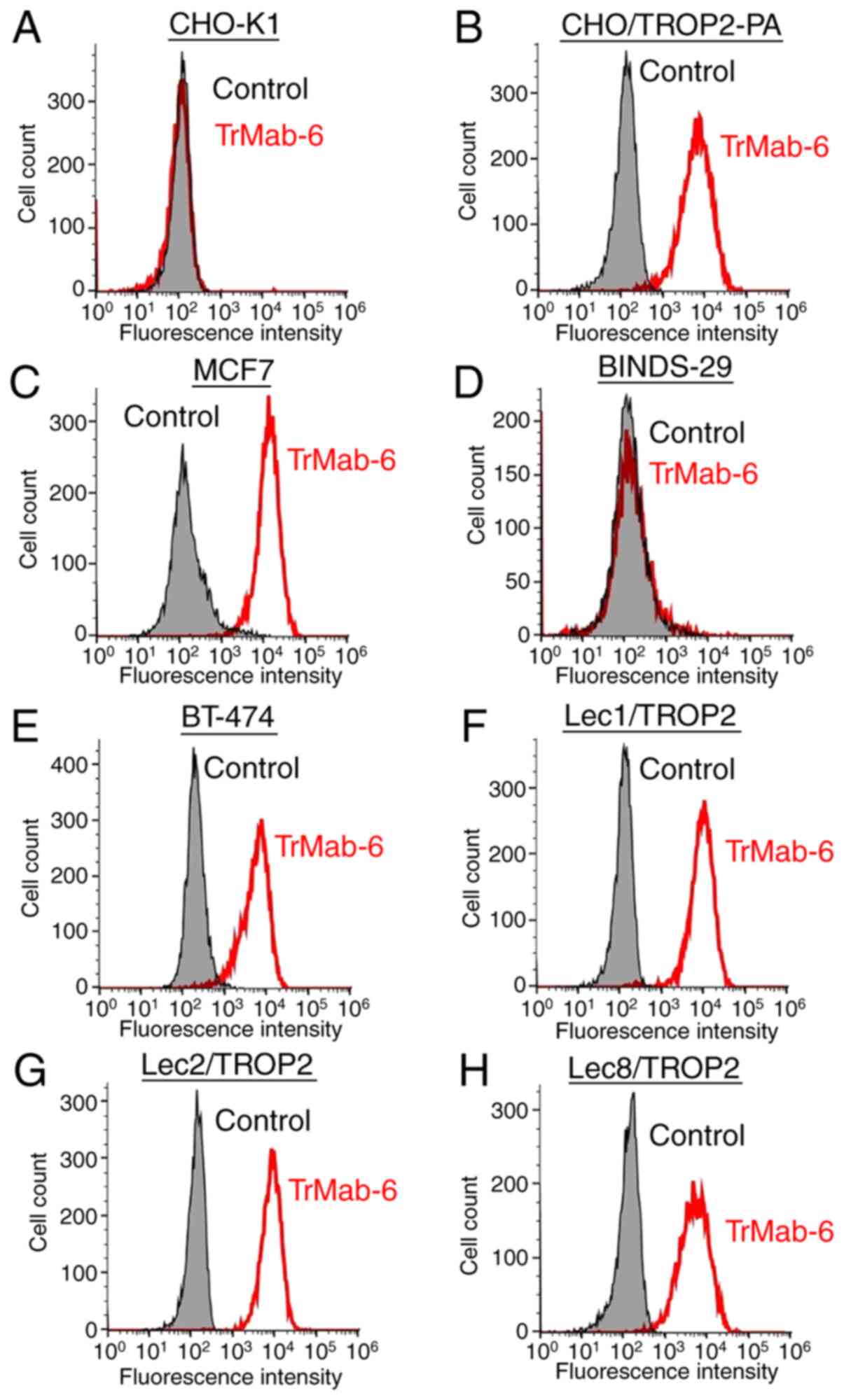
Flow cytometry analysis
We developed several transfectants, such as
CHO/TROP2-PA, Lec1/TROP2, Lec2/TROP2, and Lec8/TROP2, and the
transfection efficiency was confirmed using an anti-PA tag mAb
(NZ-1) by Western blot analysis (Fig.
S1B). Then, we performed flow cytometry targeting several
relevant cell lines in order to characterize antigen detection
using TrMab-6 (Fig. 2). TrMab-6
detected CHO/TROP2-PA cells, but not parental CHO-K1 cells. TrMab-6
also detected endogenous TROP2 on human breast cancer cell lines,
including MCF7 and BT-474. Contrarily, TrMab-6 did not react with
BINDS-29 (TROP2-gene-deleted MCF7 cells). Taken together, these
results suggested that TrMab-6 is specific for TROP2. As shown in
Fig. S2, another anti-TROP2 mAb
(clone EPR20043) weakly reacted with MCF7, but did not react with
BT-474 although TrMab-6 strongly reacted with both MCF7 and BT-474,
indicating that TrMab-6 is more useful for flow cytometry than
EPR20043 although EPR20043 was shown to be useful in all
applications, such as flow cytometry, Western blot, and
immunohistochemical analyses (Table
SI).
Next, we investigated whether the epitope of TrMab-6
is associated with glycans. Thus, we performed flow cytometry using
TROP2-transfected glycan-deficient CHO cells, including those
deficient in Lec1 (N-glycan-deficient), Lec2 (sialic
acid-deficient), and Lec8 (galactose-deficient) cells. As presented
in Fig. 2, TrMab-6 reacted with
Lec1/TROP2, Lec2/TROP2, and Lec8/TROP2 cells to an extent
indistinguishable from that observed with CHO/TROP2-PA. These
results indicated that the binding epitope recognized by TrMab-6
was unlikely to be associated with glycans.
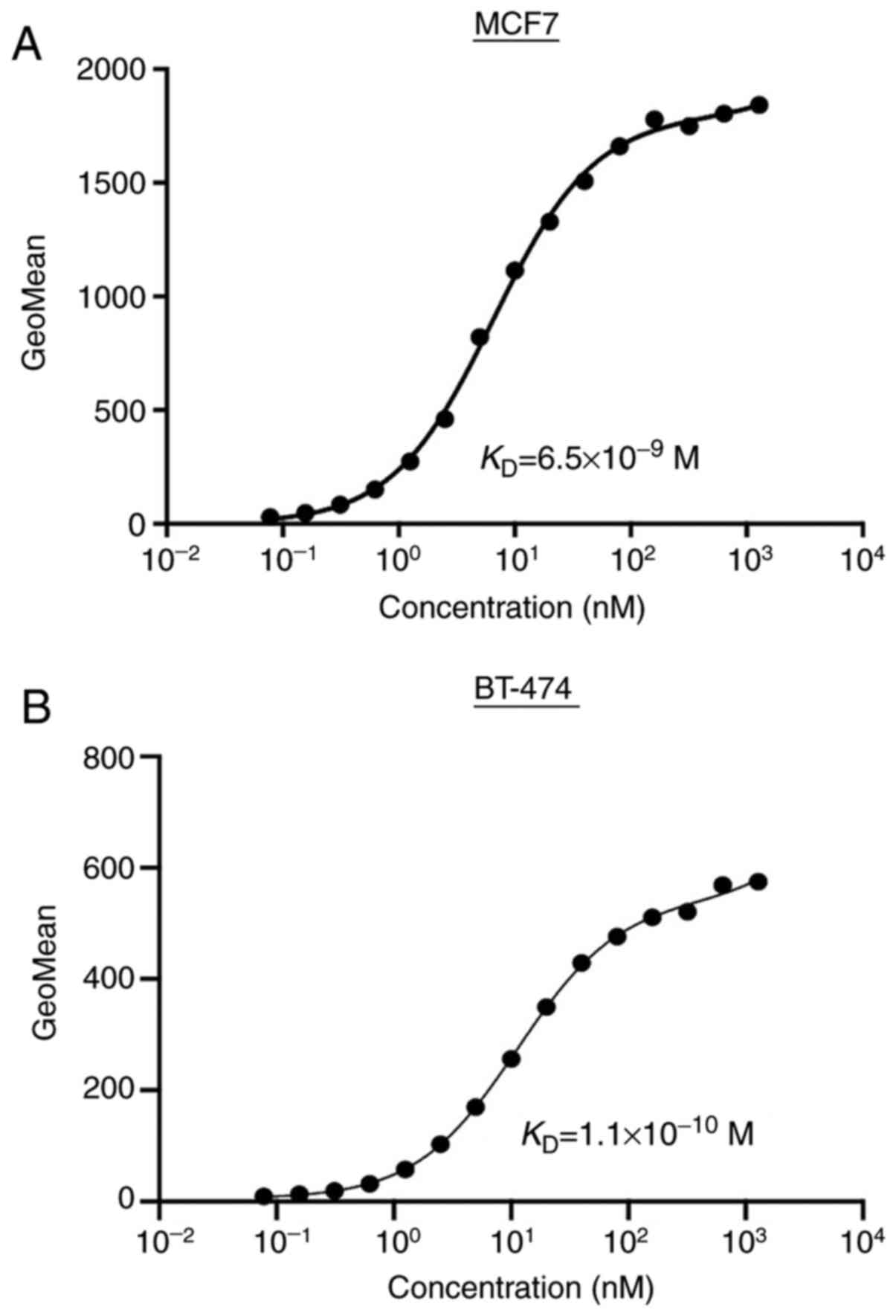
Determination of the binding affinity
using TrMab-6 against breast cancers by flow cytometry
To determine the binding affinity of TrMab-6, we
conducted kinetic analysis of the interaction of TrMab-6 with MCF7
and BT-474 cells via flow cytometry. The
KD of TrMab-6 was determined to be
6.5×10−9 M when targeting MCF7 cells and
1.1×10−10 M for BT-474 cells (Fig. 3). These results indicated that
TrMab-6 binds with high affinity to TROP2-expressing breast cancer
cells.
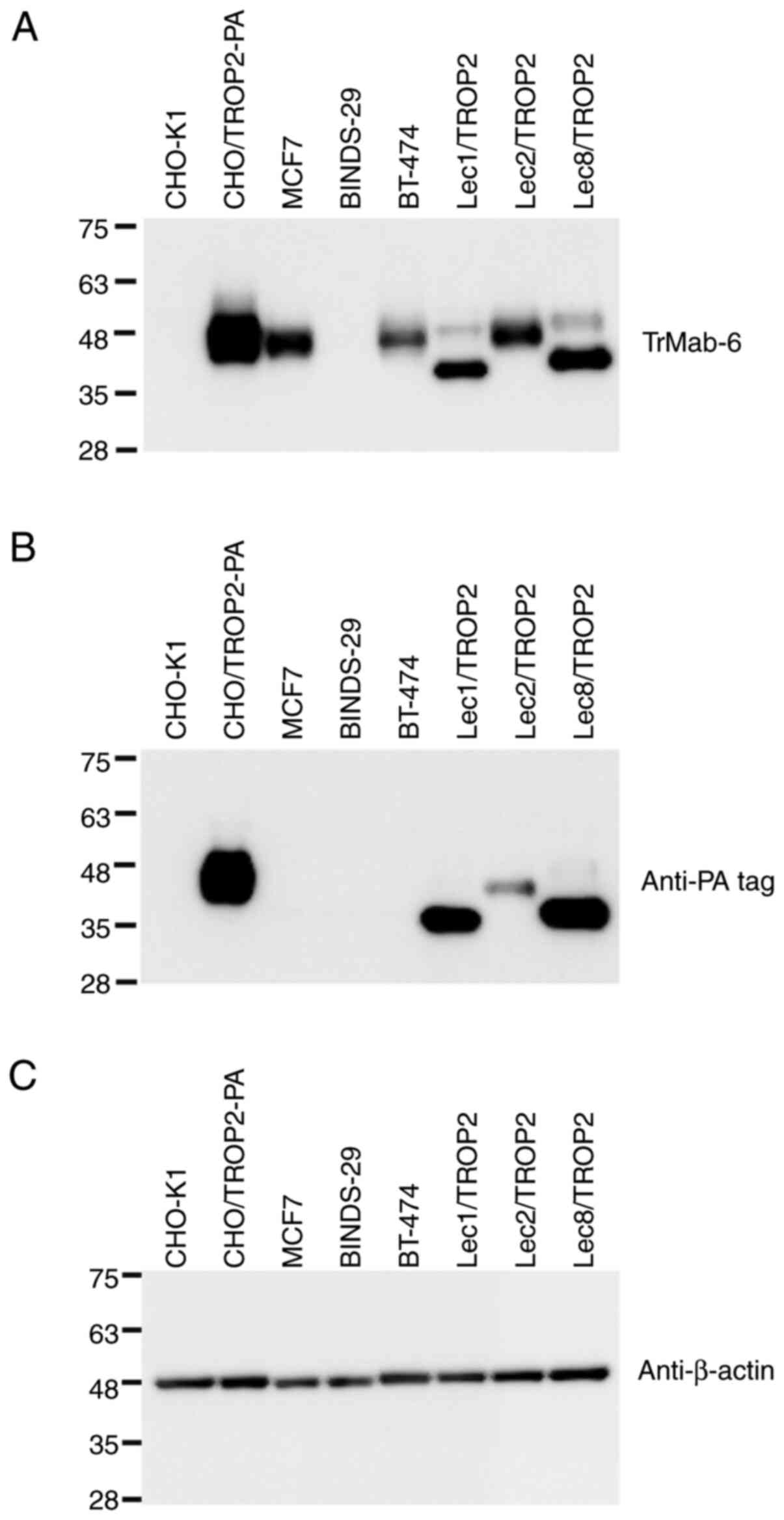
Western blot analyses
TrMab-6 binding identified TROP2 as an
immunoreactive band with an estimated 40 kDa band in lysates
prepared from CHO/TROP2-PA, MCF7, and BT-474 cells; no
immunoreactive bands were found in CHO-K1 and TROP2-gene-deleted
MCF7 (BINDS-29) cells (Fig. 4),
again confirming its specificity for TROP2. TrMab-6 also detected
TROP2 of Lec1/TROP2, Lec2/TROP2, and Lec8/TROP2. Although TROP2
proteins, which were expressed in Lec1/TROP2 and Lec8/TROP2, were
detected in lower molecular weight compared with CHO/TROP2-PA and
Lec2/TROP2, the intensity by TrMab-6 was similar among those cell
lines, indicating that the binding epitope of TrMab-6 is
independent of glycans. An anti-PA tag mAb (NZ-1) also detected
TROP2 bands in lysates of CHO/TROP2-PA, Lec1/TROP2, Lec2/TROP2, and
Lec8/TROP2 cells. These results indicated that TrMab-6 could be
used to detect TROP2 expressed by breast cancer cells via
Western blot.
We compared the reactivity of TrMab-6 and another
anti-TROP2 mAb (clone EPR20043) in Western blot analysis. As shown
in Fig. S3, both TrMab-6 and
EPR20043 strongly detected TROP2 from both MCF7 and BT-474,
indicating that both TrMab-6 and EPR20043 are useful for Western
blot analysis.
Immunohistochemical analyses against
breast cancer
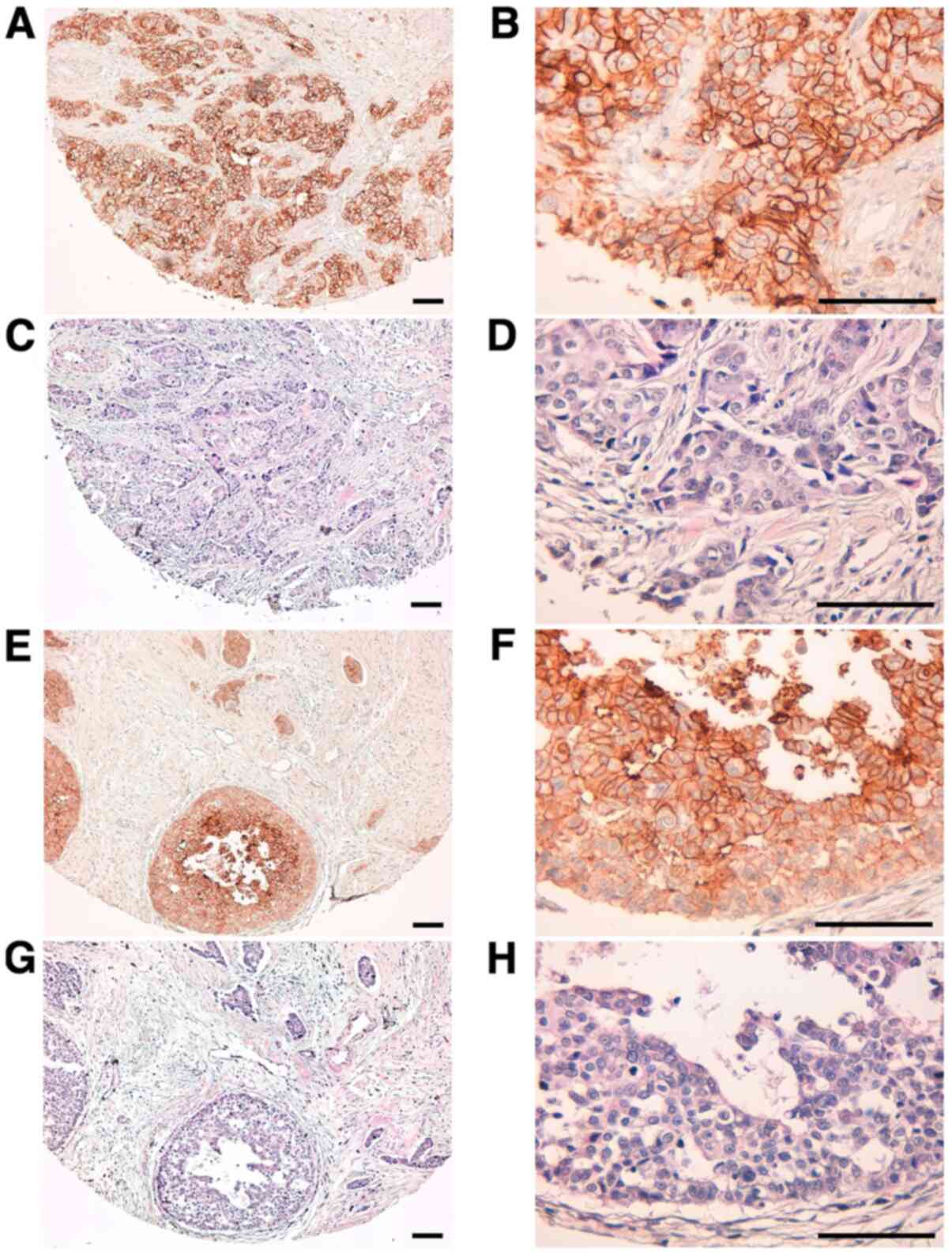
We then used TrMab-6 to target clinical specimens of
human breast cancer tissue via immunohistochemical analysis
(Table I). TrMab-6 detected TROP2
in 57/61 of the breast cancer specimens (93.4%; Table II). Among these specimens were
50/54 cases (93.1%) of invasive ductal carcinoma (Table II). Typical TrMab-6-associated
staining patterns in the specimens of invasive ductal carcinomas
are presented in Fig. 5A and B.
Hematoxylin and eosin (H&E) staining of invasive ductal
carcinoma tissue is presented in Fig.
5C and D. Furthermore, TrMab-6 detected TROP2 in 4/4 cases
(100%) of invasive lobular carcinoma, 2/2 cases (100%) of
adenocarcinoma, and 1/1 case (100%) of medullary carcinoma
(Table II); the typical staining
patterns of invasive lobular carcinoma are presented in Fig. 5E and F, and H&E staining was
performed as presented in Fig. 5G and
H. Among the 61 breast cancer cases, 30/61 cases (49.2%) were
stained strongly positive, 18/61 cases (29.5%) were stained
moderately positive, and 9/61 cases (14.8%) were stained weakly
positive by TrMab-6 (Table II). We
obtained the information about ER, PR, and HER2 (Table I). To determine HER2 expression, we
used an anti-HER2 mAb (clone H2Mab-77) (32). Among 61 breast cancers, 31 cases
(50.8%) were determined to be triple-negative (Table III). Interestingly, 28/31 (90.3%)
were stained by TrMab-6; especially, 17/31 (54.8%) were stained
strongly positive by TrMab-6 (Table
III), indicating that triple-negative breast cancers should be
an ideal target of anti-TROP2 mAbs, including TrMab-6.
 | Table I.Results of TrMab-6 and EPR20043
immunostaining in 61 breast cancers. |
Table I.
Results of TrMab-6 and EPR20043
immunostaining in 61 breast cancers.
| Case | Age | Sex | Pathological
diagnosis |
Differentiation | TNM | ER | PR |
HER2/H2Mab-77 | TrMab-6 | EPR20043 |
|---|
| 1 | 44 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M1 | – | – | – | 3+ | 3+ |
| 2 | 58 | F | Medullary
carcinoma | Moderately | T2 N2 M1 | – | – | – | 3+ | 2+ |
| 3 | 40 | F | Invasive ductal
carcinoma | Moderately | T2 N1 M0 | 1+ | – | 1+ | 2+ | 3+ |
| 4 | 52 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M1 | 1+ | 1+ | – | 1+ | 2+ |
| 5 | 60 | F | Invasive ductal
carcinoma | Moderately | T2 N1 M1 | – | – | – | 3+ | 3+ |
| 6 | 57 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 2+ | 3+ |
| 7 | 48 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | 1+ | 2+ | 2+ | 2+ | 3+ |
| 8 | 66 | F | Invasive lobular
carcinoma | Moderately | T2 N0 M0 | 2+ | 1+ | – | 2+ | 2+ |
| 9 | 58 | F | Adenocarcinoma | Moderately | T2 N2 M1 | 1+ | – | – | 3+ | 3+ |
| 10 | 63 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | 3+ | 3+ | – | 3+ | 3+ |
| 11 | 32 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 2+ | 3+ |
| 12 | 59 | F | Invasive lobular
carcinoma | Well | T2 N2 M0 | – | – | – | 1+ | 1+ |
| 13 | 44 | F | Invasive lobular
carcinoma | Well | T2 N2 M0 | 1+ | 1+ | 1+ | 2+ | 3+ |
| 14 | 60 | F | Invasive lobular
carcinoma | Moderately | T2 N1 M0 | 3+ | 2+ | – | 3+ | 3+ |
| 15 | 44 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M0 | – | 3+ | 3+ | 3+ | 3+ |
| 16 | 82 | F | Invasive ductal
carcinoma | Moderately | T2 N1 M1 | – | – | – | 3+ | 3+ |
| 17 | 58 | F | Adenocarcinoma | Moderately | T2 N1 M1 | – | – | 1+ | 3+ | 3+ |
| 18 | 57 | F | Invasive ductal
carcinoma | Well | T3 N3 M0 | 2+ | 1+ | – | 3+ | 3+ |
| 19 | 41 | F | Invasive ductal
carcinoma | Moderately | T2 N1 M0 | – | – | – | 3+ | 3+ |
| 20 | 44 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M0 | – | – | – | 3+ | 1+ |
| 21 | 78 | F | Invasive ductal
carcinoma | Moderately | T2 N1 M0 | 2+ | 1+ | – | 3+ | 3+ |
| 22 | 60 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | 1+ | 1+ | 1+ |
| 23 | N/A | F | Invasive ductal
carcinoma | Moderately | T2 N1 M1 | 3+ | – | 2+ | 3+ | 2+ |
| 24 | 46 | F | Invasive ductal
carcinoma | Moderately | T2 N3 M1 | – | – | – | 3+ | 3+ |
| 25 | 41 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M0 | – | – | – | 3+ | 3+ |
| 26 | 59 | F | Invasive ductal
carcinoma | Poorly | T2 N0 M0 | 2+ | 1+ | – | 2+ | 2+ |
| 27 | 45 | F | Invasive ductal
carcinoma | Poorly | T2 N0 M0 | 1+ | 1+ | – | – | 1+ |
| 28 | 43 | F | Invasive ductal
carcinoma | N/A | T2 N1 M1 | – | – | – | 1+ | 1+ |
| 29 | 40 | F | Invasive ductal
carcinoma | N/A | T1 N0 M0 | 1+ | 1+ | – | 2+ | 1+ |
| 30 | 51 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M0 | 1+ | 1+ | – | 2+ | 3+ |
| 31 | 45 | F | Invasive ductal
carcinoma | Poorly | T2 N0 M0 | 1+ | 2+ | 1+ | 2+ | 3+ |
| 32 | 45 | F | Invasive ductal
carcinoma | Poorly | T2 N1 M0 | 2+ | 3+ | 3+ | 3+ | 3+ |
| 33 | 47 | F | Invasive ductal
carcinoma |
Moderately-Poorly | T2 N1 M0 | – | – | – | 3+ | 3+ |
| 34 | 55 | F | Invasive ductal
carcinoma | Moderately | T2 N3 M1 | – | – | 1+ | 3+ | 3+ |
| 35 | 58 | F | Invasive ductal
carcinoma | Moderately | T3 N3 M0 | 1+ | 1+ | – | 2+ | 3+ |
| 36 | 47 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 1+ | 2+ |
| 37 | 38 | F | Invasive ductal
carcinoma | Poorly | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 38 | 40 | F | Invasive ductal
carcinoma | Poorly | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 39 | 57 | F | Invasive ductal
carcinoma | Poorly | T2 N0 M0 | – | – | – | 1+ | 1+ |
| 40 | 42 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | 2+ | 3+ | 3+ |
| 41 | 60 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 42 | 58 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | 1+ | – | 1+ | 1+ |
| 43 | 41 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | 3+ | – | 2+ | 2+ |
| 44 | 50 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 45 | 60 | F | Invasive ductal
carcinoma | Moderately | T2 N2 M1 | 1+ | 1+ | – | 3+ | 2+ |
| 46 | 53 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 47 | 65 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 1+ | 2+ |
| 48 | 43 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 49 | 57 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | 3+ | 3+ | 3+ |
| 50 | 37 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | 1+ | – | 2+ | 3+ |
| 51 | 50 | F | Invasive ductal
carcinoma | Moderately | T2 N3 M0 | – | – | – | – | 2+ |
| 52 | 48 | F | Invasive ductal
carcinoma | Poorly | T2 N1 M0 | – | – | – | 1+ | 1+ |
| 53 | 50 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 54 | 53 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 3+ | 3+ |
| 55 | 49 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 2+ | 2+ |
| 56 | 65 | F | Invasive ductal
carcinoma | Moderately | T2 N1 M0 | 1+ | 1+ | – | 2+ | 3+ |
| 57 | 43 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | – | – |
| 58 | 58 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | – | 1+ |
| 59 | 48 | F | Invasive ductal
carcinoma | Moderately | T2 N0 M0 | – | – | – | 2+ | 3+ |
| 60 | N/A | F | Invasive ductal
carcinoma | Moderately | N/A | – | – | – | 2+ | 2+ |
| 61 | N/A | F | Invasive ductal
carcinoma |
Moderately-Poorly | N/A | 1+ | 1+ | – | 2+ | 3+ |
 | Table II.Results of TrMab-6 immunostaining in
61 breast cancers. |
Table II.
Results of TrMab-6 immunostaining in
61 breast cancers.
|
|
| TrMab-6 |
|
|---|
|
|
|
|
|
|---|
| Pathological
diagnosis | No. of cases | 3+ | 2+ | 1+ | − | No. of positive
cases (%) |
|---|
| Invasive ductal
carcinoma | 54 | 26 | 16 | 8 | 4 | 50/54 (92.6) |
| Invasive lobular
carcinoma | 4 | 1 | 2 | 1 | 0 | 4/4 (100) |
| Adenocarcinoma | 2 | 2 | 0 | 0 | 0 | 2/2 (100) |
| Medullary
carcinoma | 1 | 1 | 0 | 0 | 0 | 1/1 (100) |
| Total | 61 | 30/61 (49.2%) | 18/61 (29.5%) | 9/61 (14.8%) | 4/61 (6.6%) | 57/61 (93.4%) |
 | Table III.Results of TrMab-6 immunostaining in
31 triple negative breast cancers. |
Table III.
Results of TrMab-6 immunostaining in
31 triple negative breast cancers.
|
|
| TrMab-6 |
|
|---|
|
|
|
|
|
|---|
| Breast cancer
subtype | No. of cases | 3+ (%) | 2+ (%) | 1+ (%) | - (%) | No. of positive
cases |
|---|
| Triple negative
breast cancer | 31 | 17 (54.8) | 5 (16.1) | 6 (19.4) | 3 (9.7) | 28/31 (90.3%) |
We compared the reactivity of TrMab-6 and another
anti-TROP2 mAb (clone EPR20043) in immunohistochemical analysis. As
shown in Table I, EPR20043 stained
60/61 (98.4%) breast cancer tissues, although TrMab-6 stained 57/61
(93.4%) breast cancer tissues, indicating that EPR20043 is more
useful for immunohistochemical analysis than TrMab-6.
Discussion
Generating a mAb that can be utilized for multiple
applications, including flow cytometry, Western blot, and
immunohistochemistry, is usually difficult. Using the CBIS method,
in which antigen-expressing cell lines are used for both
immunization and screening (22),
we have developed numerous useful mAbs that target membrane
proteins, including CD19 (23),
CD20 (24), CD44 (25), CD133 (22), PD-L1 (26), and podoplanin (PDPN) (33–36).
Among these unique targets, CD20 has four membrane-spanning domains
and only two small extracellular domains that include amino acids
72–80 and 142–182 (37,38). Although there are several
commercially available mAbs that interact with amino acids 142–182
of CD20 and are specifically useful in flow cytometry, there are no
available anti-CD20 mAbs that are effective not only in flow
cytometry but also in Western blot and immunohistochemical
analyses. We recently developed clone C20Mab-11, which
can detect CD20 associated with B-cell lymphoma by flow cytometry,
Western blot, and immunohistochemical analyses (24).
Likewise, we herein aimed to establish one or more
multipurpose anti-TROP2 mAbs because the applications of
commercially available anti-TROP2 mAbs were somewhat limited
(Table SI). Using the CBIS method,
we successfully developed a sensitive and specific novel anti-TROP2
mAb (clone TrMab-6) that can be used in every application,
including flow cytometry (Fig. 3),
Western blot (Fig. 4), and
immunohistochemical analyses (Fig.
5). Another anti-TROP2 mAb (clone EPR20043 from Abcam) is more
sensitive than TrMab-6 in immunohistochemical analysis (Table I), but not useful in flow cytometry
(Fig. S2). EPR20043 might react
with intracellular region of TROP2 although the immunogen was not
clearly shown in its application sheet. Clone SP293 (Abcam) was
also shown to be useful for flow cytometry, Western blot, and
immunohistochemical analyses in its application sheet; however, the
intracellular region of TROP2 was also used as immunogen (Table SI).
We conclude that TrMab-6 is more advantageous than
the other anti-TROP2 mAbs, such as EPR20043 and SP293 because
TrMab-6 is useful to detect TROP2 in all applications, such as flow
cytometry in non-fixed condition, Western blot, and
immunohistochemical analysis. However, in this study, TrMab-6 was
shown to be useful for only in vitro experiments. In the
future, we will determine whether TrMab-6 would be suitable for use
as targeted molecular therapy against breast cancers. The
subclasses of mouse IgG, IgG2a and IgG2b,
both induce antibody-dependent cellular cytotoxicity (ADCC) or
complement-dependent cytotoxicity (CDC) (39,40).
TrMab-6 was determined to be of the mouse IgG2b subclass
(Fig. S1). Although we performed
ADCC reporter assay using TrMab-6, ADCC activity was not observed
unexpectedly (data not shown). To promote antibody therapy using
TrMab-6, we will utilize antibody-drug conjugates (ADCs),
radioimmunotherapy (RIT), photoimmunotherapy (PIT), or chimeric
antigen receptor T-cell (CAR-T) therapy. About ADCs targeting
TROP2, conjugation of the irinotecan metabolite, SN-38, to a
humanized anti-TROP2 antibody (sacituzumab govitecan) promotes
broad and potent antitumor effects in human cancer xenografts and
in patients with advanced triple-negative breast, non-small-cell
and small-cell lung, and urothelial cancers (14). About RIT, van Rij et al
(41) previously reported that
TROP2-expressing prostate cancer can be targeted efficiently with
TF12 [anti-TROP2 × anti-HSG (histamine-succinyl-glycine)] and
177Lu-labeled diHSG-peptide (IMP288). Furthermore,
Nishimura et al (42)
selected TROP2 as a molecular target for PIT, and utilized a newly
developed humanized anti-TROP2 mAb conjugated to the
photosensitizer IR700 (TROP2-IR700) for the treatment of pancreatic
carcinoma and cholangiocarcinoma. Growth of tumor xenografts was
significantly inhibited in response to TROP2-targeted PIT relative
to controls, suggesting that TROP2-targeted PIT is also an
important means for improving treatment for TROP2-expressing
cancers. About CAR-T, Zhao et al (43) reported that novel bi-specific
TROP2/PD-L1 CAR-T cells could target TROP2/PD-L1 and checkpoint
blockade, resulting in cytotoxicity for gastric cancer cells. These
results also suggested that CAR-T cell therapy featuring TROP2 can
be developed to target TROP2-expressing cancers. These various
modalities will allow us to explore TrMab-6-mediated antitumor
activities in the mouse xenograft model of breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Takuro Nakamura,
Ms. Miyuki Yanaka, Ms. Saori Handa, Ms. Saki Okamoto, and Mr. Yu
Komatsu (Department of Antibody Drug Development, Tohoku University
Graduate School of Medicine) for technical assistance in the in
vitro experiments.
Funding
The present study was supported in part by Japan
Agency for Medical Research and Development (AMED; grant nos.
JP20am0401013, JP20am0101078 and JP20ae0101028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS performed experiments, and analyzed experimental
data. YS and YK wrote the manuscript. MKK and YK designed the
current study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal studies were approved by The Animal Care and
Use Committee of Tohoku University (Permit no. 2019NiA-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
CBIS
|
Cell-Based Immunization and
Screening
|
|
CHO
|
Chinese hamster ovary
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
mAb
|
monoclonal antibody
|
|
H&E
|
hematoxylin and eosin
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
TACTD2
|
tumor-associated calcium signal
transducer 2
|
|
TROP2
|
trophoblast cell-surface antigen 2
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Engl J Med.
380:741–751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anders CK, Zagar TM and Carey LA: The
management of early-stage and metastatic triple-negative breast
cancer: A review. Hematol Oncol Clin North Am. 27737–749.
(viii)2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trivers KF, Lund MJ, Porter PL, Liff JM,
Flagg EW, Coates RJ and Eley JW: The epidemiology of
triple-negative breast cancer, including race. Cancer Causes
Control. 20:1071–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plasilova ML, Hayse B, Killelea BK,
Horowitz NR, Chagpar AB and Lannin DR: Features of triple-negative
breast cancer: Analysis of 38,813 cases from the national cancer
database. Medicine (Baltimore). 95:e46142016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohler BA, Sherman RL, Howlader N, Jemal
A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et
al: Annual report to the nation on the status of cancer, 1975–2011,
featuring incidence of breast cancer subtypes by race/ethnicity,
poverty, and state. J Natl Cancer Inst. 107:djv0482015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fornaro M, Dell'Arciprete R, Stella M,
Bucci C, Nutini M, Capri MG and Alberti S: Cloning of the gene
encoding Trop-2, a cell-surface glycoprotein expressed by human
carcinomas. Int J Cancer. 62:610–618. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alberti S, Miotti S, Stella M, Klein CE,
Fornaro M, Menard S and Colnaghi MI: Biochemical characterization
of Trop-2, a cell surface molecule expressed by human carcinomas:
Formal proof that the monoclonal antibodies T16 and MOv-16
recognize Trop-2. Hybridoma. 11:539–545. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipinski M, Parks DR, Rouse RV and
Herzenberg LA: Human trophoblast cell-surface antigens defined by
monoclonal antibodies. Proc Natl Acad Sci USA. 78:5147–5150. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schon MP and Orfanos CE: Transformation of
human keratinocytes is characterized by quantitative and
qualitative alterations of the T-16 antigen (Trop-2, MOv-16). Int J
Cancer. 60:88–92. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zaman S, Jadid H, Denson AC and Gray JE:
Targeting Trop-2 in solid tumors: Future prospects. Onco Targets
Ther. 12:1781–1790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan H, Guo Z, Liang W, Li H, Wei G, Xu L,
Xiao H and Li Y: Trop2 enhances invasion of thyroid cancer by
inducing MMP2 through ERK and JNK pathways. BMC Cancer. 17:4862017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldenberg DM, Stein R and Sharkey RM: The
emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel
cancer target. Oncotarget. 9:28989–29006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldenberg DM, Cardillo TM, Govindan SV,
Rossi EA and Sharkey RM: Trop-2 is a novel target for solid cancer
therapy with sacituzumab govitecan (IMMU-132), an antibody-drug
conjugate (ADC). Oncotarget. 6:22496–22512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstein AS, Huang J, Guo C, Garraway IP
and Witte ON: Identification of a cell of origin for human prostate
cancer. Science. 329:568–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstein AS, Lawson DA, Cheng D, Sun W,
Garraway IP and Witte ON: Trop2 identifies a subpopulation of
murine and human prostate basal cells with stem cell
characteristics. Proc Natl Acad Sci USA. 105:20882–20887. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
King GT, Eaton KD, Beagle BR, Zopf CJ,
Wong GY, Krupka HI, Hua SY, Messersmith WA and El-Khoueiry AB: A
phase 1, dose-escalation study of PF-06664178, an
anti-Trop-2/Aur0101 antibody-drug conjugate in patients with
advanced or metastatic solid tumors. Invest New Drugs. 36:836–847.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cardillo TM, Govindan SV, Sharkey RM,
Trisal P, Arrojo R, Liu D, Rossi EA, Chang CH and Goldenberg DM:
Sacituzumab govitecan (IMMU-132), an anti-trop-2/SN-38
antibody-drug conjugate: Characterization and efficacy in
pancreatic, gastric, and other cancers. Bioconjug Chem. 26:919–931.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cardillo TM, Govindan SV, Sharkey RM,
Trisal P and Goldenberg DM: Humanized anti-Trop-2 IgG-SN-38
conjugate for effective treatment of diverse epithelial cancers:
Preclinical studies in human cancer xenograft models and monkeys.
Clin Cancer Res. 17:3157–3169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okajima D, Yasuda S, Yokouchi Y, Fujitani
T, Sakurai K and Yamaguchi J: Preclinical efficacy studies of
DS-1062a, a novel TROP2-targeting antibody-drug conjugate with a
novel DNA topoisomerase I inhibitor DXd. J Clinical Oncol. 36
(Suppl 15):e242062018. View Article : Google Scholar
|
|
22
|
Itai S, Fujii Y, Nakamura T, Chang YW,
Yanaka M, Saidoh N, Handa S, Suzuki H, Harada H, Yamada S, et al:
Establishment of CMab-43, a sensitive and specific anti-CD133
monoclonal antibody, for immunohistochemistry. Monoclon Antib
Immunodiagn Immunother. 36:231–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamada S, Kaneko MK, Sayama Y, Asano T,
Sano M, Yanaka M, Nakamura T, Okamoto S, Handa S, Komatsu Y, et al:
Development of novel mouse monoclonal antibodies against human
CD19. Monoclon Antib Immunodiagn Immunother. 39:45–50. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furusawa Y, Kaneko MK and Kato Y:
Establishment of C20Mab-11, a novel anti-CD20 monoclonal antibody,
for the detection of B cells. Oncol Lett. 20:1961–1967. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada S, Itai S, Nakamura T, Yanaka M,
Kaneko MK and Kato Y: Detection of high CD44 expression in oral
cancers using the novel monoclonal antibody, C(44)Mab-5. Biochem
Biophys Rep. 14:64–68. 2018.PubMed/NCBI
|
|
26
|
Yamada S, Itai S, Nakamura T, Yanaka M,
Chang YW, Suzuki H, Kaneko MK and Kato Y: Monoclonal antibody
L(1)Mab-13 detected human PD-L1 in lung cancers. Monoclon Antib
Immunodiagn Immunother. 37:110–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujii Y, Kaneko M, Neyazaki M, Nogi T,
Kato Y and Takagi J: PA tag: A versatile protein tagging system
using a super high affinity antibody against a dodecapeptide
derived from human podoplanin. Protein Expr Purif. 95:240–247.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujii Y, Kaneko MK, Ogasawara S, Yamada S,
Yanaka M, Nakamura T, Saidoh N, Yoshida K, Honma R and Kato Y:
Development of RAP Tag, a novel tagging system for protein
detection and purification. Monoclon Antib Immunodiagn Immunother.
36:68–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujii Y, Kaneko MK and Kato Y: MAP Tag: A
novel tagging system for protein purification and detection.
Monoclon Antib Immunodiagn Immunother. 35:293–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kohler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato Y, Kaneko MK, Kuno A, Uchiyama N,
Amano K, Chiba Y, Hasegawa Y, Hirabayashi J, Narimatsu H, Mishima K
and Osawa M: Inhibition of tumor cell-induced platelet aggregation
using a novel anti-podoplanin antibody reacting with its
platelet-aggregation-stimulating domain. Biochem Biophys Res
Commun. 349:1301–1307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Itai S, Fujii Y, Kaneko MK, Yamada S,
Nakamura T, Yanaka M, Saidoh N, Chang YW, Handa S, Takahashi M, et
al: H2Mab-77 is a sensitive and specific Anti-HER2 monoclonal
antibody against breast cancer. Monoclon Antib Immunodiagn
Immunother. 36:143–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Furusawa Y, Yamada S, Itai S, Nakamura T,
Yanaka M, Sano M, Harada H, Fukui M, Kaneko MK and Kato Y:
PMab-219: A monoclonal antibody for the immunohistochemical
analysis of horse podoplanin. Biochem Biophys Rep.
18:1006162019.PubMed/NCBI
|
|
34
|
Furusawa Y, Kaneko MK, Nakamura T, Itai S,
Fukui M, Harada H, Yamada S and Kato Y: Establishment of a
monoclonal antibody PMab-231 for tiger podoplanin. Monoclon Antib
Immunodiagn Immunother. 38:89–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furusawa Y, Takei J, Sayama Y, Yamada S,
Kaneko MK and Kato Y: Development of an anti-bear podoplanin
monoclonal antibody PMab-247 for immunohistochemical analysis.
Biochem Biophys Rep. 18:1006442019.PubMed/NCBI
|
|
36
|
Furusawa Y, Yamada S, Itai S, Nakamura T,
Takei J, Sano M, Harada H, Fukui M, Kaneko MK and Kato Y:
Establishment of a monoclonal antibody PMab-233 for
immunohistochemical analysis against Tasmanian devil podoplanin.
Biochem Biophys Rep. 18:1006312019.PubMed/NCBI
|
|
37
|
Polyak MJ, Li H, Shariat N and Deans JP:
CD20 homo-oligomers physically associate with the B cell antigen
receptor. Dissociation upon receptor engagement and recruitment of
phosphoproteins and calmodulin-binding proteins. J Biol Chem.
283:18545–18552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li H, Ayer LM, Lytton J and Deans JP:
Store-operated cation entry mediated by CD20 in membrane rafts. J
Biol Chem. 278:42427–42434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaneko MK, Nakamura T, Honma R, Ogasawara
S, Fujii Y, Abe S, Takagi M, Harada H, Suzuki H, Nishioka Y and
Kato Y: Development and characterization of anti-glycopeptide
monoclonal antibodies against human podoplanin, using
glycan-deficient cell lines generated by CRISPR/Cas9 and TALEN.
Cancer Med. 6:382–396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ogasawara S, Kaneko MK and Kato Y:
LpMab-19 recognizes sialylated O-Glycan on Thr76 of human
podoplanin. Monoclon Antib Immunodiagn Immunother. 35:245–253.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Rij CM, Frielink C, Goldenberg DM,
Sharkey RM, Lütje S, McBride WJ, Oyen WJG and Boerman OC:
Pretargeted radioimmunotherapy of prostate cancer with an
anti-TROP-2×Anti-HSG bispecific antibody and a (177)Lu-labeled
peptide. Cancer Biother Radiopharm. 29:323–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishimura T, Mitsunaga M, Sawada R, Saruta
M, Kobayashi H, Matsumoto N, Kanke T, Yanai H and Nakamura K:
Photoimmunotherapy targeting biliary-pancreatic cancer with
humanized anti-TROP2 antibody. Cancer Med. 8:7781–7792. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao W, Jia L, Zhang M, Huang X, Qian P,
Tang Q, Zhu J and Feng Z: The killing effect of novel bi-specific
Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res.
9:1846–1856. 2019.PubMed/NCBI
|