Estrogen receptor-associated receptor (ERR) is an
orphan nuclear receptor that exerts its biological function without
binding to a ligand. In 1988, Giguère et al (1) identified a nuclear receptor that was
highly homologous with ERα in nucleotide and amino acid sequences
using cDNA for the DNA-binding domain of estrogen receptor α (ERα)
as the probe. Both ERR and ER are type III nuclear receptors. To
date, the following three subtypes have been found, ERRα (NR3B1),
ERRβ (NR3B2) and ERRγ (NR3B3), in which ERRα is widely distributed
in various adult tissues and participates in a variety of
physiological processes, including mitochondrial biogenesis
(2), gluconeogenesis, oxidative
phosphorylation (3), fatty acid
metabolism (4) and brown adipose
tissue thermogenesis (5). It was
also identified as an important regulator of the mammalian
circadian clock, and its output pathways at both transcriptional
and physiological levels regulated the expression of transcription
factors involved in metabolic homeostasis (6). The ERRα-encoding gene is located at
site 11q13 of the human chromosome and primarily consists of the
following three functional domains: N terminal domain (NTD),
DNA-binding domain (DBD) and ligand binding domain (LBD).
Activation function 1 (AF1) is located at the NTD, while AF2 is
located at the LBD (7). The DBD of
ERRα contains two zinc fingers, which are used for identification
and binding of special sequences at the regulatory region in the
DNA of the target gene (8). AF2
regulates the transcriptional activity of nuclear receptors,
primarily through functional interactions with coactivators, such
as peroxisome proliferator-activated receptor γ coactivator-1
(PGC-1), or corepressors, such as nuclear factor RIP140 (8).
Peroxisome proliferator-activated receptor (PPAR) is
a novel steroid hormone receptor discovered by Issemann and Green
(9) in 1990, which can be activated
by fatty acid-like peroxisome proliferator. PPARs are nuclear
transcription factors activated by ligands and members of the type
II nuclear hormone receptor superfamily. There are three subtypes
of PPARs: PPARα, β/δ and γ (10).
Typically, PPARs and retinoid X receptors (RXR) form a heterodimer
and recruit a co-inhibitory protein complex to inhibit the
transcription of target genes (10). When PPARs are combined with ligands
and activated, this heterodimer may release co-inhibitor proteins
and bind to coactivator proteins, and subsequently combine with the
promoter of the target gene, upstream peroxisome proliferator
response element (PPRE), to regulate its transcription and activate
its biological function (10). The
PPARγ gene is located in the p25 region of chromosome 3 and
contains six regions known as regions A-F, which are divided into
four functional domains: Amino terminal domain, DNA binding domain,
transcriptional activity regulatory domain and ligand binding
domain (11). PPARγ regulates gene
transcription through binding of the DNA binding domain to PPRE,
and a number of nuclear factors, such as protein kinase C, protein
kinase A and 5′AMP-activated protein kinase can affect the activity
of PPARγ after binding to this domain (12,13).
ERRα is expressed in a variety of tissues from
embryonic development to adulthood. The expression of ERRα can be
detected in the heart, brain, kidney, brown adipose tissue (BAT),
intestines, bones and uterus (Table
I) (14). The expression of
ERRα is higher in metabolically active tissues, including the
heart, white adipose tissue, BAT and macrophages, while it is
relatively lower in the liver, lung and vagina (15,16).
Studies have demonstrated that ERRs play an important role in the
regulation of eukaryotic gene expression, embryonic development,
cell proliferation, bone cell production and angiogenesis (17–19).
ERRα is an orphan nuclear receptor that does not have corresponding
ligands, but may interact with and have a bypass effect on the
classical oestrogen signalling pathway through competitive binding
to the same target genes, transcription factors and coactivator
proteins with ERα (7,20,21).
Earlier studies reported the important role of ERRα in energy
metabolism of the body via the regulation of its target genes. The
metabolic processes that ERRα plays a role in include glucose
metabolism (22,23), lipid metabolism (24) and mitochondrial oxidation metabolism
(25–27). ERRα regulates the process of glucose
metabolism mainly by affecting the gluconeogenic pathway and the
derivatization of mitochondria (28,29).
ERRα influences the lipid metabolism process through targeting and
regulating genes of the fatty acid β oxidation pathway, such as
acetyl-coenzyme A dehydrogenase and malonyl coenzyme A
decarboxylase (30). ERRα regulates
mitochondrial oxidation metabolism by upregulating gene expression
related to oxidative phosphorylation through combined action with
PGC-1α as the coactivator (31).
When the body is affected by changes in the external environment,
such as hunger and cold temperatures, the upregulation of ERRα
expression may promote energy generation and the utilization of
body energy, achieving an optimal adaptive state (32).
The mRNA of PPARγ is made up of ~4,000 nucleotides.
A total of four subtypes of mRNA can be produced by different
promoters and alternative splicing: PPARγl, PPARγ2, PPARγ3 and
PPARγ4 (33). The isomers of these
four mRNA subtypes have different promoters, expression modes,
ligand affinity and tissue distribution. PPARγ1 is the main subtype
of PPARγ and is relatively widely distributed (34). It is primarily distributed in
adipose tissue, liver, heart, pancreas, intestines, kidney and
skeletal muscle. The expression levels of PPARγ2 are the highest in
adipose tissue, and lowest in skeletal muscle (35). PPARγ3 is expressed only in
macrophages and the large intestine (36). However, little is known concerning
PPARγ4 expression. PPARγ is differently expressed in a variety of
tissues (Table I) (14). PPARγ regulates the expression of
target genes through ligand-dependent mechanisms, thereby
participating in a series of physiological processes. There are two
types of PPARγ ligands: Endogenous and exogenous (37). The exogenous ligands contain insulin
sensitizers used in the treatment of clinical diabetes,
tyrosine-containing drugs, such as GW1929, and phenylacetic acid
derivatives, such as ibuprofen (38). The endogenous ligands are mainly
prostaglandin-derived metabolites (39). PPARγ forms a heterodimer with RXRα,
and then binds to a specific DNA sequence of the PPRE to activate
target genes (40). Based on
previous studies, PPARγ exerts various biological effects and plays
important roles in lipid metabolism (41), glucose metabolism (42), atherosclerosis formation (43) and inflammatory response (44). In addition, as a nuclear hormone
receptor, PPARγ can affect the function of fatty acids and its
derivatives at the transcriptional level to regulate cell survival
and control the occurrence and development of cancer in different
tissues (45).
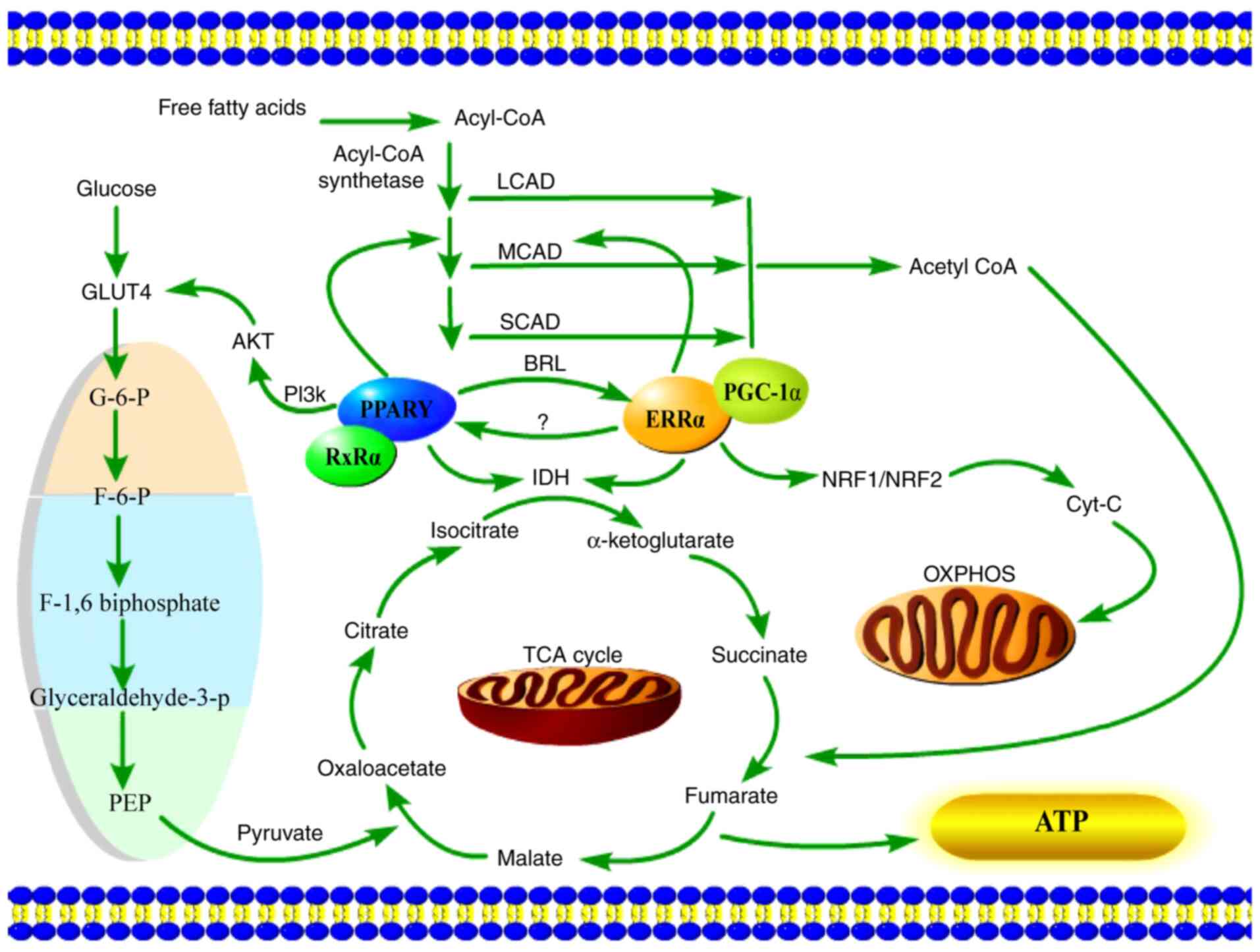
Both ERRα and PPARγ are members of the nuclear
receptor superfamily, and as ligand-dependent transcription
factors, they need to bind to co-factors to form heterodimers and
participate in the regulation of their target genes. A genome-wide
analysis of ERRα and ERRγ has confirmed their direct and
overlapping binding at the promoter regions of a large number of
mitochondrial genes, a number of which are PGC-1α targets (46). These genes cover various aspects of
mitochondrial oxidative metabolism, ranging from glucose
utilization, fatty acid oxidation, the tricarboxylic acid (TCA)
cycle and oxidative phosphorylation (OXPHOS) (46). Using laser capture techniques, Teng
et al (47) demonstrated
that the expression of the selected ERRα target gene isocitrate
dehydrogenase (IDH) was involved in the TCA cycle. PPARγ is a
master regulator of macrophage polarization. Angajala et al
(48) showed that macrophages
control the first break of the TCA cycle that occurs in the
enzymatic step involving IDH. Wei et al (49) demonstrated that
rosiglitazone-activated PPARγ can induce ERRα expression. PGC-1α
can target ERRα and transactivate nuclear factor erythroid
2-related factor (NRF)1/NRF2 target genes, which are the nuclear
respiratory factors (50). In
addition, research has revealed that the induction of NRF1
transcription factors is a prerequisite for the transcriptional
activation of cytochrome c (cyt c), which is an
important electron transporter in OXPHOS (51). ERRα was previously implicated in
regulating the gene encoding medium-chain acyl-CoA dehydrogenase
(MCAD), which catalyses the initial step in mitochondrial fatty
acid oxidation (52). Additionally,
MCAD was previously reported to be a target gene of PPARγ (53). Gandhi et al (54) demonstrated that increased PPARγ
levels can regulate insulin-mediated glucose uptake through the
translocation and activation of glucose transporter type 4 in the
PI3K/phosphorylated-Akt signalling cascade. Therefore, both ERRα
and PPARγ can regulate the amount of acetyl-CoA that will enter the
TCA cycle by affecting fatty acid metabolism. The aforementioned
findings indicated that PPARγ can also affect the production of
pyruvates associated with the TCA cycle by affecting the glycolysis
pathway. It was also suggested that ERRα expression can influence
cyt c expression, which is closely associated with the
OXPHOS process. Glycolysis, fatty acid metabolism, OXPHOS and the
TCA cycle are all ubiquitous metabolic pathways in the body that
provide the most direct energy source, ATP (Fig. 1).
ERRα recruits co-regulators, is activated in a
constitutive manner, regulates gene transcription, and serves an
important role in cell physiological functions, as well as
participates in the pathological processes of some diseases, such
as diabetes, fatty liver and hepatocellular carcinoma (55). Research has demonstrated that the
expression levels of OXPHOS-associated genes are downregulated
early in the development of insulin resistance in human diabetes
(56). ERRα is a target gene of
PGC-1, and hence can regulate the expression of OXPHOS and fatty
acid oxidation genes. Studies have reported that the expression
levels of ERRα-regulated genes are decreased in patients with
insulin resistance (57), and there
is an association between insulin sensitivity and the expression of
ERRα mRNA in human adipose tissue (58). Overaccumulation of triglycerides in
liver cells leads to non-alcoholic fatty liver disease (NAFLD).
Decreased expression of ERRα affects the intake of dietary fat,
thus inhibiting NAFLD development (59). In addition, a previous study
indicated that the absence of ERRα activity promoted the
development of rapamycin-induced NAFLD (60). Furthermore, in a mouse model of
pressure overload-induced left ventricular hypertrophy, ERRα
expression was found to be significantly downregulated, which
resulted in faster development of heart failure (61). In addition, several studies found
that in rodent models of heart failure, including models of
decompensated right ventricular hypertrophy and myocardial
infarction, and genetic models that show accelerated heart failure,
the expression of ERRα and its coactivator are reduced (62–64).
A number of studies have demonstrated the close
association between ERRα and the occurrence, development and
clinical prognosis of various tumours. In hormone-dependent
tumours, such as endometrial (65),
ovarian (20), breast (66) and prostate cancer (67), ERRα may regulate tumour development
through its effect on the ERα signalling pathway. In
non-hormone-dependent tumours, including colorectal cancer,
non-small cell lung cancer, nasopharyngeal carcinoma and glioma,
ERRα may play a role by indirectly affecting gene transcription or
proliferation of tumour cells. In endometrial cancer, a previous
study revealed that upregulated expression of ERRα was
significantly associated with tumour cell proliferation (68). Based on the findings of previous
studies, it has been proposed that ERRs and ERs are co-expressed in
ovarian cancer, and the interaction between these two families may
be the molecular basis for the complex endocrine biological
behaviour of ovarian cancer. Sun et al (20) showed that the ERRα was associated
with the occurrence of ovarian cancer and the survival rate of
patients, and could be used as a factor for poor prognosis of
ovarian cancer. In addition, breast cancer is also a
hormone-dependent tumour. Kraus et al (69) pointed out that ERRα could compete
with ERα to bind to the oestrogen response element to regulate the
transcription of target genes. Recent in vitro studies
demonstrated that ERRα promoted triple-negative breast cancer
(TNBC) cell migration and invasion, which was regulated by STAT3,
providing a potential therapeutic option against TNBC metastasis
(70). Previous studies on prostate
cancer revealed that ERR protein was highly expressed in prostatic
epithelial cells, whereas in prostate cancer cells expression was
lower, and the increase of ERRα expression levels was significantly
associated with prostate cancer development, disease prognosis and
the survival rate of patients (67,71).
ERRα-associated diseases and related tissues are shown in Table II (8).
The biological functions of PPARγ are complex and
diverse, and studies have provided a number of novel approaches for
the clinical prevention and treatment of diabetes (72), atherosclerosis, hypertension, NFLAD
(73) and kidney disease (74). For the treatment of diabetes,
thiazolidinedione (TZD) drugs can promote glucose utilization in
skeletal muscle and inhibit glucose synthesis in the liver
(75). When activated by TZD, PPARγ
can promote the expression of the PI3K subunit p85, promote c-Cbl
associated protein (CAP) transcription, promote insulin signalling
and improve insulin resistance (76). In islet α cells, activated PPARγ
improved insulin resistance by suppressing the activity of the
transcription factor Pax6 and suppressing the expression of
glucagon at the transcription level (77). Studies have reported that PPARγ
ligands can induce CD36 expression, promote the phagocytosis of
oxidized low-density lipoprotein by macrophages and cause
intracellular lipid accumulation (78,79).
In addition to enabling lipids to be taken up by macrophages, PPARγ
can also transfer excess intracellular cholesterol to the
extracellular space via ATP-binding cassette transporter A1 protein
(80). Intimal macrophages engulf
cholesterol and form foam cells during the progression of
atherosclerosis. PPARγ is expressed in the vascular endothelium,
and PPARγ agonists can lower blood pressure (81). In vitro endothelial cell
culture experiments found that TZD-like ligands can significantly
promote the secretion of vasomotor factor C-type natriuretic
peptide in bovine carotid artery endothelial cells and inhibit the
secretion of the vasoconstrictor factor endothelin (82).
PPARγ is a nuclear hormone receptor and its
transcriptional level may affect the oxidation of fatty acids and
the mitochondrial biogenesis of BAT (83). Therefore, PPARγ is most likely
involved in the development of cancer in different tissues by
regulating cell proliferation and differentiation. The expression
of PPARγ has been reported in various types of tumour cells,
including breast (84), prostate
(85) and lung cancer cells
(86), and it has been found that
the binding of PPARγ to its ligand could inhibit the growth of
tumour cells (87). However, other
studies found that the expression levels of PPARγ was significantly
increased in endometrial (88) and
epithelial ovarian cancer (89, 90). Dong (84) found that efatutazone, a PPARγ
agonist, could promote the differentiation of tumour cells in
breast cancer in a specific stage, and thus interfere with tumour
occurrence and development. In a study on ovarian cancer, Luo et
al (91) found that PPARγ could
upregulate the expression levels of microRNA-125, and thereby
inhibit the proliferation of ovarian cancer cells. In colon cancer,
studies demonstrated that patients with high PPARγ expression were
more likely to survive than those with low PPARγ expression
(92). In lung cancer, PPAR
activation may inhibit the metastasis of tumour cells by inhibiting
the epithelium-mesenchymal transition (93). In pancreatic cancer, it was revealed
that PPARγ was highly expressed in pancreatic cancer cells, and
activation of PPARγ may inhibit the growth of PANC-1 cells
(94). In gastric cancer, He et
al (95) reported that
rosiglitazone, a PPARγ agonist, could induce cell apoptosis, and
thus inhibit the growth and invasion of tumour cells, and this
effect could be reversed by GW9662, a PPARγ antagonist.
PPARγ-associated diseases and related tissues are shown in Table III (96).
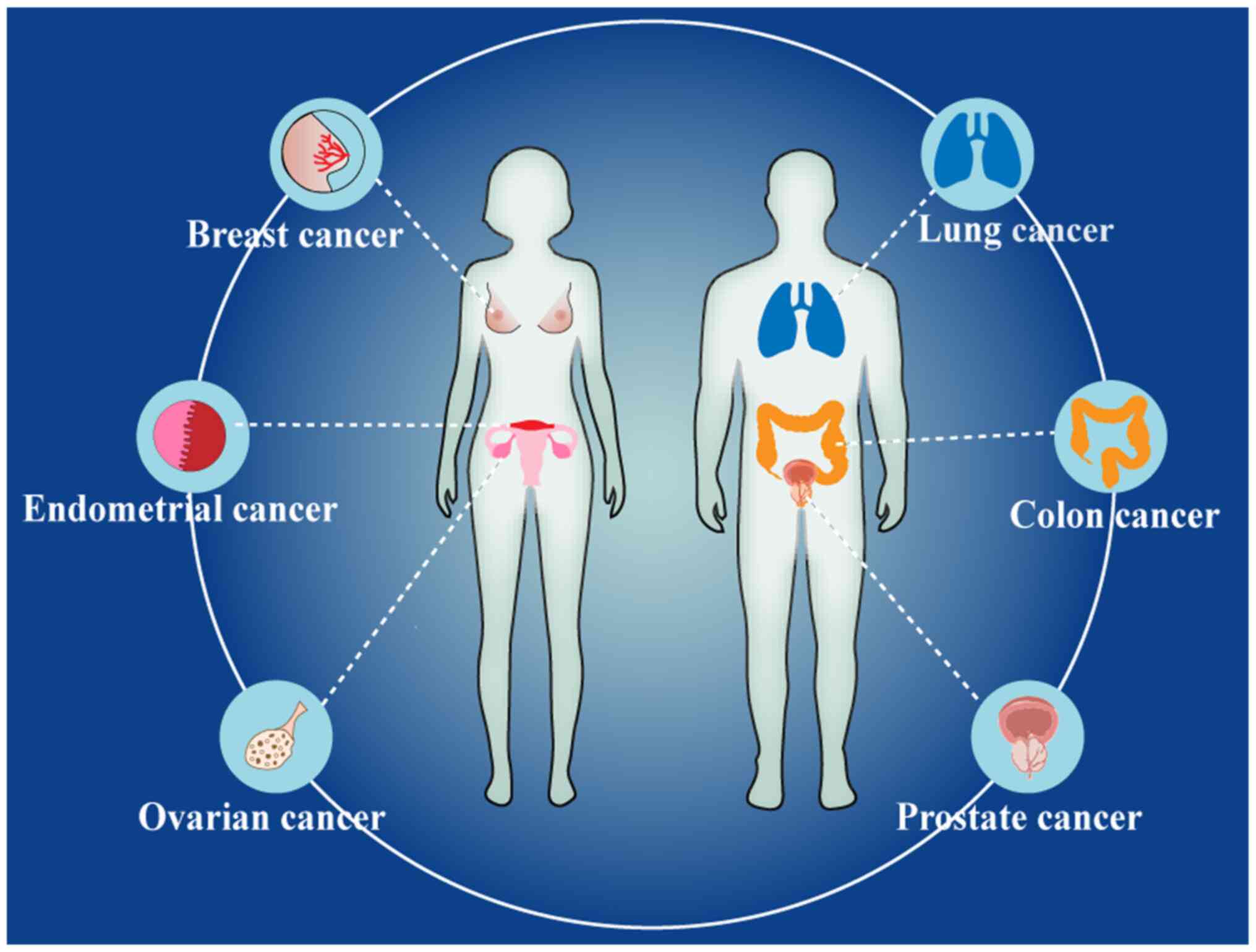
As aforementioned, both ERRα and PPARγ are involved
in tumour development. Specifically, they were reported in studies
on hormone-dependent tumours (endometrial, ovarian, breast and
prostate cancer) and hormone-independent tumours (lung and colon
cancer) (Fig. 2). Using R
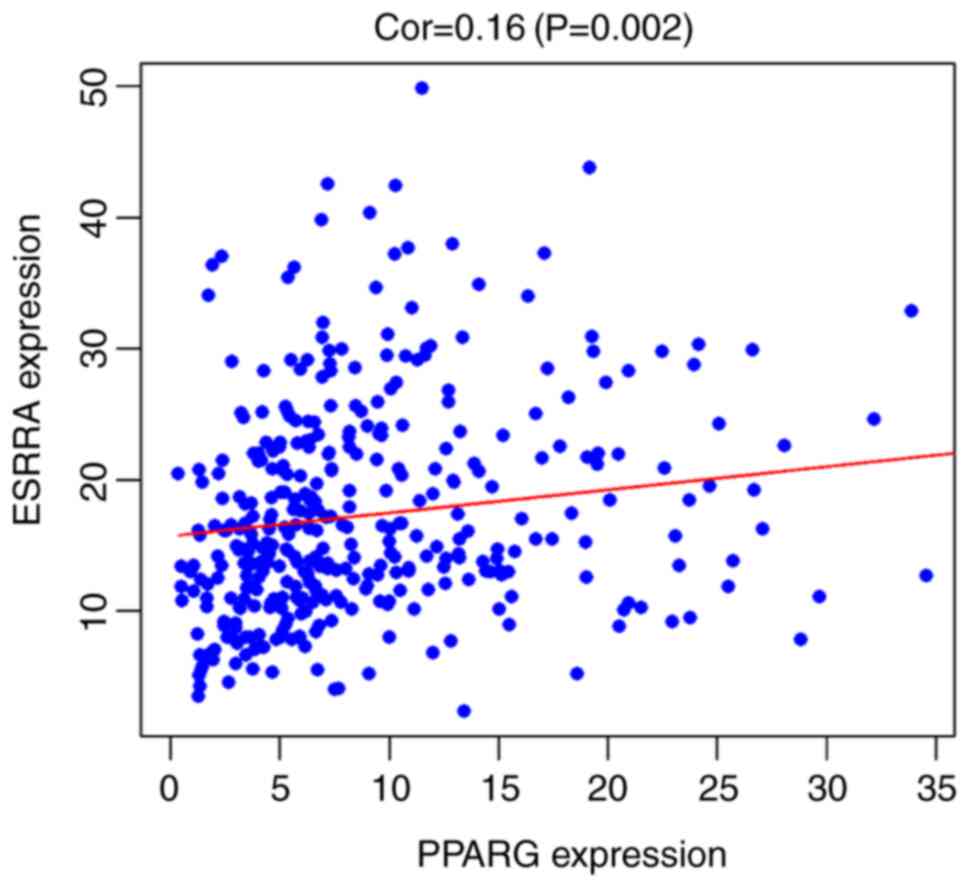
programming language (version 3.6.3; http://www.r-project.org/), based on The Cancer Genome
Atlas database (https://portal.gdc.cancer.gov/), Pearson's correlation
analysis was performed. It was found that ERRα expression was
weakly positively correlated with PPARγ expression (correlation,
r=0.16, P<0.01; Fig. 3). Using
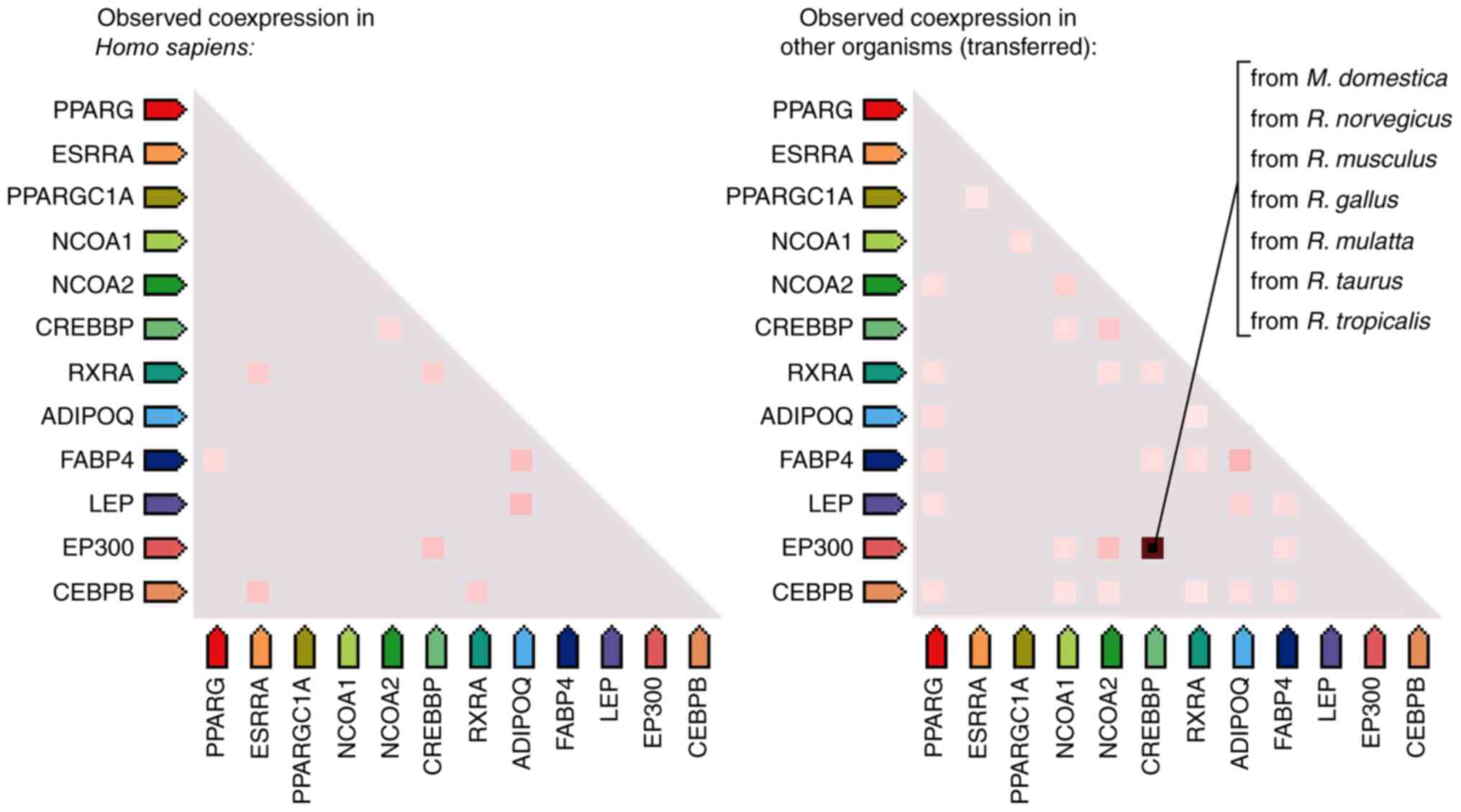
bioinformatics analysis, based on the Search Tool for the Retrieval
of Interacting Genes database (97), the co-expression analysis revealed
that ERRα and PPARγ have a co-expression relationship (Fig. 4), suggesting that the two genes may
have several similar functions. The protein-protein interaction
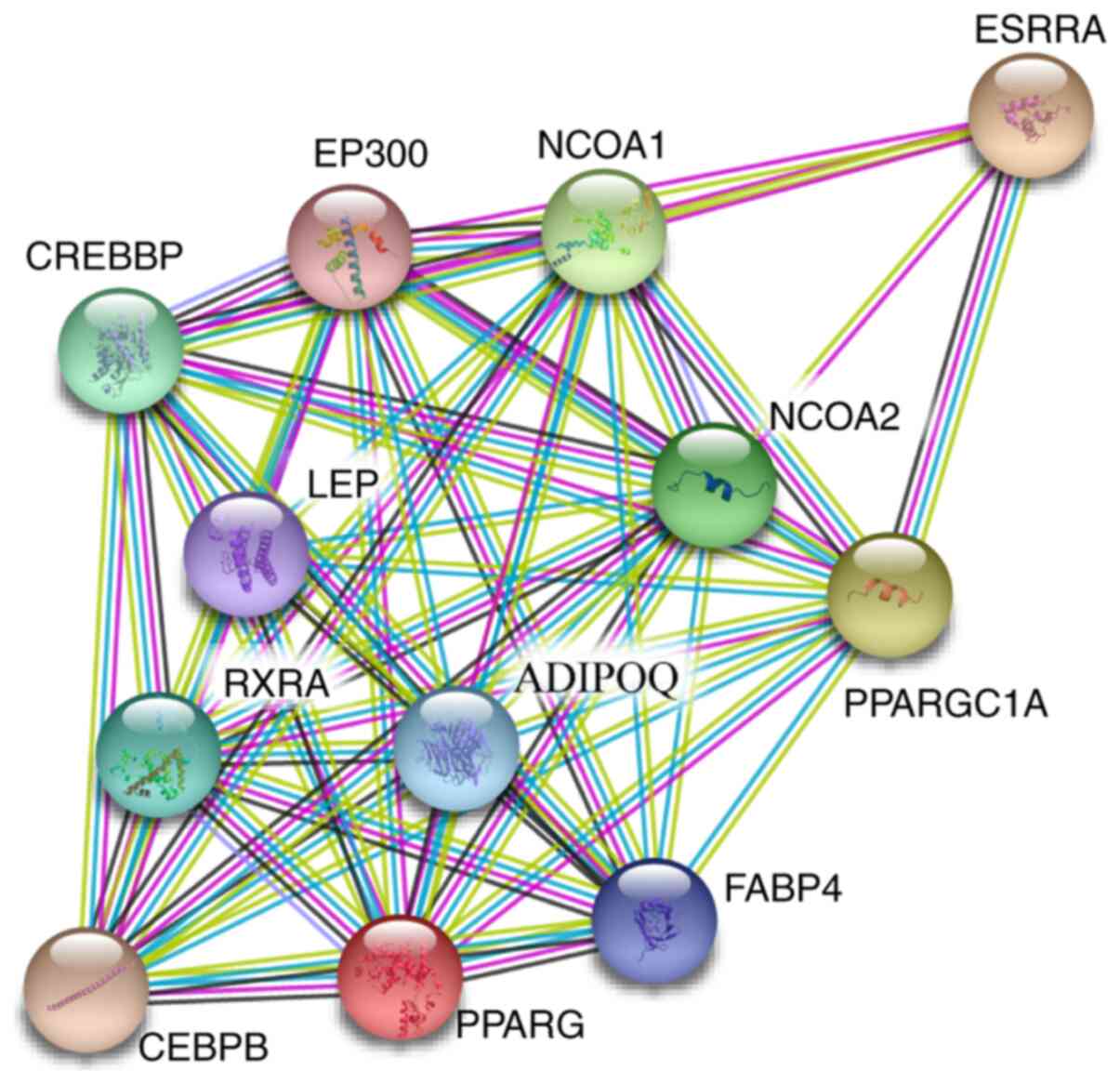
network (http://string-db.org/cgi/input.pl) between ERRα and
PPARγ showed that ERRα and PPARγ proteins interacted with nuclear
receptor coactivator 1, histone acetyltransferase p300,
CREB-binding protein, leptin, adiponectin receptor protein 1,
CCAAT/enhancer-binding protein b and fatty acid-binding protein
adipocyte. Searching UniProt database (https://www.uniprot.org/) and GeneCards database
(https://www.genecards.org/), it was
found that these interacting proteins are involved in the
activation of gene transcription, the modification of transcription
factors and cellular energy metabolism (Fig. 5).
To date, there are very few studies involving both
ERRα and PPARγ. A previous study demonstrated that ERRα knockout
with small interfering RNA resulted in decreased PPARγ expression
levels in 3T3-L1 pre-adipocytes (98). Studies have also reported that PPREs
are present at the ERRα promoter, and PPRE was the PPAR response
element (49). A previous study
revealed that rosiglitazone, as a PPARγ agonist, could induce the
expression of ERRα after activating the expression of PPARγ, thus
enhancing mitochondrial biogenesis and osteoclast function
(49). Therefore, it can be
hypothesized that there is an association between ERRα and PPARγ
expression. However, further studies are required to verify and
clarify this association.
In previous years, studies on ERRα, PPARγ and
tumorigenesis were gradually applied to clinical diagnosis and
treatment. In diseases that have been extensively studied, such as
ovarian and breast cancer, ERRα is generally considered to be a
factor closely related to the poor prognosis of tumours, and hence
is also considered to be a potential target for tumour therapy.
Meanwhile, PPARγ expression in tumours varies, and the relationship
between PPARγ and tumour prognosis is yet to be determined. In
metabolic diseases that have been comprehensively studied, such as
diabetes, PPARγ has become an important therapeutic target
(99). ERRα is also closely related
to numerous metabolic diseases. Currently, thiazolidinediones, as
PPARγ agonists, have been used in the clinical treatment of
metabolic syndromes, and they are expected to play an important
role in the treatment of inflammation and tumours (100–102).
However, there are few reports concerning the
association between ERRα and PPARγ, the underlying mechanism of
their interaction and their combined role in diseases. ERRα and
PPARγ are related to a number of diseases, and both act as
transcription factors that regulate cellular metabolic functions.
Studying the relationship between ERRα and PPARγ could help to
further understand the progress of certain diseases and will be
useful for drug research. In addition, researches on new drugs for
the ERRs have been reported (103), and thus it may be possible to
develop ERRα and PPARγ dual-targeted drugs to provide further
insight into the treatment of diseases.
Not applicable.
No funding was received.
WYH and PMS designed the study. WYH was the major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Giguère V, Yang N, Segui P and Evans RM:
Identification of a new class of steroid hormone receptors. Nature.
331:91–94. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et
al: Mechanisms controlling mitochondrial biogenesis and respiration
through the thermogenic coactivator PGC-1. Cell. 98:115–124. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mootha VK, Handschin C, Arlow D, Xie X, St
Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N,
et al: Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative
phosphorylation gene expression that is altered in diabetic muscle.
Proc Natl Acad Sci USA. 101:6570–6575. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huss JM, Torra IP, Staels B, Giguère V and
Kelly DP: Estrogen-related receptor alpha directs peroxisome
proliferator-activated receptor alpha signaling in the
transcriptional control of energy metabolism in cardiac and
skeletal muscle. Mol Cell Biol. 24:9079–9091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emmett MJ, Lim HW, Jager J, Richter HJ,
Adlanmerini M, Peed LC, Briggs ER, Steger DJ, Ma T, Sims CA, et al:
Histone deacetylase 3 prepares brown adipose tissue for acute
thermogenic challenge. Nature. 546:544–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dufour CR, Levasseur MP, Pham NH, Eichner
LJ, Wilson BJ, Charest-Marcotte A, Duguay D, Poirier-Héon JF,
Cermakian N and Giguère V: Genomic convergence among ERRα, PROX1,
and BMAL1 in the control of metabolic clock outputs. PLoS Genet.
7:e10021432011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giguère V: To ERR in the estrogen pathway.
Trends Endocrinol Metab. 13:220–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranhotra HS: The estrogen-related receptor
alpha: The oldest, yet an energetic orphan with robust biological
functions. J Recept Signal Transduct Res. 30:193–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Issemann I and Green S: Activation of a
member of the steroid hormone receptor superfamily by peroxisome
proliferators. Nature. 347:645–650. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Juge-Aubry C, Pernin A, Favez T, Burger
AG, Wahli W, Meier CA and Desvergne B: DNA binding properties of
peroxisome proliferator-activated receptor subtypes on various
natural peroxisome proliferator response elements. Importance of
the 5-flanking region. J Biol Chem. 272:25252–25259. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vamecq J and Latruffe N: Medical
significance of peroxisome proliferator-activated receptors.
Lancet. 354:141–148. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandra V, Huang P, Hamuro Y, Raghuram S,
Wang Y, Burris TP and Rastinejad F: Structure of the intact
PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature.
456:350–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burns KA and Vanden Heuvel JP: Modulation
of PPAR activity via phosphorylation. Biochim Biophys Acta.
1771:952–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bookout AL, Jeong Y, Downes M, Yu RT,
Evans RM and Mangelsdorf DJ: Anatomical profiling of nuclear
receptor expression reveals a hierarchical transcriptional network.
Cell. 126:789–799. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonnelye E, Vanacker JM, Dittmar T, Begue
A, Desbiens X, Denhardt DT, Aubin JE, Laudet V and Fournier B: The
ERR-1 orphan receptor is a transcriptional activator expressed
during bone development. Mol Endocrinol. 11:905–916. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heard DJ, Norby PL, Holloway J and Vissing
H: Human ERRgamma, a third member of the estrogen receptor-related
receptor (ERR) subfamily of orphan nuclear receptors:
Tissue-specific isoforms are expressed during development and in
the adult. Mol Endocrinol. 14:382–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonnelye E, Vanacker JM, Spruyt N, Alric
S, Fournier B, Desbiens X and Laudet V: Expression of the
estrogen-related receptor 1 (ERR-1) orphan receptor during mouse
development. Mech Dev. 65:71–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carnesecchi J and Vanacker JM:
Estrogen-related receptors and the control of bone cell fate. Mol
Cell Endocrinol. 432:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Likhite N, Yadav V, Milliman EJ,
Sopariwala DH, Lorca S, Narayana NP, Sheth M, Reineke EL, Giguère V
and Narkar V: Loss of estrogen-related receptor alpha facilitates
angiogenesis in endothelial cells. Mol Cell Biol. 39:392019.
View Article : Google Scholar
|
|
20
|
Sun P, Sehouli J, Denkert C, Mustea A,
Könsgen D, Koch I, Wei L and Lichtenegger W: Expression of estrogen
receptor-related receptors, a subfamily of orphan nuclear
receptors, as new tumor biomarkers in ovarian cancer cells. J Mol
Med (Berl). 83:457–467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnston SD, Liu X, Zuo F, Eisenbraun TL,
Wiley SR, Kraus RJ and Mertz JE: Estrogen-related receptor alpha 1
functionally binds as a monomer to extended half-site sequences
including ones contained within estrogen-response elements. Mol
Endocrinol. 11:342–352. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wende AR, Huss JM, Schaeffer PJ, Giguère V
and Kelly DP: PGC-1alpha coactivates PDK4 gene expression via the
orphan nuclear receptor ERRalpha: A mechanism for transcriptional
control of muscle glucose metabolism. Mol Cell Biol.
25:10684–10694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon JC, Puigserver P, Chen G, Donovan J,
Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al:
Control of hepatic gluconeogenesis through the transcriptional
coactivator PGC-1. Nature. 413:131–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitamura K, Erlangga JS, Tsukamoto S,
Sakamoto Y, Mabashi-Asazuma H and Iida K: Daidzein promotes the
expression of oxidative phosphorylation- and fatty acid
oxidation-related genes via an estrogen-related receptor α pathway
to decrease lipid accumulation in muscle cells. J Nutr Biochem.
77:1083152020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cartoni R, Léger B, Hock MB, Praz M,
Crettenand A, Pich S, Ziltener JL, Luthi F, Dériaz O, Zorzano A, et
al: Mitofusins 1/2 and ERRalpha expression are increased in human
skeletal muscle after physical exercise. J Physiol. 567:349–358.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soriano FX, Liesa M, Bach D, Chan DC,
Palacín M and Zorzano A: Evidence for a mitochondrial regulatory
pathway defined by peroxisome proliferator-activated receptor-gamma
coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin
2. Diabetes. 55:1783–1791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rangwala SM, Li X, Lindsley L, Wang X,
Shaughnessy S, Daniels TG, Szustakowski J, Nirmala NR, Wu Z and
Stevenson SC: Estrogen-related receptor alpha is essential for the
expression of antioxidant protection genes and mitochondrial
function. Biochem Biophys Res Commun. 357:231–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herzog B, Cardenas J, Hall RK, Villena JA,
Budge PJ, Giguère V, Granner DK and Kralli A: Estrogen-related
receptor alpha is a repressor of phosphoenolpyruvate carboxykinase
gene transcription. J Biol Chem. 281:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ranhotra HS: Estrogen-related receptor
alpha and mitochondria: Tale of the titans. J Recept Signal
Transduct Res. 35:386–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vega RB and Kelly DP: A role for
estrogen-related receptor alpha in the control of mitochondrial
fatty acid beta-oxidation during brown adipocyte differentiation. J
Biol Chem. 272:31693–31699. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
LeBleu VS, O'Connell JT, Gonzalez Herrera
KN, Wikman H, Pantel K, Haigis MC, Machado de Carvalho F, Damascena
A, Domingos Chinen LT, Rocha RM, et al: PGC-1α mediates
mitochondrial biogenesis and oxidative phosphorylation in cancer
cells to promote metastasis. Nat Cell Biol. 16:992–1003, 1001-1015.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Villena JA and Kralli A: ERRalpha: A
metabolic function for the oldest orphan. Trends Endocrinol Metab.
19:269–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aprile M, Ambrosio MR, D'Esposito V,
Beguinot F, Formisano P, Costa V and Ciccodicola A: PPARG in human
adipogenesis: Differential contribution of canonical transcripts
and dominant negative isoforms. PPAR Res. 2014:5378652014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salgia MM, Elix CC, Pal SK and Jones JO:
Different roles of peroxisome proliferator-activated receptor gamma
isoforms in prostate cancer. Am J Clin Exp Urol. 7:98–109.
2019.PubMed/NCBI
|
|
35
|
Braissant O, Foufelle F, Scotto C, Dauça M
and Wahli W: Differential expression of peroxisome
proliferator-activated receptors (PPARs): Tissue distribution of
PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology.
137:354–366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meirhaeghe A, Fajas L, Gouilleux F, Cottel
D, Helbecque N, Auwerx J and Amouyel P: A functional polymorphism
in a STAT5B site of the human PPAR gamma 3 gene promoter affects
height and lipid metabolism in a French population. Arterioscler
Thromb Vasc Biol. 23:289–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martin H: Role of PPAR-gamma in
inflammation. Prospects for therapeutic intervention by food
components. Mutat Res. 669:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanis SP, Colca JR, Parker TT, Artman GD
III, Larsen SD, McDonald WG, Gadwood RC, Kletzien RF, Zeller JB,
Lee PH, et al: PPARγ-sparing thiazolidinediones as insulin
sensitizers. Design, synthesis and selection of compounds for
clinical development. Bioorg Med Chem. 26:5870–5884. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Finch ER, Tukaramrao DB, Goodfield LL,
Quickel MD, Paulson RF and Prabhu KS: Activation of PPARγ by
endogenous prostaglandin J2 mediates the antileukemic effect of
selenium in murine leukemia. Blood. 129:1802–1810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Michalik L, Desvergne B, Dreyer C,
Gavillet M, Laurini RN and Wahli W: PPAR expression and function
during vertebrate development. Int J Dev Biol. 46:105–114.
2002.PubMed/NCBI
|
|
41
|
Lapsys NM, Kriketos AD, Lim-Fraser M,
Poynten AM, Lowy A, Furler SM, Chisholm DJ and Cooney GJ:
Expression of genes involved in lipid metabolism correlate with
peroxisome proliferator-activated receptor gamma expression in
human skeletal muscle. J Clin Endocrinol Metab. 85:4293–4297. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iwata M, Haruta T, Usui I, Takata Y,
Takano A, Uno T, Kawahara J, Ueno E, Sasaoka T, Ishibashi O, et al:
Pioglitazone ameliorates tumor necrosis factor-alpha-induced
insulin resistance by a mechanism independent of adipogenic
activity of peroxisome proliferator - activated receptor-gamma.
Diabetes. 50:1083–1092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ricote M, Huang J, Fajas L, Li A, Welch J,
Najib J, Witztum JL, Auwerx J, Palinski W and Glass CK: Expression
of the peroxisome proliferator-activated receptor gamma (PPARgamma)
in human atherosclerosis and regulation in macrophages by colony
stimulating factors and oxidized low density lipoprotein. Proc Natl
Acad Sci USA. 95:7614–7619. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamashita H, Kawasawa YI, Shuman L, Zheng
Z, Tran T, Walter V, Warrick JI, Chen G, Al-Ahmadie H, Kaag M, et
al: Repression of transcription factor AP-2 alpha by PPARγ reveals
a novel transcriptional circuit in basal-squamous bladder cancer.
Oncogenesis. 8:692019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan W and Evans R: PPARs and ERRs:
Molecular mediators of mitochondrial metabolism. Curr Opin Cell
Biol. 33:49–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Teng CT, Li Y, Stockton P and Foley J:
Fasting induces the expression of PGC-1α and ERR isoforms in the
outer stripe of the outer medulla (OSOM) of the mouse kidney. PLoS
One. 6:e269612011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Angajala A, Lim S, Phillips JB, Kim JH,
Yates C, You Z and Tan M: Diverse roles of mitochondria in immune
responses: novel insights into immuno-metabolism. Front Immunol.
9:16052018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei W, Wang X, Yang M, Smith LC, Dechow
PC, Sonoda J, Evans RM and Wan Y: PGC1beta mediates PPARgamma
activation of osteoclastogenesis and rosiglitazone-induced bone
loss. Cell Metab. 11:503–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Scarpulla RC: Metabolic control of
mitochondrial biogenesis through the PGC-1 family regulatory
network. Biochim Biophys Acta. 1813:1269–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xia Y, Buja LM, Scarpulla RC and McMillin
JB: Electrical stimulation of neonatal cardiomyocytes results in
the sequential activation of nuclear genes governing mitochondrial
proliferation and differentiation. Proc Natl Acad Sci USA.
94:11399–11404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huss JM, Kopp RP and Kelly DP: Peroxisome
proliferator-activated receptor coactivator-1alpha (PGC-1alpha)
coactivates the cardiac-enriched nuclear receptors estrogen-related
receptor-alpha and -gamma. Identification of novel leucine-rich
interaction motif within PGC-1alpha. J Biol Chem. 277:40265–40274.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fliegner D, Westermann D, Riad A, Schubert
C, Becher E, Fielitz J, Tschöpe C and Regitz-Zagrosek V:
Up-regulation of PPARgamma in myocardial infarction. Eur J Heart
Fail. 10:30–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gandhi GR, Stalin A, Balakrishna K,
Ignacimuthu S, Paulraj MG and Vishal R: Insulin sensitization via
partial agonism of PPARγ and glucose uptake through translocation
and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin
in type 2 diabetic rats. Biochim Biophys Acta. 1830:2243–2255.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xia H, Dufour CR and Giguère V: ERRα as a
bridge between transcription and function: Role in liver metabolism
and disease. Front Endocrinol (Lausanne). 10:2062019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schreiber SN, Emter R, Hock MB, Knutti D,
Cardenas J, Podvinec M, Oakeley EJ and Kralli A: The
estrogen-related receptor alpha (ERRalpha) functions in PPARgamma
coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis.
Proc Natl Acad Sci USA. 101:6472–6477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rutanen J, Yaluri N, Modi S, Pihlajamäki
J, Vänttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M,
Sinclair DA, et al: SIRT1 mRNA expression may be associated with
energy expenditure and insulin sensitivity. Diabetes. 59:829–835.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
B'chir W, Dufour CR, Ouellet C, Yan M, Tam
IS, Andrzejewski S, Xia H, Nabata K, St-Pierre J and Giguère V:
Divergent role of estrogen-related receptor α in lipid- and
fasting-induced hepatic steatosis in mice. Endocrinology.
159:2153–2164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chaveroux C, Eichner LJ, Dufour CR,
Shatnawi A, Khoutorsky A, Bourque G, Sonenberg N and Giguère V:
Molecular and genetic crosstalks between mTOR and ERRα are key
determinants of rapamycin-induced nonalcoholic fatty liver. Cell
Metab. 17:586–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sakamoto T, Matsuura TR, Wan S, Ryba DM,
Kim JU, Won KJ, Lai L, Petucci C, Petrenko N, Musunuru K, et al: A
Critical Role for Estrogen-Related Receptor Signaling in Cardiac
Maturation. Circ Res. 126:1685–1702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mitra MS, Schilling JD, Wang X, Jay PY,
Huss JM, Su X and Finck BN: Cardiac lipin 1 expression is regulated
by the peroxisome proliferator activated receptor γ coactivator
1α/estrogen related receptor axis. J Mol Cell Cardiol. 51:120–128.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gomez-Arroyo J, Mizuno S, Szczepanek K,
Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L,
Kraskauskas D, Wijesinghe DS, et al: Metabolic gene remodeling and
mitochondrial dysfunction in failing right ventricular hypertrophy
secondary to pulmonary arterial hypertension. Circ Heart Fail.
6:136–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Watson PA, Birdsey N, Huggins GS, Svensson
E, Heppe D and Knaub L: Cardiac-specific overexpression of
dominant-negative CREB leads to increased mortality and
mitochondrial dysfunction in female mice. Am J Physiol Heart Circ
Physiol. 299:H2056–H2068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sun PM, Gao M, Wei LH, Mustea A, Wang JL,
Könsgen D, Lichtenegger W and Sehouli J: An estrogen receptor
alpha-dependent regulation of estrogen receptor-related receptor
alpha in the proliferation of endometrial carcinoma cells. Int J
Gynecol Cancer. 16 (Suppl 2):564–568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stein RA, Gaillard S and McDonnell DP:
Estrogen-related receptor alpha induces the expression of vascular
endothelial growth factor in breast cancer cells. J Steroid Biochem
Mol Biol. 114:106–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cheung CP, Yu S, Wong KB, Chan LW, Lai FM,
Wang X, Suetsugi M, Chen S and Chan FL: Expression and functional
study of estrogen receptor-related receptors in human prostatic
cells and tissues. J Clin Endocrinol Metab. 90:1830–1844. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fujimoto J and Sato E: Clinical
implication of estrogen-related receptor (ERR) expression in
uterine endometrial cancers. J Steroid Biochem Mol Biol. 116:71–75.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kraus RJ, Ariazi EA, Farrell ML and Mertz
JE: Estrogen-related receptor alpha 1 actively antagonizes estrogen
receptor-regulated transcription in MCF-7 mammary cells. J Biol
Chem. 277:24826–24834. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ma JH, Qi J, Lin SQ, Zhang CY, Liu FY, Xie
WD and Li X: STAT3 targets ERR-α to promote epithelial-mesenchymal
transition, migration, and invasion in triple-negative breast
cancer cells. Mol Cancer Res. 17:2184–2195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fujimura T, Takahashi S, Urano T, Kumagai
J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M and
Inoue S: Increased expression of estrogen-related receptor alpha
(ERRalpha) is a negative prognostic predictor in human prostate
cancer. Int J Cancer. 120:2325–2330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hong F, Pan S, Guo Y, Xu P and Zhai Y:
PPARs as nuclear receptors for nutrient and energy metabolism.
Molecules. 24:25452019. View Article : Google Scholar
|
|
73
|
Fougerat A, Montagner A, Loiseau N,
Guillou H and Wahli W: Peroxisome proliferator-activated receptors
and their novel ligands as candidates for the treatment of
non-alcoholic fatty liver disease. Cells. 9:92020. View Article : Google Scholar
|
|
74
|
Shi Y, Zou Y, Shen Z, Xiong Y, Zhang W,
Liu C and Chen S: Trace elements, PPARs, and metabolic syndrome.
Int J Mol Sci. 21:212020. View Article : Google Scholar
|
|
75
|
Thangavel N, Al Bratty M, Akhtar Javed S,
Ahsan W and Alhazmi HA: Targeting peroxisome proliferator-activated
receptors using thiazolidinediones: Strategy for design of novel
antidiabetic drugs. Int J Med Chem. 2017:10697182017.PubMed/NCBI
|
|
76
|
Ribon V, Johnson JH, Camp HS and Saltiel
AR: Thiazolidinediones and insulin resistance: Peroxisome
proliferatoractivated receptor gamma activation stimulates
expression of the CAP gene. Proc Natl Acad Sci USA. 95:14751–14756.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schinner S, Dellas C, Schroder M, Heinlein
CA, Chang C, Fischer J and Knepel W: Repression of glucagon gene
transcription by peroxisome proliferator-activated receptor gamma
through inhibition of Pax6 transcriptional activity. J Biol Chem.
277:1941–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tontonoz P, Nagy L, Alvarez JG, Thomazy VA
and Evans RM: PPARgamma promotes monocyte/macrophage
differentiation and uptake of oxidized LDL. Cell. 93:241–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Maréchal L, Laviolette M, Rodrigue-Way A,
Sow B, Brochu M, Caron V and Tremblay A: The CD36-PPARγ pathway in
metabolic disorders. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
80
|
Chawla A, Barak Y, Nagy L, Liao D,
Tontonoz P and Evans RM: PPAR-gamma dependent and independent
effects on macrophage-gene expression in lipid metabolism and
inflammation. Nat Med. 7:48–52. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chandra M, Miriyala S and Panchatcharam M:
PPARγ and its role in cardiovascular diseases. PPAR Res.
2017:64046382017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fukunaga Y, Itoh H, Doi K, Tanaka T,
Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, Saito T, et
al: Thiazolidinediones, peroxisome proliferator-activated receptor
gamma agonists, regulate endothelial cell growth and secretion of
vasoactive peptides. Atherosclerosis. 158:113–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chang JS and Ha K: A truncated PPAR gamma
2 localizes to mitochondria and regulates mitochondrial respiration
in brown adipocytes. PLoS One. 13:e01950072018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dong JT: Anticancer activities of PPARγ in
breast cancer are context-dependent. Am J Pathol. 182:1972–1975.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lin SJ, Lin CY, Yang DR, Izumi K, Yan E,
Niu X, Chang HC, Miyamoto H, Wang N, Li G, et al: The differential
effects of anti-diabetic thiazolidinedione on prostate cancer
progression are linked to the TR4 nuclear receptor expression
status. Neoplasia. 17:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lakshmi SP, Reddy AT, Banno A and Reddy
RC: PPAR agonists for the prevention and treatment of lung cancer.
PPAR Res. 2017:82527962017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yousefnia S, Momenzadeh S, Seyed Forootan
F, Ghaedi K and Nasr Esfahani MH: The influence of peroxisome
proliferator-activated receptor γ (PPARγ) ligands on cancer cell
tumorigenicity. Gene. 649:14–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Holland CM, Saidi SA, Evans AL, Sharkey
AM, Latimer JA, Crawford RA, Charnock-Jones DS, Print CG and Smith
SK: Transcriptome analysis of endometrial cancer identifies
peroxisome proliferator-activated receptors as potential
therapeutic targets. Mol Cancer Ther. 3:993–1001. 2004.PubMed/NCBI
|
|
89
|
Zhang GY, Ahmed N, Riley C, Oliva K,
Barker G, Quinn MA and Rice GE: Enhanced expression of peroxisome
proliferator-activated receptor gamma in epithelial ovarian
carcinoma. Br J Cancer. 92:113–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Davidson B, Hadar R, Stavnes HT, Trope' CG
and Reich R: Expression of the peroxisome proliferator-activated
receptors-alpha, -beta, and -gamma in ovarian carcinoma effusions
is associated with poor chemoresponse and shorter survival. Hum
Pathol. 40:705–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luo S, Wang J, Ma Y, Yao Z and Pan H:
PPARγ inhibits ovarian cancer cells proliferation through
upregulation of miR-125b. Biochem Biophys Res Commun. 462:85–90.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ogino S, Shima K, Baba Y, Nosho K, Irahara
N, Kure S, Chen L, Toyoda S, Kirkner GJ, Wang YL, et al: Colorectal
cancer expression of peroxisome proliferator-activated receptor
gamma (PPARG, PPARgamma) is associated with good prognosis.
Gastroenterology. 136:1242–1250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Reka AK, Kurapati H, Narala VR, Bommer G,
Chen J, Standiford TJ and Keshamouni VG: Peroxisome
proliferator-activated receptor-gamma activation inhibits tumor
metastasis by antagonizing Smad3-mediated epithelial-mesenchymal
transition. Mol Cancer Ther. 9:3221–3232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dong YW, Wang XP and Wu K: Suppression of
pancreatic carcinoma growth by activating peroxisome
proliferator-activated receptor gamma involves angiogenesis
inhibition. World J Gastroenterol. 15:441–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
He Q, Pang R, Song X, Chen J, Chen H, Chen
B, Hu P and Chen M: Rosiglitazone suppresses the growth and
invasiveness of SGC-7901 gastric cancer cells and angiogenesis in
vitro via PPARgamma dependent and independent mechanisms. PPAR Res.
2008:6498082008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang YX: PPARs: Diverse regulators in
energy metabolism and metabolic diseases. Cell Res. 20:124–137.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ijichi N, Ikeda K, Horie-Inoue K, Yagi K,
Okazaki Y and Inoue S: Estrogen-related receptor alpha modulates
the expression of adipogenesis-related genes during adipocyte
differentiation. Biochem Biophys Res Commun. 358:813–818. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Prabhu DS and Rajeswari VD: PPAR-Gamma as
putative gene target involved in Butein mediated anti-diabetic
effect. Mol Biol Rep. 47:5273–5283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lebovitz HE: Thiazolidinediones: The
forgotten diabetes medications. Curr Diab Rep. 19:1512019.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Heneka MT, Sastre M, Dumitrescu-Ozimek L,
Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T, Van
Leuven F and Landreth GE: Acute treatment with the PPARgamma
agonist pioglitazone and ibuprofen reduces glial inflammation and
Abeta1-42 levels in APPV717I transgenic mice. Brain. 128:1442–1453.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wuertz BR, Darrah L, Wudel J and Ondrey
FG: Thiazolidinediones abrogate cervical cancer growth. Exp Cell
Res. 353:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sanghvi M, Moaddel R, Frazier C, Gandhari
M and Wainer IW: Abstract 5500: Biochromatography for the screening
of new drugs for the estrogen related receptors. Cancer Res.
70:55002010.
|