Introduction
Inflammation is an organism's defense response to
extrinsic stimuli or diseases. It is mediated by multiple processes
involving various cells and cytokines. External stimuli, such as
lipopolysaccharide (LPS), or internal stimuli, such as arachidonic
acid metabolites, induce the infiltration of inflammatory cells,
such as macrophages and granulocytes, into target sites (1).
Macrophages are inflammatory cells that protect
organisms from external pathogens by releasing inflammatory
mediators and cytokines, such as nitric oxide (NO), inducible NO
synthase (iNOS), TNF-α and IL-6 (2). Activated macrophages are involved in
the early phase of infection by producing NO or reactive oxygen
species (ROS), as well as cytokines, leading to an inflammatory
cascade (1). Under inflammatory
conditions, the excessive release of pro-inflammatory mediators and
cytokines induces injury of cells and tissues, and may lead to
chronic inflammatory diseases (2).
Therefore, the control of inflammatory responses is necessary for
the prevention of chronic inflammatory diseases (2). The currently employed non-steroidal
anti-inflammatory drugs are known to cause side effects, such as
heartburn and indigestion. Therefore, previous study has aimed to
develop anti-inflammatory drugs from natural products (2).
Heme oxygenase (HO) is an enzyme induced by
oxidative stress that promotes heme degradation. HO exists in three
isoforms: HO-1, −2 and −3 (1). HO-1
protects cells from harmful free radicals and NO, and controls
inflammatory reactions. HO-1 is regulated at the transcriptional
level and is related to nuclear factor erythroid 2-related factor 2
(Nrf2), a basic leucine zipper protein that regulates the
expression of antioxidant proteins to prevent oxidative damage
induced by wounds and inflammation (3). Under physiological conditions, Nrf2 is
bound to Kelch-like ECH-associated protein 1 (Keap1); however, it
is released from Keap1 for nuclear translocation following HO-1
stimulation through antioxidant response elements (3).
Perilla frutescens is a member of the
Labiatae family (4). It is
native to the highlands of Southeast Asia and India and
traditionally grown as crops in Korea, Japan, India and China. As a
medicinal plant, P. frutescens is used as a treatment for
cough, vomiting, cold, constipation and abdominal pain (5). P. frutescens emits a unique
odor owing to the presence of aromatic ingredients, such as
perillaketones and perillaldehydes, in addition to other compounds,
including luteolins, catechins, ferulins, rosmarinic acids and
apigenin (6). Furthermore, it
reportedly exhibits various pharmacological properties, including
antioxidant, antiallergic, and anti-inflammatory effects (6).
The plant material used in the present study was the
radiation mutant P. frutescens var. crispa. This
mutant obtained by exposing the seeds to γ-ray irradiation carries
a higher content of isoegomaketone (IK) compared with the original
cultivar (7). In previous studies,
IK was identified as an essential component of P.
frutescens, exhibiting various anti-inflammatory (8), anti-cancer (9), antioxidant (8,10),
anti-arthritic (11) and
anti-obesity (12) effects. 9-HIK
is a novel compound isolated from the radiation mutant P.
frutescens var. crispa extract, in which it is present
in 8.8-fold higher concentrations than those of the wild-type
(13). 9-HIK was shown to inhibit
the production of NO in LPS-treated RAW264.7 cells, although with
less potency than IK (13). The
9-HIK structure has a hydroxyl group attached to the carbon 9 of IK
(13).
The leaves and seeds of P. frutescens are
important for the development of natural drugs, owing to the
presence of substances with various biological activities,
including antioxidant, anti-inflammatory and antiallergic effects
(6). Therefore, the aim of the
present study was to determine whether 9-HIK could attenuate
inflammation in RAW264.7 cells.
Materials and methods
Materials
In the present study, 9-HIK was isolated as
described previously (13).
Dulbecco's modified Eagle's medium (DMEM), FBS and
penicillin-streptomycin were purchased from Hyclone (Cytiva).
Reagents, such as LPS, DMSO, Griess reagent, N-acetyl-L-cysteine
(NAC, an ROS scavenger), NP40 cell lysis buffer and protease
inhibitor cocktail were purchased from Sigma-Aldrich (Merck KGaA).
An EZ-Cytox cell viability assay kit was purchased from Daeil Lab
Service, Co., Ltd. An RNeasy kit was purchased from Qiagen GmbH. A
PrimeScript™ II 1st strand cDNA synthesis kit and TB
Green® premix Ex Taq™ (Tli RNaseH Plus) were obtained
from Takara Bio, Inc. A Chromo 4 RT-PCR detection system was
purchased from Bio-Rad Laboratories, Inc. Rabbit polyclonal
antibodies against β-tubulin (cat. no. SC-9104) and HO-1 (cat. no.
SC-10789) were obtained from Santa Cruz Biotechnology, Inc. Rabbit
polyclonal antibodies against iNOS (cat. no. 2977S), Nrf2 (cat. no.
12721S) and lamin B (cat. no. 13435S) were purchased from Cell
Signaling Technology, Inc. Goat anti-rabbit IgG HRP-conjugated
secondary antibody (cat. no. A16110) was supplied by Invitrogen
(Thermo Fisher Scientific, Inc.). ELISA kits for IL-6 detection
(cat. no. SM6000B) were purchased from R&D Systems, Inc. The
ELISA kit for interferon (IFN)-β (cat. no. 42400-2) was obtained
from Pestka Biomedical Laboratories, Inc.
Reagent preparation
9-HIK was dissolved in DMSO at a stock concentration
of 50 mM, then further diluted in DMSO for further use. Griess
reagent was dissolved in distilled water. LPS was dissolved in
distilled water to make 1 mg/ml stock solution and then further
diluted in DMSO for subsequent use. NAC was dissolved in DMSO to
make 50 mM stock solution and then further diluted in DMEM for
subsequent use. DMSO alone was used as a control and the final DMSO
concentration in the cell culture was ≤0.1% (v/v).
Cell culture
The RAW264.7 macrophage cell line was purchased from
the American Type Culture Collection. RAW264.7 cells were cultured
in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/m
streptomycin at 37°C with 5% CO2.
Cytotoxicity assay
Cell viability was measured using the EZ-Cytox assay
kit. RAW264.7 macrophages were seeded at a density of
2.0×105 cells/ml in 96-well plates. The cells were then
treated with 5, 10, 15, 20 or 40 µM 9-HIK. After 24 h of
incubation, 10 µl EZ-Cytox assay reagent was added to each well and
incubated at 37°C and 5% CO2 for 4 h. The cell viability
index was determined by using a Benchmark Plus spectrophotometer
(Bio-Rad Laboratories, Inc.) at 480 nm with a reference wavelength
of 650 nm. The results are presented as the mean ± SD of six
replicates from a single experiment.
NO production assay
RAW264.7 cells were seeded at a density of
2.0×105 cells/ml in 96-well plates. The cells were
pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with
1 µg/ml LPS for an additional 18 h at 37°C with 5% CO2
(14). The culture supernatant (100
µl) was mixed with an equal volume of Griess reagent (100 µl) in a
96-well plate and incubated for 15 min at room temperature.
Absorbance was measured at 540 nm using a spectrophotometer. The
results are presented as the mean ± SD of three replicates from one
experiment.
Western blot analysis
RAW264.7 cells were cultured in a 100-mm culture
dish at a density of 2.0×105 cells/ml for 24 h. The
cells were pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then
stimulated with 1 µg/ml LPS for 18 h to induce iNOS protein
expression. In a separate experiment, the cells were treated with
5, 10 or 20 µM 9-HIK for 12 h to determine HO-1 protein expression.
Cells were treated with 20 µM 9-HIK for 0, 1, 2 or 4 h and then
Nrf2 protein expression levels were measured.
The collected cells were washed once with cold PBS,
then lysed using NP40 cell lysis buffer (with 1 mM
phenylmethylsulfonyl fluoride and 1X protease inhibitor cocktail)
for 30 min on ice. The protein was obtained via centrifugation for
15 min at 16,853 × g at 4°C. The concentration of the cell lysate
was measured using the Bio-Rad Protein Assay kit (Bio-Rad
Laboratories, Inc.). Equal amounts of protein (30 µg) were
separated by SDS-PAGE on 10% gels, then transferred onto a
nitrocellulose membrane (Hybond ECL Nitrocellulose; GE Healthcare).
After blocking the membrane using blocking buffer (PBS with 5%
skimmed milk and 0.05% Tween 20) at room temperature for 1 h, the
membranes were incubated with primary antibodies specific for HO-1
(cat. no. sc-10789), iNOS (cat. no. 2977s), Nrf2 (cat. no. 12721s),
Lamin B (cat. no. 13435s) and β-tubulin (cat. no. sc-9104) at 4°C
overnight. Rabbit polyclonal primary antibodies against iNOS, Nrf2,
and Lamin B were diluted 1:1,000 in 0.1% TBS solution with
Tween® 20 (TBS-T) buffer. Rabbit polyclonal primary
antibodies against HO-1 and β-tubulin were diluted to 1:200 in 0.1%
TBS-T buffer. The membranes were washed three times with TBS-T
buffer for 15 min, then incubated with HRP-conjugated secondary
antibodies for 2 h on a shaker at room temperature. The goat
anti-rabbit IgG HRP-conjugated secondary antibody (cat. no. A16110)
was diluted 1:5,000 in 5% skim milk. The membranes were washed
again, and the protein bands were detected using Amersham ECL™
Prime Western Blotting Detection reagent (Cytiva). The results are
presented as the mean ± standard deviation (SD) of three replicates
for one representative experiment. Protein expression levels were
semi-quantified using ImageJ software (version 1.52; National
Institutes of Health).
Fractionation of cytosol and nuclear
extracts
RAW264.7 cells were cultured in a 60-mm culture dish
at a density of 4.0×105 cells/ml for 24 h. Cells were
pre-treated with 20 µM 9-HIK for 0, 1, 2 or 4 h. The cells were
washed once with cold PBS and harvested by pipetting. The collected
cells were mixed in buffer A (10 mM HEPES pH 7.9; 10 mM KCl; 1.5 mM
MgCl2, 0.5 mM DTT; 0.2 mM PMSF) for 10 min on ice, then
collected by centrifugation at 1,873 × g for 5 min at 4°C. To
prepare the cytosolic fraction, the collected cells were mixed with
buffer B (10 mM HEPES pH 7.9; 10 mM KCl; 1.5 mM MgCl2;
0.1% NP40; 0.5 mM DTT; 0.2 mM PMSF). After vortexing for 10 sec,
the cytosolic fractions were centrifuged at 1,873 × g for 5 min at
4°C. To collect the nuclear fraction, the pellets were re-suspended
in buffer C (20 mM HEPES pH 7.9; 420 mM NaCl; 1.5 mM
MgCl2; 25% glycerol; 0.2 mM EDTA; 0.5 mM DTT; 0.2 mM
PMSF) and maintained on ice for 30 min. The nuclear fractions were
centrifuged at 1,873 × g for 15 min at 4°C. The concentration of
the cytosolic and nuclear fraction was measured using the Bio-Rad
Protein Assay kit (Bio-Rad Laboratories, Inc.).
Reverse-transcription quantitative
(RT-q)PCR
RAW264.7 cells were cultured in 6-well plates at a
density of 2.0×105 cells/ml for 24 h. Cells were
pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with
1 µg/ml LPS for 4 h (for IL-6 and IFN-β) or 18 h (for iNOS). For
the measurement of HO-1 expression, cells were treated with 5, 10
or 20 µM 9-HIK for 4 h. To measure HO-1 mRNA expression levels,
RAW264.7 cells treated with 20 µM 9-HIK for 8 h. Also, to measure
the level of HO-1 mRNA expression in ROS scavenger treated cells,
RAW264.7 cells were co-treated with 20 µM 9-HIK and 5 mM NAC for 4
h. Total RNA was isolated using the RNeasy kit. The PrimeScript™ II
1st strand cDNA synthesis kit was used for reverse transcription at
30°C for 10 min, 42°C for 60 min and 95°C for 5 min. The Chromo 4
RT-PCR detection system and TB Green premix were used qPCR
amplification of iNOS, HO-1, IL-6, IFN-β and β-actin using 50
cycles at 94°C for 20 sec, 60°C for 20 sec and 72°C for 30 sec.
Primer sequences are listed in Table
I. The specificity of the amplified PCR products was assessed
via melting curve analysis. Real-time PCR data were calculated as
relative values using GeneXpression Macro chromo4 software program
(version 1.1; Bio-Rad Laboratories, Inc.). The results are
presented as the mean ± SD of three replicates from one
experiment.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) |
|---|
| IL-6 |
AGTTGCCTTCTTGGGACTGA |
TCCACGATTTCCCAGAGAAC |
| IFN-β |
GGAAAGATTGACGTGGGAGA |
AGGCATCAACTGACAGG |
| iNOS |
GGAAAGATTGACGTGGGAGA |
CTCCAATCTCTGCCTATCCGTCTC |
| HO-1 |
TCCTACACCACACCAAACTGTGTG |
CTCCAATCTCTGCCTATCCGTCTC |
| β-actin |
GGCTGTATTCCCCTCCATCG |
CCAGTTGGTAACAATGCCATGT |
Measurement of IL-6 and IFN-β
levels
RAW264.7 cells were cultured in 6-well plates at a
density of 2.0×105 cells/ml for 24 h. The cells were
pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with
1 µg/ml LPS for 4 h. IL-6 and IFN-β levels in the culture
supernatant were measured using ELISA kits, according to the
manufacturer's protocol. The results are presented as the mean ± SD
of three replicates from a single experiment.
Statistical methods
Data are presented as the mean ± SD of three or six
replicates from a single experiment. Differences between groups
were assessed using one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
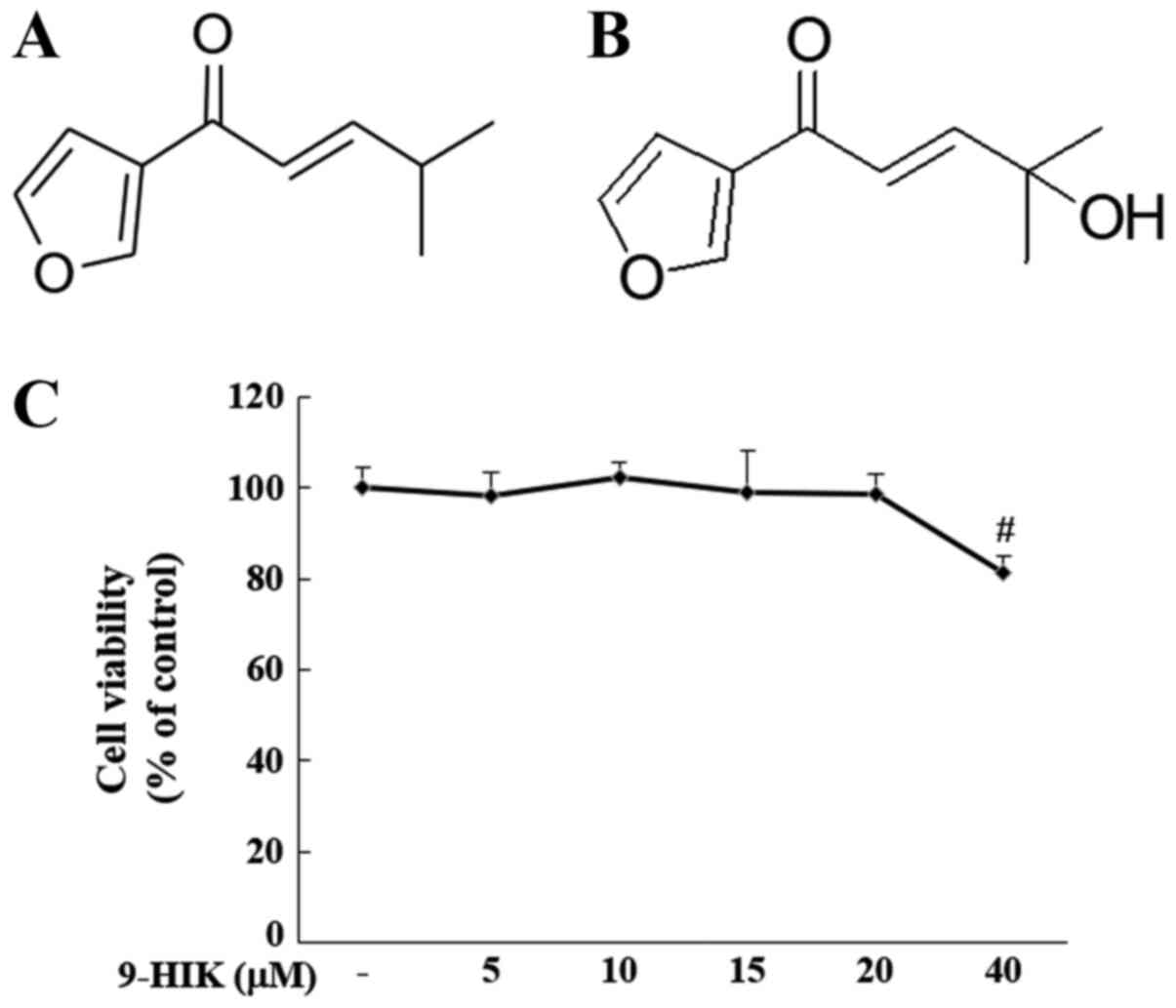
Cytotoxicity of 9-HIK
The effect of 9-HIK on cell viability was evaluated
by treating RAW264.7 cells with 5, 10, 15, 20 and 40 µM 9-HIK for
24 h. Treatment with 9-HIK did not result in cytotoxicity at
concentrations ≤20 µM but reduced cell viability to 81.27% at 40 µΜ
(Fig. 1C). Based on these results,
9-HIK was used at concentrations ≤20 µM in subsequent experiments.
Chemical structure of IK and chemical structure of 9-HIK (Fig. 1A and B).
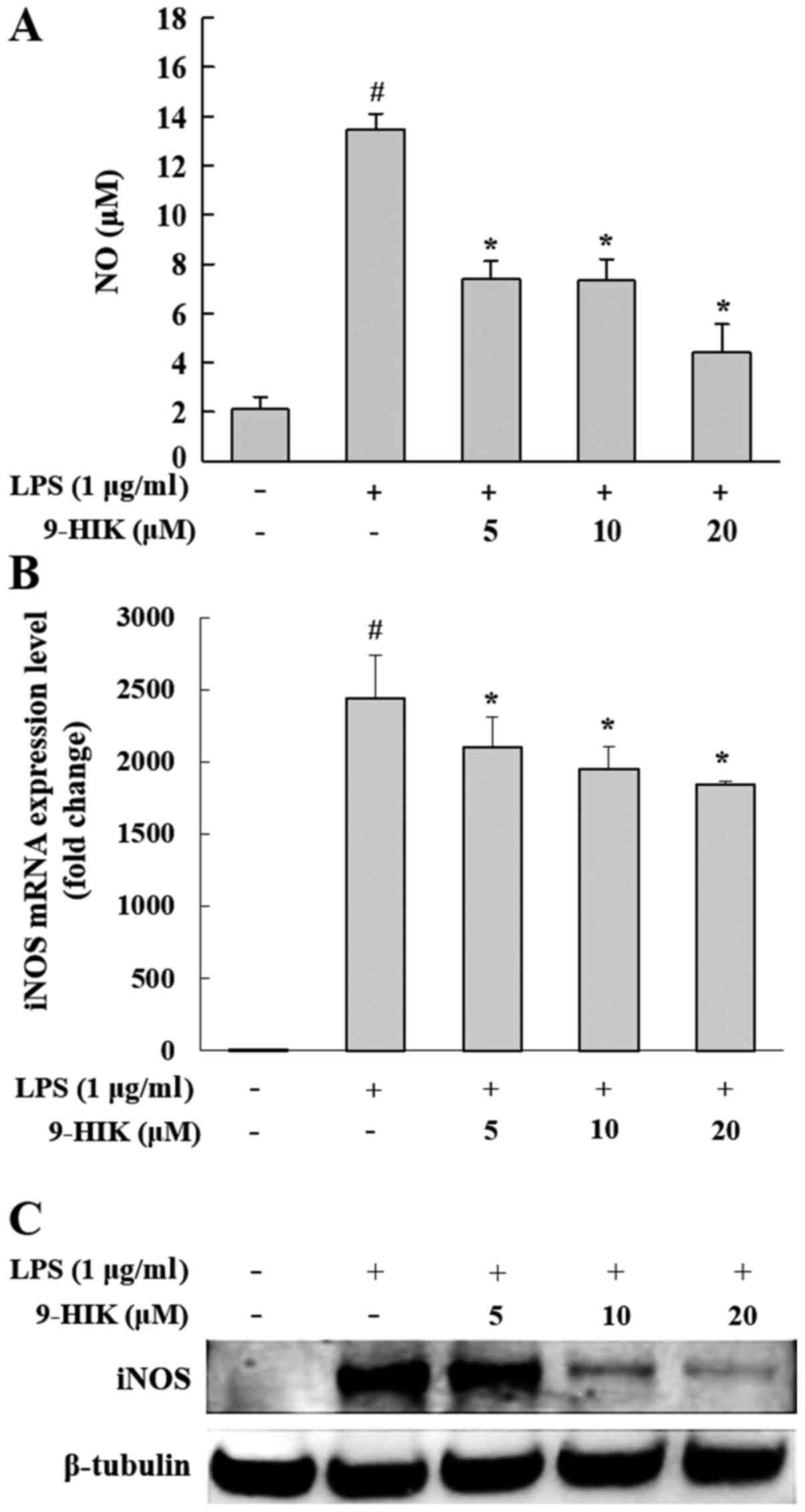
Effect of 9-HIK on NO production and
iNOS expression in LPS-stimulated RAW264.7 cells
NO production by 9-HIK was analyzed in RAW264.7
cells treated with 5, 10 or 20 µM 9-HIK for 2 h, followed by
stimulation with LPS for 18 h. LPS-treated RAW264.7 cells displayed
a 6.4-fold increase in NO production compared with the negative
control. However, NO production was inhibited with an
IC50 value of 14.4 µM by 9-HIK treatment compared with
cells treated with LPS alone (Fig.
2A). To determine whether the inhibition of NO synthesis by
9-HIK could be attributed to the suppression of iNOS expression,
the protein and mRNA expression levels of this enzyme were measured
in LPS-stimulated RAW264.7 cells. 9-HIK treatment inhibited mRNA
and protein expression of iNOS compared with LPS stimulation alone
(Fig. 2B and C). These results
suggested that 9-HIK inhibited NO production in LPS-stimulated
RAW264.7 cells via suppression of iNOS expression.
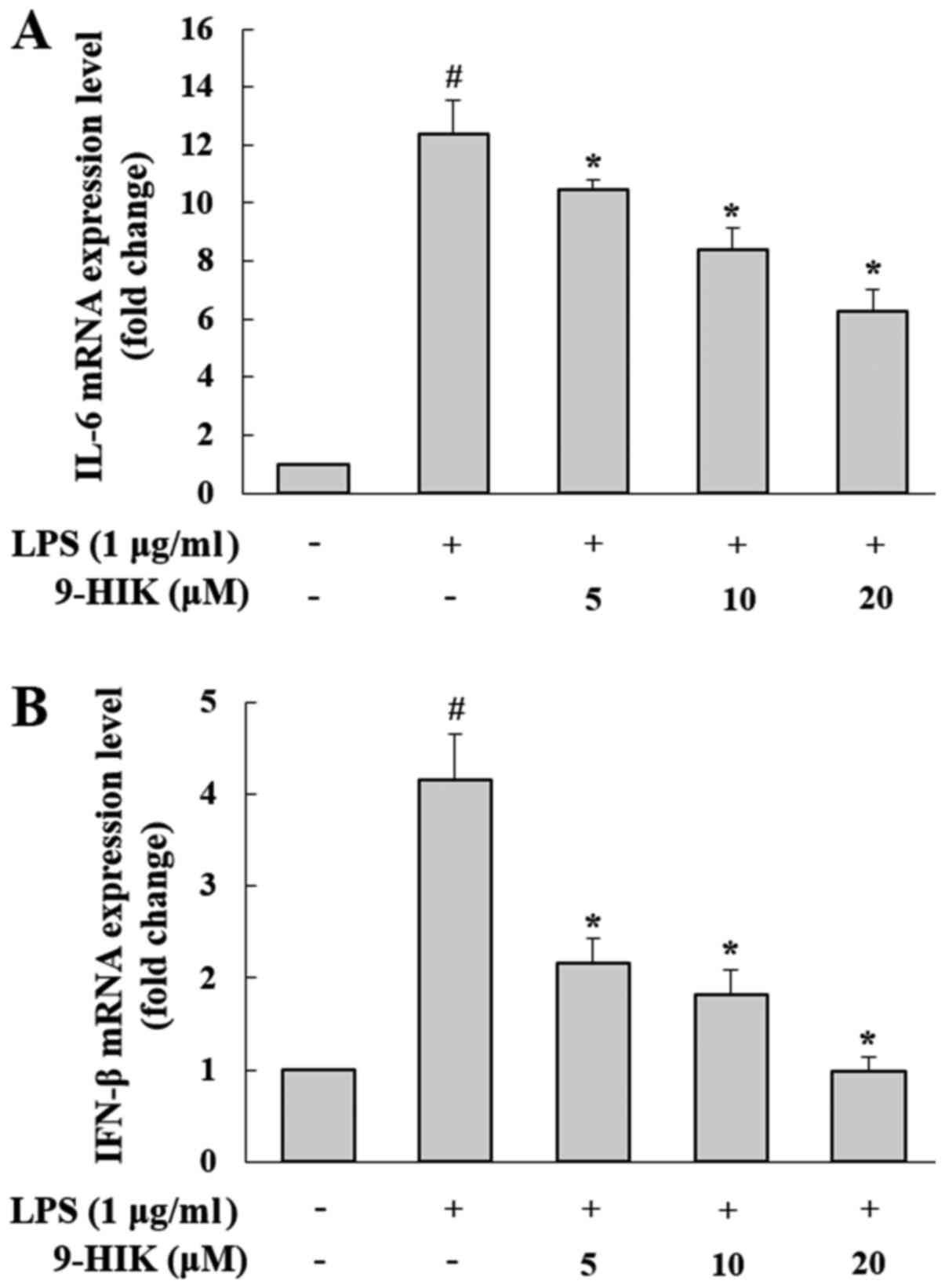
Effect of 9-HIK on LPS-induced
pro-inflammatory cytokine production in RAW264.7 cells
To determine the effects of 9-HIK on the synthesis
of pro-inflammatory mediators, such as IL-6 and IFN-β, RAW264.7
cells were pre-treated with 9-HIK for 2 h at concentrations of 5,
10 and 20 µM, then stimulated with LPS for 4 h. As shown in
Fig. 3, the mRNA expression levels
of both cytokines were increased in LPS-induced RAW264.7 cells,
compared with the control group. However, 9-HIK treatment reduced
pro-inflammatory cytokine transcriptional levels compared with LPS
challenge alone.
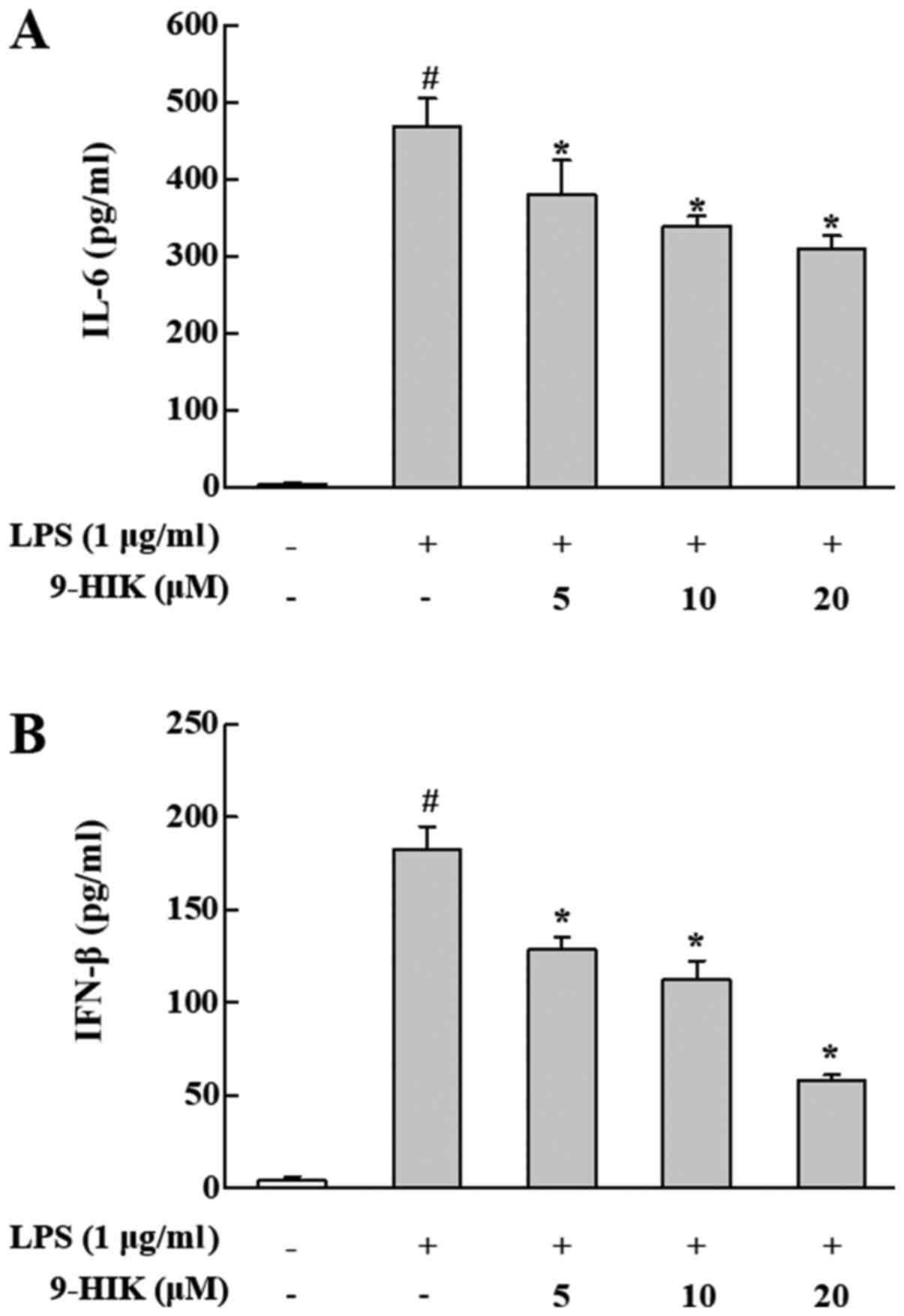
Effects of 9-HIK on IL-6 and IFN-β
protein levels in LPS-stimulated RAW264.7 cells
To further examine the inhibitory effect of 9-HIK on
inflammation in LPS-stimulated RAW264.7 cells, the protein levels
of IFN-β and IL-6 were measured in culture supernatants. RAW264.7
cells were pre-treated with 9-HIK for 2 h at concentrations of 5,
10 and 20 µM, then stimulated with LPS for 4 h. As shown in
Fig. 4, the protein levels of both
cytokines were increased in LPS-induced RAW264.7 cells, compared
with control cells. However, 9-HIK treatment attenuated protein
expression of both cytokines, compared with LPS stimulation alone.
Thus, 9-HIK inhibited the expression of pro-inflammatory cytokines
at the protein level in LPS-stimulated RAW264.7 cells.
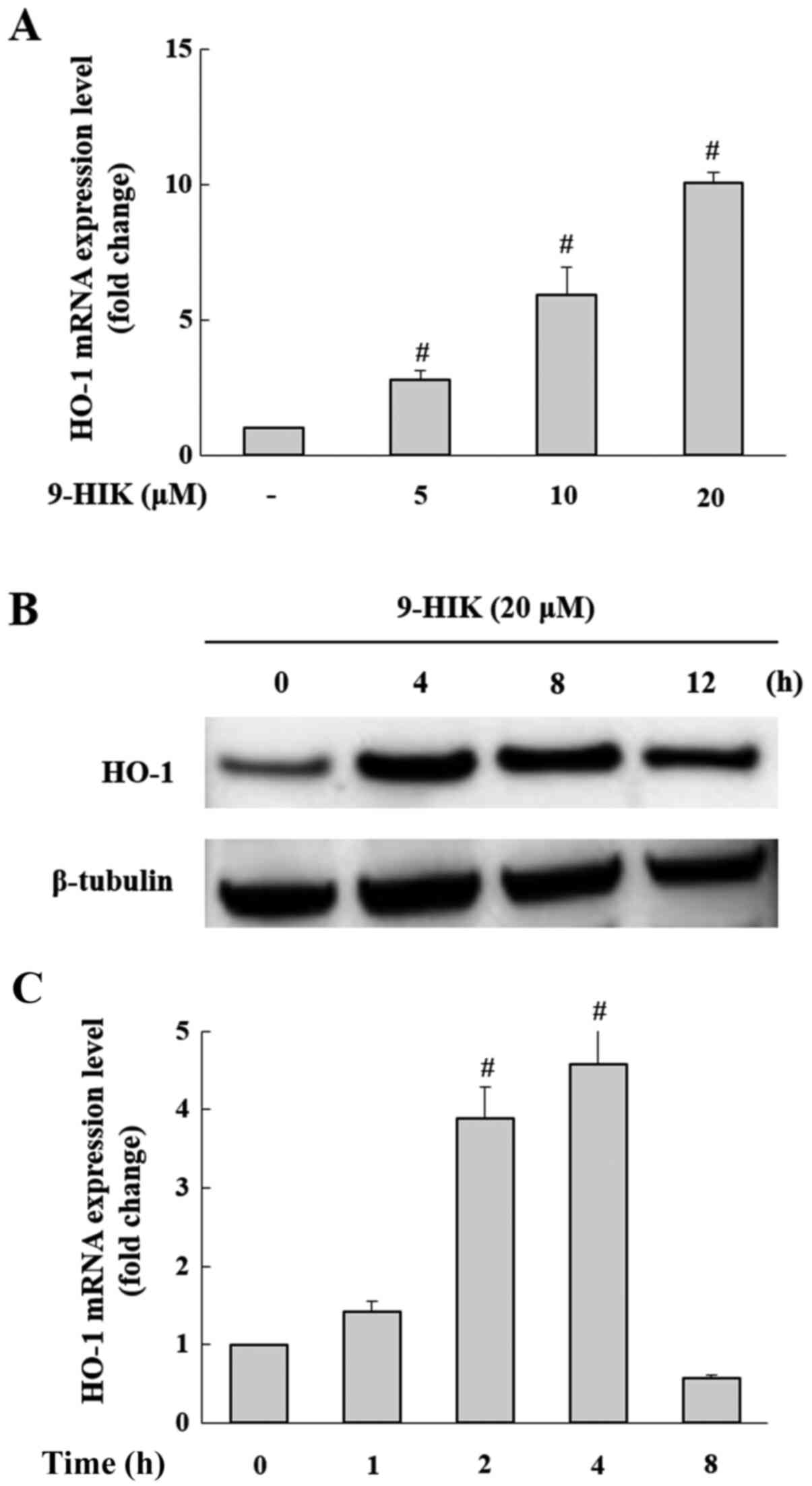
Effects of 9-HIK on HO-1 expression in
RAW264.7 cells
A previous study demonstrated that IK exerted
anti-inflammatory effects through HO-1 expression (10). Therefore, it was hypothesized that
the inhibitory effect of 9-HIK on LPS-stimulated inflammation may
also be mediated by HO-1. Treatment with 9-HIK significantly
increased the HO-1 mRNA (Fig. 5A)
and protein expression levels (Fig.
5B) compared with control cells. HO-1 mRNA and protein
expression reached a maximal increase following 9-HIK treatment for
4 h (Fig. 5C).
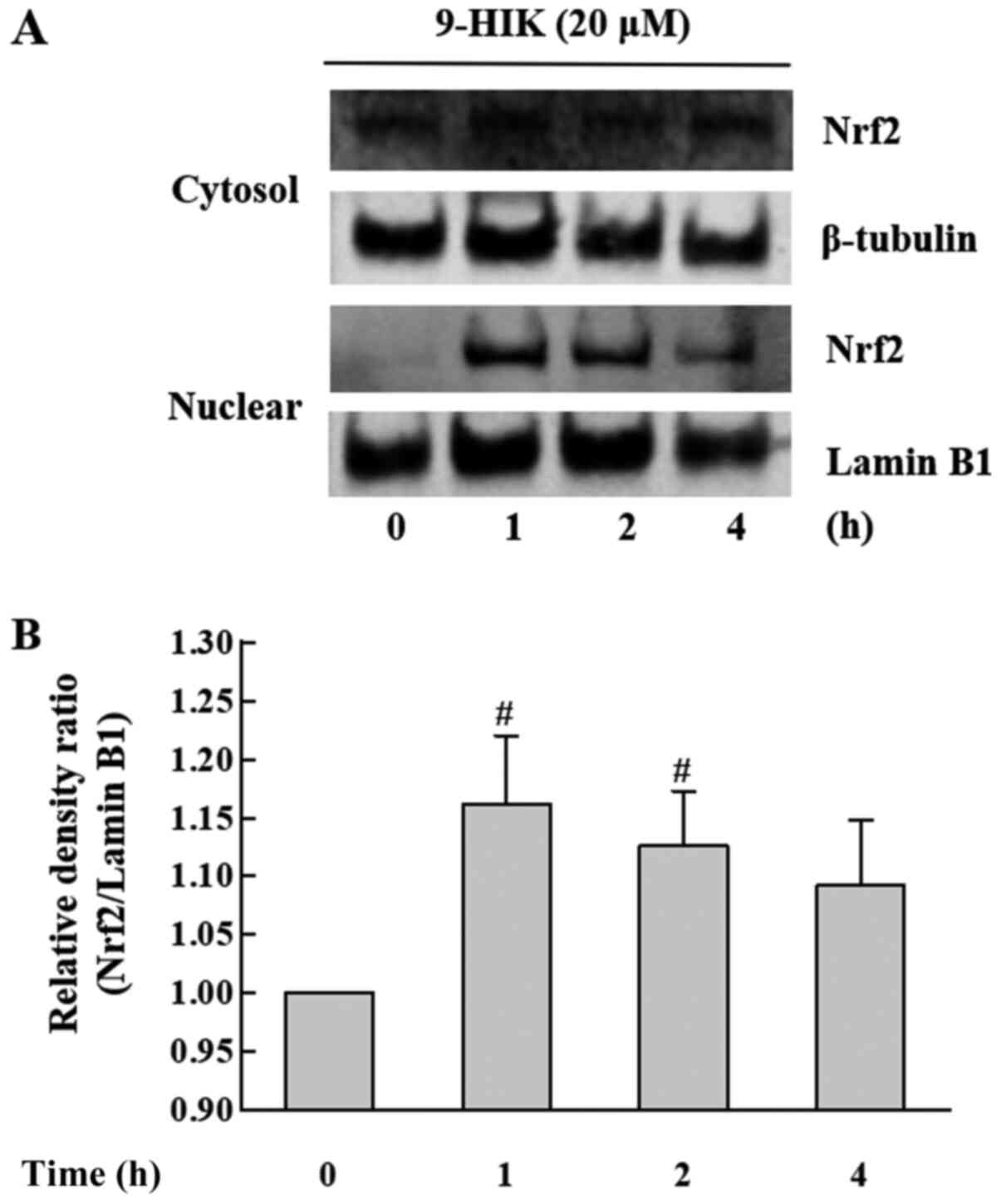
Effects of 9-HIK on Nrf2 expression
levels in RAW264.7 cells
In subsequent experiments, whether 9-HIK could
affect the expression of the Nrf2 transcription factor in RAW264.7
cells was investigated. Nrf2 plays an important role in protecting
cells from the oxidative stress (3). RAW264.7 cells were treated with 20 µM
9-HIK for 0, 1, 2 or 4 h. The subcellular localization of Nrf2 in
9-HIK-treated RAW264.7 cells was measured via western blotting. As
shown in Fig. 6, 9-HIK increased
Nrf2 protein levels in the nuclear fraction, showing a maximal
increase after 1 h of treatment. However, Nrf2 protein levels
remained unchanged in cytosolic extracts. These results indicated
that 9-HIK might induce the translocation of Nrf2 from the cytosol
to the nucleus in RAW264.7 cells. However, 9-HIK may not affect
overall Nrf2 expression levels.
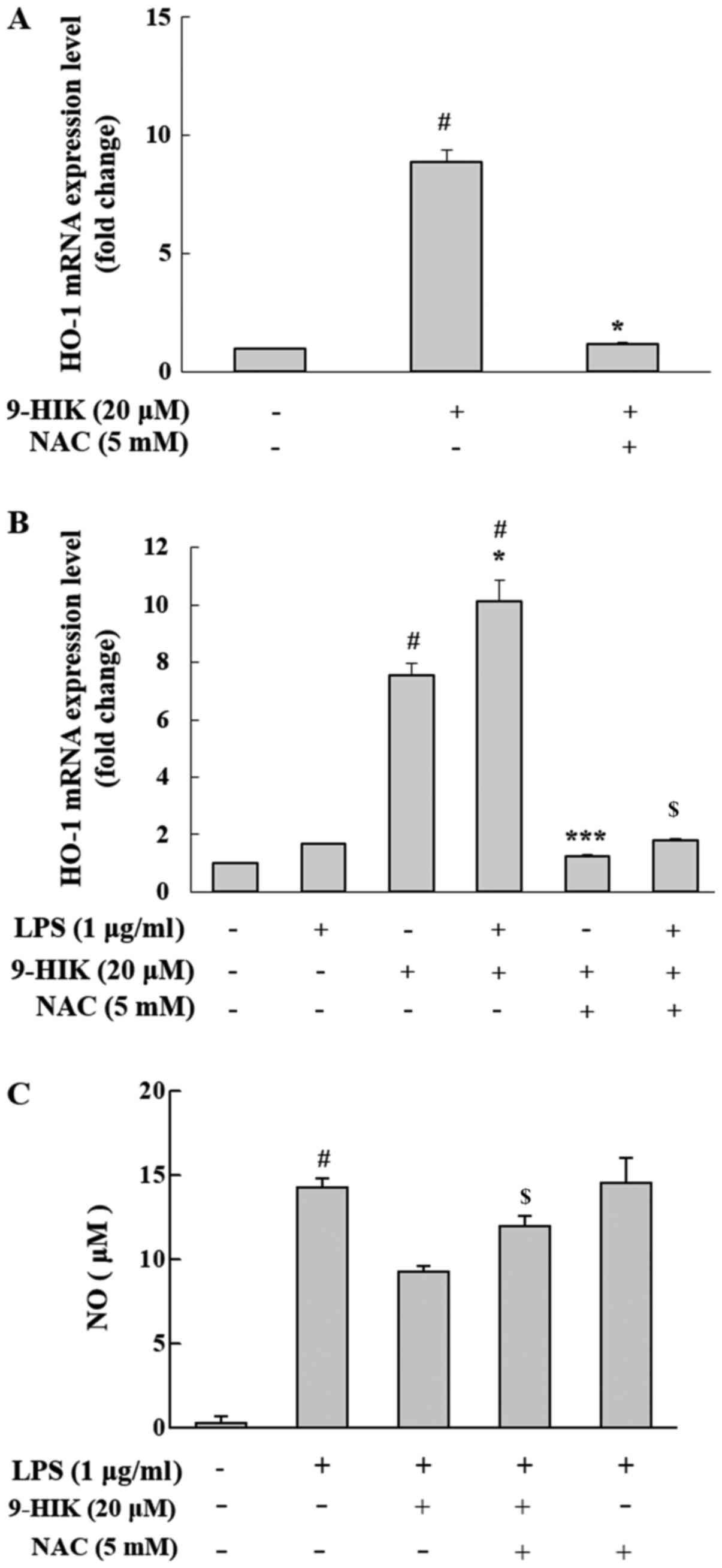
Effect of ROS scavenger on HO-1
induction in 9-HIK-treated RAW264.7 cells
Nrf2 activation is reportedly induced by ROS
(15). To measure the level of HO-1
mRNA expression in ROS scavenger treated cells, RAW264.7 cells were
co-treated with 20 µM 9-HIK and 5 mM NAC for 4 h. NAC is a potent
ROS scavenger that inhibits intracellular ROS synthesis (2). 9-HIK-induced HO-1 mRNA expression
levels decreased following NAC co-treatment in RAW264.7 cells
(Fig. 7A). Also, RAW264.7 cells
were pretreated with 20 µM 9-HIK and 5 mM NAC for 2 h, then
stimulated LPS for 4 h. Treatment with LPS alone did not affect the
mRNA expression levels of HO-1; however, treatment with LPS and
9-HIK resulted in a greater increase in HO-1 mRNA compared with
either LPS or 9-HIK alone. Additionally, treatment with NAC reduced
HO-1 mRNA levels compared with LPS and 9-HIK-treated cells
(Fig. 7B).
Furthermore, to determine whether 9-HIK could
inhibit LPS-induced NO production, RAW264.7 cells were treated with
LPS and 9-HIK in the presence or absence of NAC, and NO levels were
measured. Compared with LPS and 9-HIK co-treatment, NAC addition
increased NO levels. However, LPS and NAC treatment did not affect
NO levels compared with LPS alone (Fig.
7C). Thus, 9-HIK inhibited LPS-induced NO synthesis.
Discussion
Plant components used in traditional medicine are
proposed to have nutritional value, as well as various
physiological effects on the immune, endocrine and nervous systems
(16). Therefore, they are
frequently used in the context of inflammation, allergy and
autoimmune disease. The leaves and seeds of P. frutescens
contain small amounts of flavonoids and phenolic acids, such as
catechins, apigenin, caffeic acid and luteolin (5). The radiation mutant P.
frutescens var. crispa (cv. Antisperill) used in the
present study was generated via 200-Gy γ-ray irradiation of the
wild-type cultivar (7). Previous
studies have reported that IK, a compound found in P.
frutescens essential oil, exhibits pharmacological properties,
including anti-inflammatory, antioxidant (8), anti-cancer (9), anti-arthritic (11) and anti-obesity effects (12). The concentrations of IK, Prilla
Ketone (PK), and 9-HIK in the mutant cultivar obtained through
radiation breeding are increased relative to the wild-type, and
their predicted biosynthetic mechanisms have been suggested in a
previous study (13). All three
compounds are also present in the wild-type, albeit in extremely
small amounts (particularly IK and 9-HIK); thus, few studies have
been carried out using the wild-type cultivar. In a study using
natural resources, the results demonstrated that the smallest
amount of active ingredients offered the greatest disadvantage and
radiation breeding might be an alternative solution to these
challenges. Based on the structure of IK and PK, it is hypothesized
that the α, β-unsaturated ketone, a carbon-carbon double bond
conjugated to a ketone (Cβ=Cα-C=O) present in
IK and 9-HIK, plays an important role in mediating the
anti-inflammatory activity of these compounds (10). Moreover, the addition of a hydroxyl
group to the 9th carbon of IK does not increase the
anti-inflammatory effect, but rather marginally decreases it;
however, cytotoxicity is reduced compared with IK (13). In a previous study, 9-HIK reportedly
suppressed NO production in LPS-induced RAW264.7 cells (13), but no study on its mechanism of
action has been reported. Although 9-HIK has a structure similar to
IK, whether it inhibits NO production in a manner similar to IK in
RAW264.7 cells remains unclear. 9-HIK was separated in large
quantities using centrifugal partition chromatography (17). It has been demonstrated that 9-HIK
exerted anti-inflammatory effects indirectly through HO-1
induction, or via a direct pathway by inhibiting IFN-β production,
both pathways are similar to IK (10). Our previous study aimed to determine
the intracellular targets of IK that could mediate its
anti-inflammatory effects through a direct pathway; however, this
currently remains unknown (10).
Identification of the targets of IK may be extrapolated to 9-HIK,
as the targets of both compounds are expected to be identical.
Inflammation is a complex pathological reaction
caused by harmful stimuli, infection, or tissue damage. At the
molecular level, the inflammatory response is initiated by
cytokines, chemokines and ROS, which are water-soluble
pro-inflammatory mediators released by inflammatory cells (12). Acute inflammation is an immediate
response to external stimuli, whereby macrophages recognize the
infection and secrete pro-inflammatory cytokines to mobilize other
immune cells and trigger inflammation (2). The findings of the present study
suggested that 9-HIK was a potent agent involved in acute
inflammatory responses through inhibition of IL-6 and IFN-β
production in LPS-stimulated RAW264.7 cells. 9-HIK inhibited NO
production in LPS-treated RAW264.7 cells with an IC50
value of 14.4 µM, which was marginally less effective than IK
(IC50=8.8 µM), but demonstrated advantages over IK in
terms of cytotoxicity (13).
NO is enzymatically generated from l-arginine by NOS
(18). NO is generated by
intracellular NOS. NO produced by endothelial NOS and neuronal NOS
is beneficial for physical health, as it regulates cell signaling
and survival and has antibacterial activity (19). In contrast, NO synthesized by iNOS
is associated with inflammatory responses, as it interacts with
other free radicals to produce cytotoxic molecules (20). Therefore, agents that inhibit NO
production could be used to attenuate inflammatory disease
(21,22). In the present study, iNOS and NO
production increased in LPS-stimulated RAW264.7 cells. However,
9-HIK inhibited NO synthesis and iNOS expression. Therefore, 9-HIK
may represent a potential therapeutic candidate for inflammatory
diseases. Macrophages and neutrophils both play an important role
in the inflammatory response. Because neutrophils are short-lived,
the present study only examined macrophages. However, it is
necessary to study the anti-inflammatory effects of 9-HIK in
various cell lines, including neutrophils, in the future.
LPS can directly activate RAW264.7 macrophages via
Toll-like receptor 4, and induce the expression of pro-inflammatory
cytokines and mediators (23). NO,
prostaglandin E2 and cytokines are essential mediators of immune
responses and host responses to inflammation (24). Overproduction of pro-inflammatory
cytokines such as IL-6 and IFN-β causes fever, inflammation and
tissue destruction (25).
Therefore, studies investigating the inhibition of inflammatory
cytokine expression are crucial for the development of
anti-inflammatory agents (25). In
the current study, 9-HIK inhibited the mRNA and protein expression
levels of IL-6 and IFN-β in LPS-stimulated RAW264.7 cells. The
effect of 9-HIK on NF-κB activation was not investigated; however,
based on the present findings, it is hypothesized that the effect
of 9-HIK on NF-κB activation would be suppressed at a lower level
than the IFN-β pathway, similar to IK (10). Thus, the present data may provide
evidence of a link between Nrf2/HO-1 signaling and
anti-inflammatory activity of 9-HIK. Nevertheless, as NF-κB is an
important anti-inflammatory transcription factor, the effect of
9-HIK on this pathway will require further study.
Nrf2-mediated signaling pathways protect cells from
various external stimuli, including oxidative stress or LPS
(26). Furthermore, activated Nrf2
is considered a potential therapeutic agent for the treatment of
various inflammatory diseases, such as autoimmune diseases,
rheumatoid arthritis, gastritis and atherosclerosis (2). Nrf2 binds to Keap1 in the cytoplasm
and is readily degraded via ubiquitin (24). However, oxidative stress induces the
expression of HO-1, a gene encoding an antioxidant enzyme, by
increasing the nuclear accumulation of Nrf2 (24). In addition, HO-1 and its metabolites
exhibit important anti-inflammatory effects mediated by Nrf2
(27). Activation of NF-κB by
oxidative stress releases pro-inflammatory cytokines. Nrf2
activation plays an important role in inhibiting the transcription
of pro-inflammatory mediators through NF-κB (27). The present study demonstrated that
HO-1 expression increased with 9-HIK treatment in a
concentration-dependent manner, with maximal induction occurring at
4 h. Moreover, HO-1 mRNA expression and protein levels increased
following 9-HIK treatment, possibly via nuclear translocation of
Nrf2. However, as the observed differences were small, it may be
hypothesized that other pathways additionally participate in this
response. Furthermore, the inhibitory effect of 9-HIK on NO
production in LPS-stimulated RAW264.7 cells was attenuated
following treatment with NAC, a ROS scavenger; thus, the HO-1
induction by 9-HIK was linked to ROS generation. HO-1 expression
was inhibited by treatment with a ROS scavenger. Collectively,
these results suggested that 9-HIK may increase HO-1 mRNA and
protein levels through the translocation of Nrf2 into the nucleus.
Therefore, 9-HIK may represent a potential therapeutic candidate
for inflammatory diseases. Preclinical studies using animal models
are required to validate the anti-inflammatory properties of 9-HIK
in vivo before development as a natural anti-inflammatory
drug.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Research
Foundation of Korea grant funded by the Korean government (Ministry
of Science, ICT and Future Planning); grant no.
2017M2A2A6A05018541).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HMK designed the study, performed experiments and
wrote the manuscript. BN, SBP, ARH and JWN drafted the manuscript
and helped with understanding the structural difference between
9-HIK and IK. HGJ assisted in drafting and revising the manuscript.
CHJ designed and supervised the study. HMK and CHJ confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elbirt KK and Bonkovsky HL: Heme
oxygenase: Recent advances in understanding its regulation and
role. Proc Assoc Am Physicians. 111:438–447. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
An MY, Eo HJ, Son HJ, Geum NG, Park GH and
Jeong JB: Anti-inflammatory effects of leaf and branch extracts of
honeyberry (Lonicera caerulea) on lipopolysaccharide-stimulated
RAW264.7 cells through ATF3 and Nrf2/HO-1 activation. Mol Med Rep.
22:5219–5230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson JA, Johnson DA, Kraft AD, Calkins
MJ, Jakel RJ, Vargas MR and Chen PC: The Nrf2-ARE pathway: An
indicator and modulator of oxidative stress in neurodegeneration.
Ann NY Acad Sci. 1147:61–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seo WH and Baek HH: Characteristic
aroma-active compounds of Korean perilla (Perilla frutescens
Britton) leaf. J Agric Food Chem. 57:11537–11542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osakabe N, Yasuda A, Natsume M, Sanbongi
C, Kato Y, Osawa T and Yoshikawa T: Rosmarinic acid, a major
polyphenolic component of Perilla frutescens, reduces
lipopolysaccharide (LPS)-induced liver injury in D-galactosamine
(D-GalN)-sensitized mice. Free Radic Biol Med. 33:798–806. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toshiaki M, Furuta Y, Wakushima H, Fujii
H, Saito KI and Kano Y: Anti-allergic effect of Perilla
frutescens and its active constituents. Phytother Res.
17:240–243. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YD, Lee YM, Kang MA, Lee HJ, Jin CH,
Choi DS, Kim DS, Kang SY, Kim WG and Jeong IY: Phytochemical
profiles and in vitro anti-inflammatory properties of Perilla
frutescens cv. Chookyoupjaso mutants induced by mutagenesis
with γ-ray. Food Sci Biotechnol. 19:305–311. 2010. View Article : Google Scholar
|
|
8
|
Chung BH, Lee HY, Lee JS and Young CYF:
Perillyl alcohol inhibits the expression and function of the
androgen receptor in human prostate cancer cells. Cancer Lett.
236:222–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho BO, Jin CH, Park YD, Ryu HW, Byun MW,
Seo KI and Jeong IY: Isoegomaketone induces apoptosis through
caspase-dependent and caspase-independent pathways in human DLD1
cells. Biosci Biotechnol Biochem. 75:1306–1311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin CH, Lee HJ, Park YD, Choi DS, Kim DS,
Kang SY, Seo KI and Jeong IY: Isoegomaketone inhibits
lipopolysaccharide-induced nitric oxide production in RAW 264.7
macrophages through the heme oxygenase-1 induction and inhibition
of the interferon-beta-STAT-1 pathway. J Agric Food Chem.
58:860–867. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin CH, So Y, Nam B, Han SN and Kim JB:
Isoegomaketone alleviates the development of collagen
antibody-induced arthritis in male Balb/c Mice. Molecules.
22:12092017. View Article : Google Scholar
|
|
12
|
So YK, Jo YH, Nam BM, Lee SY, Kim JB, Kang
SY, Jeong HG and Jin CH: Anti-obesity effect of isoegomaketone
isolated from Perilla frutescens (L.) Britt. cv. Leaves.
Korean J Pharmacogn. 46:1–6. 2015.
|
|
13
|
Nam B, So Y, Kim HY, Kim JB, Jin CH and
Han AR: A New Monoterpene from the leaves of a radiation mutant
cultivar of perilla frutescens var. crispa with
Inhibitory activity on LPS-Induced NO production. Molecules.
22:14712017. View Article : Google Scholar
|
|
14
|
Baek SH, Park T, Kang MG and Park D:
Anti-inflammatory activity and ROS regulation effect of
sinapaldehyed in LPS-stimulated RAW264.7 macrophages. Molecules.
25:40892020. View Article : Google Scholar
|
|
15
|
Alam J and Cook JL: Transcriptional
regulation of the heme oxygenase-1 gene via the stress response
element pathway. Pharm Des. 9:2499–2511. 2003.
|
|
16
|
Alam J, Stewart D, Touchard C, Boinapally
S, Choi AM and Cook JL: Nrf2, a Cap‘n’Collar transcription factor,
regulates induction of the heme oxygenase-1 gene. J Biol Chem.
274:26071–26078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nam B, Paudel SB, Kim JB, Jin CH, Lee D,
Nam JW and Han AR: Preparative Separation of Three Monoterpenes
from Perilla frutescens var. crispa using centrifugal
partition chromatography. Int J Anal Chem. 2019:87513452019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo Faro ML, Fox B, Whatmore JL, Winyard PG
and Whiteman M: Hydrogen sulfide and nitric oxide interactions in
inflammation. Nitric Oxide. 41:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei ZY, Chi KQ, Wang KS, Wu J, Liu LP and
Piao HR: Design, synthesis, evaluation, and molecular docking of
ursolic acid derivatives containing a nitrogen heterocycle as
anti-inflammatory agents. Bioorg Med Chem Lett. 28:1797–1803. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Li C, Zhou C, Hong P, Zhang Y,
Sun S and Qian ZJ: 2′-Hydroxy-5′-methoxyacetophenone attenuates the
inflammatory response in LPS-induced BV-2 and RAW264.7 cells via
NF-κB signaling pathway. J Neuroimmunol. 330:143–151. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tripathi P, Tripathi P, Kashyap L and
Singh V: The role of nitric oxide in inflammatory reactions. FEMS
Immunol Med Microbiol. 51:443–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hofseth LJ: Nitric oxide as a target of
complementary and alternative medicines to prevent and treat
inflammation and cancer. Cancer Lett. 268:10–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin B and Jin H: Oxymatrine attenuates
lipopolysaccharide-induced acute lung injury by activating the
epithelial sodium channel and suppressing the JNK signaling
pathway. Exp Anim. 67:337–347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon DH, Choi EO, Hwang H, Kim KJ, Hong
SH, Lee DH and Choi YH: Socheongja and Socheong 2 extracts suppress
lipopolysaccharide-induced inflammation and oxidative stress in RAW
264.7 macrophages through activating Nrf2/HO-1 signaling and
suppressing MAPKs pathway. J Life Sci. 28:207–215. 2018.
|
|
25
|
Dinarello CA: Proinflammatory cytokines.
Chest. 118:503–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Li W, Su ZY and Kong AN: The
complexity of the Nrf2 pathway: Beyond the antioxidant response. J
Nutr Biochem. 26:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vomund S, Schafer A, Parnham MJ, Brune B
and von Knethen A: Nrf2, the master regulator of anti-oxidative
responses. Int J Mol Sci. 18:27722017. View Article : Google Scholar
|