Introduction
Severe acute pancreatitis (SAP) is a common
gastrointestinal inflammatory disease with high morbidity and
mortality. It can induce overwhelming loads of inflammatory
mediators, which leads to systemic inflammatory response syndrome
(SIRS), and even multiple organ dysfunction syndrome (1–3). Acute
kidney injury (AKI) is a serious complication of SAP, and the
morbidity of AKI induced by SAP is 74.7% and its mortality is
75–81% (4,5). Usually, AKI occurs in the early stage
of SAP and may predict the prognosis of this disease. Additionally,
it may induce the failure of other organs and promote disease
progression, eventually resulting in death. SAP-induced AKI is
associated with oxidative stress and activation of inflammatory
cytokines, including TNF-α and IL-6 (6,7).
Therefore, reduction of inflammatory cytokines may be the key
treatment strategy to attenuate AKI induced by SAP.
Isoacteoside (ISO) is a major compound separated
from Monochasma savatieri Franch. ex Maxim, which is a
Chinese herbal medicine that is a composition of Yanning particles.
A number of inflammatory diseases may be treated using Yanning
particles (8). ISO may inhibit the
production of pro-inflammatory cytokines via blocking of the
caspase-1, MAPK and NF-κB signaling pathway in vitro
(9). ISO exerts anti-inflammatory
effects that may be mediated through the NF-κB and MAPK signaling
pathways via blocking of Toll-like receptor 4 (TLR4) dimerization.
TLR4 has been reported to be involved in the myeloid
differentiation factor 88 and TIR-domain-containing
adaptor-inducing interferon signaling pathways in an AKI mouse
model (10). A number of studies
have indicated that TLR4 and NF-κB are involved in the activation
of cytokines in SAP (11–13). Toll-like receptors are transmembrane
proteins of cytokines that recognize extracellular antigens and
induce inflammation (14,15). TLR4 was the first identified member,
and also recognizes certain endogenous ligands. Activation of TLR4
triggers intracellular signal transduction cascades, leading to the
activation of NF-κB, thereby regulating the release of inflammatory
cytokines (16,17). Our previous study has demonstrated
that the inflammatory response in SAP may be attenuated via
inhibition of the TLR4/NF-κB signaling pathway (18). However, the pharmacological effects
of ISO on SAP-induced AKI remain unclear.
The present study established SAP rat models to
investigate the anti-inflammatory effects of ISO on SAP-induced
AKI. Furthermore, the present study investigated the role of the
TLR4/NF-κB signaling pathway during the course of this treatment.
The present study aimed to determine the optimal dose of ISO for
treatment of SAP-induced AKI, and to further investigate the
underlying mechanisms of ISO treatment in AKI induced by SAP.
Materials and methods
Materials
Sodium taurocholate was purchased from
Sigma-Aldrich; Merck KGaA. ISO
(C29H36O15; FW, 624.59; purity,
≥98%) was obtained from Nanjing TCM Institute of Chinese Matera
Medica. ELISA kits for rat TNF-α and IL-6, goat anti-rat TLR4
antibody, anti-rat NF-κB p65 antibody and secondary antibodies were
obtained from Abcam. Nuclear-cytosol extraction kits were purchased
from Beyotime Institute of Biotechnology. The nitric oxide (NO)
assay kit was purchased from AmyJet Scientific, Inc.
Animals
A total of 120 male Sprague Dawley (SD) rats
(weight, 230–250 g; age, 8 weeks) were obtained from the
Experimental Animal Center of Jinling Hospital (Nanjing, China).
The rats were housed at a consistent temperature of 23°C with a
12-h light-dark cycle and free access to food and water. The rats
were fasted for 12 h prior to induction of SAP; however, the rats
had free access to water. All experiments were performed according
to protocols approved by the Animal Care Committee of Jinling
Hospital (Nanjing, China) and were performed according to
established international guiding principles for animal
research.
Experimental design
In the first part of the present study, 48 SD rats
were randomly divided into six groups (8 rats/group): Sham
operation group (sham); SAP group (SAP); SAP+ISO-20 mg/kg treatment
group (SAP+ISO20); SAP+ISO-40 mg/kg treatment group (SAP+ISO40);
SAP+ISO-60 mg/kg treatment group (SAP+ISO60); and vehicle group
(saline). Surgical anesthesia was accomplished by intraperitoneal
injection of 10% chloral hydrate (300 mg/kg; Shanghai Shifeng
Technology Co., Ltd.), and none of the rats exhibited any signs of
peritonitis or discomfort. SAP was induced by retrograde infusion
of 5% sodium taurocholate (0.1 ml/100 g body weight) into the
pancreatic and biliary duct (19).
Sham group animals were administered saline instead of sodium
taurocholate into the pancreatic and biliary duct under the same
conditions. For the ISO treatment group, ISO was diluted in
dimethyl sulfoxide and was administered by intraperitoneal
injection at 30 min after the SAP model establishment. Furthermore,
the vehicle group was given the same volume of normal saline. The
rats were euthanized by exsanguination under anesthesia at 12 h
after the SAP model establishment, and anesthesia was accomplished
by intraperitoneal injection of 10% chloral hydrate (300 mg/kg),
then blood and pancreatic tissue samples were collected
immediately. Furthermore, the pancreas samples were rapidly fixed
in formalin at 35°C for 48 h for histological examination and
scoring. The effect of ISO was determined by evaluating the
pathological changes of the pancreas, and the serum levels of
amylase (AMY), lipase (LIPA), aspartate-transaminase (AST) and
alanine-aminotransferase (ALT).
Based on the first part of the present experiment,
the optimal dose of ISO was fixed as 40 mg/kg. Subsequently, 72 SD
rats were randomly divided into three groups (24 rats/group): Sham
operation group (Sham); SAP group (SAP); and SAP+ISO-40 mg/kg
treatment group (SAP+ISO40). Furthermore, 8 rats in each group were
sacrificed by exsanguination under anesthesia at 3, 6 and 12 h
after SAP model establishment. Subsequently, the blood, pancreas
and kidney tissue samples were collected immediately. The collected
blood samples were centrifuged at 12,000 × g for 10 min at 4°C, and
then the serum was collected. The pancreas and kidney samples were
rapidly fixed in formalin at 35°C for 48 h for histological
examination and scoring. For western blot analysis, three portions
of each kidney sample were stored at −80°C.
Enzyme assay
The AMY, LIPA, AST, ALT, blood urea nitrogen (BUN)
and creatinine (Cr) levels in the serum were measured using an
automated biochemical analyzer (Hitachi 7600; Hitachi, Ltd.).
ELISA
Serum concentrations of TNF-α (ab236712) and IL-6
(ab234570) were measured using commercially available ELISA kits
according to the manufacturer's protocols (R&D Systems, Inc.).
All samples were assayed three times.
Histopathological examination
The pancreas and kidney tissue samples were fixed in
4% formalin at 35°C for at least 48 h. The tissues were cut into
sections (thickness, 5 µm), and stained with hematoxylin and eosin
at 23°C for 2 h. Histopathological evaluation was performed under a
light microscope (magnification, ×100) by two experienced
laboratory pathologists in a blinded manner. The pathological
scores of the pancreas samples were evaluated according to the
extent of edema, inflammation, vacuolization and necrosis (20). Histological scores of the kidney
were assessed according to the point-counting method described by
Paller et al (21). For each
kidney, 100 cortical tubules from at least 10 different regions
were scored, and repeated scoring of different convolutions of the
same tubule was avoided. Higher scores represented more severe
damage (maximum score per tubule, 10), with points given for the
presence and degree of tubular epithelial cell flattening (1
point), brush border loss (1 point), cell membrane bleb formation
(1 or 2 points), interstitial edema (1 point), cytoplasmic
vacuolization (1 point), cell necrosis (1 or 2 points) and tubular
lumen obstruction (1 or 2 points).
Immunohistochemistry
Kidney tissues were fixed in 4% formalin at 35°C for
12 h and embedded in paraffin. Subsequently, kidney tissues were
harvested to prepare frozen sections of 6-µm thickness. Following
deparaffinization, endogenous peroxidase activity was blocked with
0.3% hydrogen peroxide for 10 min at room temperature. Non-specific
adsorption was minimized following incubation of the sections in 5%
normal goat serum in PBS (Nanjing Jiancheng Bioengineering
Institute) at 37°C for 1 h. Avidin and biotin were used to block
endogenous biotin and avidin binding sites, respectively. The
sections were incubated overnight with goat anti-rat TLR4 (cat. no.
ab22048) and anti-rat NF-κB p65 (cat. no. ab16502) monoclonal
antibodies (both 1:100; Abcam) in a humidified incubator at 4°C.
Hematoxylin was subsequently used to counterstain the sections for
2 min at room temperature. Stained sections were visualized using a
light microscope. PBS was used instead of the primary antibody as a
negative control.
NO assay
Kidney tissues were thawed and homogenized in
phosphate buffer containing 0.5% hexadecyltrimethylammonium
bromide. NO production was determined using a commercial assay kit
(Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocols.
Western blot analysis
Kidney tissue samples were homogenized, and the
lysate was boiled in sample buffer (62.5 mM Tris-HCl, pH 6.8, 2%
sodium dodecyl sulfate, 20% glycerol and 10% 2-mercaptoethanol).
The protein concentration was determined using a Bradford protein
assay kit (Thermo Fisher Scientific, Inc.) with bovine serum
albumin (Nanjing Jiancheng Bioengineering Institute) as the
standard. Proteins (50 mg) were separated via 10% SDS-PAGE and
transferred to PVDF membranes. Following the transfer of proteins,
the membrane was blocked with 5% skimmed milk in PBS with 0.1%
Tween-20 (PBST) for 2 h at room temperature, and then incubated
overnight with antibodies (all 1:1,000; Abcam) against TLR4
(ab22048), NF-κB p65 (ab16502) or β-actin (cat. no. ab8227) at 4°C.
Subsequently, the membranes were washed with PBST containing 0.1%
Tween. Following three washes in PBST, each membrane was incubated
with peroxidase-conjugated secondary antibody (anti-mouse IgG;
1:5,000; cat. no. ab131368; Abcam) for 1 h at 37°C. Labeled
proteins were visualized using an Odyssey infra-red scanner (LI-COR
Biosciences). Signals were assessed by densitometry and normalized
to the β-actin signals. An enhanced chemiluminescence detection
system (Amersham; Cytiva) was used to visualize the
antibody-specific proteins according to the manufacturer's
recommended protocol.
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance with Bonferroni's test was used to
analyze the differences among multiple groups. Statistical analysis
was performed using SPSS v19.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Surgical outcomes
The operation was completed successfully on all
rats, and no rats died during the experimental period between the
first injection and the time of euthanasia in the present
study.
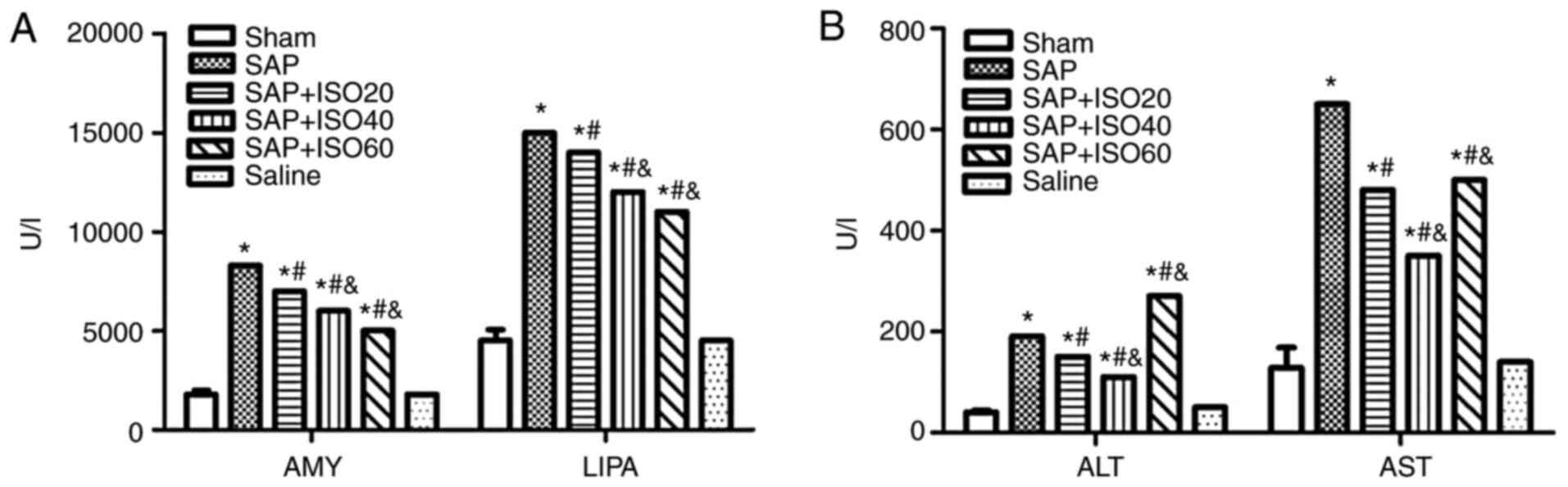
Optimum dose of ISO
The present study evaluated the serum levels of AMY,
LIPA, ALT and AST in SAP rats that were treated with multiple doses
of ISO (20, 40 and 60 mg/kg) in order to determine the optimum dose
of ISO. The three doses of ISO significantly decreased the levels
of AMY and LIPA compared with those in the SAP group (P<0.05;
Fig. 1A). However, the SAP group
treated with ISO was the only ISO-treated group in which both AST
and ALT levels were decreased significantly compared with those in
the SAP group (P<0.05; Fig. 1B).
Therefore, 40 mg/kg ISO was determined to be the optimum dose for
treatment of SAP. Additionally, 3 h following induction of the SAP
model was identified to be the best time of medication to reduce
inflammation.
Analysis of serum levels of AMY, LIPA,
BUN and Cr
Compared with those in the sham group, the serum
levels of AMY, LIPA, BUN and Cr in the SAP group were significantly
increased at either designated time point (P<0.05; Fig. 2). However, the serum levels of AMY,
LIPA, BUN and Cr in the ISO-treated group were markedly decreased
compared with those in the SAP group (P<0.05; Fig. 2). Furthermore, the serum levels of
AMY peaked at the 6 h time point in the SAP group (Fig. 2A), and the serum levels of LIPA, BUN
and Cr peaked at the 12 h time point (Fig. 2B-D).
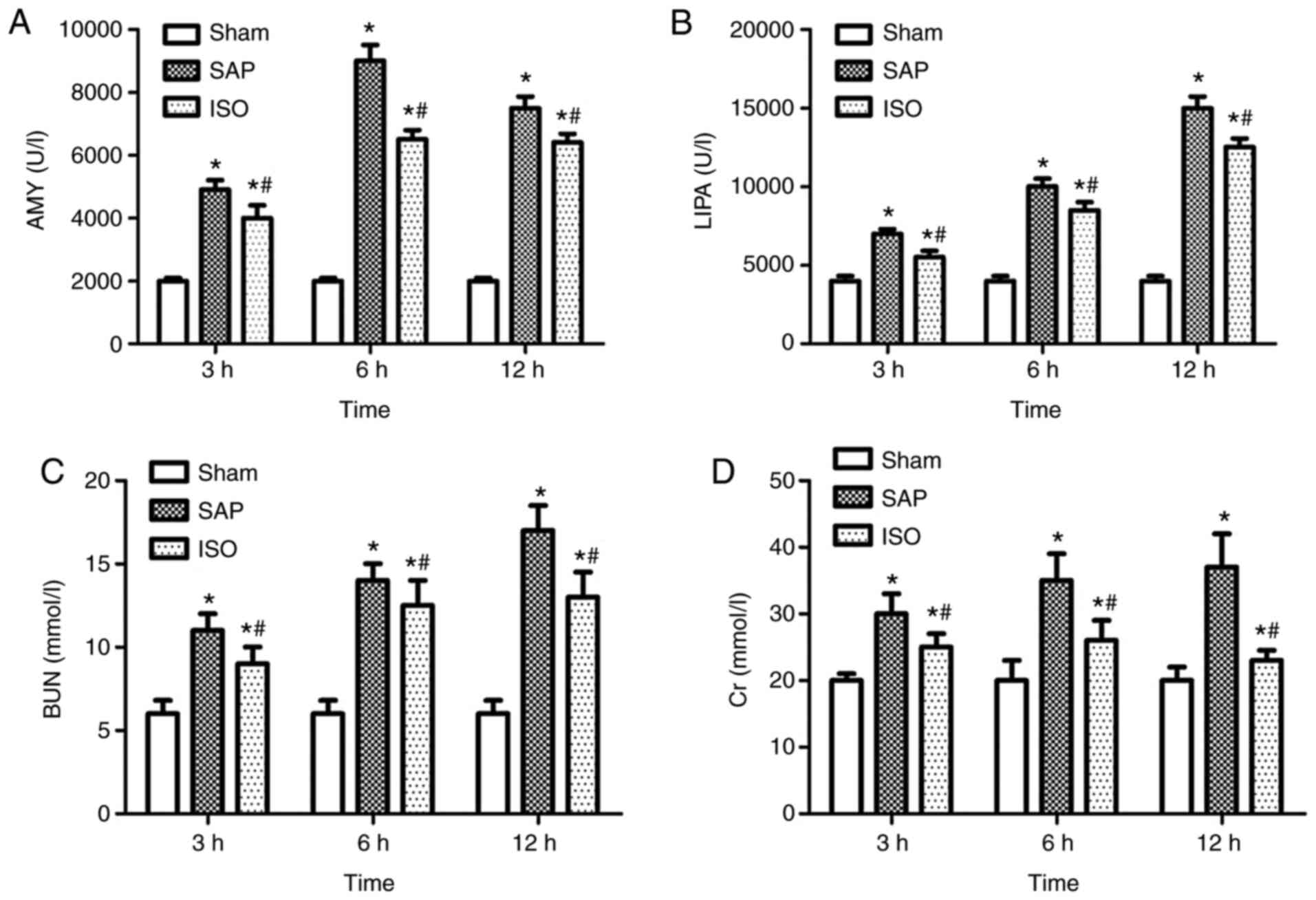 | Figure 2.AMY, LIPA, BUN and Cr activity. Serum
levels of AMY, LIPA, BUN and Cr in all groups. Serum levels of (A)
AMY, (B) LIPA, (C) BUN and (D) Cr. Data are expressed as the mean ±
standard deviation; n=8 in each group. *P<0.05 vs. the Sham
group; #P<0.05 vs. the SAP group. AMY, amylase; LIPA,
lipase; BUN, blood urea nitrogen; Cr, creatinine; SAP, severe acute
pancreatitis. |
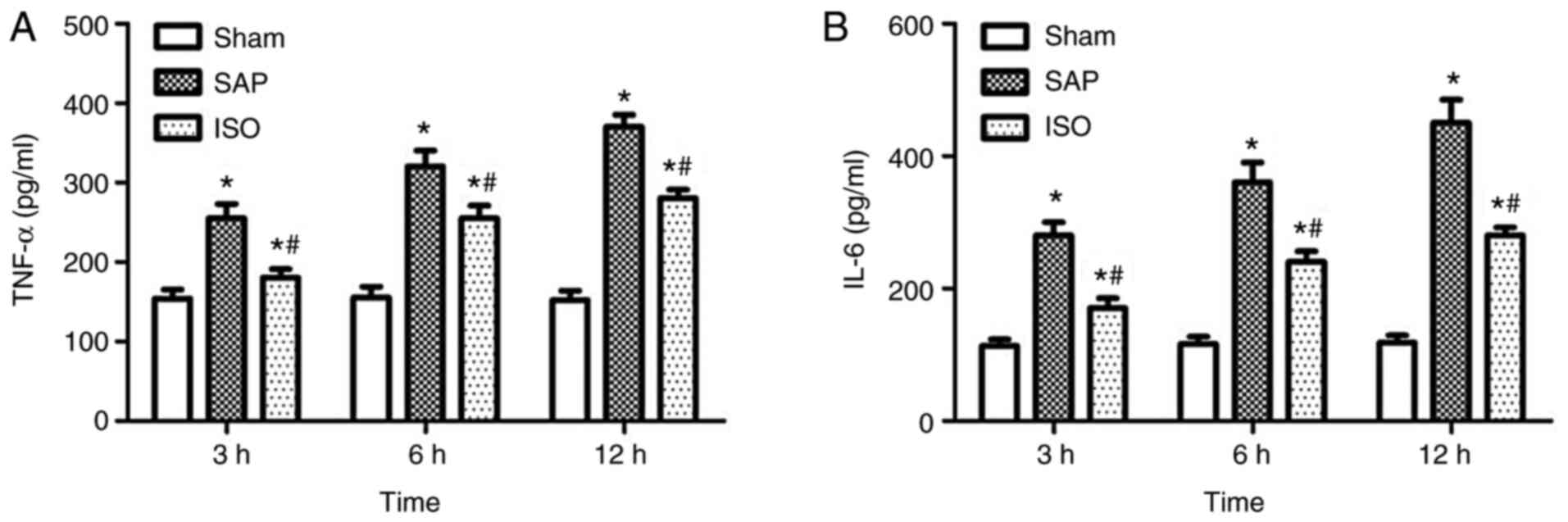
ELISA
As shown in Fig. 3,
a significant increase in serum levels of TNF-α and IL-6 was
observed in the SAP group compared with the sham group (P<0.05),
and the levels of these cytokines were progressively upregulated
between 3 and 12 h. Furthermore, the serum levels of TNF-α and IL-6
were decreased following treatment with ISO (P<0.05). However,
the levels of these cytokines in the ISO group were still higher
than those in the sham group at each time point (P<0.05).
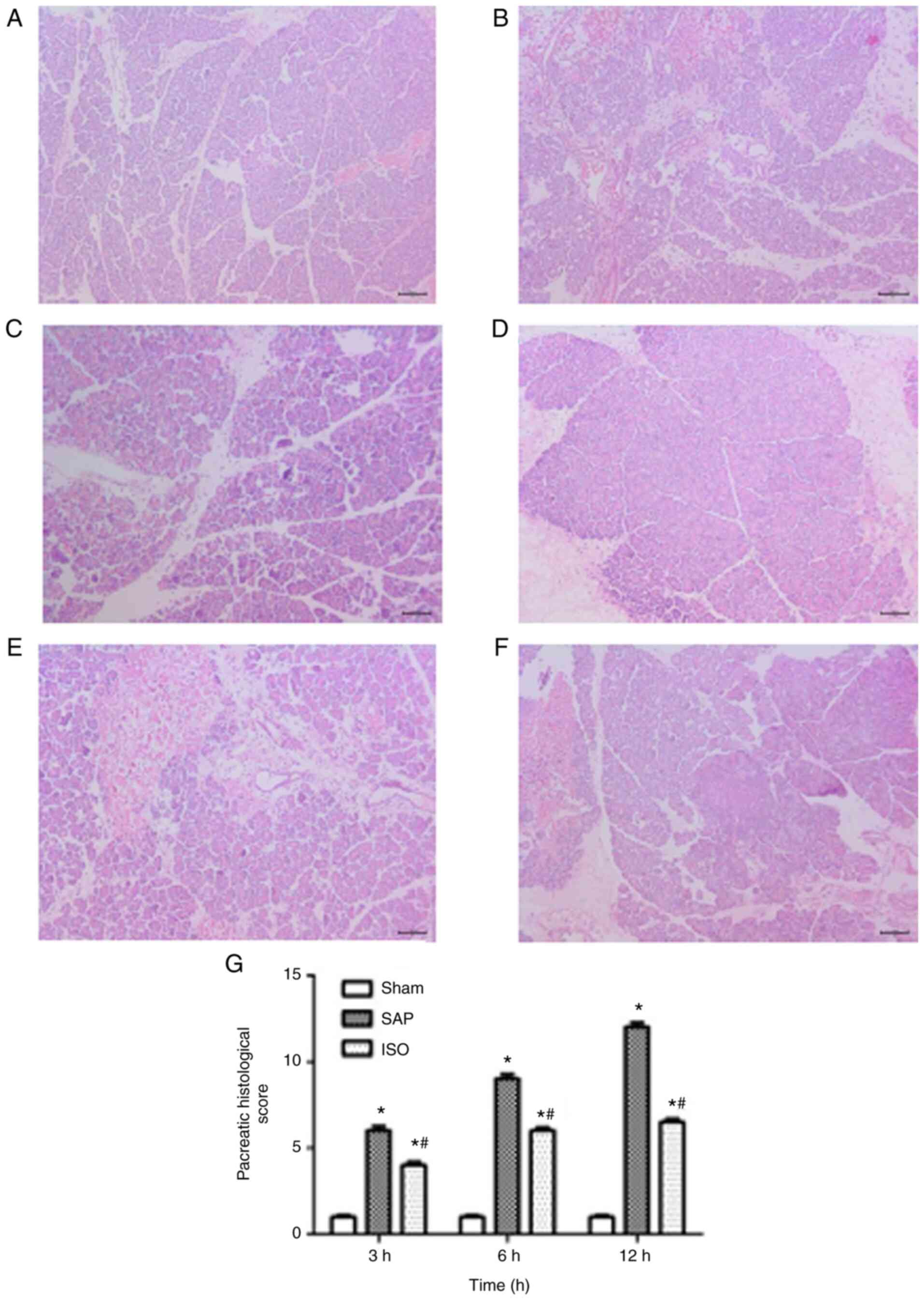
Histopathological examination
Representative histological sections of the pancreas
and kidney are shown in Figs. 4 and
5. The pancreas tissues of the sham
group exhibited a normal histological structure (Fig. 4A). Samples in the SAP group were
characterized by typical histological signs of pancreatic edema,
inflammatory leukocyte infiltration, hemorrhage and necrosis
(Fig. 4B). However, there was a
significant decrease in the severity of the pancreatic histological
injuries in the ISO-treated group (P<0.05; Fig. 4C and D).
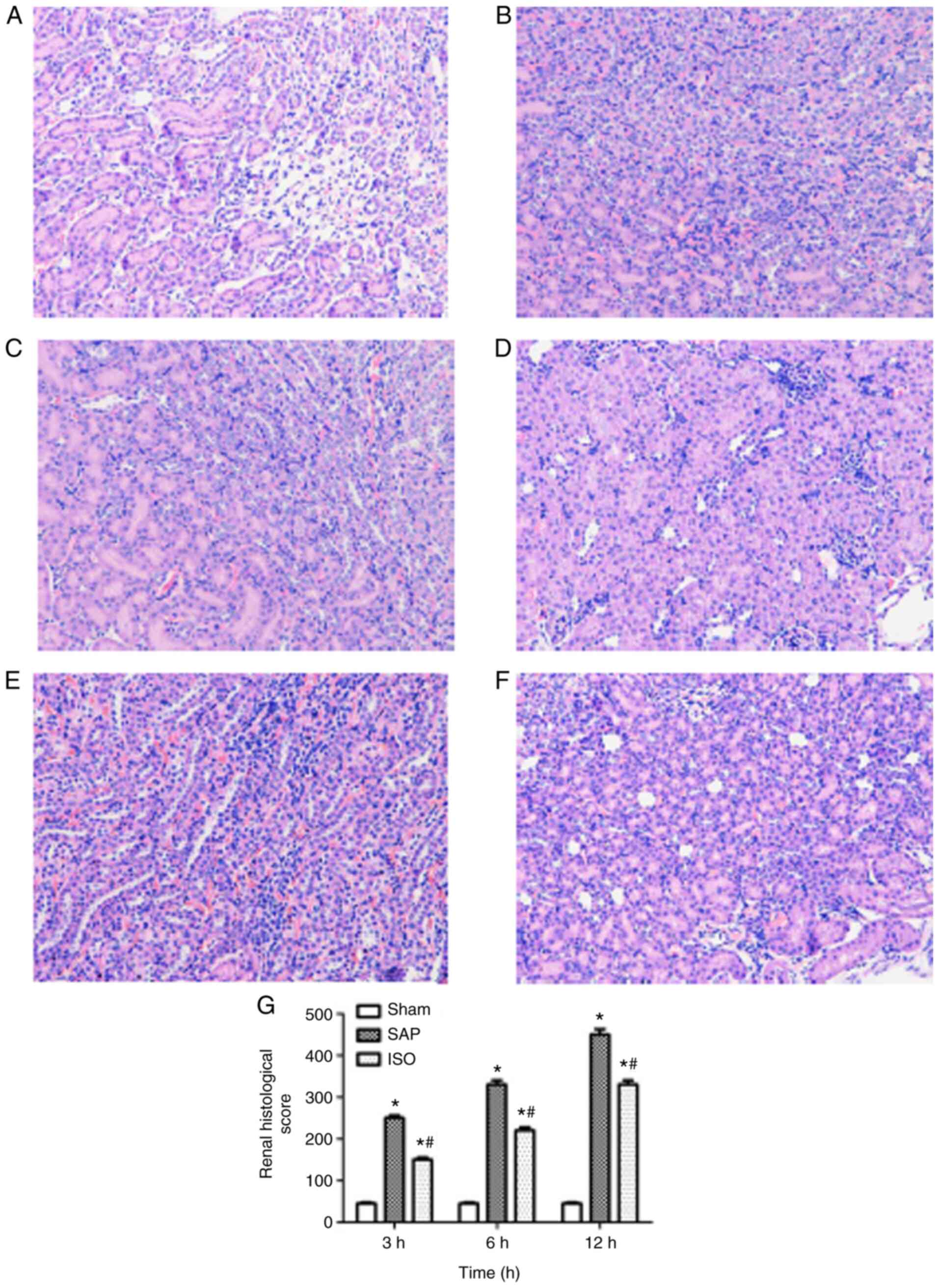
The pathological changes in kidney samples were
analyzed to examine the effect of ISO on AKI induced by SAP
(Fig. 5). The kidney samples of the
sham group exhibited a normal histological structure of the renal
glomerulus, tubules and interstitium (Fig. 5A). By contrast, samples in the SAP
group were characterized by typical histological signs of
glomerular and tubular damage, and the most severe kidney injury
was detected at the 12 h time point (Fig. 5B and D). ISO treatment attenuated
the severity of kidney injury, as demonstrated by decreased
glomerular and tubular damage and decreased inflammatory cell
infiltration (Fig. 5C).
Additionally, the kidney pathohistological scores were
significantly decreased in the ISO-treated group compared with the
SAP group at each time point (P<0.05; Fig. 5D).
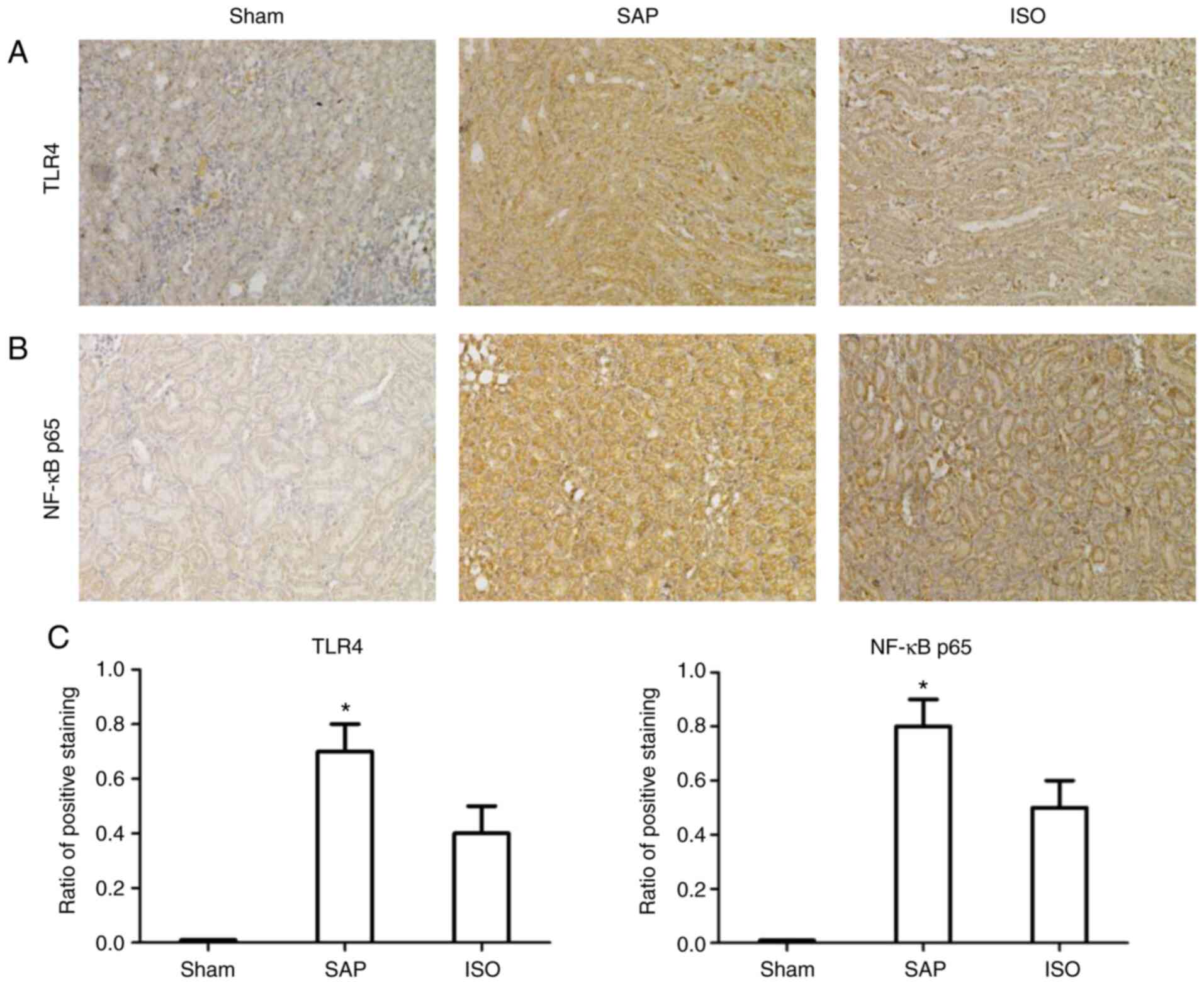
Immunohistochemical analysis of TLR4
and NF-κB p65 expression
Localization of TLR4 and NF-κB p65 in the kidney is
shown in Fig. 6. There was almost
no TLR4 and NF-κB p65 expression in the sham group. In the SAP
group, marked positive TLR4 and NF-κB p65 expression was observed
compared with the sham group. However, following treatment with
ISO, the expression levels of TLR4 and NF-κB p65 were markedly
decreased compared with those in the SAP group at the corresponding
time points (P<0.05).
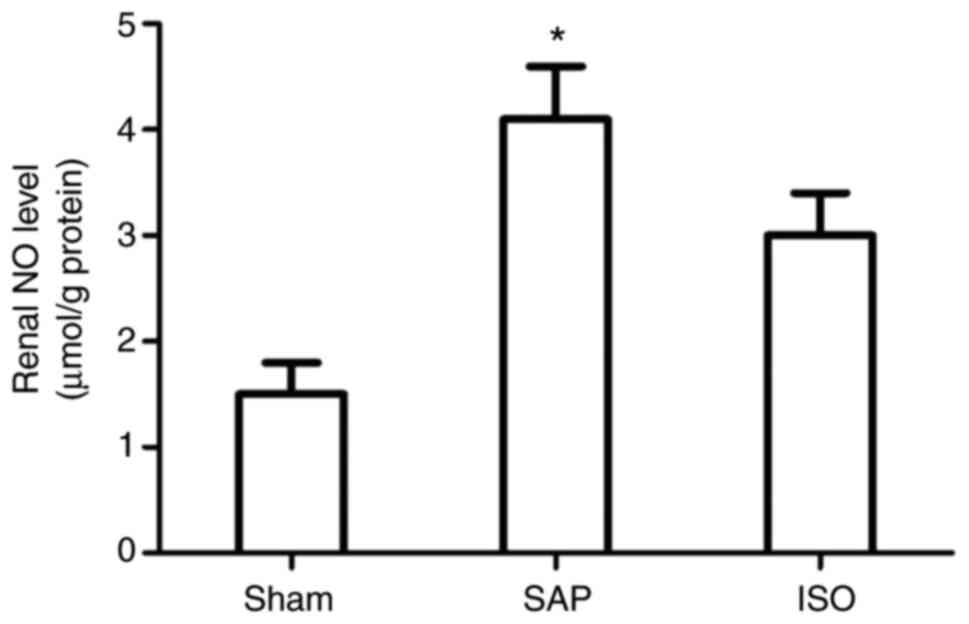
ISO treatment decreases renal NO
production
NO activity in the kidney was detected to examine
the infiltration of neutrophils (Fig.
7). NO activity was markedly elevated in the SAP group compared
with the sham group (P<0.05). However, treatment with ISO
significantly decreased NO activity in the kidney compared with the
SAP group at each time point (P<0.05).
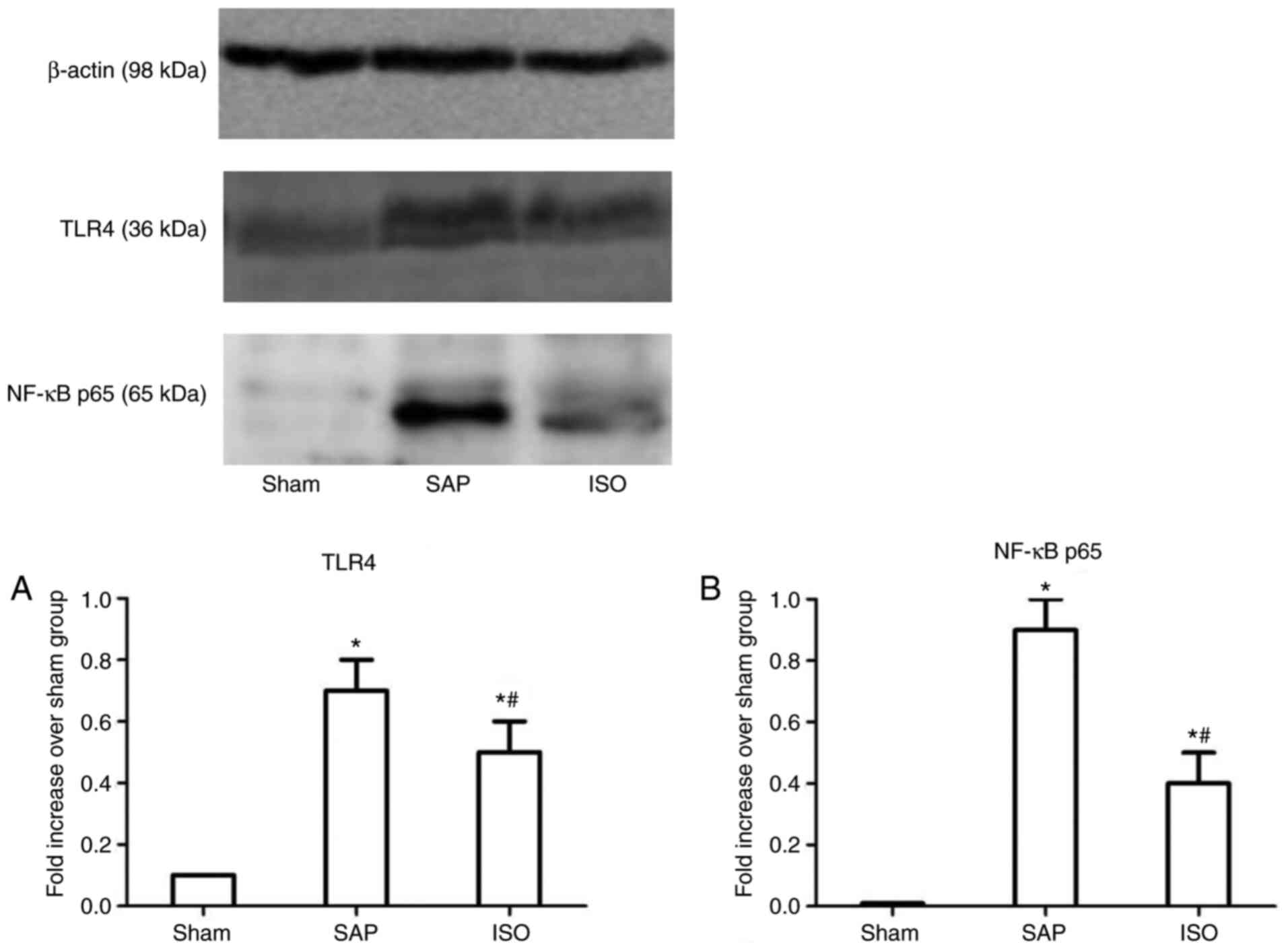
Role of the TLR4/NF-κB p65 signaling
pathway in the effects of ISO in AKI induced by SAP
Western blotting was used to determine the effects
of ISO on the expression levels of TLR4 and NF-κB p65 in the kidney
(Fig. 8). Compared with those in
the sham group, the expression levels of TLR4 and NF-κB p65 were
upregulated in the SAP group (P<0.05). Following treatment with
ISO, the expression levels of TLR4 and NF-κB p65 were downregulated
compared with those in the SAP group at each time point
(P<0.05).
Discussion
The present study investigated the effects of
treatment with ISO on SAP-induced AKI rat models. ISO is a major
ingredient of Yanning particles, and it has been used in clinical
settings for numerous years in China. It has been reported that ISO
may be a potential anti-dementia phenylethanoid glycoside and has
various memory- and behavior-improving effects against
Aβ1-42-induced behavioral dysfunction (22). Furthermore, studies have revealed
that ISO suppresses lipopolysaccharide-induced phosphorylation of
TGF-β activated kinase 1 (TAK1) while having no effects on total
TAK1 in RAW264.7 cells. ISO may attenuate inflammation by
inhibiting the production of pro-inflammatory cytokines via the
NF-κB and MAPK signaling pathway, and treatment of mice with ISO
may protect them against AKI induced by lipopolysaccharides
(10). The results of the present
study revealed that ISO may relieve SAP and AKI induced by SAP, as
demonstrated by decreased serum levels of AMY, LIPA, BUN and Cr,
and amelioration of pancreas and kidney injury. AKI is one of the
most common systemic complications, and it has been demonstrated to
have a marked impact on the clinical outcome (5,23,24).
However, the mechanism of AKI induced by SAP is complicated.
A previous study demonstrated that the inflammatory
response serves an important role in the development of SAP-induced
AKI, and that inflammatory cytokines are the key factors (25). During the inflammatory response,
numerous cytokines, including TNF-α and IL-6, are released into the
blood. It has been revealed that TNF-α and IL-6 serve an important
role in SAP and contribute toward the activation of SIRS and AKI.
TNF-α acts on glomeruli and tubular capillaries directly resulting
in ischemia and tubular necrosis of the kidney. Additionally, TNF-α
stimulates the release of other cytokines, including IL-6, which
acts on endothelial cells, resulting in ischemia, thrombosis of the
kidney and the release of oxygen free radicals (26). A clinical study reported that
patients who developed AKI had high serum levels of IL-6 and IL-8
(27). The cytokine-mediated
inflammatory response serves an important role in the
pathophysiology of AKI regardless of its cause (28). The present study demonstrated that
the serum levels of TNF-α and IL-6 were lower in the ISO treatment
group compared with the SAP group at each time point, indicating
that treatment with ISO may inhibit inflammation in SAP-induced
AKI.
A number of studies have indicated that NO may serve
an important role during the inflammatory process of SAP (29,30).
In the present study, upregulation of NO was detected in kidney
tissues in the SAP group, which indicated that NO may contribute
toward the development of AKI induced by SAP. The upregulation of
NO may lead to the production of oxygen free radicals, which
increases cytotoxic effects and aggravates AKI. However, ISO
decreased NO expression in the kidneys of SAP rats. Lower NO
expression in the ISO treatment group may lead to the reduction of
nitrotyrosine formation and lipid peroxidation in the kidneys of
SAP rats. Therefore, these results demonstrated that ISO may
ameliorate SAP-induced AKI by attenuating inflammation induced by
NO in the kidney.
As a ‘switch’ of the inflammatory response, the
TLR4/NF-κB p65 signaling pathway may be associated with the
development and progression of SAP-induced AKI. The transcription
factor NF-κB p65 serves a critical role in the regulation of
numerous genes which are responsible for the generation of
cytokines, including TNF-α and IL-6, in inflammatory diseases,
including SAP (31). One study has
demonstrated that the activation of NF-κB p65 is associated with
TLR4 expression; following stimulation by TLR4, NF-κB is activated,
phosphorylated and transferred into the nucleus (32). TLR4 may activate IκB and induce the
NF-κB signal transduction pathway. Other studies have revealed that
there are high expression levels of TLR4 in the kidney tissues of
mice models of AKI (33,34). In the present study,
immunohistochemistry revealed an increase in the expression levels
of NF-κB p65 in the kidney samples from the SAP group compared with
the sham group. Additionally, a decrease in the expression levels
of NF-κB p65 in the ISO group compared with the SAP group was
observed. Western blotting demonstrated that ISO treatment
decreased the expression levels of TLR4 in the kidney. Therefore,
ISO may attenuate the inflammatory response by inhibiting the
TLR4/NF-κB p65 signaling pathway. However, there was a limitation
to the present study. A TLR4 signaling pathway inhibitor was not
used in the present experiments. Furthermore, it remains unclear
whether ISO only works through the TLR4/NF-κBp65 signaling pathway.
Although the present study indicated that ISO protected the kidney
from SAP, the precise mechanism underlying the effects of ISO
requires further investigation.
In conclusion, ISO attenuated AKI induced by SAP by
decreasing inflammation. Therefore, ISO may be a potential
therapeutic agent for the treatment of SAP and SAP-induced AKI. The
mechanism may involve inhibition of the TLR4/NF-κB p65 signaling
pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (project no.
81904041).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW and XPZ designed and performed the research and
wrote the manuscript. XHL, MLL, XWW and XHZ performed the research.
ZS and MXG collected and analyzed data. The authenticity and
legitimacy of all the raw data have been assessed by XPZ and XHZ.
All authors read and approved the final manuscript.
Ethics statement and consent to
participate
The present study was approved by the Institutional
Review Board of Jinling Hospital and the Institutional Animal Care
and Use Committee of Jinling Hospital. All operations were
performed according to National Institutes of Health guide for the
care and use of Laboratory animals (NIH Publications no. 8023,
revised 1978) concerning the care and treatment of experimental
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS; Acute Pancreatitis
Classification Working Group, : Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu BU, Batech M, Quezada M, Lew D,
Fujikawa K, Kung J, Jamil LH, Chen W, Afghani E, Reicher S, et al:
Dynamic measurement of disease activity in acute pancreatitis: The
pancreatitis activity scoring system. Am J Gastroenterol.
112:1144–1152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buxbaum J, Quezada M, Chong B, Gupta N, Yu
CY, Lane C, Da B, Leung K, Shulman I, Pandol S and Wu B: The
pancreatitis activity scoring system predicts clinical outcomes in
acute pancreatitis: Findings from a prospective cohort study. Am J
Gastroenterol. 113:755–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devani K, Charilaou P, Radadiya D,
Brahmbhatt B, Young M and Reddy C: Acute pancreatitis: Trends in
outcomes and the role of acute kidney injury in mortality-A
propensity-matched analysis. Pancreatology. 18:870–877. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petejova N and Martinek A: Acute kidney
injury following acute pancreatitis: A review. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 157:105–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chai X, Huang HB, Feng G, Cao YH, Cheng
QS, Li SH, He CY, Lu WH and Qin MM: Baseline serum cystatin C is a
potential predictor for acute kidney injury in patients with acute
pancreatitis. Dis Markers. 19:84312192018.
|
|
7
|
Mao W, Wu J, Zhang H, Zhou J, Ye B, Li G,
Gao L, Li X, Ke L, Tong Z, et al: Increase in serum chloride and
chloride exposure are associated with acute kidney injury in
moderately severe and severe acute pancreatitis patients.
Pancreatology. 19:136–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YL, He WJ, Mo L, Shi MF, Zhu YY, Pan
S, Li XR, Xu QM and Yang SL: Antimicrobial, anti-inflammatory
activities and toxicology of phenylethanoid glycosides from
Monochasma savatieri Franch. ex Maxim. J Ethnopharmacol.
149:431–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nam SY, Kim HY, Yoou MS, Kim AH, Park BJ,
Jeong HJ and Kim HM: Anti-inflammatory effects of isoacteoside from
abeliophyllum distichum. Immunopharmacol Immunotoxicol. 37:258–264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao H, Cui Y, Kang N, Liu X, Liu Y, Zou Y,
Zhang Z, Li X, Yang S, Li J, et al: Isoacteoside, a
dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects
through blocking toll-like receptor 4 dimerization. Br J Pharmacol.
174:2880–2896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaworek J, Szklarczyk J, Kot M, Góralska
M, Jaworek A, Bonior J, Leja-Szpak A, Nawrot-Porąbka K,
Link-Lenczowski P, Ceranowicz P, et al: Chemerin alleviates acute
pancreatitis in the rat thorough modulation of NF-κB signal.
Pancreatology. 19:401–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korkmaz FT, Elsasser TH and Kerr DE:
Variation in fibroblast expression of toll-like receptor 4 and
lipopolysaccharide-induced cytokine production between animals
predicts control of bacterial growth but not severity of
Escherichia coli mastitis. J Dairy Sci. 101:10098–10115.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu GQ, Jeon SH, Lee KW, Tian WJ, Cho HJ,
Ha US, Hong SH, Lee JY, Moon MK, Moon SH, et al: Electric
stimulation hyperthermia relieves inflammation via the suppressor
of cytokine signaling 3-toll like receptor 4 pathway in a
prostatitis rat model. World J Mens Health. 38:359–369. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo L, Bokil NJ, Wall AA, Kapetanovic R,
Lansdaal NM, Marceline F, Burgess BJ, Tong SJ, Guo Z, Alexandrov K,
et al: SCIMP is a transmembrane non-TIR TLR adaptor that promotes
proinflammatory cytokine production from macrophages. Nat Commun.
8:141332017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawasaki T and Kawai T: Toll-like receptor
signaling pathways. Front Immunol. 5:4612014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cen Y, Liu C, Li X, Yan Z, Kuang M, Su Y,
Pan X, Qin R, Liu X, Zheng J and Zhou H: Artesunate ameliorates
severe acute pancreatitis (SAP) in rats by inhibiting expression of
pro-inflammatory cytokines and Toll-like receptor 4. Int
Immunopharmacol. 38:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson GB, Brunn GJ and Platt JL: Cutting
edge: An endogenous pathway to systemic inflammatory response
syndrome (SIRS)-like reactions through Toll-like receptor 4. J
Immunol. 172:20–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang B, Xu XB, Jin XX, Wu XW, Li ML, Guo
MX and Zhang XH: Effects of ω-3 fatty acids on toll-like receptor 4
and nuclear factor-κB p65 in the pancreas of rats with severe acute
pancreatitis. Pancreas. 46:1267–1274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aho HJ, Koskensalo SM and Nevalainen TJ:
Experimental pancreatitis in the rat. Sodium taurocholate-induced
acute haemorrhagic pancreatitis. Scand J Gastroenterol. 15:411–416.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiao YJ, Su MH, Lin HC and Wu CR:
Acteoside and isoacteoside protect amyloid β peptide induced
cytotoxicity, cognitive deficit and neurochemical disturbances in
vitro and in vivo. Int J Mol Sci. 18:8952017. View Article : Google Scholar
|
|
23
|
Zhao K, Chen C, Shi Q, Deng W, Zuo T, He
X, Liu T, Zhao L and Wang W: Inhibition of glycogen synthase
kinase-3β attenuates acute kidney injury in sodium
taurocholate-induced severeacute pancreatitis in rats. Mol Med Rep.
10:3185–3192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naqvi R: Acute kidney injury in
association with acute pancreatitis. Pak J Med Sci. 34:606–609.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malmstrøm ML, Hansen MB, Andersen AM,
Ersbøll AK, Nielsen OH, Jørgensen LN and Novovicet S: Cytokines and
organ failure in acute pancreatitis: Inflammatory response in acute
pancreatitis. Pancreas. 41:271–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang XP, Wang L and Zhou YF: The
pathogenic mechanism of severe acute pancreatitis complicated with
renal injury: A review of current knowledge. Dig Dis Sci.
53:297–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu J, Deng W, Wang W, Ding Y, Jin H, Chen
C, Chen X, Xiong X and Xu S: Inhibition of poly (ADPRibose)
polymerase attenuates acute kidney injury in sodium
taurocholate-induced acute pancreatitis in rats. Pancreas.
41:1299–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okusa MD, Linden J, Huang L, Rieger JM,
Macdonald TL and Huynh LP: A(2A) adenosine receptor-mediated
inhibition of renal injury and neutrophil adhesion. Am J Physiol
Renal Physiol. 279:F809–F818. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mazzon E, Impellizzeri D, Di Paola R,
Paterniti I, Esposito E, Cappellani A, Bramanti P and Cuzzocrea S:
Effects of mitogen-activated protein kinase signaling pathway
inhibition on the development of cerulean-induced acute
pancreatitis in mice. Pancreas. 41:560–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cuzzocrea S, Mazzon E, Dugo L, Serraino I,
Centorrino T, Ciccolo A, Van de Loo FAJ, Britti D, Caputi AP and
Thiemermann C: Inducible nitric oxide synthase-deficient mice
exhibit resistance to the acute pancreatitis induced by cerulein.
Shock. 17:416–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Z, Xia X, Zhang S, Zhang A, Bo W and
Zhou R: Up-regulation of Toll-like receptor 4 was suppressed by
emodin and baicalin in the setting of acute pancreatitis. Biomed
Pharmacother. 63:120–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoque R, Farooq A, Ghani A, Gorelick F and
Mehal WZ: Lactate reduces liver and pancreatic injury in Toll-like
receptor- and inflammasome-mediated inflammation via GPR81-mediated
suppression of innate immunity. Gastroenterology. 146:1763–1774.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Hartono JR, John R, Bennett M,
Zhou XJ, Wang Y, Wu Q, Winterberg PD, Nagami GT and Lu CY: Early
interleukin 6 production by leukocytes during ischemic acute kidney
injury is regulated by TLR4. Kidney Int. 80:504–515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kulkarni OP, Hartter I, Mulay SR, Hagemann
J, Darisipudi MN, Vr SK, Romoli S, Thomasova D, Ryu M, Kobold S and
Anders HJ: Toll-like receptor 4-induced IL-22 accelerates kidney
regeneration. J Am Soc Nephrol. 25:978–989. 2014. View Article : Google Scholar : PubMed/NCBI
|