Introduction
With the increasing requirement for severe medical
and respiratory support, the incidence of tracheal stenosis caused
by tracheal intubation or tracheotomy is gradually increasing in
China over the past ten years (1).
Although surgical and endoscopic interventions have several effects
on benign tracheal stenosis, secondary injury and excessive healing
following trauma can easily lead to tracheal restenosis (2). Therefore, drug treatments for the
healing process of tracheal mucosal injury is particularly
important for the prevention and treatment of benign tracheal
stenosis.
The pathogenesis of benign tracheal stenosis is
complex and involves various factors, including immunity,
inflammation, cell proliferation, differentiation, apoptosis and
oxidative stress (3–5). Inflammatory response is an important
pathogenic factor (3). Puyo and
Dahms (3) demonstrated that
patients who underwent tracheal intubations under general
anesthesia exhibited ten times the number of polymorphonuclear
cells when compared with levels prior to intubation and
significantly increased levels of inflammatory factors, including
interleukin (IL)-9, IL-6, IL-1β and tumor necrosis factor (TNF),
even if tracheal intubation was performed within 3 h. These
cytokines and inflammatory factors further aggravate the
inflammatory response of the tracheal mucosa, indicating that the
pathogenesis of tracheal stenosis following tracheal intubation is
associated with the local inflammatory reaction of the trachea and
the systemic inflammatory response (3). Following tracheal injury, repeated or
sustained inflammatory reactions promote the production of numerous
inflammatory factors (such as IL-6, IL-8) and profibrotic cytokines
[such as transforming growth factor β1 (TGF-β1), VEGF] involved in
repair activities, fibroblast cell activation and proliferation,
inhibition of apoptosis, extracellular matrix deposition and an
imbalance of collagen synthesis and degradation, and, ultimately,
excessive proliferation of granulation tissue leading to tracheal
stenosis (3–5). Histone deacetylase 2 (HDAC2), a
subtype of HDAC closely associated with chronic airway
inflammation, primarily regulates the transcriptional expression of
inflammatory genes (6). HDAC2
reduces the expression of inflammatory factors IL-8 and TNF-α by
inhibiting the transcription of NF-κB, improving the prognosis of
bronchial asthma and chronic obstructive pulmonary disease
(7,8). However, systematic research on the
associated between HDAC2 and tracheal stenosis following injury
remains unclear.
Penicillin, erythromycin and budesonide are classic
anti-inflammatory drugs and have been reported to have therapeutic
effects on tracheal stenosis (9,10).
Although these drugs are widely used for clinical control of
various inflammatory reactions, their mechanisms of action are
different. Penicillin is a highly effective, low-toxicity
antibiotic with a killing effect on sensitive bacteria (11). Erythromycin is an antibiotic with
decent effectiveness when administered orally and is involved with
a variety of biological activities, including immune regulation and
the inhibition of inflammatory factors (12). Budesonide is effective for
non-infectious inflammation; however, it is not ideal for
infectious inflammation (13).
Which drug is more powerful for protecting against tracheal
stenosis following injury remains to be elucidated. Additionally,
whether a combination of these drugs to produce a synergistic
effect following injury and whether this combination affects the
development of tracheal stenosis by regulating HDAC also remains
unclear. The current study investigated the effects of different
anti-inflammatory drugs on a model of tracheal stenosis following
injury, compared their protective effects on tracheal stenosis
following injury and explored the possible mechanisms.
Materials and methods
Experimental animals
A total of 32 New Zealand rabbits (4 weeks old; 16
males and 16 females, 2.5–3 kg) were purchased from Nanchang
Longping Rabbit Industry Co., Ltd. The rabbits were bred in a
specific pathogen-free class barrier system, maintained in a
temperature-controlled room (18–22°C) with 12-h light/dark cycles
and permitted to eat and drink freely. The present study followed
international, national and institutional guidelines for humane
animal treatment and complied with the relevant legislation
(14). Experiments were approved by
the Animal Experimental Ethics Committee of Guangxi Medical
University, Nanning, China with supervision by the facility in
which the studies were conducted (approval no. 201806020).
Experimental reagents and
instruments
Penicillin sodium (Shandong Lukang Record
Pharmaceutical Co., Ltd.) for injection, erythromycin
enteric-coated tablets (Yichang Humanwell Pharmaceutical Co.,
Ltd.), budesonide suspension (AstraZeneca) for inhalation, rabbit
anti-collagen I (Col-I) polyclonal antibodies (cat. no. bs-10423R;
BIOSS; dilution, 1:400); rabbit anti-collagen III (Col-III)
polyclonal antibodies (cat. no. bs-0549R; BIOSS; dilution, 1:400),
mouse anti-GAPDH monoclonal antibodies (cat. no. TA-08; dilution,
1:2,000); horseradish-labelled goat anti-rabbit immunoglobulin G
(IgG; H+L) (cat. no. ZB-2301; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.; dilution, 1:100), rabbit anti-HDAC2
polyclonal antibody (cat. no. bs-1813R; BIOSS; dilution, 1:400),
conjugated goat anti-rabbit IgG Cy3, (cat. no. CW0159S; CoWin
Biosciences; dilution, 1:200), ready-to-use DAPI staining solution
(cat. no. KGA215-50; Nanjing KeyGen Biotech Co., Ltd.), rabbit
TGF-β1 ELISA kit (cat. no. MM-3684001; MEIMIAN), rabbit vascular
endothelial growth factor (VEGF) ELISA kit (cat. no. MM-021001;
MEIMIAN), a rabbit IL-8 ELISA kit (cat. no. MM-030101; MEIMIAN),
rabbit polyclonal anti-TGFβ1 (cat. no. bs-0086R; BIOSS; dilution,
1:500-1:2,000), rabbit polyclonal anti-VEGF (cat. no. bs-1313R;
BIOSS; dilution, 1:500-1:2,000), rabbit monoclonal anti-IL8 (cat.
no. ab34100; Abcam; dilution, 1:1,000); rabbit polyclonal
anti-HDAC2 (cat. no. OM105905; Omnimabs; dilution, 1:500-1:2,000),
fluorescence microscope (CKX53; Olympus Corporation), microplate
reader (RT-6100; Rayto Life and Analytical Sciences Co., Ltd.),
protein vertical electrophoresis instrument (DYY-6C; Beijing Liuyi
Instrument Factory) and Ultra High Sensitivity Chemiluminescence
Imaging System (Chemi-DocTM XRS+; Bio-Rad Laboratories, Inc.) were
used in the current study.
Establishment of a tracheal stenosis
model and sampling
In total, 30 rabbits were used to establish a
tracheal stenosis model, and 2 rabbits were used as normal rabbits
to compare with the model to verify that the tracheal stenosis
model was successfully established. Modeling was performed
according to the method described by Nakagishi et al
(15). The rabbits were fasted for
8 h prior to modeling and anesthetized intravenously with 3%
pentobarbital sodium (cat. no. P11011; Merck KGaA; 30 mg/kg)
(16). To enhance analgesia, the
rabbits were injected with 2% lidocaine hydrochloride (cat. no.
T1144; TargetMol) into the anterior neck. Following anesthesia, the
rabbits were placed in the supine position and fixed onto the
operating table. The skin in the anterior cervical region was
prepared and disinfected twice with 0.5% iodophor (cat. no. YS0545;
Amresco). A longitudinal incision (~4–5 cm) was made in the skin
and the subcutaneous tissues and muscles were separated layer by
layer to expose the trachea. An annular tracheotomy was performed
in cartilage spaces 3 and 4. The length of the incision was 2/3 of
the circumference of the trachea to avoid injury to the tracheal
cartilage. The proximal end of the trachea was lifted to avoid
suffocation caused by blood flow back to the distal end of the
trachea and bleeding was stopped by compressing the tracheal
incision. A rigid nylon brush was inserted into the distal trachea
~1.5 cm through the incision and rubbed back and forth 20 times on
the front and side walls of the trachea. If intratracheal
hemorrhage occurred due to friction, a gauze was used for
hemostasis. After no obvious hemorrhage, a single no. 4.0 thread
was used to carefully suture the trachea layer by layer irregularly
over the following 3 layers: i) The muscular layer; ii) the
subcutaneous tissue layer and iii) skin. The wound was disinfected
and covered with sterile gauze. The rabbits were returned to their
cages after waking up naturally.
All rabbits were fasted for 8 h prior to sampling.
After performing anesthesia, as described above, 5 ml blood was
collected from rabbit ear veins and centrifuged at 1,400 × g for 10
min at 4°C at a centrifugation radius of 11.5 cm. The supernatant
was collected and transferred to a cryotube at −80°C for storage.
The skin was cut in the anterior cervical region and the
subcutaneous tissues and muscles were separated layer by layer to
expose the trachea. The trachea was transected 2 cm below the
original tracheal incision. The end of the trachea was compressed
with pre-prepared gauze to prevent blood from flowing into the
distal trachea and causing suffocation and contaminating the
collected bronchoalveolar lavage fluid (BALF). A syringe was
inserted into the trachea and fixed with a 4.0 surgical suture.
Left lung alveolar lavage was performed twice with 10 ml normal
saline at 4°C. At each lavage, BALF was pumped into a centrifuge
tube. Right lungs were lavaged twice using the same protocol. The
BALF of both lungs was mixed and centrifuged at 1,400 × g for 10
min at 4°C at a centrifugation radius of 11.5 cm. The supernatant
was removed and stored in a cryotube at −80°C. Once the BALF
samples were obtained, the rabbits were sacrificed via an injection
of 30 ml air/kg into the ear vein (17). The narrow trachea was collected, and
tracheal tissue specimens were stored separately at −80°C for
relevant testing.
Experimental grouping
Rabbits were randomly divided into 5 groups. All
drug doses were based on body surface area calculated using the
Meeh-Rubner formula: A=K × (W2/3/104), where
A is body surface area, the constant K is 10.6 for humans and 10.1
for rabbits W is weight (18). The
groups were as follows: i) The control (CON) group, untreated; ii)
the PEN group, animals were treated with penicillin (43500 U/kg)
twice daily via intramuscular injection; iii) the ERY group,
animals were treated with erythromycin (13.6 mg/kg) twice a day
administered via gavage; iv) the BUD group, animals were treated
with budesonide suspension (0.05 mg/kg) twice a day via atomization
inhalation; and v) the PEN + ERY + BUD group, animals were treated
with penicillin (43,500 U/kg) twice daily via intramuscular
injection, erythromycin (13.6 mg/kg) twice a day via gavage
administration and budesonide suspension (0.05 mg/kg) twice a day
via atomization inhalation. The atomization inhalation method used
was previously reported by Lei et al (19). These drugs are often used in humans
(clinical treatment) and animals (animal experiments) and are
relatively safe at these doses and time of use (11,20).
To the best of our knowledge, there are no previous reports
indicating that these drugs have obvious side effects on rabbit
organ function or body weight at these doses and time of use. The
results of preliminary experiments did not report obvious side
effects of these drugs on organs such as the liver and kidney or
body weight. A total of 6 rabbits were included in each group and
samples were collected and tested after 10 days of continuous
treatment.
Hematoxylin and eosin (H&E)
staining
The tracheal tissues of the normal and model groups
were fixed with 4% paraformaldehyde solution at 25°C for 24 h, then
rinsed for 6–8 h and dehydrated with ethanol solutions of 70, 80
and 90% and a mixture of pure alcohol and xylene at 25°C for 15
min, xylene I for 15 min and xylene II for 15 min until tissues
were transparent. Tissues were incubated in a mixture of xylene and
paraffin at 25°C for 15 min and then placed in paraffin I followed
by paraffin II at 25°C for 50–60 min each. Tissues were
paraffin-embedded and sliced (5 µm thick). The paraffinized
sections were baked at 45°C and Paraffin sections were dewaxed with
xylene I followed by xylene II for 5 min each, then placed in 100,
95, 90, 80 and 70% alcohol solutions for 3 min each, and then
placed in distilled water for 3 min. Once tissues were washed with
distilled water, each section was placed in an 0.2% aqueous
solution of hematoxylin at 25°C for 3 min and incubated in an 1%
ethanol differentiation solution at 25°C for 15 sec, washed
slightly with distilled water and returned to the blue hematoxylin
solution at 25°C for 15 sec. Sections were then rinsed with water,
stained with 0.5% eosin at 25°C for 3 min and rinsed with water
again. Sections were placed in 95 and 100% alcohol for 3 min each
for dehydration until transparent, sealed and examined by an
Olympus CX41 light microscope at ×400 magnification.
Immunofluorescence assay
Tissue sections were baked in an oven at 65°C for 2
h. Sections were placed into dimethylbenzene (cat. no. X820584;
Macklin) for 10 min and then treated with 100, 100, 95 and 80%
ethanol and purified water for 5 min each time. Antigen retrieval
was performed using citrate buffer (pH 6.0) at 121°C for 2 min.
Sections were blocked with 5% BSA (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C for 30 min and incubated with primary monoclonal
anti-HDAC2 antibody (1:400) overnight at 4°C. Sections were washed
with PBS three times and incubated with fluorescent antibodies
against Cy3 (1:200) at 37°C for 30 min. DAPI was added to the
tissue slices, which were incubated at 25°C for 5 min in the dark.
Excess DAPI was washed away with PBS and sections were washed with
water for 1 min. Sections were mounted using an ultra-clean
high-grade sealant (cat. no. BA7004; BaSO Biotech Co., Ltd.) and
observed with a fluorescence microscope (×400 at magnification).
Images were quantitatively analyzed uisng Image J software
(version: 1.52v; National Institutes of Health).
Immunohistochemistry
The tracheal tissues were fixed with 4%
paraformaldehyde solution at 25°C for 24 h, then embedded in
paraffin using a conventional method (21). Tissues were cut into 5-µm sections
and incubated with 0.3% endogenous peroxidase blocking solution
(cat. no. P0100A; Beyotime Institute of Biotechnology) at 25°C for
20 min. Sections were placed into dimethylbenzene (cat. no.
X820584; Macklin) for 10 min and then treated with 100, 100, 95 and
80% ethanol and purified water for 5 min each time at 25°C.
Sections were then incubated at room temperature for 10 min with 3%
hydrogen peroxide solution (cat. no. CS-PYJ1138; Shanghai Fusheng
Co., Ltd.; http://www.biomart.cn/infosupply/37396991.htm) and
washed with PBS three times (3 min each). Antigen retrieval was
performed using citrate buffer (pH 6.0) at 121°C for 2 min.
Sections were blocked with 5% BSA (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C for 30 min and incubated with a primary polyclonal
antibody (anti-collagen I and anti-collagen III) overnight at 4°C.
Following this, sections were incubated with horseradish
peroxide-conjugated goat anti-rabbit IgG for 30 min at 37°C,
according to the manufacturer's protocol, and mounted with epoxy
resin. Tissue sections were observed with an Olympus CX41 light
microscope at ×400 magnification.
ELISA kits
Levels of IL-8, TGF-β1 and VEGF in the serum and
BALF of the groups were detected using ELISA kits, according to the
manufacturer's protocol. Optical density values at a wavelength of
450 nm were determined using a microplate reader.
Western blotting
Total proteins were extracted using RIPA cell lysate
(cat. no. C1053; Applygen Technologies Inc.) and protein
concentrations were determined using bicinchoninic acid assays.
Proteins (50 µg/lane) were separated using 12% SDS-PAGE and
electrotransferred to PVDF membranes (Cytiva). PVDF membranes were
rinsed with TBS for 10–15 min and placed in 3% TBS-Tween-20 (TBS-T)
blocking buffer containing 5% (w/v) skim milk powder at room
temperature overnight. Membranes were incubated at room temperature
for 2 h following the addition of an appropriate dilution of
primary antibodies (anti-TGFβ1, anti-VEGF, anti-IL8, anti-HDAC2 and
anti-GAPDH). Membranes were then rinsed with TBS-T three times
(5–10 min/wash) and incubated at room temperature for 1 h with
horseradish peroxidase-labelled goat anti-rabbit IgG (1:2,000; cat.
no. ZB-2301; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) and
goat anti-mouse IgG (H+L) (1:2,000; cat. no. ZB-2305; Zhongshan
Jinqiao Biotechnology Co., Ltd.) secondary antibodies diluted with
TBS-T containing 0.05% (w/v) skim milk powder. Membranes were then
rinsed three times with TBS-T (5–10 min/wash). Protein bands were
detected using an ECL kit (PerkinElmer, Inc.) and quantified based
on ratios relative to GAPDH. Quantification was performed using
Quantity One software (version 4.6.6, Bio-Rad Laboratories,
Inc.).
Statistical analysis
Experiments were performed in triplicate for each
group. Data are presented as the mean ± SD. One-way ANOVA followed
by Tukey's post hoc test was used to analyze differences between
groups. All analyses were conducted using SPSS software (version
19.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
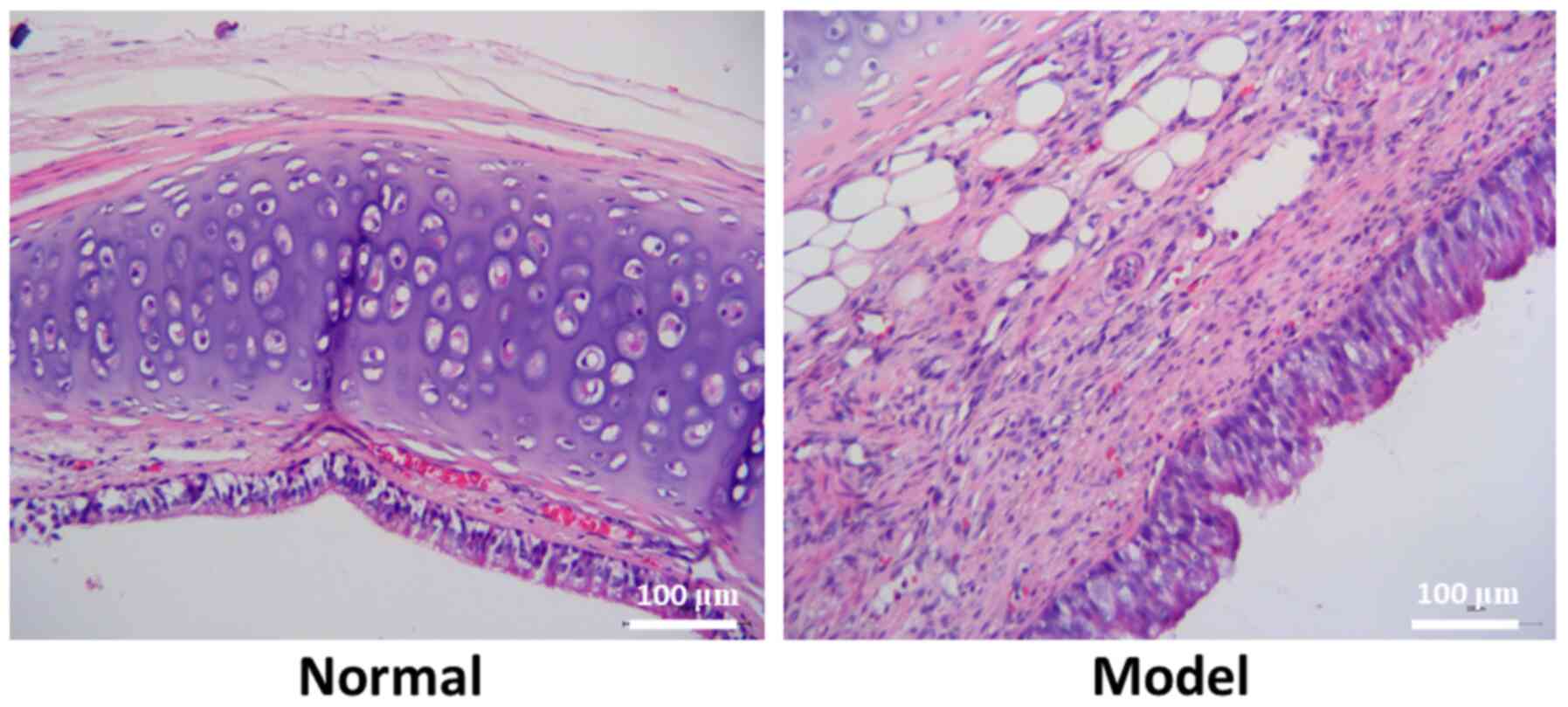
Successful establishment of the
tracheal stenosis model
In the normal rabbits, mucosa, submucosa, goblet
cells and basal cells were clearly visible, and the connective
tissues of the submucosal fibers were loose. Mucosal epithelial
hyperplasia, fibroblast proliferation, thickening of collagen
fibers, disordered collagen arrangement and high levels of
inflammatory cell infiltration were observed in the model rabbits.
Therefore, the model was successfully established (Fig. 1).
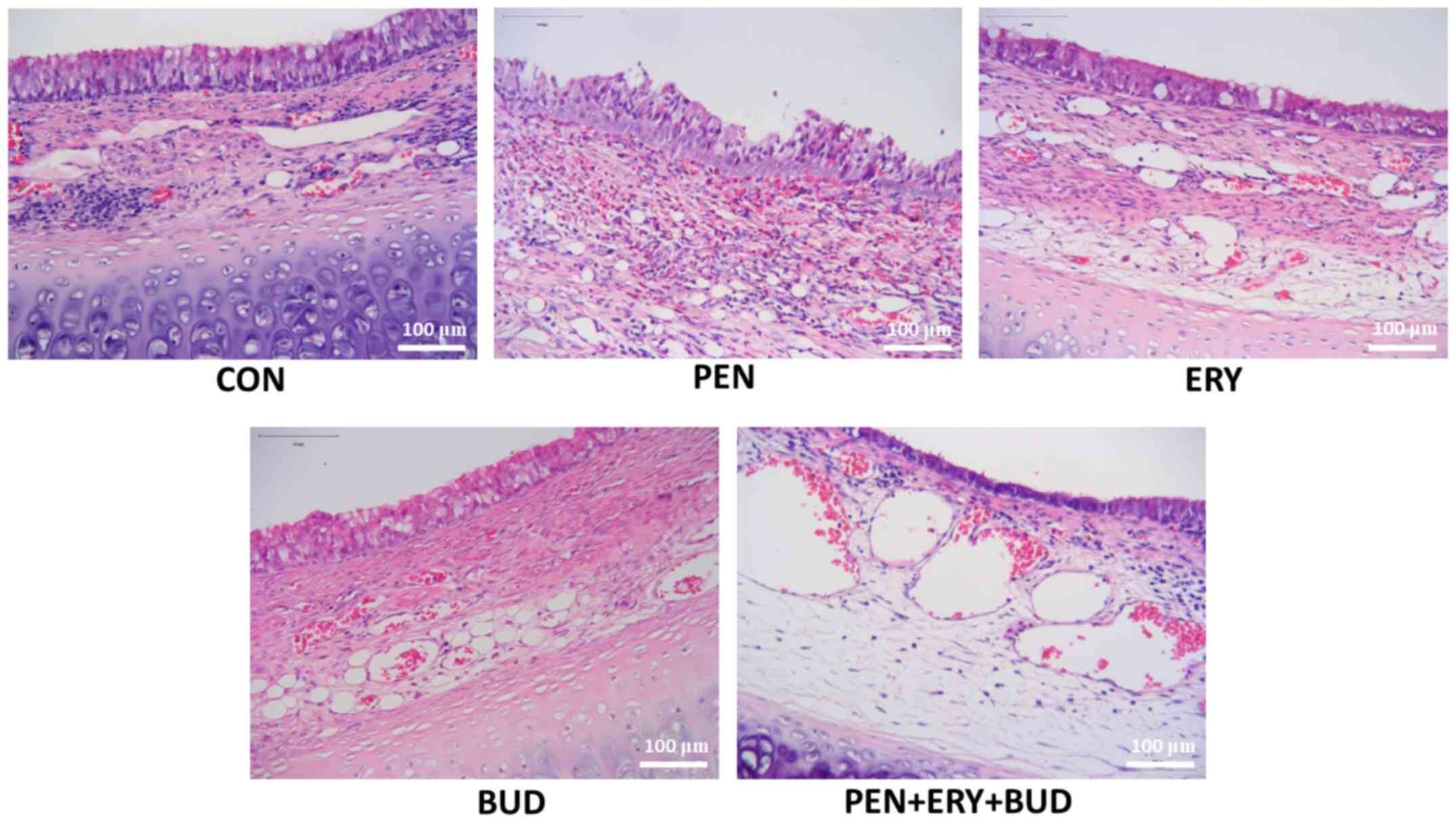
H&E staining demonstrates that
erythromycin reduces tracheal mucosal epithelial tissue
hyperplasia
Tracheal lumen stenosis, epithelial hyperplasia,
fibroblast hyperplasia, thickening of collagen fibers, disordered
arrangement and inflammatory cell infiltration were observed in the
CON, PEN and BUD groups of models (Fig.
2). In the ERY group of models, the degree of tracheal stenosis
was reduced, mucosal epithelial hyperplasia was alleviated, and
fibroblast proliferation and inflammatory cell infiltration were
markedly reduced. The effect of the drug treatment in the PEN + ERY
+ BUD group of models was more obvious compared with the ERY group,
the mucosal epithelial hyperplasia was further improved, and the
proliferation of fibroblasts was further reduced. Additionally,
compared with the PEN and BUD groups, mucosal epithelial
hyperplasia in the PEN + ERY + BUD group was significantly
relieved, and fibroblast proliferation and inflammatory cell
infiltration were significantly reduced (Fig. 2).
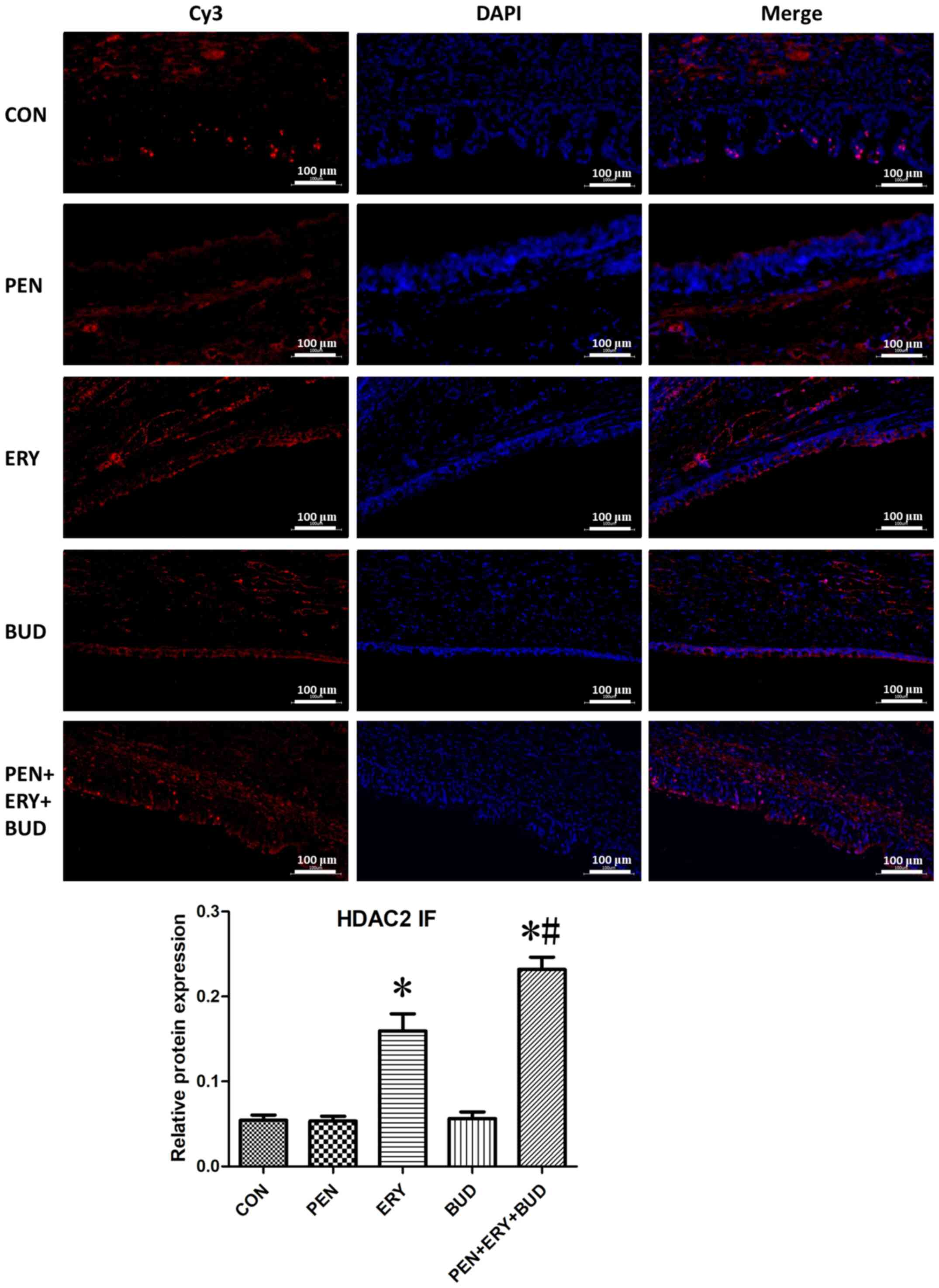
Immunofluorescence reveals that
erythromycin inhibits the expression of HDAC2 in tracheal
tissues
As shown in Fig. 3,
red fluorescence (Cy3 staining) indicates the target protein HDAC2
and blue fluorescence (DAPI) indicates the nuclei. Analysis of the
tracheal tissues indicated that HDAC2 protein expression in the ERY
and PEN + ERY + BUD groups was significantly increased compared
with the CON group (P<0.05). Additionally, HDAC2 expression in
the PEN + ERY + BUD group was significantly further increased
compared with the ERY group (P<0.05). There were no differences
in HDAC2 protein expression between the PEN, BUD and CON
groups.
Immunohistochemistry reveals that
erythromycin reduces the expression of Col-I and Col-III in
tracheal tissue
As shown in Fig. 4,
brown staining indicates collagen expression and blue staining
indicates the nuclei. In the tracheal tissues, the expression
levels of Col-I and Col-III in the ERY and PEN + ERY + BUD groups
were significantly lower compared with the CON group (P<0.05).
Moreover, levels in the PEN + ERY + BUD group were significantly
further reduced compared with the ERY group (P<0.05), indicating
that fibroblast hyperplasia was controlled and alleviated. There
were no differences in the expression levels of Col-I and Col-III
between the PEN, BUD and CON groups.
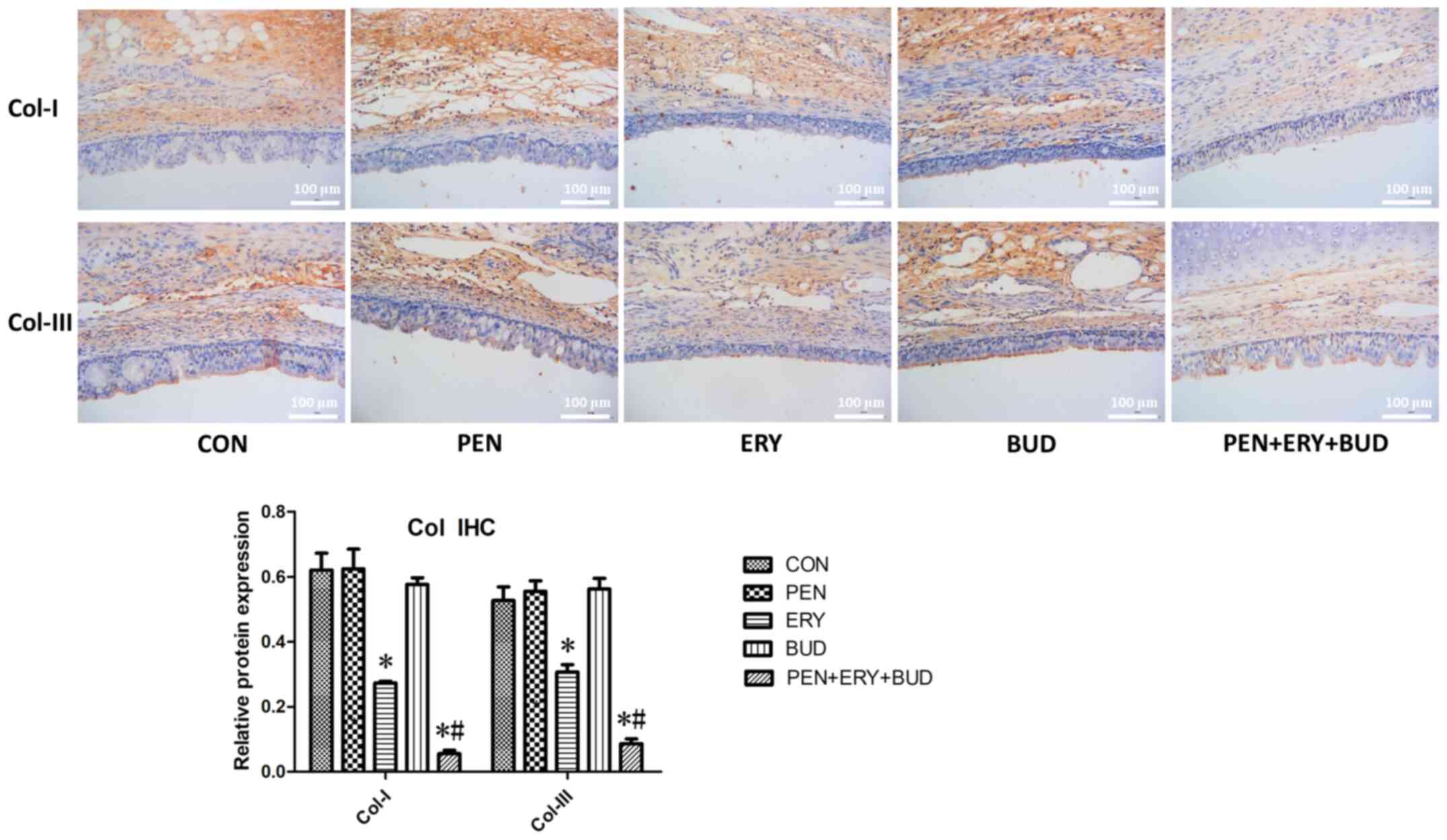 | Figure 4.Col-I and Col-III expression in the
tracheal tissues of each group was determined by IHC. Scale bar,
100 µm. Brown staining, target proteins Col-I and Col-III. Blue
staining, nucleus. Data are presented as the mean ± standard
deviation. *P<0.05 vs. CON. #P<0.05 vs. ERY.
Col-I, collagen I; Col-III, collagen III; IHC,
immunohistochemistry; CON, untreated rabbit tracheal stenosis
model; PEN, rabbit tracheal stenosis model treated with penicillin;
ERY, rabbit tracheal stenosis model treated with erythromycin; BUD,
rabbit tracheal stenosis model treated with budesonide; PEN + ERY +
BUD, rabbit tracheal stenosis model treated with penicillin,
erythromycin and budesonide. |
ELISA indicates that erythromycin
reduces the expression of IL-8, TGF-β1 and VEGF in serum and
BALF
As shown in Fig. 5,
the expression levels of IL-8, TGF-β1 and VEGF were significantly
decreased in the ERY and PEN + ERY + BUD groups compared with the
CON group in serum and BALF samples (P<0.05). Furthermore,
levels in the PEN + ERY + BUD group were significantly further
reduced compared with the ERY group (P<0.05). There were no
differences in the expression levels of IL-8, TGF-β1 and VEGF
between the PEN, BUD and CON groups.
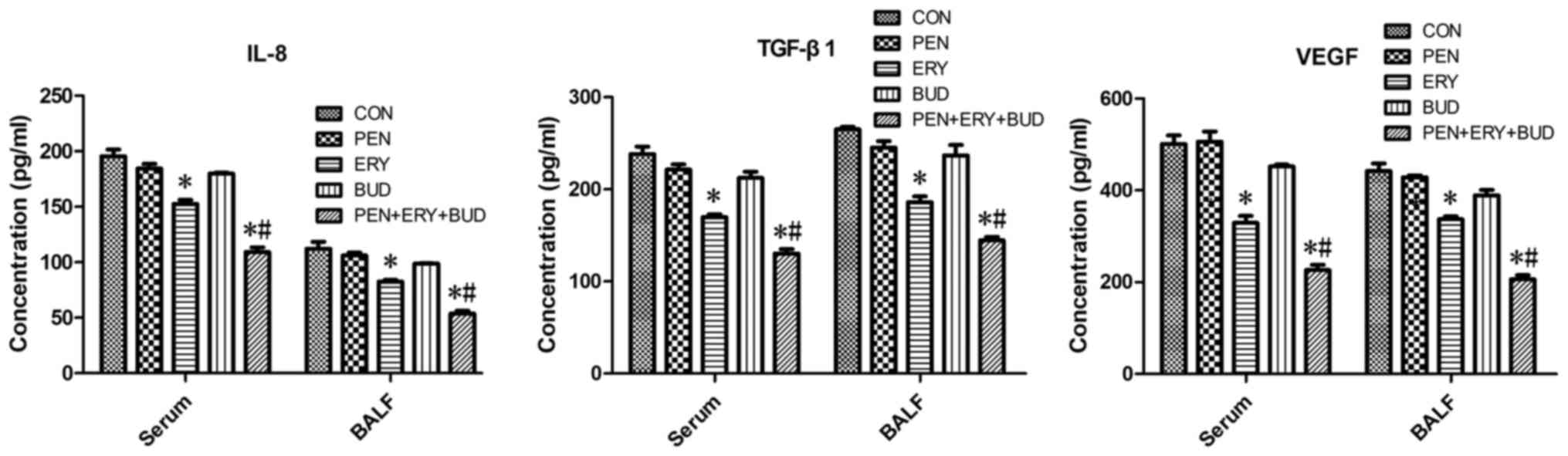 | Figure 5.IL-8, TGF-β1 and VEGF levels in the
serum and BALF of each group were determined by ELISAs. Data are
presented as the mean ± standard deviation in the corresponding
histograms. *P<0.05 vs. CON. #P<0.05 vs. ERY.
IL-8, interleukin 8; TGF-β1, transforming growth factor β1; VEGF,
vascular endothelial growth factor; BALF, bronchoalveolar lavage
fluid; CON, untreated rabbit tracheal stenosis model; PEN, rabbit
tracheal stenosis model treated with penicillin; ERY, rabbit
tracheal stenosis model treated with erythromycin; BUD, rabbit
tracheal stenosis model treated with budesonide; PEN + ERY + BUD,
rabbit tracheal stenosis model treated with penicillin,
erythromycin and budesonide. |
Western blotting shows that
erythromycin increases the expression of HDAC2 in tracheal tissues
and decreases the expression of IL-8, TGF-β1 and VEGF
In tracheal tissues, HDAC2 protein expression in the
ERY and PEN + ERY + BUD groups was significantly increased compared
with the CON group (Fig. 6;
P<0.05). Additionally, expression in the PEN + ERY + BUD group
was significantly further increased compared with the ERY group
(P<0.05). The protein expression of IL-8, TGF-β1 and VEGF in the
ERY and PEN + ERY + BUD groups were significantly decreased
compared with the CON group (P<0.05) and the expression in the
PEN + ERY + BUD group was further reduced compared with the ERY
group (P<0.05). There were no differences in the protein
expression of HDAC2, IL-8, TGF-β1 or VEGF between the PEN, BUD and
CON groups.
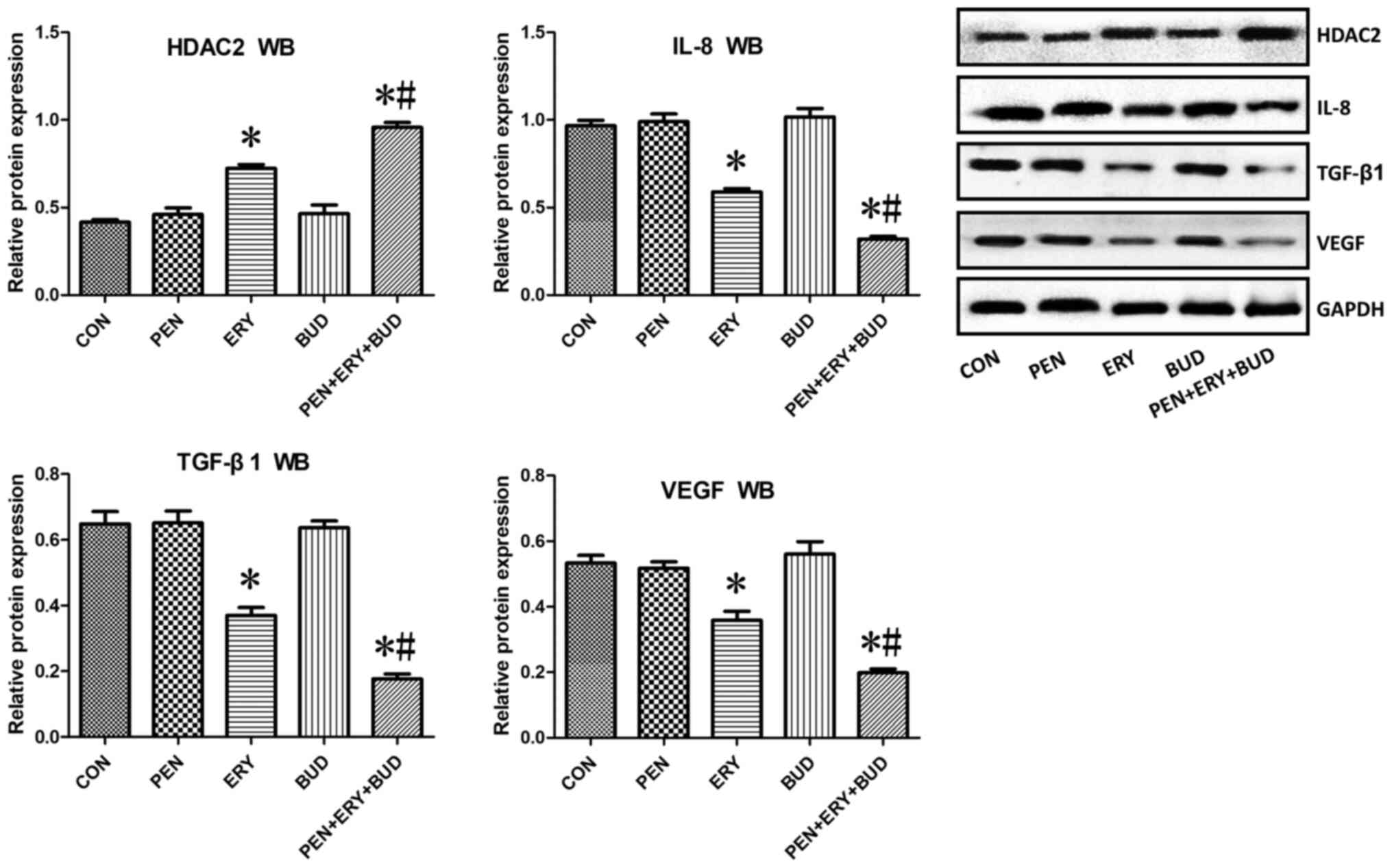 | Figure 6.HDAC2, IL-8, TGF-β1 and VEGF
expression in the tracheal tissue of each group determined by WB.
Results were normalized to the internal control GAPDH. Data are
presented as the mean ± standard deviation. *P<0.05 vs. CON.
#P<0.05 vs. ERY. HDAC2, histone deacetylase 2; IL-8,
interleukin 8; TGF-β1, transforming growth factor β1; VEGF,
vascular endothelial growth factor; WB, western blotting; CON,
untreated rabbit tracheal stenosis model; PEN, rabbit tracheal
stenosis model treated with penicillin; ERY, rabbit tracheal
stenosis model treated with erythromycin; BUD, rabbit tracheal
stenosis model treated with budesonide; PEN + ERY + BUD, rabbit
tracheal stenosis model treated with penicillin, erythromycin and
budesonide. |
Discussion
There are numerous causes of acquired benign airway
stenosis, including traumatic, infectious and inflammatory causes,
and benign and idiopathic tumors. In cases of tracheal stenosis
following injury, stenosis caused by trauma and iatrogenic factors
are the most common types (1,22).
Airway stenosis following tracheotomy and tracheal intubation is
the most common iatrogenic airway injury (23). Additionally, trauma and anastomosis
of the tracheobronchial ends following surgery are common causes.
Physical and chemical damage (acidic and alkaline toxic agents),
inhalation injury, radiation injury, bronchoscopy intervention
airway thermal ablation (laser, argon plasma coagulation,
electrocoagulation) are also important causes (24). Compared with malignant airway
stenosis, benign airway stenosis is more difficult to treat and
more likely to cause long-term complications (1). Furthermore, due to the extended
survival of patients, patients and their families have high
expectations for the quality of life and the serious short-term and
long-term complications caused by surgery are difficult to accept
(1). Therefore, the treatment of
benign airway stenosis is a difficult issue in the field of
respiratory diseases. Presently, transbronchoscopic intervention
therapy has gradually become one of the main methods for the
treatment of benign airway stenosis (25). However, due to the inevitable
secondary airway injury during the treatment of airway stenosis,
restenosis following endoscopic intervention therapy is a challenge
in the field of interventional respiratory disease (1).
The current methods for the prevention and treatment
of tracheobronchial stenosis and restenosis following endoscopic
intervention mainly include local application of mitomycin C
(26,27), intracavitary brachytherapy (28), drug-coated stents (29,30),
balloon dilation (31), argon
plasma coagulation (32),
cryotherapy (33) and adequate
anti-infection treatment (34).
Previous studies have confirmed that the formation of granulation
tissue following tracheal reconstruction and stent implantation is
closely associated with an increase in bacterial infections and
common pathogens include Pseudomonas aeruginosa, Staphylococcus
aureus, Streptococcus viridans, Haemophilus influenzae and
Neisseria (34–36). Brown and Montgomery (34) demonstrated that the use of
antibiotics significantly reduced the formation of intratracheal
granulation tissue following silicone stent implantation. The
results indicated that antibiotic therapy may have a preventive
effect on tracheal stenosis following tracheal injury. Penicillin
is an important β-lactam antibiotic with high efficiency, low
toxicity and wide clinical applications (11). The development of this antibiotic
introduced a new era of antibiotic treatment for infectious
diseases (11). In the current
study, tracheal mucosal epithelial hyperplasia and fibroblast
proliferation were not alleviated in the PEN group compared with
the CON group. Furthermore, collagen synthesis was not
significantly reduced, indicating that infection is not the only
factor in tracheal stenosis following injury and is not necessarily
the main factor. At the same time, in this study, the tracheal
stenosis model was established under strict aseptic conditions;
thus reducing the risk of infection by pathogenic microorganisms.
Therefore, the therapeutic effect of penicillin on tracheal
stenosis after injury in animal models is not as obvious as in
clinical applications (5).
Glucocorticoids are commonly used anti-inflammatory
and immunosuppressive drugs that bind to the glucocorticoid
receptor on the cell membrane and enter the nucleus to regulate the
transcription of various genes, thereby controlling cell function
(37). The mechanism by which
glucocorticoids inhibit scar formation remains unclear.
Additionally, whether topical application of glucocorticoids for
benign scar stenosis in the central airway is effective is unclear.
Yokoi et al (38) reported
that inhaled budesonide effectively treated tracheal granulation
tissue hyperplasia in patients who underwent tracheostomies
following congenital tracheal stenosis repair. The results of the
current study demonstrated that tracheal mucosal epithelial
hyperplasia and fibroblast proliferation were not significantly
improved in the BUD group and that collagen synthesis was not
significantly reduced compared with the CON group, indicating that
a single inhaled BUD cannot reduce tracheal stenosis following
tracheal injury. This is most likely due tracheal stenosis
following injury being caused by numerous factors (1). While budesonide reduces non-infectious
inflammation, it cannot address inflammation caused by infection
and may aggravate infection (39).
In addition to its antibacterial action,
erythromycin is a macrolide antibiotic with strong
anti-inflammatory and immunomodulatory effects (12). Erythromycin is widely used in
chronic inflammatory airway diseases, including asthma,
bronchiectasis and diffuse panbronchiolitis, due of its
dose-dependent, anti-inflammatory, immunomodulatory,
anti-fibroblast proliferative and inhibitory effects on the
synthesis and secretion of fibrosis-related factors (40). A previous study confirmed that
erythromycin enhanced the activity of HDAC2 in monocytes under
oxidative stress, thereby reducing the expression of NF-κB and IL-8
(41). Long-term application of
low-dose erythromycin exerted an airway anti-inflammatory effect,
which reduces the number of acute episodes of chronic obstructive
pulmonary disease (COPD) (42). In
the current study, tracheal mucosal epithelial hyperplasia and
fibroblast proliferation were significantly improved in the ERY
group and collagen synthesis was significantly reduced compared
with the CON group, indicating that erythromycin inhibited the
proliferation of tracheal granulation tissue and had preventive and
protective effects in tracheal stenosis following injury.
Furthermore, the tracheal granulation hyperplasia in the PEN + ERY
+ BUD group was further inhibited compared with the ERY group,
indicating that the drug combination exhibited synergistic
effects.
The activation and release of inflammatory cells and
inflammatory mediators are conducted by the transcription and
regulation of inflammatory genes (8). Histone acetylation and deacetylation
are the ‘switches’ which regulate the transcription of inflammatory
genes (8). HDACs remove acetyl
groups from the tails of histones and inhibit the transcription of
inflammatory genes (6). HDAC2, a
subtype of type I HDAC, is located in the nucleus and is primarily
involved in inflammatory inhibition (43,44).
Previous studies have demonstrated that HDAC2 activity in lung
inflammatory cells is reduced by tobacco smoke, leading to
transcriptional activation of NF-κB, which enhances the expression
and activity of inflammatory factors IL-8 and TNF-α (45,46).
In chronic airway inflammatory diseases, including asthma and COPD,
HDAC2 activity is significantly reduced and is closely associated
with disease progression (7,8). This
activity can be restored by inhaled corticosteroid therapy
(47). Previous studies have
reported that low concentrations of theophylline and erythromycin
upregulated the protein expression of HDAC2, enhanced HDAC2
activity (48,49) and improved the prognosis of COPD
(50). Additionally, erythromycin
may increase the activity of HDAC2 by inhibiting the
phosphoinositide 3-kinase/protein kinase B pathway and reducing the
release of inflammatory factors, including IL-8, thereby increasing
the anti-inflammatory effect of budesonide (51). IL-8 is an important pro-inflammatory
signal that promotes the migration of neutrophils to the wound site
early in wound healing, alters the local immune environment and
initiates granulation hyperplasia for wound repair (52). A previous study on biomarkers that
predict tracheal stenosis caused by metal stent implantation
demonstrated that elevated IL-8 expression levels were predictive
of the development of tracheal stenosis in rabbits (53). Furthermore, a previous study using a
murine COPD model revealed that carbocisteine improved airway
remodeling, including airway epithelium, smooth muscle thickness,
airway fibrosis and decreased levels of α-smooth muscle actin and
TGF-β1, by increasing HDAC2 expression and activity (54). In the current study, tracheal
mucosal epithelial hyperplasia and fibroblast hyperplasia were not
improved and there was no significant reduction in collagen
synthesis in the PEN and BUD groups compared with the CON group,
which was consistent with the lack of a significant upregulation
effect of PEN or BUD on the expression of HDAC2 in tracheal
tissues. However, erythromycin upregulated the expression of HDAC2
and inhibited hyperplasia in tracheal granulation tissues. Although
erythromycin may improve tracheal stenosis following injury through
a combination of multiple signaling pathways, this study
hypothesized that HDAC2 may represent an important signaling
pathway in the development of tracheal stenosis.
Granulation tissue in tracheal stenosis is
characterized by increased angiogenesis and extracellular matrix
deposition (5). TGF-β1 is a potent
extracellular matrix inducer, a chemotactic mediator of fibroblasts
and polymorphonuclear cells (55,56)
and a fibroblast mitogen (57) that
serves an important role in epithelial cell regeneration,
fibroblast proliferation and tracheal wound healing following
mechanical injury (55–57). VEGF is the most potent specific
endothelial cell mitogen and serves a crucial role in stimulating
endothelial cell mitosis, migration and angiogenesis (58,59).
The production of VEGF indicates the formation of new blood
vessels, which is characteristic of tissue repair following injury
(5). TGF-β1 enhances the repair and
reconstruction of tissues by inducing the release of VEGF (60). Lee et al (61) reported that the expression of TGF-β1
and VEGF and the number of fibroblasts increased in tracheal
granulation tissues obtained by interventional bronchoscopy
following tracheostomy. Furthermore, the submucosal layer of the
granulation tissues exhibited a further increase in the expression
of TGF-β1 and VEGF and the number of fibroblasts compared with the
epithelial layer and small blood vessels in the submucosa of the
granulation tissues were markedly increased (61). Low concentrations of erythromycin (1
mg/ml), rather than dexamethasone, inhibited TGF-β1-induced VEGF
production (61). This result is
consistent with the inhibitory effects of erythromycin on TGF-β1
and VEGF expression in the tracheal granulation tissues observed in
the current study.
In the current study, HDAC2, IL-8, TGF-β1 and VEGF
expression in the stenotic tracheal tissues of the PEN and BUD
groups were not significantly altered compared with the CON group
and there were no significant differences in IL-8, TGF-β1 and VEGF
expression in serum and BALF samples. HDAC2 expression in the
stenotic tracheal tissues of the ERY group was increased, the
expression of IL-8, TGF-β1 and VEGF was decreased and the
expression of IL-8 and TGF-β1 and VEGF in the serum and BALF was
decreased compared with the CON group. Furthermore, HDAC2
expression in the stenotic tracheal tissue was further increased,
the expression of IL-8, TGF-β1 and VEGF was further decreased and
the expression of IL-8, TGF-β1 and VEGF in the serum and BALF was
significantly decreased in the PEN + ERY + BUD group compared with
the ERY group. These results indicated that erythromycin may
inhibit the tracheal local and systemic inflammatory response by
upregulating HDAC2, promoting the downregulation of TGF-β1 and
VEGF, inhibiting the proliferation of fibroblasts and the synthesis
and secretion of fibrosis-related factors, reducing the synthesis
of Col-I and Col-III, limiting excessive repair of the damaged
trachea and protecting against tracheal stenosis following injury.
The combined use of erythromycin, budesonide and penicillin
produced a synergistic effect and protective effects are further
enhanced.
In conclusion, it is challenging to prevent tracheal
stenosis following injury and treatment efficacy is poor due to a
combination of various factors such as infection, inflammation and
oxidative stress (1). The use of
erythromycin alone significantly inhibited the proliferation of
tracheal granulation tissues and improved tracheal stenosis
following injury, while penicillin and budesonide had no
significant effect. Erythromycin upregulated the expression of
HDAC2, enhanced anti-inflammatory effect, downregulated the
expression of TGF-β1, VEGF, Col-I and Col-III and inhibited
excessive tissue repair, thereby effectively reducing tracheal
stenosis following tracheal injury. The combined use of penicillin,
erythromycin and budesonide produced a synergistic effect, which
may further upregulate the expression of HDAC2, inhibit local
airway and systemic inflammatory responses, inhibit granulation
hyperplasia and enhance the protective effect against tracheal
stenosis following injury. The main purpose of this study was to
compare the therapeutic effect of penicillin, erythromycin and
budesonide alone on tracheal stenosis after injury, and to make a
preliminary understanding on the effect of combined drug treatment
Due to the limitation of experimental time and funding, future
studies on the effects of ≥2 drug combinations will be conducted.
For the stability of the experiment, HDAC2 activators and
inhibitors were selected to initially verify the theory in this
study. Future studies will consider gene knock-in or knock-out
experiments from the perspective of genes to further verify the
theory.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
National Nature Science Foundation of China (grant no.
81760001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and GL designed the experiments. ZH, PW, LG and
WL performed the experiments. LG, WL, TZ, CQ and ZC analyzed the
data and ZH validated the analysis. ZH prepared the manuscript and
GL revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Animal
Experimental Ethics Committee of Guangxi Medical University,
Nanning, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Su ZQ, Wei XQ, Zhong CH, Chen XB, Luo WZ,
Guo WL, Wang YZ and Li SY: The cause and efficacy of benign
tracheal stenosis. Zhonghua Jie He He Hu Xi Za Zhi. 36:651–654.
2013.(In Chinese). PubMed/NCBI
|
|
2
|
Zhang J, Wang T, Wang J, Pei YH, Xu M,
Wang YL, Zhang X and Wang C: Effect of three interventional
bronchoscopic methods on tracheal stenosis and the formation of
granulation tissues in dogs. Chin Med J (Engl). 123:621–627.
2010.PubMed/NCBI
|
|
3
|
Puyo CA and Dahms TE: Innate immunity
mediating inflammation secondary to endotracheal intubation. Arch
Otolaryngol Head Neck Surg. 138:854–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lawrence DA, Branson B, Oliva I and
Rubinowitz A: The wonderful world of the windpipe: A review of
central airway anatomy and pathology. Can Assoc Radiol J. 66:30–43.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li LH, Xu MP, Gan LM, Li Y, Liang YL, Li
WT, Qin EY, Gan JH and Liu GN: Effect of low dose erythromycin on
the proliferation of granulation tissue after tracheal injury.
Zhonghua Yi Xue Za Zhi. 97:777–781. 2017.(In Chinese). PubMed/NCBI
|
|
6
|
Yao H and Rahman I: Current concepts on
oxidative/carbonyl stress, inflammation and epigenetics in
pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl
Pharmacol. 254:72–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito K, Lim S, Caramori G, Chung KF, Barnes
PJ and Adcock IM: Cigarette smoking reduces histone deacetylase 2
expression, enhances cytokine expression, and inhibits
glucocorticoid actions in alveolar macrophages. FASEB J.
15:1110–1112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barnes PJ: Role of HDAC2 in the
pathophysiology of COPD. Annu Rev Physiol. 71:451–464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croft CB, Zub K and Borowiecki B: Therapy
of iatrogenic subglottic stenosis: A steroid/antibiotic regimen.
Laryngoscope. 89:482–489. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou L, Li Y, Gan LM, Qin EY, Meng XY, Gan
JH, Li WT, Qin CC and Liu GN: Effects of different drugs on
bronchial stenosis by TGF-β/mTOR signaling pathway in rabbit model.
Zhonghua Yi Xue Za Zhi. 99:1898–1903. 2019.(In Chinese). PubMed/NCBI
|
|
11
|
Thompson SR III: Treatment of syphilis in
pregnancy. J Am Vener Dis Assoc. 3:158–167. 1976.PubMed/NCBI
|
|
12
|
Giamarellos-Bourboulis EJ: Macrolides
beyond the conventional antimicrobials: A class of potent
immunomodulators. Int J Antimicrob Agents. 31:12–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stern A, Skalsky K, Avni T, Carrara E,
Leibovici L and Paul M: Corticosteroids for pneumonia. Cochrane
Database Syst Rev. 12:CD0077202017.PubMed/NCBI
|
|
14
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. J Pharmacol
Pharmacother. 1:94–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakagishi Y, Morimoto Y, Fujita M, Ozeki
Y, Maehara T and Kikuchi M: Rabbit model of airway stenosis induced
by scraping of the tracheal mucosa. Laryngoscope. 115:1087–1092.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White WJ and Field KJ: Anesthesia and
surgery of laboratory animals. Vet Clin North Am Small Anim Pract.
17:989–1017. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harken AH, Lillo RS and Haut MJ: The
depressant influence of extracellular fluid hyperoxia on liver
slice oxygen uptake. J Lab Clin Med. 89:1269–1277. 1977.PubMed/NCBI
|
|
18
|
Spiers DE and Candas V: Relationship of
skin surface area to body mass in the immature rat: A
reexamination. J Appl Physiol Respir Environ Exerc Physiol.
56:240–243. 1984.PubMed/NCBI
|
|
19
|
Lei H, Qian GS, Wu GM and Huang GJ:
Transfection of human beta defensin 2 gene into the lung by aerosol
inhalation: Experiment with rats. Zhonghua Yi Xue Za Zhi.
88:1425–1428. 2008.(In Chinese). PubMed/NCBI
|
|
20
|
Lin JT, Zhang YM, Zhou X, Wang CZ, Huang
M, Liu CT, Wu CG, Wan HY, Yu WC and Dai YR: Chinese expert
consensus for non-antiinfective effects and clinical use of
macrolides. Zhonghua Nei Ke Za Zhi. 56:546–557. 2017.(In Chinese).
PubMed/NCBI
|
|
21
|
Ushida K, Asai N, Uchiyama K, Enomoto A
and Takahashi M: Development of a method to preliminarily embed
tissue samples using low melting temperature fish gelatin before
sectioning: A technical note. Pathol Int. 68:241–245. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stauffer JL, Olson DE and Petty TL:
Complications and consequences of endotracheal intubation and
tracheotomy. A prospective study of 150 critically ill adult
patients. Am J Med. 70:65–76. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Plojoux J, Laroumagne S, Vandemoortele T,
Astoul PJ, Thomas PA and Dutau H: Management of benign dynamic
‘A-shape’ tracheal stenosis: A retrospective study of 60 patients.
Ann Thorac Surg. 99:447–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barros CD, Fernandez-Bussy S, Folch E,
Flandes AJ and Majid A: Non-malignant central airway obstruction.
Arch Bronconeumol. 50:345–354. 2014.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Wang J, Wang T, Xu M, Dang BW,
Pei YH and Zhang CY: A pilot study on interventional bronchoscopy
in the management of airway stenosis with benign hyperplasia.
Zhonghua Jie He He Hu Xi Za Zhi. 34:334–338. 2011.(In Chinese).
PubMed/NCBI
|
|
26
|
Ferreira S, Nogueira C, Oliveira A, Neves
S, Almeida J and Moura e Sá J: Bronchoscopic dilation techniques
and topical application of mitomycin-C in the treatment of tracheal
stenosis post intubation-two case reports. Rev Port Pneumol.
16:149–156. 2010.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simpson CB and James JC: The efficacy of
mitomycin-C in the treatment of laryngotracheal stenosis.
Laryngoscope. 116:1923–1925. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tendulkar RD, Fleming PA, Reddy CA, Gildea
TR, Machuzak M and Mehta AC: High-dose-rate endobronchial
brachytherapy for recurrent airway obstruction from hyperplastic
granulation tissue. Int J Radiat Oncol Biol Phys. 70:701–706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu GH, Ng AH, Venkatraman SS, Boey FYC,
Wee ALY, Trasti SL and Lim LHY: A novel bioabsorbable drug-eluting
tracheal stent. Laryngoscope. 121:2234–2239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin JH, Song HY, Seo TS, Yuk SH, Kim YH,
Cho YM, Choi GB, Kim TH and Suh JY: Influence of a
dexamethasone-eluting covered stent on tissue reaction: An
experimental study in a canine bronchial model. Eur Radiol.
15:1241–1249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Venhaus M, Behn C, Freitag L and
Zimmermann K: Simulations and experiments of the balloon dilatation
of airway stenoses. Biomed Tech (Berl). 54:187–195. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Erelel M, Yakar F and Yakar A:
Endobronchial tuberculosis with lobar obstruction successfully
treated by argon plasma coagulation. South Med J. 102:1078–1081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krimsky WS, Rodrigues MP, Malayaman N and
Sarkar S: Spray cryotherapy for the treatment of glottic and
subglottic stenosis. Laryngoscope. 120:473–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brown MT and Montgomery WW: Microbiology
of tracheal granulation tissue associated with silicone airway
prostheses. Ann Otol Rhinol Laryngol. 105:624–627. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simoni P and Wiatrak BJ: Microbiology of
stents in laryngotracheal reconstruction. Laryngoscope.
114:364–367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matt BH, Myer CR III, Harrison CJ, Reising
SF and Cotton RT: Tracheal granulation tissue. A study of
bacteriology. Arch Otolaryngol Head Neck Surg. 117:538–541. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barnes PJ: How corticosteroids control
inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol.
148:245–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yokoi A, Nakao M, Bitoh Y, Arai H, Oshima
Y and Nishijima E: Treatment of postoperative tracheal granulation
tissue with inhaled budesonide in congenital tracheal stenosis. J
Pediatr Surg. 49:293–295, 295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toren K, Blanc PD, Qvarfordt I, Aspevall O
and Schioler L: Inhaled corticosteroids use and risk of invasive
pneumococcal disease risk in a population-based study. Ann Am
Thorac Soc. 17:1570–1575. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amsden GW: Anti-inflammatory effects of
macrolides-an underappreciated benefit in the treatment of
community-acquired respiratory tract infections and chronic
inflammatory pulmonary conditions? J Antimicrob Chemother.
55:10–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li M, Zhong X, He Z, Wen M, Li J, Peng X,
Liu G, Deng J, Zhang J and Bai J: Effect of erythromycin on
cigarette-induced histone deacetylase protein expression and
nuclear factor-kB activity in human macrophages in vitro. Int
Immunopharmacol. 12:643–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seemungal TA, Wilkinson TM, Hurst JR,
Perera WR, Sapsford RJ and Wedzicha JA: Long-term erythromycin
therapy is associated with decreased chronic obstructive pulmonary
disease exacerbations. Am J Respir Crit Care Med. 178:1139–1147.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li
X, Zhao D, Liu Y, Wang C, Zhang X, et al: Tet2 is required to
resolve inflammation by recruiting Hdac2 to specifically repress
IL-6. Nature. 525:389–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ito K, Yamamura S, Essilfie-Quaye S, Cosio
B, Ito M, Barnes PJ and Adcock IM: Histone deacetylase 2-mediated
deacetylation of the glucocorticoid receptor enables NF-kappaB
suppression. J Exp Med. 203:7–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Adenuga D, Yao H, March TH, Seagrave J and
Rahman I: Histone deacetylase 2 is phosphorylated, ubiquitinated,
and degraded by cigarette smoke. Am J Respir Cell Mol Biol.
40:464–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barnes PJ: Immunology of asthma and
chronic obstructive pulmonary disease. Nat Rev Immunol. 8:183–192.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ito K, Caramori G, Lim S, Oates T, Chung
KF, Barnes PJ and Adcock IM: Expression and activity of histone
deacetylases in human asthmatic airways. Am J Respir Crit Care Med.
166:392–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cosio BG, Tsaprouni L, Ito K, Jazrawi E,
Adcock IM and Barnes PJ: Theophylline restores histone deacetylase
activity and steroid responses in COPD macrophages. J Exp Med.
200:689–695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, He Z, Sun X, Li Z, Zhao L, Mao C,
Huang D, Zhang J and Zhong X: Erythromycin restores oxidative
stress-induced corticosteroid responsiveness of human THP-1 cells
by up-regulating the expression of histone deacetylase 2. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 30:384–387. 2014.(In Chinese).
PubMed/NCBI
|
|
50
|
Cui Y, Luo L, Li C, Chen P and Chen Y:
Long-term macrolide treatment for the prevention of acute
exacerbations in COPD: A systematic review and meta-analysis. Int J
Chron Obstruct Pulmon Dis. 13:3813–3829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miao L, Gao Z, Huang F, Huang S, Zhang R,
Ma D, Wu Q, Li F, Chen H and Wang J: Erythromycin enhances the
anti-inflammatory activity of budesonide in COPD rat model. Int J
Clin Exp Med. 8:22217–22226. 2015.PubMed/NCBI
|
|
52
|
Ellis S, Lin EJ and Tartar D: Immunology
of wound healing. Curr Dermatol Rep. 7:350–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Arellano-Orden E, Serrano C,
Montes-Worboys A, Sánchez-López V, Laborda A, Lostalé F, Lahuerta
C, Rodríguez-Panadero F and de Gregorio MA: Stent-induced tracheal
stenosis can be predicted by IL-8 expression in rabbits. Eur J Clin
Invest. 47:84–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song Y, Yu P, Lu JJ, Lu HZ, Zhu L, Yu ZH,
Chen HZ and Cui YY: A mucoactive drug carbocisteine ameliorates
steroid resistance in rat COPD model. Pulm Pharmacol Ther.
39:38–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hannigan M, Zhan L, Ai Y and Huang CK: The
role of p38 MAP kinase in TGF-beta1-induced signal transduction in
human neutrophils. Biochem Biophys Res Commun. 246:55–58. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Postlethwaite AE, Keski-Oja J, Moses HL
and Kang AH: Stimulation of the chemotactic migration of human
fibroblasts by transforming growth factor beta. J Exp Med.
165:251–256. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Perng DW, Wu YC, Chang KT, Wu MT, Chiou
YC, Su KC, Perng RP and Lee YC: Leukotriene C4 induces TGF-beta1
production in airway epithelium via p38 kinase pathway. Am J Respir
Cell Mol Biol. 34:101–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ferrara N: Vascular endothelial growth
factor and the regulation of angiogenesis. Recent Prog Horm Res.
55:15–35, 35–36. 2000.PubMed/NCBI
|
|
59
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang J, Li Q, Bai C, Han Y and Huang Y:
Inhalation of TGF-beta1 antibody: A new method to inhibit the
airway stenosis induced by the endobronchial tuberculosis. Med
Hypotheses. 73:1065–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee YC, Hung MH, Liu LY, Chang KT, Chou
TY, Wang YC, Wu YC, Lai CL, Tsai CC, Su KC and Perng DW: The roles
of transforming growth factor-beta(1) and vascular endothelial
growth factor in the tracheal granulation formation. Pulm Pharmacol
Ther. 24:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|