Introduction
Salvia miltiorrhiza (SM), known as Danshen in
China (1–3), is a popular traditional herbal
medicine owing to its anti-inflammatory (1), antioxidative (2), and antiosteoporotic (3) activity. SM is often used alone or in
conjunction with other traditional herbal medicines to treat
dementia and cardiovascular diseases, such as stroke, myocardial
infarction, and angina pectoris (4,5). SM
also affects the gastrointestinal (GI) tract. SM pretreatment
inhibits the increase in cholecystokinin (CCK) and vasoactive
intestinal peptide (VIP), thereby promoting the recovery of
impaired GI motility from digestive diseases caused by liver
ischemia (6). SM suppresses the
colon inflammation induced by dextran sulfate sodium in rats
(7) and alleviates the pathological
changes in the intestine, thymus, and spleen and promotes recovery
in rats with acute pancreatitis (8). SM causes intestinal contraction owing
to an intracellular Ca2+ concentration- and
Ca2+-calmodulin-dependent mechanism (9) and the contraction of the lower
esophageal sphincter via an extracellular Ca2+
influx-dependent mechanism (10).
Estrogen and estrogen receptors (ERs) control
various GI activities (11).
Additionally, SM is involved in the activation of ERs (12). SM is clinically used to increase
estrogen-like efficacy and treat postmenopausal symptoms (13). Subtypes of the ER include ERα and
ERβ (14). ERα is mainly present in
the uterus, mammary glands, adipose tissue, and bone and ERβ in the
ovary, prostate, and cardiovascular and central nervous systems
(15). Many studies are currently
investigating the role of estrogen and ERs in the GI tract
(11,12,16–19)
but there is still a lack of knowledge on the physiological,
pharmacological, and molecular biological processes involved.
Interstitial cells of Cajal (ICCs) are pacemaker
cells located throughout the GI tract (20,21).
Slow waves are generated by ICCs, which are electrically connected
to nearby ICCs and smooth muscle cells via gap junctions; thus, the
slow waves are conducted (22).
However, few studies on the effect of SM on ICCs and GI motility
have been conducted to date.
In the present study, we investigated the effects of
SM on the pacemaker potentials of ICCs in vitro and GI
motility in vivo.
Materials and methods
Preparation of the sample and
high-performance liquid chromatography (HPLC) analysis
Dried SM was purchased from Boncho Co. Tanshinone I
(T), tanshinone IIA (TA), and cryptotanshinone (CT) were purchased
from Sigma-Aldrich; Merck KGaA. The plant sample was identified by
Dr Yun Tai Kim according to a previous study (23) and a voucher specimen (#NP-0103) was
deposited with the Research Group of Innovative Functional Foods,
Korea Food Research Institute. Dried SM (600 g) was extracted with
70% ethanol (6,000 ml) for 4 h at 80°C. The extract was filtered
through a membrane filter (0.45 µm; EMD Millipore). After removing
the solvents via rotary evaporation, the remaining extracts were
freeze-dried, yielding 33.6% of the dried weight (w/w). The
freeze-dried extract powder (100 mg) was dissolved in 5 ml of
methanol:dimethyl sulfoxide (DMSO; 1:1, v/v), before it was
filtered through a 0.45-µm regenerated cellulose membrane filter
(Sartorius Stedim) and diluted in methanol/DMSO (1:1, v/v) to a
final concentration of 100 µg/ml prior to the injection of 10 µl of
the solution for HPLC. Analytical HPLC was performed using a Jasco
HPLC system (Jasco), comprising a PU-980 pump, an AS-950-10
autosampler, and an MD-2010 Plus multi-wavelength detector.
Chromatographic separation was conducted at 30°C using a Waters
Symmetry® C18 (4.6×250 mm, particle size 5 µm) column
with gradient elution using a mobile phase composed of 100%
methanol (mobile phase A) and water containing 0.5% (v/v) glacial
acetic acid (mobile phase B). The mobile phase change was carried
out with a linear gradient system from 20:80 (mobile phase A:mobile
phase B, v/v) to 80:20 (mobile phase A:mobile phase B, v/v) over 30
min with a 1 ml/min flow rate, before the samples were detected at
254 nm. Quantitative analysis was replicated four times. The
regression equation and correlation coefficient
(r2) of each standard curve were automatically
determined using the Jasco HPLC system. The regression equations
for T, TA, and CT were y=70277.0913x + 103899.0348
(R2 was 0.99847), y=65183.0492× +
76181.5418 (R2 was 0.99937), and
y=85154.2548x + 84310.5739 (R2 was
0.99981), respectively, indicating that a high linear correlation
was achieved for all standard curves. The concentrations of T, TA,
and CT were determined to be 2.61±0.131, 30.84±0.324, and
2.92±0.096 mg/g, respectively, using the peak area in the
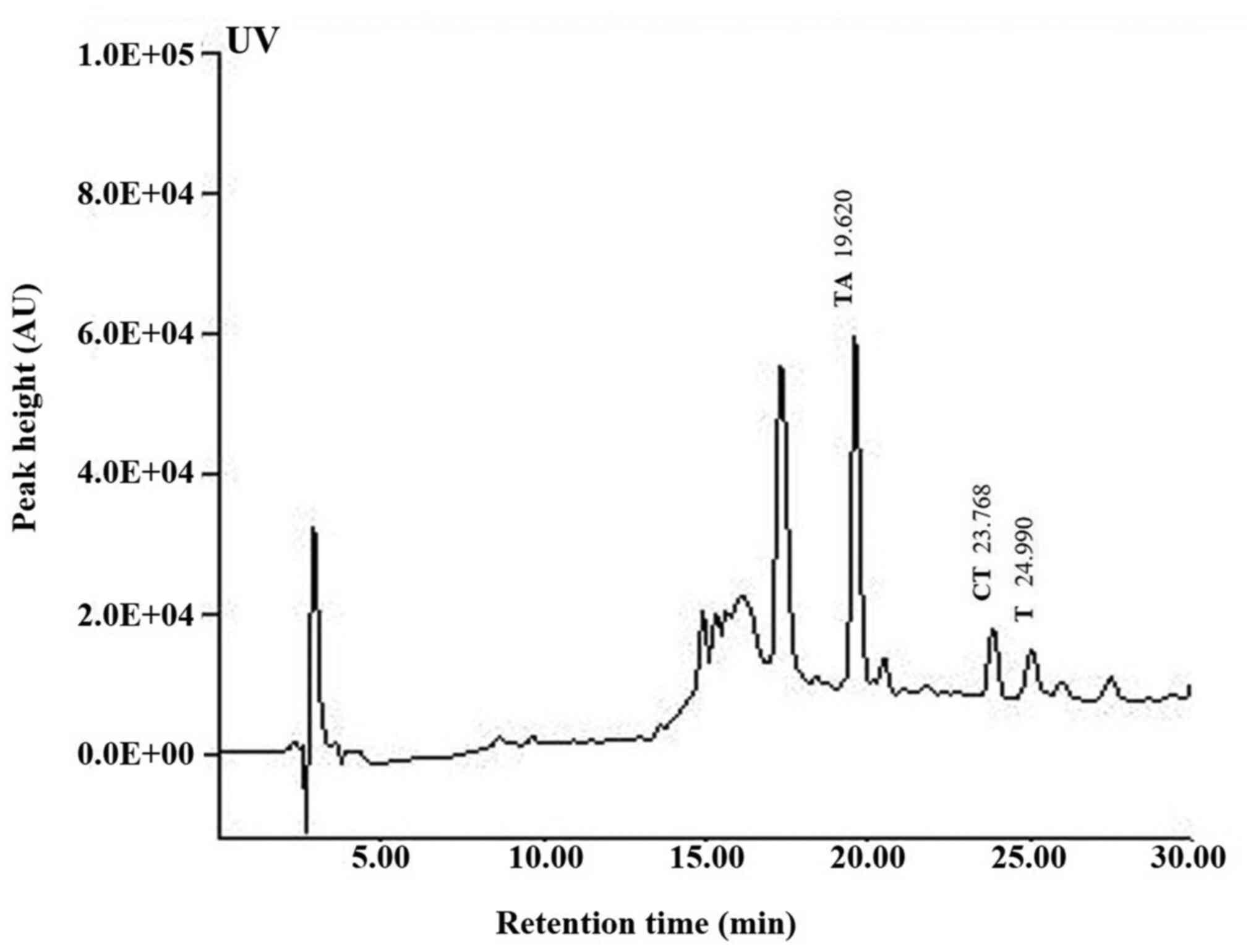
chromatogram and regression equation (Fig. 1).
Animals
A total of 82 mice (40 male and 42 female; 4-8 days
old; weighing 2.0-2.3 g) of the Institute of Cancer Research (ICR)
mice from the Samtako Bio Korea Co., Ltd. (Osan) were used for the
ICCs experiments, 32 mice (male; 5-6 weeks old; weighing 20-25 g)
for the intestinal transit rate (ITR) experiments, and 21 mice
(male; 5-6 weeks old; weighing 20-25 g) for the intestinal hormones
and protein expression experiments. ICCs experiments were completed
within 12 h after culture and ITR experiments within 1 h. In the
hormone measurement experiments, it took about 1 min to draw blood
from the tail vein after immobilizing the mouse using a holder.
Also, in the protein expression experiments, the mice were
anesthetized and then sacrificed. Subsequently, the small intestine
was removed. The whole process took ~3 min. All mice were housed in
a specific pathogen-free laboratory environment under a controlled
temperature (21–23°C) and humidity (50–60%) with day and night
cycles (light on at 7:00 a.m. and light off at 7:00 p.m) and ad
libitum access to normal diet and autoclaved water. During the
study, indicators of the general condition of the mice were
observed daily, such as fur brightness, food and water intake,
defecation and behavior. Furthermore, body weight was measured
every day. According to the cellular survival status and
experimental repetitions, 135 ICR mice were sacrificed in this
research. There were no other causes of mortality of mice other
than execution for the experiment. Before execution, their skin
condition and autonomous movements were monitored, and a warm
environment was required. Euthanasia of mice was performed by
decapitation after anesthesia. Mice were anesthetized by
intraperitoneal injection of pentobarbital sodium (50 mg/kg). Brain
palsy was considered effective when spontaneous or stimulating
movements and squeaks were not detected, and then, the mice were
decapitated. The Institutional Animal Care and Use Committee at
Pusan National University (approval no. PNU-2019-2462) approved all
animal care and experiments (Busan, Republic of Korea).
Additionally, animals were treated in accordance with the Guide for
the Care and Use of Laboratory Animals (24).
Preparation of ICCs and ICC
clusters
The small intestine was removed, and the luminal
contents were removed using Krebs-Ringer bicarbonate solution. As
reported previously, mucosae were removed by sharp dissection and
small tissue strips of intestinal muscle were equilibrated for 30
min in Ca2+-free Hank's solution. Then, cells were
dispersed in an enzyme solution and cultured at 37°C in a 95%
O2−5% CO2 incubator in a smooth muscle growth
medium (Clonetics). Finally, ICCs were identified (25). The patch-clamp technique was used on
ICCs that showed the network-like structures in culture.
Patch-clamp experiments
We used the whole-cell patch-clamp method to record
the effects of SM on the pacemaker potentials of ICCs. For the bath
solution, 5 mM KCl, 135 mM NaCl, 2 mM CaCl2, 10 mM
glucose, 1.2 mM MgCl2, and 10 mM HEPES (pH 7.4) were
used and, for the pipette solution, 140 mM KCl, 5 mM
MgCl2, 2.7 mM K2ATP, 0.1 mM NaGTP, 2.5 mM
creatine phosphate disodium, 5 mM HEPES, and 0.1 mM EGTA (pH 7.2)
were used. Patch-clamping was conducted using Axopatch I-D and
Axopatch 200B amplifiers (Axon Instruments). Command pulses were
applied using an IBM-compatible personal computer and pClamp
software (version 6.1 and version 10.0; Axon Instruments). All
experiments were performed at 30-31°C. In case of Ca2+
free experiments, the experiment was conducted after removing 2 mM
of Ca2+ in bath solution. In case of Na+ 5 mM
experiments, the experiment was carried out by changing 130 mM
Na+ to 130 mM N-Methyl-D-glucamine (NMDG).
Drugs
1,3-Dihydro-3,3-bis(4-hydroxyphenyl)-7-methyl-2H-indol-2-one (BHPI;
selective ERα antagonist) and MA2029 (MTL receptor antagonist) were
purchased from Tocris Bioscience and fulvestrant (ERα and ERβ
antagonist),
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
(PHTPP; selective ERβ antagonist), guanosine
5′-O-(2-thiodiphosphate) (GDP-β-S; for the inactivation of G
protein-binding proteins) and all other drugs were obtained from
Sigma-Aldrich; Merck KGaA.
ITR measurement using Evans blue in
vivo
Evans blue (5%, w/v; 0.1 ml/kg) was administered
through an orogastric tube 30 min after the intragastric
administration of SM to ICR mice. After 30 min of Evans blue
administration, the ITR was measured (the distance that Evans blue
traveled from the pylorus to the most distal point).
Measurement of serum gut hormone
levels
After SM (0.5 g/kg) was fed once a day for 5 days,
serum levels of gut hormones, such as MTL, substance P (SP),
somatostatin (SS), and vasoactive intestinal polypeptide (VIP),
were detected by radioimmunoassay using commercial kits purchased
from Abbkine Scientific Co., Ltd.
Western blotting
After feeding SM (0.5 g/kg) for 5 days, small
intestine samples were collected. The samples were prepared by
incubation with in RIPA buffer containing protease and phosphatase
inhibitor cocktail (Calbiochem). The total protein extracted from
the samples was quantified using the Bradford method (Bio-Rad
Laboratories, Inc.). An equal amount of protein (35 µg per lane)
from the samples was resolved using 8% SDS-PAGE and then
transferred to polyvinylidene difluoride membranes. The membranes
were blocked with 5% skim milk in TBS + 0.1% Tween-20 for 1 h at
room temperature and probed with the indicated antibodies.
Anti-transmembrane protein 16A (TMEM16A; Abcam), anti-c-kit (Cell
Signaling Technology, Inc.), anti-transient receptor potential
melastatin 7 (TRPM7; Abcam), and anti-β-actin (Santa Cruz
Biotechnology, Inc.) antibodies were used. An enhanced
chemiluminescence reagent kit (Advansta) was used for detection.
All other procedures were carried out as previously described
(26).
Statistical analysis
Results are expressed as mean ± SEM. For multiple
comparison analysis, we used one-way analysis of variance (ANOVA)
with Bonferroni's post hoc comparison and when only two groups were
compared, Student's t-test for unpaired data was used. For
statistical analyses, we used Prism 6.0 (GraphPad Software Inc.)
and Origin version 8.0 (OriginLab Corporation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Functional constituents of SM
The presence of T, TA, and CT in SM was established
by HPLC and their levels were quantified using calibration curves
obtained from purchased standards (Fig.
1). Validation of the method used confirmed its reliability and
stability.
Effects of SM on the pacemaker
potentials of ICCs
ICC clusters generated spontaneous rhythmic
contractions (21,22,27).
Under the current clamp mode (I=0), ICCs generated pacemaker
potentials with a mean resting membrane potential of −57.6±1.4 mV
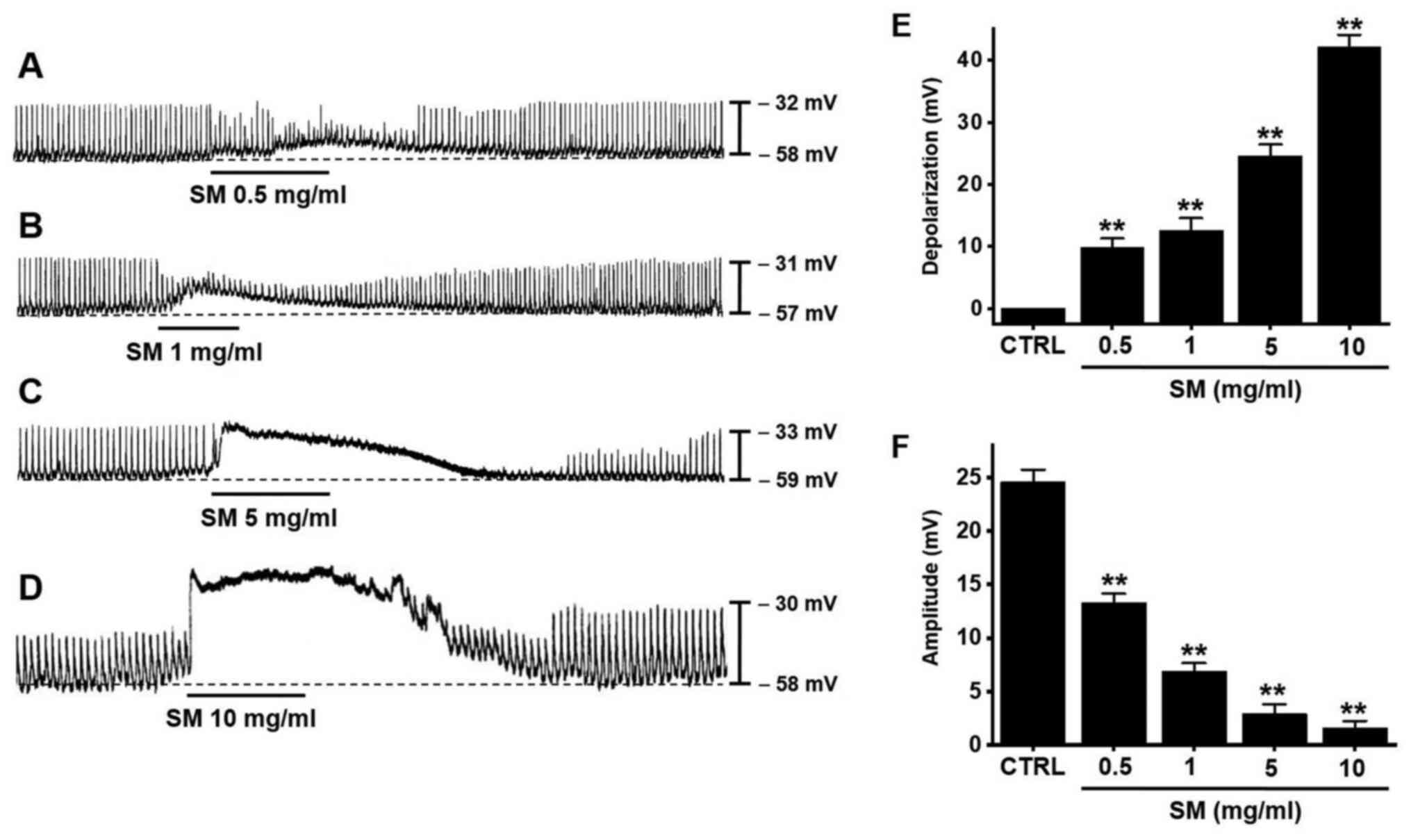
and mean amplitude of 24.6±1.1 mV (Fig.
2). SM (0.5-10 mg/ml) depolarized pacemaker potentials and
decreased their amplitudes in a concentration-dependent manner
(Fig. 2A-D). The mean degrees of
depolarization by SM were 9.8±1.5 mV (P<0.01) at 0.5
mg/ml, 12.6±1.9 mV (P<0.01) at 1 mg/ml, 24.6±1.8 mV
(P<0.01) at 5 mg/ml, and 42.2±1.9 mV (P<0.01)
at 10 mg/ml (Fig. 2E, n=13) and the
mean amplitudes were 13.3±0.8 mV (P<0.01) at 0.5 mg/ml,
6.9±0.7 mV (P<0.01) at 1 mg/ml, 2.9±0.9 mV
(P<0.01) at 5 mg/ml, and 1.6±0.7 mV (P<0.01) at
10 mg/ml (Fig. 2F, n=13). These
results show that SM dose-dependently depolarizes ICC pacemaker
potentials.
Effects of ER antagonist on SM-induced
pacemaker potential depolarization in ICCs
SM is involved in the activation of ERs (12). Various ER agonists and antagonists
were used to confirm the relevance of the ER in the response of
ICCs to SM. Daidzein (ER agonist) depolarizes pacemaking activity
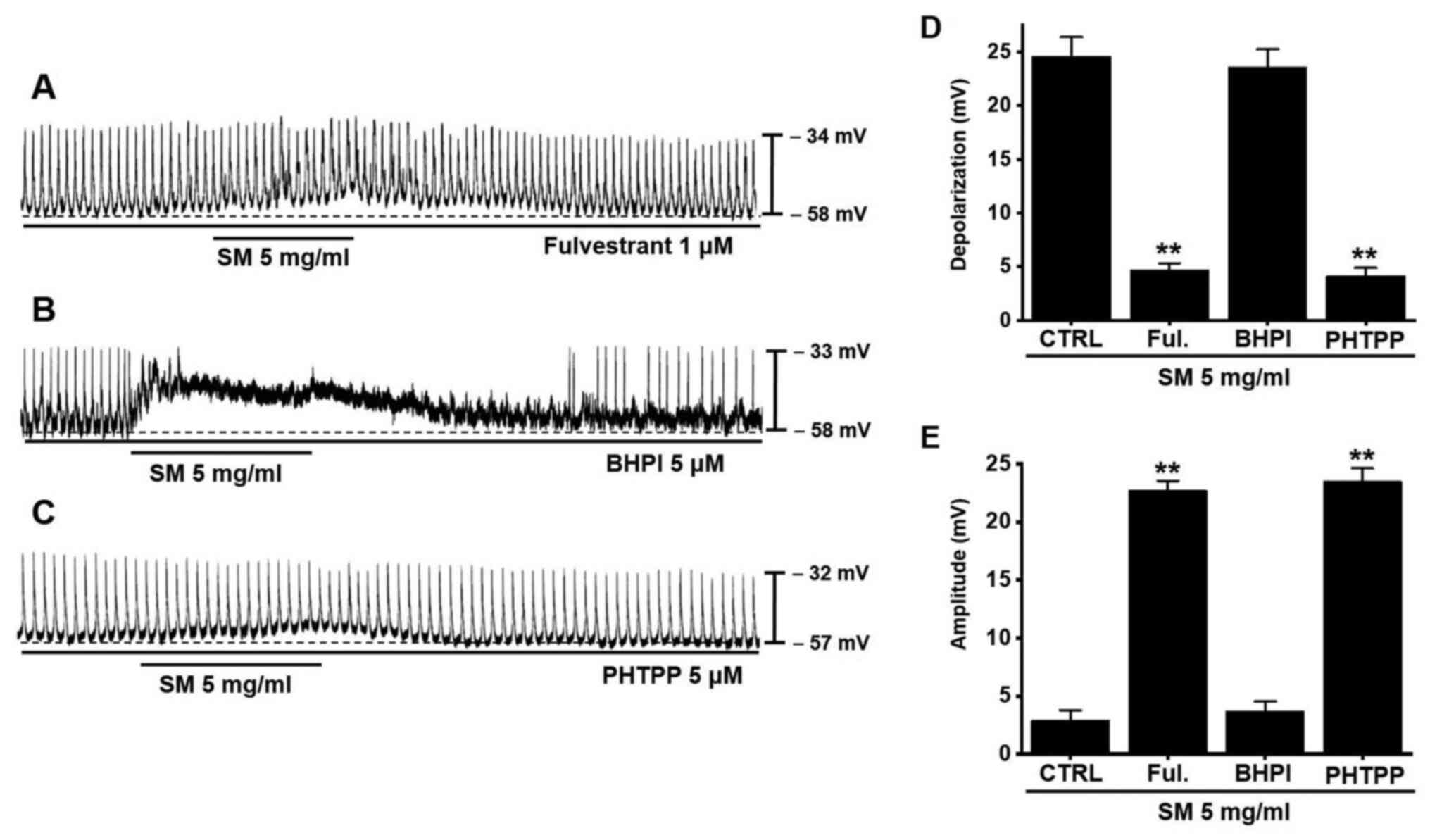
(25). Pretreatment with
fulvestrant (both an ERα and ERβ antagonist) blocked SM-induced
effects (Fig. 3A). However,
pretreatment with BHPI (ERα antagonist) did not (Fig. 3B). Additionally, pretreatment with
PHTPP (ERβ antagonist) blocked SM-induced effects (Fig. 3C). The mean degrees of
depolarization by SM were 4.7±0.7 mV (P<0.01) with
fulvestrant, 23.6±1.7 mV with BHPI, and 4.1±0.8 mV
(P<0.01) with PHTPP (Fig.
3D) and the mean amplitudes were 22.7±0.8 mV (P<0.01)
with fulvestrant, 3.7±0.8 mV with BHPI, and 23.5±1.1 mV
(P<0.01) with PHTPP (Fig.
3E). The results show that SM affects ICC pacemaker potentials
via ERβ.
Effects of GDP-β-S and extracellular
Ca2+ and Na+ on SM-induced pacemaker
potential depolarization in ICCs
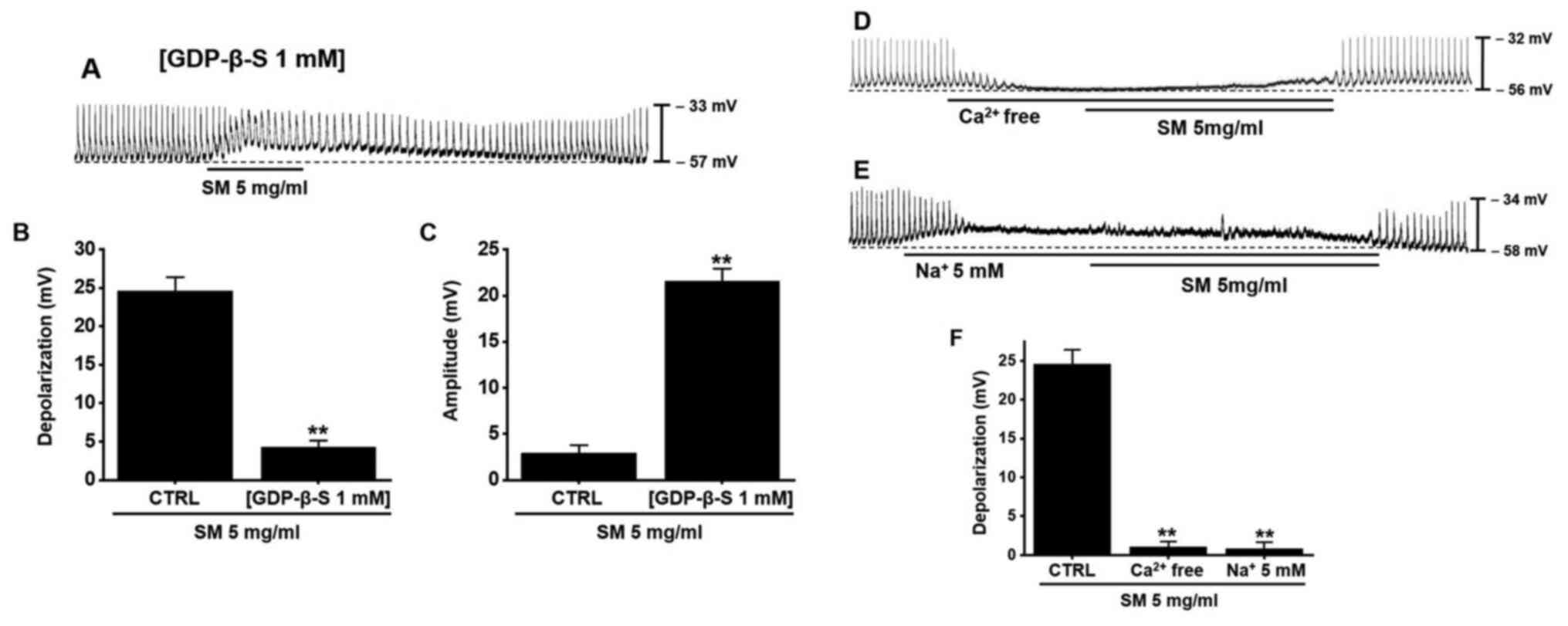
GDP-β-S was used to identify the relevance of the G
protein in the response of ICCs to SM. GDP-β-S disables G
protein-binding proteins (28,29).
When GDP-β-S (1 mM) was injected intracellularly, SM had a slight
pacemaker potential depolarization reaction (Fig. 4A). The mean degree of depolarization
by SM was 4.3±0.8 mV (P<0.01) with GDP-β-S (Fig. 4B) and the mean amplitude was
21.6±1.3 mV (P<0.01) with GDP-β-S (Fig. 4C). Extracellular Ca2+ and
Na+ have important roles in GI motility (30,31).
To investigate the involvement of extracellular Ca2+ and
Na+ in the SM-induced response in ICCs, we conducted
experiments with no extracellular Ca2+ or
Na+. Pre-treatment with no extracellular Ca2+
solution (Fig. 4D) or no
extracellular Na+ (Fig.
4E) abolished the pacemaker potentials and inhibited the
SM-induced response in ICCs. The mean degree of depolarization by
SM was 1.1±0.7 mV (P<0.01) with no extracellular
Ca2+ and 0.8±0.7 mV (P<0.01) with no
extracellular Na+ (Fig.
4F). The results suggest that G protein and extracellular
Ca2+ and Na+ influx are involved in the
SM-induced response in ICCs.
Effects of SM on ITR and intestinal
hormones of normal mice
ITR was measured 30 min after the administration of
Evans blue in normal mice. In normal mice, the average ITR was
34.5±1.5% (Fig. 5A). After the
administration of SM, ITR was 37.1±2.3% at 0.01 g/kg, 54.4±2.5%
(P<0.01) at 0.1 g/kg, and 48.9±3.8% (P<0.01) at
1 g/kg (Fig. 5A). In comparison,
Poncirus trifoliata Raf., which is another herbal medicine
commonly used in GI motility studies, showed an average ITR of
55.7±2.2% (P<0.01), similar to the results of a past
study (32). GI hormone levels in
the mouse serum were evaluated by radioimmunoassay. The level of
MTL in the GI was significantly elevated (Fig. 5B) but the levels of SP (Fig. 5C), SS (Fig. 5D), and VIP (Fig. 5E) showed no significant changes
after SM administration. Furthermore, feeding SM (0.5 g/kg) and
MA2029, MTL receptor antagonist together for 5 days reduced ITR
(Fig. 5F). These results suggest
that the SM increases ITR through an increase in MTL.
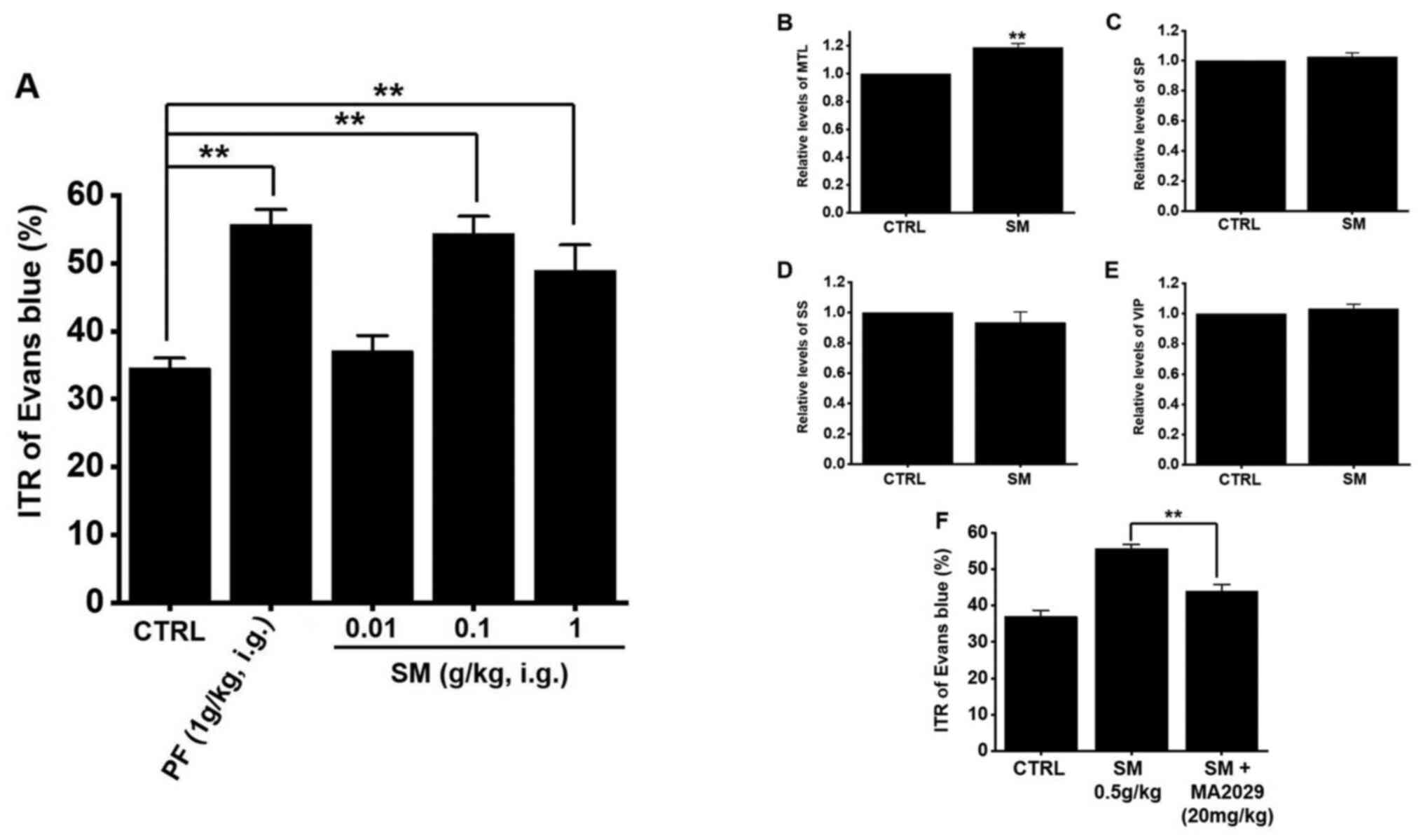 | Figure 5.Effects of SM on ITR and intestinal
hormones in mice. (A) SM increased ITR. Levels of GI hormones such
as (B) MTL, (C) SP, (D) SS, and (E) VIP in the serum were measured
using a radioimmunoassay technique. (F) Feeding SM and MA2029,
motilin receptor antagonist together for 5 days reduced ITR.
Results represent the mean ± SEM. **P<0.01 vs. CTRL or as
indicated. SM, Salvia miltiorrhiza; ITR, intestinal transit
rate; GI, gastrointestinal; MTL, motilin; SP, substance P; SS,
somatostatin; VIP, vasoactive intestinal peptide; CTRL, control;
PF, Poncirus trifoliata Raf. |
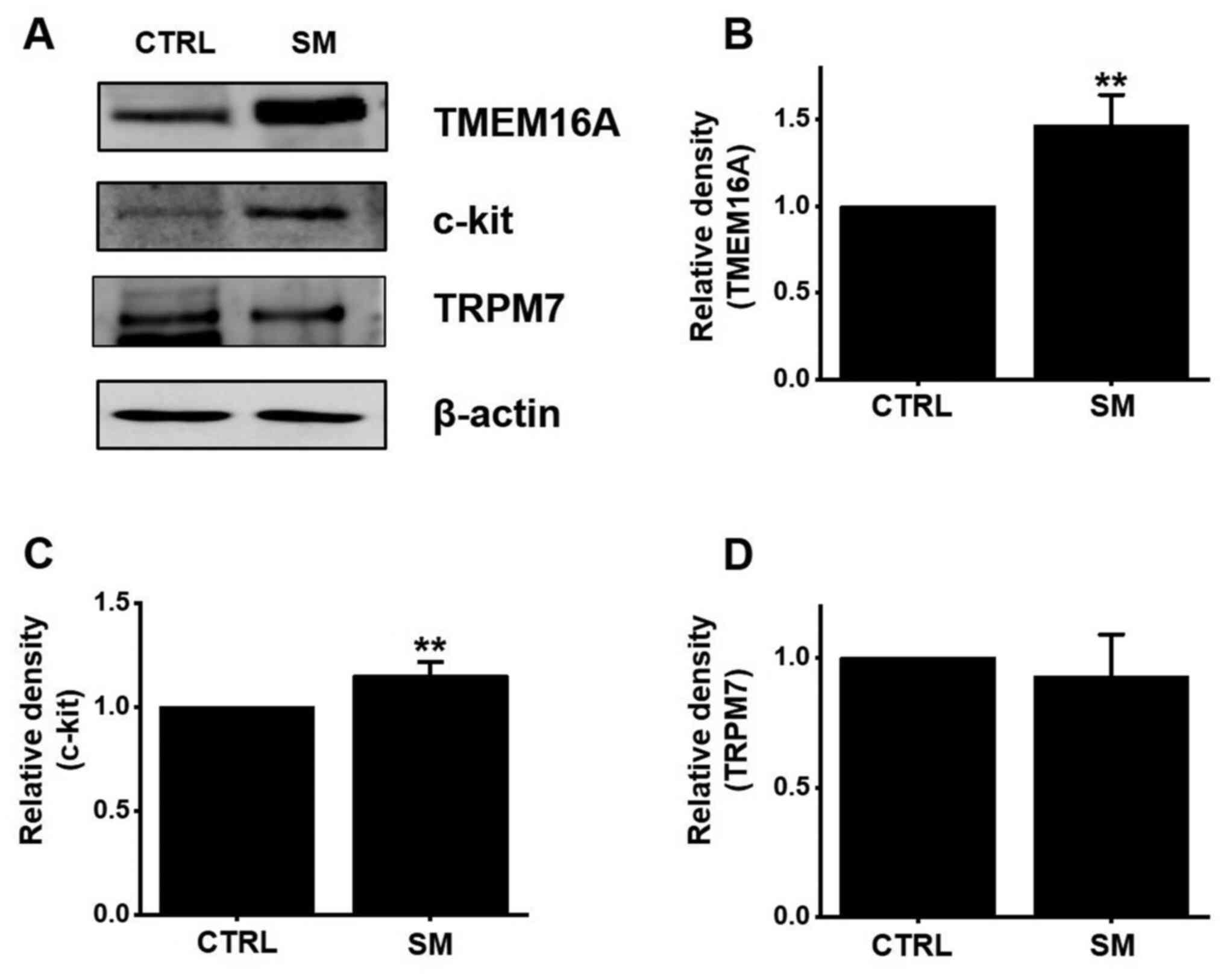
Effects of SM on the protein
expression of TMEM16A, c-kit, and TRPM7
The TMEM16A (33,34)
and TRPM7 (21) channels are
involved in ICC activity. Furthermore, c-Kit is a transmembrane
protein associated with the density of ICCs (35). Therefore, TMEM16A, TRPM7, and c-Kit
may play a role in the treatment of GI motility disorders. After
treatment with SM, the expression of TMEM16A, TRPM7, and c-Kit was
evaluated using western blotting. The expression of TMEM16A and
c-kit increased considerably after SM treatment (Fig. 6A). TMEM16A and c-kit expression
significantly increased by 46.7 and 15.3%, respectively
(P<0.01) after SM treatment (Fig. 6B and C). The expression of TRPM7 was
almost unchanged (Fig. 6D). The
results suggest that the SM-induced ITR increase is mediated
through an increase in TMEM16A and c-kit expression.
Discussion
SM, a plant belonging to the Lamiaceae family, is
widely used as a traditional herbal medicine to promote blood
circulation, slow blood congestion, suppress edema, and maintain
cognitive equilibrium (36).
Additionally, it has anti-inflammatory (1), antioxidative (2), and antiosteoporotic (3) activities. Furthermore, SM is often
used alone or in conjunction with other traditional herbal
medicines to treat dementia and cardiovascular diseases, such as
stroke, myocardial infarction, and angina pectoris (4,5). SM
also affects the GI tract. SM pretreatment inhibits the increase in
CCK and VIP levels, thereby promoting the recovery of impaired GI
motility from digestive diseases caused by liver ischemia (6). SM suppresses colon inflammation
induced by dextran sulfate sodium in rats (7) and alleviates the pathological changes
in the intestine, thymus, and spleen and promotes recovery in rats
with acute pancreatitis (8). SM
causes intestinal contraction owing to intracellular
Ca2+ concentration- and
Ca2+-calmodulin-dependent mechanisms (9) and contraction of the lower esophageal
sphincter via an extracellular Ca2+ influx-dependent
mechanism (10). Although there are
many reports that SM is effective in the treatment of many
digestive tract diseases, few have reported the effect of SM on GI
motility.
ICCs provide the pacemaker activity needed for
electrical slow waves in GI muscles. Slow waves are generated by
ICCs, which are electrically connected to nearby ICCs and smooth
muscle cells via gap junctions; thus, the slow waves are conducted
(22). Slow waves are caused by
phasic contraction in most areas of the GI tract. In the present
study, we elucidated the mechanism by which SM depolarizes murine
small intestinal ICC pacemaker potentials. We demonstrated that SM
depolarized pacemaker potentials of ICCs from the murine small
intestine in a dose-dependent manner (Fig. 2). We then showed that this occurred
via ERβ, as this effect was inhibited by the ERβ antagonists
fulvestrant and PHTPP (Fig. 3). We
also demonstrated that intracellular GDP-β-S and extracellular
Ca2+ and Na+ inhibited SM-induced
depolarization (Fig. 4). Moreover,
ITR values were increased by SM in vivo (Fig. 5A) and SM elevated the level of MTL
but had no effect on SP, SS, and VIP levels (Fig. 5B-E). Additionally, the SM-induced
ITR increase was related to the increase in the protein expression
of c-kit and the TMEM16A channel (Fig.
6). Therefore, SM may have the ability to control GI motility
and could be used as a GI motility regulator in the future.
SM increases estrogenic effects and is a safe and
effective complementary or alternative treatment for menopause
(3,12). In this study, fulvestrant (both an
ERα and ERβ antagonist) and PHTPP (ERβ antagonist) blocked
SM-induced effects but BHPI (ERα antagonist) did not. SM could play
a major role in the regulation of estrogen-related GI motility
through the pacemaking activity of ICCs. Gender-related differences
in GI motility have been broadly studied in the clinical and in
numerous animal models (16,17).
Female hormones, mainly estrogens, were found to affect visceral
sensitivity, GI motility, and intestinal permeability leading to
the conclusion that female sex hormones may play an important role
in the pathophysiology of GI dysmotility diseases such as irritable
bowel syndrome (IBS) (18,19). The physiological effects of female
sex hormones on the body systems and on the regulation of GI
homeostasis are predominantly moderated via ERs (37). G protein-coupled estrogen receptor
(GPER) belongs to the seven transmembrane G protein-coupled
receptor (GPCR) family and mediates the effects of estrogen on GI
motility (38). The classical
nuclear ERs (ERα and ERβ) appear to have the function of cellular
and physiological responses (growth, differentiation, and
proliferation) (39,40). Therefore, ERs are involved in the
regulation of GI motility and they may be an important
pharmacological target in the GI dysmotility therapy.
In this study, pre-treatment with no extracellular
Ca2+ solution abolished the ICCs pacemaker potentials
(Fig. 4D). It is well known that
the underlying pacemaker potential in ICCs is spontaneous transient
inward currents (STICs) and STICs generate depolarization, activate
Ca2+ entry, and synchronize the openings of channels
responsible for STICs (34,41). Therefore, reduced extracellular
Ca2+ concentrations and T-type Ca2+ channel
blockers decreased the number of STICs and firing probability of
Ca2+ transients in ICCs (42). Also, intracellular Ca2+
release from endoplasmic reticulum depend on ryanodine receptors as
well as amplification from IP3 receptors and is
important in generating pacemaker activity in ICCs (22,43).
Therefore, when there is no extracellular Ca2+, the
pacemaker potential of ICCs does not occur. At this time, there was
no effect by SM in this study (Fig.
4D). In addition, Tsai et al (9) suggested that Ca2+-free
Krebs solution plus EGTA also had no significant effect on
SM-induced rat ileal segment contractions. There are many cells
such as ICCs, smooth muscles, and platelet-derived growth factor
receptor-α positive (PDGFRα+) interstitial cells in the
GI tissue (35). Therefore, the
effects of SM in the small intestine segments are thought to be the
results of the reactions of these various cells, although it is not
known exactly which cell reactions among these many cells. However,
in this paper, it can be seen that when there is no extracellular
Ca2+, pacemaker potential does not occur in ICCs and at
this time, there is no response of SM. In the future, it is thought
that in-depth studies are needed on the effects of SM in each of
these various cells in GI tract. In addition, both Gd3+
and flufenamic acid, nonselective cation channel blockers,
abolished pacemaker current generation, as did a reduction in
external Na+ concentrations (to 5 mM) (Fig. 4E) (44). These results strongly suggest that
nonselective cationic channels are involved in generating the
pacemaker potentials of ICCs. Furthermore, the bidirectional nature
of Na+/Ca2+ exchanger (NCX) is exploited
during the slow wave cycle in ICCs (45). NCX facilitates removal of
Ca2+ during the inter-slow wave interval and provides
Ca2+ for sustained activation of ANO1 during the slow
wave plateau phase (45).
Therefore, extracellular Na+ also is involved in
generating the pacemaker potentials of ICCs.
SM depolarized the pacemaker potentials in ICCs and
increased ITR (Figs. 2 and 5A). ICCs have a pacemaker function to
generate the GI motility and signaling through the tyrosine kinase
receptor c-Kit in ICCs is important for development and
differentiation in ICCs (20–22).
GI motility patterns are achieved through coordinated contractions
and relaxations of smooth muscle in the gut wall which are
controlled by a number of intrinsic and extrinsic mechanisms
including various humoral factors such as GI hormones (20–22).
The GI hormones can be divided into main groups based upon their
chemical structure. i) Gastrin-cholecystokinin family: Gastrin and
cholecystokinin. ii) Secretin family: Secretin, glucagon, and VIP.
iii) Somatostatin family. iv) Motilin family. v) Substance P
(46). We selected 4 representative
GI hormones such as MTL, SP, SS, and VIP (47,48)
and conducted the experiments. These hormones are secreted by
endocrine cells and islet cells. They stimulate smooth muscle cells
and play key roles in the control of GI motility (49,50).
In GI abnormal motility states, ICCs dysfunction may result in
abnormal motility and GI hormones, leading to further GI
dysfunction. Thus, altering the hormone level would promote GI
motility. Therefore, changes in hormone levels are involved in the
control of GI motility. In this study, MTL level increased
considerably (Fig. 5B) but the
levels of SP (Fig. 5C), SS
(Fig. 5D), and VIP (Fig. 5E) remained unchanged after the
administration of SM. In addition, feeding SM and MA2029, MTL
receptor antagonist together for 5 days reduced ITR (Fig. 5F). Therefore, we think that SM may
increase MTL and increased MTL may stimulate c-kit to increase ICCs
activity, resulting in increased ANO1 activity rather than TRPM7 in
ICCs cell membranes. Therefore, the increase in the secretion of
the GI hormone MTL could be a key mechanism involved in the
SM-mediated control of intestinal motility.
Organized smooth muscle contraction, such as
peristalsis and segmentation in GI motility, is caused by the
integrated regulation of smooth muscle cells, ICCs, enteric motor
neurons, and hormones (22,35). Slow waves in ICCs are caused by
Ca2+-activated Cl− channels, such asTMEM16A
(34,51,52)
and TRPM7 channels (21).
Additionally, the expression of c-kit, a
proto-oncogene, and the mechanism through the receptor kinase gene
product, KIT, are essential to the conductance of the electrical
rhythm (53,54). In the present study, western
blotting revealed the higher expression of TMEM16A and c-kit in the
murine small intestine after SM treatment (Fig. 6A-C). However, the expression of
TRPM7 was almost unchanged following exposure to SM (Fig. 6A and D). Therefore, we believe that
the SM-induced ITR increase may be associated with the upregulation
of TMEM16A and c-kit in ICCs.
In summary, the results of this study show that: i)
SM depolarized the pacemaker potentials of ICCs; ii) PHTPP, an ERβ
antagonist, inhibited SM-induced depolarization; iii) Intracellular
GDP-β-S inhibited SM-induced depolarization; iv) Extracellular
Ca2+- and Na+-free solutions blocked
SM-induced depolarization; v) ITR values were increased by SM in
vivo; vi) SM elevated the level of MTL but had no effect on SP,
SS, and VIP levels; vii) SM-Induced ITR increase was related to the
increase in the protein expression of c-kit and the TMEM16A
channel. Taken together, the results show that SM induces pacemaker
potential depolarization through ERβ in a G protein-dependent
manner via extracellular Ca2+ and Na+
regulation in the murine small intestine in vitro. Moreover,
SM increased the ITRs in vivo through MTL hormone via c-kit-
and TMEM16A-dependent pathways. SM may control GI motility through
ICCs; thus, SM could be used as a GI regulator in the future,
although further research is required.
Acknowledgements
Not applicable.
Funding
This study was supported by a Korean National
Research Foundation (NRF) grant funded by the Korean government
(MSIP) (grant no. 2017R1A2B2003764) and by the Main Research
Program (grant no. E0164502-05) of the Korea Food Research
Institute (KFRI).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
DH and BJK designed the research. DH, JNK, MJK, TH
and YTK conducted experiments. DH, JNK and BJK analyzed the data.
BJK wrote the manuscript. DH and BJK confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee at
Pusan National University (approval no. PNU-2019-2462) approved all
animal care and experiments (Busan, Republic of Korea).
Additionally, animals were treated in accordance with the Guide for
the Care and Use of Laboratory Animals (23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim SY, Moon TC, Chang HW, Son KH, Kang SS
and Kim HP: Effects of tanshinone I isolated from Salvia
miltiorrhiza bunge on arachidonic acid metabolism and in vivo
inflammatory responses. Phytother Res. 16:616–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu YJ, Hong CY, Lin SJ, Wu P and Shiao MS:
Increase of vitamin E content in LDL and reduction of
atherosclerosis in cholesterol-fed rabbits by a water-soluble
antioxidant-rich fraction of Salvia miltiorrhiza.
Arterioscler Thromb Vasc Biol. 18:481–486. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo Y, Li Y, Xue L, Severino RP, Gao S,
Niu J, Qin LP, Zhang D and Bromme D: Salvia miltiorrhiza: An
ancient Chinese herbal medicine as a source for antiosteoporotic
drugs. J Ethnopharmacol. 155:1401–1416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji XY, Tan BK and Zhu YZ: Salvia
miltiorrhiza and ischemic diseases. Acta Pharmacol Sin.
21:1089–1094. 2000.PubMed/NCBI
|
|
5
|
Chan K, Chui SH, Wong DY, Ha WY, Chan CL
and Wong RN: Protective effects of danshensu from the aqueous
extract of Salvia miltiorrhiza (Danshen) against
homocysteine-induced endothelial dysfunction. Life Sci.
75:3157–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang ZY, Chen XP and Lu QP: Effect of
Salvia miltiorrhiza pretreatment on the CCK and VIP
expression in hepatic ischemia-reperfusion-induced digestive tract
congestion. Front Med China. 4:317–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen XD, Wang CZ, Yu C, Zhang Z, Calway T,
Wang Y, Li P and Yuan CS: Salvia miltiorrhiza (Danshen)
significantly ameliorates colon inflammation in dextran sulfate
sodium induced colitis. Am J Chin Med. 41:1097–1108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiping Z, Yan P, Xinmei H, Guanghua F,
Meili M, Jie N and Fangjie Z: Effects of dexamethasone and
Salvia miltiorrhizae on the small intestine and immune
organs of rats with severe acute pancreatitis. Inflammation.
33:259–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai CC, Huang SC, Liu JK, Wang HC, Tsai
TR, Tsai PJ, Liu CW and Chang LC: Salvia miltiorrhiza causes
tonic contraction in rat ileum through Ca2+-calmodulin
pathway. J Ethnopharmacol. 142:694–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai CC, Chang LC, Huang SC, Tey SL, Hsu
WL, Su YT, Liu CW and Tsai TR: Salvia miltiorrhiza induces
tonic contraction of the lower esophageal sphincter in rats via
activation of extracellular Ca2+ influx. Molecules.
20:14504–14521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang X, Guo Y, He J, Zhang F, Sun X, Yang
S and Dong H: Estrogen and estrogen receptors in the modulation of
gastrointestinal epithelial secretion. Oncotarget. 8:97683–97692.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang JM, Li J, Liu EW, Wang H, Fan GW,
Wang YF, Zhu Y, Ma SW and Gao XM: Danshen enhanced the estrogenic
effects of qing E formula in ovariectomized rats. BMC Complement
Altern Med. 16:1812016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan G, Zhu Y, Guo H, Wang X, Wang H and
Gao X: Direct vasorelaxation by a novel phytoestrogen tanshinone
IIA is mediated by nongenomic action of estrogen receptor through
endothelial nitric oxide synthase activation and calcium
mobilization. J Cardiovasc Pharmacol. 57:340–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evans RM: The steroid and thyroid hormone
receptor superfamily. Science. 240:889–895. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasar P, Ayaz G, User SD, Güpür G and
Muyan M: Molecular mechanism of estrogen-estrogen receptor
signaling. Reprod Med Biol. 16:4–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mapplebeck JC, Beggs S and Salter MW: Sex
differences in pain: A tale of two immune cells. Pain. 157
(Suppl):S2–S6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulak A and Bonaz B: Irritable bowel
syndrome: A model of the brain-gut interactions. Med Sci Monit.
10:RA55–RA62. 2004.PubMed/NCBI
|
|
18
|
Sorge RE, Mapplebeck JC, Rosen S, Beggs S,
Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D,
et al: Different immune cells mediate mechanical pain
hypersensitivity in male and female mice. Nat Neurosci.
18:1081–1083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meleine M and Matricon J: Gender-Related
differences in irritable bowel syndrome: Potential mechanisms of
sex hormones. World J Gastroenterol. 20:6725–6743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huizinga JD, Thuneberg L, Klüppel M,
Malysz J, Mikkelsen HB and Bernstein A: W/kit gene required for
interstitial cells of cajal and for intestinal pacemaker activity.
Nature. 373:347–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY,
Park CS, So I, Stanfield PR and Kim KW: Melastatin-Type transient
receptor potential channel 7 is required for intestinal pacemaking
activity. Gastroenterology. 129:1504–1517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanders KM, Ward SM and Koh SD:
Interstitial cells: Regulators of smooth muscle function. Physiol
Rev. 94:859–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a
systematic review. Med Res Rev. 34:768–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care Use of Laboratory Animals:
Guide for the Care and Use of Laboratory Animals, 8th edition.
National Academies Press (US); Washington, DC: 2011
|
|
25
|
Kim JH and Kim BJ: Depolarization of
pacemaker potentials by caffeic acid phenethyl ester in
interstitial cells of cajal from the murine small intestine. Can J
Physiol Pharmacol. 98:201–210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang M, Kim JN, Lee JR, Kim SC and Kim
BJ: Effects of Chaihu-Shugan-san on small intestinal interstitial
cells of Cajal in mice. Biol Pharm Bull. 43:707–715. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koh SD, Sanders KM and Ward SM:
Spontaneous electrical rhythmicity in cultured interstitial cells
of cajal from the murine small intestine. J Physiol. 513:203–213.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komori S, Kawai M, Takewaki T and Ohashi
H: GTP-Binding protein involvement in membrane currents evoked by
carbachol and histamine in guinea-pig ileal muscle. J Physiol.
450:105–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogata R, Inoue Y, Nakano H, Ito Y and
Kitamura K: Oestradiol-Induced relaxation of rabbit basilar artery
by inhibition of voltage-dependent Ca channels through GTP-binding
protein. Br J Pharmacol. 117:351–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ward SM: Interstitial cells of cajal in
enteric neurotransmission. Gut. 47:40–43. 2000. View Article : Google Scholar
|
|
31
|
Liao QS, Du Q, Lou J, Xu JY and Xie R:
Roles of Na+/Ca2+ exchanger 1 in digestive
system physiology and pathophysiology. World J Gastroenterol.
25:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim H, Kim I, Lee MC, Kim HJ, Lee GS, Kim
H and Kim BJ: Effects of hwangryunhaedok-tang on gastrointestinal
motility function in mice. World J Gastroenterol. 23:2705–2715.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang F, Rock JR, Harfe BD, Cheng T, Huang
X, Jan YN and Jan LY: Studies on expression and function of the
TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci USA.
106:21413–21418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh
SD and Sanders KM: A Ca(2+)-activated Cl(−)conductance in
interstitial cells of cajal linked to slow wave currents and
pacemaker activity. J Physiol. 587:4905–4918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sanders KM, Koh SD, Ro S and Ward SM:
Regulation of gastrointestinal motility-insights from smooth muscle
biology. Nat Rev Gastroenterol Hepatol. 9:633–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lam FFY, Deng SY, Ng ESK, Yeung JHK, Kwan
YW, Lau CBS, Loon JCM, Zhou L, Zuo Z, Leung PC and Fung KP:
Mechanisms of the relaxant effect of a danshen and gegen
formulation on rat isolated cerebral basilar artery. J
Ethnopharmacol. 132:186–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eyster KM: The estrogen receptors: An
overview from different perspectives. Methods Mol Biol. 1366:1–10.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Xu J, Jiang F, Jiang Z, Liu C, Li L,
Luo Y, Lu R, Mu Y, Liu Y and Xue B: G protein-coupled estrogen
receptor is involved in modulating colonic motor function via
nitric oxide release in C57BL/6 female mice. Neurogastroenterol
Motil. 28:432–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vivacqua A, Bonofiglio D, Recchia AG,
Musti AM, Ando DPS and Maggiolini M: The G protein-coupled receptor
GPR30 mediates the proliferative effects induced by
17beta-estradiol and hydroxytamoxifen in endometrial cancer cells.
Mol Endocrinol. 20:631–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zielińska M, Fichna J, Bashashati M,
Habibi S, Sibaev A, Timmermans JP and Storr M: G protein-coupled
estrogen receptor and estrogen receptor ligands regulate colonic
motility and visceral pain. Neurogastroenterol Motil. 29:1–11.
2017. View Article : Google Scholar
|
|
41
|
Zhu MH, Sung IK, Zheng H, Sung TS, Britton
FC, O'Driscoll K, Koh SD and Sanders KM: Muscarinic activation of
Ca2+-activated Cl− current in interstitial
cells of cajal. J Physiol. 589:4565–4582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu MH, Sung TS, O'Driscoll K, Koh SD and
Sanders KM: Intracellular Ca(2+) release from endoplasmic reticulum
regulates slow wave currents and pacemaker activity of interstitial
cells of cajal. Am J Physiol Cell Physiol. 308:C608–C620. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Drumm BT, Hennig GW, Battersby MJ,
Cunningham EK, Sung TS, Ward SM, Sanders KM and Baker SA:
Clustering of Ca2+ transients in interstitial cells of
cajal defines slow wave duration. J Gen Physiol. 149:703–725. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jun JY, Choi S, Yeum CH, Chang IY, You HJ,
Park CK, Kim MY, Kong ID, Kim MJ, Lee KP, et al: Substance P
induces inward current and regulates pacemaker currents through
tachykinin NK1 receptor in cultured interstitial cells of cajal of
murine small intestine. Eur J Pharmacol. 495:35–42. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng H, Drumm BT, Zhu MH, Xie Y,
O'Driscoll KE, Baker SA, Perrino BA, Koh SD and Sanders KM:
Na+/Ca2 + exchange and pacemaker activity of
interstitial cells of cajal. Front Physiol. 11:2302020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ahmed M and Ahmed S: Functional,
Diagnostic and Therapeutic Aspects of Gastrointestinal Hormones.
Gastroenterology Res. 12:233–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Thomas PA, Akwari OE and Kelly KA:
Hormonal control of gastrointestinal motility. World J Surg.
3:545–552. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peeters TL: Gastrointestinal hormones and
gut motility. Curr Opin Endocrinol Diabetes Obes. 22:9–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rehfeld JF: The new biology of
gastrointestinal hormones. Physiol Rev. 78:1087–1108. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dockray GJ: Gastrointestinal hormones and
the dialogue between gut and brain. J Physiol. 592:2927–2941. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hirst GD, Bramich NJ, Teramoto N, Suzuki H
and Edwards FR: Regenerative component of slow waves in the
guinea-pig gastric antrum involves a delayed increase in
[Ca(2+)](i) and Cl(−) channels. J Physiol. 540:907–919. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hwang SJ, Blair PJ, Britton FC, O'Driscoll
KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM and Ward
SM: Expression of anoctamin 1/TMEM16A by interstitial cells of
cajal is fundamental for slow wave activity in gastrointestinal
muscles. J Physiol. 587:4887–4904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Maeda H, Yamagata A and Nishikawa S,
Yoshinaga K, Kobayashi S, Nishi K and Nishikawa S: Requirement of
c-kit for development of intestinal pacemaker system. Development.
116:369–753. 1992.PubMed/NCBI
|
|
54
|
Torihashi S, Ward SM, Nishikawa S, Nishi
K, Kobayashi S and Sanders KM: C-Kit-Dependent development of
interstitial cells and electrical activity in the murine
gastrointestinal tract. Cell Tissue Res. 280:97–111. 1995.
View Article : Google Scholar : PubMed/NCBI
|