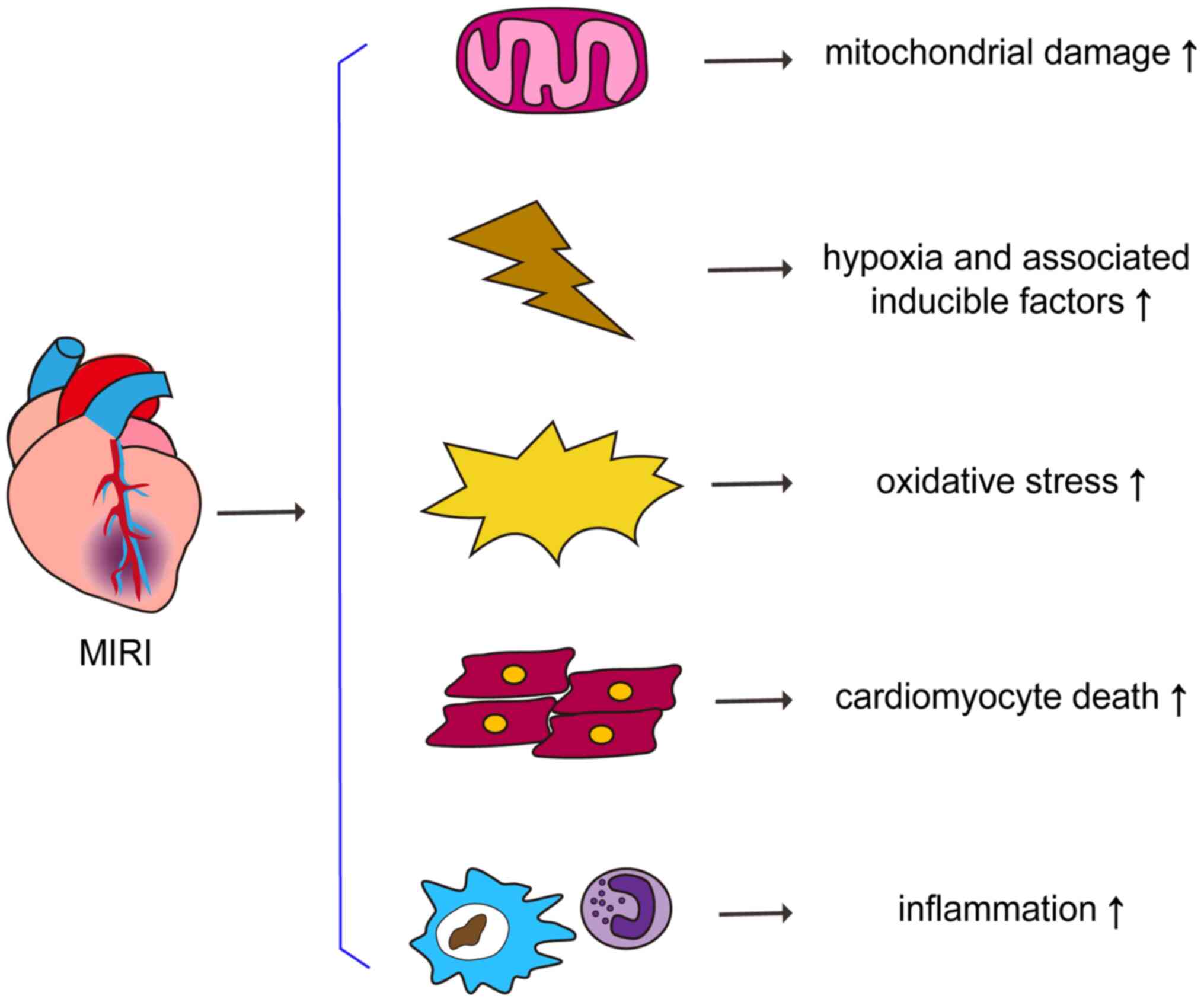
Introduction
Ischemic heart disease is a major cause of mortality
and disability in high-income countries, followed by
cerebrovascular disease (1). The
mortality rate for ischemic heart disease in China is increasing at
an annual rate of 5.05% (2,3). The recanalization of obstructed
vessels (reperfusion therapy) by thrombolytic drug treatment,
percutaneous coronary intervention (PCI) and coronary artery bypass
grafting are considered to be the most effective therapeutic
strategies for myocardial ischemia (4). In 2017, 753,142 patients in mainland
China accepted coronary artery intervention, representing a 13%
increase compared with 2016. The vast majority of patients undergo
interventional surgery through the radial artery, and the mortality
rate following PCI is low (0.23%) (2). However, there is accumulating evidence
that reperfusion therapy may cause further tissue damage, referred
to as myocardial ischemia-reperfusion injury (MIRI) (5). Oxidative stress, inflammatory
responses, mitochondrial damage and calcium overload, as well as
cell death and cell survival-associated signaling pathways are
involved in the pathophysiology of MIRI (6). Therefore, the development of therapies
targeting the molecular mechanism underlying MIRI development is of
significance for the treatment of ischemic heart disease and the
prevention of MIRI.
Hypoxia-inducible factor (HIF) is a heterodimeric
transcription factor that serves a pivotal role in mediating
adaptive responses to hypoxia (7).
It consists of an oxygen-sensitive HIF-α subunit and a HIF-β
subunit. The latter is also termed aryl hydrocarbon receptor
nuclear translocator (ARNT). Mammals have three isoforms of the
HIF-α subunit (HIF-1α, HIF-2α and HIF-3α). HIF-1α is a key factor
in the oxygen-sensing pathway initially identified by Semenza et
al (8) in 1991 and is
particularly important for the maintenance of oxygen homeostasis in
mammalian cells (9). In cancer,
ischemic heart disease or chronic obstructive pulmonary disorder,
tissue partial pressure of oxygen is decreased, resulting in the
activation of HIF-1α (10). Under
hypoxic conditions, HIF-1α protein is does not undergo degradation
by the oxygen-dependent ubiquitin proteasome system and is stably
expressed. HIF-1α accumulates in the cytoplasm and is translocated
to the nucleus and subsequently forms a dimer with ARNT to regulate
target gene transcription (11).
The activation of HIF-1α improves cell survival in a hypoxic
environment by altering energy metabolism, proliferation,
angiogenesis and vascular remodeling. HIF-1α is essential for
cardioprotection against MIRI (12). Stable expression stabilization of
HIF-1α allows cells or tissues to adapt to hypoxic responses during
MIRI, protects cardiomyocytes from ischemic heart disease and
improves patient prognosis.
Molecular characteristics of HIF-1α
HIF-1 is the first identified nuclear transcription
factor with a highly specific regulatory role in oxygen
homeostasis. Both HIF-1α and HIF-1β exhibit basic helix-loop-helix
(bHLH) motifs and belong to the bHLH-Per-ARNT-Sim (PAS) homology
protein family. The bHLH domain is a DNA-binding domain that can
bind to hypoxia response elements (HRE) of target genes. The HLH
motif mediates dimerization with other proteins. The PAS domain is
the only conserved domain among all members of the bHLH-PAS protein
family, including HIF-1α, ARNT, aryl hydrocarbon receptor (AhR) and
PAS (13,14).
The protein stability, subcellular localization and
transcriptional activity of HIF-1α can be regulated by the
intracellular oxygen concentration. This regulatory association is
primarily due to the unique structure of the oxygen-dependent
degradation domain (ODDD) (Fig. 1).
Under normoxic conditions, the two proline sites (Pro402 and
Pro564) of the ODDD of HIF-1α are hydroxylated by oxygen-dependent
prolyl hydroxylase domain (PHD)-containing proteins including the
prolyl hydroxylases PHD1, 2 and PHD3 (15). Hydroxylated HIF-1α is recognized by
von Hippel-Lindau (VHL) and degraded by the oxygen-dependent
ubiquitin-proteasome pathway (15,16).
Therefore, the half-life of HIF-1α is very short under normal
oxygen levels, <5 min.
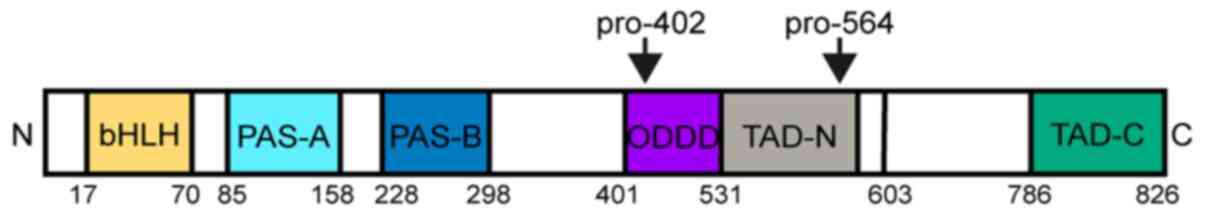 | Figure 1.Schematic illustration of the domain
structure of HIF-1α. HIF-1α consists of a bHLH motifs and a PAS
domain in the NH2-terminal, which are necessary for
heterodimerization and DNA binding to hypoxia response elements.
The two TADs, which stimulate transcription, are present in the
COOH-terminal of HIF-1α. TAD-C interacts with coactivators such as
CREB-binding protein/p300 to activate gene transcription. HIF-1α
also contains an ODDD, which promotes proteasomal degradation of
HIF-1α by PHD-containing enzymes and factor inhibiting HIF. HIF-1α,
hypoxia-inducible factor-1α; bHLH, basic helix-loop-helix; PAS,
Per-ARNT-Sim homology; TAD, transactivation domains; TAD-N,
transactivation domain N terminal; TAD-C, transactivation domain C
terminal; ODD, oxygen-dependent-degradation; PHD, prolyl
hydroxylase domain. |
HIF-1α-mediated transcriptional responses to
hypoxia
Under hypoxic conditions, the inhibition of
oxygen-dependent PHD1, −2 and −3 enzyme activity results in the
degradation of HIF-1α via the ubiquitin-proteasome pathway. HIF-1α
accumulates and is translocated to the nucleus, where it binds to
ARNT to form a heterodimeric complex that binds to HRE. Thus,
transcription of target genes is regulated by HIF-1α via the core
sequence of HRE (5′-RCGTG-3′) contained in the promoter region
(17). The recruitment of
CREB-binding protein/p300 results in the induction of the
transcription of >100 downstream target genes, such as vascular
endothelial growth factor (VEGF), erythropoietin, induced
nitric oxide synthase (iNOS) and glucose transporter
(GLUT), thereby regulating the response to hypoxia at the
cellular and systemic levels (7,18–20).
In addition to hypoxia, HIF-1α can be activated by other factors,
such as growth factors, acetylcholine and angiotensin II (10) (Fig.
2). HIF-1α target genes associated with ischemic heart disease
and their roles are listed in Table
I.
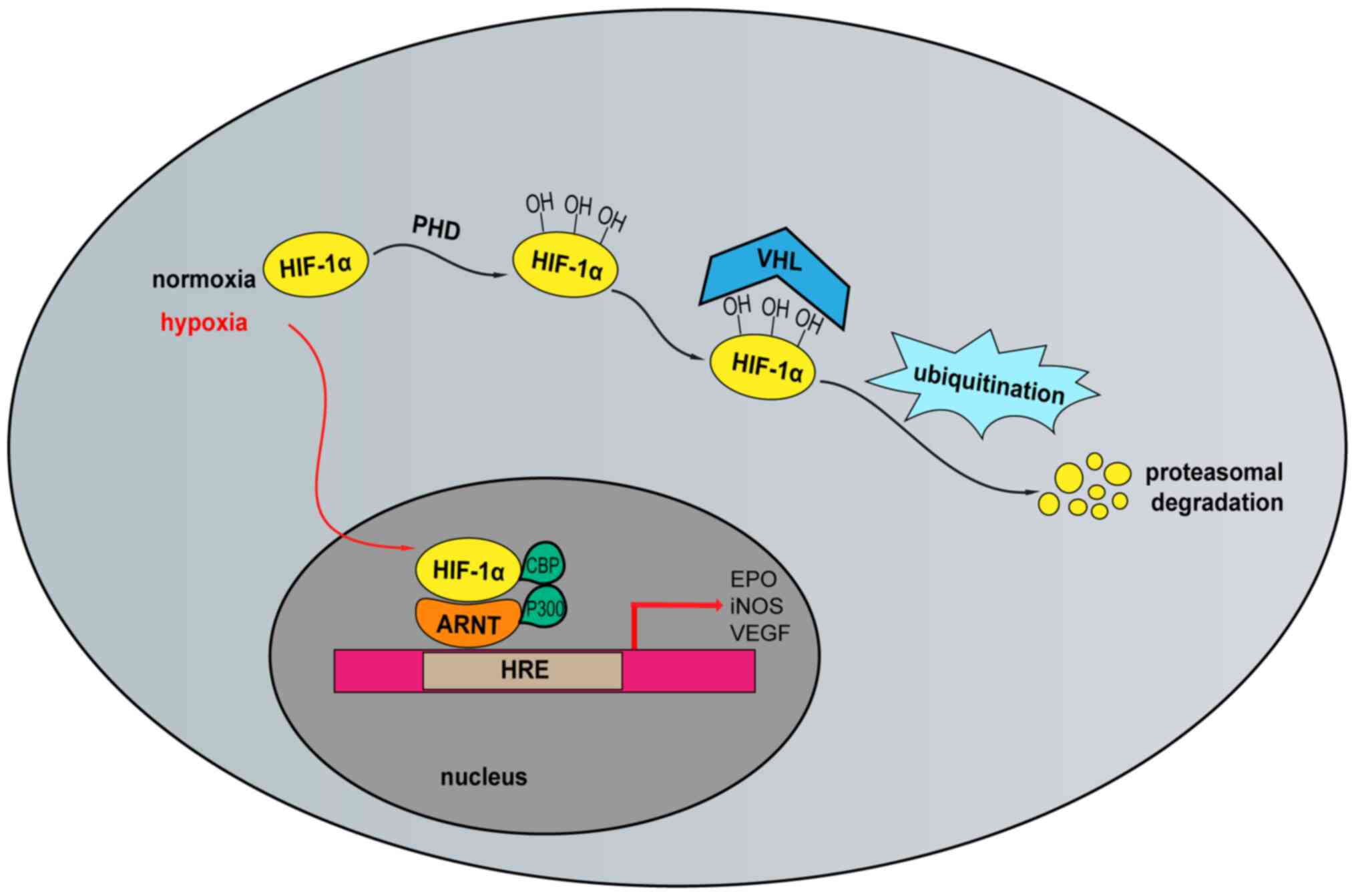 | Figure 2.Oxygen-dependent regulation of
HIF-1α. In normoxic conditions, HIF-1α protein is hydroxylated by
prolyl hydroxylases PHD1, PHD2, and PHD3. Hydroxylated HIF-1α is
recognized by VHL and degraded by the ubiquitin-proteasome pathway.
In hypoxic conditions, HIF-1α accumulates and translocates to the
nucleus where it binds to ARNT to form a heterodimeric complex that
binds to the promoter region of the HRE. CBP/p300 recruitment
induces transcription of downstream target genes. HIF-1α,
hypoxia-inducible factor-1α; PHD, prolyl hydroxylases; VHL, von
Hippel-Lindau; ARNT, aryl hydrocarbon receptor nuclear
translocator; HRE, hypoxia-response element; CBP, CREB-binding
protein; EPO, erythropoietin; iNOS, inductible nitric oxide
synthase; VEGF, vascular endothelial cell growth factor; PHD,
prolyl hydroxylase domain. |
 | Table I.HIF-1α target genes. |
Table I.
HIF-1α target genes.
| Function | HIF-1α target | (Refs.) |
|---|
| Angiogenesis | Vascular
endothelial growth factor | (7,48) |
| Erythropoiesis | Erythropoietin | (19) |
| Vascular tone | Induced nitric
oxide synthase | (20) |
|
| Heme
oxygenase-1 | (47) |
| Glucose
metabolism | Glucose
transporter | (18) |
| Mitochondrial
function | Frataxin | (29) |
| Mitochondrial
function | Bcl-2 and
adenovirus E1B 19-kDa-interacting protein 3 | (52) |
| Anti-oxidation | Nuclear factor
erythroid 2-related factor 2; superoxide dismutase 2 | (39) |
|
| Nox2, Nox4 | (38) |
|
| GSK3β | (53) |
| Apoptosis | Phosphorylated-P38;
Bcl-2 | (58) |
| Remote ischemic
preconditioning | Interleukin-10 | (82) |
Roles of HIF-1α in MIRI
Matsushima et al (21) demonstrated that HIF-1α may protect
cardiac fibroblasts from apoptosis and represent a potential
therapeutic target for heart remodeling following hypoxic injury.
Additionally, a number of studies have confirmed that HIF-1α
prevents MIRI and has a cardioprotective effect (22,23).
Protective effect on mitochondrial
function
Mitochondria are the main sites for aerobic
respiration and are also primary targets for ischemic injury.
Mitochondrial dysfunction plays a crucial role in MIRI (24,25).
During ischemia, decreased oxygen levels impair mitochondrial ATP
production and induce an increase in intracellular Ca2+.
During reperfusion, the concentration of intracellular
Ca2+ increases further, resulting in a calcium overload
in the cytoplasm and mitochondria. Simultaneously, hypoxia damages
the mitochondrial electron transport chain (ETC), resulting in
increased reactive oxygen species (ROS) production. The increases
in ROS and Ca2+ levels lead to the opening of
non-selective, highly conductive permeability transition pores
(PTPs) in the mitochondrial inner membrane, as well as changes in
mitochondrial membrane permeability (26). PTP opening further increases
mitochondrial Ca2+ and ROS levels and stimulates the
oxidation of proteins and lipids in mitochondria (27). Calcium overload and oxidative stress
may cause mitochondrial dysfunction, which in turn induces
cardiomyocyte apoptosis or necrosis. On the one hand, the decrease
in the intracellular oxygen concentration can activate HIF-1α by
inhibiting PHD proteins during ischemia. On the other hand, HIF-1α
can regulate the expression levels of mitochondria-specific genes
to adapt to hypoxic stress and improve mitochondrial function
(28). Nanayakkara et al
(29) suggested that HIF-1α could
transcriptionally regulate frataxin expression levels in response
to hypoxia and acted as a cardioprotective factor against ischemic
injury. Increased levels of frataxin can mitigate mitochondrial
iron overload and subsequent ROS production, thereby preserving
mitochondrial membrane integrity and the viability of
cardiomyocytes. Thus, HIF-1α preserves the integrity of the
mitochondrial membrane, promotes cell survival and protects against
MIRI. Moreover, HIF-1α can also improve mitochondrial respiratory
function by activating different cardioprotective signaling
pathways, such as the PI3K/AKT and Janus kinase 2/STAT3 pathways,
to protect the heart following IRI (30). Additionally, some studies have
suggested that mitochondria can regulate HIF-1α stability and that
ROS produced by the mitochondrial ETC can stabilize HIF-1α under
hypoxic conditions (16).
Mitochondria-derived ROS have multiple, opposing effects. They can
impair mitochondrial function and promote cardiomyocyte death or
stabilize HIF-1α to improve mitochondrial function (31). However, this finding is
controversial and may be associated with oxygen levels, as oxygen
concentration affects both mitochondrial function and HIF-1α
stability and activity. The determination of the oxygen levels
required to optimize mutual beneficial effects of mitochondria and
HIF-1α may provide a novel direction for the treatment of MIRI.
Maintenance of cellular redox
balance
ROS are generally considered to be toxic by-products
of aerobic metabolism and are the primary cause of macromolecular
destruction (32,33). However, it has been demonstrated
that ROS also contribute substantially to numerous physiological
and pathological conditions (34).
In MIRI, ROS generation begins during ischemia, and large amounts
of ROS are produced during reperfusion. Excessive ROS accumulation
in cells is one of the primary causes of MIRI. This deleterious
effect is mediated by the oxidative modification of proteins,
lipids and histone-free mitochondrial DNA (35). Therefore, the elimination of
excessive ROS in cells and the alleviation of oxidative stress in
cardiomyocytes can promote myocardial cell survival and decrease
the severity of MIRI (36,37).
The mitochondrial ETC is an important source of ROS
and may contribute to MIRI (38).
In ischemia, an abnormal increase in ROS causes the accumulation of
succinate, resulting in decreased ETC complex activity. Following
reperfusion, succinate is rapidly oxidized to generate large
amounts of ROS. A notable increase in ROS and IR-induced
Ca2+ influx leads to mitochondrial PTP opening, which
decreases ETC activity and further increases the formation of ROS.
In addition, the NADPH oxidase (Nox) family produces large amounts
of ROS. The Nox2 and Nox4 isoforms are major components of the Nox
family that produce reactive oxidants in the heart, leading to
MIRI. Decreased ROS production by mitochondria and Nox can
attenuate the severity of MIRI, whereas low levels of ROS can also
modulate HIF-1α expression levels (32,39).
HIF-1α contributes to MIRI through numerous
mechanisms. It can activate the HIF pathway, thereby activating
target genes involved in the regulation of the redox state of cells
as well as decreasing ROS production and apoptosis in the
cardiomyocytes of patients with IRI (6). Additionally, HIF-1α stabilizes
mitochondrial function and promotes the production of mitochondrial
antioxidants in cells. For instance, HIF-1α can enhance the
antioxidant capacity of cells through the antioxidant transcription
factor nuclear factor erythroid 2-related factor 2 (Nrf2) and by
upregulating the synthesis of the antioxidant tripeptide
glutathione and superoxide dismutase 2 (32,40,41).
Furthermore, HIF-1α mediates a shift from oxidative metbolism to
glycolysis (thus decreasing the production of mitochondrial
oxidants), decreases ETC activity and attenuates mitochondrial ROS
production, thereby avoiding cell death (42). HIF-1α mediates ROS production by
mitochondria and the Nox family and regulates the redox state of
cells, which decrease the severity of MIRI (36).
A number of studies support the beneficial effects
of HIF-1α on the maintenance of cellular redox balance (43–45).
However, this view is also somewhat controversial. Tang et
al (46) demonstrated that
during MIRI, the polyol pathway increases the cytosolic
NADH/NAD+ ratio, resulting in HIF-1α activation and
transferrin receptor upregulation, which exacerbates oxidative
damage and increases lipid peroxidation. However, the effect of
HIF-1α on oxidative stress was not evaluated separately in this
previous study. Further investigation is needed to determine the
dynamic regulatory effects of HIF-1α on different types of redox
indicators at different time points in MIRI.
HIF-1α signaling pathway
Since the first reported target gene of HIF-1α
(erythropoietin), hundreds of downstream targets have been
identified, demonstrating the complexity and importance of the
HIF-1α signaling pathway (15). In
MIRI, HIF-1α can regulate and participate in a number of signaling
pathways that protect the heart (47). MIRI initially leads to hypoxia,
resulting in AKT phosphorylation and activation of HIF-1α and
numerous protective genes (48).
Dong et al (49)
demonstrated that sevoflurane pretreatment can increase the
expression levels of VEGF by activating the AKT/HIF-1α/VEGF
signaling pathway. VEGF is closely associated with angiogenesis. In
MIRI, increased angiogenesis can effectively improve hypoxia in
lesions, thereby protecting the heart (50). iNOS acts downstream of HIF-1α
and has a cardioprotective effect in MIRI (51,52).
In addition, heme oxygenase-1 (HO-1), adiponectin, insulin-like
growth factor-2, GLUT and other loci are involved in the protective
effect of HIF-1α against MIRI (18).
In MIRI, HIF-1α can also act by directly or
indirectly regulating numerous signaling pathways (42,49).
In terms of mitochondria, HIF-1α can directly or indirectly
influence mitochondrial function, decrease mitochondrial damage and
attenuate the severity of MIRI (53). HIF-1α can induce myocardial
mitochondrial autophagy via the HIF-1α/Bcl2 and adenovirus E1B
19-kDa-interacting protein 3 (BNIP3) signaling pathway, thereby
promoting myocardial cell survival following MIRI. However, this is
limited to HIF-1α-mediated mitochondrial autophagy in the early
stage of IR, which may lead to protective responses, whereas
prolonged autophagy may promote cardiomyocyte death. In terms of
oxidative stress, HIF-1α also plays a significant role. For
instance, HIF-1α can upregulate Nrf2, which then activates
antioxidant enzymes to protect cells by enhancing intrinsic ROS
clearance (23,53). In terms of inflammation, the
phosphorylation of IκBα during hypoxia results in the degradation
of IκBα and activation of NF-κB (48). However, HIF-1α activation can
inhibit the NF-κB pathway and induce HO-1, thereby attenuating the
production of pro-inflammatory cytokines, inhibiting tissue
inflammation and decreasing the severity of MIRI. In addition,
HIF-1α can regulate numerous signaling pathways, such as
GSK3β/mitochondrial PTP, β-catenin, ERK1/2, Bcl-2, PI3K/AKT and
mTOR, which are involved in the regulation of broad-spectrum cell
functions, and thus can decrease the severity of MIRI (54–57).
Crosstalk between HIF-1α and
non-coding RNAs
MicroRNAs (miRNAs or miRs) are small non-coding RNA
molecules ~22 nucleotides in length. They primarily bind to the 3′
untranslated region of mRNAs to control stability and translation,
thereby decreasing protein levels. More than 30% of genes in the
human genome are regulated by miRNAs. A number of studies have
reported that miRNAs serve a vital role in cardiovascular disease
(58). Sheng et al (59) suggested that the overexpression of
miR-7b inhibits IR-induced apoptosis in H9C2 cells by targeting the
HIF-1α/phosphorylated-P38 pathway. In vivo experiments have
demonstrated that overexpression of miR-335 enhances the
transcriptional activity of HIF-1α, increases the expression levels
of HO-1 and iNOS and inhibits the opening of mitochondrial PTP,
thereby decreasing myocardial infarct size and myocardial apoptosis
as well as improving MIRI (41).
Liu et al (60) demonstrated
that sirtuin 1 (SIRT1) also acts as a transcriptional repressor to
suppress the expression levels of miR-138 in adult sensory neurons
in response to peripheral nerve injury. Therefore, miR-138 and
SIRT1 can form a mutual negative feedback regulatory loop, which
provides a novel mechanism for controlling intrinsic axon
regeneration. The overexpression of miRNA-138 can decrease
apoptosis following myocardial IR by decreasing the expression
levels of HIF-1α. This may be explained by the difference between
early and prolonged hypoxia (60).
During early hypoxia, changes in miRNA levels may contribute to the
accumulation of HIF-1α and maintain the steady-state levels of
HIF-2 and HIF-3 (61). In addition,
HIF-1α can act as a modulator of miRNA function. Wu et al
(62) demonstrated that the
accumulation of HIF-1α following myocardial infarction leads to a
decrease in miRNA-10b-5p, which mediates the apoptosis of
cardiomyocytes. Although the number of known miRNAs associated with
HIF-1α is increasing, the majority of studies have concentrated on
cancer cell lines, and relatively little is known about their role
in cardiovascular disease, particularly in MIRI (63,64).
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length and have no protein-coding properties; they
mediate numerous biological processes, such as cell proliferation,
cell differentiation and apoptosis (65). lncRNAs negatively or positively
regulate the expression levels of protein-coding genes by numerous
modes of action. For example, one type of lncRNA, referred to as
competitive endogenous RNAs (ceRNAs) (66) decrease the availability of
functional miRNAs by acting as a complementary sequence for miRNA
binding. lncRNAs are closely associated with a number of biological
functions and pathological processes (e.g. cardiovascular diseases)
(67). There is increasing evidence
that lncRNAs serve a significant role in the regulation of the
myocardial IR process (68). For
example, Ren et al (69)
investigated the effect of lncRNA nuclear enriched abundant
transcript 1 (lnc-NEAT1) on cell proliferation and apoptosis in
MIRI and observed that lnc-NEAT1 is overexpressed in MIRI compared
with levels in normal cardiomyocytes. Downregulation of lnc-NEAT1
enhances cell proliferation and inhibits cell apoptosis by
targeting miR-193a in IR injury H9C2 cells (69). Li et al (70) assessed the role of lncRNA H19 in the
regulation of MIRI and suggested that H19 expression levels are
downregulated in IR hearts of mice and cardiomyocytes treated with
H2O2. They also demonstrated that H19
functions as a ceRNA, decreasing the expression levels of
miR-877-3p via the aforementioned base-pairing mechanism. However,
the association between HIF-1α and lncRNAs has not yet been fully
elucidated. Yang et al (71)
assessed the association between HIF-1α and hypoxia-responsive
lncRNA-p21 and demonstrated that lncRNA-p21 is essential for
enhancing cell glycolysis under hypoxic conditions. VHL-mediated
HIF-1α ubiquitination is attenuated and leads to the accumulation
of HIF-1α, promoting glycolysis under hypoxic conditions (72). Xue and Luo (73) investigated the mechanism by which
lncRNA HIF-1α-antisense RNA 1 (AS1) regulates cytokine signaling
inhibitor 2 (SOCS2) via miR-204 in MIRI ventricular remodeling: The
silencing of HIF1α-AS1 and upregulation of miR-204 inhibited
apoptosis, and lncRNA HIF1A-AS1 served as a ceRNA to adsorb
miR-204, thereby inhibiting miR-204 and increasing SOCS2 expression
levels. The downregulation of HIF1A-AS1 and upregulation of miR-204
can attenuate ventricular remodeling and improve cardiac function
in mice following MIRI by regulating SOCS2. There is extensive
evidence that interactions between HIF-1α and lncRNA play pivotal
roles in many diseases (73);
however, further research on their involvement in MIRI is needed.
In-depth studies of the specific mechanisms of action may provide a
new direction for the treatment of MIRI.
Involvement of HIF-1α in the myocardial
protective effects of natural compounds against MIRI
Several studies have demonstrated that certain
natural compounds alleviate MIRI, and the therapeutic effect is
mediated by HIF-1α. For example, Liu et al (74) demonstrated that saponins of Panax
notoginseng had protective effects against MIRI via the
HIF-1α/Bcl-2/BNIP3 pathway, which increases mitochondrial
autophagy. In addition, Shen et al (75) suggested that Panax
notoginseng saponin Ft1 could increase the expression levels of
HIF-1α and growth factor secretion, thereby activating the PI3K/AKT
and Raf/MEK/ERK signaling pathways. Asiatic acid is another natural
compound that decreases ROS accumulation, enhances mitochondrial
membrane potential and decreases the intracellular Ca2+
concentration to improve mitochondrial function (76). In H9C2 oxygen/glucose
deprivation/reoxygenation in vitro models, asiatic acid
protects against MIRI, which is mediated by the AKT/GSK-3β/HIF-1α
signaling pathway (76). Moreover,
asiatic acid decreases HIF-3α by regulating miR-1290 targeting,
thereby increasing HIF-1α expression levels, resulting in decreased
hypoxia-induced apoptosis (62).
Lastly, several other natural compounds, such as ginsenoside Rg1,
paclitaxel, dihydrotanshinone I and protocatechuic aldehyde, can
protect against MIRI by affecting mitochondria, ROS, angiogenesis
and cell survival through increases in HIF-1α expression levels
(77,78).
Roles of HIF-1α in myocardial ischemic pre-
and post-conditioning
Ischemic preconditioning (IPC) aims to enhance
resistance to subsequent ischemic injury by transient ischemia.
Post-IPC protection is divided into two time periods: Immediately
following IPC and 12–24 h after IPC. Cai et al (79) evaluated the role of HIF-1α in the
acute phase of IPC and suggested that compared with wild-type mice,
cardiac function was improved in mice expressing the HIF-1α gene
following IPC, and that infarct size and cell death decreased.
These findings indicated that the reduced severity of MIRI
following IPC is HIF-1α-dependent. Jia et al (80) demonstrated that IPC could activate
HIF-1α, upregulating miRNA-21 at the transcriptional level and
ultimately decreasing the production of proinflammatory cytokines
and apoptosis in target organs. IPC can also protect the heart by
upregulating myocardial iNOS expression levels, which is also
mediated by HIF-1α. Furthermore, HIF-1α plays a key role in remote
IPC (81). In remote IPC, transient
IR in the arm or leg protects the heart from long-term coronary
occlusion and reperfusion. Cai et al (82) demonstrated that remote IPC increases
plasma IL-10 levels and decreases myocardial infarct size in
wild-type mice, but not in HIF-1α knockout mice. Their study
further revealed that HIF-1 is necessary and sufficient for
induction of IL-10 gene transcription in cultured mouse myocytes.
indicating that the protective effect of remote IPC on MIRI depends
on HIF-1α (82). Late IPC can also
increase the expression levels of HIF-1α and IL-10 in the heart,
thereby activating the cardiomyocyte anti-apoptosis and survival
signals and thus serving an anti-MIRI role (83).
Previous studies have suggested that ischemic
postconditioning can improve myocardial function, decrease
myocardial infarct size, and protect cardiomyocytes against MIRI.
For example, Wan et al (84)
reported that ischemic postconditioning can enhance HIF-1α activity
in the heart of rats following MIRI, and that HIF-1α can act on
miR-214 to relieve MIRI. In addition, remote ischemic
postconditioning can notably decrease the degree of MIRI severity.
Wang et al (85)
demonstrated that the cardioprotective effects of remote ischemic
postconditioning are primarily associated with macrophage migration
inhibitory factor (MIF). Remote ischemic postconditioning confers
protection against MIRI through the MIF-AMPK signaling pathway.
However, MIF also plays a role in remote ischemic postconditioning
through the HIF-1α-dependent humoral pathway, which inhibits HIF-1α
and leads to a decrease in plasma MIF and concomitant increase in
cardiac MIF, thereby reducing its cardioprotective effects. In
summary, HIF-1α serves critical roles in IPC, including remote IPC,
late IPC and their post-conditioning, and may thus represent an
important target for MIRI treatment.
Future perspectives
With the rapid development of biomedical research in
the past decade, the mechanisms underlying MIRI have been
extensively studied. MIRI is mediated by multiple factors.
Cardiomyocyte death may be promoted by a number of synergistic
mechanisms throughout the pathophysiological process. Numerous
molecular targets associated with MIRI and cardioprotection have
been identified (86,87). HIF-1α is one of the most promising
targets (88). HIF-1α can mitigate
MIRI by various complex mechanisms (Figs. 3 and 4). However, although a previous study has
demonstrated that inhibiting HIF-1α in the early stage of cerebral
IR injury in rats can decrease infarction volume and mortality by
inhibiting apoptosis, it is unclear whether a similar process might
occur in MIRI (89). The mechanisms
underlying the protective effect of HIF-1α in MIRI, including the
roles of genes involved in glycolysis, mitochondrial function, cell
survival, apoptosis and oxidative stress, are still poorly
characterized. HIF-1α regulates multiple target genes and may thus
play different roles at different stages of MIRI progression via
different mechanisms. Further research on the role of HIF-1α in the
pathophysiology of MIRI and the underlying mechanisms is therefore
essential.
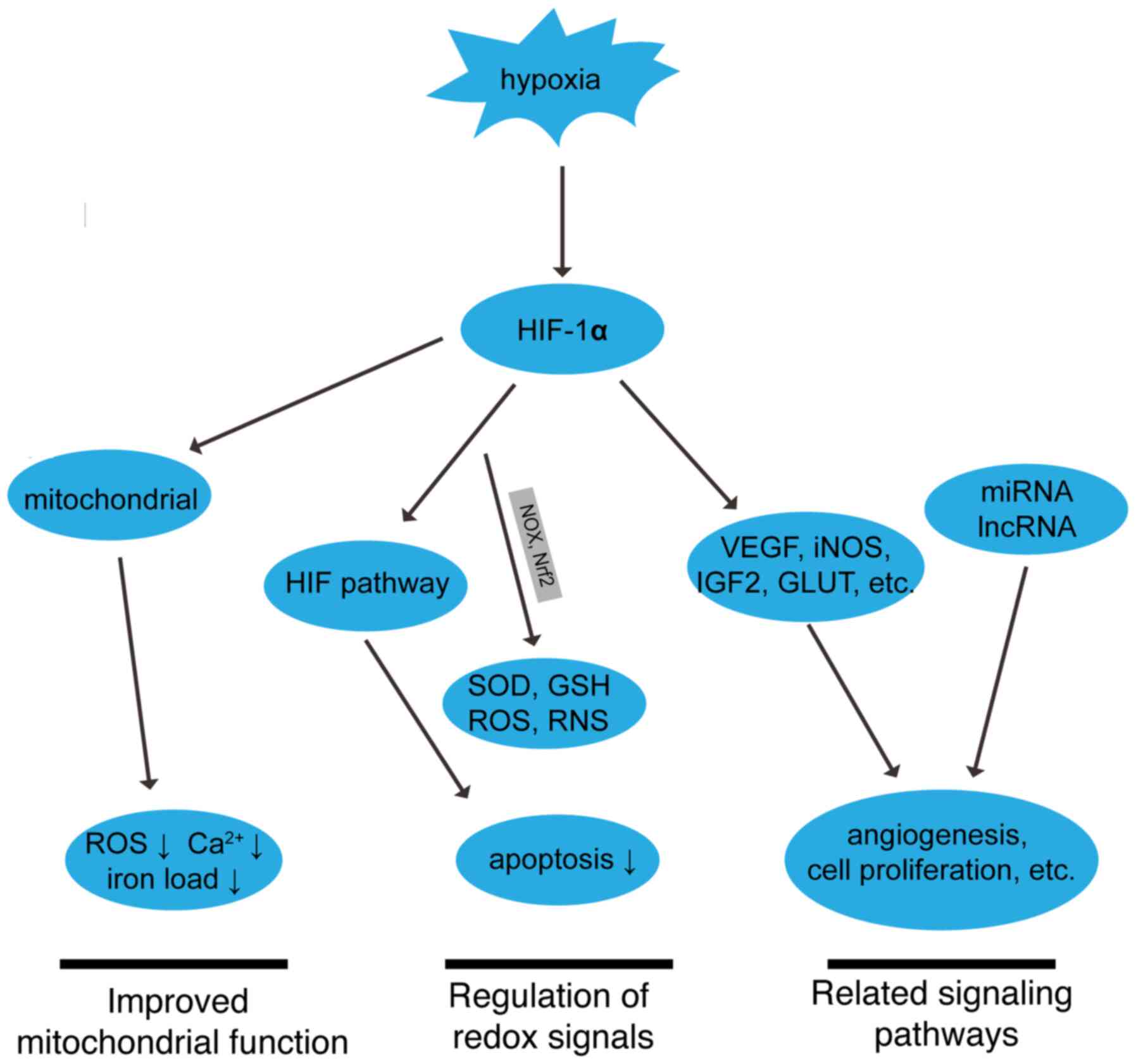 | Figure 4.Roles of HIF-1α in MIRI. The
upward-facing arrow indicates enhancement. The downward-facing
arrow indicates inhibition. MIRI, myocardial ischemia reperfusion
injury; HIF-1α, hypoxia-inducible factor-1α; miRNA, microRNA;
lncRNA, long non-coding RNA; ROS, reactive oxygen species; VEGF,
vascular endothelial growth factor; iNOS, induced nitric oxide
synthase; IGF2, insulin-like growth factor 2; GLUT, glucose
transporter; Nox, NADPH oxidase; Nrf2, nuclear factor erythroid
2-related factor 2; SOD, superoxide dismutase; glutathione; ROS,
reactive oxygen species; RNS, reactive nitrogen species, RNS. |
Acknowledgements
Not applicable.
Funding
The present paper was supported by the National
Natural Science Foundation of China (grant no. 81700269).
Availability of data and materials
Not applicable.
Authors' contributions
CC and YH conceived and designed the review. JZhe
retrieved the relevant literature and wrote the manuscript. HC
constructed the figures. PC, JZho and YC reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaski JC, Crea F, Gersh BJ and Camici PG:
Reappraisal of ischemic heart disease. Circulation. 138:1463–1480.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma LY, Chen WW, Gao RL, Liu LS, Zhu ML,
Wang YJ, Wu ZS, Li HJ, Gu DF, Yang YJ, et al: China cardiovascular
diseases report 2018: An updated summary. J Geriatr Cardiol.
17:1–8. 2020.PubMed/NCBI
|
|
3
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: Current features
and implications. Nat Rev Cardiol. 16:203–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel MR, Calhoon JH, Dehmer GJ, Grantham
JA, Maddox TM, Maron DJ and Smith PK:
ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria
for coronary revascularization in patients with stable ischemic
heart disease: A report of the American college of cardiology
appropriate use criteria task force, American association for
thoracic surgery, American heart association, American society of
echocardiography, American society of nuclear cardiology, society
for cardiovascular angiography and interventions, society of
cardiovascular computed tomography, and society of thoracic
surgeons. J Am Coll Cardiol. 69:2212–2241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ibáñez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellanti F: Hypoxia-inducible factor-1 in
myocardial ischaemia/reperfusion injury. Acta Physiol (Oxf).
221:93–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choudhry H and Harris AL: Advances in
hypoxia-inducible factor biology. Cell Metab. 27:281–298. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semenza GL, Nejfelt MK, Chi SM and
Antonarakis SE: Hypoxia-inducible nuclear factors bind to an
enhancer element located 3′ to the human erythropoietin gene. Proc
Natl Acad Sci USA. 88:5680–5684. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chee NT, Lohse I and Brothers SP:
mRNA-to-protein translation in hypoxia. Mol Cancer. 18:49. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eckle T, Köhler D, Lehmann R, El Kasmi K
and Eltzschig HK: Hypoxia-inducible factor-1 is central to
cardioprotection: A new paradigm for ischemic preconditioning.
Circulation. 118:166–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang BH, Rue E, Wang GL, Roe R and
Semenza GL: Dimerization, DNA binding, and transactivation
properties of hypoxia-inducible factor 1. J Biol Chem.
271:17771–17778. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sousa Fialho MDL, Abd Jamil AH, Stannard
GA and Heather LC: Hypoxia-inducible factor 1 signalling,
metabolism and its therapeutic potential in cardiovascular disease.
Biochim Biophys Acta Mol Basis Dis. 1865:831–843. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ong SG and Hausenloy DJ: Hypoxia-inducible
factor as a therapeutic target for cardioprotection. Pharmacol
Ther. 136:69–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mole DR, Blancher C, Copley RR, Pollard
PJ, Gleadle JM, Ragoussis J and Ratcliffe PJ: Genome-wide
association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha
DNA binding with expression profiling of hypoxia-inducible
transcripts. J Biol Chem. 284:16767–16775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh L, Aldosary S, Saeedan AS, Ansari MN
and Kaithwas G: Prolyl hydroxylase 2: A promising target to inhibit
hypoxia-induced cellular metabolism in cancer cells. Drug Discov
Today. 23:1873–1882. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abe H, Semba H and Takeda N: The roles of
hypoxia signaling in the pathogenesis of cardiovascular diseases. J
Atheroscler Thromb. 24:884–894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsushima S, Kuroda J, Ago T, Zhai P,
Ikeda Y, Oka S, Fong GH, Tian R and Sadoshima J: Broad suppression
of NADPH oxidase activity exacerbates ischemia/reperfusion injury
through inadvertent downregulation of hypoxia-inducible factor-1α
and upregulation of peroxisome proliferator-activated receptor-α.
Circ Res. 112:1135–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou YH, Han QF, Wang LH, Liu T, Meng XY,
Wu L, Li T, Jiao YR, Yao HC and Zhang DY: High mobility group box 1
protein attenuates myocardial ischemia reperfusion injury via
inhibition of the p38 mitogen-activated protein kinase signaling
pathway. Exp Ther Med. 14:1582–1588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu N, Li J, Li Y, Zhang Y, Du Q, Hao P,
Li J, Cao X and Li L: Berberine protects against simulated
ischemia/reperfusion injury-induced H9C2 cardiomyocytes apoptosis
in vitro and myocardial ischemia/reperfusion-induced apoptosis in
vivo by regulating the mitophagy-mediated HIF-1α/BNIP3 pathway.
Front Pharmacol. 11:3672020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lesnefsky EJ, Chen Q, Tandler B and Hoppel
CL: Mitochondrial dysfunction and myocardial ischemia-reperfusion:
Implications for novel therapies. Annu Rev Pharmacol Toxicol.
57:535–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hausenloy DJ, Garcia-Dorado D, Bøtker HE,
Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J,
Madonna R, et al: Novel targets and future strategies for acute
cardioprotection: Position paper of the european society of
cardiology working group on cellular biology of the heart.
Cardiovasc Res. 113:564–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bernardi P and Di Lisa F: The
mitochondrial permeability transition pore: Molecular nature and
role as a target in cardioprotection. J Mol Cell Cardiol.
78:100–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang S, Lewis TS, Powers C, Khuchua Z,
Baines CP, Wipf P and Javadov S: Elucidating mitochondrial electron
transport chain supercomplexes in the heart during
ischemia-reperfusion. Antioxid Redox Signal. 27:57–69. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chowdhury A, Aich A, Jain G, Wozny K,
Lüchtenborg C, Hartmann M, Bernhard O, Balleiniger M, Alfar EA,
Zieseniss A, et al: Defective mitochondrial cardiolipin remodeling
dampens HIF-1α expression in hypoxia. Cell Rep. 25:561–570. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nanayakkara G, Alasmari A, Mouli S,
Eldoumani H, Quindry J, McGinnis G, Fu X, Berlin A, Peters B, Zhong
J and Amin R: Cardioprotective HIF-1α-frataxin signaling against
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
309:H867–H879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fuhrmann DC and Brüne B: Mitochondrial
composition and function under the control of hypoxia. Redox Biol.
12:208–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cadenas S: ROS and redox signaling in
myocardial isch-emia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deshwal S, Antonucci S, Kaludercic N and
Di Lisa F: Measurement of mitochondrial ROS formation. Methods Mol
Biol. 1782:403–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garlick PB, Davies MJ, Hearse DJ and
Slater TF: Direct detection of free radicals in the reperfused rat
heart using electron spin resonance spectroscopy. Circ Res.
61:757–760. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boengler K, Bornbaum J, Schlüter KD and
Schulz R: P66shc and its role in ischemic cardiovascular diseases.
Basic Res Cardiol. 114:292019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boengler K, Lochnit G and Schulz R:
Mitochondria ‘THE’ target of myocardial conditioning. Am J Physiol
Heart Circ Physiol. 315:H1215–H1231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Luo Y, Wang S, Zhu H and Li D:
Roles and mechanisms of SUMOylation on key proteins in myocardial
ischemia/reperfusion injury. J Mol Cell Cardiol. 134:154–164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurian GA, Rajagopal R, Vedantham S and
Rajesh M: The role of oxidative stress in myocardial ischemia and
reperfusion injury and remodeling: Revisited. Oxid Med Cell Longev.
2016:16564502016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bertero E and Maack C: Calcium signaling
and reactive oxygen species in mitochondria. Circ Res.
122:1460–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang L, Zeng H, Ni L, Qi L, Xu Y, Xia L,
Yu Y, Liu B, Yang H, Hao H and Li P: HIF-1α preconditioning
potentiates antioxidant activity in ischemic injury: The role of
sequential administration of dihydrotanshinone I and protocatechuic
aldehyde in cardioprotection. Antioxid Redox Signal. 31:227–242.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Semenza GL: Hypoxia-inducible factors:
Coupling glucose metabolism and redox regulation with induction of
the breast cancer stem cell phenotype. EMBO J. 36:252–259. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thomas LW and Ashcroft M: Exploring the
molecular interface between hypoxia-inducible factor signalling and
mitochondria. Cell Mol Life Sci. 76:1759–1777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adeyemi OS, Eseola AO, Plass W, Otuechere
CA and Elebiyo TC: New imidazoles cause cellular toxicity by
impairing redox balance, mitochondrial membrane potential, and
modulation of HIF-1α expression. Biochem Biophys Res Commun.
529:23–27. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gyongyosi A, Terraneo L, Bianciardi P,
Tosaki A, Lekli I and Samaja M: The impact of moderate chronic
hypoxia and hyperoxia on the level of apoptotic and autophagic
proteins in myocardial tissue. Oxid Med Cell Longev.
2018:57867422018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watanabe Y, Cohen RA and Matsui R: Redox
regulation of ischemic angiogenesis-another aspect of reactive
oxygen species. Circ J. 80:1278–1284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang WH, Wu S, Wong TM, Chung SK and Chung
SS: Polyol pathway mediates iron-induced oxidative injury in
ischemic-reperfused rat heart. Free Radic Biol Med. 45:602–610.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang S, Ma K, Liu Y, Pan X, Chen Q, Qi L
and Li S: Stabilization of hypoxia-inducible factor by DMOG
inhibits development of chronic hypoxia-induced right ventricular
remodeling. J Cardiovasc Pharmacol. 67:68–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee JW, Ko J, Ju C and Eltzschig HK:
Hypoxia signaling in human diseases and therapeutic targets. Exp
Mol Med. 51:1–13. 2019. View Article : Google Scholar
|
|
49
|
Dong J, Xu M, Zhang W and Che X: Effects
of sevoflurane pretreatment on myocardial ischemia-reperfusion
injury through the Akt/hypoxia-inducible factor 1-alpha
(HIF-1α)/vascular endothelial growth factor (VEGF) signaling
pathway. Med Sci Monit. 25:3100–3107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li J, Zhou W, Chen W, Wang H, Zhang Y and
Yu T: Mechanism of the hypoxia inducible factor 1/hypoxic response
element pathway in rat myocardial ischemia/diazoxide
post-conditioning. Mol Med Rep. 21:1527–1536. 2020.PubMed/NCBI
|
|
51
|
Wu N, Zhang X, Du S, Chen D and Che R:
Upregulation of miR-335 ameliorates myocardial ischemia reperfusion
injury via targeting hypoxia-inducible factor 1-alpha subunit
inhibitor. Am J Transl Res. 10:4082–4094. 2018.PubMed/NCBI
|
|
52
|
Si J, Wang N, Wang H, Xie J, Yang J, Yi H,
Shi Z, Ma J, Wang W, Yang L, et al: HIF-1α signaling activation by
post-ischemia treatment with astragaloside IV attenuates myocardial
ischemia-reperfusion injury. PLoS One. 9:e1078322014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Y, Liu D, Hu H, Zhang P, Xie R and
Cui W: HIF-1α/BNIP3 signaling pathway-induced-autophagy plays
protective role during myocardial ischemia-reperfusion injury.
Biomed Pharmacother. 120:1094642019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu DW, Zhang YN, Hu HJ, Zhang PQ and Cui
W: Downregulation of microRNA-199a-5p attenuates
hypoxia/reoxygenation-induced cytotoxicity in cardiomyocytes by
targeting the HIF-1α-GSK3β-mPTP axis. Mol Med Rep. 19:5335–5344.
2019.PubMed/NCBI
|
|
55
|
Adluri RS, Thirunavukkarasu M, Dunna NR,
Zhan L, Oriowo B, Takeda K, Sanchez JA, Otani H, Maulik G, Fong GH
and Maulik N: Disruption of hypoxia-inducible transcription
factor-prolyl hydroxylase domain-1 (PHD-1−/−) attenuates
ex vivo myocardial ischemia/reperfusion injury through
hypoxia-inducible factor-1α transcription factor and its target
genes in mice. Antioxid Redox Signal. 15:1789–1797. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yao HC, Zhou M, Zhou YH, Wang LH, Zhang
DY, Han QF, Liu T, Wu L, Tian KL and Zhang M: Intravenous high
mobility group box 1 upregulates the expression of HIF-1α in the
myocardium via a protein kinase B-dependent pathway in rats
following acute myocardial ischemia. Mol Med Rep. 13:1211–1219.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tekin D, Dursun AD and Xi L:
Hypoxia-inducible factor 1 (HIF-1) and cardioprotection. Acta
Pharmacol Sin. 31:1085–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' action through
miRNA editing. Int J Mol Sci. 20:62492019. View Article : Google Scholar
|
|
59
|
Sheng Z, Lu W, Zuo Z, Wang D, Zuo P, Yao Y
and Ma G: MicroRNA-7b attenuates ischemia/reperfusion-induced H9C2
cardiomyocyte apoptosis via the hypoxia-inducible factor-1/p-p38
pathway. J Cell Biochem. 120:9947–9955. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Y, Zou J, Liu X and Zhang Q:
MicroRNA-138 attenuates myocardial ischemia reperfusion injury
through inhibiting mitochondria-mediated apoptosis by targeting
HIF1-α. Exp Ther Med. 18:3325–3332. 2019.PubMed/NCBI
|
|
61
|
Serocki M, Bartoszewska S,
Janaszak-Jasiecka A, Ochocka RJ, Collawn JF and Bartoszewski R:
miRNAs regulate the HIF switch during hypoxia: A novel therapeutic
target. Angiogenesis. 21:183–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu L, Chen Y, Chen Y, Yang W, Han Y, Lu L,
Yang K and Cao J: Effect of HIF-1α/miR-10b-5p/PTEN on
hypoxia-induced cardiomyocyte apoptosis. J Am Heart Assoc.
8:e0119482019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu K, Hu M, Chen Z, Xiang F, Chen G, Yan
W, Peng Q and Chen X: Asiatic acid enhances survival of human AC16
cardiomyocytes under hypoxia by upregulating miR-1290. IUBMB Life.
69:660–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bavelloni A, Ramazzotti G, Poli A, Piazzi
M, Focaccia E, Blalock W and Faenza I: MiRNA-210: A current
overview. Anticancer Res. 37:6511–6521. 2017.PubMed/NCBI
|
|
65
|
Long B, Li N, Xu XX, Li XX, Xu XJ, Guo D,
Zhang D, Wu ZH and Zhang SY: Long noncoding RNA FTX regulates
cardiomyocyte apoptosis by targeting miR-29b-1-5p and Bcl2l2.
Biochem Biophys Res Commun. 495:312–318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang X and Ning Q: The emerging roles of
long noncoding RNAs in common cardiovascular diseases. Hypertens
Res. 38:375–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ong SB, Katwadi K, Kwek XY, Ismail NI,
Chinda K, Ong SG and Hausenloy DJ: Non-coding RNAs as therapeutic
targets for preventing myocardial ischemia-reperfusion injury.
Expert Opin Ther Targets. 22:247–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ren L, Chen S, Liu W, Hou P, Sun W and Yan
H: Downregulation of long non-coding RNA nuclear enriched abundant
transcript 1 promotes cell proliferation and inhibits cell
apoptosis by targeting miR-193a in myocardial ischemia/reperfusion
injury. BMC Cardiovasc Disord. 19:1922019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li X, Luo S, Zhang J, Yuan Y, Jiang W, Zhu
H, Ding X, Zhan L, Wu H, Xie Y, et al: lncRNA H19 alleviated
myocardial I/RI via suppressing miR-877-3p/Bcl-2-mediated
mitochondrial apoptosis. Mol Ther Nucleic Acids. 17:297–309. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang F, Zhang H, Mei Y and Wu M:
Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the
Warburg effect. Mol Cell. 53:88–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yue X, Wang R, Li W, Wang C, Lu H and
Zhang J: Research progress of long chain non-coding RNA H19 in
anoxic environment mechanism. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
43:1151–1158. 2018.(In Chinese). PubMed/NCBI
|
|
73
|
Xue X and Luo L: LncRNA HIF1A-AS1
contributes to ventricular remodeling after myocardial
ischemia/reperfusion injury by adsorption of microRNA-204 to
regulating SOCS2 expression. Cell Cycle. 18:2465–2480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu XW, Lu MK, Zhong HT, Wang LH and Fu
YP: Panax notoginseng saponins attenuate myocardial
ischemia-reperfusion injury through the HIF-1α/BNIP3 pathway of
autophagy. J Cardiovasc Pharmacol. 73:92–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shen K, Ji L, Gong C, Ma Y, Yang L, Fan Y,
Hou M and Wang Z: Notoginsenoside Ft1 promotes angiogenesis via
HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and
Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 84:784–792.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang X, Zuo L, Lv Y, Chen C, Yang Y, Xin
H, Li Y and Qian Y: Asiatic acid attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β/HIF-1α signaling in rat
H9c2 cardiomyocytes. Molecules. 21:12482016. View Article : Google Scholar
|
|
77
|
Veloso CD, Belew GD, Ferreira LL, Grilo
LF, Jones JG, Portincasa P, Sardão VA and Oliveira PJ: A
mitochondrial approach to cardiovascular risk and disease. Curr
Pharm Des. 25:3175–3194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Guo H, Zheng M, Jiao YB and Zheng H:
Paclitaxel enhances the protective effect of myocardial ischemia
preconditioning on ischemia/reperfusion injury in aged rat.
Zhonghua Xin Xue Guan Bing Za Zhi. 46:719–724. 2018.(In Chinese).
PubMed/NCBI
|
|
79
|
Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot
K, Wang L, Wei C, Trush MA and Semenza GL: Complete loss of
ischaemic preconditioning-induced cardioprotection in mice with
partial deficiency of HIF-1 alpha. Cardiovasc Res. 77:463–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jia P, Wu X, Dai Y, Teng J, Fang Y, Hu J,
Zou J, Liang M and Ding X: MicroRNA-21 is required for local and
remote ischemic preconditioning in multiple organ protection
against sepsis. Crit Care Med. 45:e703–e710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu X, Ge L, Niu L, Lian X, Ma H and Pang
L: The dual role of inducible nitric oxide synthase in myocardial
ischemia/reperfusion injury: Friend or foe? Oxid Med Cell Longev.
2018:83648482018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cai Z, Luo W, Zhan H and Semenza GL:
Hypoxia-inducible factor 1 is required for remote ischemic
preconditioning of the heart. Proc Natl Acad Sci USA.
110:17462–17467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen H, Jing XY, Shen YJ, Wang TL, Ou C,
Lu SF, Cai Y, Li Q, Chen X, Ding YJ, et al: Stat5-dependent
cardioprotection in late remote ischaemia preconditioning.
Cardiovasc Res. 114:679–689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wan DY, Zhang Z and Yang HH:
Cardioprotective effect of miR-214 in myocardial ischemic
postconditioning by down-regulation of hypoxia-inducible factor 1,
alpha subunit inhibitor. Cell Mol Biol (Noisy-le-grand). 61:1–6.
2015.PubMed/NCBI
|
|
85
|
Wang C, Zuo B and Wu X: The role of
macrophage migration inhibitory factor in remote ischemic
postconditioning. Can J Cardiol. 35:501–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Davidson SM, Ferdinandy P, Andreadou I,
Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM,
Hausenloy DJ, et al: Multitarget strategies to reduce myocardial
ischemia/reperfusion injury: JACC review topic of the week. J Am
Coll Cardiol. 73:89–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li D, Ni H, Rui Q, Gao R and Chen G: Mst1:
Function and mechanism in brain and myocardial ischemia reperfusion
injury. Curr Neuropharmacol. 16:1358–1364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang S, Shao X, Li X, Su X, Huo Y and Yang
C: HIF-1α may provide only short-term protection against
ischemia-reperfusion injury in Sprague-Dawley myocardial cultures.
Mol Clin Oncol. 4:579–583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen C, Hu Q, Yan J, Yang X, Shi X, Lei J,
Chen L, Huang H, Han J, Zhang JH and Zhou C: Early inhibition of
HIF-1alpha with small interfering RNA reduces ischemic-reperfused
brain injury in rats. Neurobiol Dis. 33:509–517. 2009. View Article : Google Scholar : PubMed/NCBI
|