Introduction
The transcription of genetic information from DNA to
RNA and the subsequent translation into proteins constitutes the
core of molecular biology. It has been long established that the
key factor in gene regulatory networks is the protein-coding gene.
Non-coding RNAs (ncRNAs) were first identified in the early 1990s
with the advent of RNA interference technology. However, it took
~10 years until the fundamental roles of ncRNAs in gene silencing
and biological functions were recognized (1). MicroRNAs (miRNAs/miRs) are a major
family of ncRNAs, and numerous studies have demonstrated that most
miRNAs play key roles in biological processes and cellular
metabolism, which are tightly regulated at multiple levels.
Aberrant miRNA expression is involved in various pathophysiological
processes, such as spinal cord injury, neurodegenerative and
cardiovascular diseases, and aging (2–5).
However, to the best of our knowledge, the potential mechanisms
have not yet been fully elucidated.
Pyroptosis is a form of programmed cell removal as a
result of various factors. Firstly, it is through a CARD-containing
inflammasome that a directly-activated inflammatory caspase
triggers the removal of cells (6).
Secondly, the pores, 1–2 nm in diameter, develop in the plasma
membrane of cells due to the activation of the inflammatory
caspase, resulting in cell swelling due to water uptake and
subsequent cell lysis through rapid disruption of the plasma
membrane. Thirdly, the local or systemic inflammatory effects are
amplified by membrane rupture and various cytosolic contents
entering the extracellular environment, for example, interleukin
(IL)-1β and IL-18 (7,8). Pyroptosis processes function as a
double-edged sword through both rapidly eliminating intracellular
pathogens by coordinating antimicrobial host defenses, and
deleteriously amplifying local destructive pathways (9,10). The
regulatory mechanisms of pyroptosis involve a variety of molecular
mechanisms and signaling pathways, but there has been little
research investigating the effects of miRNAs on the regulatory
mechanisms of pyroptosis. In the present review, the expression of
miRNAs and the association between miRNAs and pyroptosis are
summarized in order to provide a novel insight into the prevention
and treatment of diseases associated with pyroptosis.
Overview of ncRNAs
ncRNAs fall into the category of functional RNA
molecules responsible for the coding of substances other than
protein (11). In 1970, most
scholars widely accepted that humans have >10,000 genes, the
majority of which possess protein-coding functions (12). By the 1990s, the existence of
numerous more genes had been revealed in the Human Genome Project
and Encyclopedia of DNA Elements. Genomic transcription is common,
but >80% of genes are transcribed into ncRNAs, which lack the
ability to encode proteins (13,14).
Nevertheless, a number of recent studies have demonstrated that
numerous ncRNAs not only regulate DNA expression, but are also
involved in several complex biological processes (4,15,16).
The ncRNAs are classified into three major
subclasses according to their sequence length and structure: Short
ncRNAs (<200 nucleotides in length), long ncRNAs (lncRNAs;
>200 nucleotides in length) and circular RNAs (17). Based on their localization and
function, ncRNAs can also be divided into lncRNAs, miRNAs,
ribosomal RNAs (17), transfer RNAs
(18), piwi-interacting RNAs
(19), exosomal RNAs (20), small interfering RNAs (21), small nucleolar RNAs (22) and small nuclear RNAs (23). Due to the limited number of
protein-coding genes, miRNAs, of which there are several in the
non-coding transcriptome, are attracting much attention as
potential therapeutic targets for human diseases. However, the
functions, target specificity and molecular mechanisms of numerous
miRNAs remain to be determined. Therefore, the ways in which miRNAs
can be utilized in the clinical setting remain to be further
studied (24).
Overview of miRNAs
Discovery and origin of miRNAs and
communication of miRNAs between cells
As small ncRNA molecules (19–25 nucleotides in
length), miRNAs can regulate the way in which protein-coding genes
are negatively expressed (25).
Since miRNAs were first identified in Caenorhabditis elegans
in 1993 (26), with the continuous
maturity of sequencing technologies, scholars have discovered
>1,000 types of miRNA genes within the human body (27,28).
As much as ~30% of the human genome is suspected to be subject to
regulation by miRNAs, thus implying their significance in
regulating gene expression. Biological activities such as growth,
cell multiplication, apoptosis, the immune response and pyroptosis
are all associated with miRNAs (29).
The biogenesis of miRNAs and various other
small-size RNAs are different. miRNAs are obtained by creating
distinctive hairpin structures after folding back transcripts
(30). A two-step cleavage process
is required for miRNA biogenesis. The first step is miRNA cleavage
by the ribonucleases Drosha and DiGeorge syndrome critical region
gene 8 (DGCR8). A miRNA duplex is obtained from the second cleavage
event performed by Dicer and argonaute protein (31). The processes are shown in Fig. 1. However, there are some endogenous
small RNAs stemming from the hairpins with a far greater length,
thus making small RNAs, bimolecular RNA duplexes or the precursors
lacking double-stranded character even more diversified (25).
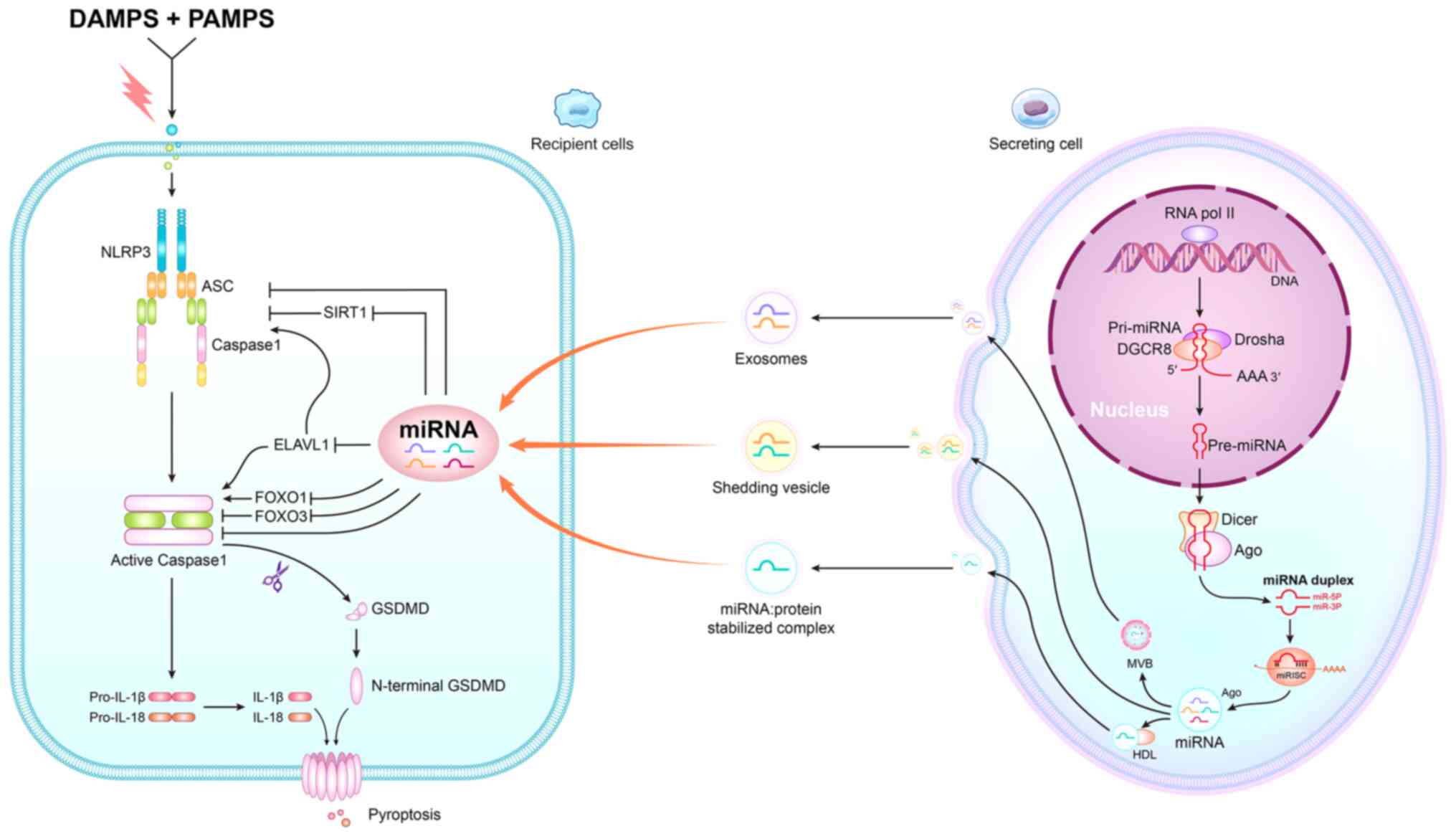 | Figure 1.Graphical depiction of the process of
miRNA regulating pyroptosis. On secreting cells, miRNAs are first
transcribed as pri-miRNAs, typically by RNA polymerase II.
Pri-miRNAs are processed by the Drosha-DGCR8 complex in the nucleus
to generate pre-miRNAs, which are then exported to the cytoplasm to
be cleaved by Dicer, producing duplexes containing both Ago and
guide miRNA strands. The passenger strand is degraded and the guide
strand is loaded onto an Ago to form the miRISC. On recipient
cells, DAMPs and PAMPs can activate the NLRP3 inflammasome, which
includes NLRP3, ASC and pro-caspase-1 and cleavage of the caspase-1
precursor, which activates caspase-1. Caspase-1 then regulates the
production of the inflammatory cytokines IL-1β and IL-18.
Horizontal transfer of miRNAs from secreting cell to receiving cell
includes three pathways: i) First pathway, active secretion via
MVBs, such as exosomes; ii) second pathway, shedding vesicles are
another active secretion pathway; and iii) third pathway, uses
RNA-binding protein to secrete miRNA. For instance, HDL can
associate with exogenous miRNAs and deliver them to recipient
cells. The received miRNAs regulate the activity of NLRP3 by
targeting SIRT1. In addition, the expression of ELAVL1, which is a
target of miRNAs, can affect the activity of caspase-1 and NLRP3 to
regulate pyroptosis. In addition, miRNAs also can regulate the
activity of caspase-1 by affecting FOXO3 and FOXO1 expression.
miRNA, microRNAs; pri-miRNA, primary miRNA; pre-miRNA, precursor
miRNAs; miRISC, miRNA-induced silencing complex; DAMPs;
danger-associated molecular patterns; PAMPS pathogen-associated
molecular patterns; NLRP3, NLR family pyrin domain containing 3;
ASC, apoptosis-associated speck-like protein; SIRT1, silencing
information regulator 2-related enzyme 1; GSDMD, gasdermin D;
ELAVL, ELAV-like RNA binding protein 1; FOXO, forkhead box O; IL,
interleukin; HDL, high density lipoprotein; MVBs, multivesicular
bodies. |
There are three major miRNA communication pathways
between cells that inhibit pyroptosis, including exosomes, shedding
vesicles and RNA-binding proteins (Fig.
1). In exosome pathways, studies have identified that miR-148a
derived from the M2 exosome, which is secreted by macrophages, can
inhibit thioredoxin interacting protein and the toll-like receptor
4/NF-κB/NLR family pyrin domain-containing 3 (NLRP3) inflammasome
signaling pathway (32,33). Wang et al (34) also reported that macrophages secrete
exosomes that release miR-155 into the cytosol, which can directly
target forkhead box O3 (FOXO3a) to inhibit pyroptosis in uremic
cardiomyopathy. Regarding the shedding of vesicles, it was revealed
that extracellular vesicles carrying miR-21-5p affect podocyte
pyroptosis in diabetic nephropathy (35). Another communication pathway is
associated with RNA-binding proteins (36), such as RNA-Binding Protein Dnd1. It
has been reported that Dnd1 can stabilize miR-221, which can
further suppress activation of the NLRP3/apoptosis-associated
speck-like protein containing a CARD (ASC)/pro-caspase-1
inflammasome pathway (37,38).
miRNA maturation pathways and
regulatory mechanisms of miRNAs
The maturation of miRNA is a tightly regulated
multistep procedure. In most cases, the respective facilitators are
what the transcription of intergenic miRNAs with gene regulatory
regions is reliant on. In addition, the expression of host mRNAs
determines the transcription of intronic miRNAs. Following
transcription, primary miRNAs undergo two processes that form
mature miRNAs of 21–22 nucleotides in length (39,40).
When liberating a 60–70 nucleotide-length stem loop intermediate,
the first process is the nuclear cleavage of the primary miRNA in
the nucleus, which is then referred to as the precursor miRNA. The
cleavage occurs through the use of the Drosha RNase III
endonuclease (41). As performed by
the enzyme Dicer, which is also an RNase III endonuclease, the
second step occurs in the cytoplasm, whereby the protein Dicer acts
with argonaute protein to cleave the pre-miRNA into ~22 nucleotide
miRNA duplexes (double-stranded RNA) (42,43).
The mechanism by which miRNAs regulate gene expression is
relatively simple. It requires an ideal base pairing between the
seed and target sequences. The direct interaction between miRNA and
mRNA can silence the majority of mRNAs targeted by miRNAs, thus
inducing mRNA degradation and/or inhibiting mRNA translation
(44,45). It is common that miRNAs are able to
bind to multiple mRNA species and inhibit the expression of several
different transcripts simultaneously (46). Individual miRNAs target mRNAs, which
frequently encode the proteins performing relevant functions
(47). However, miRNA inhibitory
effects on individual mRNAs are generally modest when it comes to
regulating critical biological events, and the combined effects of
several miRNAs on multiple mRNAs can induce strong biological
responses (48,49).
Pyroptosis
Origin and characteristics of
pyroptosis
Cell death is not only the end of life, it is also
necessary to sustain life. There are three different types of cell
removal that have been widely studied: Apoptosis (50), autophagic cell death (51) and necrosis (52). Among the most classical types of
cell death, apoptosis features a number of morphological changes:
Cell shrinkage, cytoplasm condensation, chromatin condensation and
apoptotic body formation. In contrast to necrotic cells, apoptotic
cells do not release intracellular contents into the extracellular
environment upon death (53).
Cytoplasmic vacuolization, phagocytic uptake and consequent
lysosomal degradation are the manifestations of autophagic cell
removal (54). Pyroptosis, as the
other form of programmed cell death, has been widely investigated.
Pyroptosis was first observed in Shigella flexneri-infected
macrophages in 1992 by Zychlinsky et al (55). In 2001, caspase-1-dependent cell
removal was termed pyroptosis, combining the Greek roots ‘pyro’,
associated with fire or fever, and ‘ptosis’, signifying decline
(56). The characteristics of
pyroptosis include pore formation in plasma membranes, cell
swelling and discharge of pro-inflammatory cytokines (IL-1β and
IL-18) (57,58). The process is mediated by Nod-like
receptors with C-terminal leucine-rich repeats (LRRs) that can
detect the pathogen-associated molecular patterns (PAMPs) or the
damage-associated molecular patterns (DAMPs). Next, through the
homotypic interaction of NACHT domains, NLR monomers oligomerize
before attaching to an adapter protein known as ASC/PYCARD, by
means of PYD-PYD interaction. Subsequently, procaspase-1 is
recruited by adaptor proteins and cleaved into caspase-1.
Caspase-1-mediated pyroptosis requires pores to develop in the cell
membrane, thus causing water influx and the discharge of
pro-inflammatory factors, such as IL-18 and IL-1β (59,60).
Canonical and non-canonical pathways
of pyroptosis
Signaling pathways for pyroptosis mainly include a
canonical pathway that depends on caspase-1 activation, and a
non-canonical pathway that relies on caspase-4/5 (human) or
caspase-11 (mouse) activation.
In the canonical pathway, LRR recognition of DAMPs
and PAMPs can activate the NLRP3 inflammasome, which includes
NLRP3, ASC and pro-caspase-1 and cleavage of the caspase-1
precursor, which activates caspase-1. Caspase-1 then regulates the
production of the inflammatory cytokines IL-1β and IL-18 (61). Following the activation of caspase-1
acting on gasdermin-D (GSDMD), GSDMD is cleaved to generate a
reactive amino (N) end and a carboxyl (C) end. The formation of 10
to 15-nm pore-like structures inside the membrane lipid bilayer is
preceded by the oligomerization of an N-terminal domain (62). These pores are assumed to play the
role as conduits in the discharge of small molecules, such as IL-1β
and IL-18. These mechanisms are shown in Fig. 1. Furthermore, a local inflammatory
response is prompted by stimulating various target cells, such as
monocytes, macrophages and dendritic cells. In the meantime,
systemic inflammatory functions, such as neutrophil recruitment,
are initiated (63).
The non-canonical pathway of inflammasome activation
is dependent on human caspase-4/-5 and murine caspase-11. Caspase-4
is linked to pyroptotic cell removal in monomyelocytic cell lines
(THP1 and U937) as a result of delivering lipopolysaccharide (LPS)
within cells (64). In addition,
the activated stimuli and function of caspase-5 have been revealed.
Both caspase-4 and −5 were verified by Viganò et al
(65) as critical downstream
targets for activating LPS in human monocytes. Furthermore,
intracellular LPS can be sensed by caspases-4 and −5, both of which
contribute to self-activation (64). Caspase-11 has two different effects.
Not only does caspase-11 activation directly lead to macrophage
pyroptosis, but it also acts as a binding partner in regulating how
caspase-1 is activated, leading to the production of IL-1β and
IL-18, and subsequent pyroptosis (66–68).
Association between miRNAs and
pyroptosis
miRNA pathways regulating cell
pyroptosis
Post-transcriptional modifications
negatively regulate inflammasome activation
Generally involved in the pathological processes of
various diseases, miRNAs can bind to complementary target mRNAs,
thus regulating gene expression in a negative way (25,69).
Thus, numerous mRNAs that encode proteins with shared biological
processes are regulated by one miRNA. In addition, one mRNA can
also be regulated by a number of other miRNAs. It was indicated
that miRNAs perform regulation in two ways. One way is inhibiting
mRNA translation. Another way is decreasing target mRNA amounts
(70,71). As revealed by some previous studies,
NLRP3 is a direct target for miR-223, which negatively regulates
the development of inflammasomes (69,72).
Bauernfeind et al (69)
identified miR-223 as playing a vital role in regulating the
activity performed by NLRP3 inflammasomes in macrophages. For
suppressed expression, miR-233 gets attached to a preserved binding
site within the 3′-untrnaslated region (UTR) of NLRP3 (69). Thus, miR-223 plays a crucial role in
NLRP3 inflammasome activity for rheostat control considering the
strict transcriptional regulation of NLRP3 mRNA. Furthermore, a
previous study revealed that the same site in the NLRP3 mRNA 3′-UTR
was targeted by EBV miR-BART15 for hindering the inflammasome from
being activated (72).
Transcription factors negatively
regulating inflammasome activation
Silencing information regulator 2-related enzyme 1
(SIRT1) and FOXO3a expression levels were silenced by the
transcription factors negatively regulating pyroptosis (73). SIRT1 is essential for suppressing
apoptosis, decreasing inflammatory reactions, preserving
mitochondrial function and oxidative stress. STAT1 hinders
pyroptosis by making the NLRP1 and NLRP3 inflammasomes less active
(74). In a bioinformatics
analysis, Wang et al (75)
revealed that miR-9-5p could bind to the 3′-UTR of SIRT1 for the
negative regulation of SIRT1 expression. In addition, Ding et
al (76) revealed that SIRT1
was targeted by miR-29a in H9c2 cardiomyocytes using a dual
luciferase assay. The inhibition of SIRT1 resulted from miR-29a
binding to SIRT1, which promoted pyroptosis.
Another transcription factor is FOXO3a, which was
reportedly associated with the negative regulation of pyroptosis.
As revealed in a previous study, miRNAs enhanced the downregulation
of FOXO3a before the decreased suppression of apoptosis, as
regulated by FOXO3a (77). This led
to the upregulation of caspase-1 and the induction of pyroptosis
(78).
miRNA suppresses pyroptosis by
inhibiting caspase-1
As a major enzyme involved in regulating pyroptosis,
caspase-1 processes pro-IL-1β and pro-IL-18 into mature
inflammatory cytokines (57,79).
The activation of caspase-1 and subsequent cleavage of GSDMD
contributes to the formation of pores on the cell membrane, thus
causing pyroptosis. Jin et al (80) demonstrated caspase-1 to be a
functional downstream target of miR-214, revealing that partial
sequences of miR-214 could bind to sites in the caspase-1 3′-UTR
(80). This may be evidence to
support that caspase-1 is targeted by miRNA to regulate pyroptosis.
These regulating pathways are presented in Fig. 1.
Association between miRNAs and
pyroptosis in disease. Cardiovascular disease. i) Myocardial
infarction (MI)
The various types of miRNAs that target pyroptosis
following MI have been extensively studied. Mezzaroma et al
(81) demonstrated the presence of
the NLRP3 inflammasome in the heart in an experimental mouse model
of MI. However, whether miRNAs inhibit pyroptosis in such cases
should be further investigated and validated. Thus, Li et al
(82) revealed that miR-135b
targeted and regulated caspase-1, as assessed by a luciferase
assay. By detecting the expression of mRNA, the study further
discovered that miR-135b downregulated the mRNA expression of
caspase-1, suggesting that miR-135b is associated with MI and that
its expression can assist with the diagnosis and treatment of MI
(82).
ii) Diabetic cardiomyopathy (DCM)
Some studies have shown that miRNAs regulate
pyroptosis over the course of DCM. A study by Yang et al
(83) reported that miR-214-3p
targets caspase-1 to regulate the expression of NLRP3, IL-1β and
IL-18 in DCM. Cell dysfunction in vitro was triggered, and
the pathological process of DCM in vivo was facilitated as a
result of inflammatory cytokine enhancement (83). Furthermore, Li et al
(84) revealed that miR-30d
increased the downregulation of FOXO3a in a diabetic rat model.
Thus, miR-30d directly represses FOXO3a expression, which leads to
the inhibition of its downstream proteins. Subsequently, the
upregulation of caspase-1 occurred, which contributed to
pyroptosis. These findings provide another potential mechanism of
cardiomyocyte pyroptosis: The upregulation of miR-30d promotes
pyroptosis via the downregulation of FOXO3a, which may increase
apoptosis repressor with caspase recruitment domain, thus promoting
caspase-1 expression and subsequently increasing IL-1β and IL-18
levels, ultimately increasing the levels of pyroptosis (84). In addition, Jeyabal et al
(85) revealed that in human
cardiomyocytes, hyperglycemic conditions enhance the expression of
ELAV-like RNA binding protein 1 (ELAVL1), and the expression levels
of caspase-1 and IL-1β are increased. Furthermore, ELAVL1-knockdown
inhibited pyroptosis through NLRP3, caspase-1 and inflammatory
cytokine inhibition. In addition, direct targeting of ELAVL1 by
miR-9 was confirmed via bioinformatics analysis and target
validation assays (85). Thus, the
application of miR-9 can inhibit not only the ELAVL1 overexpression
caused by hyperglycemia but also cardiomyocyte pyroptosis. Overall,
these studies show that miRNA can inhibit caspase-1-induced
pyroptosis, and their results may identify novel therapeutic
targets in the pyroptosis signaling pathway in DCM.
iii) Atherosclerosis
Atherosclerotic plaques result in inflammatory
processes and lipid metabolism abnormalities (86). Furthermore, several studies have
revealed that cholesterol crystals and oxidized low-density
lipoproteins (ox-LDLs) can cause inflammasome activation, and have
also demonstrated the role of pyroptosis in atherosclerosis
(87,88). In addition, miRNAs play a crucial
role in treating endothelial dysfunction and have potential for
treating atherosclerosis (89).
Thus, miRNAs may contribute to the progression of atherosclerosis
via pyroptosis. In human aortic endothelial cells, ox-LDL-activated
pyroptosis was indicated by Li et al (90) as capable of suppressing miR-30c-5p.
Furthermore, FOXO3 is considered to be a target gene of miR-30c-5p;
however, whether it promotes or inhibits FOXO3 expression remains
controversial. These findings provide an alternative method for
treating atherosclerosis (90).
Furthermore, the impact of lncRNA metastasis-associated lung
adenocarcinoma transcript 1 on high glucose-induced cell pyroptosis
can be offset by the overexpression of miR-22 (91). Functioning as a DNA demethylase, tet
methylcytosine dioxygenase 2 (TET2) is effective in decreasing
atherosclerosis (92). In a study
by Zhaolin et al (93), a
bioinformatics analysis was performed to determine whether
miR-125a-5p can bind to the 3′-UTR of TET2 mRNA. As revealed by a
luciferase reporter gene assay, the expression of TET2 could be
suppressed by an miR-125a-5p mimic and enhanced by an miR-125a-5p
inhibitor, implying that targeting the TET2 3′-UTR may result in
abnormal mitochondrial DNA methylation levels and mitochondrial
dysfunction, which induces the production of reactive oxygen
species and activates NF-κB, and subsequently, induces the
formation of the NLRP3 inflammasome (93). Thus, miR-125a-5p may regulate TET2
expression from the perspective of post-transcription.
With regard to other cardiovascular diseases, such
as viral myocarditis, Tong et al (94) revealed that the downregulation of
NLRP3 and caspase-1 expression could decrease pyroptosis following
the inhibition of miR-15 (94).
iv) Ischemia-reperfusion (I/R)
injury
According to previous studies, small miRNAs are
associated with I/R injury (95,96).
In addition, pyroptosis plays a crucial role in the tissue
impairments caused by I/R injury (97). Thus, there may be an association
between miRNAs and pyroptosis in I/R injury. As revealed by Wu
et al (98), the direct
binding of FOXO3a with miR-155 could enable the induction of
pyroptosis in renal tubular cells, which plays a vital role in the
regulation of various cellular activities. Capable of inhibiting
apoptosis, the proteins downstream of FOXO3a cause both the
intrinsic and extrinsic pathways of cell death to be antagonized,
thus producing an inhibitory effect (98). Thus, miR-155 has a significant role
in renal tubular cell pyroptosis. Furthermore, in a study on blood
perfusion following myocardial ischemia, Lin et al (99) revealed that miR-149 can bind to the
3′-UTR to negatively regulate FOXO3 expression, whereas silencing
of FOXO3 promoted pyroptosis in I/R-treated cells.
A previous study demonstrated that the suppression
of pyroptosis and alleviation of inflammatory reactions were
largely affected by SIRT1 (100).
In addition, Ding et al (76) revealed that myocardial I/R injury
can be alleviated by inhibiting miR-29a, targeting SIRT1 and
decreasing NLRP3-mediated pyroptosis.
Neurodegenerative disease
Individuals aged >90 years have a high risk of
developing Parkinson's disease (PD) (101). An increasing number of studies
have shown that the pathophysiological process of PD is closely
associated with miRNAs (102). In
a recent study, Zeng et al (103) demonstrated that FOXO1 expression
in patients with PD can be enhanced by downregulating miR-135b,
which can also affect the activation of the NLRP3 inflammasome and
pyroptosis. With respect to PD, one of the complicated mechanisms
of its progression is miR-135b-mediated cell death (103). Fan et al (104) confirmed that the expression of
Renilla luciferase can be decreased by miR-7 via the NLRP3
3′-UTR as analyzed using a luciferase assay, which enabled the
assessment of NLRP3 protein translation levels (104). In addition, miR-7 overexpression
significantly downregulated NLRP3 protein expression levels. By
contrast, miR-7 silencing upregulated the expression of NLRP3. The
protein levels of caspase-1 or IL-1β production were unaffected by
miR-7 overexpression or silencing, suggesting that miR-7 targets
NLRP3 (104,105). This may represent a novel
therapeutic avenue for neurodegenerative diseases, including
PD.
Cancer
As revealed by a previous study, both glioma tissues
and cell lines had significantly upregulated caspase-1 expression
levels, but significantly downregulated miR-214 expression levels
(106). This same studied
demonstrated via a luciferase reporter assay that caspase-1 was a
target gene of miR-214, and intervention with pyroptosis was found
to render miR-214 effective in restricting cell migration and
multiplication (106). In
addition, miR-181a could enhance the growth and invasiveness of
osteosarcoma cells by blocking the activation of NLRP3-dependent
pyroptosis, as proposed by Tian et al (107). These findings suggested that
miR-181a could serve as a therapeutic target in osteosarcoma
progression. In summary, miR-214 and miR-181 are associated with
the regulation of pyroptosis in cancer.
Other diseases
In LPS-induced septic shock, Xue et al
(108) revealed that the knockdown
of miR-21 downregulated NLRP3, ASC and caspase-1 protein levels and
inflammasome activation in myeloid cells. In acute lung injury,
Ying et al (109) showed
that alveolar macrophage inflammation and pyroptosis can be
decreased by the overexpression of miR-495, while negative
regulation of the NLRP3 gene rendered the NLRP3 inflammasome less
active. The association between miRNAs and pyroptosis is summarized
in Table I.
 | Table I.Association between miRNAs and
pyroptosis. |
Table I.
Association between miRNAs and
pyroptosis.
| miRNA | Mechanism | Regulatory effect
on pyroptosis | Disease | (Refs.) |
|---|
| miR-223 | Inhibiting NLRP3
activation | Negative | Unclear | (69) |
| miR-7 | Inhibiting NLRP3
activation | Negative | PD | (104,105) |
| miR-495 | Inhibiting NLRP3
activation | Negative | Acute lung
injury | (109) |
| miR-9-5p | Activating
SIRT1/NLRP3 pathway | Positive | PD | (75) |
| miR-29a | Activating
SIRT1/NLRP3 pathway | Positive | Myocardial I/R
injury | (76) |
| miR-214 | Inhibiting
caspase-1 activation | Negative | Glioma | (80,83,106) |
| miR-135b | Inhibiting
FOXO1/caspase-1 pathway | Negative | PD | (82,103) |
| miR-181a | Unclear | Negative | Osteosarcoma | (107) |
| miR-30d | Activating
FOXO3/caspase-1 pathway | Positive | DCM | (84) |
| miR-155 | Activating
FOXO3/caspase-1 pathway | Positive | Renal I/R
injury | (98) |
| miR-149 | Activating
FOXO3/caspase-1 pathway | Positive | Myocardial I/R
injury | (99) |
| miR-30c-5p | Inhibiting
FOXO3/caspase-1 pathway | Negative |
Atherosclerosis | (90) |
| miR-22 | Unclear | Negative |
Atherosclerosis | (92) |
| miR-125a-5p | Activating tet
methylcytosine dioxygenase 2/NLRP3 pathway | Positive |
Atherosclerosis | (93) |
| miR-15 | Unclear | Negative | Viral
myocarditis | (94) |
| miR-9 | Inhibiting
ELAV-like RNA binding protein 1/caspase-1 and NLRP3 pathways | Negative | DCM | (85) |
| miR-21 | Unclear | Negative |
Lipopolysaccharide-induced septic
shock | (108) |
Conclusion
Recently, the regulation of pyroptosis in different
pathological situations has attracted significant attention.
Considering the complex functions of miRNAs in regulating cell
proliferation, survival and death, it is sensible to predict that
miRNAs are also associated with biological functions, such as
pyroptosis. The present review discussed the maturation process of
miRNAs and the process of pyroptosis, with a focus on the transport
of miRNA to damaged cells through exosomes, shedding vesicles and
protein stabilized complexes. Currently, these miRNA communication
pathways between cells that regulate pyroptosis are less studied in
diseases. This needs to be a focus of attention in future research.
The review also determined the different miRNAs that specifically
regulate the process of pyroptosis through different genes and
protein targets. In addition, the review aimed to summarize the
current evidence available to verify the mechanisms underlying
miRNA regulation in pyroptosis. Moreover, it provided evidence of
the regulatory role of miRNAs on pyroptosis in the cardiovascular
system, nervous system and cancer, which indicates that miRNAs may
play an important role in the regulation of pyroptosis. Apart from
contributing evidence that miRNAs mediate cell death, an attempt
was made to provide recommendations for further research into
investigating other mechanisms by which miRNAs may regulate cell
death. It is expected that the current understanding of
miRNA-dependent regulation of pyroptosis can be improved by
performing further research. The present review provides a novel
insight into potential targets for the development of novel
therapeutic strategies to alter miRNAs in vivo to treat
pyroptosis-associated diseases.
Acknowledgements
The authors of the present study would like to thank
Dr Chang Jia (Pediatric Research Institute, the Second Affiliated
Hospital and Yuying Children's Hospital of Wenzhou Medical
University, Wenzhou, China), who made valuable suggestions
regarding this manuscript.
Funding
This study was supported by grants from the Natural
Science Foundation of China (grant nos. 8207219, 81601705 and
81873992), the Zhejiang Provincial Medicine and Health Technology
Project (grant nos. 2017KY472 and 2015RCB022), the Public Welfare
Technology Research Project of Zhejiang Province (grant no.
LGF20H150003) and the Zhejiang Provincial Natural Science
Foundation (grant no. LY17H060009).
Availability of data and materials
Not applicable.
Authors' contributions
XH, CW, JL, HM and YX searched and reviewed the
literature, and drafted and revised the manuscript; YC, SS, HuaX
and XiaW provided important interpretation of content; XinW and
HuiX designed the figure. WN and KZ designed and formulated the
review theme, and revised and finalized the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASC
|
apoptosis-associated speck-like
protein containing a CARD
|
|
DAMPs
|
danger-associated molecular
patterns
|
|
DCM
|
diabetic cardiomyopathy
|
|
FOXO3a
|
forkhead box O3
|
|
GSDMD
|
gasdermin D
|
|
IL-1β
|
interleukin-1β
|
|
IL-18
|
interleukin-18
|
|
lncRNAs
|
long non-coding RNAs
|
|
LPS
|
lipopolysaccharide
|
|
LRR
|
leucine-rich repeats
|
|
MI
|
myocardial infarction
|
|
miRNAs
|
microRNAs
|
|
ncRNAs
|
non-coding RNAs
|
|
NLRP3
|
NOD-like receptor protein 3
|
|
NLRs
|
Nod-like receptors
|
|
PAMPs
|
pathogen-associated molecular
patterns
|
|
PD
|
Parkinson's disease
|
|
SIRT1
|
silencing information regulator
2-related enzyme 1
|
|
TET2
|
tet methylcytosine dioxygenase 2
|
References
|
1
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Ye L, Zhang K, Gao L, Xiao J and
Zhang Y: Upregulation of microRNA-200a in bone marrow mesenchymal
stem cells enhances the repair of spinal cord injury in rats by
reducing oxidative stress and regulating Keap1/Nrf2 pathway. Artif
Organs. 44:744–752. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang D, Fei Z, Luo S and Wang H:
MiR-335-5p inhibits β-Amyloid (Aβ) accumulation to attenuate
cognitive deficits through targeting c-jun-N-terminal kinase 3 in
Alzheimer's disease. Curr Neurovasc Res. 17:93–101. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirzadeh Azad F, Arabian M, Maleki M and
Malakootian M: Small molecules with big impacts on cardiovascular
diseases. Biochem Genet. 58:359–383. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Q, Wu J, Wang X and Song C: Noncoding
RNAs in vascular aging. Oxid Med Cell Longev. 2020:79149572020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jorgensen I and Miao EA: Pyroptotic cell
death defends against intracellular pathogens. Immunol Rev.
265:130–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zychlinsky A, Fitting C, Cavaillon JM and
Sansonetti PJ: Interleukin 1 is released by murine macrophages
during apoptosis induced by Shigella flexneri. J Clin
Invest. 94:1328–1332. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sangiuliano B, Perez NM, Moreira DF and
Belizario JE: Cell death-associated molecular-pattern molecules:
Inflammatory signaling and control. Mediators Inflamm.
2014:8210432014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zendedel A, Monnink F, Hassanzadeh G,
Zaminy A, Ansar MM, Habib P, Slowik A, Kipp M and Beyer C: Estrogen
attenuates local inflammasome expression and activation after
spinal cord injury. Mol Neurobiol. 55:1364–1375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mortezaee K, Khanlarkhani N, Beyer C and
Zendedel A: Inflammasome: Its role in traumatic brain and spinal
cord injury. J Cell Physiol. 233:5160–5169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ning S and Li X: Non-coding RNA resources.
Adv Exp Med Biol. 1094:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lander ES: Initial impact of the
sequencing of the human genome. Nature. 470:187–197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paukstelis PJ, Chen JH, Chase E, Lambowitz
AM and Golden BL: Structure of a tyrosyl-tRNA synthetase splicing
factor bound to a group I intron RNA. Nature. 451:94–97. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
International Human Genome Sequencing
Consortium, . Finishing the euchromatic sequence of the human
genome. Nature. 431:931–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao MS, Ai Y and Wilusz JE: Biogenesis
and functions of Circular RNAs come into focus. Trends Cell Biol.
30:226–240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veneziano D, Nigita G and Ferro A:
Computational approaches for the analysis of ncRNA through deep
sequencing techniques. Front Bioeng Biotechnol. 3:772015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nie A, Sun B, Fu Z and Yu D: Roles of
aminoacyl-tRNA synthetases in immune regulation and immune
diseases. Cell Death Dis. 10:9012019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nigita G, Marceca GP, Tomasello L,
Distefano R, Calore F, Veneziano D, Romano G, Nana-Sinkam SP,
Acunzo M and Croce CM: ncRNA editing: Functional characterization
and computational resources. Methods Mol Biol. 1912:133–174. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tielking K, Fischer S, Preissner KT,
Vajkoczy P and Xu R: Extracellular RNA in Central Nervous System
Pathologies. Front Mol Neurosci. 12:2542019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu AM, Batra N, Tu MJ and Sweeney C: Novel
approaches for efficient in vivo fermentation production of
noncoding RNAs. Appl Microbiol Biotechnol. 104:1927–1937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang D, Zhou J, Gao J, Wu RY, Huang YL,
Jin QW, Chen JS, Tang WZ and Yan LH: Targeting snoRNAs as an
emerging method of therapeutic development for cancer. Am J Cancer
Res. 9:1504–1516. 2019.PubMed/NCBI
|
|
23
|
Ferlita A, Battaglia R, Andronico F,
Caruso S, Cianci A, Purrello M and Pietro CD: Non-Coding RNAs in
endometrial physiopathology. Int J Mol Sci. 19:21202018. View Article : Google Scholar
|
|
24
|
Lu Q, Wu R, Zhao M, Garcia-Gomez A and
Ballestar E: MiRNAs as therapeutic targets in inflammatory disease.
Trends Pharmacol Sci. 40:853–865. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cummins JM and Velculescu VE: Implications
of micro-RNA profiling for cancer diagnosis. Oncogene.
25:6220–6227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shendure J and Ji H: Next-generation DNA
sequencing. Nat Biotechnol. 26:1135–1145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Croce CM and Calin GA: MiRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gaudet AD, Fonken LK, Watkins LR, Nelson
RJ and Popovich PG: MicroRNAs: Roles in regulating
neuroinflammation. Neuroscientist. 24:221–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Liang H, Zhang J, Zen K and Zhang
CY: Secreted microRNAs: A new form of intercellular communication.
Trends Cell Biol. 22:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai Y, Wang S, Chang S, Ren D, Shali S, Li
C, Yang H, Huang Z and Ge J: M2 macrophage-derived exosomes carry
microRNA-148a to alleviate myocardial ischemia/reperfusion injury
via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome
signaling pathway. J Mol Cell Cardiol. 142:65–79. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang B, Wang ZM, Ji JL, Gan W, Zhang A,
Shi HJ, Wang H, Lv L, Li Z, Tang T, et al: Macrophage-Derived
Exosomal Mir-155 Regulating cardiomyocyte pyroptosis and
hypertrophy in uremic cardiomyopathy. JACC Basic Transl Sci.
5:148–166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding X, Jing N, Shen A, Guo F, Song Y, Pan
M, Ma X, Zhao L, Zhang H, Wu L, et al: MiR-21-5p in
macrophage-derived extracellular vesicles affects podocyte
pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol
Invest. Sep 15–2020.(Epub ahead of print). View Article : Google Scholar
|
|
36
|
Vickers KC, Palmisano BT, Shoucri BM,
Shamburek RD and Remaley AT: MicroRNAs are transported in plasma
and delivered to recipient cells by high-density lipoproteins. Nat
Cell Biol. 13:423–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng F, Pan Y, Lu YM, Zhu L and Chen S:
RNA-binding protein dnd1 promotes breast cancer apoptosis by
stabilizing the Bim mRNA in a miR-221 binding site. Biomed Res Int.
2017:95961522017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rong J, Xu J, Liu Q, Xu J, Mou T, Zhang X,
Chi H and Zhou H: Anti-inflammatory effect of up-regulated
microRNA-221-3p on coronary heart disease via suppressing
NLRP3/ASC/pro-caspase-1 inflammasome pathway activation. Cell
Cycle. 19:1478–1491. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akkoc Y and Gozuacik D: MicroRNAs as major
regulators of the autophagy pathway. Biochim Biophys Acta Mol Cell
Res. 1867:1186622020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and its crosstalk with other
cellular pathways. Nat Rev Mol Cell Biol. 20:5–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee Y, Jeon K, Lee JT, Kim S and Kim VN:
MicroRNA maturation: Stepwise processing and subcellular
localization. EMBO J. 21:4663–4670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barrett SP and Salzman J: Circular RNAs:
Analysis, expression and potential functions. Development.
143:1838–1847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Santarpia L, Nicoloso M and Calin GA:
MicroRNAs: A complex regulatory network drives the acquisition of
malignant cell phenotype. Endocr Relat Cancer. 17:F51–F75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Negrini M, Nicoloso MS and Calin GA:
MicroRNAs and cancer-new paradigms in molecular oncology. Curr Opin
Cell Biol. 21:470–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li G, Shen F, Fan Z, Wang Y, Kong X, Yu D,
Zhi X, Lv G and Cao Y: Dynasore improves motor function recovery
via inhibition of neuronal apoptosis and astrocytic proliferation
after spinal cord injury in rats. Mol Neurobiol. 54:7471–7482.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang P, Hou H and Zhang L, Lan X, Mao Z,
Liu D, He C, Du H and Zhang L: Autophagy reduces neuronal damage
and promotes locomotor recovery via inhibition of apoptosis after
spinal cord injury in rats. Mol Neurobiol. 49:276–287. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bao Z, Fan L, Zhao L, Xu X, Liu Y, Chao H,
Liu N, You Y, Liu Y, Wang X and Ji J: Silencing of A20 aggravates
neuronal death and inflammation after traumatic brain injury: A
potential trigger of necroptosis. Front Mol Neurosci. 12:2222019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zychlinsky A, Prevost MC and Sansonetti
PJ: Shigella flexneri induces apoptosis in infected
macrophages. Nature. 358:167–169. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fink SL and Cookson BT:
Caspase-1-dependent pore formation during pyroptosis leads to
osmotic lysis of infected host macrophages. Cell Microbiol.
8:1812–1825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu F, Lan Z, Xin Z, He C, Guo Z, Xia X and
Hu T: Emerging insights into molecular mechanisms underlying
pyroptosis and functions of inflammasomes in diseases. J Cell
Physiol. 235:3207–3221. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Johnson DC, Taabazuing CY, Okondo MC, Chui
AJ, Rao SD, Brown FC, Reed C, Peguero E, de Stanchina E, Kentsis A
and Bachovchin DA: DPP8/DPP9 inhibitor-induced pyroptosis for
treatment of acute myeloid leukemia. Nat Med. 24:1151–1156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu C, Lu W, Zhang Y, Zhang G, Shi X,
Hisada Y, Grover SP, Zhang X, Li L, Xiang B, et al: Inflammasome
activation triggers blood clotting and host death through
pyroptosis. Immunity. 50:1401–1411.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bergsbaken T, Fink SL and Cookson BT:
Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol.
7:99–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Spel L and Martinon F: Inflammasomes
contributing to inflammation in arthritis. Immunol Rev. 294:48–62.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li
P, Hu L and Shao F: Inflammatory caspases are innate immune
receptors for intracellular LPS. Nature. 514:187–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Viganò E, Diamond CE, Spreafico R,
Balachander A, Sobota RM and Mortellaro A: Human caspase-4 and
caspase-5 regulate the one-step non-canonical inflammasome
activation in monocytes. Nat Commun. 6:87612015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kayagaki N, Warming S, Lamkanfi M, Vande
Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al:
Non-canonical inflammasome activation targets caspase-11. Nature.
479:117–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Broz P, Ruby T, Belhocine K, Bouley DM,
Kayagaki N, Dixit VM and Monack DM: Caspase-11 increases
susceptibility to Salmonella infection in the absence of caspase-1.
Nature. 490:288–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vigano E and Mortellaro A: Caspase-11: The
driving factor for noncanonical inflammasomes. Eur J Immunol.
43:2240–2245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bauernfeind F, Rieger A, Schildberg FA,
Knolle PA, Schmid-Burgk JL and Hornung V: NLRP3 inflammasome
activity is negatively controlled by miR-223. J Immunol.
189:4175–4181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Baek D, Villen J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Haneklaus M, Gerlic M, Kurowska-Stolarska
M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA and
Masters SL: Cutting edge: MiR-223 and EBV miR-BART15 regulate the
NLRP3 inflammasome and IL-1β production. J Immunol. 189:3795–3799.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen C, Zhou M, Ge Y and Wang X: SIRT1 and
aging related signaling pathways. Mech Ageing Dev. 187:1112152020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Khan RS, Fonseca-Kelly Z, Callinan C, Zuo
L, Sachdeva MM and Shindler KS: SIRT1 activating compounds reduce
oxidative stress and prevent cell death in neuronal cells. Front
Cell Neurosci. 6:632012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang Z, Sun L, Jia K, Wang H and Wang X:
MiR-9-5p modulates the progression of Parkinson's disease by
targeting SIRT1. Neurosci Lett. 701:226–233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting MicroRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lu D, Liu J, Jiao J, Long B, Li Q, Tan W
and Li P: Transcription factor Foxo3a prevents apoptosis by
regulating calcium through the apoptosis repressor with caspase
recruitment domain. J Biol Chem. 288:8491–8504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee S, Choi E, Cha MJ and Hwang KC:
Looking for pyroptosis-modulating miRNAs as a therapeutic target
for improving myocardium survival. Mediators Inflamm.
2015:2548712015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bergsbaken T and Cookson BT: Macrophage
activation redirects yersinia-infected host cell death from
apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog.
3:e1612007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jin X, Jin H, Shi Y, Guo Y and Zhang H:
Long Non-Coding RNA KCNQ1OT1 promotes cataractogenesis via miR-214
and activation of the caspase-1 pathway. Cell Physiol Biochem.
42:295–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mezzaroma E, Toldo S, Farkas D, Seropian
IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and
Abbate A: The inflammasome promotes adverse cardiac remodeling
following acute myocardial infarction in the mouse. Proc Natl Acad
Sci USA. 108:19725–19730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li A, Yu Y, Ding X, Qin Y, Jiang Y, Wang
X, Liu G, Chen X, Yue E, Sun X, et al: MiR-135b protects
cardiomyocytes from infarction through restraining the
NLRP3/caspase-1/IL-1β pathway. Int J Cardiol. 307:137–145. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X,
Che H, Han T, Meng S, Bai Y and Wang L: LncRNA KCNQ1OT1 mediates
pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem.
50:1230–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5:e14792014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jeyabal P, Thandavarayan RA, Joladarashi
D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R
and Krishnamurthy P: MicroRNA-9 inhibits hyperglycemia-induced
pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1.
Biochem Biophys Res Commun. 471:423–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hoseini Z, Sepahvand F, Rashidi B,
Sahebkar A, Masoudifar A and Mirzaei H: NLRP3 inflammasome: Its
regulation and involvement in atherosclerosis. J Cell Physiol.
233:2116–2132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xu YJ, Zheng L, Hu YW and Wang Q:
Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta.
476:28–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chang W, Lin J, Dong J and Li D:
Pyroptosis: An inflammatory cell death implicates in
atherosclerosis. Med Hypotheses. 81:484–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao Y, Peng J, Ren Z, He NY, Li Q, Zhao
XS, Wang MM, Wen HY, Tang ZH, Jiang ZS, et al: Functional
regulatory roles of microRNAs in atherosclerosis. Clin Chim Acta.
460:164–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li P, Zhong X, Li J, Liu H, Ma X, He R and
Zhao Y: MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated
endothelial cell pyroptosis through FOXO3 down-regulation in
atherosclerosis. Biochem Biophys Res Commun. 503:2833–2840. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Song Y, Yang L, Guo R, Lu N, Shi Y and
Wang X: Long noncoding RNA MALAT1 promotes high glucose-induced
human endothelial cells pyroptosis by affecting NLRP3 expression
through competitively binding miR-22. Biochem Biophys Res Commun.
509:359–366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Peng J, Tang ZH, Ren Z, He B, Zeng Y, Liu
LS, Wang Z, Wei DH, Zheng XL and Jiang ZS: TET2 protects against
oxLDL-induced HUVEC dysfunction by upregulating the
CSE/H2S system. Front Pharmacol. 8:4862017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhaolin Z, Jiaojiao C, Peng W, Yami L,
Tingting Z, Jun T, Shiyuan W, Jinyan X, Dangheng W, Zhisheng J and
Zuo W: OxLDL induces vascular endothelial cell pyroptosis through
miR-125a-5p/TET2 pathway. J Cell Physiol. 234:7475–7491.
2019.PubMed/NCBI
|
|
94
|
Tong R, Jia T, Shi R and Yan F: Inhibition
of microRNA-15 protects H9c2 cells against CVB3-induced myocardial
injury by targeting NLRX1 to regulate the NLRP3 inflammasome. Cell
Mol Biol Lett. 25:62020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liu X, Hong Q, Wang Z, Yu Y, Zou X and Xu
L: MiR-21 inhibits autophagy by targeting Rab11a in renal
ischemia/reperfusion. Exp Cell Res. 338:64–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ma N, Bai J, Zhang W, Luo H, Zhang X, Liu
D and Qiao C: Trimetazidine protects against cardiac
ischemia/reperfusion injury via effects on cardiac miRNA21
expression, Akt and the Bcl2/Bax pathway. Mol Med Rep.
14:4216–4222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
An P, Xie J, Qiu S, Liu Y, Wang J, Xiu X,
Li L and Tang M: Hispidulin exhibits neuroprotective activities
against cerebral ischemia reperfusion injury through suppressing
NLRP3-mediated pyroptosis. Life Sci. 232:1165992019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wu H, Huang T, Ying L, Han C, Li D, Xu Y,
Zhang M, Mou S and Dong Z: MiR-155 is involved in renal
ischemia-reperfusion injury via direct targeting of FoxO3a and
regulating renal tubular cell pyroptosis. Cell Physiol Biochem.
40:1692–1705. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lin J, Lin H, Ma C, Dong F, Hu Y and Li H:
MiR-149 aggravates pyroptosis in myocardial ischemia-reperfusion
damage via silencing FoxO3. Med Sci Monit. 25:8733–8743. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Corpas R, Revilla S, Ursulet S,
Castro-Freire M, Kaliman P, Petegnief V, Giménez-Llort L, Sarkis C,
Pallàs M and Sanfeliu C: SIRT1 overexpression in mouse hippocampus
induces cognitive enhancement through proteostatic and neurotrophic
mechanisms. Mol Neurobiol. 54:5604–5619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ran C, Wirdefeldt K, Brodin L, Ramezani M,
Westerlund M, Xiang F, Anvret A, Willows T, Sydow O, Johansson A,
et al: Genetic variations and mRNA expression of NRF2 in
Parkinson's disease. Parkinsons Dis. 2017:40201982017.PubMed/NCBI
|
|
102
|
Sharma S and Lu HC: MicroRNAs in
neurodegeneration: Current findings and potential impacts. J
Alzheimers Dis Parkinsonism. 8:4202018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zeng R, Luo DX, Li HP, Zhang QS, Lei SS
and Chen JH: MicroRNA-135b alleviates MPP+-mediated
Parkinson's disease in in vitro model through suppressing
FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci.
65:125–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fan Z, Lu M, Qiao C, Zhou Y, Ding JH and
Hu G: MicroRNA-7 enhances subventricular zone neurogenesis by
inhibiting nLRP3/Caspase-1 axis in adult neural stem cells. Mol
Neurobiol. 53:7057–7069. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cao B, Wang T, Qu Q, Kang T and Yang Q:
Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson's
disease via regulating miR-7/NLRP3 pathway. Neuroscience.
388:118–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jiang Z, Yao L, Ma H, Xu P, Li Z, Guo M,
Chen J, Bao H, Qiao S, Zhao Y, et al: MiRNA-214 inhibits cellular
proliferation and migration in glioma cells targeting caspase 1
involved in pyroptosis. Oncol Res. 25:1009–1019. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tian BG, Hua Z, Wang ZJ and Li J:
Knockdown of microRNA-181a inhibits osteosarcoma cells growth and
invasion through triggering NLRP3-dependent pyroptosis. Eur Rev Med
Pharmacol Sci. 24:1030–1040. 2020.PubMed/NCBI
|
|
108
|
Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang
Z, Li Y, Yang G, Zhou D, Yang H, et al: MiR-21 promotes NLRP3
inflammasome activation to mediate pyroptosis and endotoxic shock.
Cell Death Dis. 10:4612019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ying Y, Mao Y and Yao M: NLRP3
inflammasome activation by MicroRNA-495 promoter methylation may
contribute to the progression of acute lung injury. Mol Ther
Nucleic Acids. 18:801–814. 2019. View Article : Google Scholar : PubMed/NCBI
|















