Introduction
Adjuvant radiotherapy is an effective treatment for
thoracic malignancies and has resulted in a significant improvement
in the chances of cancer patient's survival over the past decades
(1–4). However, while high-energy radiotherapy
treatment successfully kills cancer cells, unavoidable radiation
exposure to the heart and large arteries occurs during treatment,
resulting in radiation-induced secondary cardiovascular disease
(CVD) in cancer survivors (5–16).
Indeed, epidemiological, clinical and experimental data have
established the link between radiation exposure at high and medium
doses that are received during radiotherapy (>0.5 Gy) and the
risk for CVD (6,9,10,17–20).
In addition, recent data suggest that radiation-induced CVD occurs
at much lower doses than previously thought (<0.5 Gy) (7,10,19,21–24).
However, the underlying cellular and molecular mechanisms of
radiation-induced CVD are not fully understood, possibly resulting
in improper radiation protection. Most of the clinical and
experimental data focus on identifying the late cardiovascular
response after radiation exposure, although physiological changes
and cellular and molecular damage in the cardiovascular system may
happen directly after radiation exposure (7,18,25–32).
Moreover, there are currently no validated biomarkers that predict
the risk to develop CVD after radiotherapy. Therefore, experimental
animal studies of acute and early cardiovascular response after
thoracic irradiation may improve our knowledge on early
asymptomatic changes in the cardiovascular system after ionizing
exposure. This may help in identifying biomarkers indicating
developing cardiovascular complications after radiotherapy, which
may help in screening patients at risk for developing CVD, thus
countermeasures and early medical intervention might be applied to
prevent further cardiac toxicity.
Atherosclerosis, a progressive inflammatory disease
of the arterial wall, is the main underlying cause of major CVD.
Multiple inflammatory markers have been correlated with the
pathogenesis of atherosclerosis (33–36).
Radiation exposure is also known to trigger the release of several
inflammatory and adhesion molecules, involved in the pathogenesis
of atherosclerosis (18,26,37–42).
Here, we explored a large panel of inflammatory markers in the
serum of atherosclerosis-prone ApoE−/− mice
at 24 h (acute response) and 1 month (early term response) of
thoracic X-ray irradiation. The inflammatory markers were further
verified in vitro in coronary artery and microvascular
endothelial cell lines exposed to low and high dose X-rays. Serum
triglyceride and cholesterol levels, which are known to be strongly
associated with atherogenesis (43,44),
were also evaluated at 24 h and 1 month post-irradiation. We
identified two inflammatory markers, growth differentiation
factor-15 (GDF-15) and C-X-C motif chemokine ligand 10 (CXCL10),
which were significantly elevated 24 h after irradiation in both
female and male mice, which were found to be also increased in
irradiated coronary artery and microvascular endothelial cells.
Interestingly, we observed gender-specific responses in
triglyceride and cholesterol levels 1 month post-irradiation, and
in the assessed inflammatory markers at 24 h and 1 month
post-irradiation.
Materials and methods
Animals
Animal experiments were approved by the Ethical
Committee Animal Studies of the Faculty of Medicine and Health
Sciences, Ghent University (ECD 17/60), and were performed in
compliance with the Belgian laboratory animal legislation and the
European Communities Council Directive of 22 September 2010
(2010/63/EU). Apolipoprotein E-deficient
ApoE−/−) mice were purchased from Charles
River Laboratories, and animals were housed at the animal facility
of UGent. Pups were weaned at the age of three weeks and housed in
temperature-controlled, individually ventilated cages, with a 12 h
light-dark cycle. They received a standardized mouse chow diet
(3.7% fat) and water ad libitum. At the age of 10–12 weeks,
ApoE−/− mice were randomly allocated to
receive irradiation or sham treatment. In total 72 female and male
ApoE−/− mice were used (6 mice per group,
except for the control group at 1 month, which were 3 for male and
9 for female mice).
Mouse irradiation
The Small Animal Radiation Research Platform (SARRP;
Xstrahl®; in collaboration with INFINITY lab, UGent) was
used to irradiate ApoE−/− mice. Mice were
anesthetized with isoflurane (5% induction and 2% maintenance) and
subjected to a full body CT-scan (50 kV, 1.5 mA, 360 projections
>360°, 1 mm aluminium filter) prior to irradiation. CT images
were analyzed by using Muriplan software (Xstrahl®) to
determine the coordinates of the isocenter for subsequent
irradiation. Thoracic irradiation was performed in a ventro-dorsal
direction, with 220-kV X-rays, operating at 13 mA and filtered with
0.15 mm of copper, operating with a dose rate of 3.4 Gy/min. The
field size, created by the collimator of 10×10 mm, was encompassing
both carotid arteries, the aortic arch and apical portion of the
heart. The doses that were delivered to the heart region are 0 Gy
(sham treatment; also referred to as ‘controls’), 0.1 and 10
Gy.
Cell culture
Telomerase Immortalized human Microvascular
Endothelial cells (TIME) from the American Type Cell Culture (ATCC,
France; ATCC® CRL-4025™) (https://www.lgcstandards-atcc.org/Products/All/CRL-4025)
was used. In addition, TICAE cells, which are primary human
coronary artery endothelial cells from the European Collection of
Authenticated Cell Cultures (ECACC; HCAECs cat. no. 300-05a) that
were transduced with retroviruses bearing the est2 gene, a yeast
homologue of the human TERT protein (45,46),
were used. TIME and TICAE cells are from a male human donor.
MesoEndo Cell Growth Medium (Sigma-Aldrich; Merck KGaA) was used
for TIME and TICAE cells. The passage number that was used in the
experiments is 38 for TIME cells and 33 for TICAE cells. Cells were
grown at 37°C in a humidified incubator supplemented with 5%
CO2 and were split with a 0.05% trypsin supplemented
with 0.02% ethylenediaminetetraacetic acid (EDTA) every 3–4 days.
Moxi Z Mini Automated Cell Counter (Orflo Technologies) was used to
count the cells. For the cytokine detection experiment, TIME and
TICAE cells were seeded in 6-well plate at a density of
2.5×105 per well in 6 biological replicates. Three days
later, cells reached 100% confluence. The medium was changed before
irradiation, and at 24 and 72 h after irradiation the supernatant
was collected.
Cell irradiation
Both TIME and TICAE cells were irradiated at 100%
confluence. Single X-rays doses [0 Gy (also referred to as
‘control’), 0.1 and 5 Gy] were applied to the cells using a
vertical X-ray beam using a Xstrahl RX generator (320 kV,
Filtration: 3.8 mm Al and 1 mm Cu, tube current: 12 mA)
(Camberley), at a dose rate of 0.5 Gy/min. X-irradiation was
performed in accordance to ISO 4037 and under ISO 17025
accreditation of the Laboratory for Nuclear Calibrations (LNK) of
the Belgian Nuclear Research Centre (SCK•CEN). Cells were moved to
the irradiation facility using a mobile incubator.
Blood sampling
The blood collection from each mouse was done at two
different time points: 1) One week before irradiation and 2) a
specific time period after radiation exposure (24 h or 1 month).
The mice were anesthetized intraperitoneally with xylazine (10
mg/kg) and ketamine (80 mg/kg) using a 30G needle, and anesthetic
depth was assessed using the pedal withdrawal reflex. Retro-orbital
puncture was performed to take a 100 µl blood sample with a Brand
micropipette one week before irradiation, and at the specific time
points after irradiation, where the eye was pulled out to collect
at least 500 µl blood. Immediately after the second blood sampling,
anesthetized mice were euthanized due to exsanguination via
transcardial perfusion. To obtain serum, the collected blood was
allowed to clot in eppendorf tubes for 1.5 h at room temperature
and was subsequently centrifuged at 1,500 g for 15 min at 4°C. The
supernatant was removed and centrifuged again at the same
conditions to obtain pure serum. The latter was collected in
eppendorf tubes and stored at −80°C.
Triglyceride quantification
The Triglyceride Quantification assay kit (Abcam,
ab65336) was used according to the manufacturer's instructions.
Briefly, a 50 µl triglyceride standard curve was prepared and 1 µl
serum was added to 49 µl assay buffer and all samples were
performed in duplicate. Subsequently, 2 µl of lipase, which
converts the triglycerides to free fatty acids and glycerol, was
added to each well and incubated for 20 min at room temperature.
Then, 50 µl reaction mix consisting of 46 µl triglyceride assay
buffer, 2 µl triglyceride probe and 2 µl triglyceride enzyme mix,
was added to each well and incubated at room temperature for 60 min
protected from light. The glycerol molecules are oxidized to
generate hydrogen peroxide (H2O2), which
reacts with the probe to generate fluorescence that was measured at
Ex/Em 535/587 nm with a microplate reader (Victor3, 1420 multilabel
counter, PerkinElmer).
Cholesterol quantification
The Cholesterol Assay kit (Abcam, ab65390) was used
according to the manufacturer's instructions. A 50 µl cholesterol
standard curve was prepared. The serum samples were diluted 1/400
in assay buffer and 50 µl of this diluted serum was used. Then, 50
µl of a cholesterol reaction mix, consisting of 44 µl assay buffer,
2 µl cholesterol probe, 2 µl enzyme mix, and 2 µl cholesterol
esterase was added to each well for an incubation period of 60 min
at 37°C. The cholesterol esterase hydrolyzes cholesteryl esters to
free cholesterol. Subsequently, cholesterol oxidase specifically
recognizes free cholesterol and produce H2O2
that reacts with the probe to generate fluorescence (Ex/Em=538/587
nm) that was measured with a microplate reader (Victor3, 1420
multilabel counter, PerkinElmer).
Inflammatory cytokine detection
Simultaneous detection of multiple inflammatory
markers in mice serum samples (Adiponectin, CRP, CXCL10, Endoglin,
FGF-basic, GDF-15, ICAM-1, IL-6, IL-1β, MCP-1, P-selectin, PAI-1,
PCSK9, and uPAR) or in cell supernatant samples (GDF-15, CXCL10,
ICAM-1, MCP-1, uPAR, PAI-1, P-selectin, FGF-basic and IL-6) was
performed using multiplex bead assay (Luminex® MAGPIX
Assay, R&D Systems) following manufacturer's instructions.
Luminex® MAGPIX technology is based on the use of a
mixture of magnetic microspheres that is added to the sample in a
pre-coated plate with analyte-specific capture antibodies, followed
by adding specific biotinylated detection antibodies and
phycoerythrin that binds to the biotinylated antibodies. Magnetic
beads are captured and held in a monolayer by a magnet, while the
beads are illuminated by two spectrally distinct light-emitting
diodes (LEDs), one to identify the analyte and the other to
determine amount of analyte based on the magnitude of the
phycoerythrin-derived signal. Signals are captured and imaged with
a charge-coupled device (CDD) camera (47–50).
Briefly, standards, serum or supernatant samples, and magnetic
microparticles cocktail were incubated into cytokine-specific
antibodies in a pre-coated 96-well plate. After 2-h incubation in
the dark, the plate was washed, and a biotinylated detection
antibody cocktail was added. After applying a second wash to remove
the unbound biotinylated antibodies, a streptavidin-phycoerythrin
conjugate was added to each well to bind to the biotinylated
antibodies. Further, a final wash was performed and the
microparticles were detected using the Luminex® MAGPIX
Analyzer. Inflammatory markers concentration in the supernatant of
TIME and TICAE cells was normalized to cell count performed using
IncuCyte ZOOM™ phase contrast imaging with ×10 magnification.
Statistical analysis
Analysis of cholesterol, triglyceride and
supernatant inflammatory cytokine data were performed using
Kruskal-Wallis test followed by Dunn's test, and the P-value was
adjusted using Benjamini-Hochberg procedure to control the False
Discovery Rate. Data are presented as mean ± standard error of the
mean. GraphPad Prism 5.01 (GraphPad Software Inc.) was used for
these statistical analysis. For in vivo serum inflammatory
cytokines, the fold change in inflammatory marker levels before and
after irradiation was first calculated, followed by statistical
analysis. Statistical analysis was done with a Kruskal-Wallis test
followed by Dunn's test and the P-value was adjusted using
Benjamini-Hochberg procedure, using ggsignif R package. The
results are considered significant if the P-value is <0.05. Data
are presented as boxplot, ggplot2 R package, showing the
median with the lower and upper hinges that correspond to the first
and third quartiles (the 25 and 75th percentiles) and upper/lower
whisker extends from the hinges to the largest/smallest value no
further than 1.5 * IQR from the corresponding upper and lower
hinge, respectively. Data beyond the end of the whiskers are called
‘outlying’ points and are plotted individually. In vivo data
are represented separately in female ApoE−/−
mice and male ApoE−/− mice, or as the sum of
female and male data, which are referred to as combined data.
Results
Thoracic irradiation induces early term
alterations in triglyceride and cholesterol levels in
ApoE−/− mice
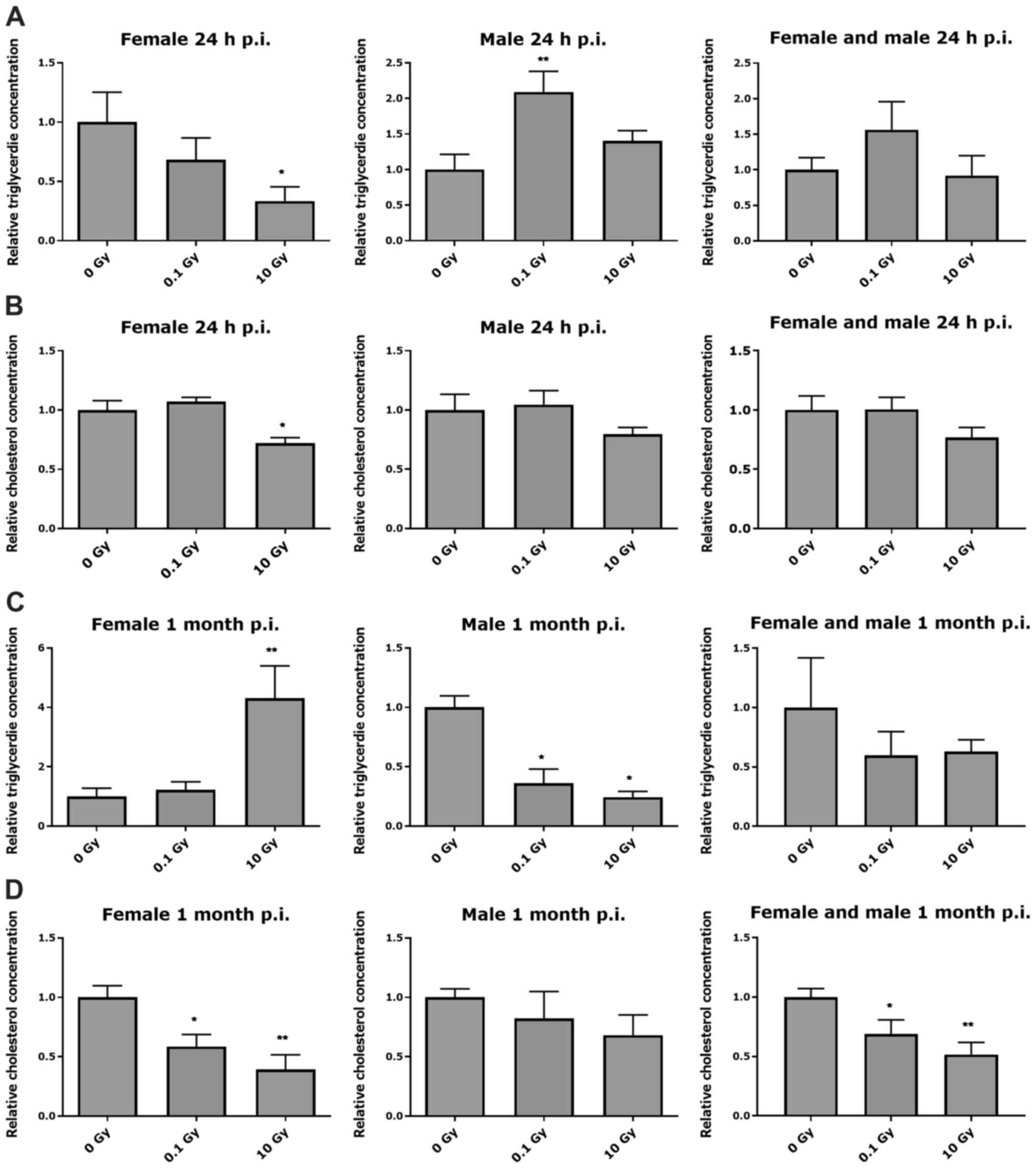
At 24 h post-thoracic irradiation
Thoracic irradiation of female
ApoE−/− mice with 10 Gy induced a significant
decrease in triglyceride and cholesterol levels at 24 h after
irradiation, compared to age-matched controls (Fig. 1A and B), (summarized in Table SI). In male
ApoE−/− mice, thoracic irradiation induced a
significant increase in triglyceride level only at 0.1 Gy, but no
significant changes in cholesterol level was observed at 24 h after
irradiation (Fig. 1A and B). Female
and male ApoE−/− mice combined data showed no
significant changes in triglyceride and cholesterol levels at 24 h
after irradiation (Fig. 1A and
B).
At 1 month post thoracic
irradiation
At 1 month after irradiation, a significant increase
in triglyceride levels in female ApoE−/− mice
was observed at 10 Gy, whereas total cholesterol levels were
significantly decreased at 0.1 and 10 Gy, compared to age-matched
controls (Fig. 1C and D). In male
ApoE−/− mice, a significant decrease in
triglyceride level was observed in a dose-dependent manner at 1
month after irradiation, but no significant changes in cholesterol
level was observed (Fig. 1C and D).
Female and male ApoE−/− mice combined data
showed no significant changes in triglyceride levels, while a
significant dose-dependent decrease in cholesterol level at 1 month
after irradiation was observed (Fig. 1C
and D).
Thoracic irradiation induces acute and
early term systemic inflammatory response in
ApoE−/− mice
To assess the inflammatory response, fourteen
different cytokines, which are involved in the pathogenesis of
atherosclerosis (Adiponectin, CRP, CXCL10, Endoglin, FGF-basic,
GDF-15, IGMA-1, IL-6, IL-1β, MCP-1, P-selectin, PAI-1, PCSK9, and
uPAR), have been assessed in serum samples of female and male
ApoE−/− mice at 24 h and 1 month after local
thoracic X-ray irradiation. Fold changes between the baseline
cytokines levels and at 24 h or at 1 month post irradiation
cytokine levels were analyzed (summarized in Table SII).
At 24 h post thoracic irradiation
At 24 h post thoracic irradiation, a significant
increase in GDF-15 at 0.1 and 10 Gy in female
ApoE−/− mice and a significant increase at 10
Gy in male ApoE−/− mice were observed
(Fig. 2A). Female and male
ApoE−/− mice combined data showed a
significant increase in GDF-15 at 10 Gy. A significant increase in
CXCL10 was also observed at 10 Gy in female and male
ApoE−/− mice, as well as in the combined
female and male data (Fig. 2B). In
addition, a significant increase in ICAM-1 was observed at 10 Gy
only in male ApoE−/− mice (Fig. 2C). Whereas, thoracic irradiation
induced a significant decrease in MCP-1 at 0.1 and 10 Gy only in
female ApoE−/− mice (Fig. 2D). Moreover, a significant decrease
in female uPAR at 10 Gy was observed, and female and male uPAR
combined data showed a significant decrease at 0.1 and 10 Gy
(Fig. 2E). Next to that, in male
ApoE−/− mice, there was a significant
increase in PCSK9 at 0.1 Gy (Fig.
2F), and an increase in P-selectin at 10 Gy (Fig. 2G). Nonetheless, no significant
changes were observed in adiponectin, PAI-1, CRP, endoglin,
FGF-basic, IL-1β and IL-6 at 24 h post thoracic irradiation
(Fig. S1).
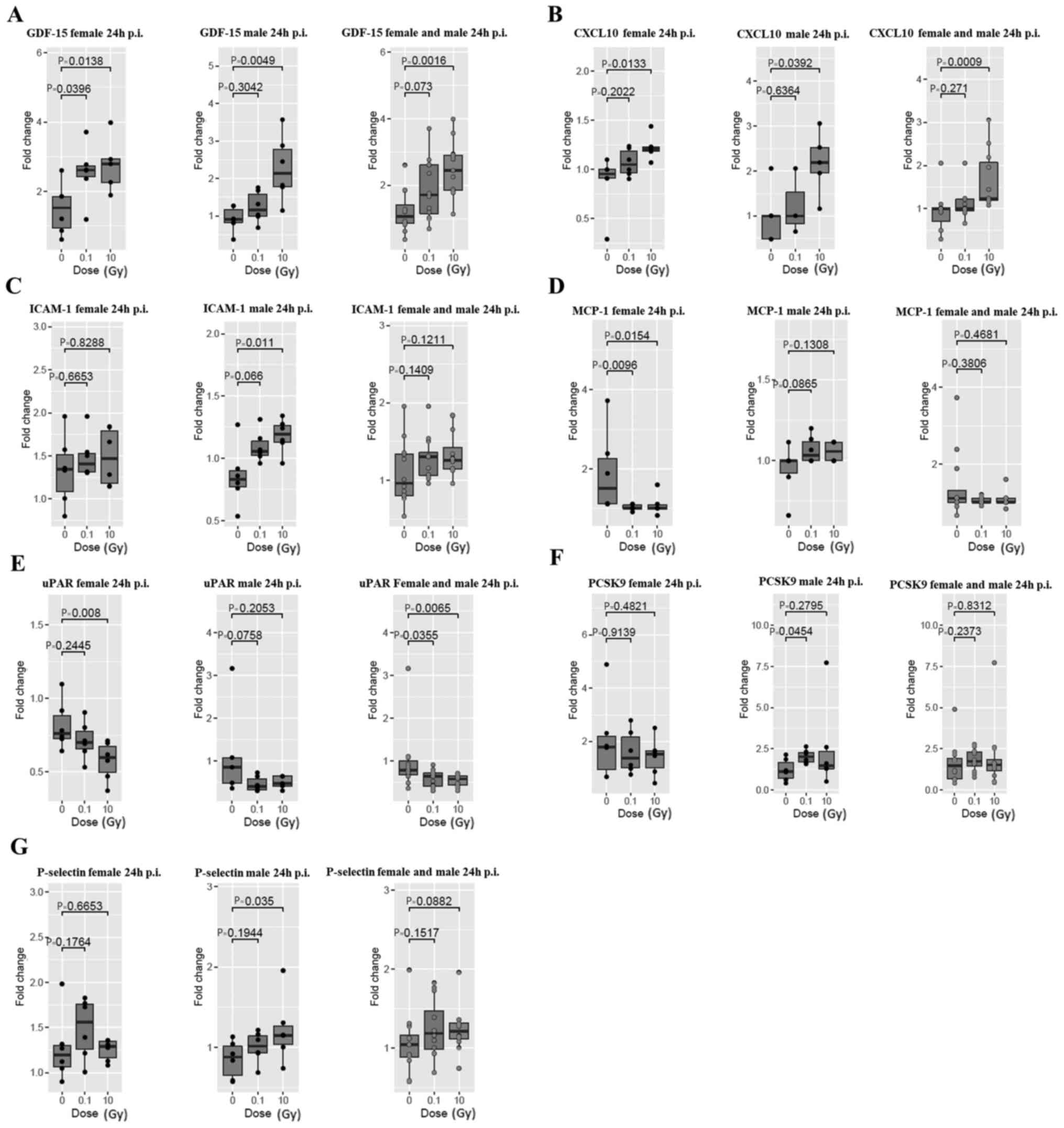 | Figure 2.Systemic inflammatory response in
female (left panels), male (middle panels) and combined female and
male ApoE−/− mice (right panels) at 24 h post
thoracic irradiation. (A) GDF-15, (B) CXCL10, (C) ICAM-1, (D)
MCP-1, (E) uPAR, (F) PCSK9 and (G) P-selectin cytokines were
analysed. The remaining are listed in Fig. S1. Data are presented as the fold
change of the post-irradiation cytokine level relative to the
baseline level. Statistical analysis was performed using a
Kruskal-Wallis test, with the P-value being adjusted using the
Benjamini-Hochberg method. Data are presented as a boxplot showing
the median value of 6 mice per group. p.i., post irradiation;
GDF-15, growth differentiation factor-15; CXCL10, C-X-C motif
chemokine ligand 10; ICAM-1, intercellular adhesion molecule-1;
MCP-1, monocyte chemoattractant protein-1; uPAR, urokinase-type
plasminogen activator receptor; PCSK9, proprotein convertase
subtilisin/kexin type 9. |
At 1 month post thoracic
irradiation
The systemic inflammatory response at 1 month was
different from the acute response at 24 h post thoracic
irradiation. Adiponectin was significantly increased at 10 Gy
post-irradiation only in male ApoE−/− mice
(Fig. 3A). CXCL10 and IL-6 were
significantly decreased at 0.1 Gy in female
ApoE−/− mice, and in combined female and male
data (Fig. 3B and E). FGF-basic
level was significantly decreased at 10 Gy in female mice, and in
the female and male mice combined data (Fig. 3C). Furthermore, PAI-1 showed a
significant increase at 10 Gy in male ApoE−/−
mice (Fig. 3D). No significant
changes were observed in Endoglin, P-selectin, GDF-15, IL-1β,
ICAM-1, MCP-1, PCSK-9, and uPAR at 1 month post thoracic
irradiation (Fig. S2).
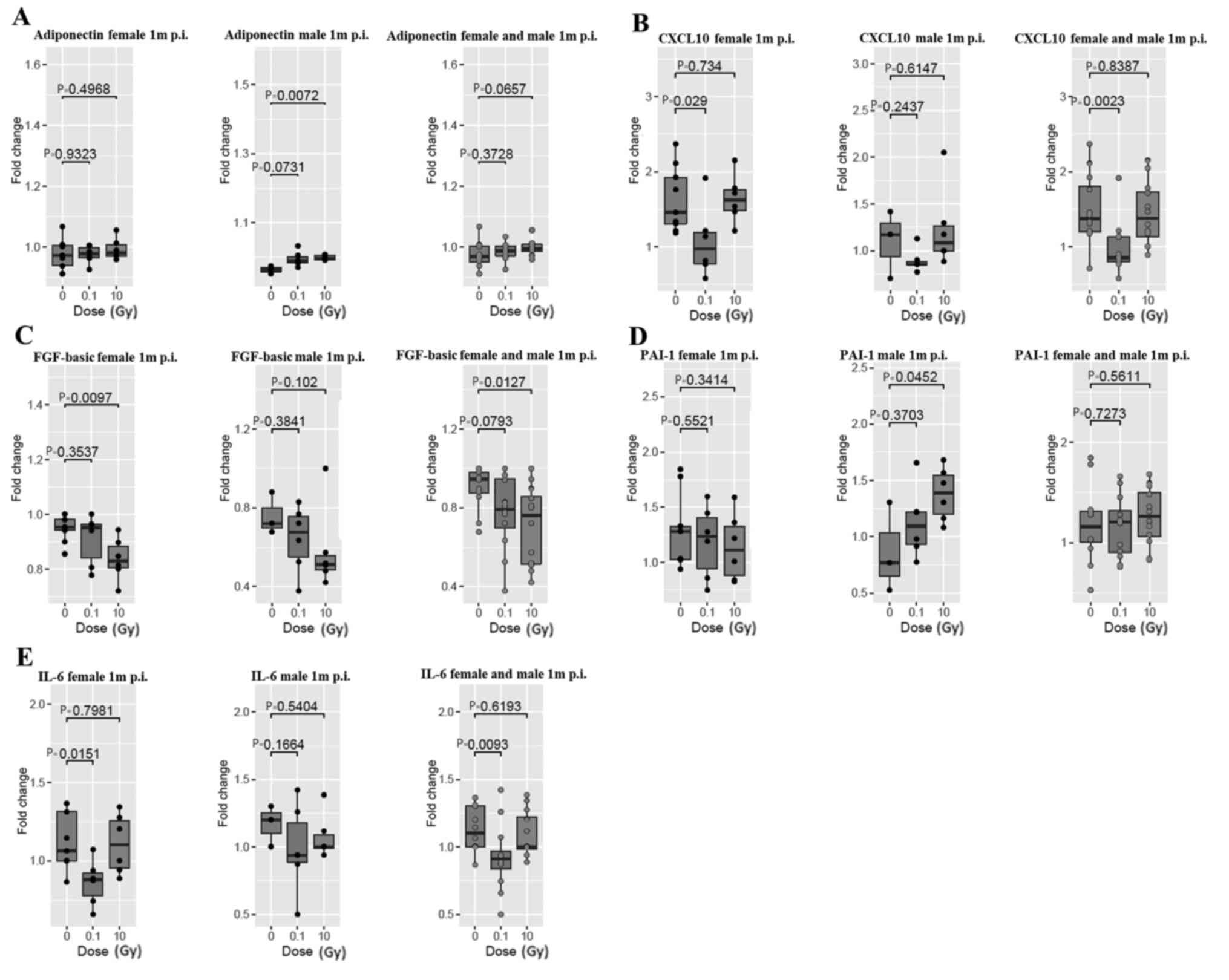 | Figure 3.Systemic inflammatory response in
female (left panels), male (middle panels) and combined female and
male ApoE−/− mice (right panels) at 1 month
post thoracic irradiation. (A) Adiponectin, (B) CXCL10, (C)
FGF-basic, (D) PAI-1 and (E) IL-6 cytokines were assessed. The
remaining are listed in Fig. S2.
Data are presented as the fold change of the post-irradiation
cytokine level relative to the baseline inflammatory cytokine
level. Data are considered significant when P<0.05. Data are
presented as boxplots showing the median of 6 mice per group,
except for the male 0 Gy group, which had 3 mice. p.i., post
irradiation; m, month; CXCL10, C-X-C motif chemokine ligand 10;
FGF-basic, basic fibroblast growth factor; PAI-1, plasminogen
activator inhibitor-1. |
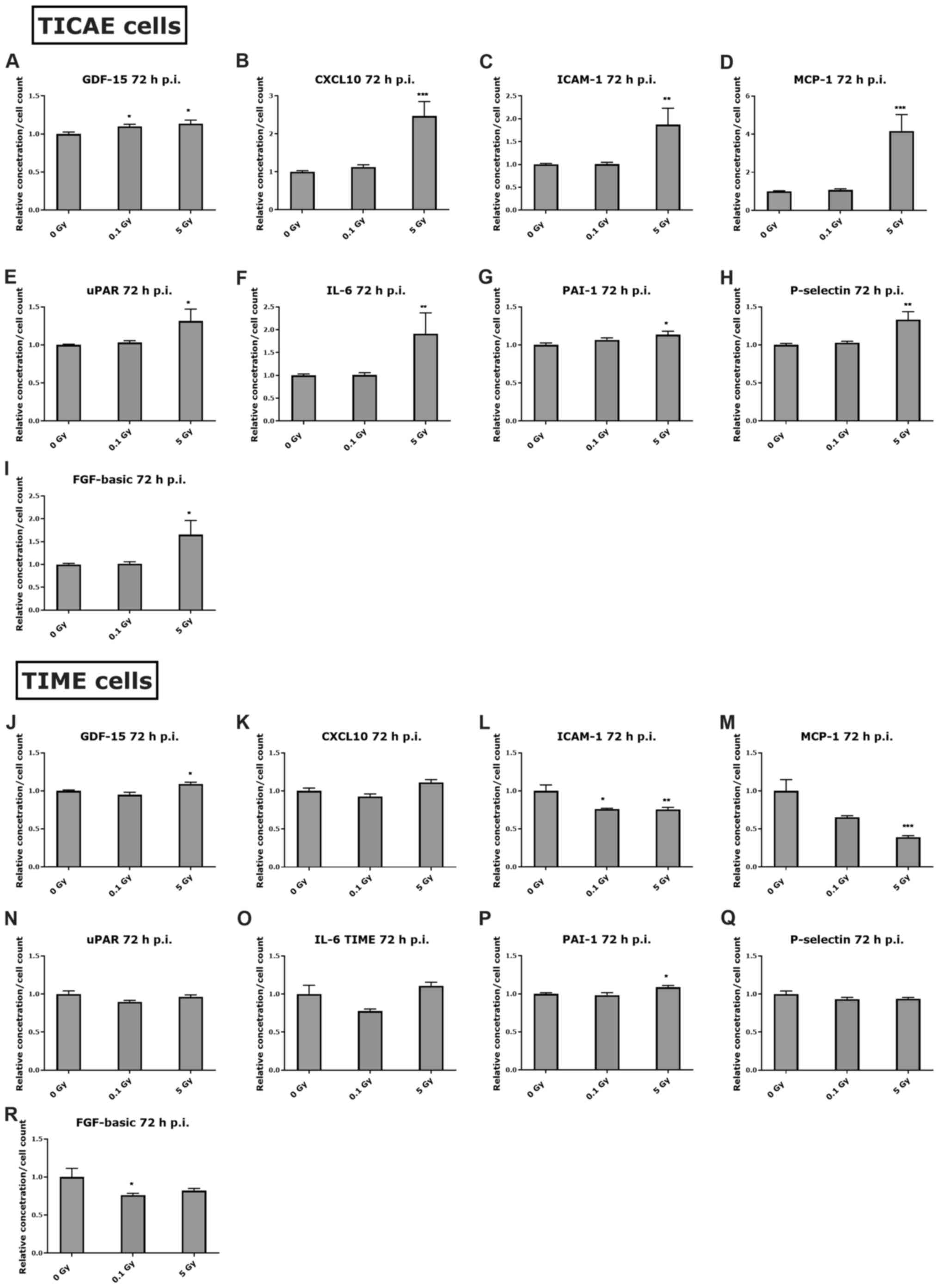
X-irradiation induces inflammatory
response in coronary artery and microvascular endothelial
cells
To verify whether the observed inflammatory
responses in serum cytokines at 24 h and 1 month after irradiation
is linked to endothelial cell responses, two endothelial cell lines
(TICAE and TIME cells, originating from a male donor) were
irradiated at low dose (0.1 Gy) or high dose (5 Gy), with
supernatant being collected at 24 h or 72 h after irradiation. The
72 h time point was chosen since it is not possible to keep cells
in culture for 1 month post-irradiation without passaging them. In
addition, the high dose was chosen to be 5 Gy in vitro,
since we previously reported that 5 Gy induced significant
apoptotic cell death from 4 h after X-irradiation in TICAE and TIME
cells, which increased with time (51), and also considering that we are
irradiating a monolayer of endothelial cells (not tissue like the
in vivo situation). The cytokines tested were GDF-15,
CXCL10, ICAM-1, MCP-1, uPAR, IL-6, PAI-1, P-selectin, and FGF-basic
(summarized in Table SIII).
At 24 h post endothelial
irradiation
At 24 h after irradiation, GDF-15 and CXCL10
increased significantly at 5 Gy in TICAE and TIME cells (Fig. 4A, B, J and K) which correspond to
the increase in these markers in female and male mice at 24 h post
irradiation (Fig. 2A and B). A
significant increase in ICAM-1 was observed at 5 Gy in TIME cells
(Fig. 4L), which may correlate with
the increase in serum ICAM-1 in male mice at 10 Gy for the 24 h
time point (Fig. 2C). Additionally,
MCP-1 level was not changed in response to radiation in TICAE and
TIME cells at 24 h post irradiation (Fig. 4D and M), and this was the case in
the irradiated male mice at the same time point (Fig. 2D). Moreover, a significant increase
in P-selectin was observed at 5 Gy in TICAE and TIME cells at 24 h
post irradiation (Fig. 4H and Q),
which corresponds to the observed trend of increase in male mice
data (Fig. 2G). The levels of uPAR,
IL-6 and PAI-1 were significantly increased in TICAE and TIME
cells, and FGF-basic increased in TICAE cells at 5 Gy after 24 h of
irradiation (Fig. 4E-G, I, N, O and
P), while this was not the case in the irradiated female and
male mice at the same time point.
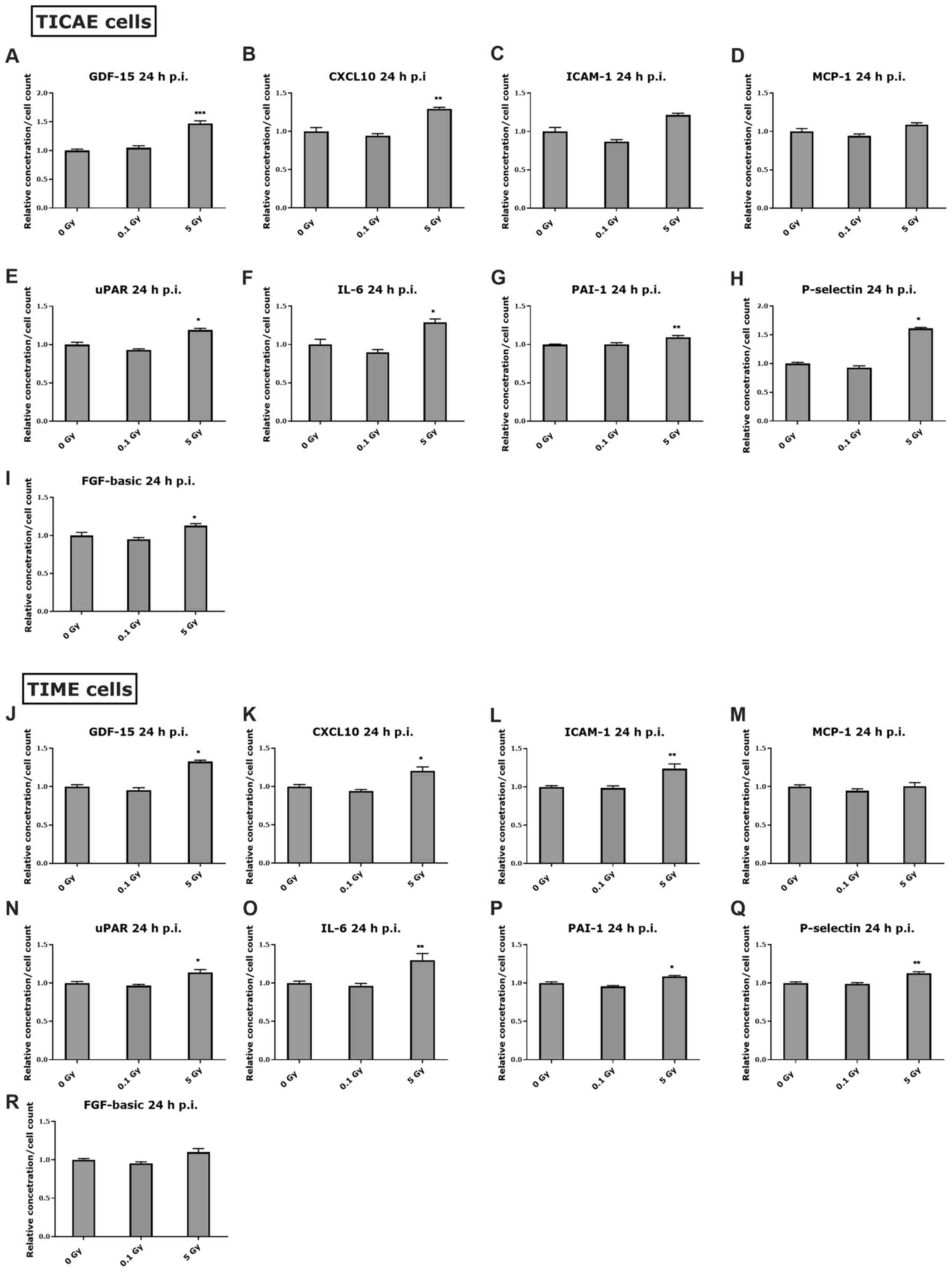 | Figure 4.Inflammatory response in endothelial
cells at 24 h after irradiation. The response of various
inflammatory markers at 24 h after 0.1 and 5 Gy irradiation is
represented in (A-I) TICAE cells and (J-R) TIME cells, normalized
to cell count. The Kruskal-Wallis test was used to analyse the data
and the P-value was adjusted using the Benjamini-Hochberg method.
Values represent the average ± SEM of 6 biological replicates.
*P<0.05, **P<0.01 and ***P<0.001 vs. 0 Gy. p.i., post
irradiation; GDF-15, growth differentiation factor-15; CXCL10,
C-X-C motif chemokine ligand 10; ICAM-1, intercellular adhesion
molecule-1; MCP-1, monocyte chemoattractant protein-1; uPAR,
urokinase-type plasminogen activator receptor; PAI-1, plasminogen
activator inhibitor-1; FGF-basic, basic fibroblast growth
factor. |
At 72 h post endothelial
irradiation
At 72 h after irradiation, GDF-15 was still
significantly elevated in TICAE and TIME cells at 5 Gy (Fig. 5A and J). CXCL10, ICAM-1, MCP-1,
uPAR, IL-6 and FGF-basic were also significantly elevated at 5 Gy
in the irradiated TICAE cells (Fig.
5B-E, F and I), while ICAM-1 and MCP-1 significantly decreased
in a dose-dependent manner in the irradiated TIME cells at 72 h
post irradiation (Fig. 5L and M).
Moreover, there was a significant increase in P-selectin at 5 Gy in
TICAE cells, and significant increase in PAI-1 at 5 Gy in TICAE and
TIME cells (Fig. 5G, H and P).
Discussion
Thoracic cancer radiotherapy significantly increases
the risk for developing CVD (6,9,10,52,53).
The underlying pathophysiology is complex and the mechanisms are
not fully understood (6,7,9,17–21).
Previous in vivo studies have identified late cardiovascular
responses to ionizing radiation exposure, which was mainly
manifested by pro-inflammatory responses and accelerated formation
of atherosclerotic lesions/plaques 2–15 months after irradiation
(17,18,26–29,54).
Most of these pre-clinical studies were performed in a single
gender, with limited data available related to possible gender
differences. Here, we investigated acute (24 h) and early term (1
month) cardiovascular responses to ionizing radiation exposure, by
assessing triglyceride and total cholesterol levels, and exploring
a large panel of inflammatory markers in serum of female and male
ApoE−/− mice that received low or high doses
of local thoracic X-ray irradiation. We report, for the first time,
that local thoracic irradiation of ApoE−/−
mice increases serum GDF-15 and CXCL10 in both female and male mice
24 h after high dose irradiation (10 Gy). GDF-15 and CXCL10 levels
were also significantly elevated at 24 h in irradiated coronary
artery and microvascular endothelial (TICAE and TIME) cells in
vitro after high dose of X-ray irradiation. In addition, we
report gender-specific responses in the tested triglyceride and
cholesterol levels at 1 month after irradiation, and in the
assessed inflammatory markers at 24 h and 1 month post-irradiation.
Below we discuss these findings in more detail.
Increased cholesterol and triglyceride levels in
atherosclerosis have been shown in several clinical studies
(55), and it was suggested that
triglyceride level can be used as independent risk factor biomarker
for coronary artery disease (56).
Increased cholesterol and triglyceride levels may contribute to a
large number of biological actions and consequences, including
inflicting endothelial cell injury, increasing adhesion molecule
expression, increasing leukocytes recruitment, as well as the
formation of foam cells, therefore contributing to the
atherosclerotic process (43,44).
We observed different responses in cholesterol and triglyceride
levels in the irradiated female and male
ApoE−/− mice, especially at 1 month after
exposure. Although triglyceride levels were increased in 10
Gy-irradiated female mice, levels dose-dependently decreased in
male mice, demonstrating that triglyceride levels display gender
specific responses. In contrast, cholesterol levels
dose-dependently decreased in female mice, and showed a decreasing
trend in male mice, resulting in a significant dose-dependent
decrease when combining female and male responses 1 month
post-irradiation. A similar observation for the decrease in total
cholesterol and the increase in triglyceride levels in female mice
was reported in female breast cancer patients after 50–60 Gy of
radiotherapy which were given over 5 weeks (57). Previous studies performed 2–5 months
after irradiation of ApoE−/− mice, revealed
variable responses in cholesterol and triglyceride levels depending
mainly on the time point after exposure, gender used in the
experiment, and on radiation doses/quality used (17,18,27,29).
Cholesterol and triglyceride changes can perhaps be secondary to
the inflammatory or oxidative stress responses after radiation
exposure, though further investigations are needed to unveil the
involved mechanisms.
Our results further demonstrate that local thoracic
irradiation induced an increase in serum GDF-15, which was
significant at low and high doses (0.1 and 10 Gy) in female mice
and at high dose in male mice 24 h after irradiation. In
vitro, GDF-15 was secreted from irradiated TICAE and TIME cells
at 24 h after high dose (5 Gy) irradiation. An increased GDF-15
gene expression was previously observed in human aortic endothelial
cells at 4–24 h after 4 Gy (58),
and in carotid arteries of male ApoE−/− mice
at 1 week after 14 Gy of local neck X-ray irradiation (59). GDF-15 is a member of the
transforming growth factor β superfamily that increases its
expression under inflammatory conditions (60,61).
Elevated GDF-15 serum levels have been associated with an increased
risk for a range of CVD, including atherosclerosis, and currently
being evaluated as a biomarker in CVD (62–65).
Previous studies revealed that GDF-15 may contribute to the
initiation and the progression of atherosclerotic lesions by
regulating apoptosis and IL-6-dependent inflammatory responses
(66), promoting migration of
macrophage, and by contributing to plaque instability (67). In line with this, our in
vitro data showed an increased IL-6 level in TICAE and TIME
cells 24 h after 5 Gy irradiation. GDF-15 may play an important
role in ionizing radiation-induced endothelial cell senescence
through an oxidative stress-mediated p16 pathway (58). Another inflammatory marker of
potential interest is CXCL10, also known as Interferon-γ-inducible
protein 10 (IP-10), which acts as a chemoattractant cytokine.
CXCL10 was shown to promote atherosclerosis by recruitment and
retention of activated T lymphocytes to vascular wall lesions
during the atherogenesis process (68,69),
and also is being investigated as a potential biomarker for CVD
(70–73). In our study, the serum level of
CXCL10 was significantly elevated in female and male
ApoE−/− mice 24 h after high dose thoracic
irradiation, and was found to be secreted from both TICAE and TIME
cells 24 h post-irradiation. In line with our in vitro data,
CXCL10 gene expression was previously reported to be upregulated in
human coronary artery endothelial cells after 10 Gy of fractionated
X-irradiation (74), and in human
umbilical vascular endothelial cells following exposure to 20 Gy of
acute gamma irradiation (75).
One of the earliest responses to endothelial cell
injury, which is considered one potential initiating event of the
atherosclerotic process, is upregulation of adhesion molecules
including ICAM-1 and P-selectin, leading to leukocyte adherence to
the endothelium (30,76,77).
Multiple in vitro and in vivo studies have reported
post-irradiation increases in ICAM-1 and P-selectin levels, thereby
promoting leucocyte adhesion to the endothelium (26,78–83).
However, most of the in vivo studies were performed in
endothelial cells of irradiated arteries weeks to months after
radiation exposure. Here, we report an acute dose-dependent
increase in serum ICAM-1 level 24 h after thoracic irradiation in
male ApoE−/− mice, and this increase was
stabilized 1 month later. Previous observations have shown that
local irradiation of carotid arteries in female and male
ApoE−/− mice did not induce ICAM-1 changes at
1 and 22 weeks after 14 Gy X-ray exposure (17,18),
indicating that the early 24 h response reported here may be
transient in nature. We further found a corresponding ICAM-1
increase in the irradiated TIME cells at 24 h for the 5 Gy dose,
which is in line with previous in vitro studies performed at
the same time window (39,84). Moreover, we showed that the serum
level of P-selectin was increased 24 h after irradiation, again
only in male ApoE−/− mice. This elevated
P-selectin level was also observed in irradiated TICAE and TIME
cells, which confirms previous in vitro findings (79). The inflammatory responses observed
at high irradiation dose (5 Gy) in TICAE and TIME cells could be an
injury response, since an increased DNA damage and persistent cell
death were previously observed in these cells after 5 Gy
X-irradiation (51).
Our data further demonstrate a significant decrease
in atheroprotective basic fibroblast growth factor (FGF-basic), in
female ApoE−/− mice at 10 Gy, and a
decreasing trend in male ApoE−/− mice 1 month
after irradiation, resulting is a significant dose-dependent
decrease in the combined female and male data. A previous study
performed in non-Hodgkin lymphoma cancer patients showed a
significant decrease in FGF-2 serum level after doses ranging
between 6 and 52 Gy of radiotherapy (85), and another study performed in
patients with different tumour histotypes, also reported that FGF-2
serum level decreased after radiotherapy (86). FGF-basic was observed to have a
protective effect against irradiation (87), since it inhibited radiation-induced
apoptosis of endothelial cells under both in vitro and in
vivo conditions (88,89), and was also reported to decrease
VCAM-1 expression and macrophage presence in atherosclerotic
plaques from rabbits fed with a high cholesterol diet (90).
In contrast to the proatherogenic inflammatory
response after thoracic irradiation, our results showed an acute
decrease in the proatherogenic cytokine MCP-1, only observed in
female mice 24 h after irradiation. MCP-1 is involved in
atherosclerosis initiation and progression by recruiting monocytes
and contributing to macrophage infiltration into the subendothelial
cell layer (91,92). Interestingly, it has been shown that
post-irradiation MCP-1-mediated chemoattraction is regulated by the
proatherogenic uPAR expression (93,94).
In line with this observation, our results showed a decrease in
uPAR serum level only in female ApoE−/− mice
24 h after 10 Gy exposure. It is worth mentioning that the observed
alterations in inflammatory markers, as discussed, are limited to
multiplex bead assay assessment, and further validation using other
approaches, such as western blotting or RT-qPCR, are required.
The gender difference in response to radiation
exposure could be explained by hormone differences between male and
female. Several groups have reported that estrogen is protective
against vascular dysfunction and atherosclerotic lesions in mice
(95–98). Estrogen is known to exert protective
and beneficial effects in the cardiovascular system by improving
vascular function, increasing NO production and by inhibiting
proliferation and migration of vascular smooth muscle cells
(99–101). It was also shown that
atherosclerotic lesions were significantly less extensive in female
ApoE−/− mice than in male mice (101). Indeed, in our experiment, we
observed an increase in the pro-inflammatory markers ICAM-1,
P-selectin in male mice while a decrease in the pro-inflammatory
MCP-1 and uPAR levels in female mice. Though, further
investigations are required to unveil mechanisms behind the
observed gender specific response after irradiation in
ApoE−/− mice, by scrutinizing the effect of
sex steroid hormones on cytokine response in vitro, or by
using knock-down/out techniques in vivo.
Taken together, our results reveal acute and early
term inflammatory responses after X-ray exposure of
ApoE−/− mice and of coronary artery and
microvascular endothelial cells. Future research is needed to fully
grasp the scope of early changes in the cardiovascular system after
thoracic irradiation, and to determine whether GDF-15 and CXCL10
could be used as potential biomarkers for early detection of
cardiovascular risks in thoracic radiotherapy-treated patients,
thus identifying patients who may benefit from early medical
intervention.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Kenneth Raj
(Centre for Radiation, Chemical and Environmental Hazards, Public
Health England, Didcot, UK) who donated the TICAE cell line.
Funding
This work was supported by the Fund for Scientific
Research Flanders, Belgium (grant no. G040720N). RR was supported
by a doctoral grant obtained from Belgian Nuclear Research
Centre/Ghent University.
Availability of data and materials
The datasets used and/or analysed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
RR conducted ex-vivo and in vitro
experiments, guided and supervised the in vivo irradiation
and blood collection experiments, and wrote the manuscript. MC and
EC conducted in vivo irradiation and blood sampling, as well
as the cholesterol and triglyceride experiments. MM analysed the
in vivo experiments. RR and AA confirm the authenticity of
all the raw data. ED, SB, AA and LL designed the experiments and
supervised the study. All authors, except MC, contributed equally
in reviewing the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Mice were treated according to the European Ethics
Committee guidelines and the study protocol was approved by the
Animal Experiment Ethical Committee of the Faculty of Medicine and
Health Sciences, Ghent University, Belgium (approval no. ECD
17/60).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kong FM, Zhao L and Hayman JA: The role of
radiation therapy in thoracic tumors. Hematol Oncol Clin North Am.
20:363–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delaney G, Jacob S, Featherstone C and
Barton M: The role of radiotherapy in cancer treatment: Estimating
optimal utilization from a review of evidence-based clinical
guidelines. Cancer. 104:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darby S, McGale P, Peto R, Granath F, Hall
P and Ekbom A: Mortality from cardiovascular disease more than 10
years after radiotherapy for breast cancer: Nationwide cohort study
of 90 000 Swedish women. BMJ. 326:256–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Darby SC, Ewertz M, McGale P, Bennet AM,
Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante
B, et al: Risk of ischemic heart disease in women after
radiotherapy for breast cancer. N Engl J Med. 368:987–998. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselet B, Rombouts C, Benotmane AM,
Baatout S and Aerts A: Cardiovascular diseases related to ionizing
radiation: The risk of low-dose exposure (review). Int J Mol Med.
38:1623–1641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yusuf SW, Sami S and Daher IN:
Radiation-induced heart disease: A clinical update. Cardiol Res
Pract. 2011:3176592011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Authors on behalf of ICRP, ; Stewart FA,
Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ,
Aleman BM, Edgar AB, Mabuchi K, et al: ICRP publication 118: ICRP
statement on tissue reactions and early and late effects of
radiation in normal tissues and organs-threshold doses for tissue
reactions in a radiation protection context. Ann ICRP. 41:1–322.
2012. View Article : Google Scholar
|
|
10
|
Little MP: Radiation and circulatory
disease. Mutat Res. 770:299–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eftekhari M, Anbiaei R, Zamani H, Fallahi
B, Beiki D, Ameri A, Emami-Ardekani A, Fard-Esfahani A,
Gholamrezanezhad A, Seid Ratki KR and Roknabadi AM:
Radiation-induced myocardial perfusion abnormalities in breast
cancer patients following external beam radiation therapy. Asia
Ocean J Nucl Med Biol. 3:3–9. 2015.PubMed/NCBI
|
|
12
|
Kole TP, Aghayere O, Kwah J, Yorke ED and
Goodman KA: Comparison of heart and coronary artery doses
associated with intensity-modulated radiotherapy versus
three-dimensional conformal radiotherapy for distal esophageal
cancer. Int J Radiat Oncol Biol Phys. 83:1580–1586. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tillman GF, Pawlicki T, Koong AC and
Goodman KA: Preoperative versus postoperative radiotherapy for
locally advanced gastroesophageal junction and proximal gastric
cancers: A comparison of normal tissue radiation doses. Dis
Esophagus. 21:437–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong JC, Rahimy E, Gross CP, Shafman T, Hu
X, Yu JB, Ross R, Finkelstein SE, Dosoretz A, Park HS, et al:
Radiation dose and cardiac risk in breast cancer treatment: An
analysis of modern radiation therapy including community settings.
Pract Radiat Oncol. 8:e79–e86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Kindi SG and Oliveira GH: Incidence and
trends of cardiovascular mortality after common cancers in young
adults: Analysis of surveillance, epidemiology and end-results
program. World J Cardiol. 8:368–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drost L, Yee C, Lam H, Zhang L, Wronski M,
McCann C, Lee J, Vesprini D, Leung E and Chow E: A systematic
review of heart dose in breast radiotherapy. Clin Breast Cancer.
18:e819–e824. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoving S, Heeneman S, Gijbels MJ, te Poele
JA, Russell NS, Daemen MJ and Stewart FA: Single-dose and
fractionated irradiation promote initiation and progression of
atherosclerosis and induce an inflammatory plaque phenotype in
ApoE(−/−) mice. Int J Radiat Oncol Biol Phys. 71:848–857. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stewart FA, Heeneman S, Te Poele J, Kruse
J, Russell NS, Gijbels M and Daemen M: Ionizing radiation
accelerates the development of atherosclerotic lesions in
ApoE−/− mice and predisposes to an inflammatory plaque
phenotype prone to hemorrhage. Am J Pathol. 168:649–658. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kreuzer M, Auvinen A, Cardis E, Hall J,
Jourdain JR, Laurier D, Little MP, Peters A, Raj K, Russell NS, et
al: Low-dose ionising radiation and cardiovascular
diseases-strategies for molecular epidemiological studies in
Europe. Mutat Res Rev Mutat Res. 764:90–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu Y, Kodama K, Nishi N, Kasagi F,
Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, et
al: Radiation exposure and circulatory disease risk: Hiroshima and
Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 340:b53492010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada M, Naito K, Kasagi F, Masunari N
and Suzuki G: Prevalence of atherosclerosis in relation to atomic
bomb radiation exposure: An RERF adult health study. Int J Radiat
Biol. 81:821–826. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azizova TV, Grigoryeva ES, Haylock RG,
Pikulina MV and Moseeva MB: Ischaemic heart disease incidence and
mortality in an extended cohort of Mayak workers first employed in
1948–1982. Br J Radiol. 88:201501692015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kashcheev VV, Chekin SY, Karpenko SV,
Maksioutov MA, Menyaylo AN, Tumanov KA, Kochergina EV, Kashcheeva
PV, Gorsky AI, Shchukina NV, et al: Radiation risk of
cardiovascular diseases in the cohort of Russian emergency workers
of the chernobyl accident. Health Phys. 113:23–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramadan R, Vromans E, Anang DC, Decrock E,
Mysara M, Monsieurs P, Baatout S, Leybaert L and Aerts A: Single
and fractionated ionizing radiation induce alterations in
endothelial connexin expression and channel function. Sci Rep.
9:46432019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mathias D, Mitchel RE, Barclay M, Wyatt H,
Bugden M, Priest ND, Whitman SC, Scholz M, Hildebrandt G, Kamprad M
and Glasow A: Low-dose irradiation affects expression of
inflammatory markers in the heart of ApoE−/− mice. PLoS
One. 10:e01196612015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sievert W, Trott KR, Azimzadeh O, Tapio S,
Zitzelsberger H and Multhoff G: Late proliferating and inflammatory
effects on murine microvascular heart and lung endothelial cells
after irradiation. Radiother Oncol. 117:376–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitchel RE, Hasu M, Bugden M, Wyatt H,
Little MP, Gola A, Hildebrandt G, Priest ND and Whitman SC:
Low-dose radiation exposure and atherosclerosis in ApoE(−)/(−)
mice. Radiat Res. 175:665–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mancuso M, Pasquali E, Braga-Tanaka I III,
Tanaka S, Pannicelli A, Giardullo P, Pazzaglia S, Tapio S, Atkinson
MJ and Saran A: Acceleration of atherogenesis in ApoE−/−
mice exposed to acute or low-dose-rate ionizing radiation.
Oncotarget. 6:31263–31271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumarathasan P, Vincent R, Blais E,
Saravanamuthu A, Gupta P, Wyatt H, Mitchel R, Hannan M, Trivedi A
and Whitman S: Cardiovascular changes in atherosclerotic
ApoE-deficient mice exposed to Co60 (ү) radiation. PLoS One.
8:e654862013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Massberg S, Brand K, Gruner S, Page S,
Müller E, Müller I, Bergmeier W, Richter T, Lorenz M, Konrad I, et
al: A critical role of platelet adhesion in the initiation of
atherosclerotic lesion formation. J Exp Med. 196:887–896. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heidenreich PA, Hancock SL, Lee BK,
Mariscal CS and Schnittger I: Asymptomatic cardiac disease
following mediastinal irradiation. J Am Coll Cardiol. 42:743–749.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yusuf SW, Venkatesulu BP, Mahadevan LS and
Krishnan S: Radiation-induced cardiovascular disease: A clinical
perspective. Front Cardiovasc Med. 4:662017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Corrado E, Rizzo M, Coppola G, Fattouch K,
Novo G, Marturana I, Ferrara F and Novo S: An update on the role of
markers of inflammation in atherosclerosis. J Atheroscler Thromb.
17:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soeki T and Sata M: Inflammatory
biomarkers and atherosclerosis. Int Heart J. 57:134–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Libby P: Inflammation in atherosclerosis.
Arterioscler Thromb Vasc Biol. 32:2045–2051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murabito JM, Keyes MJ, Guo CY, Keaney JF
Jr, Vasan RS, D'Agostino RB Sr and Benjamin EJ: Cross-sectional
relations of multiple inflammatory biomarkers to peripheral
arterial disease: The Framingham offspring study. Atherosclerosis.
203:509–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Halle M, Gabrielsen A, Paulsson-Berne G,
Gahm C, Agardh HE, Farnebo F and Tornvall P: Sustained inflammation
due to nuclear factor-kappa B activation in irradiated human
arteries. J Am Coll Cardiol. 55:1227–1236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kiyohara H, Ishizaki Y, Suzuki Y, Katoh H,
Hamada N, Ohno T, Takahashi T, Kobayashi Y and Nakano T:
Radiation-induced ICAM-1 expression via TGF-β1 pathway on human
umbilical vein endothelial cells; comparison between X-ray and
carbon-ion beam irradiation. J Radiat Res. 52:287–292. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hallahan D, Kuchibhotla J and Wyble C:
Cell adhesion molecules mediate radiation-induced leukocyte
adhesion to the vascular endothelium. Cancer Res. 56:5150–5155.
1996.PubMed/NCBI
|
|
40
|
Di Maggio FM, Minafra L, Forte GI,
Cammarata FP, Lio D, Messa C, Gilardi MC and Bravatà V: Portrait of
inflammatory response to ionizing radiation treatment. J Inflamm
(Lond). 12:142015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baluna RG, Eng TY and Thomas CR: Adhesion
molecules in radiotherapy. Radiat Res. 166:819–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Min X, Lu M, Tu S, Wang X, Zhou C, Wang S,
Pang S, Qian J, Ge Y, Guo Y, et al: Serum cytokine profile in
relation to the severity of coronary artery disease. Biomed Res
Int. 2017:40136852017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peng J, Luo F, Ruan G, Peng R and Li X:
Hypertriglyceridemia and atherosclerosis. Lipids Health Dis.
16:2332017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sloop GD: A critical analysis of the role
of cholesterol in atherogenesis. Atherosclerosis. 142:265–268.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lowe D and Raj K: Premature aging induced
by radiation exhibits pro-atherosclerotic effects mediated by
epigenetic activation of CD44 expression. Aging Cell. 13:900–910.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lowe D, Horvath S and Raj K: Epigenetic
clock analyses of cellular senescence and ageing. Oncotarget.
7:8524–8531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leligdowicz A, Conroy AL, Hawkes M, Zhong
K, Lebovic G, Matthay MA and Kain KC: Validation of two multiplex
platforms to quantify circulating markers of inflammation and
endothelial injury in severe infection. PLoS One. 12:e01751302017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vitkova V, Panek M, Janec P, Šibíková M,
Vobruba V, Haluzík M, Živný J and Janota J: Endothelial
microvesicles and soluble markers of endothelial injury in
critically Ill newborns. Mediators Inflamm. 2018:19750562018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bahlas S, Damiati L, Dandachi N, Sait H,
Alsefri M and Pushparaj PN: Rapid immunoprofiling of cytokines,
chemokines and growth factors in patients with active rheumatoid
arthritis using luminex multiple analyte profiling technology for
precision medicine. Clin Exp Rheumatol. 37:112–119. 2019.PubMed/NCBI
|
|
50
|
Reslova N, Michna V, Kasny M, Mikel P and
Kralik P: xMAP technology: Applications in detection of pathogens.
Front Microbiol. 8:552017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ramadan R, Vromans E, Anang DC,
Goetschalckx I, Hoorelbeke D, Decrock E, Baatout S, Leybaert L and
Aerts A: Connexin43 hemichannel targeting with TAT-Gap19 alleviates
radiation-induced endothelial cell damage. Front Pharmacol.
11:2122020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Darby SC, Cutter DJ, Boerma M, Constine
LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce
LJ, et al: Radiation-related heart disease: Current knowledge and
future prospects. Int J Radiat Oncol Biol Phys. 76:656–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aleman BM, Moser EC, Nuver J, Suter TM,
Maraldo MV, Specht L, Vrieling C and Darby SC: Cardiovascular
disease after cancer therapy. EJC Suppl. 12:18–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Monceau V, Meziani L, Strup-Perrot C,
Morel E, Schmidt M, Haagen J, Escoubet B, Dörr W and Vozenin MC:
Enhanced sensitivity to low dose irradiation of ApoE−/−
mice mediated by early pro-inflammatory profile and delayed
activation of the TGFβ1 cascade involved in fibrogenesis. PLoS One.
8:e570522013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brunner D, Altman S, Loebl K, Schwartz S
and Levin S: Serum cholesterol and triglycerides in patients
suffering from ischemic heart disease and in healthy subjects.
Atherosclerosis. 28:197–204. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sarwar N, Danesh J, Eiriksdottir G,
Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT and
Gudnason V: Triglycerides and the risk of coronary heart disease:
10,158 incident cases among 262,525 participants in 29 Western
prospective studies. Circulation. 115:450–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ozmen HK, Erdemci B, Askin S and Sezen O:
Carnitine and adiponectin levels in breast cancer after
radiotherapy. Open Med (Wars). 12:189–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park H, Kim CH, Jeong JH, Park M and Kim
KS: GDF15 contributes to radiation-induced senescence through the
ROS-mediated p16 pathway in human endothelial cells. Oncotarget.
7:9634–9644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hoving S, Heeneman S, Gijbels MJ, Te Poele
JA, Visser N, Cleutjens J, Russell NS, Daemen MJ and Stewart FA:
Irradiation induces different inflammatory and thrombotic responses
in carotid arteries of wildtype C57BL/6J and atherosclerosis-prone
ApoE(−/−) mice. Radiother Oncol. 105:365–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bootcov MR, Bauskin AR, Valenzuela SM,
Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor
K, et al: MIC-1, a novel macrophage inhibitory cytokine, is a
divergent member of the TGF-beta superfamily. Proc Natl Acad Sci
USA. 94:11514–11519. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hsiao EC, Koniaris LG, Zimmers-Koniaris T,
Sebald SM, Huynh TV and Lee SJ: Characterization of
growth-differentiation factor 15, a transforming growth factor beta
superfamily member induced following liver injury. Mol Cell Biol.
20:3742–3751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wollert KC and Kempf T: Growth
differentiation factor 15 in heart failure: An update. Curr Heart
Fail Rep. 9:337–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen J, Luo F, Fang Z and Zhang W: GDF-15
levels and atherosclerosis. Int J Cardiol. 257:362018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kempf T and Wollert KC: Growth
differentiation factor-15: A new biomarker in cardiovascular
disease. Herz. 34:594–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu X, Li Z and Gao W: Growth
differentiation factor 15 in cardiovascular diseases: From bench to
bedside. Biomarkers. 16:466–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bonaterra GA, Zugel S, Thogersen J, Walter
SA, Haberkorn U, Strelau J and Kinscherf R: Growth differentiation
factor-15 deficiency inhibits atherosclerosis progression by
regulating interleukin-6-dependent inflammatory response to
vascular injury. J Am Heart Assoc. 1:e0025502012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
de Jager SC, Bermudez B, Bot I, Koenen RR,
Bot M, Kavelaars A, de Waard V, Heijnen CJ, Muriana FJ, Weber C, et
al: Growth differentiation factor 15 deficiency protects against
atherosclerosis by attenuating CCR2-mediated macrophage chemotaxis.
J Exp Med. 208:217–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Heller EA, Liu E, Tager AM, Yuan Q, Lin
AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, et al:
Chemokine CXCL10 promotes atherogenesis by modulating the local
balance of effector and regulatory T cells. Circulation.
113:2301–2312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mach F, Sauty A, Iarossi AS, Sukhova GK,
Neote K, Libby P and Luster AD: Differential expression of three T
lymphocyte-activating CXC chemokines by human atheroma-associated
cells. J Clin Invest. 104:1041–1050. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
van den Borne P, Quax PH, Hoefer IE and
Pasterkamp G: The multifaceted functions of CXCL10 in
cardiovascular disease. Biomed Res Int. 2014:8931062014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ardigo D, Assimes TL, Fortmann SP, Go AS,
Hlatky M, Hytopoulos E, Iribarren C, Tsao PS, Tabibiazar R and
Quertermous T; ADVANCE Investigators, : Circulating chemokines
accurately identify individuals with clinically significant
atherosclerotic heart disease. Physiol Genomics. 31:402–409. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Orn S, Breland UM, Mollnes TE, Manhenke C,
Dickstein K, Aukrust P and Ueland T: The chemokine network in
relation to infarct size and left ventricular remodeling following
acute myocardial infarction. Am J Cardiol. 104:1179–1183. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tavakolian Ferdousie V, Mohammadi M,
Hassanshahi G, Khorramdelazad H, Khanamani Falahati-Pour S, Mirzaei
M, Allah Tavakoli M, Kamiab Z, Ahmadi Z, Vazirinejad R, et al:
Serum CXCL10 and CXCL12 chemokine levels are associated with the
severity of coronary artery disease and coronary artery occlusion.
Int J Cardiol. 233:23–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Palayoor ST, John-Aryankalayil M, Makinde
AY, Falduto MT, Magnuson SR and Coleman CN: Differential expression
of stress and immune response pathway transcripts and miRNAs in
normal human endothelial cells subjected to fractionated or
single-dose radiation. Mol Cancer Res. 12:1002–1015. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Heinonen M, Milliat F, Benadjaoud MA,
François A, Buard V, Tarlet G, d'Alché-Buc F and Guipaud O:
Temporal clustering analysis of endothelial cell gene expression
following exposure to a conventional radiotherapy dose fraction
using Gaussian process clustering. PLoS One. 13:e02049602018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hallahan DE and Virudachalam S:
Accumulation of P-selectin in the lumen of irradiated blood
vessels. Radiat Res. 152:6–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tu J, Hu Z and Chen Z: Endothelial gene
expression and molecular changes in response to radiosurgery in in
vitro and in vivo models of cerebral arteriovenous malformations.
Biomed Res Int. 2013:4082532013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hallahan DE and Virudachalam S: Ionizing
radiation mediates expression of cell adhesion molecules in
distinct histological patterns within the lung. Cancer Res.
57:2096–2099. 1997.PubMed/NCBI
|
|
81
|
Gaugler MH, Squiban C, van der Meeren A,
Bertho JM, Vandamme M and Mouthon MA: Late and persistent
up-regulation of intercellular adhesion molecule-1 (ICAM-1)
expression by ionizing radiation in human endothelial cells in
vitro. Int J Radiat Biol. 72:201–209. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Haubner F, Leyh M, Ohmann E, Pohl F,
Prantl L and Gassner HG: Effects of external radiation in a
co-culture model of endothelial cells and adipose-derived stem
cells. Radiat Oncol. 8:662013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Azimzadeh O, Sievert W, Sarioglu H,
Merl-Pham J, Yentrapalli R, Bakshi MV, Janik D, Ueffing M, Atkinson
MJ, Multhoff G and Tapio S: Integrative proteomics and targeted
transcriptomics analyses in cardiac endothelial cells unravel
mechanisms of long-term radiation-induced vascular dysfunction. J
Proteome Res. 14:1203–1219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cervelli T, Panetta D, Navarra T,
Andreassi MG, Basta G, Galli A, Salvadori PA, Picano E and Del
Turco S: Effects of single and fractionated low-dose irradiation on
vascular endothelial cells. Atherosclerosis. 235:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ria R, Cirulli T, Giannini T, Bambace S,
Serio G, Portaluri M, Ribatti D, Vacca A and Dammacco F: Serum
levels of angiogenic cytokines decrease after radiotherapy in
non-Hodgkin lymphomas. Clin Exp Med. 8:141–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ria R, Portaluri M, Russo F, Cirulli T, Di
Pietro G, Bambace S, Cucci F, Romano T, Vacca A and Dammacco F:
Serum levels of angiogenic cytokines decrease after antineoplastic
radiotherapy. Cancer Lett. 216:103–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang X, Liaw L, Prudovsky I, Brooks PC,
Vary C, Oxburgh L and Friesel R: Fibroblast growth factor signaling
in the vasculature. Curr Atheroscler Rep. 17:5092015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fuks Z, Persaud RS, Alfieri A, McLoughlin
M, Ehleiter D, Schwartz JL, Seddon AP, Cordon-Cardo C and
Haimovitz-Friedman A: Basic fibroblast growth factor protects
endothelial cells against radiation-induced programmed cell death
in vitro and in vivo. Cancer Res. 54:2582–2590. 1994.PubMed/NCBI
|
|
89
|
Zhang S, Qiu X, Zhang Y, Fu K, Zhao X, Wu
J, Hu Y, Zhu W and Guo H: Basic fibroblast growth factor
ameliorates endothelial dysfunction in radiation-induced bladder
injury. Biomed Res Int. 2015:9676802015.PubMed/NCBI
|
|
90
|
Six I, Mouquet F, Corseaux D, Bordet R,
Letourneau T, Vallet B, Dosquet CC, Dupuis B, Jude B, Bertrand ME,
et al: Protective effects of basic fibroblast growth factor in
early atherosclerosis. Growth Factors. 22:157–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Aiello RJ, Bourassa PA, Lindsey S, Weng W,
Natoli E, Rollins BJ and Milos PM: Monocyte chemoattractant
protein-1 accelerates atherosclerosis in apolipoprotein E-deficient
mice. Arterioscler Thromb Vasc Biol. 19:1518–1525. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Harrington JR: The role of MCP-1 in
atherosclerosis. Stem Cells. 18:65–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nalla AK, Gogineni VR, Gupta R, Dinh DH
and Rao JS: Suppression of uPA and uPAR blocks radiation-induced
MCP-1 mediated recruitment of endothelial cells in meningioma. Cell
Signal. 23:1299–1310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Farris SD, Hu JH, Krishnan R, Emery I, Chu
T, Du L, Kremen M, Dichek HL, Gold E, Ramsey SA and Dichek DA:
Mechanisms of urokinase plasminogen activator (uPA)-mediated
atherosclerosis: Role of the uPA receptor and S100A8/A9 proteins. J
Biol Chem. 286:22665–22677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kimura M, Sudhir K, Jones M, Simpson E,
Jefferis AM and Chin-Dusting JP: Impaired acetylcholine-induced
release of nitric oxide in the aorta of male aromatase-knockout
mice: Regulation of nitric oxide production by endogenous sex
hormones in males. Circ Res. 93:1267–1271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Adams MR, Golden DL, Register TC, Anthony
MS, Hodgin JB, Maeda N and Williams JK: The atheroprotective effect
of dietary soy isoflavones in apolipoprotein E−/− mice
requires the presence of estrogen receptor-alpha. Arterioscler
Thromb Vasc Biol. 22:1859–1864. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox
D, Hodgin J, Shaul PW, Thoren P, Smithies O, et al: Abnormal
vascular function and hypertension in mice deficient in estrogen
receptor beta. Science. 295:505–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hodgin JB, Krege JH, Reddick RL, Korach
KS, Smithies O and Maeda N: Estrogen receptor alpha is a major
mediator of 17beta-estradiol's atheroprotective effects on lesion
size in Apoe−/− mice. J Clin Invest. 107:333–340. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hurtado R, Celani M and Geber S: Effect of
short-term estrogen therapy on endothelial function: A
double-blinded, randomized, controlled trial. Climacteric.
19:448–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zheng S, Chen X, Hong S, Long L, Xu Y,
Simoncini T and Fu X: 17β-Estradiol inhibits vascular smooth muscle
cell migration via up-regulation of striatin protein. Gynecol
Endocrinol. 31:618–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang G, Li C, Zhu N, Chen Y, Yu Q, Liu E
and Wang R: Sex differences in the formation of atherosclerosis
lesion in apoE−/−mice and the effect of 17β-estrodiol on
protein S-nitrosylation. Biomed Pharmacother. 99:1014–1021. 2018.
View Article : Google Scholar : PubMed/NCBI
|