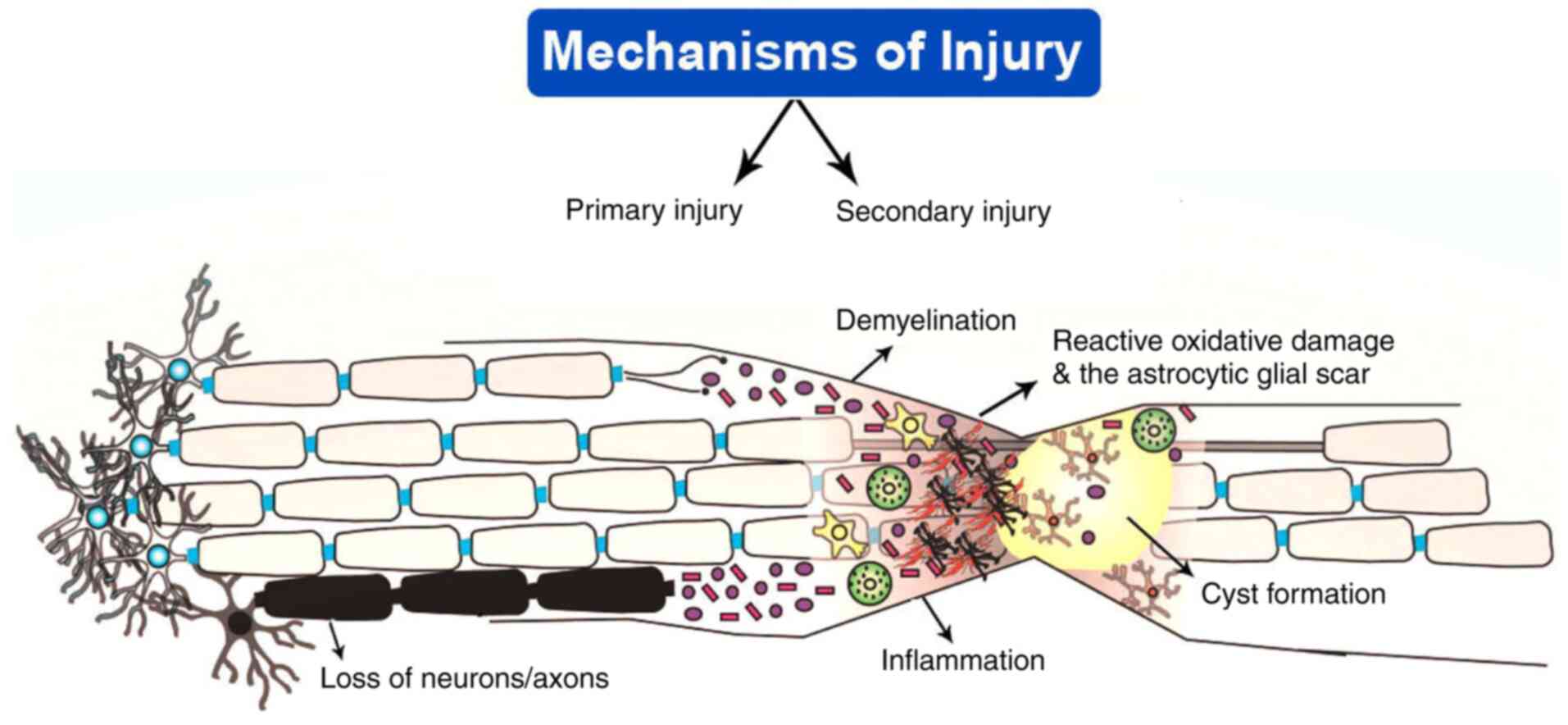
Spinal cord injury (SCI) is caused by a degenerative
loss of motor, sensory and autonomic function (1). As a result, trauma occurs either due
to partial or complete damage to the spinal cord (2). It poses physical, psychosocial and
vocational implications for patients and caregivers alike. SCI can
therefore pose a threat to the health of the patient. Long-time
treatment, cost of care and economic losses can affect the patients
and their families, raising social and physiological issues.
Typically, >50% of patients may not regain their normal function
and daily life (3). Healthy young
individuals of 15–25 years are most commonly affected; because of
this, SCI is a serious worldwide health concern (3). Extensive attempts for SCI treatment
have so far been aimed at developing treatments for SCI effects
(1). However, an active or
permanent cure in the treatment of this condition has yet to be
developed.
Central nervous system function cannot be regained
following SCI and this is a crucial clinical concern (4). For a number of years, researchers and
clinicians have been trying to determine various viable options
based on the pathophysiology of SCI to improve neuronal function.
This has led to efforts aimed at developing pharmacological
treatments for SCI to reduce neuronal damage and improve neuronal
function (5). Several
pharmacological and non-pharmacological treatments show improvement
or even recovery of motor functions and minimization of
neurological damages (2,6). In particular, preventing secondary
injury, enhancing regeneration and replacing destroyed spinal
tissue are the current primary aims to treat SCI (7). Additionally, some potential
pharmacological candidates including mynocycline, are already being
studied in clinical trials for the treatment of SCI (8).
Spinal cord compression is the most frequent
mechanism of SCI and can continue after the injury (9). Penetrating injuries and strain to the
neural tissues or vascular structures are caused by dislocation,
flexion, extension or distraction forces related to rotation
(9). Other mechanical damage to
bone structures and ligaments can result, or consequences related
to compression can give rise to hematomas in the spinal cord
channel (10). Bleeding during
spinal trauma begins during the early period of SCI and is later
followed by the interruption of blood supply (11). Hypoxia and local ischemic infraction
are both consequences of the disruption of blood flow following SCI
(12). Specifically, these two
conditions damage the grey matter, where metabolic function is
high. Neurons in the damaged area are physically fractured and the
myelin thickness is reduced (13).
In addition, deterioration in neuronal transmission can be
augmented by edema and the accumulation of macrophages in the
damaged tissue (14).
Secondary damage can be initiated by primary damage,
whereas a number of pathophysiological mechanisms can come into
play even hours and days after developing SCIs (15). The most notable mechanism is a lack
of energy due to ischemia and impaired perfusion at the cellular
level (16). Ischemia can result
immediately after traumatic SCI and, if left untreated, additional
damage may commence within the first 3 h and continue for at least
24 h (8,17). Several crucial changes are found,
such as hemorrhage, demyelination, edema, and cavity formation with
axonal and neuronal necrosis, as well as a series of pathological
changes in the nerve tissues following SCI, which can further
increase infarction (18). High
levels of glutamate can cause excitotoxicity, oxidative damage and
ischemia, while Ca2+-dependent nitric oxide synthesis
can cause secondary spinal cord damage (19,20).
Following secondary injuries, increased free radical damage and
lipid peroxidation in the cell membrane and secondary injury
signaling cascades at the injured tissue areas can eventually lead
to neuronal death (12,21).
Nevertheless, extensive experiments show that the
spinal cord has excellent healing properties (22). Proper blood flow is an essential
factor in ensuring that progressive tissue damage precedes and
promotes necrosis during the healing process (23). SCI is therefore regarded as a
pathological condition involving injury to the nerve tissue.
Pathological cascades, ranging from atrophy to apoptosis or
necrosis, can be result in the deterioration of neurons in the
brain due to local injuries to the spinal cord (6). The axons in the injured area may be
regenerated due to the well-vascularized astrocytic environment
(24). Secondary injury may also
cause neuronal death (25). Insight
into the mechanisms of secondary damage can be useful in developing
advanced therapies. Systemic and local effects contribute to the
development of secondary damage (15).
Systemic factors causing acute SCI include
hypotension resulting from neurological shocks, minimized cardiac
output and respiratory failure (9).
In such cases, the supply of essential metabolites and oxygen to
the nervous tissue is restricted. Low blood pressure must be
promptly controlled in patients with spinal shock (19,26).
Since perfusion pressure is mainly related to systemic blood
pressure, damage to the spinal cord is aggravated (26,27).
Hence, issues arising in the cardiopulmonary system are liable to
increase the severity of SCI by damaging the spinal cord (18). Normal blood pressure must be
maintained to avoid intramedullary hyperemia and hemorrhage
(28). In addition, post-traumatic
hypotension can occur following SCI and last for a few days or even
months (29). In animal models,
blood transfusion and dopamine can provide normotension, which can
lead to an increase in blood flow to the spinal cord (23). However, since the local
micro-circulation is affected, the functions of the spinal cord
cannot be improved.
Peripheral immune cells, including macrophages,
neutrophils and T cells, can initiate an inflammatory response
following SCI, which may gradually increase within a few days
(22). Macrophages and neutrophils
can cause the growth of lesions and lead to tissue damage (Fig. 1) (30). The release of inflammatory cytokines
(TNF, IL-1, IL-6 and IL-10) and macrophages results in inflammatory
reactions and subsequent pathological changes to the microglia
(4). The lesion sites exhibit an
increase in the levels of several inflammatory mediators including
leukotrienes, bradykinin, prostaglandins, platelet-activating
factors and serotonin (4). Central
nervous system (CNS) inflammatory responses may be promoted by
several cytokines, chemokines, oxygen, nitric oxide and other
nitrogen-containing molecules (31). IL-10 is a neuroprotective cytokine
and can improve motor function (32). The inflammation levels are
significantly decreased in an animal model after 30 min of IL-10
administration (8).
There is a direct influence of the excitatory
neurotransmitter in the spinal cord by N-methyl-D-aspartate
(NMDA) receptors (39). Studies
reveal that blocking the NMDA receptor results in protection from
secondary damage due to trauma and ischemia in animal models
(39,40). NMDA antagonists can significantly
improve neurological functions and decrease the incidence of edema
(41,42). Magnesium ions can block the ion
channels of the NMDA receptors (14). A previous study identified a
reduction in the injured area and indicates that functional
improvement can be achieved by the administration of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
antagonists (32). Glutamate is an
important excitatory neurotransmitter located in the CNS. Neuronal
damage occurs due to the overactivation of the glutamate receptors
(43). Shortly following SCI, there
is an increase in the levels of excitatory amino acids, including
glutamate and aspartate (44). The
extracellular excitatory amino acids reach toxic levels within 15
min of injury to the spinal cord and can last ≤120 min (45).
Apoptosis is activated following SCI due to the
release of inflammatory cytokines and free radicals, which lead to
inflammation and excitotoxicity (46). Between 3 h and 8 weeks following
SCI, apoptosis occurs in the areas surrounding the injured spinal
cord tissue (47). Several studies
indicate that after a few weeks of injury, demyelination is
intensified by apoptosis of the oligodendrocytes (47,48).
David et al (48) report
that oligodendrocytic changes occur in response to SCI.
Furthermore, apoptosis adversely affects the situation by
increasing neuronal loss. Other studies reveal that apoptosis
results in deterioration of the microglia and enhances secondary
inflammatory injury (15,49). Previous studies also show that
several caspase components are activated following SCI (48,50).
Several stimuli are known to activate three major apoptosis
pathways through caspases (51).
Apoptosis is induced in specialized cells, including neurons,
astrocytes, oligodendrocytes and microglia (48). Therefore, neuronal protection is a
crucial and challenging concern, as neurons in the spinal cord
cannot be reproduced. Based on the regenerative ability of the
glial cells, preventing glial death can enhance neuronal protection
by two mechanisms (52). In the
first mechanism, glial cells provide neurotrophic and metabolic
support to the injured neurons to promote recovery (53). The second mechanism is based on the
ability of glial cells to scavenge apoptotic mediators, including
cytokines, and prevent the leak of free radicals from dying cells,
which are toxic to the adjacent cells (54).
Severe SCI causes a substantial decrease in blood
supply, resulting in the initiation of ischemia following the
trauma (62). When the spinal cord
autoregulation is disrupted, abnormal changes in systemic
hemodynamics may be reflected in blood flow in the spinal cord
(63). Therefore, the ischemia
produced by SCI is enhanced by systemic hypotension and hypoxia.
The transportation of glucose and oxygen to tissues and ATP
generation are substantially reduced due to ischemia (63). The exact cause of post-traumatic
ischemia remains to be elucidated (2). However, focal narrowing of sulcal
arterioles and intramedullary capillaries, fragmentation,
aneurysmal dilatation or occlusion have been reported in
experimental studies (64,65). A study demonstrated progressive
vascular damage in a spinal cord contusion injury model for the
first time (66). The uptake of
Evans blue increases by ~76% in the injury area at 24 h compared
with those at 2 h (67). Low pH of
tissue due to lactic acidosis, as well as the accumulation of
fibrin and platelets that cause congestion of venous stasis, may
contribute to ischemia (68). In
addition, ischemia is caused by the damaging of capillary
endothelium, edema, petechial hemorrhages and vasoactive cytokines
(69). Under these conditions, the
systems adopt anaerobic respiration. Various pathophysiological
processes can be affected by ischemia and activation of anaerobic
respiration (70). Edema is formed
at the injured area and around peripheral tissues due to
proteinaceous leakage from intrinsic spinal cord veins (71). Spinal cord pressure is increased by
edema and the disruption of blood flow of the spinal cord occurs
(72). Magnesium can diminish edema
and vascular permeability in SCI (71).
Abnormal changes in the concentration of
electrolytes are observed following spinal cord trauma (14,73,74).
Formation of glutamate and free radicals is significantly increased
due to ischemia-reperfusion injury (75). Antagonism of glutamate in
endothelium can prevent the devastation of the blood spinal cord
barrier. Evidence demonstrates that glutamate receptor blockers
improve neurological results in SCI (60). Magnesium ions can decrease the lipid
peroxidation process via the antagonism of glutamate receptors
(76). Calcium ions can also serve
a role in cell death, as calcium ions can activate phospholipases,
proteases and phosphorylases in cells (77). Another experimental study reported
that calcium channel blockers can significantly prevent secondary
SCI (77). Extensive experimental
studies suggest that blood flow to the spinal cord is improved by
those agents, such as gacyclidine, which have significantly
positive activity on healing (18,77,78).
COX inhibitors are a preferred choice for the
treatment of SCI in the damaging secondary pathway (79). Ibuprofen and meclofenamate are two
common non-steroidal anti-inflammatory drugs that can be applied to
maintain spinal cord blood flow following SCI in an animal model
(80,81). The combination of a thromboxane
inhibitor with a prostacyclin analogue shows a similar effect
(82). In a rat model, COX-2
expression levels are greatly augmented in the injured spinal cord
tissue following contusion injury and the pharmacological
inhibition of COX-2 isoform enhances functional results in
moderately acute spinal injuries (80,83).
Although the widespread use and clinical applications of COX-1 or
COX-2 inhibitors for SCI in humans have not yet been indicated in
the aforementioned previous studies, their wide use as well as
application in the musculoskeletal and rheumatologic conditions
assuages several of the safety and pharmacokinetics concerns.
Activation of NMDA and non-NMDA can serve a crucial
role in the excitotoxic damage following SCI (84). Along with traumatic disorders,
non-traumatic CNS disorders have quickened the development of
associated pharmacological interventions. NMDA-receptor
antagonists, including MK801 and gacyclidine, demonstrate
substantial neuroprotective effects following SCI in animal
experiments (76). The development
of NMDA-targeting systemic clinical therapies and applications has
been impeded by the extensive distribution of glutamate and its
receptor throughout the entire human CNS, which makes it a
challenge to avoid possible side effects (76,85).
Gacyclidine, an NMDA receptor antagonist, has been evaluated in
France in a phase-II double-blind, randomized evaluation of 280
patients with SCI (86). However,
this study reveals no remarkable improvement in American Spinal
Injury Association (ASIA) scores compared with placebo treatment.
White matter glial loss is reduced and locomotor function can be
improved by focal microinjections of NBQX, an AMPA receptor
blocker, into the injured spinal cord areas after blunt injury
(87). Unfortunately, this invasive
form of receptor antagonism has not been developed into
pharmacological therapies due to several side effects (87). Magnesium is an NMDA receptor
antagonist that reduces excitotoxicity and inflammatory factors
(88). However, cerebrospinal fluid
levels are increased by the combination of magnesium and polyethene
glycol (PEG) without large doses of magnesium (86). The enhancement of tissue sparing and
the action of magnesium produces an improvement of functional motor
recovery with PEG in the treatment of SCI in animal models
(86). However, a phase I/II
clinical trial for the combination of magnesium and PEG was ended
in 2015, due to several difficulties in enrolling patients.
Calcium channel blockers display significant effects
on reducing the pathological influx of calcium into cells via
non-glutamate receptors (78).
However, the potent physiological impacts of calcium blockers are
regulated via their pharmacological activity on vascular smooth
muscle, rather than inducing calcium flux across neuronal and glial
cell membranes (78). In
experimental SCI models, nimodipine (NDP) can improve spinal blood
flow and rescue hypoperfusion (77). NDP administration can significantly
improve neurological recovery following spinal cord trauma or
ischemia (Table I) (89). NDP was first studied in France in a
potential randomized clinical trial of 160 patients within 8 h of
injury: Methylprednisolone (MPSS), NDP 0.15 mg/kg/h for 2 h, then
0.03 mg/kg/h for 7 days; both MPSS and NDP or placebo (90–92).
Studies demonstrate that blood flow and neurological recoveries are
promoted by dopamine, adrenaline, NPD and dextran following SCI
(77,93–95).
It is difficult to prove any firm effects in neurological outcomes
after 1 year of injury. The administration of calcium channel
blockers in acute SCI raises concerns regarding the potential for
systemic hypotension that, in the context of the injured spinal
cord, could be harmful due to autoregulation (18). The administration of sodium channel
blockers notably improves the neuroprotection of white matter and
functional outcomes with microinjections of tetrodotoxin after
blunt experimental SCI (96). Even
though surgery is required, similar neuroprotective effects have
been observed for the administration of riluzole, another sodium
channel blocker, after a clip compaction injury to a rodent spinal
cord (97). The US Food and Drug
Administration has recently approved riluzole for the treatment of
amyotrophic lateral sclerosis. Notably, riluzole has an important
role in protecting against excitotoxic cell death by blocking the
sodium channel in the injured neurons constraining the presynaptic
toggle of glutamate (97). Neuronal
loss and cavity size have been reduced in SCI animal models
(98). A significant improvement of
sensorimotor and results of electrophysiological were demonstrated
in a previous study (66). A
phase-I trial has been completed in which 36 patients (50 mg orally
every 12 h for 14 days) participated (99). This study demonstrates a substantial
increase in motor recovery following cervical injury at 3 months
compared with matched registry control patients. Enhanced motor
scores were not observed at 6 months follow-up. Regarding the
neurological results, an improvement in ASIA motor score was
demonstrated in patients with cervical SCI treated with riluzole
compared with non-riluzole-treated patients. Although a transient
increase in liver enzyme levels was found, a number of
pharmacokinetics and toxicity effects have not been investigated
(99). Nevertheless, the
therapeutic application of riluzole has yet to be documented.
Further studies should be carried out for its therapeutic use in
patients with SCI. A phase-II/III multicenter randomized controlled
trial of riluzole is currently underway by the AO Spine North
America Research Network (100).
Blocking potassium channels may be a potential
therapeutic target for the treatment of SCI. A patient with chronic
SCI taking 4-aminopyridine (4-AP) demonstrated an increase of the
axonal regeneration with mild levels of recovery (101). Similarly, an animal model of SCI
supports the potential effects of 4-AP on blocking the fast
potassium channels. 4-AP exhibits the most significant improvement
in conduction in chronic SCI (102). Fampiridine is a fast potassium
channel blocker, which has completed phase-II clinical trials and
is co-developed by Elan and Acorda Therapeutics (103). In some small clinical trials,
chronic SCI patients with incomplete injury have demonstrated
improved patient satisfaction, quality of life scores, sensory and
motor scores, decreased spasm and increased amplitude following
fampiridine administration (103,104). In addition, this potent drug has
good tolerance and few side effects (102). In another study, patients treated
with 4-AP exhibit less impact on functional status (104). This beneficial mechanism
demonstrates that potassium channel blockade enhances axonal
transmission between demyelinating nodes, and enhances neuronal and
neuromuscular transmission in the preserved axons (103). Thus, further investigations in
greater detail are urgently required to explore the potential
benefits of potassium channel blockers in improving axon conduction
in patients with the chronic injury. If more axons are retained in
the acute phase following SCI, the potential of this treatment may
be more substantial.
Glibenclamide (GLC) is a sulfonylurea receptor
1-regulated, transient receptor potential cation channel subfamily
M member 4 channel, Ca2+-activated, non-specific cation
channel blocker that is commonly used to treat diabetes by
increasing insulin release (105).
It exhibits substantial neuroprotective effects in ischemic
hemorrhagic stroke and traumatic brain injury models (106,107). GLC reduces hemorrhagic necrosis,
edema and inflammation in SCI (108,109). In animal models, GLC reduces
hemorrhage at 24 h after injury and minimized the lesion size at
1–6 weeks (110). GLC treatment
significantly improves functional recovery following SCI within 6
weeks. In one animal study, the improvement of bilateral injury
(33% reduction in lesion size) was more limited compared with
unilateral injury (57% reduction in lesion size) at 6 weeks
following GLC treatment (111).
These findings illustrate that the severity of the damage may play
significant roles in the functional recovery outcomes. In
conclusion, GLC can be potentially useful in the treatment of
SCI.
The National Acute Spinal Cord Injury Study (NASCIS)
is a large randomized clinical trial of SCI, which has investigated
the therapeutic role of corticosteroids (CSs) in SCI and produced
high-quality data (112). CSs can
significantly reduce various cellular stresses, including oxidative
stress associated with hypoperfusion, calcium influx and
excitotoxicity, and immune-regulated neuronal phagocytosis in the
injured spinal cord (113). All
NASCIS trials have yet to demonstrate benefits ascertained by
primary outcome measures.
Numerous investigations reveal the potential effects
of CSs. NASCIS-I published a paper in 1984, compared low-dose and
high-dose MPSS (100 mg bolus and 100 mg/day vs. 1,000 mg bolus and
1,000 mg/day) following SCI (114). No significant differences in
neurological improvement were observed in this study, but high-dose
MPSS administration can increase several physical complications
including gastrointestinal bleeding, sepsis, wound infection,
pulmonary embolism and even mortality (115). Notably, a placebo was not provided
in the NASCIS-I study, because CS was considered effective and to
not apply them raised ethical concerns. NASCIS-II assessed
high-dose MPSS within 24 h of SCI compared with naloxone, opioid
antagonists and placebo (115,116). A pre-planned subgroup analysis
illustrated that the patients administered with MPSS within 8 h of
injury could demonstrate substantially enhance motor function
recovery. NASCIS-III assessed MPSS and tirilazad mesylate (21
aminosteroids with antioxidant effect) within 8 h after injury and
is the first NASCIS study to use functional measurement results. CS
(30+5.4 mg/kg) were compared at 24 and 48 h with tirilazad mesylate
(2.5 mg/kg every 6 h) at 48 h. Subgroup analysis demonstrated that
patients who received MPSS bolus 3 to 8 h after injury and were
infused within 48 h exhibit improved neurological functions within
1 year. Following this study, MPSS was recommended for 24 h in
patients treated within 3 h of injury and 48 h in patients treated
within 3–8 h of injury. The treatment of acute SCI with MPSS is
still controversial. Recent guidelines give first-class evidence
against the application of CSs in SCI (117).
A randomized controlled trial reported that MPSS
administration within 8 h of injury is significantly effective in
SCI (118). This study
demonstrated the lack of other therapeutic strategies and MPSS is
the only drug to be investigated in phase-III trials. Notably, a
previous study also suggested that CS administration has limited
efficacy and increases several physical complications, including
septicemia and pneumonia (119). A
study of 77 patients with SCI demonstrates that despite limited
risk and efficacy, most patients prefer to take CS (120). By contrast, 59.4% of the patients
considered that the chance of neurological recovery was improved
and that it was worth using CS. Only a few (1.4%) hypothesized that
it was inappropriate to use CS. These findings should be explored
by health practitioners caring for the treatment of SCI patients.
The upcoming AO Spine-guidelines for the management of acute SCI
will reinstate the NASCIS-II dosing of MPSS administered within 3
to 8 h after injury to level-III treatment strategy (121).
Primary supportive care during acute SCI maintains
spinal cord perfusion and oxygenation. Injuries above the T6
vertebra can cause sympathetic nerve damage and neurogenic shock.
Liquid administration and vasoactive drugs are widely used in the
treatment of SCI (122). Following
volume resuscitation, vasoactive drugs can be used to increase
blood pressure, aimed at improving spinal cord perfusion (123). Patients with acute SCI can limit
tolerance for intravascular volume expansion in the context of
impaired sympathetic outflow. Several pharmacological agents,
including dopamine (1–10 mg/kg/min), dobutamine (5–15 mg/kg/min),
epinephrine (1–8 mg/min), norepinephrine (1–20 mg/min) and
phenylephrine (10–100 mg/min), can be considered (124). Norepinephrine and dopamine result
in vasoconstriction and enhance cardiac activity. Inoue et
al (125) found that dopamine
is most commonly used as a vasoactive drug, followed by
epinephrine, phenylephrine, norepinephrine and vasopressin. The
study suggested that both dopamine and phenylephrine administration
can increase cardiovascular complications. Patients with a mean
arterial pressure >85 mm Hg enhance their ASIA-score recovery
(126). This study also suggests
that this important therapeutic strategy might be effective soon
after injury.
Following traumatic SCI, the preservation of axons
can significantly enhance their overall function, even in a small
number. Studies have demonstrated that the activity of caspase-1
and caspase-3 in neurons and non-neuronal cells increases following
SCI (127,128). Treatment using a broad-spectrum
caspase inhibitor, such as Z-VAD-FMK, can reduce the area of
post-traumatic injury and neurological deficit, as the therapeutic
window of Z-VAD-FMK is extended by 9 h following transient (30 min)
cerebral ischemia (128). It can
be inferred that this treatment regimen may have a neuroprotective
effect and prolong the therapeutic window of the drug following
SCI. Bcl-2 gene therapy and cephalon, a protease/calpain inhibitor,
have completed pre-clinical studies and appear beneficial to
patients with SCI (129,130). However, targeting long-term
delayed caspase activation in oligodendrocytes may also be a
potential treatment, as it may save the degenerated white matter
and significantly improve patient prognosis.
MIN is a tetracycline antibiotic and previous
studies have shown that it exhibits significant neuroprotective
activities in Huntington's disease and multiple sclerosis (131,132). MIN can significantly impede the
activation of IL-1β, TNF-α, COX-2 and MMPs following SCI (132). In addition, MIN administration can
substantially hinder the expression of caspase-1 and caspase-3
levels following SCI (133). MIN
also inhibits inducible nitric oxide synthase, leading to
microglial activation following SCI (134). MIN protects neurons from glutamate
excitotoxicity at the injured spinal cord tissue area (135). Thus, MIN is a useful drug, since
it targets multiple processes involved in mediating cell death and
prevents the progression of secondary injury following SCI. A
previous study demonstrated that MIN can enhance significant
long-term functional outcomes in a mouse model (136). MIN-treated mice showed continual
and substantial improvements over a 4-week recovery period. MIN
administration could significantly enhance the Basso, Beattie, and
Bresnahan scores at 20 days post-injury. In the injured mouse model
of SCI, consistent weight support and considerable plantar stepping
were found, while some mice indicated evidence of forelimb-hindlimb
coordination. MIN could also improve neurological and histological
outcomes, impede neuronal and oligodendroglial apoptosis, minimize
microglial activation and inhibit inflammation in animal SCI models
(131). MIN significantly reduces
the lesion size and promotes tissue sparing following acute SCI
(131). Patients with acute
incomplete cervical SCI can benefit from early MIN administration
(128). These findings were
supported by a phase-II clinical trial where a 14-point ASIA motor
score recovery was achieved compared with the placebo (137). The development of a phase-III
trial entitled ‘Minocycline in Acute Spinal Cord Injury’ has been
driven by these initial outcomes and was evaluated for 7 days
(compared with placebo) using an intravenous administration of MIN
(138).
The glycosphingolipid GM-1, stimulates tyrosine
kinase receptors, which increases neuronal plasticity and
regeneration (139). Successful
tissue sparing has been developed to study neuroprotection in an
animal model of SCI (42,140). A successfully completed phase-II
trial shows enhanced 1-year ASIA motor scores after daily
administration of GM-1 for 18–32 days post-injury (141). Additionally, a landmark trial
indicates a substantial improvement in ASIA motor score and Frankel
grade following GM-1 administration in 37 patients with SCI
(142); lower limb function was
markedly improved at 48 h following treatment with GM-1. Thus, this
study demonstrated that GM-1 serves an important role in the repair
of neurons. Although these initial findings led to a large-scale
phase-III trial involving >750 patients in 28 centers, the major
findings of the study failed to meet the primary outcomes (2-point
improvement in the improved Benz walking scale) (137). The two groups showed a substantial
improvement in bowel/bladder recovery and sacral function, as well
as a remarkable improvement in functional independence scores and
an enhanced Barthel index. Intensive physical therapy was also
combined with pharmacological therapy to improve the prognosis of
patients in the study. A valid criticism of this study is the
delayed therapeutic effects of GM-1 treatment, as most of the
patients received MPSS for the first time in their clinical
treatment (137). Meta-analyses to
evaluate the potential therapeutic benefits of GM-1 in the
treatment of SCI failed to support its extensive use (136,142).
FGFs are heparin-binding proteins that have
significant neuroprotective activity against excitotoxicity and in
impeding the generation of oxygen free radicals (143). FGFs determine the fate of neuronal
cells, including migration and differentiation (144,145). The administration of FGFs can
reduce neuronal motor loss and improve respiratory deficits
(146,147). A phase-I/II trial of an FGF analog
is successfully completed (148).
Several growth factors possess neuroprotective effects and can
enhance functional recovery in SCI (144). FGF1 administration can improve the
survival and growth of various neuronal cell types, including
neocortical, hippocampal, cerebellar, dopaminergic, isolated
sensory neuronal and spinal cord cells (145,149). In addition, basic FGFs and/or
keratinocyte growth factor possess significant neuroprotective
effects in SCI (150). Although
preliminary studies of FGFs in animals are obligatory and
essential, reviews on SCI should focus more on human trials
(151). Ko et al (152) were the first to demonstrate a
clinical trial involving a patient suffering from chronic SCI, who
was treated with four survival nerve grafts coupled with fibrin
glue containing acidic FGF (aFGF). The authors suggest that
patients with acute SCI can significantly enhance functional
recovery from their wheelchair-bound status and independently
ambulate using a walker, 2.5 years after surgery (152). The authors also report that ASIA
motor and sensory scores are significantly enhanced in
postoperative functional status patients compared with presurgical
patients. Wu et al (153)
conducted a clinical trial on nine patients with cervical SCI, who
received direct spinal cord implantation of fibrin glue containing
aFGF for >6 months. The 6-month postoperative follow-up
indicated a marked enhancement in the ASIA motor and sensory scale
scores of patients. After 3 years, the authors published an
open-labeled, prospective, uncontrolled human clinical trial
involving 60 patients with SCI (comprising 30 cervical and 30
thoracolumbar SCIs) and significant improvement in ASIA motor and
sensory scale scores was observed in these patients at 24 months,
following FGF treatment (147).
Detailed studies are required to explore the potential therapeutic
effects and long-term outcomes.
G-CSF is a cytokine glycoprotein located in
numerous tissues in the body. Several studies report that G-CSF can
significantly promote the proliferation, survival and mobilization
of neuronal cells (147–149). Increasing evidence suggests that
G-CSF can enhance neurogenesis, reduce apoptotic-mediated neuronal
death, and reduce TNF-α and IL-1β expression levels at the injured
spinal cord area (154–156). White matter sparing and improved
hind-limb function are the positive effects associated with this
therapy (154). Non-randomized
phase-I/IIa trials have indicated ASIA grade improvement, without
any adverse effects, associated with G-CSF therapy (155–157). However, additional randomized
controlled trials are needed to establish the potential therapeutic
benefits of G-CSF in SCI. Recently, a phase-III clinical trial of
G-CSF in Japan has been completed, but the results are pending.
The ρ-signaling pathway is detrimental to axonal
regeneration and neurite growth (162). In a rodent model of SCI,
ρ-mediated inhibition of axonal growth enhances neuronal
regeneration and recovers the motor function induced by C3
transferase (Cethrin), a toxin produced by Clostridium
botulinum (163). During
decompressive surgery in the acute phase, Cethrin is injected into
the dura mater due to its high permeability at regions of the
injured spinal cord tissue.A phase-I/IIa multicenter trial of
VX-210 in patients with cervical or thoracic SCI showed a
significant improvement of 1.8±5.1 points in the ASIA motor score
for thoracic injury and 18.6±19.3 points for cervical injury
(164). In addition, 31 and 6% of
patients showed an improvement in ASIA C or D classification of
cervical or thoracic injury, respectively. The largest and most
significant improvement was observed at 12 months in the treatment
of patients with cervical injury using 3 mg Cethrin (27.3 points)
(165). It is worth noting that
RhoA impedance and subsequent functional enhancement are also
reported when non-steroidal anti-inflammatory drugs, such as
ibuprofen, are used for therapy, indicating that targeting COX may
be a potential therapeutic option in treating SCI (166).
ATI-355 is one type of monoclonal antibody against
Nogo-A and can significantly inhibit the adult CNS myelin component
(167). It can markedly impede
neurite growth and plasticity in adult CNS, enhance axon growth and
regeneration, and stabilize neuronal circuits (168). Blocking myelin protein Nogo-A
function or its signaling pathway is an emerging strategy to
suppress the release of neurite growth inhibitory factor of the
adult central CNS and improve the axonal regeneration
micro-environment and plasticity following SCI (168). Anti-Nogo-A antibody treatment
following SCI can significantly enhance axonal regeneration at the
injured tissue area, and improve compensatory sprouting and
functional recovery (165,169). Similar therapeutic effects are
found in a SCI macaque monkey model (170). A study evaluated the potential
therapeutic effects of acute, as well as 1 or 2 weeks delayed
intrathecal anti-Nogo-A antibody infusions, on corticospinal tract
(CST) axon regeneration and motor function recovery following
thoracic SCI in experimental rat models (165). Lesioned CST fibers regenerated
over several mm following acute or 1 week-delayed treatments, but
not when the antibody treatment was started following a delay of 2
weeks. Notably, Anti-Nogo-A antibodies administration can
significantly neutralize inhibitory effects on neurite growth of
purified or recombinant Nogo-A, oligodendrocytes and CNS myelin
in vitro (171). A phase-I
human clinical trial with humanized anti-Nogo antibody in patients
with SCI has been completed in Europe and the United States
(172).
Cyclosporin A and FK-506 are neuroimmunophilin
ligands, which exhibit neuroprotective activity in experimental
models, including those of ischemia, trauma and neurogenerative CNS
disorders (173–176). These drugs are commonly used as
immunosuppressants and are capable of crossing the BBB (173,176). GP-1046 is a novel neuroimunophilin
ligand, which was initially synthesized by Gold et al
(177) but was then replaced with
NIL-A, which binds with FK506 binding protein (FKBP)-12 in neuronal
tissues. NIL-A is currently in phase-II clinical trials for the
treatment of SCI (177). Vertex
Pharmaceuticals has conducted a preclinical trial on the
neurophilin ligand, V10367, which can significantly bind to the
FK-506 binding protein, FKBP-12 (178). Results and opinions on the
clinical use of neuroimmunophilin ligands to enhance neuronal
outgrowth are conflicting; therefore, additional studies are needed
for further validation (178).
There are different ways to administer neurotrophic
factors to the injured spinal cord. Direct injection, continuous
injection and growth factor-saturated gel are typically used,
although these methods are not that efficient (179). However, collagenases are currently
used, which involve collagen-based drug therapy as an improved
method for the effective delivery of neurotrophic factors (180). An alternative approach that can
overcome these shortcomings is ex vivo therapy. This
involves removing cells (including Schwann cells and fibroblasts)
from the host, genetically modifying them in vitro to
synthesize specific neurotrophic factors, expanding the cells in
culture and re-transplanting them into the host (180,181). The technique provides a
significant long-term, localized, high-dose growth factor upon
delivery. Gene therapies are currently being pursued by Selective
Genetics, Cell Genesys, Biovex, Oxford Biomedica and Amsterdam
Molecular Therapeutics (182).
Ex vivo therapies are not very effective in promoting distal
axonal growth after the initial axonal growth, although only a few
studies have been conducted so far (183,184). The adverse effects, complications
and potential risks of gene therapy must be carefully considered
before implementation. However, Proneuron Biotechnologies provides
autotherapy of the larger arteries, where the cells of the patient
are removed, activated and returned to stimulate nerve regeneration
(185). Some preclinical studies
show that macrophages or cytokines can be injected to promote
inflammatory responses and functional outcomes (186,187). Efforts should be made to determine
the potentially destructive role of macrophages in removing cell
debris, promoting tissue blood flow, reconstruction, restoring
phagocytic capacity and inducing cellular proliferation around the
injured spinal cord by releasing factors to stimulate scar
formation (30). This technology is
currently in phase-I clinical trials.
Following SCI, axons of the CNS in adult mammals
cannot regenerate correctly, resulting in permanent paralysis.
Glial reaction occurs at the injured area, forming a glial scar
(188). The glial response leads
to the recruitment of microglia, oligodendrocyte precursors,
meningeal cells, astrocytes and stem cells, as well as
oligodendrocytes and myelin fragments (189,190). The majority of these cells release
molecules and inhibit axon regeneration, which leads to
regeneration failure (177,178).
The glial scar also contains chondroitin sulfate proteoglycan
(CSPG), a recovery inhibitor (191). However, the natural bacterial
enzyme ChABC can degrade the inhibitory carbohydrate side chains on
CSPG. Indeed, ChABC administration following SCI can promote
corticospinal cord and sensory axon regeneration, and enhance
functional outcomes (192). These
growth-promoting effects of ChABC are due to the elimination of
perineuronal nets, increased germination of spare axons and the
formation of new synaptic connections under the injured sites
(180). ChABC may enhance axonal
regeneration in the severed axon on the bridge (192). The potential therapeutic effects
of ChABC administration have been identified in rodent models of
SCI, nigrostriatal injury and stroke, and cats with SCI (193–195). In a study in male Wistar rats,
using a combination of low-level laser therapy (LLLT) as an
anti-inflammatory agent and ChABC as a CSPG digesting factor after
inducing SCI by clip compression, the combination of LLLT and ChABC
produced beneficial effects in the form of reductions in cavity
size, increased myelination and number of axons around the cavity,
and reduced glycogen synthase kinase-3β, CSPG and aquaporin 4
expression compared with LLLT and ChABC alone, resulting in greater
functional recovery in the combination group (196). ChABC treatment in rats can restore
postsynaptic activity under injury, which is significant for the
electrical stimulation of corticospinal neurons and enhances the
functional recovery of motor and proprioceptive behavior (197).
After 4 weeks of acute SCI, ChABC combined with
rehabilitation therapy can also promote functional recovery
(198). In a recent study on dogs
with severe chronic SCI, intracerebral injection of ChABC
demonstrates impressive results (199) and the authors suggest that human
trials be commenced. The effect of ChABC has been evaluated in
rhesus monkeys that had undergone C7-spinal cord hemisection
(200,201). At 4 weeks after hemisection,
multiple-intraparenchymal ChABC injections were administered below
the lesion area, targeting spinal cord circuits, which regulate
hand functions (202,203). Compared with the vehicle injection
control group, the hand function of the monkeys treated with ChABC
was significantly improved. In addition, ChABC can substantially
increase the regeneration of corticospinal axons and synapses
formed by corticospinal terminals in the gray matter caudal to the
lesion (202). No harmful effects
were identified. This method may be helpful for the clinical
treatment of SCI. Although no human trial has been carried out thus
far, the results of animal studies are promising and point to the
potential benefits of ChABC in SCI treatment.
Previous studies show that recovery of motor and
sensory function following SCI is the most crucial and challenging
step in rehabilitation (194,195). In addition, axonal regeneration
and functional recovery following SCI are limited and challenging,
especially in patients with SCI, whose paralysis duration can last
a year or more. ACP is a simple, inexpensive and safe treatment,
which is widely used for improving the recovery of motor and
sensory function in patients with SCI (204). ACP can not only improve motor
function but also promote nerve recovery (205,206). Electroacupuncture can inhibit the
proliferation of astrocytes, downregulate the levels of
platelet-derived growth factor and enhance motor neurons in the
hind limbs of rats by inhibiting neuronal apoptosis and regulating
the expression of related genes (207,208). ACP can significantly enhance the
recovery of the function of motor neurons in the anterior horn of
rats with SCI by augmenting the activity of acetylcholinesterase,
which can upregulate the activity of neurotrophic factor mRNA
(208). A study investigating the
benefits of ACP therapy in neurological recovery following SCI
reported that ACP therapy can substantially improve neurological
and motor function recovery (204). The study also indicated that
studies involving acute cases of SCI and those using different ACP
treatments can achieve more significant therapeutic results in
treating SCI.
Another study evaluated the beneficial effects of
massage and exercise therapy on C5-C7 SCI (209). MT can improve upper body muscle
strength and range of motion, and enhance function (209). Previous studies suggested that MT
increases the range of activity and reduces the level of pain in
patients with lower back pain, such as dancers (202,210). MT also reduces stiffness, pain and
fatigue in patients with fibromyalgia (209,211). Furthermore, MT can reduce the
symptoms of anxiety and depression in patients with SCI (212). Notably, MT reduces self-reported
anxiety and depression, decreases the levels of stress hormones
(cortisol and noradrenaline) and increases serotonin levels in
adults (210). Exercise programs
can enhance muscle strength, the range of motion and function and
reduce depression and anxiety in patients with SCI (213). Patients with SCI who receive MT
twice a week for 5 weeks demonstrate a significant enhancement in
muscle strength, as well as in fine motor (wrist) range, compared
with those in an exercise group (210). It was previous reported that an
improvement in muscle strength and range of motion could
significantly reduce depression and anxiety in patients with SCI
(201). A study that evaluated the
psychological aspects of patients show that MT can significantly
mitigate depression and anxiety (209). Depression is common in patients
with SCI and negatively affects their quality of life (214). Therefore, these findings indicate
that patients with SCI may benefit from MT. A detailed
investigation is required to evaluate MT for other SCI issues, such
as spasticity and pain. Additionally, further studies are required
to assess the potential effect of MT in the lower extremities of
patients with SCI, especially those with affected C5-C7 vertebrae,
in improving circulation and reducing muscle atrophy.
Electrotherapy is a non-invasive and inexpensive
technique. Thus, a number of physical therapists recommend it to
treat patients with SCI (215).
TENS is generally used to relieve pain during electrotherapy
(211). Increasing evidence
reveals that TENS is a safe treatment intervention with fewer side
effects for most patients compared with other existing therapies
(204). Several clinical
investigations report that TENS has clear benefits in the
management of SCI; however, there are controversies regarding
treatment conditions and the appropriate parameters that should be
adhered to during therapy using TENS (216,217). Some randomized controlled trials
have explored the pain relief that can potentially be achieved
using TENS and investigated its potential benefits in patients with
SCI (218,219). A randomized clinical trial
reported that TENS could significantly relieve pain in patients
with SCI (220). Based on the
visual analog scale, present pain intensity-T, pain rating index-S,
pain rating index-A, present pain intensity and number of words
chosen outcomes, a study indicated that 12 weeks of TENS could
relieve pain in patients with SCI (220). Notably, the pain levels were
significantly reduced in the TENS-treated patients with SCI
(219,221). These results also indicate that
TENS induces substantial effects in patients with SCI. A total of
33 patients with SCI were enrolled and were randomly assigned to
the TENS and control groups (222). Patients in the TENS groups
received 30 min of TENS, while those in the control group were
assigned 30 min of sham TENS, once a day for 10 days. Hagen and
Rekand (223) demonstrated that
TENS could efficaciously relieve the pain of patients with SCI. In
conclusion, TENS can be a useful approach to treat patients with
SCI.
EXO using robotic suits is widely used for the
rehabilitation of patients with SCI (224). EXO provides an alternative
opportunity for patients with SCI to experience standing and
walking at a low metabolic cost (224). EXO-supported walking can
considerably reduce spasticity and enhance bowel movement (225). A frequency of 2–3 times or more
per week for 1–2 h can be beneficial to the rehabilitation of
patients with SCI (224). Using
EXO-supported walking to enhance physical activity level may be
attractive for patients with SCI (224). Before the development of EXO,
mobility options beyond a wheelchair were few for the majority of
patients with SCI lacking leg movement (226). Robotic EXOs have become
increasingly popular, and it is now possible to use them for
personal purposes in families and communities (227). However, it is required for users
to set realistic expectations. Robotic EXOs may allow individuals
with SCI with diverse levels of injury to safely and functionally
walk for personal mobility or exercise (226). In addition to the potential
cardiovascular benefits, physical activities and proper standing
may minimize the risk of contracture, osteoporosis, cramps,
pressure sores and edema in patients with SCI, especially when used
early after injury (228). There
is evidence that the use of EXOs affects other health aspects
(229). Karelis et al
(230) reported that after 6 weeks
of EXO training the BMD of the tibia increased by 14.5%, which may
have clinical significance, and the BMD of subcutaneous and
muscular adipose tissue decreased by 5%. EXO can significantly
improve the physical aspects, including body composition and bone
health condition (231). Similar
to the way the amount of physical labor depends on individual
factors, individual factors may also affect the degree of influence
of exoskeleton on bone health (232). The effects on bone health can also
be affected by different factors. For example, individuals who use
EXO without the physical support of others may experience a
significant effect on bone health compared with those who require
physical support, because the ground reaction force of walking with
the help of SCI exoskeletons is similar to healthy walking
(233).
The pathophysiological processes following SCI are
highly complex and the extent of our knowledge concerning these
processes is limited. This is evident from the slow advancement of
currently available neuroprotective methods compared with rapid
trauma revitalization and other clinical interventions. Emerging
studies continue to be added to the existing literature, comprising
studies on inflammation, dysregulation, lipid peroxidation and
apoptosis, which may be considered while developing suitable
pharmacological therapies. Although drugs such as
methylprednisolone, GM-1ganglioside and sodium channel blockers
have undergone several clinical trials, other pharmacological
agents and therapies have been reported to be efficacious in animal
models of SCI. Additionally, some drugs show potential as
candidates that can be further pursued as viable treatment options
in the management of SCIs. Thus, this review considered and
discussed various factors that are involved in the etiology,
detection and management of SCIs, with a focus on the availability
and use of current pharmacological and non-pharmacological
therapies for this debilitating condition.
Not applicable.
The present study was partly supported by research
grants from the National Natural Science Funding of China (grant
nos. 81801233 and 81870842) and the Zhejiang Provincial Natural
Science Foundation of China (grant no. LQ18H090011).
Not applicable.
YZ and AAM wrote the paper. YY, QL, JX and SY
participated in literature collection and producing the figures. CW
participated in literature collection and edited the manuscript. YW
and JW conceived the study and wrote the manuscript. All authors
reviewed and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hamid R, Averbeck MA, Chiang H, Garcia A,
Al Mousa RT, Oh SJ, Patel A, Plata M and Del Popolo G: Epidemiology
and pathophysiology of neurogenic bladder after spinal cord injury.
World J Urol. 36:1517–1527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu HZ, Granger N and Jeffery ND:
Pathophysiology, clinical importance, and management of neurogenic
lower urinary tract dysfunction caused by suprasacral spinal cord
injury. J Vet Intern Med. 30:1575–1588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elliott CS, Dallas KB, Zlatev D, Comiter
CV, Crew J and Shem K: Volitional voiding of the bladder after
spinal cord injury: Validation of bilateral lower extremity motor
Function as a key predictor. J Urol. 200:154–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma HS: Early microvascular reactions
and blood-spinal cord barrier disruption are instrumental in
pathophysiology of spinal cord injury and repair: Novel therapeutic
strategies including nanowired drug delivery to enhance
neuroprotection. J Neural Transm (Vienna). 118:155–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tator CH: Experimental and clinical
studies of the pathophysiology and management of acute spinal cord
injury. J Spinal Cord Med. 19:206–214. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hulsebosch CE: Recent advances in
pathophysiology and treatment of spinal cord injury. Adv Physiol
Educ. 26:238–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tator CH and Deecke L: Studies of the
treatment and pathophysiology of acute spinal cord injury in
primates. Paraplegia. 10:344–345. 1973.PubMed/NCBI
|
|
8
|
Xiong Y and Hall ED: Pharmacological
evidence for a role of peroxynitrite in the pathophysiology of
spinal cord injury. Exp Neurol. 216:105–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wasner G, Naleschinski D and Baron R: A
role for peripheral afferents in the pathophysiology and treatment
of at-level neuropathic pain in spinal cord injury? A case report.
Pain. 131:219–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaidyanathan S, Soni BM, Sett P, Watt JW,
Oo T and Bingley J: Pathophysiology of autonomic dysreflexia:
Long-term treatment with terazosin in adult and paediatric spinal
cord injury patients manifesting recurrent dysreflexic episodes.
Spinal Cord. 36:761–770. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Austin JW, Afshar M and Fehlings MG: The
relationship between localized subarachnoid inflammation and
parenchymal pathophysiology after spinal cord injury. J
Neurotrauma. 29:1838–1849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yezierski RP: Pain following spinal cord
injury: Pathophysiology and central mechanisms. Prog Brain Res.
129:429–449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nickel M and Gu C: Regulation of central
nervous system myelination in higher brain functions. Neural Plast.
2018:64364532018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fehlings MG and Agrawal S: Role of sodium
in the pathophysiology of secondary spinal cord injury. Spine
(Phila Pa 1976). 20:2187–2191. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwartz G and Fehlings MG: Secondary
injury mechanisms of spinal cord trauma: A novel therapeutic
approach for the management of secondary pathophysiology with the
sodium channel blocker riluzole. Prog Brain Res. 137:177–190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma HS, Patnaik R, Sharma A, Sjoquist
PO and Lafuente JV: Silicon dioxide nanoparticles (SiO2, 40–50 nm)
exacerbate pathophysiology of traumatic spinal cord injury and
deteriorate functional outcome in the rat. An experimental study
using pharmacological and morphological approaches. J Nanosci
Nanotechnol. 9:4970–4980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valles M and Mearin F: Pathophysiology of
bowel dysfunction in patients with motor incomplete spinal cord
injury: Comparison with patients with motor complete spinal cord
injury. Dis Colon Rectum. 52:1589–1597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agrawal SK, Nashmi R and Fehlings MG: Role
of L- and N-type calcium channels in the pathophysiology of
traumatic spinal cord white matter injury. Neuroscience.
99:179–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furlan JC and Fehlings MG: Cardiovascular
complications after acute spinal cord injury: Pathophysiology,
diagnosis, and management. Neurosurg Focus. 25:E132008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayta E and Elden H: Acute spinal cord
injury: A review of pathophysiology and potential of non-steroidal
anti-inflammatory drugs for pharmacological intervention. J Chem
Neuroanat. 87:25–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hall ED: Pathophysiology of spinal cord
injury. Current and future therapies. Minerva Anestesiol. 55:63–66.
1989.PubMed/NCBI
|
|
22
|
Segal JL: Immunoactivation and altered
intercellular communication mediate the pathophysiology of spinal
cord injury. Pharmacotherapy. 25:145–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delamarter RB, Sherman J and Carr JB:
Pathophysiology of spinal cord injury. Recovery after immediate and
delayed decompression. J Bone Joint Surg Am. 77:1042–1049. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santos-Nogueira E, López-Serrano C,
Hernandez J, Lago N, Astudillo AM, Balsinde J, Estivill-Torrús G,
de Fonseca FR, Chun J and López-Vales R: Activation of
Lysophosphatidic acid receptor type 1 contributes to
pathophysiology of spinal cord injury. J Neurosci. 35:10224–10235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma HS, Badgaiyan RD, Alm P, Mohanty S
and Wiklund L: Neuroprotective effects of nitric oxide synthase
inhibitors in spinal cord injury-induced pathophysiology and motor
functions: An experimental study in the rat. Ann N Y Acad Sci.
1053:422–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karimi-Abdolrezaee S, Eftekharpour E and
Fehlings MG: Temporal and spatial patterns of Kv1.1 and Kv1.2
protein and gene expression in spinal cord white matter after acute
and chronic spinal cord injury in rats: Implications for axonal
pathophysiology after neurotrauma. Eur J Neurosci. 19:577–589.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tator CH: Acute spinal cord injury: A
review of recent studies of treatment and pathophysiology. Can Med
Assoc J. 107:143–145. 1972.PubMed/NCBI
|
|
28
|
Sharma HS, Muresanu DF, Sharma A, Patnaik
R and Lafuente JV: Chapter 9-nanoparticles influence
pathophysiology of spinal cord injury and repair. Prog Brain Res.
180:154–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neal JM, Kopp SL, Pasternak JJ, Lanier WL
and Rathmell JP: Anatomy and pathophysiology of spinal cord injury
associated with regional anesthesia and pain medicine: 2015 update.
Reg Anesth Pain Med. 40:506–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun
L, Guo L, Bai P, Sun D, Fan J, et al: Macrophages in spinal cord
injury: Phenotypic and functional change from exposure to myelin
debris. Glia. 63:635–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eldahan KC and Rabchevsky AG: Autonomic
dysreflexia after spinal cord injury: Systemic pathophysiology and
methods of management. Auton Neurosci. 209:59–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sharma HS: Neurotrophic factors in
combination: A possible new therapeutic strategy to influence
pathophysiology of spinal cord injury and repair mechanisms. Curr
Pharm Des. 13:1841–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siddiqui TA, Lively S and Schlichter LC:
Complex molecular and functional outcomes of single versus
sequential cytokine stimulation of rat microglia. J
Neuroinflammation. 13:662016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou LH, Ouyang L, Lin SZ, Chen S, Liu Y,
Zhou W and Wang X: Protective role of β-carotene against oxidative
stress and neuroinflammation in a rat model of spinal cord injury.
Int Immunopharmacol. 61:92–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao X, Zhang Y, Hao J, Duan HQ, Zhao CX,
Sun C, Li B, Fan BY, Wang X, Li WX, et al: Deferoxamine promotes
recovery of traumatic spinal cord injury by inhibiting ferroptosis.
Neural Regen Res. 14:532–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang D, Wang F, Zhai X, Li XH and He XJ:
Lithium promotes recovery of neurological function after spinal
cord injury by inducing autophagy. Neural Regen Res. 13:2191–2199.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou LY, Song ZF, Zhou LW, Qiu Y, Hu N, Hu
Y and Hu X: Protective role of astragalus injection in spinal cord
ischemia-reperfusion injury in rats. Neurosciences (Riyadh).
23:116–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma HS: Pathophysiology of blood-spinal
cord barrier in traumatic injury and repair. Curr Pharm Des.
11:1353–1389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aarabi B, Olexa J, Chryssikos T, Galvagno
SM, Hersh DS, Wessell A, Sansur C, Schwartzbauer G, Crandall K, et
al: Extent of Spinal Cord Decompression in Motor Complete (American
Spinal Injury Association Impairment Scale Grades A and B)
Traumatic Spinal Cord Injury Patients: Post-Operative Magnetic
Resonance Imaging Analysis of Standard Operative Approaches. J
Neurotrauma. 36:862–876. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akhmetzyanova ER, Mukhamedshina YO,
Zhuravleva MN, Galieva LR, Kostennikov AA, Garanina EE and Rizvanov
AA: Transplantation of microglia in the area of spinal cord injury
in an acute period increases tissue sparing, but not functional
recovery. Front Cell Neurosci. 12:5072018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aguiar SA, Baker SN, Gant K, Bohorquez J
and Thomas CK: Spasms after spinal cord injury show low-frequency
intermuscular coherence. J Neurophysiol. 120:1765–1771. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang Y, Liu HL, Min LX, Yuan HS, Guo L,
Han PB, Lu YX, Zhong JF and Wang DL: Serum and cerebrospinal fluid
tau protein level as biomarkers for evaluating acute spinal cord
injury severity and motor function outcome. Neural Regen Res.
14:896–902. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alizadeh A, Santhosh K, Kataria H and
Karimi-Abdolrezaee S: Neuregulin-1 positively modulates
neuroinflammation and improves functional recovery following
traumatic spinal cord injury. J Neurotrauma. 35:A32. 2018.
|
|
44
|
Baklaushev VP, Durov OV, Kim SV, Gulaev
EV, Gubskiy IL, Konoplyannikov MA, Zabozlaev FG, Zhang C, Agrba VZ,
Orlov SV, et al: Development of a motor and somatosensory evoked
potentials-guided spinal cord Injury model in non-human primates. J
Neurosci Methods. 311:200–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bonizzato M, Pidpruzhnykova G, DiGiovanna
J, Shkorbatova P, Pavlova N, Micera S and Courtine G:
Brain-controlled modulation of spinal circuits improves recovery
from spinal cord injury. Nat Commun. 9:30152018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Burnside ER, De Winter F, Didangelos A,
James ND, Andreica EC, Layard-Horsfall H, Muir EM, Verhaagen J and
Bradbury EJ: Immune-evasive gene switch enables regulated delivery
of chondroitinase after spinal cord injury. Brain. 141:2362–2381.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Casper DS, Zmistowski B, Schroeder GD,
McKenzie JC, Mangan J, Vatson J, Hilibrand AS, Vaccaro AR and
Kepler CK: Preinjury patient characteristics and postinjury
neurological status are associated with mortality following spinal
cord injury. Spine (Phila Pa 1976). 43:895–899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
David S, Kroner A, Greenhalgh AD, Zarruk
JG and Lopez-Vales R: Myeloid cell responses after spinal cord
injury. J Neuroimmunol. 321:97–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chhaya SJ, Quiros-Molina D,
Tamashiro-Orrego AD, Houle JD and Detloff MR: Exercise-induced
changes to the macrophage response in the dorsal root ganglia
prevent neuropathic pain after spinal cord injury. J Neurotrauma.
36:877–890. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chi LY, Yu J, Zhu H, Li XG, Zhu SG and
Kindy MS: The dual role of tumor necrosis factor-alpha in the
pathophysiology of spinal cord injury. Neurosci Lett. 438:174–179.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lv RX, Du LL, Zhang LX and Zhang ZQ:
Polydatin attenuates spinal cord injury in rats by inhibiting
oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway.
Life Sci. 217:119–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ziegler G, Grabher P, Thompson A, Altmann
D, Hupp M, Ashburner J, Friston K, Weiskopf N, Curt A and Freund P:
Progressive neurodegeneration following spinal cord injury
Implications for clinical trials. Neurology. 90:e1257–e1266. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou QS, Xiang HK, Li A, Lin W, Huang Z,
Guo J, Wang P, Chi Y, Xiang K, Xu Y, et al: Activating adiponectin
signaling with exogenous AdipoRon reduces myelin lipid accumulation
and suppresses macrophage recruitment after spinal cord injury. J
Neurotrauma. 36:903–918. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cizkova D, Cubinkova V, Smolek T, Murgoci
AN, Danko J, Vdoviakova K, Humenik F, Cizek M, Quanico J, Fournier
I, Salzet M; Correction, ; Cizkova D..et al: Localized intrathecal
delivery of mesenchymal stromal cells conditioned media improves
functional recovery in a rat model of contusive spinal cord injury.
Int. J. Mol. Sci. 2018.19, 870. Int J Mol Sci 19: 1942, 2018.
View Article : Google Scholar
|
|
55
|
de Menezes MF, Nicola F, Vital da Silva
IR, Vizuete A, Elsner VR, Xavier LL, Gonçalves CAS, Netto CA and
Mestriner RG: Glial fibrillary acidic protein levels are associated
with global histone H4 acetylation after spinal cord injury in
rats. Neural Regen Res. 13:1945–1952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Faden AI and Holaday JW: A role for
endorphins in the pathophysiology of spinal cord injury. Adv
Biochem Psychopharmacol. 28:435–446. 1981.PubMed/NCBI
|
|
57
|
O'Hare Doig RL, Santhakumar S, Fehily B,
Raja S, Solomon T, Bartlett CA, Fitzgerald M and Hodgetts SI: Acute
cellular and functional changes with a combinatorial treatment of
ion channel inhibitors following spinal cord injury. Front Mol
Neurosci. 13(85)https://doi.org/10.3389/fnmol.2020.000852020.
|
|
58
|
Durdag E, Yildirim Z, Unlu NL, Kale A and
Ceviker N: Neuroprotective effects of vigabatrin on spinal cord
ischemia-reperfusion injury. World Neurosurg. 120:e33–e41. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dietrich WD: Clinical significance and
potential translation of neural regeneration and functional
recovery in monkeys after spinal cord injury. Sci China Life Sci.
61:1291–1292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Olsson Y, Sharma HS, Nyberg F and Westman
J: The opioid receptor antagonist naloxone influences the
pathophysiology of spinal cord injury. Prog Brain Res. 104:381–399.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Young W, Flamm ES, Demopoulos HB, Tomasula
JJ and DeCrescito V: Effect of naloxone on posttraumatic ischemia
in experimental spinal contusion. J Neurosurg. 55:209–219. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yadollahi M, Kashkooe A, Habibpour E and
Jamali K: Prevalence and risk factors of spinal trauma and spinal
cord injury in a trauma center in Shiraz, Iran. Iran Red Crescent
Med J. Feb;2018.doi: 10.5812/ircmj.14238. View Article : Google Scholar
|
|
63
|
Hogan MK, Zhao TY, Kondiles B, Sellers D,
Pun SZ and Horner P: Controlled release of thrombin-inhibitor from
injectable hydrogel modulates gliosis after spinal cord injury. J
Neurotrauma. 35:A37. 2018.
|
|
64
|
Huber E, David G, Thompson AJ, Weiskopf N,
Mohammadi S and Freund P: Dorsal and ventral horn atrophy is
associated with clinical outcome after spinal cord injury.
Neurology. 90:e1510–e1522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang WH, Bai XS, Stopper L, Catalin B,
Cartarozzi LP, Scheller A and Kirchhoff F: During development NG2
glial cells of the spinal cord are restricted to the
oligodendrocyte lineage, but generate astrocytes upon acute injury.
Neuroscience. 385:154–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hupp M, Pavese C, Bachmann LM, Koller R
and Schubert M; EMSCI Study Group, : Electrophysiological
multimodal assessments improve outcome prediction in traumatic
cervical spinal cord injury. J Neurotrauma. 35:2916–2923. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kaptanoglu E, Okutan O, Akbiyik F,
Solaroglu I, Kilinc A and Beskonakli E: Correlation of injury
severity and tissue Evans blue content, lipid peroxidation and
clinical evaluation in acute spinal cord injury in rats. J Clin
Neurosci. 11:879–885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Irrera N, Arcoraci V, Mannino F, Vermiglio
G, Pallio G, Minutoli L, Bagnato G, Anastasi GP, Mazzon E, Bramanti
P, et al: Activation of A2A receptor by PDRN reduces neuronal
damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal
cord injury. Front Pharmacol. 9:5062018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huo J, Ma R, Chai X, Liang HJ, Jiang P,
Zhu XL, Chen X and Su BX: Inhibiting a spinal cord signaling
pathway protects against ischemia injury in rats. J Thorac
Cardiovasc Surg. 157:494–503.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Werner C and Engelhard K: Pathophysiology
of traumatic brain injury. Br J Anaesth. 99:4–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li Y, Gu R, Zhu QS and Liu JC: Changes of
spinal edema and expression of aquaporin 4 in
methylprednisolone-treated rats with spinal cord injury. Ann Clin
Lab Sci. 48:453–459. 2018.PubMed/NCBI
|
|
72
|
Jaja B, Jiang F, Badhiwala J, Fehlings MG
and Wilson J: Neurological recovery and functional outcomes
following acquired infections after acute spinal cord injury. J
Neurotrauma. 35:A177–A178. 2018.
|
|
73
|
Lanza M, Campolo M, Casili G, Filippone A,
Cuzzocrea S and Esposito E: Sodium butyrate exerts neuroprotective
effects in spinal cord injury. FASEB J. 32:8242018.
|
|
74
|
DeForge D, Nymark J, Lemaire E, Gardner S,
Hunt M, Martel L, Curran D and Barbeau H: Effect of 4-aminopyridine
on gait in ambulatory spinal cord injuries: A double-blind,
placebo-controlled, crossover trial. Spinal Cord. 42:674–685. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hall ED: Antioxidant therapies for acute
spinal cord injury. Neurotherapeutics. 8:152–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang HJ, Cahoon R, Cahoon EB, Zheng H,
Patel KP and Zucker IH: Glutamatergic receptor dysfunction in
spinal cord contributes to the exaggerated exercise pressor reflex
in heart failure. Am J Physiol Heart Circ Physiol. 308:H447–H455.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Winkler T, Sharma HS, Stalberg E,
Badgaiyan RD, Gordh T and Westman J: An L-type calcium channel
blocker, nimodipine influences trauma induced spinal cord
conduction and axonal injury in the rat. Acta Neurochir Suppl.
86:425–432. 2003.PubMed/NCBI
|
|
78
|
Kusuyama K, Tachibana T, Yamanaka H, Okubo
M, Yoshiya S and Noguchi K: Upregulation of calcium channel
alpha-2-delta-1 subunit in dorsal horn contributes to spinal cord
injury-induced tactile allodynia. Spine J. 18:1062–1069. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen YM, Wang BL and Zhao H: Thymoquinone
reduces spinal cord injury by inhibiting inflammatory response,
oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways.
Exp Ther Med. 15:4987–4994. 2018.PubMed/NCBI
|
|
80
|
Damon Y, Kitano Y and Makino M: Analgesic
effects of the novel α2δ ligand mirogabalin in a rat
model of spinal cord injury. Pharmazie. 73:659–661. 2018.PubMed/NCBI
|
|
81
|
Chaves RHD, de Souza CC, Furlaneto IP,
Teixeira RKC, Oliveira CP, Rodrigues EM, Santos DASD, Silva RC,
Penha NEAD and Lima AR: Influence of tramadol on functional
recovery of acute spinal cord injury in rats. Acta Cir Bras.
33:1087–1094. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harada N, Taoka Y and Okajima K: Role of
prostacyclin in the development of compression trauma-induced
spinal cord injury in rats. J Neurotrauma. 23:1739–1749. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sohn S: Ursodeoxycholic acid inhibits
inflammatory responses and promotes functional recovery after
spinal cord injury in rats. J Neurotrauma. 35:A172. 2018.
|
|
84
|
Pallottie A, Ratnayake A, Ni L, Acioglu C,
Li L, Mirabelli E, Heary RF and Elkabes S: A toll-like receptor 9
antagonist restores below-level glial glutamate transporter
expression in the dorsal horn following spinal cord injury. Sci
Rep. 8:87232018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cai J, Sun Y, Yin Z, Wang D, Shi K, Fu Y,
Cao X and Ge Y: Analysis of FK506-mediated functional recovery and
neuroprotection in a rat model of spinal cord injury indicates that
EGF is modulated in astrocytes. Exp Ther Med. 16:501–510.
2018.PubMed/NCBI
|
|
86
|
Kim YH, Ha KY and Kim SI: Spinal cord
injury and related clinical trials. Clin Orthop Surg. 9:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mori E, Ueta T, Maeda T, Ideta R, Yugué I,
Kawano O and Shiba K: Sequential neurological improvements after
conservative treatment in patients with complete motor paralysis
caused by cervical spinal cord injury without bone and disc injury.
J Neurosurg Spine. 29:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W,
Lin W, Li Y, Fu H and Li S: Valproic acid attenuates traumatic
spinal cord injury-induced inflammation via STAT1 and NF-κB pathway
dependent of HDAC3. J Neuroinflammation. 15:1502018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mongardon N, Kohlhauer M, Lidouren F,
Barretto M, Micheau P, Adam C, Dhonneur G, Ghaleh B and Tissier R:
Targeted temperature management with total liquid ventilation after
ischemic spinal cord injury. Ann Thorac Surg. 106:1797–1803. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chang MC: Spinal Cord injury by direct
damage during CT-Guided C7 transforaminal epidural steroid
injection. Am J Phys Med Rehabil. 97:e62–e64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sámano C and Nistri A: Mechanism of
neuroprotection against experimental spinal cord injury by riluzole
or methylprednisolone. Neurochem Res. 44:200–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ye JC, Qin Y, Tang Y, Ma M, Wang P, Huang
L, Yang R, Chen K, Chai C, Wu Y and Shen H: Methylprednisolone
inhibits the proliferation of endogenous neural stem cells in
nonhuman primates with spinal cord injury. J Neurosurg Spine.
29:199–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chu TH, Cummins K and Stys PK: The triple
monoamine re-uptake inhibitor DOV 216,303 promotes functional
recovery after spinal cord contusion injury in mice. Neurosci Lett.
675:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ko MY, Jang EY, Lee JY, Kim SP, Whang SH,
Lee BH, Kim HY, Yang CH, Cho HJ and Gwak YS: The role of ventral
tegmental area gamma-aminobutyric acid in chronic neuropathic pain
after spinal cord injury in rats. J Neurotrauma. 35:1755–1764.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fehlings MG, Tator CH and Linden RD: The
effect of nimodipine and dextran on axonal function and blood flow
following experimental spinal cord injury. J Neurosurg. 71:403–416.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Rosenberg LJ, Teng YD and Wrathall JR:
Effects of the sodium channel blocker tetrodotoxin on acute white
matter pathology after experimental contusive spinal cord injury. J
Neurosci. 19:6122–6133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Caglar YS, Demirel A, Dogan I, Huseynov R,
Eroglu U, Ozgural O, Cansiz C, Bahadir B, Kilinc MC and Al-Beyati
ESM: Effect of riluzole on spinal cord regeneration with
hemisection method before injury. World Neurosurg. 114:e247–e253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Verma R, Virdi JK, Singh N and Jaggi AS:
Animals models of spinal cord contusion injury. Korean J Pain.
32:12–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nagoshi N, Nakashima H and Fehlings MG:
Riluzole as a neuroprotective drug for spinal cord injury: From
bench to bedside. Molecules. 20:7775–7789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fehlings MG, Nakashima H, Nagoshi N, Chow
DS, Grossman RG and Kopjar B: Rationale, design and critical end
points for the Riluzole in acute spinal cord injury study (RISCIS):
A randomized, double-blinded, placebo-controlled parallel
multi-center trial. Spinal Cord. 54:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Grijalva I, García-Pérez A, Díaz J,
Aguilar S, Mino D, Santiago-Rodríguez E, Guizar-Sahagún G,
Castañeda- Hernández G, Maldonado-Julián H and Madrazo I: High
doses of 4-aminopyridine improve functionality in chronic complete
spinal cord injury patients with MRI evidence of cord continuity.
Arch Med Res. 41:567–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Page JC, Park J, Chen Z, Cao P and Shi R:
Parallel evaluation of two potassium channel blockers in restoring
conduction in mechanical spinal cord injury in rat. J Neurotrauma.
35:1057–1068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Darlington C: Fampridine acorda
therapeutics. Curr Opin Investig Drugs. 1:375–379. 2000.PubMed/NCBI
|
|
104
|
Hayes KC: Impact of extended-release
dalfampridine on walking ability in patients with multiple
sclerosis. Neuropsychiatr Dis Treat. 7:229–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Proks P, Reimann F, Green N, Gribble F and
Ashcroft F: Sulfonylurea stimulation of insulin secretion.
Diabetes. 51 (Suppl 3):S368–S376. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kurland DB, Tosun C, Pampori A, Karimy JK,
Caffes NM, Gerzanich V and Simard JM: Glibenclamide for the
treatment of acute CNS injury. Pharmaceuticals (Basel).
6:1287–1303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Caffes N, Kurland DB, Gerzanich V and
Simard JM: Glibenclamide for the treatment of ischemic and
hemorrhagic stroke. Int J Mol Sci. 16:4973–4984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Popovich PG, Lemeshow S, Gensel JC and
Tovar CA: Independent evaluation of the effects of glibenclamide on
reducing progressive hemorrhagic necrosis after cervical spinal
cord injury. Exp Neurol. 233:615–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Simard JM, Tsymbalyuk O, Ivanov A, Ivanova
S, Bhatta S, Geng Z, Woo SK and Gerzanich V: Endothelial
sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate
progressive hemorrhagic necrosis following spinal cord injury. J
Clin Invest. 117:2105–2113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Simard JM, Popovich PG, Tsymbalyuk O and
Gerzanich V: Spinal cord injury with unilateral versus bilateral
primary hemorrhage-effects of glibenclamide. Exp Neurol.
233:829–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Simard JM, Woo SK, Schwartzbauer GT and
Gerzanich V: Sulfonylurea receptor 1 in central nervous system
injury: A focused review. J Cereb Blood Flow Metab. 32:1699–1717.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cheung V, Hoshide R, Bansal V, Kasper E
and Chen CC: Methylprednisolone in the management of spinal cord
injuries: Lessons from randomized, controlled trials. Surg Neurol
Int. 6:1422015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Anwar MA, Al Shehabi TS and Eid AH:
Inflammogenesis of secondary spinal cord injury. Front Cell
Neurosci. 10:982016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bracken MB, Shepard MJ, Hellenbrand KG,
Collins WF, Leo LS, Freeman DF, Wagner FC, Flamm ES, Eisenberg HM,
Goodman JH, et al: Methylprednisolone and neurological function 1
year after spinal cord injury. Results of the national acute spinal
cord injury study. J Neurosurg. 63:704–713. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sayer FT, Kronvall E and Nilsson OG:
Methylprednisolone treatment in acute spinal cord injury: The myth
challenged through a structured analysis of published literature.
Spine J. 6:335–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bracken MB, Shepard MJ, Collins WF,
Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers
L, Maroon J, et al: A randomized, controlled trial of
methylprednisolone or naloxone in the treatment of acute
spinal-cord injury. Results of the second national acute spinal
cord injury study. N Engl J Med. 322:1405–1411. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bracken MB, Shepard MJ, Holford TR,
Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW,
Marshall LF, et al: Administration of methylprednisolone for 24 or
48 hours or tirilazad mesylate for 48 hours in the treatment of
acute spinal cord injury. Results of the third national acute
spinal cord injury randomized controlled trial. National acute
spinal cord injury study. JAMA. 277:1597–1604. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bracken MB: Steroids for acute spinal cord
injury. Cochrane Database Syst Rev. 1:CD0010462012.PubMed/NCBI
|
|
119
|
Bowers CA, Kundu B, Rosenbluth J and
Hawryluk GW: Patients with spinal cord injuries favor
administration of methylprednisolone. PLoS One. 11:e01459912016.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ferrara G, Petrillo MG, Giani T, Marrani
E, Filippeschi C, Oranges T, Simonini G and Cimaz R: Clinical use
and molecular action of corticosteroids in the pediatric age. Int J
Mol Sci. 20:4442019. View Article : Google Scholar
|
|
121
|
Fehlings MG, Wilson JR, Tetreault LA,
Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K,
Dettori JR, et al: A clinical practice guideline for the management
of patients with acute spinal cord injury: Recommendations on the
use of methylprednisolone sodium succinate. Global Spine J. 7
(Suppl 3):203S–211S. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yue JK, Tsolinas RE, Burke JF, Deng H,
Upadhyayula PS, Robinson CK, Lee YM, Chan AK, Winkler EA and Dhall
SS: Vasopressor support in managing acute spinal cord injury:
Current knowledge. J Neurosurg Sci. 63:308–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Resnick DK: Updated guidelines for the
management of acute cervical spine and spinal cord injury.
Neurosurgery. 72 (Suppl 2):S12013. View Article : Google Scholar
|
|
124
|
Mojtahedzadeh M, Taghvaye-Masoumi H,
Najafi A, Dianatkhah M, Sharifnia H and Shahrokhi M: Management of
hypotension and bradycardia caused by spinal cord injury. The
usefulness of midodrine and methylxanthines. Iran J Pharm Res.
18:2131–2135. 2019.PubMed/NCBI
|
|
125
|
Inoue T, Manley GT, Patel N and Whetstone
WD: Medical and surgical management after spinal cord injury:
Vasopressor usage, early surgerys, and complications. J
Neurotrauma. 31:284–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hawryluk G, Whetstone W, Saigal R,
Ferguson A, Talbott J, Bresnahan J, Dhall S, Pan J, Beattie M and
Manley G: Mean arterial blood pressure correlates with neurological
recovery after human spinal cord injury: Analysis of high frequency
physiologic data. J Neurotrauma. 32:1958–1967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yakovlev AG and Faden AI: Mechanisms of
neural cell death: Implications for development of neuroprotective
treatment strategies. NeuroRx. 1:5–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Knoblach SM, Alroy DA, Nikolaeva M, Cernak
I, Stoica BA and Faden AI: Caspase inhibitor z-DEVD-fmk attenuates
calpain and necrotic cell death in vitro and after traumatic brain
injury. J Cereb Blood Flow Metab. 24:1119–1132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hall ED and Springer JE: Neuroprotection
and acute spinal cord injury: A reappraisal. NeuroRx. 1:80–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Squair JW, Ruiz I, Phillip AA, Zheng MMZ,
Sarafis ZK, Sachdeva R, Gopaul R, Liu J, Tetzlaff W, West CR and
Krassioukov AV: Minocycline reduces the severity of autonomic
dysreflexia after experimental spinal cord injury. J Neurotrauma.
35:2861–2871. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhang H, Xiang Z, Duan X, Jiang JL, Xing
YM, Zhu C, Song Q and Yu QR: Antitumor and anti-inflammatory
effects of oligosaccharides from Cistanche deserticola extract on
spinal cord injury. Int J Biol Macromol. 124:360–367. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Scholz R, Sobotka M, Caramoy A, Stempfl T,
Moehle C and Langmann T: Minocycline counter-regulates
pro-inflammatory microglia responses in the retina and protects
from degeneration. J Neuroinflammation. 12:2092015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tikka TM and Koistinaho JE: Minocycline
provides neuroprotection against N-methyl-D-aspartate neurotoxicity
by inhibiting microglia. J Immunol. 166:7527–7533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wells JE, Hurlbert RJ, Fehlings MG and
Yong VW: Neuroprotection by minocycline facilitates significant
recovery from spinal cord injury in mice. Brain. 126:1628–1637.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Curtis E, Martin JR, Gabel B, Sidhu N,
Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T,
Marsala S, et al: A first-in-human, phase I study of neural stem
cell transplantation for chronic spinal cord injury. Cell Stem
Cell. 22:941–950.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Casha S, Zygun D, McGowan MD, Bains I,
Yong VW and Hurlbert RJ: Results of a phase II placebo-controlled
randomized trial of minocycline in acute spinal cord injury. Brain.
135:1224–1236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Barros TE Jr, Araujo FF, Higino Lda P,
Marcon RM and Cristante AF: The effect of monosialoganglyoside
(GM-1) administration in spinal cord injury. Acta Ortop Bras.
24:123–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Marcon RM, Cristante AF, de Barros Filho
TE, de Oliveira RP and dos Santos GB: Potentializing the effects of
GM1 by hyperbaric oxygen therapy in acute experimental spinal cord
lesion in rats. Spinal Cord. 48:808–813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Badhiwala JH, Wilson JR, Kwon BK, Casha S
and Fehlings MG: A Review of clinical trials in spinal cord injury
including biomarkers. J Neurotrauma. 35:1906–1917. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Geisler FH, Dorsey FC and Coleman WP:
Recovery of motor function after spinal-cord injury-a randomized,
placebo- controlled trial with GM-1 ganglioside. N Engl J Med.
324:1829–1838. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lee Y, Kim CY, Lee HJ, Kim JG, Han DW and
Ko K, Walter J, Chung HM, Schöler HR, Bae YM and Ko K: Two-step
generation of oligodendrocyte progenitor cells from mouse
fibroblasts for spinal cord injury. Front Cell Neurosci.
12:1982018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Awad BI, Carmody MA and Steinmetz MP:
Potential role of growth factors in the management of spinal cord
injury. World Neurosurg. 83:120–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ma DN, Zhang XQ, Ying J, Chen ZJ and Li
LX: Efficacy and safety of 9 nonoperative regimens for the
treatment of spinal cord injury: A network meta-analysis. Medicine
(Baltimore). 96:e86792017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Teng YD, Mocchetti I and Wrathall JR:
Basic and acidic fibroblast growth factors protect spinal motor
neurones in vivo after experimental spinal cord injury. Eur J
Neurosci. 10:798–802. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wu JC, Huang WC, Chen YC, Tu TH, Tsai YA,
Huang SF, Huang HC and Cheng H: Acidic fibroblast growth factor for
repair of human spinal cord injury: A clinical trial. J Neurosurg
Spine. 15:216–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gupta V, Mesa RA, Deininger MW, Rivera CE,
Sirhan S, Brachmann CB, Collins H, Kawashima J, Xin Y and
Verstovsek S: A phase 1/2, open-label study evaluating twice-daily
administration of momelotinib in myelofibrosis. Haematologica.
102:94–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Clarke WE, Berry M, Smith C, Kent A and
Logan A: Coordination of fibroblast growth factor receptor 1
(FGFR1) and fibroblast growth factor-2 (FGF-2) trafficking to
nuclei of reactive astrocytes around cerebral lesions in adult
rats. Mol Cell Neurosciences. 17:17–30. 2001. View Article : Google Scholar
|
|
150
|
Raballo R, Rhee J, Lyn-Cook R, Leckman JF,
Schwartz ML and Vaccarino FM: Basic fibroblast growth factor (Fgf2)
is necessary for cell proliferation and neurogenesis in the
developing cerebral cortex. J Neuroscience. 20:5012–5023. 2000.
View Article : Google Scholar
|
|
151
|
Zhou Y, Wang Z, Li J, Li X and Xiao J:
Fibroblast growth factors in the management of spinal cord injury.
J Cell Mol Med. 22:25–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ko CC, Tu TH, Wu JC, Huang WC, Tsai YA,
Huang SF, Huang HC and Cheng H: Functional improvement in chronic
human spinal cord injury: Four years after acidic fibroblast growth
factor. Sci Rep. 8:126912018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wu JC, Huang WC, Tsai YA, Chen YC and
Cheng H: Nerve repair using acidic fibroblast growth factor in
human cervical spinal cord injury: A preliminary phase I clinical
study. J Neurosurg Spine. 8:208–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wallner S, Peters S, Pitzer C, Resch H,
Bogdahn U and Schneider A: The Granulocyte-colony stimulating
factor has a dual role in neuronal and vascular plasticity. Front
Cell Dev Biol. 3:482015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Nishio Y, Koda M, Kamada T, Someya Y,
Kadota R, Mannoji C, Miyashita T, Okada S, Okawa A, Moriya H and
Yamazaki M: Granulocyte colony-stimulating factor attenuates
neuronal death and promotes functional recovery after spinal cord
injury in mice. J Neuropathol Exp Neurol. 66:724–731. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Derakhshanrad N, Saberi H, Yekaninejad MS,
Joghataei MT and Sheikhrezaei A: Granulocyte-colony stimulating
factor administration for neurological improvement in patients with
postrehabilitation chronic incomplete traumatic spinal cord
injuries: A double-blind randomized controlled clinical trial. J
Neurosurg Spine. 29:97–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Koda M, Yamazaki M, Furuya T, Hanaoka H
and Grp GSS: Phase 3 clinical trial of granulocyte colony
stimulating factor-for acute spinal cord injury (G-Spirit Trial). J
Neurotrauma. 35:A162–A163. 2018.
|
|
158
|
Kitamura K, Iwanami A, Nakamura M, Yamane
J, Watanabe K, Suzuki Y, Miyazawa D, Shibata S, Funakoshi H,
Miyatake S, et al: Hepatocyte growth factor promotes endogenous
repair and functional recovery after spinal cord injury. J Neurosci
Res. 85:2332–2342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ohta Y, Takenaga M, Hamaguchi A, Ootaki M,
Takeba Y, Kobayashi T, Watanabe M, Iiri T and Matsumoto N:
Isolation of adipose-derived stem/stromal cells from cryopreserved
fat tissue and transplantation into rats with spinal cord injury.
Int J Mol Sci. 19:19632018. View Article : Google Scholar
|
|
160
|
Kitamura K, Fujiyoshi K, Yamane J, Toyota
F, Hikishima K, Nomura T, Funakoshi H, Nakamura T, Aoki M, Toyama
Y, et al: Human hepatocyte growth factor promotes functional
recovery in primates after spinal cord injury. PLoS One.
6:e277062011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Kitamura K, Nagoshi N, Tsuji O, Matsumoto
M, Okano H and Nakamura M: Application of hepatocyte growth factor
for acute spinal cord Injury: The road from basic studies to human
treatment. Int J Mol Sci. 20:10542019. View Article : Google Scholar
|
|
162
|
Dergham P, Ellezam B, Essagian C,
Avedissian H, Lubell WD and Mckerracher L: Rho signaling pathway
targeted to promote spinal cord repair. J Neurosci. 22:6570–6577.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Siddiqui AM, Khazaei M and Fehlings MG:
Translating mechanisms of neuroprotection, regeneration, and repair
to treatment of spinal cord injury. Prog Brain Res. 218:15–54.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Fehlings MG, Theodore N, Harrop J, Maurais
G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A and
McKerracher L: A phase I/IIa clinical trial of a recombinant Rho
protein antagonist in acute spinal cord injury. J Neurotrauma.
28:787–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Gonzenbach RR, Zoerner B, Schnell L,
Weinmann O, Mir AK and Schwab ME: Delayed anti-nogo-a antibody
application after spinal cord injury shows progressive loss of
responsiveness. J Neurotrauma. 29:567–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Xing B, Li H, Wang H, Mukhopadhyay D,
Fisher D, Gilpin CJ and Li S: RhoA-inhibiting NSAIDs promote axonal
myelination after spinal cord injury. Exp Neurol. 231:247–260.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Iannitti RG, Napolioni V, Oikonomou V, De
Luca A, Galosi C, Pariano M, Massi-Benedetti C, Borghi M, Puccetti
M, Lucidi V, et al: IL-1 receptor antagonist ameliorates
inflammasome-dependent inflammation in murine and human cystic
fibrosis. Nat Commun. 7:107912016. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liebscher T, Schnell L, Schnell D, Scholl
J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, et
al: Nogo-A antibody improves regeneration and locomotion of spinal
cord-injured rats. Ann Neurol. 58:706–719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Freund P, Wannier T, Schmidlin E, Bloch J,
Mir A, Schwab ME and Rouiller EM: Anti-Nogo-A antibody treatment
enhances sprouting of corticospinal axons rostral to a unilateral
cervical spinal cord lesion in adult macaque monkey. J Comp Neurol.
502:644–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wiessner C, Bareyre FM, Allegrini PR, Mir
AK, Frentzel S, Zurini M, Schnell L, Oertle T and Schwab ME:
Anti-Nogo-A antibody infusion 24 hours after experimental stroke
improved behavioral outcome and corticospinal plasticity in
normotensive and spontaneously hypertensive rats. J Cereb Blood
Flow Metab. 23:154–165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zörner B and Schwab ME: Anti-Nogo on the
go: From animal models to a clinical trial. Ann N Y Acad Sci. 1198
(Suppl 1):E22–E34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Kawakami M: Molecular dissection of
cyclosporin A's neuroprotective effect reveals potential
therapeutics for ischemic brain injury. Brain Sci. 3:1325–1356.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Liu J, Farmer JD Jr, Lane WS, Friedman J,
Weissman I and Schreiber SL: Calcineurin is a common target of
cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell.
66:807–815. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sharkey J and Butcher SP: Immunophilins
mediate the neuroprotective effects of FK506 in focal cerebral
ischaemia. Nature. 371:336–339. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Arii T, Kamiya T, Arii K, Ueda M, Nito C,
Katsura KI and Katayama Y: Neuroprotective effect of
immunosuppressant FK506 in transient focal ischemia in rat:
Therapeutic time window for FK506 in transient focal ischemia.
Neurol Res. 23:755–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Gold BG, Densmore V, Shou W, Matzuk MM and
Gordon HS: Immunophilin FK506-binding protein 52 (not FK506-binding
protein 12) mediates the neurotrophic action of FK506. J Pharmacol
Exp Ther. 289:1202–1210. 1999.PubMed/NCBI
|
|
178
|
Kang CB, Hong Y, Dhe-Paganon S and Yoon
HS: FKBP family proteins: Immunophilins with versatile biological
functions. Neurosignals. 16:318–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Jones LL, Oudega M, Bunge MB and Tuszynski
MH: Neurotrophic factors, cellular bridges and gene therapy for
spinal cord injury. J Physiol. 533:83–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Weissmiller AM and Wu C: Current advances
in using neurotrophic factors to treat neurodegenerative disorders.
Transl Neurodegener. 1:142012. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Barakat DJ, Gaglani SM, Neravetla SR,
Sanchez AR, Andrade CM, Pressman Y, Puzis R, Garg MS, Bunge MB and
Pearse DD: Survival, integration, and axon growth support of glia
transplanted into the chronically contused spinal cord. Cell
Transplant. 14:225–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Jiang DJ, Xu CL and Tsang SH: Revolution
in gene medicine therapy and genome surgery. Genes. 9:5752018.
View Article : Google Scholar
|
|
183
|
Boyce VS and Mendell LM: Neurotrophic
factors in spinal cord injury. Handb Exp Pharmacol. 220:443–460.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hodgetts SI and Harvey AR: Neurotrophic
factors used to treat spinal cord injury. Vitam Horm. 104:405–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Campbell JD and Burnett AL:
Neuroprotective and nerve regenerative approaches for treatment of
erectile dysfunction after cavernous nerve injury. Int J Mol Sci.
18:17942017. View Article : Google Scholar
|
|
186
|
Doll DN, Barr TL and Simpkins JW:
Cytokines: Their role in stroke and potential use as biomarkers and
therapeutic targets. Aging Dis. 5:294–306. 2014.PubMed/NCBI
|
|
187
|
Emsley HC and Tyrrell PJ: Inflammation and
infection in clinical stroke. J Cereb Blood Flow Metab.
22:1399–1419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Huebner EA and Strittmatter SM: Axon
regeneration in the peripheral and central nervous systems. Results
Probl Cell Differ. 48:339–351. 2009.PubMed/NCBI
|
|
189
|
Barritt AW, Davies M, Marchand F, Hartley
R, Grist J, Yip P, McMahon SB and Bradbury EJ: Chondroitinase ABC
promotes sprouting of intact and injured spinal systems after
spinal cord injury. J Neurosci. 26:10856–10867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Caggiano AO, Zimber MP, Ganguly A, Blight
AR and Gruskin EA: Chondroitinase ABCI improves locomotion and
bladder function following contusion injury of the rat spinal cord.
J Neurotrauma. 22:226–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Iaci JF, Vecchione AM, Zimber MP and
Caggiano AO: Chondroitin sulfate proteoglycans in spinal cord
contusion injury and the effects of chondroitinase treatment. J
Neurotrauma. 24:1743–1759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Iseda T, Okuda T, Kane-Goldsmith N, Mathew
M, Ahmed S, Chang YW, Young W and Grumet M: Single, high-dose
intraspinal injection of chondroitinase reduces glycosaminoglycans
in injured spinal cord and promotes corticospinal axonal regrowth
after hemisection but not contusion. J Neurotrauma. 25:334–349.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kwok JC, Afshari F, Garcia-Alias G and
Fawcett JW: Proteoglycans in the central nervous system:
Plasticity, regeneration and their stimulation with chondroitinase
ABC. Restor Neurol Neurosci. 26:131–145. 2008.PubMed/NCBI
|
|
194
|
Grimpe B and Silver J: A novel DNA enzyme
reduces glycosaminoglycan chains in the glial scar and allows
microtransplanted dorsal root ganglia axons to regenerate beyond
lesions in the spinal cord. J Neurosci. 24:1393–1397. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
McRae PA and Porter BE: The perineuronal
net component of the extracellular matrix in plasticity and
epilepsy. Neurochem Int. 61:963–972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Moon LD, Asher RA, Rhodes KE and Fawcett
JW: Regeneration of CNS axons back to their target following
treatment of adult rat brain with chondroitinase ABC. Nat Neurosci.
4:465–466. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
197
|
Bradbury EJ, Moon LD, Popat RJ, King VR,
Bennett GS, Patel PN, Fawcett JW and McMahon SB: Chondroitinase ABC
promotes functional recovery after spinal cord injury. Nature.
416:636–640. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Wang D, Ichiyama RM, Zhao R, Andrews MR
and Fawcett JW: Chondroitinase combined with rehabilitation
promotes recovery of forelimb function in rats with chronic spinal
cord injury. J Neurosci. 31:9332–9344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Hu HZ, Granger N, Pai SB, Bellamkonda RV
and Jeffery ND: Therapeutic efficacy of microtube-embedded
chondroitinase ABC in a canine clinical model of spinal cord
injury. Brain. 141:1017–1027. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Rosenzweig ES, Courtine G, Jindrich DL,
Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM,
Beattie MS, et al: Extensive spontaneous plasticity of
corticospinal projections after primate spinal cord injury. Nat
Neurosci. 13:1505–1510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Lawrence DG and Kuypers HG: The functional
organization of the motor system in the monkey. I. The effects of
bilateral pyramidal lesions. Brain. 91:1–14. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Nout YS, Ferguson AR, Strand SC, Moseanko
R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Zhong H,
Rosenzweig ES, et al: Methods for functional assessment after C7
spinal cord hemisection in the rhesus monkey. Neurorehabil Neural
Repair. 26:556–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Jefferson SC, Tester NJ and Howland DR:
Chondroitinase ABC promotes recovery of adaptive limb movements and
enhances axonal growth caudal to a spinal hemisection. J
Neuroscience. 31:5710–5720. 2011. View Article : Google Scholar
|
|
204
|
Ma R, Liu X, Clark J, Williams GM and Doi
SA: The impact of acupuncture on neurological recovery in spinal
cord injury: A systematic review and Meta-analysis. J Neurotrauma.
32:1943–1957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Wang J, Zhai Y, Wu J, Zhao S, Zhou J and
Liu Z: Acupuncture for chronic urinary retention due to spinal cord
injury: A systematic review. Evid Based Complement Alternat Med.
2016:92451862016.PubMed/NCBI
|
|
206
|
Dyson-Hudson TA, Kadar P, LaFountaine M,
Emmons R, Kirshblum SC, Tulsky D and Komaroff E: Acupuncture for
chronic shoulder pain in persons with spinal cord injury: A
small-scale clinical trial. Arch Phys Med Rehabil. 88:1276–1283.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Liu F, Zou Y, Liu S, Liu J and Wang T:
Electro-acupuncture treatment improves neurological function
associated with downregulation of PDGF and inhibition of
astrogliosis in rats with spinal cord transection. J Mol Neurosci.
51:629–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Tu WZ, Jiang SH, Zhang L, Li SS, Gu PP, He
R, Hu J, Gao LP and Sun QS: Electro-acupuncture at Governor Vessel
improves neurological function in rats with spinal cord injury.
Chin J Integr Med. Jul 31–2017.(Epub ahead of print). doi:
10.1007/s11655-017-2968-9. View Article : Google Scholar
|
|
209
|
Lovas J, Tran Y, Middleton J, Bartrop R,
Moore N and Craig A: Managing pain and fatigue in people with
spinal cord injury: A randomized controlled trial feasibility study
examining the efficacy of massage therapy. Spinal Cord. 55:162–166.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Diego MA, Field T, Hernandez-Reif M, Hart
S, Brucker B, Field T and Burman I: Spinal cord patients benefit
from massage therapy. Int J Neurosci. 112:133–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Field T: Massage therapy research review.
Complement Ther Clin Pract. 24:19–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Moyer CA, Rounds J and Hannum JW: A
meta-analysis of massage therapy research. Psychol Bull. 130:3–18.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Field T, Hernandez-Reif M, Diego M,
Schanberg S and Kuhn C: Cortisol decreases and serotonin and
dopamine increase following massage therapy. Int J Neurosci.
115:1397–1413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Campeau MP, Gaboriault R, Drapeau M, Van
Nguyen T, Roy I, Fortin B, Marois M and Nguyen-Tân PF: Impact of
massage therapy on anxiety levels in patients undergoing radiation
therapy: Randomized controlled trial. J Soc Integr Oncol.
5:133–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Sliwinski MM, Smith R and Wood A: Spinal
cord injury rehabilitation patient and physical therapist
perspective: A pilot study. Spinal Cord Ser Cases. 2:150362016.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
DeSantana JM, Walsh DM, Vance C, Rakel BA
and Sluka KA: Effectiveness of transcutaneous electrical nerve
stimulation for treatment of hyperalgesia and pain. Curr Rheumatol
Rep. 10:492–499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Gozani SN: Remote analgesic effects of
conventional transcutaneous electrical nerve stimulation: A
scientific and clinical review with a focus on chronic pain. J Pain
Res. 12:3185–3201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Gibson W, Wand BM and O'Connell NE:
Transcutaneous electrical nerve stimulation (TENS) for neuropathic
pain in adults. Cochrane Database Syst Rev.
9:CD0119762017.PubMed/NCBI
|
|
219
|
Bi X, Lv H, Chen BL, Li X and Wang XQ:
Effects of transcutaneous electrical nerve stimulation on pain in
patients with spinal cord injury: A randomized controlled trial. J
Phys Ther Sci. 27:23–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Dailey DL, Rakel BA, Vance CG, Liebano RE,
Amrit AS, Bush HM, Lee KS, Lee JE and Sluka KA: Transcutaneous
electrical nerve stimulation reduces pain, fatigue and hyperalgesia
while restoring central inhibition in primary fibromyalgia. Pain.
154:2554–2562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Vance CG, Dailey DL, Rakel BA and Sluka
KA: Using TENS for pain control: The state of the evidence. Pain
Manage. 4:197–209. 2014. View Article : Google Scholar
|
|
222
|
Zeb A, Arsh A, Bahadur S and Ilyas SM:
Effectiveness of transcutaneous electrical nerve stimulation in
management of neuropathic pain in patients with post traumatic
incomplete spinal cord injuries. Pak J Med Sci. 34:1177–1180. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Hagen EM and Rekand T: Management of
neuropathic pain associated with spinal cord injury. Pain Ther.
4:51–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Guanziroli E, Cazzaniga M, Colombo L,
Basilico S, Legnani G and Molteni F: Assistive powered exoskeleton
for complete spinal cord injury: Correlations between walking
ability and exoskeleton control. Eur J Phys Rehabil Med.
55:209–216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Louie DR, Eng JJ and Lam T: Gait speed
using powered robotic exoskeletons after spinal cord injury: A
systematic review and correlational study. J Neuroeng Rehabil.
12:822015. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Miller LE, Zimmermann AK and Herbert WG:
Clinical effectiveness and safety of powered exoskeleton-assisted
walking in patients with spinal cord injury: Systematic review with
meta-analysis. Med Devices. 9:455–466. 2016. View Article : Google Scholar
|
|
227
|
Lajeunesse V, Vincent C, Routhier F,
Careau E and Michaud F: Exoskeletons' design and usefulness
evidence according to a systematic review of lower limb
exoskeletons used for functional mobility by people with spinal
cord injury. Disabil Rehabil Assist Technol. 11:535–547. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Nas K, Yazmalar L, Şah V, Aydın A and Öneş
K: Rehabilitation of spinal cord injuries. World J Orthopedics.
6:8–16. 2015. View Article : Google Scholar
|
|
229
|
Grasmücke D, Zieriacks A, Jansen O, Fisahn
C, Sczesny-Kaiser M, Wessling M, Meindl RC, Schildhauer TA and Aach
M: Against the odds: What to expect in rehabilitation of chronic
spinal cord injury with a neurologically controlled Hybrid
Assistive Limb exoskeleton. A subgroup analysis of 55 patients
according to age and lesion level. Neurosurg Focus. 42:E152017.
View Article : Google Scholar
|
|
230
|
Karelis AD, Carvalho LP, Castillo MJ,
Gagnon DH and Aubertin-Leheudre M: Effect on body composition and
bone mineral density of walking with a robotic exoskeleton in
adults with chronic spinal cord injury. J Rehabil Med. 49:84–87.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Gorgey AS, Wade R, Sumrell R, Villadelgado
L, Khalil RE and Lavis T: Exoskeleton training may improve level of
physical activity after spinal cord injury: A case series. Top
Spinal Cord Inj Rehabil. 23:245–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Cirnigliaro CM, Myslinski MJ, La Fountaine
MF, Kirshblum SC, Forrest GF and Bauman WA: Bone loss at the distal
femur and proximal tibia in persons with spinal cord injury:
Imaging approaches, risk of fracture, and potential treatment
options. Osteoporosis Int. 28:747–765. 2017. View Article : Google Scholar
|
|
233
|
Escalona MJ, Brosseau R, Vermette M,
Comtois AS, Duclos C, Aubertin-Leheudre M and Gagnon DH:
Cardiorespiratory demand and rate of perceived exertion during
overground walking with a robotic exoskeleton in long-term manual
wheelchair users with chronic spinal cord injury: A cross-sectional
study. Ann Phys Rehabil Med. 61:215–223. 2018. View Article : Google Scholar : PubMed/NCBI
|