Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is
a nasal inflammatory disease, characterized by symptoms including
nasal obstruction, drainage, smell loss and facial pain or pressure
(1). CRSwNP affects a large
proportion of the population world-wide and is associated with high
cost of management and low quality of life (2). The prevalence of CRSwNP in Europe is
estimated to be between 2.1 and 4.4%, while it is 4.2% in the
United States and 1.1% in China (3). Lourijsen et al (4) found that the total direct costs were
1,501 Euros per year per patient with CRSwNP. CRS is currently
classified into eosinophilic CRS (ECRS) and non-ECRS (NECRS)
subtypes based on the presence or absence of tissue eosinophilic
infiltration (1). Therefore, CRSwNP
may also be subclassified into eosinophilic CRSwNP (ECRSwNP) and
non-eosinophilic CRSwNP (NECRSwNP) (5), with the former having a higher
recurrence rate and asthma incidence (6). Over the past 40 years, advances in
functional endoscopic sinus surgery and pre- and postoperative drug
therapy have greatly improved the cure rate of CRSwNP; however,
disease recurrence ranges from 40 to 78.9% in CRSwNP, and the rate
of revision surgery is as high as 36.8% (7,8). There
is still a lack of effective mechanism-based treatments in clinical
practice.
NOD-like receptor pyrin domain containing 3 (NLRP3)
can assemble with apoptosis-associated speck-like protein
containing a CARD (ASC) and pro-caspase-1 to form a multimeric
protein complex called the NLRP3 inflammasome. Recently, the NLRP3
inflammasome was demonstrated to be implicated in the pathogenesis
of CRSwNP (9). It was found that
the NLRP3 inflammasome was activated in nasal mucosa in a murine
acute bacterial rhinosinusitis model (10). This suggested that the NLRP3
inflammasome contributed to nasal inflammation. Activated
caspase-1, generated when pro-caspase-1 is cleaved by the NLRP3
inflammasome, proteolytically cleaves the inflammatory cytokines
IL-1β and IL-18 into their mature forms (11). Elevated IL-1β and IL-18 levels are
found in nasal polyps of CRSwNP patients (9,12).
Furthermore, increased IL-18 levels was significantly associated
with the radiological severity of sinusitis and local eosinophilia
(12). Therefore, a mechanism for
decreasing the levels of inflammatory cytokines may provide a
clinical benefit. The formation of the NLRP3 inflammasome can be
induced by several microbial and nonmicrobial stimuli. Nonmicrobial
stimuli include substances which cause specific diseases such as
uric acid, silica fibres and extracellular adenosine triphosphate
(ATP) (13,14). The purinergic 2X7 receptor (P2X7R)
can be triggered by ATP which then results in the assembly of the
NLRP3 inflammasome (15).
The ATP-gated ionotropic P2X receptor subfamily
consists of seven (1–7) members, and several P2X receptors,
including P2X7, are expressed in normal human nasal epithelial
cells (16). An increased ATP
concentration induced by infection or inflammation can act on the
P2X7 receptor leading to NLRP3 inflammasome and caspase-1
activation (17). However, the
specific role of P2X7R in CRSwNP has not yet been established, and
the interaction between P2X7R and the NLRP3 inflammasome in the
development of CRSwNP remains unclear. Therefore, it was
hypothesized that P2X7R is upregulated in nasal polyps and
modulation of the P2X7R/inflammasome axis would attenuate
lipopolysaccharide (LPS)-induced inflammation in cultured human
nasal epithelial cells.
Materials and methods
Patients
A total of 32 patients with CRSwNP (16 ECRSwNP and
16 NECRSwNP) and 16 control subjects were included in the present
study. NP specimens were obtained from patients diagnosed with
CRSwNP according to the criteria of the European Position Statement
updated in 2020 (1). Human nasal
mucosa of the middle turbinate were collected from patients that
underwent neurosurgery, who had undergone surgery because of a
pituitary tumour, as the control. The visual analogue scale and
computed tomography scores were graded according to the method
previously described (18). The
clinical characteristics of patients are listed in Table I. Subjects who had used oral or
nasal corticosteroids, anti-histamines, antibiotics or
antileukotrienes within the preceding 4 weeks before sample
collection were excluded. The collected samples were used for
haematoxylin-eosin (HE) staining, western blotting (WB),
immunofluorescence (IF) staining, and primary human nasal
epithelial cell (HNECs) culture. The present experimental study was
approved by the ethical committees of Tong-ji Medical College,
Huazhong University of Science and Technology (permit no. S135).
All participants of the study were informed and signed a consent
form.
 | Table I.Characteristics of included
subjects. |
Table I.
Characteristics of included
subjects.
|
Characteristics | ECRSwNP | nECRSwNP | Controls |
P-valuea |
|---|
| Subjects, n | 16 | 16 | 16 | N/A |
| Sex, male, n
(%) | 11 (68.75) | 14 (87.5) | 12 (75) | 0.394 |
| Age, years, median
(IQR) | 50
(24.26–66.25) | 37.5
(26.25–63) | 36.5
(27.5–48.75) | 0.926 |
| With smoking
exposure, n (%) | 8 (50) | 10 (62.5) | 10 (62.5) | 0.722 |
| With allergic
rhinitis, n (%) | 6 (37.5) | 4 (25) | 1 (6.25) | 0.704 |
| With asthma, n
(%) | 2 (12.5) | 0 | 0 | 0.484 |
| Prior sinus
surgery | 3 (18.75) | 1 (6.25) | 0 | 0.6 |
| VAS score, median
(IQR) | 11.5 (9.25–16) | 9.5
(7.25–11.75) | 3 (3–4.75) | 0.403 |
| CT score, median
(IQR) | 17.5 (12.5–20) | 15.5 (12.5–18) | 7 (6–8) | 0.402 |
Histological and immunofluorescence
observation
The NP tissues collected from patients were
immediately fixed overnight in 4% formaldehyde-phosphate buffered
saline solution, then dehydrated through a graded ethanol series
(70, 80, 90, 95 and 100%) for 5 min each, before samples were
embedding in paraffin, and finally sectioned at 5-µm thickness. In
order to quantify the eosinophilic infiltration of NP, haematoxylin
and eosin staining was performed. The NP sections were observed at
high power (HP) (magnification, ×400; Leica GmbH; cat. no. DM2500)
and then, 10 HP fields were randomly selected and eosinophil
numbers were microscopically counted. A tissue eosinophil count of
10 or more eosinophils per high-power field (HPF) was defined as
ECRSwNP (1,19). After deparaffinization and
rehydration, the tissue sections underwent heat-induced epitope
retrieval followed by blocking with 10% bovine serum albumin
protein at room temperature for 30 min. The blocked sections were
incubated with rabbit anti-human P2X7R (1:200; GeneTex; cat no.
GTX104288) at 4°C overnight. The next day, the sections were rinsed
three times with PBS, and incubated with secondary anti-rabbit
antibody (1:300; antGene; cat no. ANT032) in the dark at room
temperature for 1 h and counterstained with DAPI (Beyotime
Institute of Biotechnology; cat. no. C1005). Images were captured
with a confocal laser scanning microscope (Nikon-A1-Si; Nikon
Corporation).
Cell culture
HNECs isolated from seven patients with CRSwNP were
cultured according to a previously reported method (9). In brief, the tissues were transferred
and digested with 0.1% protease from Streptomyces griseus
(cat no. 9036-06-0; Sigma-Aldrich; Merck KGaA) and 0.1 mg/ml
deoxyribonuclease (cat no. D5025; Sigma-Aldrich; Merck KGaA).
Separated epithelial cells were collected and seeded onto PureCol™
EZ Gel solution (Sigma-Aldrich; Merck KGaA; cat no. 5074)-coated
12-well culture plates, and cultured in PneumaCult™-Ex Plus Medium
(Stemcell Technologies, Inc.; cat. no. #05040) at 37°C in 5%
CO2. To induce inflammation, one part of the adherent
epithelial cells were incubated under the following conditions: i)
no additions (control); ii) 10 µg/ml LPS for 24 h (LPS from
Pseudomonas aeruginosa; Sigma-Aldrich; Merck KGaA; cat. no.
L8643); iii) 10 µg/ml LPS for 24 h, 300 µM
2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate
triethylammonium salt (BzATP) (Sigma-Aldrich; Merck KGaA; cat. no.
B6396) was supplemented 1 h after LPS administration; iv) 20 µg/ml
LPS for 24 h, 300 µM BzATP was supplemented 1 h after LPS
administration. At the end of the incubation, the cells were
collected, centrifuged and frozen at −80°C until use. To confirm
the role of the P2X7 receptor in the inflammatory response of
HNECs, the other part of the adherent epithelial cells were treated
with LPS and A740003 (P2X7 receptor blocker) were incubated under
the following conditions: i) no additions (control); ii) 10 µg/ml
LPS for 24 h, 300 µM BzATP was added 1 h after LPS administration;
iii) 10 µg/ml LPS for 24 h, 300 µM BzATP was added 1 h after LPS
administration, with supplementation with A740003 (10 µM;
MedChemExpress; cat no. HY-50697) 15 min before BzATP stimulation.
At the end of the incubation, the supernatants and cells were
collected, centrifuged and frozen at −80°C until use.
WB
NP specimens were collected and stored in liquid
nitrogen at −80°C until use. Total cellular protein was extracted
from NP tissue and cultured cells using RIPA lysis buffer (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions, and the protein concentration was measured using a
BCA protein assay kit (Beyotime Institute of Biotechnology). Then,
12% SDS-polyacrylamide gels were used to separate the protein (30
µg), after which the proteins were transferred onto
polyvinylidenedifluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were blocked with 5% non-fat milk at 4°C for 1 h and
then incubated with the working dilution of primary antibodies:
Rabbit anti-human P2X7R (1:1,000; GeneTex; cat no. GTX16827);
rabbit anti-human NLRP3 (1:1,000; Abcam; cat no. ab260017); mouse
anti-human IL-1β (1:5,000; Arigo Biolaboratories; cat. no.
ARG66285); rabbit anti-human GAPDH (1:5,000; AntGene; cat. no.
ANT012) at 4°C overnight. Subsequently, the membranes were washed
in Tris-buffered saline mixed with 0.1% Tween four times for 10 min
each, and then incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody: HRP goat anti-rabbit IgG
(H+L) (1:4,000; cat. no. ANT020; AntGene) and HRP goat anti-mouse
IgG (H+L) (1:4,000; cat. no. ANT019; AntGene) for 1 h at room
temperature. The immunolabelled proteins were detected using
BeyoECL Plus (Beyotime Institute of Biotechnology). The gel images
were analysed using Quantity One software (version 4.6; Bio-Rad
Laboratories, Inc.) to estimate the relative quantitative density
of the protein bands. GAPDH was used as an internal control.
Real-time PCR
The total mRNA of primary HNECs was extracted using
an E.Z.N.A™ Total RNA kit (Omega Bio-Tek, Inc.) according to the
manufacturers' instructions. A PrimeScript™ RT Reagent kit with a
gDNA Eraser (Takara Biotechnology Co., Ltd.) was used to conduct
the reverse transcription reaction (37°C for 15 min, 85°C for 5 sec
and stop at 4°C) to obtain cDNA for real-time PCR analysis.
Real-time PCR was performed using SYBR® Premix Ex Taq™
II (TliRNaseH Plus) (X2 concentration) X1 (Takara Biotechnology
Co., Ltd.) according to the manufacturer's instructions.
Sequence-specific primers for P2X7R, IL-1β, and GADPH were as
follows: P2X7R, 5′-TCTGTACTTTGCAGCCAATCAGAAC-3′ (forward primer);
P2X7R, 5′-CCAACTCTAGTGACCAAACCAGGAA-3′ (reverse primer); IL-1β,
5′-CCAGGGACAGGATATGGAGCA-3′ (forward primer); IL-1β,
5′-TTCAACACGCAGGACAGGTACAG-3′ (reverse primer); GAPDH,
5′-GCACCGTCAAGGCTGAGAAC-3′ (forward primer), GAPDH,
5′-TGGTGAAGACGCCAGTGGA-3′ (reverse primer). The real-time PCR
protocol was as follows: Denaturation at 95°C for 30 sec, followed
by 40 cycles of amplification at 95°C for 5 sec, 60°C for 30 sec
and annealing at 60°C for 30 sec. Relative mRNA level was
determined by the 2−ΔΔCq method (20).
Enzyme-linked immunosorbent assay
(ELISA)
Human IL-1β was measured in cell culture
supernatants from HNECs under different culture conditions using an
ELISA kit (Arigo; cat. no. ARG80101) following the manufacturers'
instructions. Samples were run at least in duplicate.
Statistical analysis
For continuous clinical variables, the data are
expressed as median and interquartile ranges, or as box and whisker
plots displaying medians and interquartile ranges, which were
analysed by the Kruskal-Wallis H-test and Mann-Whitney U test. For
dichotomous parameters, the χ2 test or Fisher's exact
test was performed to determine the difference between groups. Data
were analysed using the Statistical Package for the Social Sciences
(version 22.0; SPSS Inc.). For tissue samples and in vitro
experiments, the data are expressed as mean ± standard deviation
and were analysed by one-way ANOVA. Tukey's post hoc test was also
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
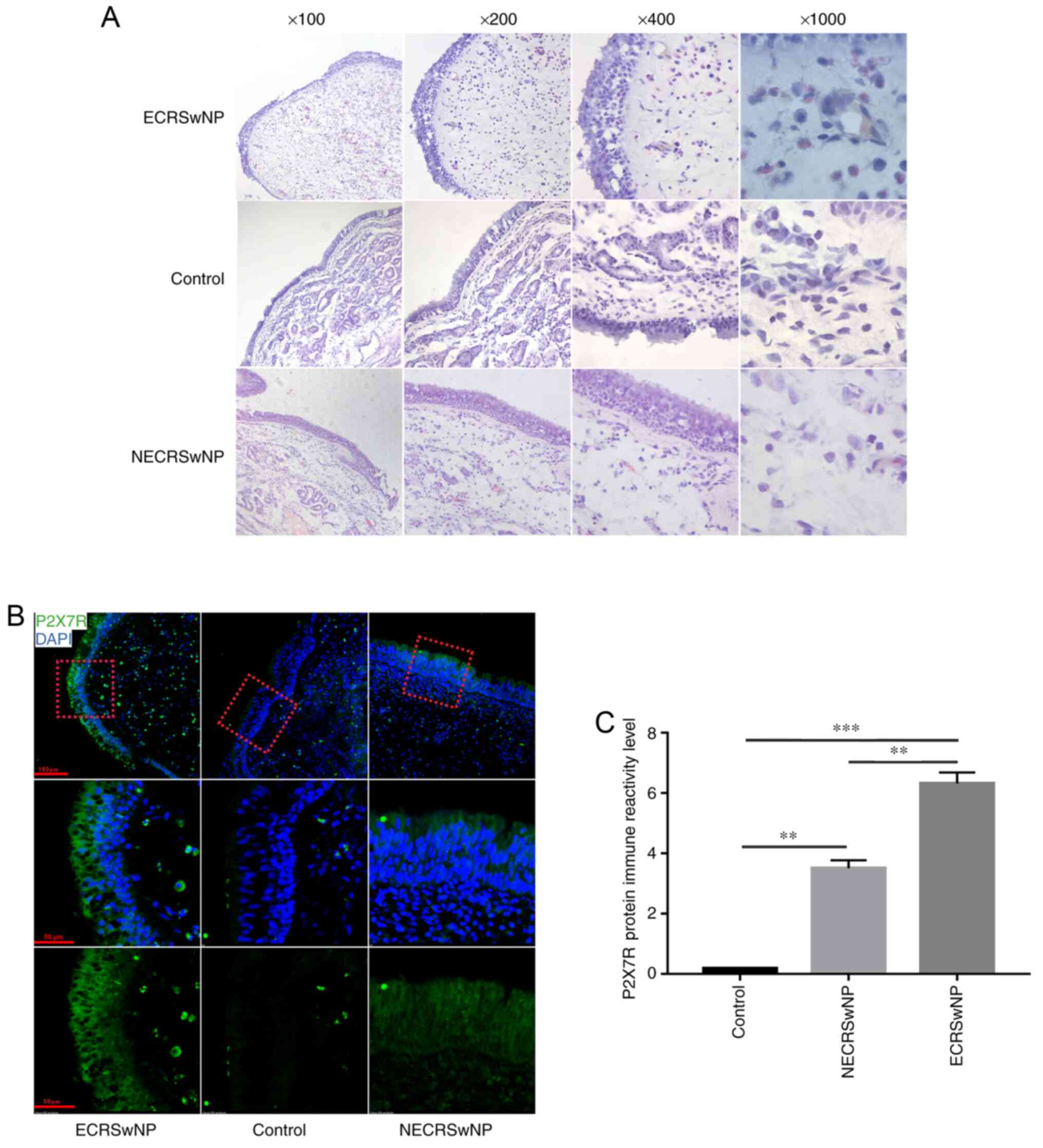
Histological changes and localisation
of P2X7R in nasal mucosa
Overall, all patients, including 16 control subjects
and 32 patients with CRSwNP, underwent H&E staining, which
showed that numerous eosinophils infiltrated the nasal mucosa of
patients with ECRSwNP. Furthermore, an intense oedematous stroma
and subepithelial and perivascular inflammatory cell infiltration
was also observed in CRSwNP (Fig.
1A). Immunofluorescence (Fig.
1B) showed that P2X7R was predominantly expressed in epithelial
cells. It is worth noting that the level of receptor in the ECRSwNP
group was higher compared with that of the other two groups.
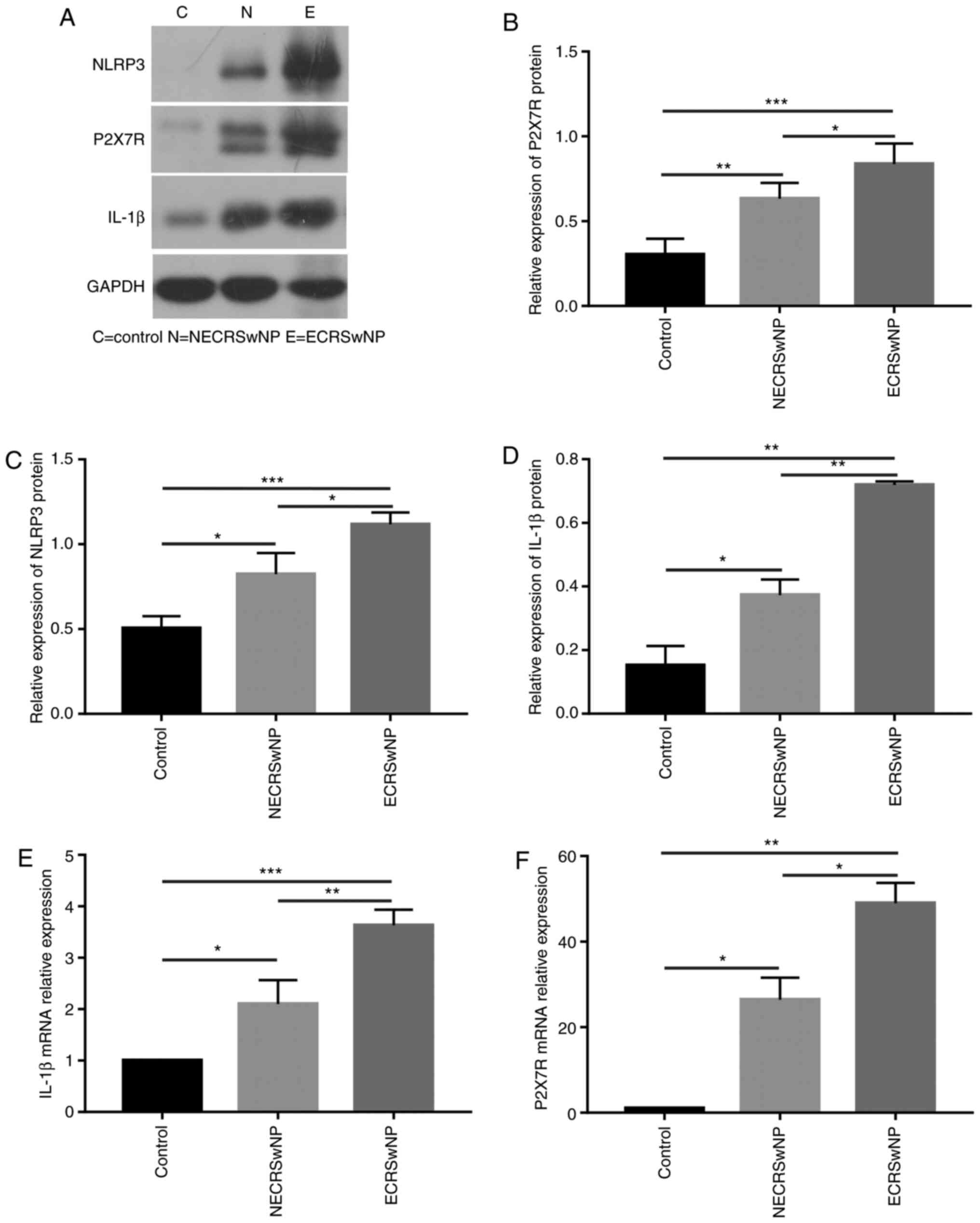
Expression of P2X7R is higher in
ECRSwNP compared with the control group
Protein content was analysed in the nasal mucosa of
ECRSwNP, NECRSwNP and control groups. As shown by WB (Fig. 2A), P2X7R (Fig. 2B), NLRP3 (Fig. 2C) and IL-1β (Fig. 2D) were significantly overexpressed
in CRSwNP (P<0.05), especially in the ECRSwNP group. The mRNA
expression of P2X7R and IL-1β were elevated in the CRSwNP groups
compared with the control group, and these increases were also
found in the ECRSwNP group compared with the NECRSwNP group
(Fig. 2E and F).
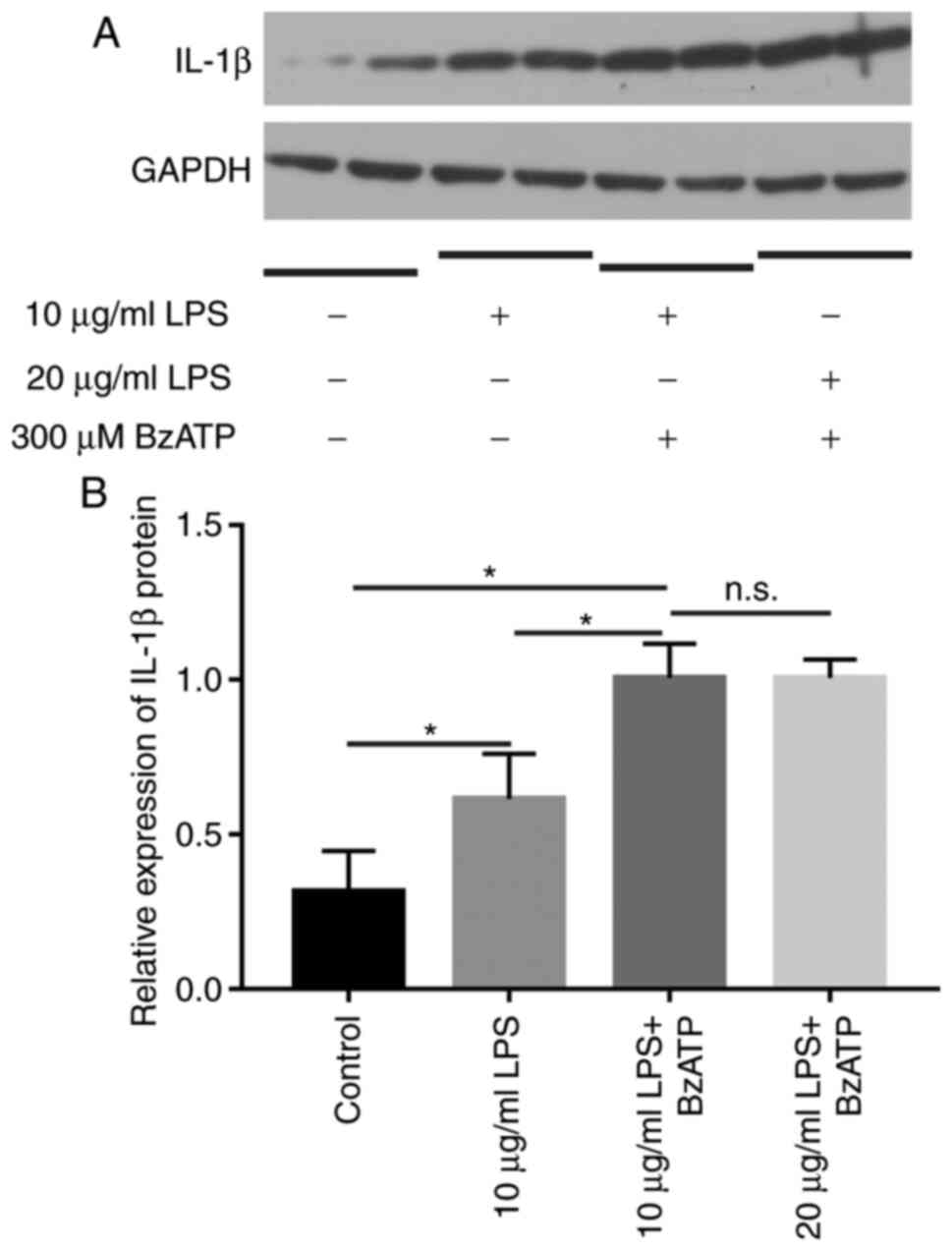
IL-1β is upregulated after incubation
with LPS combined with BzATP
LPS induces a cellular inflammatory response in
vitro. The expression of IL-1β was evaluated under different
conditions by WB (Fig. 3). When
treated with LPS alone, the expression of IL-1β increased relative
to controls. When LPS was combined with BzATP, IL-1β markedly
increased, and the increase was statistically significant compared
with the control group (P<0.05). There was no significant
difference in the expression of IL-1β between the 10 µg/ml LPS
group and the 20 µg/ml group, thus, 10 µg/ml LPS combined with
BzATP was chosen as the inflammatory stimulation condition.
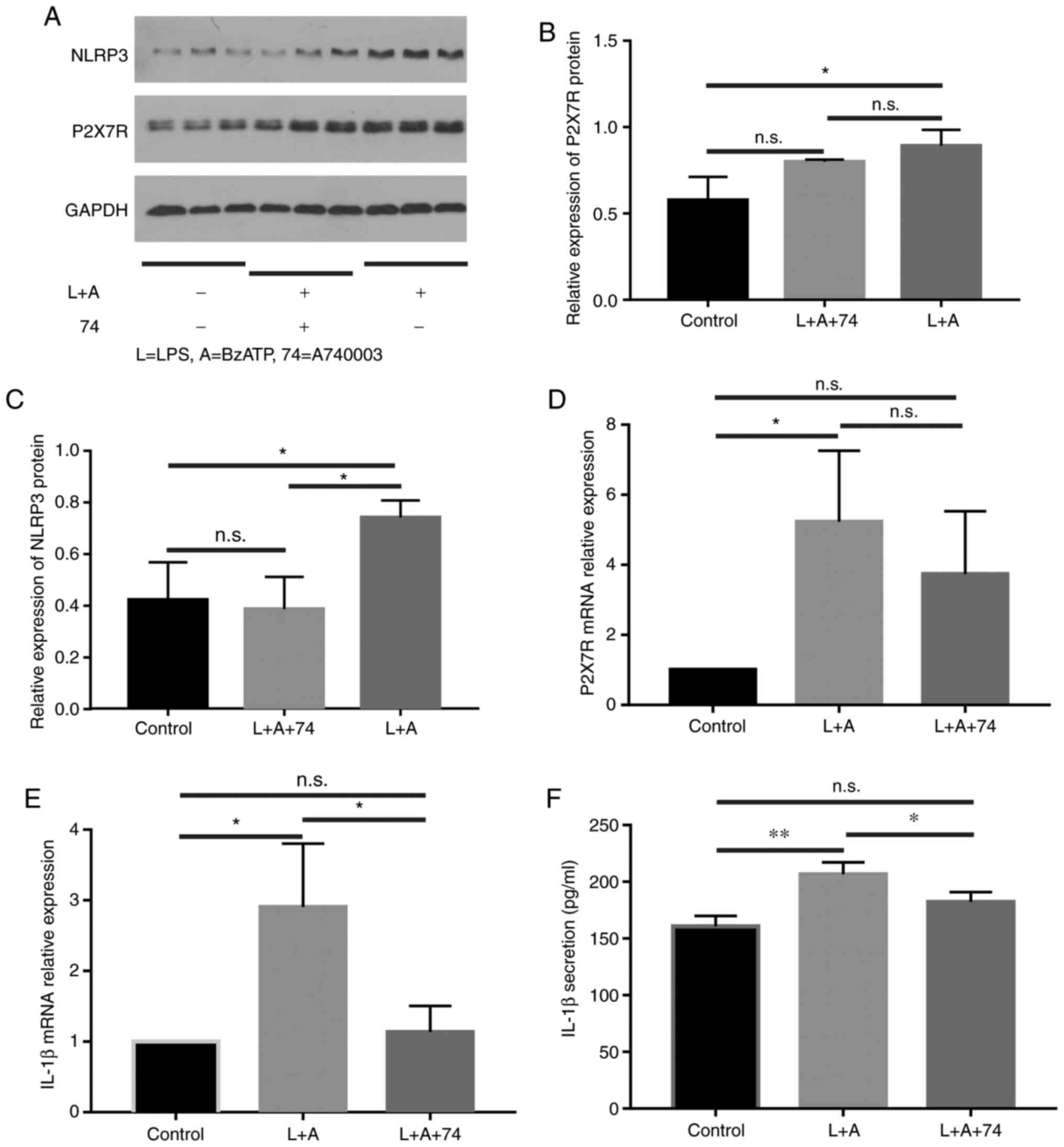
Expression of P2X7R shows no
significant change, but NLRP3 is downregulated after stimulation
with A740003
A740003 significantly blocks the sustained phase of
the BzATP-induced response. The expression levels of P2X7R and
NLRP3 were evaluated by WB (Fig.
4). The expression of P2X7R (Fig.
4B) showed no significant change, while the expression of NLRP3
(Fig. 4C) was significantly
downregulated after A740003 treatment in HNECs after stimulation
with LPS+BzATP. Similarly, after treatment of HNECs with LPS
combined with BzATP, the expression of both P2X7R and IL-1β mRNA
increased (P<0.05). After addition of the inhibitor A74003,
compared with the inflammatory stimulation group, the expression of
P2X7R decreased somewhat, but this was not statistically
significant, while IL-1β mRNA significantly decreased (Fig. 4D and E). The level of IL-1β in the
culture supernatant increased after LPS+BzATP stimulation and was
inhibited by A740003 (Fig. 4F),
suggesting that the blockade of P2X7R might prevent NLRP3
inflammasome activation.
Discussion
The present study found that P2X7R, NLRP3 and IL-1β
protein levels were significantly increased in the CRSwNP groups
compared with the control group and expression was further
significantly higher in the ECRSwNP group compared with the
NECRSwNP group. Thus, the inflammatory form of ECRSwNP is more
severe compared with NECRSwNP. There are obvious regional
differences in the inflammatory characteristics of CRS.
Approximately 80% of nasal polyps in Western patients are
eosinophilic, while the rate in Asia is <50% (21). However, recent studies have shown
that the incidence of ECRS in East Asian countries is increasing. A
Chinese study showed that the proportion of eosinophilic CRSwNP
significantly increased from 59.1 to 73.7% over 11 years (19). ECRS is considered a special and
recalcitrant subtype of CRS (21).
ATP concentration is maintained at a low level in
healthy tissues (22).
Extracellular ATP is involved in the release of various
pro-inflammatory cytokines including thymic stromal lymphopoietin,
IL-25 and IL-33 in nasal mucosal inflammation (23,24).
In pathological situations, cell injury leads to a substantial
increase in extracellular ATP as a key danger alarmin that
initiates inflammation and further amplifies immune responses
(25). As the effects of
extracellular ATP are mediated by P2 receptors, the role of P2X7
was investigated, which is the most involved of the P2 receptors in
inflammation and infection and exhibits a high binding affinity for
ATP (22).
In the present study, P2X7 was expressed in
epithelial cells in control subjects; this result was consistent
with previous reports (16).
Furthermore, it was found that the expression of P2X7R was
increased in ECRSwNP compared with controls and the protein was
mainly located in epithelial cells. Since P2X7R has been
demonstrated to be highly expressed in immune cells, the increase
in P2X7R expression may be partly associated with enhanced
infiltration of macrophages into nasal polyps (26). It was also found that LPS increased
the expression level of IL-1β in a dose-dependent manner when
combined with BzATP in primary human HNECs. LPS stimulation can
result in the accumulation of cytoplasmic IL-1β through Toll-like
receptor 4 (27). Meanwhile, LPS
may cause pannexin-1 opening, allowing ATP release, which then
triggers K+ efflux, to promote inflammasome-mediated
caspase-1 activation. The activated NLRP3 inflammasome catalyses
pro-IL-1β cleavage. NLRP3 inflammasomes have been proven to be
activated in both ECRSwNP and NECRSwNP (9) and the present study findings confirmed
this. The downstream inflammatory cytokines released after NLRP3
inflammasome assembly include IL-1β and IL-18, which were elevated
in CRSwNP (9). Elevated IL-1β
expression was found in HNECs after LPS inoculation. Further
examination using the P2X7-selective antagonist A740003 confirmed
that the LPS-induced effects are P2X7 specific. A740003 is a
selective competitive antagonist of P2X7R (28). In addition, A740003 can block
P2X7R-mediated calcium influx, pore formation and IL-1β release
(29). This effect was also found
in HNECs in the present study. This suggested that P2X7R may be
implicated in the release of IL-1β in CRSwNP via the activation of
the NLRP3 inflammasome (Fig. 5).
P2X7R expression after antagonist administration in HNECs was
slightly decreased compared with LPS alone. This effect may be
associated with membrane internalization stimulated by P2X7R under
inflammatory conditions. The administration of antagonists could
decrease P2X7R activation and membrane internalization, which could
induce receptor relocation, degradation and replenishment in the
membrane, resulting in decreased receptor expression (30).
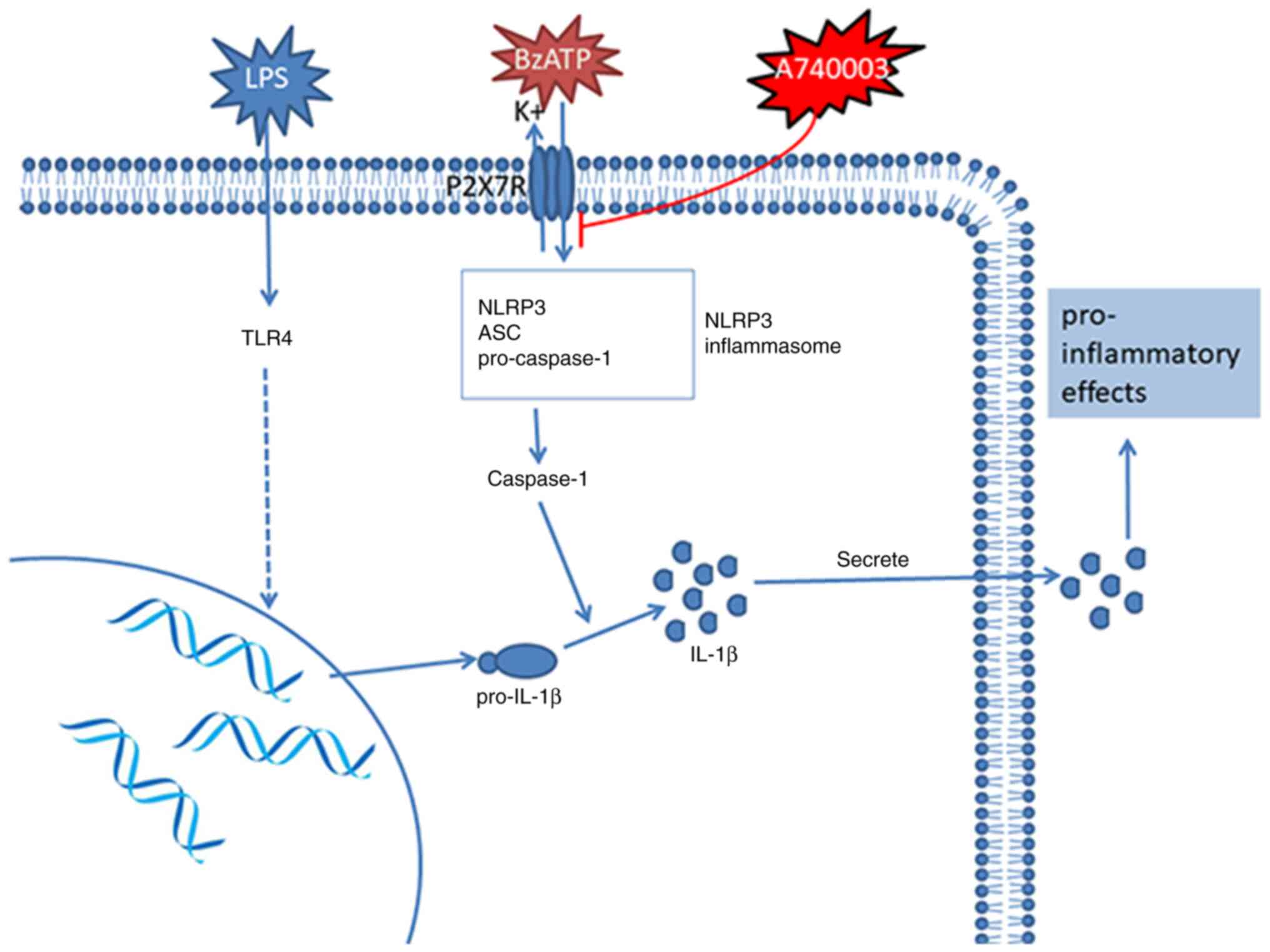 | Figure 5.LPS activates Toll-like receptor 4,
leading to transcription of the IL-1β gene and translation of the
31-kDa pre-cytokine (pro-IL-1β). P2X7R exposure to its agonist,
BzATP, triggers depolarization via the opening of cation channels,
which triggers K+ efflux, leading to stimulation of the
NLRP3 inflammasome. Activated caspase-1 converts pro-IL-1β to the
17-kDa mature protein (IL-1β), which is then secreted to the
extracellular space. A740003, a selective competitive antagonist of
P2X7R, blocks P2X7R-mediated calcium influx, pore formation and
IL-1β release. TLR4, Toll-like receptor 4; IL-1β, interleukin-1β;
P2X7R, purinergic 2X7 receptor; BzATP,
2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate
triethylammonium salt; NLRP3, NLR family pyrin domain-containing 3;
ASC, apoptosis-associated speck-like protein containing a CARD. |
Nasal epithelial cells exist at the surface of the
nasal mucosa and are capable of detecting microbial products and
endogenous molecules associated with cellular damage via an
assortment of pattern recognition receptors (31). NLRP3, a member of the NLR family,
induces an increase in the expression of IL-1β, and can be
activated by pathogens (9). The
present study found that after blocking P2X7R, the expression of
NLRP3 decreased, indicating that P2X7R is upstream of NLRP3 in
nasal polyps. When persistent inflammation in nasal mucosa results
in tissue damage, a variety of molecules are released that are
normally sequestered intracellularly or within the extracellular
matrix. The nasal mucosa is exposed to harmful elements, such as
pathogens and air pollutants, leading to an enhanced and sustained
stream of danger signals (32,33).
Studies examining the nasal mucosa of patients with CRSwNP have
demonstrated the infiltration of immune cells including
CD8+ T-cells, eosinophils, neutrophils and macrophages,
and such inflammatory cells have been implicated in the
pathogenesis of CRSwNP (3).
Therefore, it was speculate that P2X7R may regulate the occurrence
and development of nasal polyps by activating the NLRP3
inflammasome and mediating the release of IL-1β.
There are some limitations to the present study,
which should be pointed out. Firstly, the experiment only included
32 patients with CRSwNP and 16 control subjects, thus the sample
size is small. A larger sample is needed to confirm the present
findings. Secondly, the experiment only compared patients with
CRSwNP with a control group; consequently the role of P2X7R in the
pathogenesis of CRSsNP and chronic refractory rhinosinusitis needs
further research and analysis.
In summary, the present study found that the
expression of P2X7R in the nasal mucosa of patients with CRSwNP and
HNECs under inflammatory conditions was higher compared with that
of the control group. P2X7R-NLRP3 inflammasome signaling pathway
can be augmented by LPS combined with BzATP, but suppressed by
A740003.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81570898 and 18020067) and
Health Commission of Hubei Province Scientific research project
(no. WJ2021M250).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and SC performed the majority of the experiments,
the statistical analysis and prepared the manuscript. WW and JC
performed experiments and helped to draft the manuscript. WW, JC
and YJW analysed the data. WK and YJW participated in the
conception and design of the study. YW and YJW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethical
committees of Tong-ji Medical College, Huazhong University of
Science and Technology (permit no. S135). All participants of the
study were informed and signed a consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fokkens WJ, Lund VJ, Hopkins C, Hellings
PW, Kern R, Reitsma S, Toppila-Salmi S, Bernal-Sprekelsen M, Mullol
J, Alobid I, et al: European position paper on rhinosinusitis and
nasal polyps 2020. Rhinology. 58 (Suppl S29):S1–S464. 2020.
View Article : Google Scholar
|
|
2
|
Yim MT and Orlandi RR: Evolving Rhinology:
Understanding the burden of chronic rhinosinusitis today, tomorrow,
and beyond. Curr Allergy Asthma Rep. 20:72020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Gevaert E, Lou H, Wang X, Zhang
L, Bachert C and Zhang N: Chronic rhinosinusitis in Asia. J Allergy
Clin Immunol. 140:1230–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lourijsen ES, Fokkens WJ and Reitsma S:
Direct and indirect costs of Dutch adult patients with Chronic
Rhinosinusitis with nasal polyps. Rhinology. 58:213–217.
2020.PubMed/NCBI
|
|
5
|
Czerny MS, Namin A, Gratton MA and
Antisdel JL: Histopathological and clinical analysis of chronic
rhinosinusitis by subtype. Int Forum Allergy Rhinol. 4:463–469.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Zele T, Holtappels G, Gevaert P and
Bachert C: Differences in initial immunoprofiles between recurrent
and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J
Rhinol Allergy. 28:192–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calus L, Van Bruaene N, Bosteels C,
Dejonckheere S, Van Zele T, Holtappels G, Bachert C and Gevaert P:
Twelve-year follow-up study after endscopic sinus surgery in
patients with chronic rhinosinusitis with nasal polyposis. Clin
Transl Allergy. 9:302019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DeConde AS, Mace JC, Levy JM, Rudmik L,
Alt JA and Smith TL: Prevalence of polyp recurrence after
endoscopic sinus surgery for chronic rhinosinusitis with nasal
polyposis. Laryngoscope. 127:550–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin H, Li ZP, Lin D, Zheng CQ and Zhang
WT: Role of NLRP3 inflammasome in eosinophilic and non-eosinophilic
chronic rhinosinusitis with nasal polyps. Inflammation.
39:2045–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YJ, Gong GQ, Chen S, Xiong LY, Zhou
XX, Huang X and Kong WJ: NLRP3 inflammasome sequential changes in
Staphylococcus aureus-induced mouse model of acute rhinosinusitis.
Int J Mol Sci. 15:15806–15820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yazdi AS, Guarda G, Riteau N, Drexler SK,
Tardivel A, Couillin I and Tschopp J: Nanoparticles activate the
NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause
pulmonary inflammation through release of IL-1α and IL-1β. Proc
Natl Acad Sci USA. 107:19449–19454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okano M, Fujiwara T, Makihara S, Fujiwara
R, Higaki T, Kariya S, Noda Y, Haruna T and Nishizaki K:
Characterization of IL-18 expression and release in the
pathogenesis of chronic rhinosinusitis. Int Arch Allergy Immunol.
160:275–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robbins GR, Wen H and Ting JP:
Inflammasomes and metabolic disorders: Old genes in modern
diseases. Mol Cell. 54:297–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dostert C, Pétrilli V, Bruggen RV, Steele
C, Mossman BT and Tschopp J: Innate immune activation through Nalp3
inflammasome sensing of asbestos and silica. Science. 320:674–677.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu Y, Franchi L, Nunez G and Dubyak GR:
Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is
dependent on inflammasome activation and correlated with exosome
release in murine macrophages. J Immunol. 179:1913–1925. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim CH, Kim SS, Choi JY, Shin JH, Kim JY,
Namkung W, Lee JG, Lee MG and Yoon JH: Membrane-specific expression
of functional purinergic receptors in normal human nasal epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 287:L835–L842. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Virgilio F: Liaisons dangereuses:
P2X(7) and the inflammasome. Trends Pharmacol Sci. 28:465–472.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao B, Cao PP, Zeng M, Zhen Z, Wang H,
Zhang YN, Hu CY, Ma J, Li ZY, Song J, et al: Interaction of thymic
stromal lymphopoietin, IL-33, and their receptors in epithelial
cells in eosinophilic chronic rhinosinusitis with nasal polyps.
Allergy. 70:1169–1180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang WQ, Gao YL, Zhu ZZ, Zha Y, Wang XW,
Qi F, Zhou LG, Pang JY, Gao ZQ and Lv W: Changes in the clinical
and histological characteristics of Chinese chronic rhinosinusitis
with nasal polyps over 11 years. Int Forum Allergy Rhinol.
9:149–157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ET, Zheng Y, Liu PF and Guo LJ:
Eosinophilic chronic rhinosinusitis in East Asians. World J Clin
Cases. 2:873–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lohman AW, Billaud M and Isakson BE:
Mechanisms of ATP release and signalling in the blood vessel wall.
Cardiovasc Res. 95:269–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato T, Kouzaki H, Matsumoto K, Hosoi J
and Shimizu T: The effect of calprotectin on TSLP and IL-25
production from airway epithelial cells. Allergol Int. 66:281–289.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paris G, Pozharskaya T, Asempa T and Lane
AP: Damage-associated molecular patterns stimulate interleukin-33
expression in nasal polyp epithelial cells. Int Forum Allergy
Rhinol. 4:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cauwels A, Rogge E, Vandendriessche B,
Shiva S and Brouckaert P: Extracellular ATP drives systemic
inflammation, tissue damage and mortality. Cell Death Dis.
5:e11022014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ZC, Yao Y, Wang N, Liu JX, Ma J, Chen
CL, Deng YK, Wang MC, Liu Y, Zhang XH and Liu Z: Deficiency in
interleukin-10 production by M2 macrophages in eosinophilic chronic
rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol.
8:1323–1333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weigt SS, Palchevskiy V and Belperio JA:
Inflammasomes and IL-1 biology in the pathogenesis of allograft
dysfunction. J Clin Inves. 127:2022–2029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nelson DW, Gregg RJ, Kort ME,
Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT,
Donnelly-Roberts DL, Niforatos W, et al: Structure-activity
relationship studies on a series of novel, substituted
1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem.
49:3659–3666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donnelly-Roberts DL and Jarvis MF:
Discovery of P2X7 receptor-selective antagonists offers new
insights into P2X7 receptor function and indicates a role in
chronic pain states. Br J Pharmacol. 151:571–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng YH, Wang LQ, Wang QF, Li X, Zeng RB
and Gorodeski GI: ATP stimulates GRK-3 phosphorylation and
beta-arrestin-2-dependent internalization of P2X7 receptor. Am J
Physiol Cell Physiol. 288:C1342–C1356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stevens WW, Schleimer RP and Kern RC:
Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol
Pract. 4:565–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gallucci S and Matzinger P: Danger
signals: SOS to the immune system. Curr Opin Immunol. 13:114–119.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heffler E, Malvezzi L, Boita M, Brussino
L, De Virgilio A, Ferrando M, Puggioni F, Racca F, Stomeo N,
Spriano G and Canonica GW: Immunological mechanisms underlying
chronic rhinosinusitis with nasal polyps. Expert Rev Clin Immunol.
14:731–737. 2018. View Article : Google Scholar : PubMed/NCBI
|