Introduction
Diabetes mellitus (DM) is a major public health
concern globally. In 2015, there were 415 million diabetic patients
in total and it is estimated that this will increase to 642 million
by 2040 (1). Despite notable
progress in the management of blood glucose in diabetic patients
(2), the complications of diabetes
are often the cause of death in affected patients, and the
clinically available strategies for the management of complications
of diabetes are limited (3).
Diabetic cardiomyopathy (DCM) is a common
macrovascular complication that is characterized by ventricular
hypertrophy, heart failure and cardiac fibrosis (4–6).
Increasing evidence has demonstrated that, in addition to chronic
inflammation, mitochondrial dysfunction and oxidative stress also
contribute to the development of DCM (7–9).
Although certain therapies, including zinc supplementation
(10), advanced glycation end
product breakers (aminoguanidine) (11), copper chelators (trientine)
(12), metformin (13,14),
angiotensin-converting enzyme inhibitors (15,16),
β-blockers (timolol) (17) and
dipeptidyl peptidase-4 inhibitors (18), have been assessed in animal studies,
none of these therapies have been reported to be effective in
patients with DCM due to their side effects, and the underlying
mechanisms of action in the modulation of DCM are not completely
understood.
The nuclear factor erythroid 2-related factor 2
(Nrf2) signaling pathway is crucial for the regulation of
endogenous antioxidant enzyme protein expression levels (19), including heme oxygenase-1 (HO-1) and
γ-glutamylcysteine synthetase heavy subunit (γ-GCS). Nrf2-mediated
antioxidant enzymes are closely associated with inhibition of
oxidative stress and protection against DCM (20,21).
Therefore, enhancement of Nrf2 signaling may serve as a promising
therapeutic strategy for preventing the development of DCM.
The Guan Xin Dan Shen formulation (GXDSF) is
primarily composed of Panax notoginseng Radix et Rhizoma,
Dalbergiae odoriferae Lignum and Salviae
miltiorrhizae Radix et Rhizoma, and is a herbal prescription of
Traditional Chinese Medicine (22).
GXDSF has been used clinically for the treatment of cardiovascular
diseases (23). Our previous study
demonstrated that GXDSF prevented left ventricular remodeling
induced by myocardial ischemia/reperfusion injury (22). High-performance liquid
chromatography (HPLC) has been used to identify the contents of the
chemical components of GXDSF, demonstrating that it is composed of
notoginsenoside R1 (2.34%), ginsenoside Rb1 (8.63%), salvianolic
acid B (0.50%), ginsenoside Rg1 (9.51%), cryptotanshinone (0.84%),
tanshinone I (0.55%) and tanshinone IIA (1.71%) (22). The key components have been reported
to exert cardioprotective effects, including protecting against
ischemia in rats following acute myocardial infarction (24), preventing cardiac hypertrophy in
ApoE−/− mice (25) and
attenuating high glucose-induced endothelial cell injury (26,27).
Ginsenoside Rg1 ameliorates DCM by inhibiting endoplasmic reticulum
stress (28). However, to the best
of our knowledge, no previous studies have assessed the protective
roles of GXDSF in DCM. Furthermore, the drugs currently available
for the management of DCM are limited and are often accompanied by
adverse effects (29). Therefore,
assessing the protective effects of GXDSF in DCM is important.
In the present study, db/db mice were used to
investigate whether GXDSF exerted a protective effect against DCM
and to further determine the underlying mechanism.
Materials and methods
Drugs
Guan Xin Dan Shen dripping pills (40 mg/pill; cat.
no. 20160426) were obtained from Harbin Pharmaceutical Group Co.,
Ltd. In our previous study, the aforementioned components of GXDSF
were determined via HPLC (22).
Metformin was provided by Sino-American Shanghai Squibb
Pharmaceuticals, Ltd. Valsartan was provided by Novus Biologicals,
Ltd.
Animals
Mice were obtained from GemPharmatech Co., Ltd. In
total, 60 leptin receptor-deficient db/db 8-week-old male
mice (weight, 40±2 g; BKS.Cg+/+ Leprdb NJU) and 10
non-diabetic 8-week-old male mice (weight, 20±2 g; C57BLKS/J) were
used in the present study. The mice were maintained at 22–24°C and
40% humidity with 12-h light/dark cycles and ad libitum
access to food and water. All animal experiments were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (30). The present study
was approved by the Laboratory Animal Ethics Committee of the
Institute of Medicinal Plant Development, Peking Union Medical
College and Chinese Academy of Medical Sciences (license no.
SLXD-20190406017).
Groups and drug administration
After 4 weeks of acclimatization, the db/db
mice were randomly divided into the following six groups (n=10 per
group): i) db/db group (model); ii) db/db + metformin
(140 mg/kg/day) group; iii) db/db + valsartan (48 mg/kg/day)
group; iv) db/db + GXDSF high dose (180 mg/kg) group; v)
db/db + GXDSF moderate dose (90 mg/kg) group; and vi)
db/db + GXDSF low dose (45 mg/kg) group. The control group
consisted of the C57BLKS/J mice (n=10). The clinic dose of GXDSF
for patients with coronary heart disease is 20 mg/kg day. The doses
of GXDSF used in the present study were based on the recommended
clinical dose of GXDSF (31). Over
a period of 10 weeks, the mice were administered drugs via gavage
daily (Fig. 1A). Mice in the
control and model groups were orally administered distilled
water.
Fasting blood glucose (FBG) and
insulin tolerance test (ITT) assay
The blood glucose of mice fasted overnight was
measured using an automatic glucometer (Roche Diagnostics). For
assessing insulin tolerance, an ITT assay was performed via
intraperitoneal injection of 1 U/kg insulin (Sigma-Aldrich; Merck
KGaA). The tail blood glucose concentration was measured every 30
min after injection for a total of 120 min, and is expressed as the
area under the curve (AUC).
Echocardiography
Following treatment for 10 weeks, the mice were
anesthetized with 1.2% avertin (300 mg/kg; intraperitoneal) and
echocardiography was performed. The echocardiograms were obtained
using a Vevo770 high-resolution imaging system
(VisualSonics, Inc.). To calculate percentage ejection fraction (%
EF) and percentage fractional shortening (% FS), M-mode tracing of
the left ventricle (LV) based on the parasternal long-axis view was
used. To evaluate left ventricular end diastolic diameter (LVIDd)
and left ventricular end systolic diameter (LVIDs), the pulse-wave
and tissue Doppler in mice at baseline and after treatment were
used. LV end-diastolic volume (LVVd) and LV end-systolic volume
(LVVs) were automatically calculated by an ultrasound machine
(VisualSonics, Inc.).
Determination of serum lipids
After 10 weeks of treatment, the db/db mice
were anesthetized using 4% chloral hydrate (400 mg/kg;
intraperitoneal). Subsequently, ~0.75 ml blood was collected from
the eye socket vein of mice fasted overnight. The anesthetized mice
were then sacrificed via cervical dislocation. To obtain the serum
samples, the blood was centrifuged at 3,000 × g for 15 min at room
temperature. An automatic biochemical analysis system (Beckman
Coulter, Inc.) was used to measure serum triglyceride (TG, cat. no.
A110-1-1), low-density lipoprotein (LDL, cat. no. A113-1-1) and
total cholesterol (TC, cat. no. A111-1-1) levels according to the
manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute).
Assessing myocardial enzyme
activity
The serum myocardial enzyme activities of aspartate
transaminase (AST; cat. no. C010-1-1), lactate dehydrogenase (LDH;
cat. no. A020-1-2) and creatine kinase MB (CK-MB; cat. no.
E006-1-1) were measured using specific detection kits (Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
protocol.
Histological analysis
After the mice were sacrificed, five heart tissue
samples in each group were fixed in 4% buffered paraformaldehyde
for 48 h at room temperature. Following embedding in paraffin,
tissues were cut into 4-µm sections. Subsequently, sections were
stained with hematoxylin and eosin (H&E) at room temperature as
previously (32). Stained sections
were examined by a blinded pathologist using a BX53 light
microscope (Olympus Corporation; magnification, ×200).
TUNEL staining
The aforementioned fixed and embedded heart samples
were cut into 4-µm sections and then deparaffinized using xylene.
The antigen retrieval was performed by heating the tissues to 80°C.
Subsequently, the sections were incubated with Protease K for 15
min at room temperature before pre-incubation with terminal
deoxynucleotidyl transferase buffer (Sigma-Aldrich; Merck KGaA) at
37°C for 60 min. After washing with PBS, the sections were
incubated with an anti-digoxin and anti-serum alkaline phosphatase
complex (Sigma-Aldrich; Merck KGaA) at 37°C overnight. Following
washing in Tris buffer, sections were counterstained with
hematoxylin at 25°C for 2 min and washed again in Tris buffer. In
total, five visual fields were randomly selected in each group to
observe the apoptosis under a light microscope (BX53; Olympus
Corporation; magnification, ×200). The results were quantified
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from heart tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA purity and concentration were assessed using a
Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific, Inc.).
Total RNA (1 µg) was reverse transcribed into cDNA using
PrimeScript RT MasterMix (37°C for 15 min; 85°C for 5 sec, held at
4°C; Takara Biotechnology Co., Ltd.). Subsequently, qPCR was
performed to measure the mRNA expression levels of atrial
natriuretic peptide (ANP), brain natriuretic peptide (BNP),
α-myosin heavy polypeptide (MHC), β-MHC, Bcl-2, Bax, caspase-3,
caspase-9 and 18s using a CFX-96 Touch Thermocycler PCR system and
SYBR Green Master Mix. The thermocycling conditions used for qPCR
were as follows: Initial denaturation at 94°C for 30 sec, followed
by 45 cycles of 94°C for 5 sec, 58°C for 30 sec and 72°C for 30
sec, then a dissociation stage (95°C for 15 sec, 65°C for 30 sec
and 95°C for 15 sec). The sequences of the primers used for qPCR
are listed in Table I. mRNA
expression levels were quantified using the 2−ΔΔCq
method (33) and normalized to the
internal reference gene 18s.
 | Table I.Sequences of primers used for
quantitative PCR. |
Table I.
Sequences of primers used for
quantitative PCR.
| Gene | Sequence
(5′→3′) | Product size,
bp |
|---|
| Caspase-3 | F:
TGGTGATGAAGGGGTCATTTATG | 105 |
|
| R:
TTCGGCTTTCCAGTCAGACTC |
|
| Caspase-9 | F:
TCCTGGTACATCGAGACCTTG | 109 |
|
| R:
AAGTCCCTTTCGCAGAAACAG |
|
| Bcl-2 | F:
GCTACCGTCGTGACTTCGC | 147 |
|
| R:
CCCCACCGAACTCAAAGAAGG |
|
| Bax | F:
TGAAGACAGGGGCCTTTTTG | 140 |
|
| R:
AATTCGCCGGAGACACTCG |
|
| ANP | F:
GCTTCCAGGCCATATTGGAG | 126 |
|
| R:
GGGGGCATGACCTCATCTT |
|
| BNP | F:
GAGGTCACTCCTATCCTCTGG | 100 |
|
| R:
GCCATTTCCTCCGACTTTTCTC |
|
| α-MHC | F:
TGAGTGGGAGTTTATCGACTTCG | 194 |
|
| R:
CCTTGACATTGCGAGGCTTC |
|
| β-MHC | F:
CCTGCGGAAGTCTGAGAAGG | 119 |
|
| R:
CTCGGGACACGATCTTGGC |
|
| 18s | F:
CATGCAGAACCCACGACAGTA | 119 |
|
| R:
CCTCACGCAGCTTGTTGTCTA |
|
Western blotting
A standard western blotting protocol was used to
measure protein expression, as previously described (32). Briefly, cytoplasmic and nuclear
proteins were extracted from heart tissues using a protein
extraction kit (cat. no. CW0199S; CoWin Bioscience Co., Ltd.).
Protein concentrations were measured using a BCA protein
quantification kit (CWBio). Proteins (50 µg) were separated via 12%
SDS-PAGE and transferred to nitrocellulose membranes. Following
blocking in 5% fat-free milk in TBS-0.1% Tween-20 (TBST) for 2 h at
room temperature, the membranes were incubated at 4°C overnight
with primary antibodies targeted against: Bcl-2 (1:500; cat. no.
sc-7382; Santa Cruz Biotechnology, Inc.), Bax (1:500; cat. no.
sc-7480; Santa Cruz Biotechnology, Inc.), cleaved caspase-3
(1:1,000; cat. no. ab214430; Abcam), cleaved caspase-9 (1:1,000;
cat. no. 9509; Cell Signaling Technology, Inc.), phosphorylated
(p)-Akt (1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.),
Akt (1:1,000; cat. no. 9272; Cell Signaling Technology, Inc.), Nrf2
(1:500; cat. no. sc-13032; Santa Cruz Biotechnology, Inc.), HO-1
(1:1,000; cat. no. ab68477; Abcam), NAD(P)H quinone
oxidoreductase-1 (NQO-1; 1:500; cat. no. sc-32793; Santa Cruz
Biotechnology, Inc.), γ-GCS (1:500; cat. no. sc-166603; Santa Cruz
Biotechnology, Inc.), lamin B1 (1:500; cat. no. sc-374015; Santa
Cruz Biotechnology, Inc.) and β-actin (1:1,000; cat. no. CW0096M;
CWBio). Following washing with TBST, the membranes were incubated
with a HRP-conjugated secondary antibody (1:10,000; anti-rabbit
IgG, cat. no. 31460 or anti-mouse IgG, cat. no. 31430; Thermo
Fisher Scientific, Inc.) at room temperature for 2 h. Protein bands
were visualized using SuperSignal™ West Pico PLUS chemiluminescent
substrate (cat. no. 34580; Thermo Fisher Scientific, Inc.). Protein
expression was semi-quantified using Image Lab software (version
3.0; Bio-Rad Laboratories, Inc.). β-actin and lamin B1 were used as
the loading controls for cytoplasmic and nuclear proteins,
respectively.
Statistical analysis
All experiments were performed ≥3 times. Data are
presented as the mean ± SD. Statistical analyses were performed
using SPSS software (version 19.0; IBM Corp.). Comparisons among
multiple groups were analyzed using one-way or mixed two-way ANOVA
follow by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
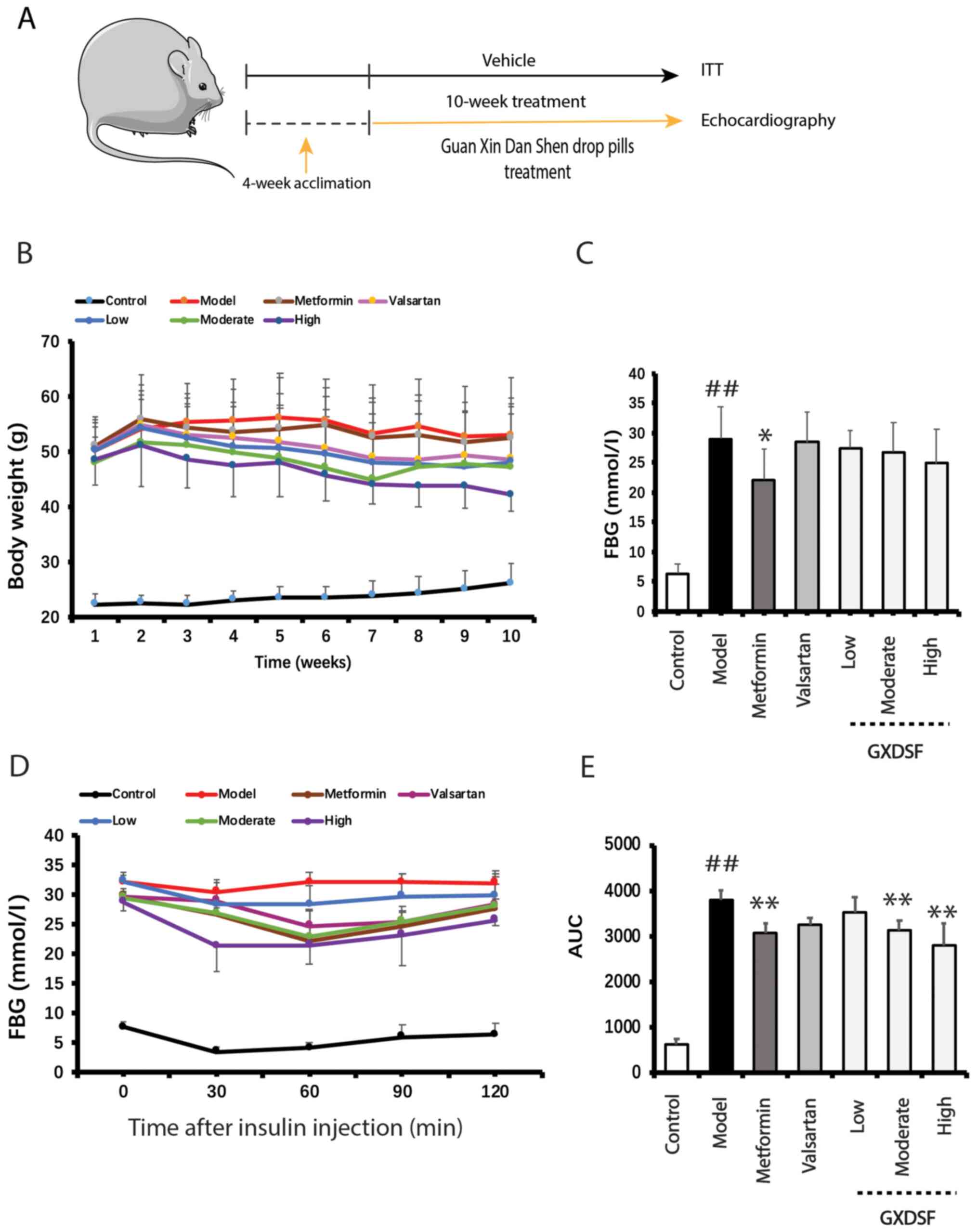
GXDSF reduces the increase in body
weight and enhances insulin sensitivity in db/db mice
The body weight of db/db mice was measured
over the 10-week treatment period. Compared with the model group,
the body weight of the mice in the GXDSF high-dose groups was
reduced, but this trend was not significant (Fig. 1B). Compared with the model group,
metformin significantly decreased elevated FBG levels in
db/db mice (P<0.05; Fig.
1C), whereas the effect of GXDSF on FBG was not significant
compared with the model group, which suggested that GXDSF displayed
no distinct hypoglycemic effect in db/db mice. Subsequently,
insulin sensitivity following GXDSF treatment was assessed. The ITT
results demonstrated that both moderate- and high-dose GXDSF
enhanced insulin sensitivity, as indicated by significantly
decreased AUC values compared with the model group (P<0.01;
Fig. 1D and E).
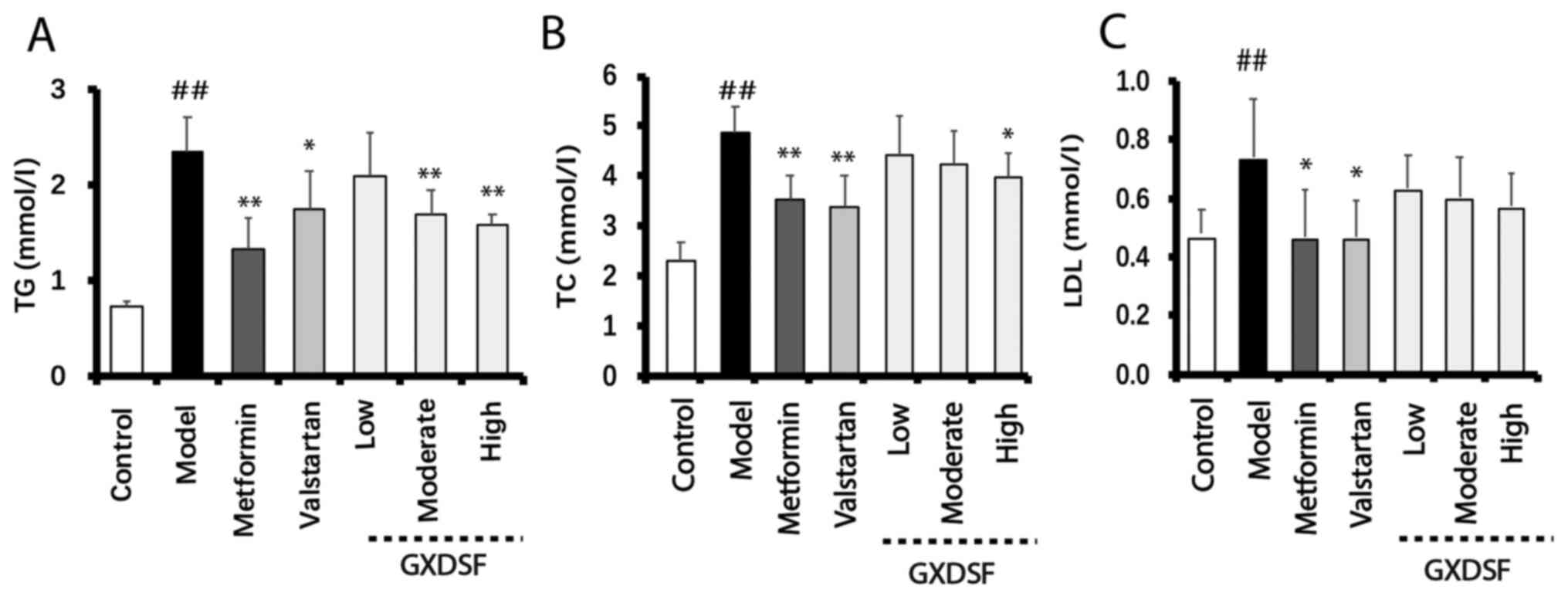
GXDSF decreases serum lipid levels in
db/db mice
Compared with the control group, db/db mice
displayed significantly increased serum lipid levels in the model
group (Fig. 2). Administration of
metformin or high-dose GXDSF significantly reduced serum TG and TC
levels in db/db mice compared with the model group
(P<0.05; Fig. 2A and B).
Additionally, increased serum LDL levels in db/db mice were
significantly reduced by metformin or valsartan administration
compared with the model group (both P<0.05; Fig. 2C). Serum LDL levels in the GXDSF
groups were slightly reduced, but the differences were not
significant compared with the model group.
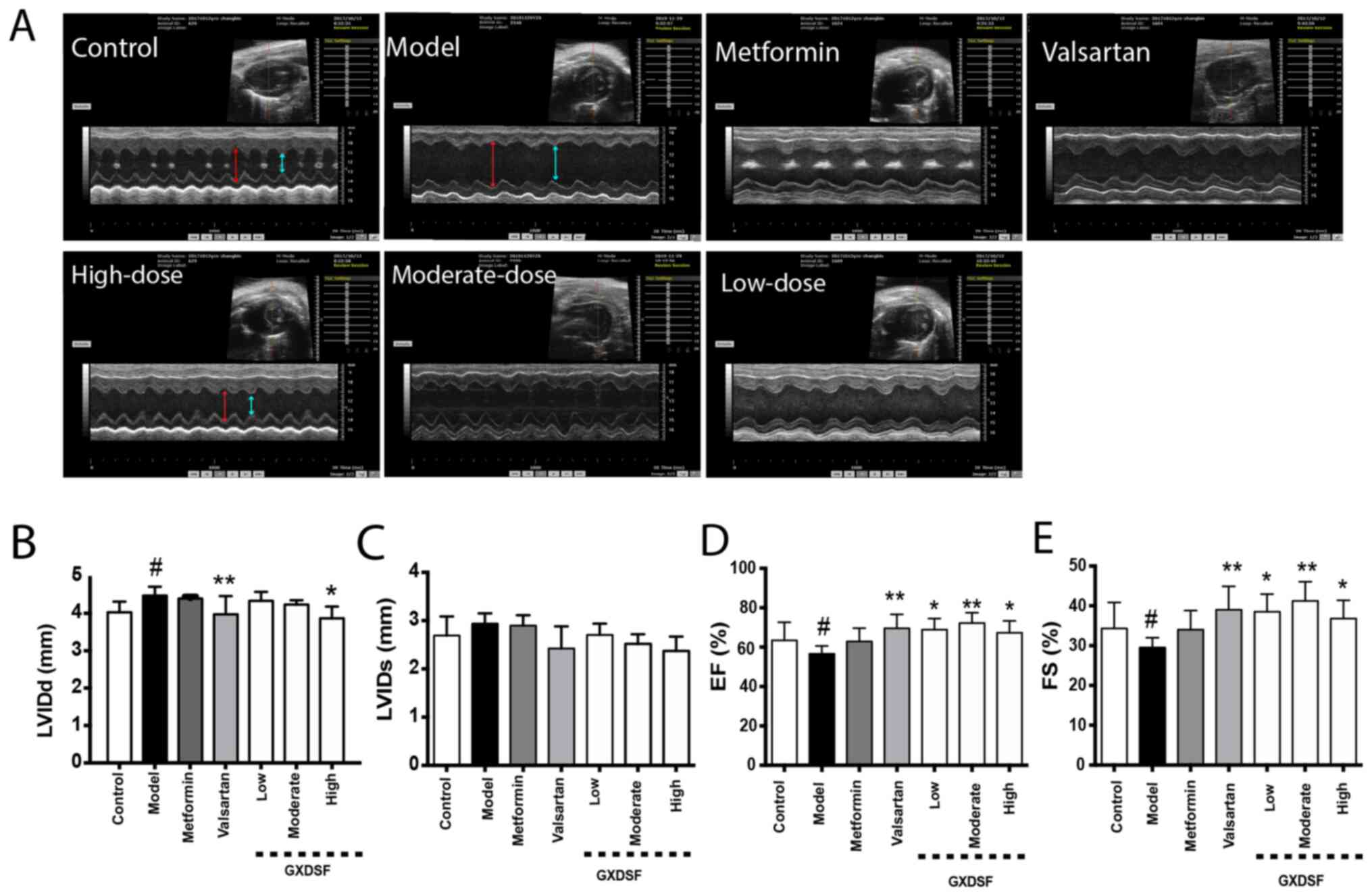
GXDSF ameliorates the cardiac
dysfunction of db/db mice
After 10 weeks of GXDSF treatment, M-mode
echocardiography was used to assess cardiac function. Compared with
the control group, db/db mice in the model group displayed a
significantly larger LVIDd (Fig.
3A). Administration of valsartan or high-dose GXDSF
significantly reduced LVIDd compared with the model group
(P<0.01). Compared with the model group, the effects of
metformin and moderate- and low-dose GXDSF on the LVIDd and LVIDs
were not significant (Fig. 3B and
C). Additionally, compared with the control group, the LV
end-systolic volume and LV end-diastolic volume of the db/db
mice in the model group were significantly reduced (P<0.01),
whereas valsartan, moderate-dose GXDSF and high-dose GXDSF
treatment significantly increased these volumes in db/db
mice (P<0.05; Fig. S1A and B).
Additionally, compared with the model group, valsartan or GXDSF
treatment significantly improved the impaired left ventricular EF
and FS in db/db mice (P<0.05; Fig. 3D and E).
GXDSF attenuates diabetes-induced
cardiac hypertrophy in db/db mice
After GXDSF treatment, the heart weight was
initially measured. Compared with the control group, the heart
weight of the model group was significantly increased, which was
significantly reduced by treatment with metformin, moderate-dose
GXDSF or high-dose GXDSF (P<0.05; Fig. 4A and B). In the model group, the
db/db mice developed notable myocardial hypertrophy, as
evidenced by H&E staining, which demonstrated disordered
cardiac fibers (Fig. 4C). However,
compared with the model group, treatment with metformin or GXDSF
for 10 weeks markedly reduced myocardial hypertrophy in
db/db mice. Moreover, compared with the control group, the
mRNA expression levels of cardiac hypertrophy-related genes ANP,
BNP and β-MHC/α-MHC were significantly increased in the heart
tissues of the model group (P<0.01), which was significantly
reversed by treatment with metformin, valsartan or GXDSF
(P<0.05; Fig. 4D). Furthermore,
treatment with moderate- or high-dose GXDSF significantly decreased
the serum levels of CK-MB and LDH compared with the model group
(Fig. 4E). Additionally, high-dose
GXDSF could reduce the increased serum AST level. Collectively, the
aforementioned results indicated that GXDSF prevented
diabetes-induced cardiac hypertrophy.
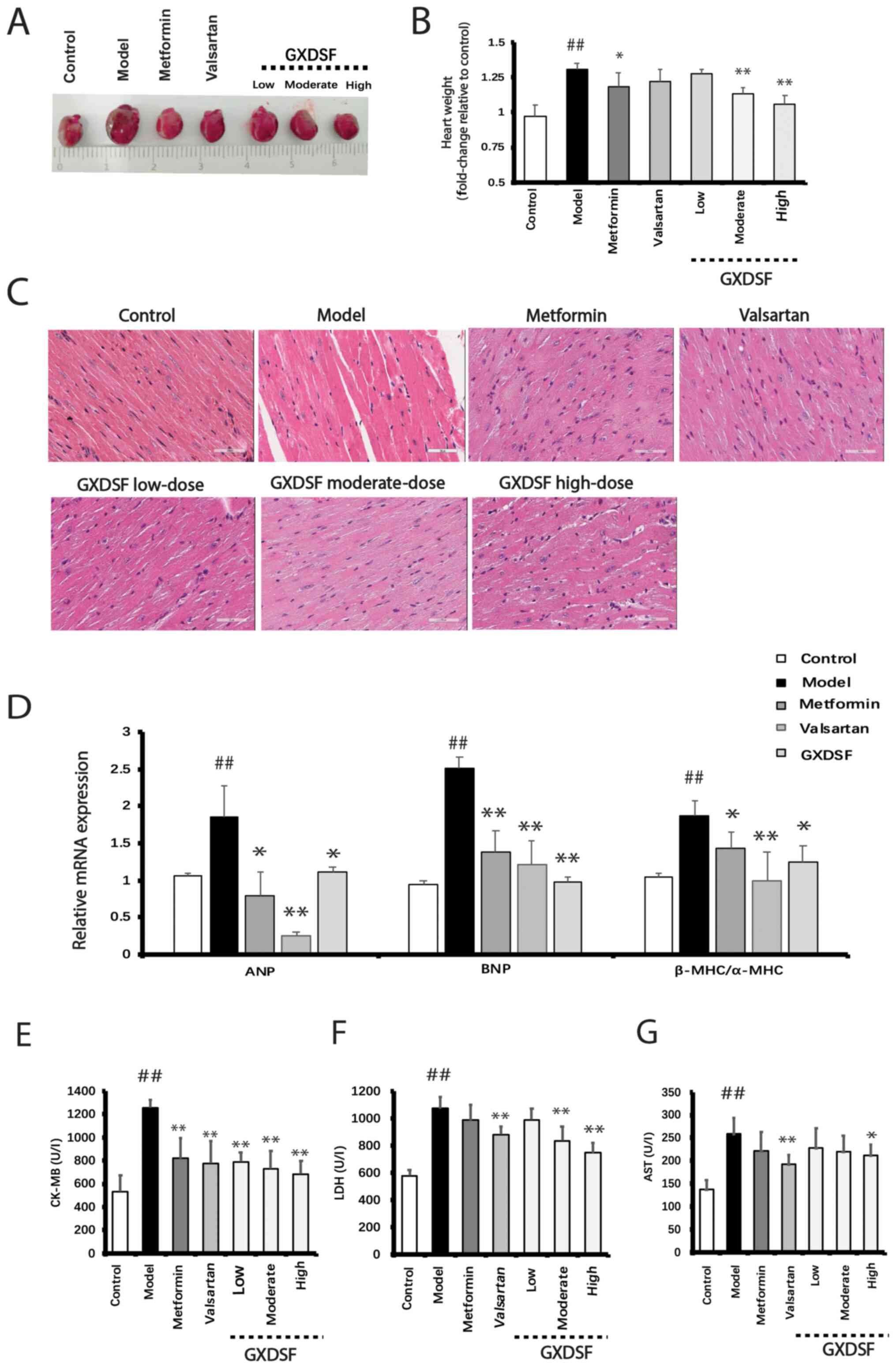 | Figure 4.Effect of GXDSF on pathological
alterations to the heart in db/db mice. (A) Representative
images of hearts isolated from db/db mice in each group. (B)
Heart weight of db/db mice in each group. (C) Representative
images of hematoxylin and eosin staining for identification of the
pathological structure of the heart (n=5; scale bar, 50 µm). (D)
Reverse transcription-quantitative PCR was performed to measure the
impact of GXDSF (high-dose) on the mRNA expression levels of
cardiac hypertrophy-related genes. Serum (E) CK-MB, (F) LDH and (G)
AST levels in db/db mice. Data are presented as the mean ±
SD (n=10). ##P<0.01 vs. control; *P<0.05 and
**P<0.01 vs. model. GXDSF, Guan Xin Dan Shen formulation; CK-MB,
creatine kinase MB; LDH, lactate dehydrogenase; AST, aspartate
transaminase; ANP, atrial natriuretic peptide; BNP, brain
natriuretic peptide; α-MHC, α-myosin heavy chain; β-MHC, β-myosin
heavy chain. |
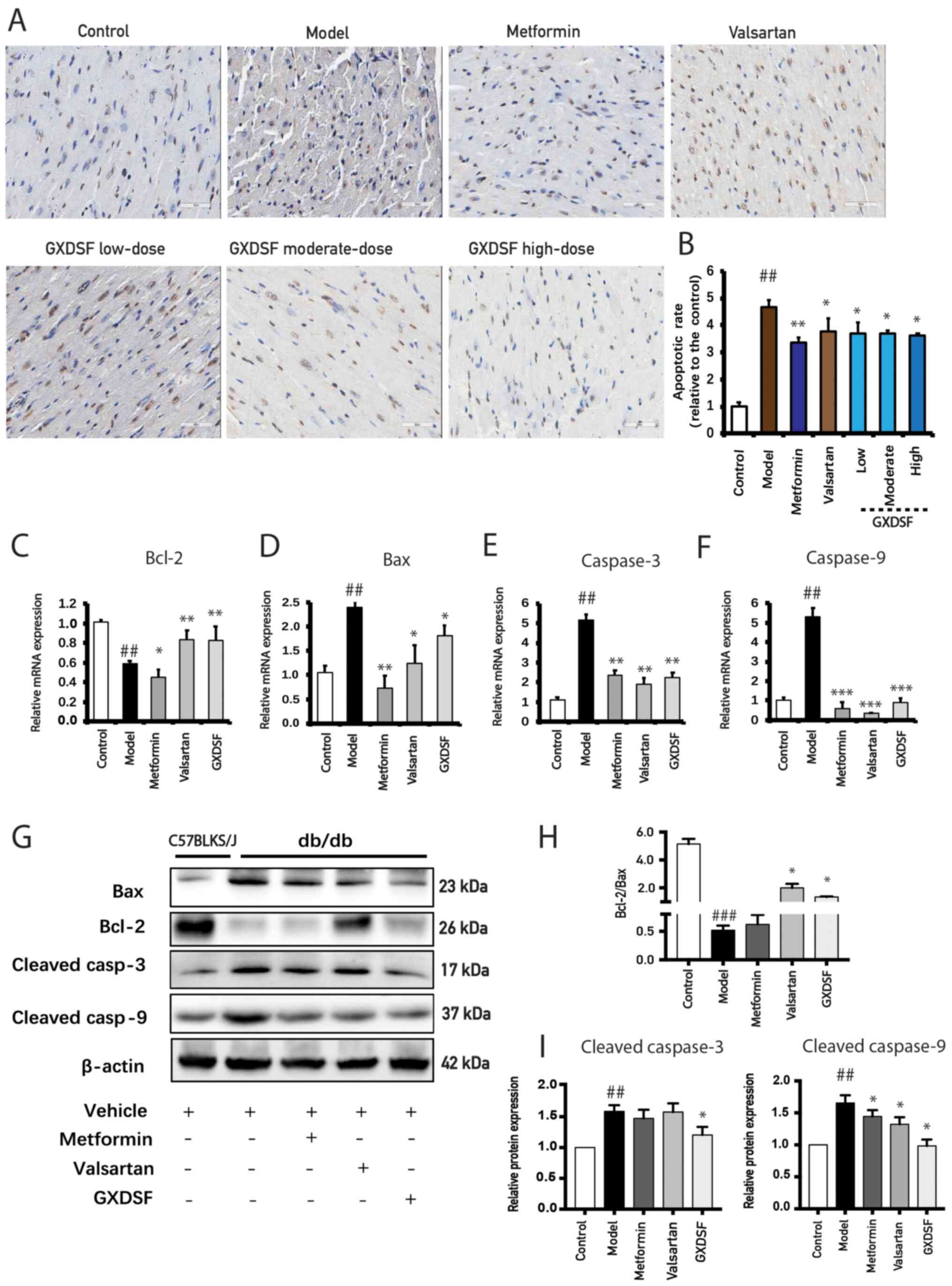
GXDSF attenuates diabetes-induced
cardiomyocyte apoptosis in db/db mice
Diabetes-induced cardiomyocyte apoptosis is closely
associated with cardiac hypertrophy (34). The TUNEL assay results demonstrated
that there was a significantly higher proportion of apoptotic cells
in the hearts of the model mice compared with the control group
(Fig. 5A and B). After 10 weeks of
treatment with metformin or GXDSF, the ratio of apoptotic cells was
notably decreased. The mRNA and protein expression levels of
apoptosis-related markers were further examined. In line with the
results of the TUNEL assay, compared with the model group, GXDSF
significantly decreased the mRNA expression levels of Bax,
caspase-3 and caspase-9 (Fig. 5D and
E), but significantly upregulated the mRNA expression levels of
Bcl-2 (Fig. 5C). The western
blotting results revealed that the Bcl-2/Bax ratio significantly
decreased, whereas the protein expression levels of cleaved
caspase-3 and cleaved caspase-9 were significantly upregulated in
the heart tissues of db/db mice in the model group compared
with the control group (Fig. 5G-I).
Diabetes-induced alterations in protein expression were
significantly reversed by GXDSF treatment (Fig. 5G-I), indicating that GXDSF protected
the diabetic heart against apoptosis.
GXDSF promotes translocation of Nrf2
to the nucleus to increase the expression of antioxidant
enzymes
The PI3K/Akt signaling pathway and Nrf2-mediated
antioxidant enzymes serve key anti-apoptotic roles (35), and our previous study demonstrated
that GXDSF increased Akt phosphorylation (22). Therefore, it was hypothesized that
GXDSF may protect against diabetes-induced cardiomyocyte apoptosis
by increasing Akt phosphorylation and Nrf2 signaling. The western
blotting results demonstrated that Akt phosphorylation, Nrf2
nuclear translocation and the expression of antioxidant enzymes
(γ-GCS, NQO-1 and HO-1) were significantly decreased in the heart
tissues of db/db mice in the model group compared with the
control group (Fig. 6A-F). After
treatment with GXDSF, the expression levels of p-Akt, nuclear Nrf2,
HO-1 and NQO-1 were significantly upregulated compared with the
model group. The results suggested that GXDSF promoted Akt and Nrf2
signaling, which subsequently upregulated the expression of
antioxidant enzymes (Fig. 6G).
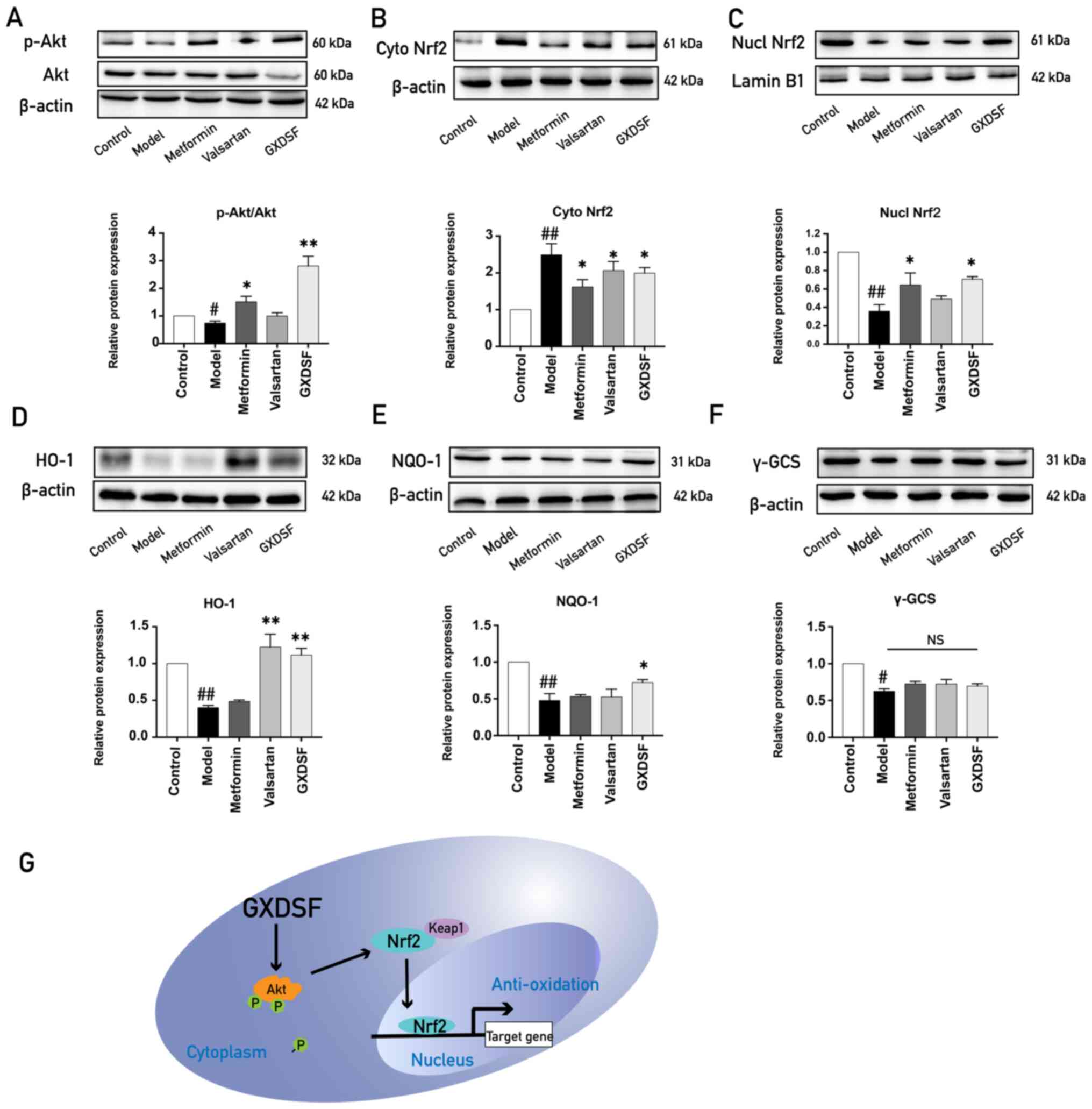 | Figure 6.GXDSF-induced activation of the
Akt-mediated Nrf2 signaling pathway. Western blotting was performed
to determine the impact of high dose of GXDSF on the protein
expression of (A) p-Akt, (B) cytoplasmic Nrf2, (C) nuclear Nrf2,
(D) HO-1, (E) NQO-1 and (F) γ-GCS (n=3). (G) Schematic illustration
indicating the mechanism underlying the protective effects of GSDXF
against diabetic cardiomyopathy. Data are presented as the mean ±
SD. #P<0.05 and ##P<0.01 vs. control;
*P<0.05 and **P<0.01 vs. model. GXDSF, Guan Xin Dan Shen
formulation; p, phosphorylated; Nrf2, nuclear factor-erythroid
2-related factor 2; HO-1, heme oxygenase-1; γ-GCS,
γ-glutamylcysteine synthetase heavy subunit; NQO-1, NAD(P)H quinone
oxidoreductase-1; NS, not significant; cyto, cytoplasmic; nucl,
nuclear; Keap1, kelch like ECH associated protein. |
Discussion
With an increasing prevalence of diabetes worldwide,
the incidence of DCM is also becoming an increasingly more common
problem. DCM is characterized by myocardial left ventricular
dysfunction, cardiac injury, cardiomyocyte hypertrophy,
cardiomyocyte apoptosis and microvascular abnormalities (36,37),
which may be the result of hyperglycemia, oxidative stress,
Ca2+ overload, ischemia and hypoxia (24,38,39).
According to previous studies, LV diastolic dysfunction is closely
related to myocardial cell apoptosis, and inhibition of apoptosis
may improve cardiac function (40).
Increased myocardial cell apoptosis leads to loss of contractile
units and cardiac remodeling, which ultimately results in cardiac
dysfunction (41,42). The present study demonstrated that
GXDSF may prevent the development of DCM by enhancing insulin
sensitivity, decreasing diabetic heart weight, improving cardiac
function and inhibiting cardiac hypertrophy. Mechanistically, the
results indicated that GXDSF may exert cardioprotective effects via
Akt/Nrf2-mediated upregulation of antioxidant enzymes. Therefore,
the present study suggested that GXDSF may serve as a therapeutic
agent for the prevention of DCM.
Chronic hyperglycemia, hyperlipidemia and insulin
resistance collectively lead to the development of DCM (43–45).
These risk factors not only result in metabolic dysfunction, but
also in severe oxidative stress and mitochondrial injury (46,47).
Currently, both db/db mice and streptozotocin (STZ) with
high-fat diet-induced mice are used as models of diabetes, and each
model displays different characteristics. The STZ with high-fat
diet-induced mouse model is more economical, but STZ might have
some unknown impacts on the mice. Another problem of the STZ with
high-fat diet-induced model is that the fasting blood glucose is
not as stable compared with db/db mice (48). The majority of previous studies
investigating DCM used db/db mice as a model of DCM
(49,50). Therefore, the present study selected
db/db mice as a model to study the protective effect of
GXDSF. Metformin was selected as a positive control for examining
the hyperglycemic effects of GXDSF. Compared with the model group,
the effect of GXDSF on FBG was insignificant; however, moderate and
high doses of GXDSF significantly improved insulin sensitivity and
decreased serum TG levels. The antilipemic effects of GXDSF may be
related to notoginseng, a key component of GXDSF that is
closely associated with fat metabolism according to recent studies
(51,52).
Mitochondria are crucial organelles that mediate
cardiomyocyte apoptosis (53). Loss
of the mitochondrial membrane potential is a key step in activating
mitochondrial-mediated apoptosis, which is primarily controlled by
the Bcl-2 family of proteins (54),
vital mediators of apoptosis (55).
Mitochondrial permeability is regulated by Bcl-2 and Bax (56). An increase in the Bax/Bcl-2 ratio
results in release of cytochrome c and activation of
caspase-3, leading to apoptosis (57). Compared with the model group, GXDSF
treatment significantly reduced the expression levels of the
proapoptotic protein Bax, increased the expression of the
antiapoptotic protein Bcl-2, and reduced the protein expression
levels of cleaved caspase-3 and cleaved caspase-9. Collectively,
the aforementioned results demonstrated that GXDSF inhibited
metabolic dysfunction-mediated cardiomyocyte apoptosis.
Numerous stimuli, including hypoxia, oxidative
stress and mechanical stress, can activate the PI3K/Akt signaling
pathway (58). Activation of Akt
signaling is sufficient to inhibit cardiomyocyte apoptosis and
maintain the function of viable cardiomyocytes (59). Promoting Akt signaling inhibits high
glucose-induced cardiomyocyte apoptosis (60). Consistent with the aforementioned
studies, the present study demonstrated that GXDSF significantly
upregulated p-Akt in the diabetic heart of db/db mice
compared with the model group. Cytoplasmic Nrf2 separates with
kelch like ECH associated protein and subsequently enters into the
nucleus to participate in controlling gene expressions. Our
previous study demonstrated that upregulation of p-Akt expression
promoted Nrf2-mediated expression of HO-1 and NQO-1 (61), an endogenic antioxidant enzyme with
anti-apoptotic and anti-inflammatory properties (62). As GXDSF can regulate p-Akt
expression, it was hypothesized that it may increase HO-1
expression by regulating translocation of Nrf2. In the present
study, compared with the control group, a significant increase in
the cytoplasmic protein expression levels of Nrf2 and a significant
decrease in the nuclear protein expression levels of Nrf2 in the
diabetic heart of model mice was observed, suggesting that Nrf2
nuclear translocation was inhibited. Furthermore, the results
indicated that GXDSF facilitated the nuclear translocation of Nrf2,
reversing the downregulation of the expression levels of the
antioxidant enzymes HO-1 and NQO-1 in db/db mice.
The present study preferentially evaluated the
protective effects of GXDSF on DCM in db/db mice because 90%
of cases of diabetes are type 2 DM (63). However, the present study did not
investigate whether GXDSF attenuated the progression of DCM in type
1 DM. Cardiac hypertrophy and fibrosis serve key roles in DCM
(64). However, diabetes-induced
cardiac hypertrophy and myocardial fibrosis appeared in different
periods of DCM (65). The impact of
GXDSF on diabetes-induced myocardial fibrosis is not completely
understood. Moreover, to further clarify the exact mechanism
underlying GXDSF-mediated prevention of DCM, future studies should
utilize a H9c2 cell model with or without Nrf2 knockdown.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate the protective function
of GXDSF against DCM via upregulation of Nrf2-mediated antioxidant
enzyme expression. The results of the present study highlighted the
potential therapeutic properties of GXDSF for the management of DCM
and glucose control.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the CAMS Innovation Fund
for Medical Sciences (grant no. 2019-I2M-1-005), the Drug
Innovation Major Project (grant no. 2018ZX09711001-009) and the
Central Public-Interest Scientific Institution Basal Research Fund
(grant no. 2018PT35030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XBS and GBS contributed to the conception and design
of the present study. BZ, CYZ and XLZ performed the experiments.
CYZ participated in performing the histological and western
blotting procedures. XLZ collected the data and performed
statistical analyses. BZ drafted the manuscript. BZ and XBS
confirmed the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Laboratory Animal Ethics Committee of the Institute of Medicinal
Plant Development, Peking Union Medical College and Chinese Academy
of Medical Sciences (license no. SLXD-20190406017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barooti A, Kamran M, Kharazmi F, Eftakhar
E, Malekzadeh K, Talebi A and Soltani N: Effect of oral magnesium
sulfate administration on blood glucose hemostasis via inhibition
of gluconeogenesis and FOXO1 gene expression in liver and muscle in
diabetic rats. Biomed Pharmacother. 109:1819–1825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perry BD, Caldow MK, Brennan-Speranza TC,
Sbaraglia M, Jerums G, Garnham A, Wong C, Levinger P, Asrar Ul Haq
M, Hare DL, et al: Muscle atrophy in patients with type 2 diabetes
mellitus: Roles of inflammatory pathways, physical activity and
exercise. Exerc Immunol Rev. 22:94–109. 2016.PubMed/NCBI
|
|
3
|
Papatheodorou K, Banach M, Bekiari E,
Rizzo M and Edmonds M: Complications of diabetes 2017. J Diabetes
Res. 2018:30861672018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai TH, Lin CJ, Chua S, Chung SY, Chen
SM, Lee CH and Hang CL: Deletion of RasGRF1 attenuated interstitial
fibrosis in streptozotocin-induced diabetic cardiomyopathy in mice
through affecting inflammation and oxidative stress. Int J Mol Sci.
19:30942018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lam CS: Diabetic cardiomyopathy: An
expression of stage B heart failure with preserved ejection
fraction. Diab Vasc Dis Res. 12:234–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai B, Li H, Fan J, Zhao Y, Yin Z, Nie X,
Wang DW and Chen C: MiR-21 protected against diabetic
cardiomyopathy induced diastolic dysfunction by targeting gelsolin.
Cardiovasc Diabetol. 17:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ni R, Cao T, Xiong S, Ma J, Fan GC,
Lacefield JC, Lu Y, Le Tissier S and Peng T: Therapeutic inhibition
of mitochondrial reactive oxygen species with mito-TEMPO reduces
diabetic cardiomyopathy. Free Radic Biol Med. 90:12–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo W, Jin Y, Wu G, Zhu W, Qian Y, Zhang
Y, Li J, Zhu A and Liang G: Blockage of ROS and MAPKs-mediated
inflammation via restoring SIRT1 by a new compound LF10 prevents
type 1 diabetic cardiomyopathy. Toxicol Appl Pharmacol. 370:24–35.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kayama Y, Raaz U, Jagger A, Adam M,
Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM and Tsao
PS: Diabetic cardiovascular disease induced by oxidative stress.
Int J Mol Sci. 16:25234–25263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Song Y, Elsherif L, Song Z, Zhou
G, Prabhu SD, Saari JT and Cai L: Cardiac metallothionein induction
plays the major role in the prevention of diabetic cardiomyopathy
by zinc supplementation. Circulation. 113:544–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu MS, Liang JT, Lin YD, Wu ET, Tseng YZ
and Chang KC: Aminoguanidine prevents the impairment of cardiac
pumping mechanics in rats with streptozotocin and
nicotinamide-induced type 2 diabetes. Br J Pharmacol. 154:758–764.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Pontré B, Pickup S, Choong SY, Li M,
Xu H, Gamble GD, Phillips AR, Cowan BR, Young AA and Cooper GJ:
Treatment with a copper-selective chelator causes substantive
improvement in cardiac function of diabetic rats with
left-ventricular impairment. Cardiovasc Diabetol. 12:282013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forcheron F, Basset A, Abdallah P, Del
Carmine P, Gadot N and Beylot M: Diabetic cardiomyopathy: Effects
of fenofibrate and metformin in an experimental model-the Zucker
diabetic rat. Cardiovasc Diabetol. 8:162009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie Z, Lau K, Eby B, Lozano P, He C,
Pennington B, Li H, Rathi S, Dong Y, Tian R, et al: Improvement of
cardiac functions by chronic metformin treatment is associated with
enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes.
60:1770–1778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rösen R, Rump AF and Rösen P: The
ACE-inhibitor captopril improves myocardial perfusion in
spontaneously diabetic (BB) rats. Diabetologia. 38:509–517. 1995.
View Article : Google Scholar
|
|
16
|
Al-Shafei AI, Wise RG, Gresham GA, Bronns
G, Carpenter TA, Hall LD and Huang CL: Non-invasive magnetic
resonance imaging assessment of myocardial changes and the effects
of angiotensin-converting enzyme inhibition in diabetic rats. J
Physiol. 538:541–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Turan B: A comparative summary on
antioxidant-like actions of timolol with other antioxidants in
diabetic cardiomyopathy. Curr Drug Deliv. 13:418–423. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Savarese G, D'Amore C, Federici M, De
Martino F, Dellegrottaglie S, Marciano C, Ferrazzano F, Losco T,
Lund LH, Trimarco B, et al: Effects of dipeptidyl peptidase 4
inhibitors and sodium-glucose linked coTransporter-2 inhibitors on
cardiovascular events in patients with type 2 diabetes mellitus: A
meta-analysis. Int J Cardiol. 220:595–601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki T and Yamamoto M: Molecular basis
of the Keap1-Nrf2 system. Free Radic Biol Med. 88:93–100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ge ZD, Lian Q, Mao X and Xia Z: Current
status and challenges of NRF2 as a potential therapeutic target for
diabetic cardiomyopathy. Int Heart J. 60:512–520. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo J, Yan D, Li S, Liu S, Zeng F, Cheung
CW, Liu H, Irwin MG, Huang H and Xia Z: Allopurinol reduces
oxidative stress and activates Nrf2/p62 to attenuate diabetic
cardiomyopathy in rats. J Cell Mol Med. 24:1760–1773. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng X, Xing X, Sun G, Xu X, Wu H, Li G
and Sun X: Guanxin danshen formulation protects against myocardial
ischemia reperfusion injury-induced left ventricular remodeling by
upregulating estrogen receptor β. Front Pharmacol. 8:7772017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Han L, Zhu, Wang D and Shang Z:
Guanxin Danshen Pills in Treatment of 200 Cases of Patients with
Coronary Heart Disease Angina Pectoris. China &Foreign Medical
Treatment. 24:126–128. 2017.
|
|
24
|
Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang
J, Ai Q, Xing N and Sun X: Cardioprotective effects of
Notoginsenoside R1 against ischemia/reperfusion injuries by
regulating oxidative stress- and endoplasmic reticulum stress-
related signaling pathways. Sci Rep. 6:217302016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao J, Zhu T, Yin YZ and Sun B:
Notoginsenoside R1, a unique constituent of Panax
notoginseng, blinds proinflammatory monocytes to protect
against cardiac hypertrophy in ApoE−/− mice. Eur J
Pharmacol. 833:441–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan C, Qiao Y and Tang M: Notoginsenoside
R1 attenuates high glucose-induced endothelial damage in rat
retinal capillary endothelial cells by modulating the intracellular
redox state. Drug Des Devel Ther. 11:3343–3354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu LJ, Zhang KJ, Zhu JZ, Zheng Q, Bao XY,
Thapa S, Wang Y and Chu MP: Salvianolic acid exerts
cardioprotection through promoting angiogenesis in animal models of
acute myocardial infarction: Preclinical evidence. Oxid Med Cell
Longev. 2017:81923832017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu H, Zhen J, Yang Y, Gu J, Wu S and Liu
Q: Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by
inhibiting endoplasmic reticulum stress-induced apoptosis in a
streptozotocin-induced diabetes rat model. J Cell Mol Med.
20:623–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paolillo S, Marsico F, Prastaro M, Renga
F, Esposito L, De Martino F, Di Napoli P, Esposito I, Ambrosio A,
Ianniruberto M, et al: Diabetic cardiomyopathy: Definition,
diagnosis, and therapeutic implications. Heart Fail Clin.
15:341–347. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press; Washington, DC:
1996
|
|
31
|
Xie W, Meng X, Zhai Y, Ye T, Zhou P, Nan
F, Sun G and Sun X: Antidepressant-like effects of the Guanxin
Danshen formula via mediation of the CaMK II-CREB-BDNF signalling
pathway in chronic unpredictable mild stress-induced depressive
rats. Ann Transl Med. 7:5642019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan T, Liang X, Bi P and Kuang S:
Myostatin knockout drives browning of white adipose tissue through
activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J.
27:1981–1989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Wang F, Zhang Y, Kang Y, Wang H,
Si M, Su L, Xin X, Xue F, Hao F, et al: Celecoxib prevents pressure
overload-induced cardiac hypertrophy and dysfunction by inhibiting
inflammation, apoptosis and oxidative stress. J Cell Mol Med.
20:116–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chai C, Song LJ, Han SY, Li XQ and Li M:
MicroRNA-21 promotes glioma cell proliferation and inhibits
senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT
signaling pathway. CNS Neurosci Ther. 24:369–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Zhang T, Yang Z, Lin J, Cai B, Ke
Q, Lan W, Shi J, Wu S and Lin W: Heme oxygenase-1 protects spinal
cord neurons from hydrogen peroxide-induced apoptosis via
suppression of Cdc42/MLK3/MKK7/JNK3 signaling. Apoptosis.
22:449–462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boudina S, Bugger H, Sena S, O'Neill BT,
Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ,
et al: Contribution of impaired myocardial insulin signaling to
mitochondrial dysfunction and oxidative stress in the heart.
Circulation. 119:1272–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dludla PV, Joubert E, Muller CJF, Louw J
and Johnson R: Hyperglycemia-induced oxidative stress and heart
disease-cardioprotective effects of rooibos flavonoids and
phenylpyruvic acid-2-O-β-D-glucoside. Nutr Metab (Lond). 14:452017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gencoglu H, Tuzcu M, Hayirli A and Sahin
K: Protective effects of resveratrol against streptozotocin-induced
diabetes in rats by modulation of visfatin/sirtuin-1 pathway and
glucose transporters. Int J Food Sci Nutr. 66:314–320. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu R, Sun H, Yu K, Zhong Y, Shi H, Wei Y,
Su X, Xu W, Luo Q, Zhang F, et al: Interleukin-37 and dendritic
cells treated with interleukin-37 plus troponin I ameliorate
cardiac remodeling after myocardial infarction. J Am Heart Assoc.
5:e0044062016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang WX, He BM, Wu Y, Qiao JF and Peng
ZY: Melatonin protects against sepsis-induced cardiac dysfunction
by regulating apoptosis and autophagy via activation of SIRT1 in
mice. Life Sci. 217:8–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamada S, Ding Y, Tanimoto A, Wang KY, Guo
X, Li Z, Tasaki T, Nabesima A, Murata Y, Shimajiri S, et al:
Apoptosis signal-regulating kinase 1 deficiency accelerates
hyperlipidemia-induced atheromatous plaques via suppression of
macrophage apoptosis. Arterioscler Thromb Vasc Biol. 31:1555–1564.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guo Y, Yin HJ and Shi DZ: Effect of xinnao
shutong capsule on cardiac muscle cell apoptosis and protein
expressions of Bcl-2 and Bax in hyperlipidemia rats after
myocardial infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi.
26:541–544. 2006.(In Chinese). PubMed/NCBI
|
|
45
|
Wang Y, Xue J, Li Y, Zhou X, Qiao S and
Han D: Telmisartan protects against high glucose/high lipid-induced
apoptosis and insulin secretion by reducing the oxidative and ER
stress. Cell Biochem Funct. 37:161–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vásquez-Trincado C, García-Carvajal I,
Pennanen C, Parra V, Hill JA, Rothermel BA and Lavandero S:
Mitochondrial dynamics, mitophagy and cardiovascular disease. J
Physiol. 594:509–525. 2016. View Article : Google Scholar
|
|
48
|
Cheng L, Shen ZF, Sun GB and Sun XB:
Advances in diabetic animal models and its application in the
traditional Chinese medicine research. Yao Xue Xue Bao. 50:951–958.
2015.(In Chinese). PubMed/NCBI
|
|
49
|
Zhang X and Hao Y: Beneficial effects of
echinacoside on diabetic cardiomyopathy in diabetic Db/Db mice.
Drug Des Devel Ther. 14:5575–5587. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang S, Wang B, Wang Y, Tong Q, Liu Q, Sun
J, Zheng Y and Cai L: Zinc prevents the development of diabetic
cardiomyopathy in db/db Mice. Int J Mol Sci. 18:5802017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Quan LH, Zhang C, Dong M, Jiang J, Xu H,
Yan C, Liu X, Zhou H, Zhang H, Chen L, et al: Myristoleic acid
produced by enterococci reduces obesity through brown adipose
tissue activation. Gut. 69:1239–1247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu Y, Wang N, Tan HY, Li S, Zhang C, Zhang
Z and Feng Y: Panax notoginseng saponins modulate the gut
microbiota to promote thermogenesis and beige adipocyte
reconstructionvia leptin-mediated AMPKα/STAT3 signaling in
diet-induced obesity. Theranostics. 10:11302–11323. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tan PP, Zhou BH, Zhao WP, Jia LS, Liu J
and Wang HW: Mitochondria-mediated pathway regulates C2C12 cell
apoptosis induced by fluoride. Biol Trace Elem Res. 185:440–447.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lu Q and Hong W: Bcl2 enhances
c-Myc-mediated MMP-2 expression of vascular smooth muscle cells.
Cell Signal. 21:1054–1059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang G, Zeng X, Zhang R, Liu J, Zhang W,
Zhao Y, Zhang X, Wu Z, Tan Y, Wu Y and Du B: Dioscin suppresses
hepatocellular carcinoma tumor growth by inducing apoptosis and
regulation of TP53, BAX, BCL2 and cleaved CASP3. Phytomedicine.
23:1329–1336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Birkinshaw RW and Czabotar PE: The BCL-2
family of proteins and mitochondrial outer membrane
permeabilisation. Semin Cell Dev Biol. 72:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Matsui T and Rosenzweig A: Convergent
signal transduction pathways controlling cardiomyocyte survival and
function: The role of PI 3-kinase and akt. J Mol Cell Cardiol.
38:63–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hong HJ, Liu JC, Chen PY, Chen JJ, Chan P
and Cheng TH: Tanshinone IIA prevents doxorubicin-induced
cardiomyocyte apoptosis through Akt-dependent pathway. Int J
Cardiol. 157:174–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Su D, Zhou Y, Hu S, Guan L, Shi C, Wang Q,
Chen Y, Lu C, Li Q and Ma X: Role of GAB1/PI3K/AKT signaling high
glucose-induced cardiomyocyte apoptosis. Biomed Pharmacother.
93:1197–1204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang B, Chen Y, Shen Q, Liu G, Ye J, Sun
G and Sun X: Myricitrin attenuates high glucose-induced apoptosis
through activating Akt-Nrf2 signaling in H9c2 cardiomyocytes.
Molecules. 21:8802016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mahalanobish S, Saha S, Dutta S and Sil
PC: Mangiferin alleviates arsenic induced oxidative lung injury via
upregulation of the Nrf2-HO1 axis. Food Chem Toxicol. 126:41–55.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Unnikrishnan R, Anjana RM and Mohan V:
Diabetes mellitus and its complications in India. Nat Rev
Endocrinol. 12:357–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Athithan L, Gulsin GS, McCann GP and
Levelt E: Diabetic cardiomyopathy: Pathophysiology, theories and
evidence to date. World J Diabetes. 10:490–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hu X, Bai T, Xu Z, Liu Q, Zheng Y and Cai
L: Pathophysiological fundamentals of diabetic cardiomyopathy.
Compr Physiol. 7:693–711. 2017. View Article : Google Scholar : PubMed/NCBI
|