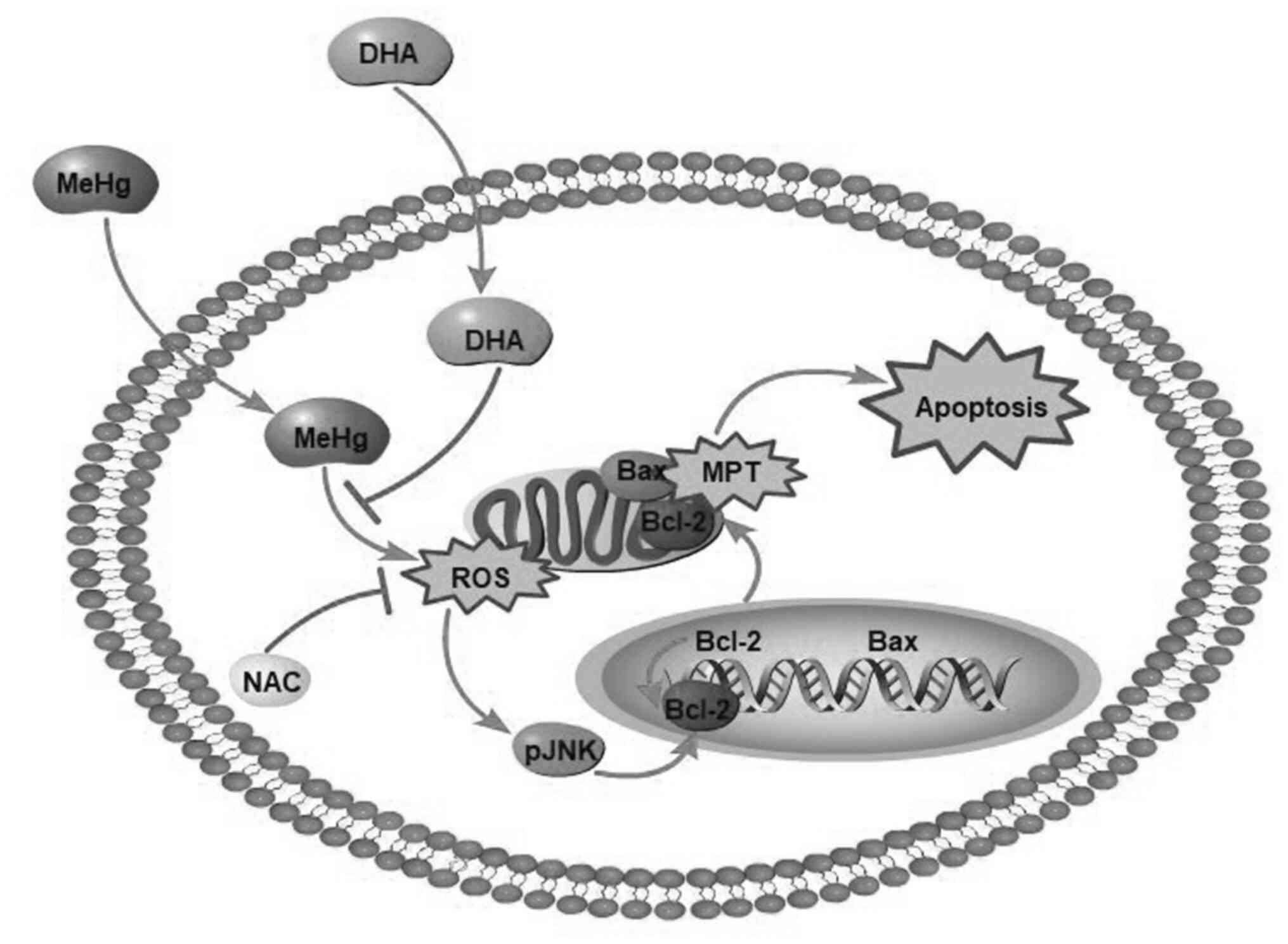
Introduction
Epidemiological studies have reported that global
methylmercury (MeHg) pollution has become increasingly serious in
recent years and humans are suffering from the effects of MeHg,
which has become a concern for several countries (1). The development of industry and
agriculture; the discharge of wastewater, residue and exhaust gas
in the process of smelting mercury ores; the combustion of fossil
fuel; and the irresponsible use of medical devices, such as use of
amalgam as filling material have led to increasingly serious
environmental mercury pollution (2,3).
Moreover, mercury released into the environment can be converted
into MeHg under certain conditions, such as transformation by
aquatic microorganisms, thereby becoming enriched in the aquatic
food chain. Once food containing MeHg is consumed, MeHg derivatives
are formed and accumulate in the body, particularly in the brain,
severely affecting human health (4).
The toxic effects of MeHg have been characterized by
a long incubation period before symptoms appear in humans. The main
symptoms include blurred vision, weight loss, ataxia and
neurodevelopmental abnormalities (5). In addition, MeHg has been reported to
serve an important role in early fetal neurodevelopment. It has
been reported that the brain MeHg content of exposed individuals
may be 3–6 times higher compared with that in the blood (6). Furthermore, maternal exposure to MeHg
from the consumption of fish and seafood may have irreversible
effects on the neurobehavioral development of children, including
cognitive impairment, memory impairment and motor developmental
abnormalities (7,8).
It was recently demonstrated that the neurotoxicity
produced by MeHg is closely associated with cell apoptosis, and
oxidative stress induced by reactive oxygen species (ROS) is the
most likely predisposing factor (9). As a secondary messenger, ROS serve a
dual role in the body. Stable physiological ROS levels can suppress
harmful cellular processes; however, high concentrations of ROS can
cause cell apoptosis and death (10). A previous study reported that
excessive ROS may directly damage DNA and activate the MAPK
signaling pathway, initiate mitochondria-related apoptosis and
depolarize the mitochondrial membrane, thus damaging the integrity
of the mitochondrial membrane (11). Eventually, cytochrome c may
be released into the cytoplasm from the mitochondrial intermembrane
space, thereby activating caspases to induce cell apoptosis
(12).
JNK, also known as stress-activated protein kinase,
is a member of the MAPK superfamily, and is involved in various
stress responses, particularly oxidative damage (13,14).
It has previously been reported that MeHg can increase
intracellular ROS levels, causing changes in glutathione content
and lipid peroxidation, resulting in oxidative damage (9). Therefore, MeHg-induced apoptosis may
be associated with activation of the ROS-regulated JNK signaling
pathway.
Docosahexaenoic acid (DHA) is an essential n-3
long-chain polyunsaturated fatty acid that is mainly present in the
brain, and is involved in neurotransmitter pathways, synaptic
transmission and signal transduction (15). DHA is essential for the development
of the nervous system in children. Epidemiological studies have
identified that the presence of more n-3 unsaturated fatty acids
may promote in utero fetal neurodevelopment in women who are
exposed to low levels of MeHg (16,17).
In addition, consumption of certain fish, such as salmon, with a
higher DHA content may effectively decrease the neurotoxicity of
MeHg in children (18).
Previous studies have revealed that DHA can
effectively reduce MeHg-induced oxidative damage and inhibit
apoptosis in different types of cells (19–21).
Kaur et al (20) reported
that DHA pretreatment effectively reduced cell-associated MeHg and
the prooxidant response from MeHg in cerebellar astrocytes and
neurons, thus supporting the hypothesis that fish-derived nutrients
offer possible neuroprotection from MeHg. However, there is limited
research on the mechanism underlying the effects of DHA against
MeHg-induced apoptosis. Therefore, as PC12 cells are widely used in
neurological research, a MeHg-induced PC12 cell apoptosis model was
established in the present study to further examine the
anti-apoptotic mechanism of DHA against MeHg based on regulation of
the JNK signaling pathway.
Materials and methods
Materials
MeHg chloride and cis-4,7,10,13,16,19-DHA were
obtained from Sigma-Aldrich (Merck KGaA). Cell Counting Kit-8
(CCK-8), TUNEL Apoptosis Assay kit, Annexin V-FITC Apoptosis Assay
kit, Reactive Oxygen Assay kit, cell lysis buffer for western
blotting and N-acetyl-l-cysteine (NAC) were purchased from Beyotime
Institute of Biotechnology. Antibodies against Bax (cat. no.
2772S), JNK (cat. no. 9252S) and phosphorylated (p)JNK (cat. no.
4668S) were purchased from Cell Signaling Technology, Inc.
Anti-Bcl-2 (cat. no. AB112) was purchased from Beyotime Institute
of Biotechnology and anti-GAPDH (cat. no. 30202ES40) was purchased
from Shanghai Yeasen Biotechnology Co., Ltd. The gene-specific
primers were designed by Sangon Biotech Co., Ltd.
Cell culture
The PC12 rat pheochromocytoma cell line (serial no.
TCR8) was purchased from The Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences. The purchased cells were
commercialized poorly differentiated PC12 cells. The cells were
incubated in a 60-mm culture dish in DMEM (HyClone; Cytiva)
supplemented with 5% horse serum (Sigma-Aldrich; Merck KGaA), 105%
FBS (Sigma-Aldrich; Merck KGaA) and 100 U/ml
penicillin-streptomycin in a humidified atmosphere containing 55%
CO2/955% air at 37°C.
Cell viability assay
The CCK-8 reagent was applied to evaluate the
viability ability of cells. The cells were treated with MeHg (1.25,
2.5, 5, 10 and 20 µmol/l) for 24, 48 and 72 h in a 55%
CO2 incubator at 37°C, and with different concentrations
of DHA (5, 10, 20, 40 and 80 µmol/l) for 24 h in a 55%
CO2 incubator at 37°C to screen for the suitable drug
treatment concentration. In addition, to evaluate the protective
effect of DHA against MeHg-induced damage in PC12 cells, the cells
pretreated with DHA (5, 10, 20, 40 and 80 µmol/l) for 24 h were
treated with MeHg (2.5 µmol/l) for 48 h. After treatment, PC12
cells were cultured in a 55% CO2 incubator at 37°C, then
incubated with 105% CCK-8 reagent for 1 h at 37°C. The optical
density of PC12 cells was measured at a wavelength of 450 nm using
a microplate reader. A cell growth curve was drawn with GraphPad
Prism 5 (GraphPad Software, Inc.).
TUNEL staining assay
A TUNEL apoptosis detection kit was used to observe
cell apoptosis under a fluorescence microscope. Firstly, cells were
grown on culture plates until they reached 805% confluence and were
then treated with DHA followed by MeHg. Cells were washed with PBS
and incubated with the 10% TUNEL solution at 37°C in the dark for
60 min. The samples were immediately observed under a fluorescence
microscope, where the excitation wavelength was 450–500 nm and the
emission wavelength range was 515–565 nm. Green fluorescence
represented apoptotic cells. In addition, nuclei were stained by
100 ng/ml DAPI (Beyotime Institute of Biotechnology) for 5 min at
25°C.
Flow cytometric analysis of
apoptosis
A total of 1×105 PC12 cells were added to
100 µl binding buffer for 20 min at 25°C following centrifugation
at 1,000 × g for 5 min at 25°C and were then added to the Annexin
V-FITC and PI mixture, with separate Annexin V-FITC and PI control
tubes and an unstained tube. Finally, each sample tube was placed
on ice in the dark for 20 min, and 500 µl 1X binding buffer was
added. The samples were analyzed on a LSR Fortessa flow cytometer
(BD Biosciences) to detect the apoptotic rate within 30 min, and
the upper- and lower-right quadrants show apoptotic cells with
FlowJo V10.0.7 (BD Biosciences). The position of the gates of other
groups was relative to the control plot in the flow cytometric
analysis.
Assessment of ROS
Accumulation of intracellular ROS was detected using
the peroxide-sensitive fluorescent probe DCFH-DA. PC12 cells
(1×105) were cultured for 48 h, after which MeHg and DHA
were added to the culture media. At the end of the treatment, the
cells were incubated with 10 µmol/l DCFH-DA for 40 min at 37°C.
Fluorescence detection was performed using a fluorescence
microplate reader at an excitation wavelength of 488 nm and an
emission wavelength of 525 nm, in order to determine the generation
of ROS.
Western blot analysis
After treatment for the indicated duration, the
cells were washed three times with cold PBS and lysed. Total
cytoplasmic and nuclear protein was collected using cell lysis
buffer for western blotting, according to the manufacturer's
instructions. The protein concentration was measured using a BCA
assay. Equal amounts of protein (25 µg) were separated by 10%
SDS-PAGE, followed by transfer to PVDF membranes. The membranes
were blocked in TBS containing 55% skimmed milk and 0.15% Tween-20
at 25°C for 1 h, and then incubated with primary antibodies against
Bcl-2 (1:1,000), Bax (1:1,000), JNK (1:1,000), pJNK (1:1,000) and
GAPDH (1:10,000) at 4°C overnight. Anti-GAPDH antibody was selected
as an internal reference. Subsequently, the membranes were washed
with TBS and incubated with HRP-labeled secondary antibodies
(1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) at
room temperature for 1 h. Finally, the blots were visualized using
ECL (BeyoECL Moon; cat. no. P0018FS; Beyotime Institute of
Biotechnology) and the results were analyzed using Image Lab
(5.2.1; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from PC12 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The extracted RNA was then reverse transcribed to obtain
stable cDNA as follows: 37°C for 15 min, 85°C for 5 sec, 4°C. RT
primers, including Oligo dT primer and random hexamer primers, were
used to generate cDNA with the PrimeScript™ RT reagent kit with
gDNA Eraser (Takara Bio, Inc.). The ABI 7500 real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
amplify the specific PCR products and analyze the threshold cycle
number (Cq value) with TB Green Premix Ex Taq kit from Takara Bio,
Inc. The thermocycling conditions as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec. The
primers for the amplification of Bax, Bcl-2 and GAPDH mRNA were
designed and synthesized by Sangon Biotech Co., Ltd., as follows:
Bax forward, 5′-AGGACGCATCCACCAAGAAG-3′ and reverse,
5′-CAGTTGAAGTTGCCGTCTGC-3′; Bcl-2 forward,
5′-TGGCCTTCTTTGAGTTCGGT-3′ and reverse, 5′-GATGCCGGTTCAGGTACTCA-3′;
and GAPDH forward, 5′-GGGTCCCAGCTTAGGTTCATC-3′ and reverse,
5′-TGAGGTCAATGAAGGGGTCG-3′. GAPDH was used as an internal standard.
The qPCR results were normalized to the Cq value of GAPDH from the
same sample, and the 2−∆∆Cq (22) method was used to calculate the fold
change of each gene expression. The amplification reaction for each
sample was repeated three times (23). A non-template control was included
in each experiment.
Statistical analysis
SPSS 20.0 (version 20.0; IBM Corp.) was used for
data analysis. Measurement data are presented as the mean ± SD
(n=3), and one-way ANOVA was performed among multiple groups. When
meeting the homogeneity of variance, the data were subsequently
analyzed with a Dunnett's T3 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of DHA on MeHg-induced PC12
cell viability
PC12 cells were treated with DHA (5, 10, 20, 40 or
80 µmol/l) for 24 h to select the appropriate concentration. In
addition, the appropriate MeHg concentration was screened; PC12
cells were treated with MeHg (1.25, 2.5, 5, 10 and 20 µmol/l) for
24, 48 and 72 h. As presented in Fig.
1A, compared with the untreated control group, cell viability
was significantly decreased in the 2.5 µmol/l MeHg group treated
for 48 h. Moreover, it was revealed that treatment with 5 and 10
DHA increased cell viability (Fig.
1B). According to the principle of minimizing the dose and
maximizing the curative effect, treatments with 2.5 µmol/l MeHg for
48 h, and 10 and 20 µmol/l DHA for 24 h were selected as the
conditions for subsequent experiments. The results demonstrated
that MeHg significantly inhibited the viability of PC12 cells,
whereas DHA could effectively alleviate this effect, which
suggested a significant dose-response relationship (Fig. 1C).
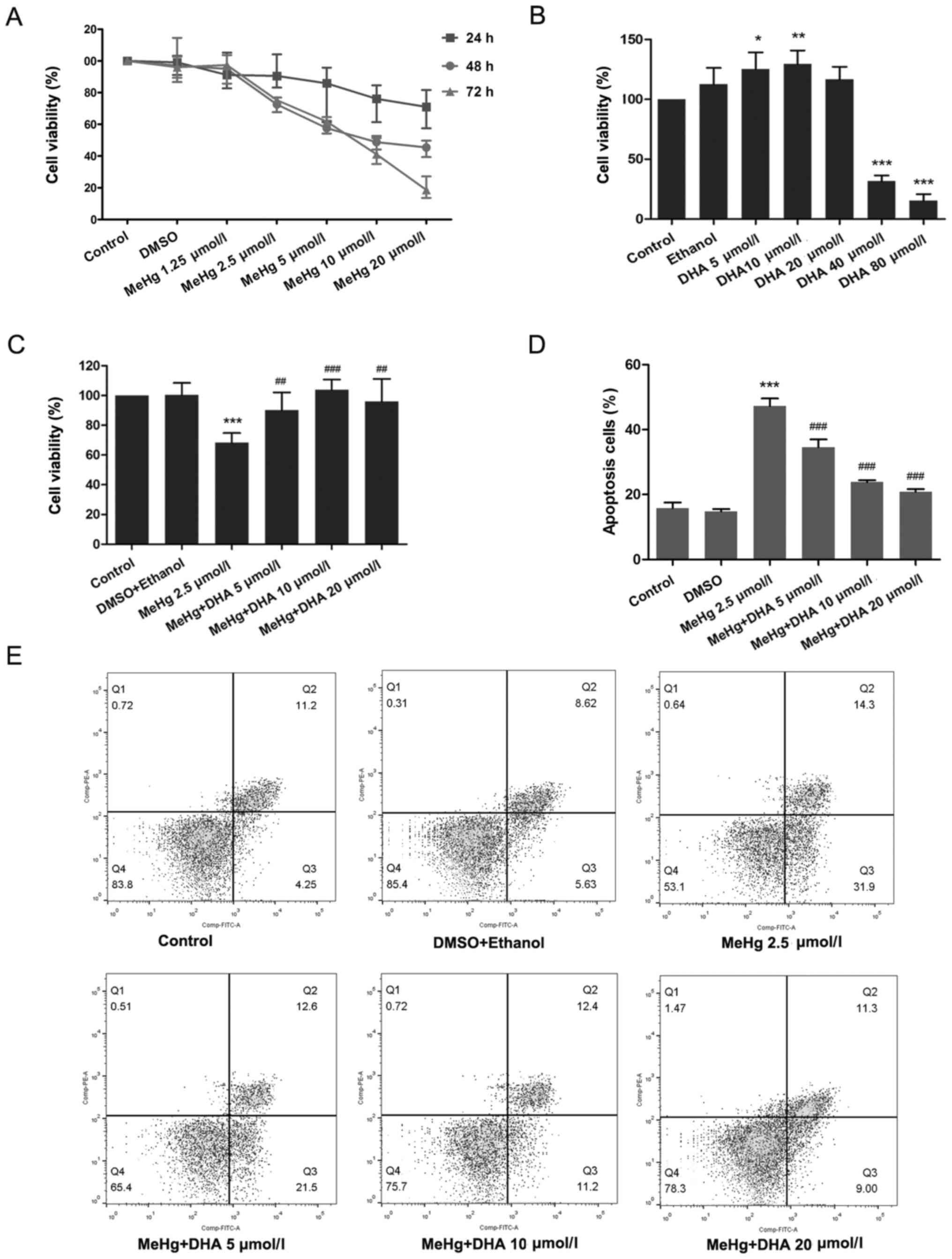 | Figure 1.Effects of DHA on MeHg-induced
proliferation inhibition and apoptosis of PC12 cells. (A) Changes
in cell viability following treatment with MeHg (1.25, 2.5, 5, 10
and 20 µmol/l) for 24, 48 or 72 h. (B) Changes in cell viability
following treatment with DHA (5, 10, 20, 40 and 80 µmol/l) for 24
h. (C) PC12 cells were treated with 2.5 µmol/l MeHg for 48 h and
were pretreated with DHA (5, 10 and 20 µmol/l) for 24 h; cell
viability was detected using a Cell Counting Kit-8 assay. (D and E)
Annexin V-FITC apoptosis detection of PC12 cells pretreated with
DHA followed by exposure to MeHg. The apoptosis rate was
subsequently analyzed (n=3). Data are presented as the mean ± SD.
*P<0.05, **P<0.01 and ***P<0.001 vs. control group;
##P<0.01 and ###P<0.001 vs. MeHg group.
MeHg, methylmercury; DHA, docosahexaenoic acid. |
Effect of DHA on MeHg-induced PC12
cell apoptosis
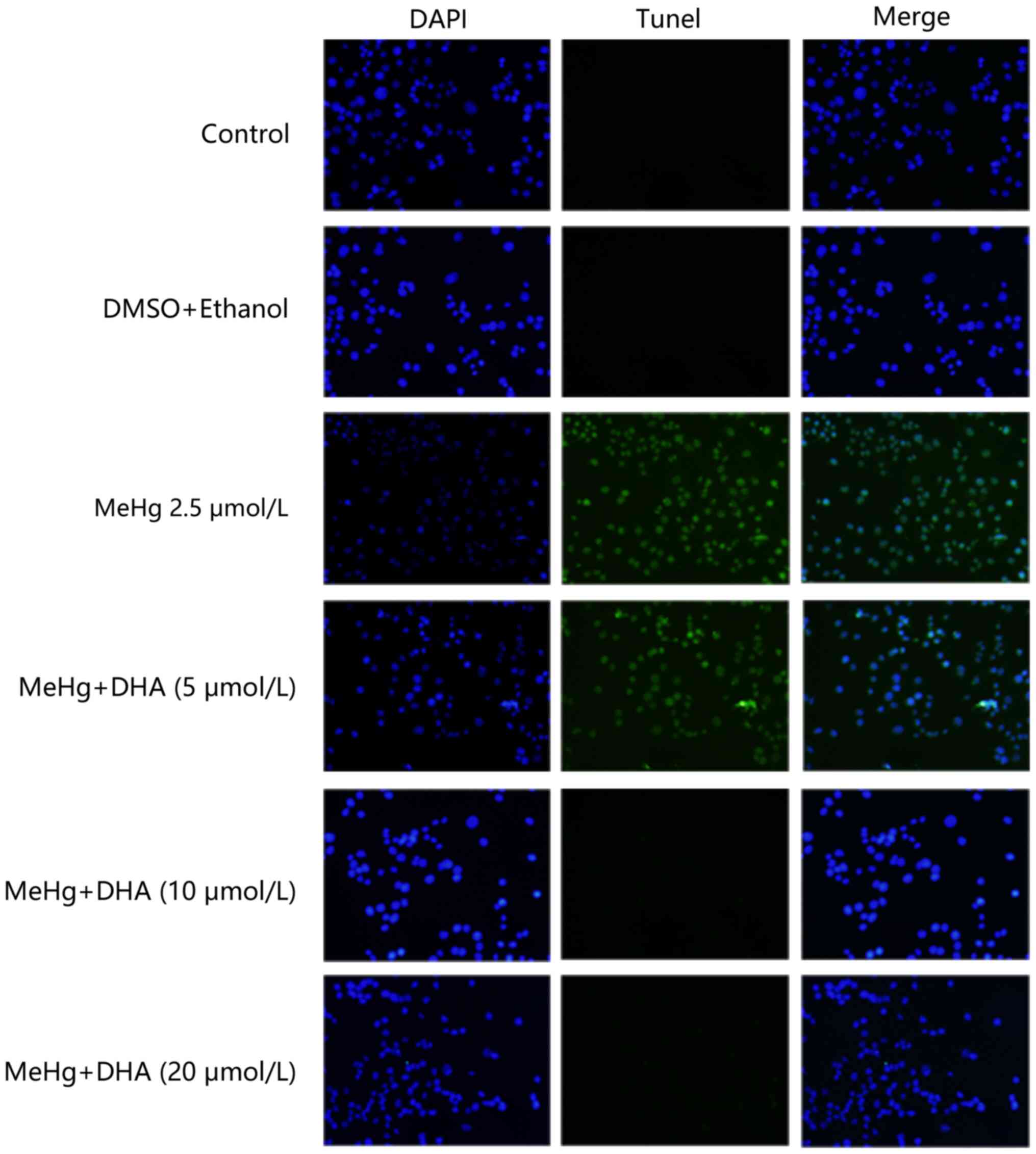
TUNEL staining was conducted to examine the
protective effect of DHA on MeHg-induced PC12 cell apoptosis. PC12
cells were treated with 2.5 µmol/l MeHg and marked apoptosis was
observed; however, the fluorescence intensity of DHA was markedly
decreased (Fig. 2). In addition,
the results of flow cytometry revealed similar findings, and there
was a significant dose-response relationship (Fig. 1D and E).
Effects of DHA and MeHg on the
expression levels of apoptosis-associated genes and proteins
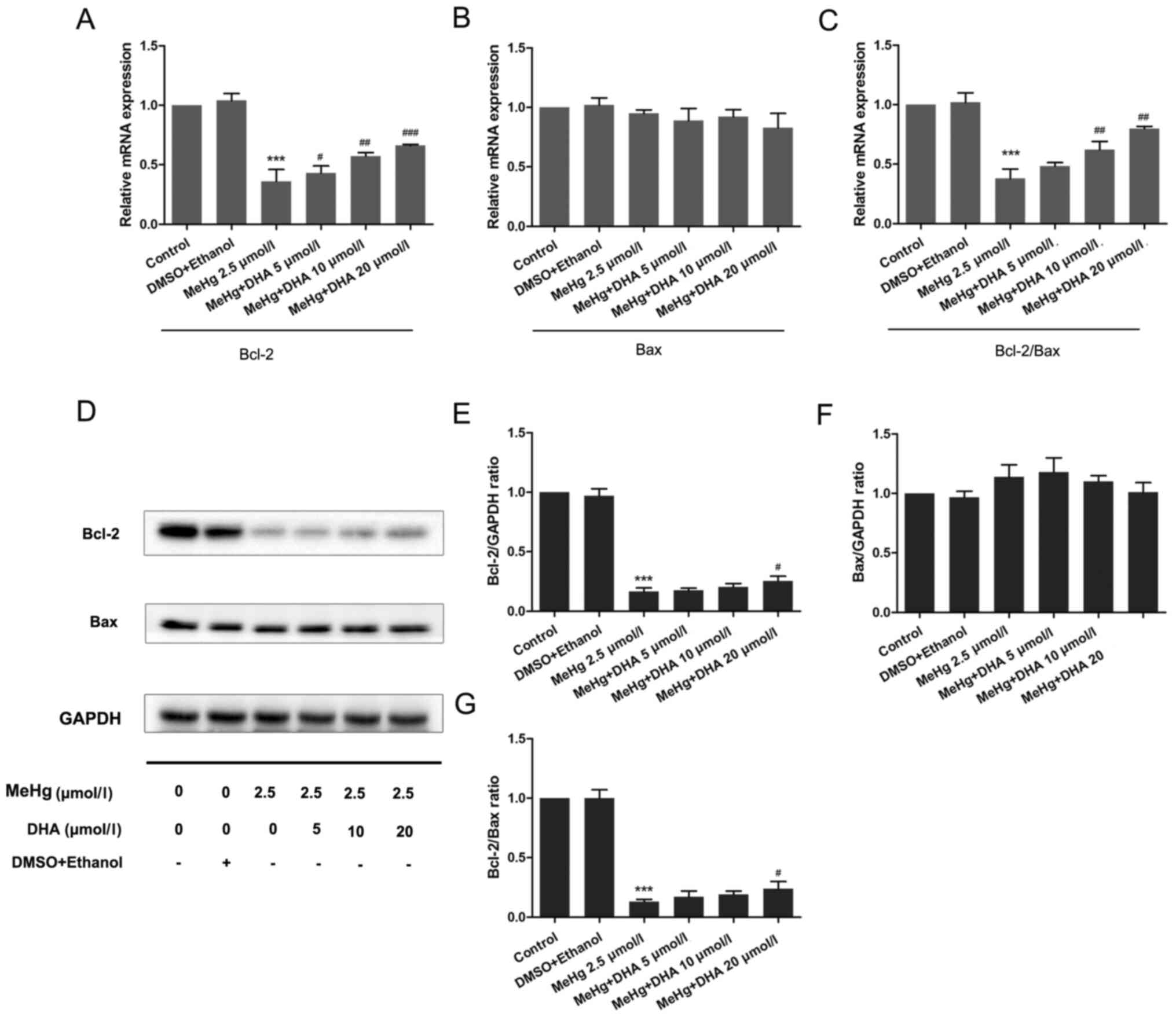
To further confirm the mechanism underlying
MeHg-induced PC12 cell apoptosis, RT-qPCR and western blotting were
performed to observe the changes in the mRNA and protein expression
levels of apoptosis-related genes. As presented in Fig. 3, the mRNA and protein expression
levels of the anti-apoptosis factor Bcl-2 were significantly
inhibited after PC12 cells were treated with MeHg compared with
those in the control group (Fig. 3A, D
and E). However, pretreatment with DHA could markedly rescue
the MeHg-induced decrease in Bcl-2. Notably, it was revealed that
the relative mRNA and protein expression levels of Bax were
unchanged in all groups (Fig. 3B, D and
F).
The Bcl-2/Bax ratio has an important role in cell
survival and apoptosis, with a larger ratio associated with more
extensive apoptosis (24). The
present study demonstrated that the Bcl-2/Bax ratio was
significantly decreased in the MeHg group compared with that in the
control group, and was increased in the DHA (5, 10 and 20 µmol/l) +
MeHg (2.5 µmol/l) group compared with that in the MeHg group
(Fig. 3C, D and G).
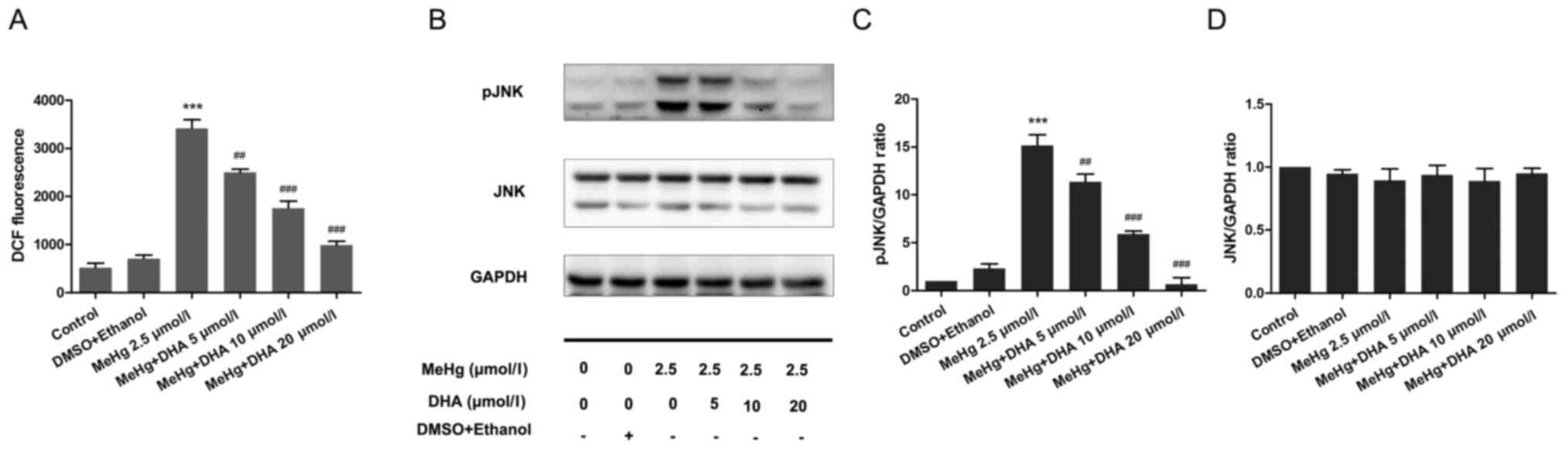
Effect of DHA on ROS production in
PC12 cells
PC12 cells were stained with the DCFH-DA fluorescent
probe to examine the DCF fluorescence intensity using a
fluorescence microplate reader. Compared with that in the control
group, the ROS level was significantly increased in the MeHg group.
After DHA pretreatment, the ROS level was decreased in a
dose-dependent manner compared with that in the MeHg group
(Fig. 4A).
Effect of DHA on the JNK signaling
pathway in MeHg-induced PC12 cells
Western blotting was performed to examine
phosphorylation of the JNK pathway. The results demonstrated that
MeHg significantly activated pJNK compared with in the control
group; however, DHA significantly inhibited the effect of MeHg
(Fig. 4B-D).
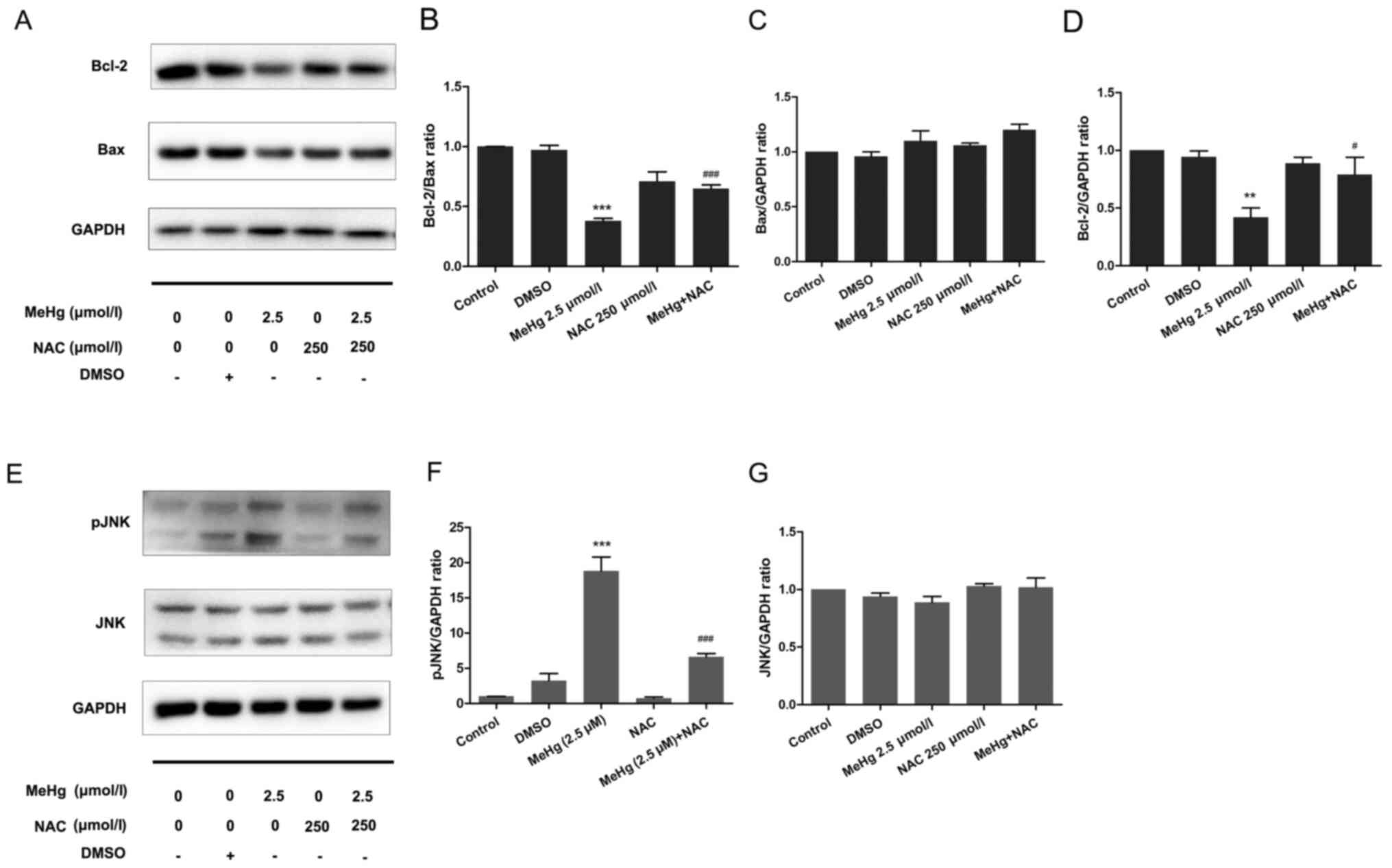
Inhibitory effect of NAC on
MeHg-induced PC12 cell apoptosis
To further support the results of the mechanistic
research, NAC was used as an ROS inhibitor to pretreat PC12 cells
to further confirm the relationship between ROS and the JNK
pathway. The results demonstrated that, after pretreatment with 500
µmol/l NAC at 37°C for 30 min, the expression levels of the
anti-apoptotic protein Bcl-2 were significantly increased compared
with those in the MeHg group (Fig. 5A
and B). Moreover, the expression levels of Bax were not
affected (Fig. 5A and C), whereas
the Bcl-2/Bax ratio was significantly increased (Fig. 5A and B). In addition, in PC12 cells
exposed to MeHg, the expression levels of pJNK were significantly
increased (Fig. 5E-G). By contrast,
NAC significantly downregulated the expression levels of pJNK.
These findings indicated that ROS may mediate the expression levels
of the downstream protein pJNK, and NAC may exert a significant
protective effect against MeHg-induced apoptosis. These findings
also suggested that DHA may protect PC12 cells from apoptosis
induced by MeHg via antioxidant activity.
Discussion
Oxidative stress has been widely recognized as an
initiator of apoptosis, in which it plays a key role (25,26).
Kuo et al (27) reported
that oxidative stress caused by ROS may activate the JNK pathway
and regulate the transcriptional expression of related downstream
genes, leading to apoptosis. Moreover, studies have shown that
ROS-induced apoptosis is closely associated with activation of the
JNK signaling pathway, which can regulate the expression of
apoptosis-related genes and ultimately lead to cell apoptosis
(28–30). Therefore, the JNK/ROS signaling
pathway was selected as the focus of the mechanistic research in
the present study.
MeHg has been recognized as a highly toxic pollutant
in the environment, which is mainly absorbed by humans in the
gastrointestinal tract, and easily crosses the blood-brain barrier
and the placental barrier, exerting adverse effects on
neurodevelopment (31), including
IQ reduction and language impairment (32). It has been reported that a low dose
of MeHg may exert a toxic effect on nerve precursor cells (33). In addition, Petroni et al
(34) observed that low
concentrations of MeHg significantly decreased the viability of
SY-SY5Y neuroblastoma cells, resulting in permanent cell damage. As
an essential unsaturated fatty acid in humans, DHA has been
reported to possess antioxidant and antiapoptotic effects (35). DHA has been demonstrated to be
crucial for the development of the nervous system, particularly
vision and cognition of infants and young children (36). Therefore, the present study
investigated the potential protective effect of DHA against MeHg
poisoning and the possible molecular mechanisms. In the current
study, PC12 cells, which are widely applied in neuronal cell models
in vitro, were used to identify the mechanism of
MeHg-induced PC12 cell apoptosis via the ROS/JNK signaling pathway
and to examine the protective role of DHA in this process.
First, the MeHg poisoning model was established
in vitro. DHA pretreatment efficiently rescued MeHg-induced
cell viability inhibition and apoptosis. Notably, MeHg at 1.25
µmol/l had no toxic effect on PC12 cells, and it was suggested that
low-dose MeHg may initiate the toxic excitatory effect of cells and
trigger a series of repair activation mechanisms, which was
verified in the research of Tan et al (37). In addition, it is well known that
DHA has significant antioxidant effects; however, the present study
revealed that PC12 cell survival was reduced following treatment
with high concentrations of DHA, which was consistent with the
finding of Iuchi et al (38). In addition, Srikanth et al
(39) revealed that higher
concentrations of DHA induced profound cell swelling and a
reduction in viability, which was accompanied by increased
expression levels of inflammatory cytokine and lipoxygenase genes,
activation of caspase-1 activity and release of IL1β, indicating
that cells were undergoing a proinflammatory cell death program
known as pyroptosis; this may be one of the reasons that high
concentrations of DHA become toxic.
A number of studies have demonstrated that oxidative
stress may serve an important role in MeHg-induced apoptosis
(40,41). Therefore, the present study examined
the effect of MeHg and DHA on intracellular ROS levels to evaluate
the potential mechanism underlying the effects of DHA on
MeHg-induced apoptosis of PC12 cells. The results demonstrated that
MeHg significantly increased the levels of ROS, whereas DHA
effectively inhibited MeHg-induced oxidative injury to PC12 cells.
As members of the MAPK superfamily, JNKs serve a crucial role in
cell survival and apoptosis (42,43).
Therefore, the protective effects of DHA on MeHg-induced apoptosis
at the level of the JNK signaling pathway were further
investigated. The results demonstrated that the JNK signaling
pathway was markedly activated and that the expression of pJNK was
significantly increased after PC12 cells were exposed to MeHg.
The mitochondrial pathway is the most important
pathway that mediates cell apoptosis. The change in mitochondrial
outer membrane permeability (MOMP) is considered to be the main
switch of the mitochondrial apoptosis pathway, and MOMP is strictly
regulated by the Bcl-2 family and promotes apoptosis (44). Some members of the Bcl-2 family,
such as Bax, can directly promote changes in MOMP. When apoptosis
is induced, Bax migrates from the cytosol to the mitochondria and
nuclear membrane, and initiates the caspase cascade via cytochrome
c and second mitochondria-derived activator caspase pathways
(45). The present study
demonstrated that the mRNA and protein expression levels of
anti-apoptotic Bcl-2 were almost completely inhibited by MeHg;
however, there was no significant change in the mRNA or protein
expression levels of pro-apoptotic Bax, which was similar to other
research findings (46). Notably,
Hou et al (47) revealed
that Bax may not always be a potent inducer of apoptosis in tumor
cells. It has been reported that the Bcl-2/Bax ratio is essential
for determining whether cells undergo apoptosis, which is
consistent with the current study (24). Additionally, the present study
revealed that restoration of Bcl-2 transcription by DHA seemed to
be more obvious than that of Bcl-2 protein expression, which may be
related to the length of administration time. This phenomenon was
also reported in a previous study (28).
To further confirm the protective mechanism of DHA
and to elucidate the relationship between ROS and signaling
pathways, NAC, an efficient ROS scavenger, was used to pretreat
cells in the present study. The results demonstrated that all of
the adverse effects of MeHg were improved; notably, NAC
pretreatment inhibited JNK signaling, and increased the expression
levels of the anti-apoptosis protein Bcl-2 and the ratio of Bcl-2
to Bax. Therefore, these results suggested that DHA may ameliorate
MeHg-induced apoptosis via the ROS-regulated JNK signaling
pathway.
In conclusion, MeHg significantly increased the ROS
content in PC12 cells and induced oxidative stress, whereas DHA
effectively decreased levels of intracellular ROS to relieve
oxidative stress-induced PC12 cell apoptosis (Fig. 6). Moreover, DHA may reverse the
MeHg-induced adverse effects, including the elevated
phosphorylation of JNK, and decreased mRNA and protein expression
levels of anti-apoptotic Bcl-2. To the best of our knowledge, the
present study was the first to attempt to elucidate the protective
effects of DHA against MeHg-induced PC12 cell apoptosis via the
ROS/JNK signaling pathway, which may provide a theoretical basis
for the treatment of MeHg poisoning. Our future studies will focus
on MeHg target gene prediction by full transcriptome sequencing of
samples, in order to further elucidate the protective mechanism of
DHA on MeHg-induced apoptosis of PC12 cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Science Foundation of China (grant no. 81973062) and the
National Key R&D Program of China (grant no.
2017YFC1600500).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to privacy of
laboratory content but are available from the corresponding author
on reasonable request.
Authors' contributions
CY made substantial contributions to the conception
and design of the study, analysis and interpretation of data,
acquisition of funding and supervision of the research. HZ and SW
performed the experiments. YW, AL and CH made substantial
contributions to the analysis and interpretation of data. HZ and SW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jacobs S, Sioen I, Jacxsens L, Domingo JL,
Sloth JJ, Marques A and Verbeke W: Risk assessment of methylmercury
in five European countries considering the national seafood
consumption patterns. Food Chem Toxicol. 104:26–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hylander LD: Global Mercury Pollution and
its Expected Decrease after a Mercury Trade Ban. Water Air Soil
Pollut. 125:331–344. 2001. View Article : Google Scholar
|
|
3
|
Borowska S and Brzóska MM: Metals in
cosmetics: Implications for human health. J Appl Toxicol.
35:551–572. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim W, Kim DW, Yoo DY, Jung HY, Kim JW,
Kim DW, Choi JH, Moon SM, Yoon YS and Hwang IK: Antioxidant effects
of Dendropanax morbifera Léveille extract in the hippocampus
of mercury-exposed rats. BMC Complement Altern Med. 15:2472015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarkson TW, Magos L and Myers GJ: The
toxicology of mercury - current exposures and clinical
manifestations. N Engl J Med. 349:1731–1737. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Syversen T and Kaur P: The toxicology of
mercury and its compounds. J Trace Elem Med Biol. 26:215–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bellinger DC, Devleesschauwer B, O'Leary K
and Gibb HJ: Global burden of intellectual disability resulting
from prenatal exposure to methylmercury, 2015. Environ Res.
170:416–421. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sheehan MC, Burke TA, Navas-Acien A,
Breysse PN, McGready J and Fox MA: Global methylmercury exposure
from seafood consumption and risk of developmental neurotoxicity: A
systematic review. Bull World Health Organ. 92:F254–F269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke T, Gonçalves FM, Gonçalves CL, dos
Santos AA, Rocha JBT, Farina M, Skalny A, Tsatsakis A, Bowman AB
and Aschner M: Post-translational modifications in MeHg-induced
neurotoxicity. Biochim Biophys Acta Mol Basis Dis. 1865:2068–2081.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ying Z, Chen K, Zheng L, Wu Y, Li L, Wang
R, Long Q, Yang L, Guo J, Yao D, et al: Transient Activation of
Mitoflashes Modulates Nanog at the Early Phase of Somatic Cell
Reprogramming. Cell Metab. 23:220–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu L, Liu Z, Qiu L, Hao L and Guo J:
Ipatasertib sensitizes colon cancer cells to TRAIL-induced
apoptosis through ROS-mediated caspase activation. Biochem Biophys
Res Commun. 519:812–818. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan BL, Norhaizan ME and Chan LC:
ROS-Mediated Mitochondrial Pathway is Required for Manilkara
Zapota (L.) P. Royen Leaf Methanol Extract Inducing Apoptosis
in the Modulation of Caspase Activation and EGFR/NF-κ B Activities
of HeLa Human Cervical Cancer Cells. Evid Based Complement Alternat
Med. 2018:65786482018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mangali S, Bhat A, Udumula MP, Dhar I,
Sriram D and Dhar A: Inhibition of protein kinase R protects
against palmitic acid-induced inflammation, oxidative stress, and
apoptosis through the JNK/NF-κB/NLRP3 pathway in cultured H9C2
cardiomyocytes. J Cell Biochem. 120:3651–3663. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Feng X, Hu X, Sha J, Li B, Zhang H
and Fan H: Dexmedetomidine Ameliorates Acute Stress-Induced Kidney
Injury by Attenuating Oxidative Stress and Apoptosis through
Inhibition of the ROS/JNK Signaling Pathway. Oxid Med Cell Longev.
2018:40353102018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niu S-L, Mitchell DC, Lim S-Y, Wen ZM, Kim
HY, Salem N Jr and Litman BJ: Reduced G protein-coupled signaling
efficiency in retinal rod outer segments in response to n-3 fatty
acid deficiency. J Biol Chem. 279:31098–31104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken E, Wright RO, Kleinman KP, Bellinger
D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW and Gillman MW:
Maternal fish consumption, hair mercury, and infant cognition in a
U.S. Cohort. Environ Health Perspect. 113:1376–1380. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oken E, Østerdal ML, Gillman MW, Knudsen
VK, Halldorsson TI, Strøm M, Bellinger DC, Hadders-Algra M,
Michaelsen KF and Olsen SF: Associations of maternal fish intake
during pregnancy and breastfeeding duration with attainment of
developmental milestones in early childhood: A study from the
Danish National Birth Cohort. Am J Clin Nutr. 88:789–796. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cardoso C, Bernardo I, Bandarra NM, Louro
Martins L and Afonso C: Portuguese preschool children: Benefit
(EPA+DHA and Se) and risk (MeHg) assessment through the consumption
of selected fish species. Food Chem Toxicol. 115:306–314. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nøstbakken OJ, Bredal IL, Olsvik PA, Huang
TS and Torstensen BE: Effect of marine omega 3 fatty acids on
methylmercury-induced toxicity in fish and mammalian cells in
vitro. J Biomed Biotechnol. 2012:4176522012. View Article : Google Scholar
|
|
20
|
Kaur P, Heggland I, Aschner M and Syversen
T: Docosahexaenoic acid may act as a neuroprotector for
methylmercury-induced neurotoxicity in primary neural cell
cultures. Neurotoxicology. 29:978–987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takanezawa Y, Nakamura R, Hamaguchi M,
Yamamoto K, Sone Y, Uraguchi S and Kiyono M: Docosahexaenoic acid
enhances methylmercury-induced endoplasmic reticulum stress and
cell death and eicosapentaenoic acid potentially attenuates these
effects in mouse embryonic fibroblasts. Toxicol Lett. 306:35–42.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Huang H, Wang S, Chen F and Zheng
G: Dehydrocorydaline inhibits the tumorigenesis of breast cancer
MDA MB 231 cells. Mol Med Rep. 22:43–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Gu Q, Xiang L, Dong X, Li H, Ni J,
Wan L, Cai G and Chen G: Curcumin inhibits apoptosis by modulating
Bax/Bcl-2 expression and alleviates oxidative stress in testes of
streptozotocin-induced diabetic rats. Ther Clin Risk Manag.
13:1099–1105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Angelis C, Galdiero M, Pivonello C,
Salzano C, Gianfrilli D, Piscitelli P, Lenzi A, Colao A and
Pivonello R: The environment and male reproduction: The effect of
cadmium exposure on reproductive function and its implication in
fertility. Reprod Toxicol. 73:105–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wang M, Teng S and Wang D, Li X,
Wang X, Liao W and Wang D: Indolyl-chalcone derivatives induce
hepatocellular carcinoma cells apoptosis through oxidative stress
related mitochondrial pathway in vitro and in vivo. Chem Biol
Interact. 293:61–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuo LM, Chen PJ, Sung PJ, Chang YC, Ho CT,
Wu YH and Hwang TL: The Bioactive Extract of Pinnigorgia sp.
Induces Apoptosis of Hepatic Stellate Cells via
ROS-ERK/JNK-Caspase-3 Signaling. Mar Drugs. 16(19)2018.
|
|
28
|
Ren X, Wang S, Zhang C, Hu X, Zhou L, Li Y
and Xu L: Selenium ameliorates cadmium-induced mouse leydig TM3
cell apoptosis via inhibiting the ROS/JNK /c-jun signaling pathway.
Ecotoxicol Environ Saf. 192:1102662020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Wu W, Gong B, Hou L, Dong X, Xu C,
Zhao R, Yu Q, Zhou Z, Huang S, et al: Metformin attenuates
cadmium-induced neuronal apoptosis in vitro via blocking
ROS-dependent PP5/AMPK-JNK signaling pathway. Neuropharmacology.
175:1080652020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Yu K, Hu Y, Su F, Gao Z, Hu T,
Yang Y, Cao X and Qian F: Schisantherin A induces cell apoptosis
through ROS/JNK signaling pathway in human gastric cancer cells.
Biochem Pharmacol. 173:1136732020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zareba G, Cernichiari E, Hojo R, Nitt SM,
Weiss B, Mumtaz MM, Jones DE and Clarkson TW: Thimerosal
distribution and metabolism in neonatal mice: Comparison with
methyl mercury. J Appl Toxicol. 27:511–518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poling A and LeSage M: Evaluating
psychotropic drugs in people with mental retardation: Where are the
social validity data? Am J Ment Retard. 100:193–200.
1995.PubMed/NCBI
|
|
33
|
Watanabe J, Nakamachi T, Ohtaki H,
Naganuma A, Shioda S and Nakajo S: Low dose of methylmercury (MeHg)
exposure induces caspase mediated-apoptosis in cultured neural
progenitor cells. J Toxicol Sci. 38:931–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petroni D, Tsai J, Agrawal K, Mondal D and
George W: Low-dose methylmercury-induced oxidative stress,
cytotoxicity, and tau-hyperphosphorylation in human neuroblastoma
(SH-SY5Y) cells. Environ Toxicol. 27:549–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calder PC: Omega-3 polyunsaturated fatty
acids and inflammatory processes: nutrition or pharmacology? Br J
Clin Pharmacol. 75:645–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuratko CN, Barrett EC, Nelson EB and
Salem N Jr: The relationship of docosahexaenoic acid (DHA) with
learning and behavior in healthy children: a review. Nutrients.
5:2777–2810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan Q, Zhang M, Geng L, Xia Z, Li C, Usman
M, Du Y, Wei L and Bi H: Hormesis of methylmercury-human serum
albumin conjugate on N9 microglia via ERK/MAPKs and STAT3 signaling
pathways. Toxicol Appl Pharmacol. 362:59–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iuchi K, Ema M, Suzuki M, Yokoyama C and
Hisatomi H: Oxidized unsaturated fatty acids induce apoptotic cell
death in cultured cells. Mol Med Rep. 19:2767–2773. 2019.PubMed/NCBI
|
|
39
|
Srikanth M, Chandrasaharan K, Zhao X,
Chayaburakul K, Ong W-Y and Herr DR: Metabolism of Docosahexaenoic
Acid (DHA) Induces Pyroptosis in BV-2 Microglial Cells.
Neuromolecular Med. 20:504–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xue D-F, Pan S-T, Huang G and Qiu J-X: ROS
enhances the cytotoxicity of cisplatin by inducing apoptosis and
autophagy in tongue squamous cell carcinoma cells. Int J Biochem
Cell Biol. 122:1057322020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hassani S, Ghaffari P, Chahardouli B,
Alimoghaddam K, Ghavamzadeh A, Alizadeh S and Ghaffari SH:
Disulfiram/copper causes ROS levels alteration, cell cycle
inhibition, and apoptosis in acute myeloid leukaemia cell lines
with modulation in the expression of related genes. Biomed
Pharmacother. 99:561–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Li C, Wu G, Li W, Zhang X, Zhou J,
Zhang L, Tao J, Shen M and Liu H: Involvement of JNK/FOXO1 pathway
in apoptosis induced by severe hypoxia in porcine granulosa cells.
Theriogenology. 154:120–127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Li Y, Ma P, Chen J and Xie W:
Ficus carica leaves extract inhibited pancreatic β-cell apoptosis
by inhibiting AMPK/JNK/caspase-3 signaling pathway and
antioxidation. Biomed Pharmacother. 122:1096892020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kalkavan H and Green DR: MOMP, cell
suicide as a BCL-2 family business. Cell Death Differ. 25:46–55.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qi H, Jiang Y, Yin Z, Jiang K, Li L and
Shuai J: Optimal pathways for the assembly of the Apaf-1·cytochrome
c complex into apoptosome. Phys Chem Chem Phys.
20:1964–1973. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pouraminaei M, Mirzaiey MR, Khoshdel A,
Hajizadeh MR, Mahmoodi M and Fahmidehkar MA: The effect of Cressa
Cretica hydroalcoholic extract on apoptosis and the expression of
Bcl2, Bax and P53 genes in hepatoma cell line HepG2. Gene Rep.
20:1006922020. View Article : Google Scholar
|
|
47
|
Hou D, Che Z, Chen P, Zhang W, Chu Y, Yang
D and Liu J: Suppression of AURKA alleviates p27 inhibition on Bax
cleavage and induces more intensive apoptosis in gastric cancer.
Cell Death Dis. 9:7812018. View Article : Google Scholar : PubMed/NCBI
|