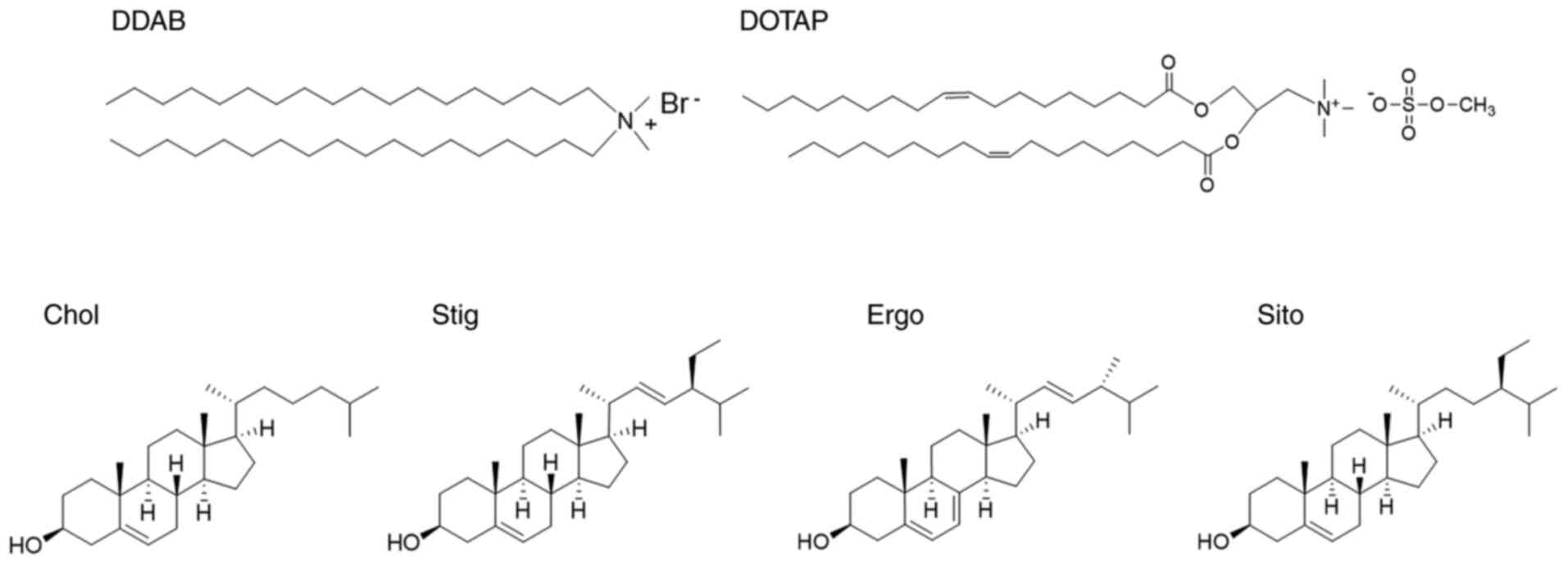
Introduction
Small interfering RNA (siRNA) therapeutics are a new
class of drugs comprising synthetic RNA duplexes that target a
specific mRNA for degradation. The lungs are promising targets of
siRNA therapy in terms of pathological states such as infectious
diseases, asthma, and cancer (1,2).
Therapy with siRNA reduced the formation of lung metastases and
increased the life span of mouse models of lung metastasis
(3), although few siRNA
therapeutics are in advanced clinical trials for pulmonary
diseases. The main routes of administering siRNA therapeutics for
pulmonary delivery are inhalation and intravenous injection
(4). Inhalation is less invasive
and the lung epithelium can be targeted. However, several barriers
must be surmounted, such as being swept away by respiratory cilia
and the need to penetrate the pulmonary mucus layer (5). The intravenous administration of siRNA
therapeutics is limited by instability in the blood circulation,
ineffective delivery to the target, and the potential for renal and
hepatic toxicity. These limitations can be addressed using siRNA
delivery systems for targeting to the lungs via systemic injection.
Cationic liposomes have recently attracted attention as a means of
delivering siRNA to the lungs. The systemic injection with cationic
liposomes aids successful siRNA delivery to the lungs (1,5) and
induces the specific gene silencing in pulmonary endothelial cells
(6,7). Cationic liposomes consisting of the
cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)
have served as vectors for siRNA delivery to the pulmonary
epithelium and endothelium via systemic injection (6). Previously, for evaluation of
gene-silencing efficacy in lung, we used 11 kinds of dialkyl or
trialkyl cationic lipids and 6 kinds of cationic cholesterol
derivatives as cationic lipids, and prepared 17 kinds of cationic
liposomes composed of a type of cationic lipid and neutral helper
lipid (1,2-dioleoyl-L-α-glycero-3-phosphatidylethanolamine, DOPE)
(8). We found that cationic
liposomes composed of dimethyldioctadecylammonium bromide (DDAB)
and DOPE resulted in abundant siRNA accumulation in the lung and
the significant suppression of targeted mRNA expression after
systemic injection of siRNA lipoplexes (8). We also showed that including
cholesterol (Chol) in DDAB or DOTAP liposomes increased the
accumulation of systemically injected siRNA lipoplexes and gene
silencing effects in the lungs compared with DOPE (9). However, to the best of our knowledge,
the effects of sterol derivatives in liposomal formulations
containing cationic liposomes for siRNA delivery to the lungs have
never been determined.
Here, we examined the effects of sterol derivatives
in cationic liposomes on siRNA delivery to the lungs. We
systemically injected siRNA lipoplexes comprising the cationic
lipids DOTAP and DDAB combined with Chol, β-sitosterol (Sito),
ergosterol (Ergo), or stigmasterol (Stig), then evaluated the
biodistribution and gene knockdown effects in the lungs. The
systemically injected DDAB/Chol or DDAB/Ergo lipoplexes resulted in
efficient gene-silencing in the lungs.
Materials and methods
Materials
We obtained the following from the respective
suppliers: 1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate
salt (DOTAP; Avanti Polar Lipids Inc.), dimethyldioctadecylammonium
bromide (DDAB, product name: DC-1-18; Sogo Pharmaceutical Co.,
Ltd.), cholesterol (Chol; Wako Pure Chemical Industries, Ltd.),
β-sitosterol (Sito; Tama Biochemical Co., Ltd.), Ergosterol (Ergo)
and stigmasterol (Stig; Tokyo Chemical Industry Co., Ltd.). All
other chemicals were of the highest available grade.
Small interfering RNAs
The siRNAs targeting the nucleotides of pGL3 and
pGL4 Firefly luciferase siRNA (Luc siRNA), negative control (Cont)
siRNA for Luc siRNA, and cyanine 5.5 (Cy5.5)-labeled siRNA
(Cy5.5-siRNA) were synthesized by Sigma Genosys. Mouse Tie2 siRNA
and luciferase siRNA (Cont siRNA) as a negative control for Tie2
siRNA were obtained from Japan Bio Services Co., Ltd. The siRNA
sequences of the pGL3 Luc siRNA, pGL4 Luc siRNA, and Cont siRNA for
Luc siRNA are described elsewhere (10,11),
as is Cy5.5-siRNA (12). Tie2 siRNA
and Cont siRNA as a negative control for Tie2 siRNA were
alternating 2′-O-methyl-modified, blunt-ended siRNA, and siRNA
sequences as described (3,13).
Preparation of cationic liposomes and
siRNA lipoplexes
Cationic liposomes were prepared from cationic
lipid/sterol derivatives at a molar ratio of 1:1. Cationic lipid
and sterol derivatives were dissolved in chloroform to prepare
cationic liposomes using thin-film hydration. The chloroform was
then evaporated under vacuum on a rotary evaporator at 60°C to
obtain a thin film, which was then hydrated with water at 60°C by
vortex mixing. The liposomes were disrupted in a
Bransonic® 2510 J-MTH, 100 W bath-type sonicator
(Branson Utrasonics Corp.) for 5–10 min at room temperature.
Liposomes were vortex-mixed for 10 sec with siRNA at
a charge ratio (+:-) of 4:1, then incubated at room temperature for
15 min to prepare siRNA/cationic liposome complexes (siRNA
lipoplexes). The charge ratio (+:-) of cationic liposomes to siRNA
is expressed as the molar ratio of cationic lipid to siRNA
phosphate.
Size and ζ-potential of cationic
liposomes and siRNA lipoplexes
The particle size distributions and ζ-potentials of
cationic liposomes and siRNA lipoplexes were measured using an
ELS-Z2 light-scattering photometer (Otsuka Electronics Co., Ltd.)
at 25°C after dispersion in ~1.5 ml of water as described (14).
Free siRNA in siRNA lipoplex
suspensions
Lipoplexes carrying siRNA (1 µg) were formed at
charge ratios (+:-) of 1:1, 2:1, 3:1 and 4:1. Amounts of free siRNA
in siRNA lipoplex suspensions were measured using exclusion assays
with SYBR® Green I Nucleic Acid Gel Stain (Takara Bio
Inc.) as described (15). The
fluorescence value obtained by adding free siRNA was set as
100%.
Cell culture
Murine colon adenocarcinoma Colon 26 cells stably
expressing pGL3-luciferase (Colon 26-Luc) were donated by Professor
Takashi Murakami (Department of Microbiology, Saitama Medical
University, Saitama, Japan) (16).
Mouse Lewis lung carcinoma LLC cells were obtained from the Cell
Resource Center for Biomedical Research, Institute of Development,
Aging, and Cancer, Tohoku University (Sendai, Japan). The cells
were cultured in RPMI-1640 medium with 10% heat-inactivated fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and
kanamycin (100 µg/ml) at 37°C in a 5% CO2 humidified
atmosphere.
A DNA construct for Luc2 expression was amplified by
PCR using plasmid DNA encoding the pGL4 luciferase gene (Promega
Corp.). This construct served as a template to prepare LLC cells
stably expressing pGL4-luciferase (LLC-Luc). The following primers
were included in the PCR reaction:
5′-ATTTCCGGTGAATTCATGGAAGATGCCAAAAAC-3′ and
5′-AGTCTCGAGGAATTCTTACACGGCGATCTTGCC-3′. The product was cloned
into the EcoRI restriction site of the pLVSIN-CMV Pur vector
(pLVSIN-Luc2; Takara Bio Inc.) using the In-Fusion HD cloning
system (Takara Bio Inc.). Lentiviral particles were co-transfected
with pLVSIN-Luc2 and ViraPower (three packaging plasmids,
Invitrogen) using polyethylenimine ‘MAX’ (Polysciences Inc.),
produced in 293FT cells (Invitrogen; Thermo Fisher Scientific,
Inc.), and concentrated by PEG precipitation as described (17,18).
The virus solution was mixed with hexadimethrine bromide (final
concentration, 8 µg/ml), then the mixture was added to cultured LLC
cells (1×105/well in 6-well plates). The plates were
centrifuged (1,800 × g, 90 min) at 32°C and incubated at 37°C for
24 h. The medium was replaced with RPMI-1640 medium supplemented
with 10% FBS cells, then the cells were successively cultured for
48 h by selection with puromycin (2.5 µg/ml).
Gene-silencing effects of siRNA
lipoplexes in cultured cells
We seeded LLC-Luc or Colon 26-Luc cells
(3×105/well) in 6-well plates before transfection. siRNA
lipoplexes were formed at a charge ratio (+:-) of 4:1 by
vortex-mixing cationic liposomes with 50 pmol of Cont siRNA or Luc
siRNA for 10 sec, then incubating the mixture at room temperature
for 15 min. The cells were then transfected with siRNA lipoplexes
diluted in 1 ml of medium supplemented with 10% FBS (final 50 nM
siRNA concentration). Forty-eight hours after transfection, the
cells were lysed by addition of 250 µl of cell lysis buffer
(Pierce™ Luciferase Cell Lysis Buffer; Thermo Fisher Scientific,
Inc.) after washing with PBS, and subjected to one cycle of
freezing (−80°C) and thawing at 37°C, followed by centrifugation at
15,000 × g for 10 sec. Aliquots (10 µl) of supernatants were mixed
with 50 µl of PicaGene MelioraStar-LT Luminescence Reagent (Toyo
Ink Mfg. Co. Ltd.), and luminescence was measured as described
(14). The protein concentration in
the supernatants was determined using Pierce™ BCA Protein Assay
Kits (Thermo Fisher Scientific, Inc.). Luciferase activity (%) was
calculated relative to the luciferase activity (cps/μg protein) of
untransfected cells.
Cytotoxicity of siRNA lipoplexes
Colon 26-Luc or LLC-Luc cells were seeded in 96-well
plates 24 h before transfection. Each siRNA lipoplex with 50 pmol
Cont siRNA was diluted in 1 ml of medium supplemented with 10% FBS.
The mixture (100 µl) was then added to the cells at 50% confluency
in the well (final 50 nM siRNA concentration). After a 24 h
incubation period, cell numbers were determined using a Cell
Counting Kit-8 (Dojindo Laboratories). Cell viability was expressed
as relative to the absorbance at 450 nm of untransfected cells.
Agglutination assays
Erythrocytes were separated as described (14) from whole blood (0.3 ml) collected
from the jugular vein of an 8-week-old female BALB/c mouse (Sankyo
Labo Service Corp., Inc.) under anesthesia induced by isoflurane
inhalation (4% for induction and 1.5–2% for maintenance; FUJIFILM
Wako Pure Chemical Corporation). Lipoplexes containing siRNA (2 µg)
were then incubated with 100 µl of 2% (v/v) erythrocyte suspension
for 15 min at 37°C, then samples on glass plates were assessed by
phase-contrast microscopy.
Biodistribution of siRNA after
systemic injection of siRNA lipoplexes into mice
All animal experiments were conducted in accordance
with ‘Guide for the Care and Use of Laboratory Animals’ published
by the U.S. National Institutes of Health and ‘Guide for the Care
and Use of Laboratory Animals’ adopted by the Institutional Animal
Care and Use Committee of Hoshi University (Tokyo, Japan), which is
accredited by the Ministry of Education, Culture, Sports, Science,
and Technology, Japan. The Institutional Animal Care and Use
Committee of Hoshi University approved this study (permission no.
20-018).
Seven female BALB/c mice (18–20 g, 8 weeks of age;
Sankyo Labo Service Corp. Inc.) were housed at 24°C and 55%
humidity with a 12/12 h light/dark cycle (lights on at 8:00 a.m.)
and access to food and water ad libitum. Lipoplexes carrying
20 µg of Cy5.5-siRNA were injected into the lateral tail veins of
mice (n=1 per siRNA lipoplex), then all mice were sacrificed by
cervical dislocation 1 h later as described (19), and cervical dislocation was
confirmed by careful assessment of the mice for explicit signs of
death such as cardiac arrest. Tissues were analyzed by Cy5.5
fluorescence imaging using a NightOWL LB981 NC100 system (Berthold
Technologies GmbH & Co. KG., Bad Wildbad, Germany) as described
(14).
Tie2 mRNA levels in the lungs after
systemically injecting mice with siRNA lipoplexes
Lipoplexes with 5, 10 or 20 µg of Cont siRNA or Tie2
siRNA were injected into the lateral tail veins of 8-week-old
female BALB/c mice (n=3–4 per siRNA lipoplex), then Tie2 mRNA
expression in the lungs was analyzed 48 h later. Complementary DNA
was synthesized from total RNA isolated using Isogen II (Nippon
Gene Co., Ltd.), then real-time quantitative PCR proceeded using
TaqMan Gene expression assays (Tek [Tie-2], Mm00443243_m1,
phosphatase, and tensin homolog [PTEN]: Mm00477208_m1; Applied
Biosystems®) on a Roche Light Cycler 96 system. Levels
of Tie2 mRNA expression were normalized to those of PTEN mRNA in
the same sample as described (3)
and analyzed using the comparative Cq (2−ΔΔCq) method
(20).
Determination of serum transaminase
activity
Lipoplexes carrying 20 µg of Cont siRNA were
injected into the lateral tail veins of mice (n=4 per siRNA
lipoplex), then serum was separated from whole blood collected from
the mice and coagulated 24 h later. We then measured aspartate
aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT)
levels in the serum using Transaminase CII-test kits (Wako Pure
Chemicals). Normal values were determined in blood from age-matched
untreated mice.
Statistical analysis
Data are presented as means ± standard deviation
(SD) of triplicate determinations. The statistical significance of
differences between means was determined by Student t-tests using
GraphPad Prism 4.0 (GraphPad Software Inc.). Multiple comparisons
were assessed by analysis of variance followed by one-way analysis
of variance on ranks with post-hoc Tukey tests using GraphPad Prism
4.0. Statistical significance was set at P≤0.05.
Results and Discussion
Characterization of cationic liposomes
and siRNA lipoplexes
Cationic liposomes comprising dialkyl cationic
lipids with neutral helper lipids, such as DOPE or Chol, have been
studied extensively as vectors for siRNA delivery (8,9). Here,
we investigated the biodistribution and gene knockdown effects of
siRNA in the lungs of mice when systemically injected as lipoplexes
containing the cationic lipids DOTAP and DDAB, and the sterol
derivatives Chol, Stig, Ergo and Sito (Fig. 1). Cholesterol is a major component
of animal cell membranes, whereas Ergo is the primary sterol in
yeasts and other fungi (21). Both
Sito and Stig are derived from plants (22). The main structural difference
between Chol and the other three sterols is the presence of extra
methyl or ethyl group and/or double bond in side chain of Chol
(Fig. 1).
Cationic liposomes were prepared from cationic
lipid/sterol derivatives at a molar ratio of 1:1. However,
DOTAP/Stig at a molar ratio of 1:1 aggregated during the
preparation of cationic liposomes (Table I), so this formulation was excluded
from further study. The sizes of cationic liposomes prepared in
this study were approximately 100–190 nm. The ζ-potentials were
approximately +40–50 mV (Table I).
When the liposomes were mixed with siRNA, their lipoplex sizes were
about 160–220 nm and their ζ-potentials were about +29–38 mV
(Table I).
 | Table I.Particle size and ζ-potential of
cationic liposomes and siRNA lipoplexes. |
Table I.
Particle size and ζ-potential of
cationic liposomes and siRNA lipoplexes.
|
|
| Liposome |
Lipoplexb |
|---|
|
|
|
|
|
|---|
| Liposome | Formulation, mol
ratio | Sizea, nm | PDI |
ζ-potentiala, mV | Sizea, nm | PDI |
ζ-potentiala, mV |
|---|
| LP-DDAB/Chol | DDAB:Chol =
1:1 | 105.1±0.8 | 0.27±0.01 | 41.5±0.6 | 157.6±1.8 | 0.22±0.01 | 28.7±1.1 |
| LP-DDAB/Stig | DDAB:Stig =
1:1 | 110.6±2.5 | 0.23±0.01 | 50.2±0.9 | 214.5±4.6 | 0.26±0.00 | 31.7±0.7 |
| LP-DDAB/Ergo | DDAB:Ergo =
1:1 | 191.0±19.4 | 0.17±0.05 | 45.7±1.0 | 195.2±2.4 | 0.20±0.00 | 30.6±1.1 |
| LP-DDAB/Sito | DDAB:Sito =
1:1 | 100.5± 0.8 | 0.22±0.01 | 47.0±0.9 | 223.7±0.6 | 0.24±0.00 | 27.1±1.8 |
| LP-DOTAP/Chol | DOTAP:Chol =
1:1 | 108.7±0.8 | 0.24±0.00 | 49.1±1.3 | 197.1±1.5 | 0.23±0.01 | 38.3±0.2 |
| LP-DOTAP/Stig | DOTAP:Stig =
1:1 | Aggregation | N.D. | N.D. | N.D. | N.D. | N.D. |
| LP-DOTAP/Ergo | DOTAP:Ergo =
1:1 | 148.3±1.1 | 0.27±0.00 | 40.7±1.7 | 171.1±1.1 | 0.17±0.00 | 38.4±1.0 |
| LP-DOTAP/Sito | DOTAP:Sito =
1:1 | 127.6±0.9 | 0.25±0.02 | 44.3±0.2 | 174.5±1.3 | 0.21±0.01 | 33.9±0.6 |
We also examined associations of siRNA with each
type of cationic liposome using exclusion assays with
SYBR®-Green I. The amount of free siRNA in the siRNA
lipoplex suspension was largely decreased by adding DDAB- and
DOTAP-cationic liposomes to siRNA beyond a charge ratio (+:-) of
2:1 (Fig. 2). This phenomenon
suggested that the cationic liposomes were completely bound to
siRNA at this ratio regardless of the sterol derivative.
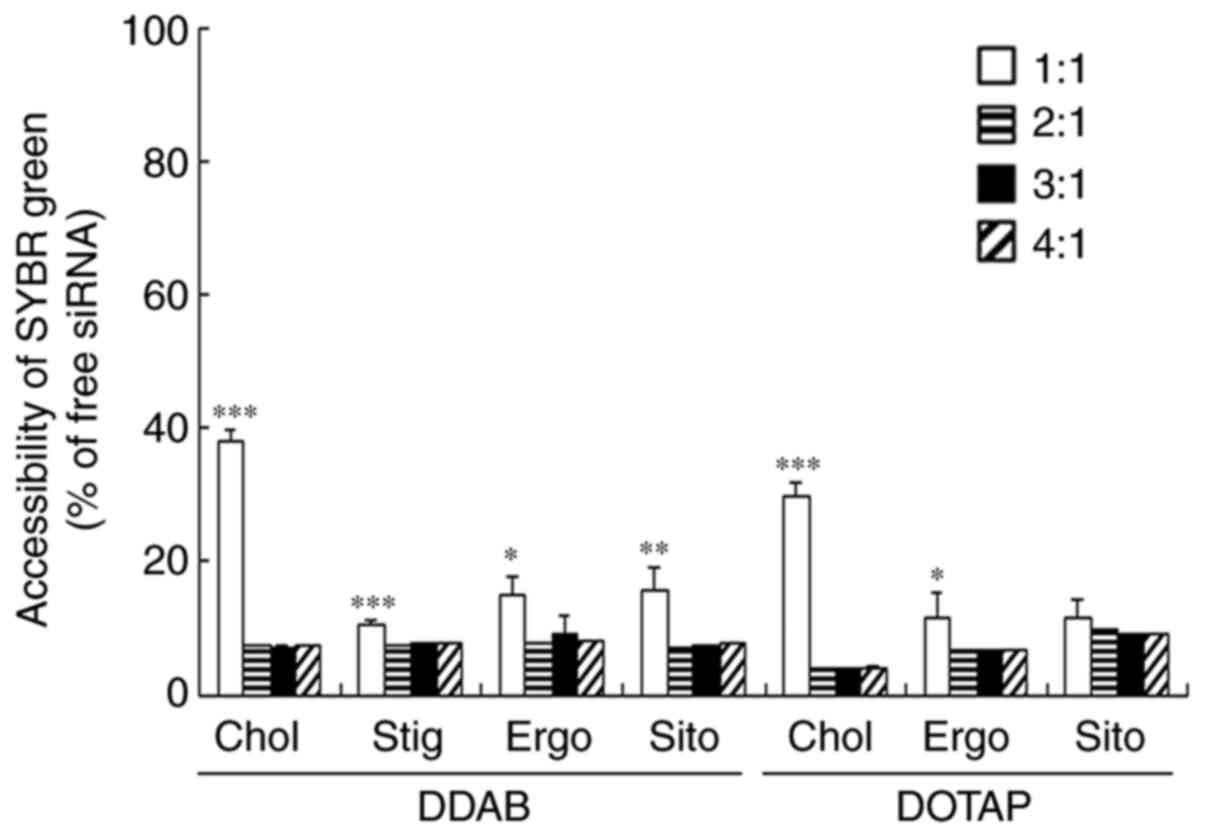 | Figure 2.Effects of sterol derivatives in
cationic liposomes on the association between siRNA and cationic
liposomes. siRNA lipoplexes were formed at charge ratios (+:-) of
1:1, 2:1, 3:1 and 4:1. Amounts of free siRNA in the siRNA lipoplex
suspensions were measured using exclusion assays with
SYBR® Green I Nucleic Acid Gel Stain and calculated
based on standard curves of free siRNA. Data are presented as the
mean ± standard deviation (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. charge ratio (+:-) of 4:1. si, short interfering;
lipoplex, cationic liposome complexes; DDAB,
dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; Chol,
cholesterol; Stig, stigmasterol; Ergo, ergosterol; Sito,
β-sitosterol. |
Effects of sterol derivatives in
cationic liposomes on gene knockdown in vitro
We previously showed that including DOPE in cationic
liposomal formulations increased, whereas increased Chol content
decreased gene-silencing in vitro (9). Here, we investigated the effects of
sterol derivatives in cationic liposomes on gene knockdown by siRNA
lipoplexes in Colon 26-Luc and LLC-Luc cells. Luciferase activity
evaluated using Lipofectamine™ RNAiMax, revealed that Luc siRNA
knocked down 90% of luciferase genes in LLC-Luc and Colon 26-Luc
cells compared with Cont siRNA (data not shown). In contrast,
DDAB-based liposomes induced moderate gene silencing when combined
with Stig and Sito, but not with Chol and Ergo (Fig. 3A and B). Cholesterol formed a more
rigid and ordered liposomal membrane, suggesting that stabilizing
the liposomal membrane might decrease gene-silencing effects by
inhibiting the escape of siRNA lipoplexes from endosomes after
cellular uptake. In contrast, an extra ethyl group in the side
chains of Sito and Stig might enhance the effect of steric
hindrance and weaken molecular interactions in DDAB-based liposomal
membranes, resulting in improved gene silencing by increasing
membrane fluidity. However, DOTAP-based liposomes moderately
suppressed luciferase expression regardless of the sterol
derivative (Fig. 3A and B). These
findings suggested that unsaturation of the dialkyl chains in DOTAP
enhances transfection efficiency when combined with sterol
derivatives. These results indicated that gene-silencing activities
in vitro were affected by the types of cationic lipids and
sterol derivatives in the liposomes, although inclusion of sterol
derivatives into liposomal formulation did not exhibit strong gene
silencing effects in the cells overall.
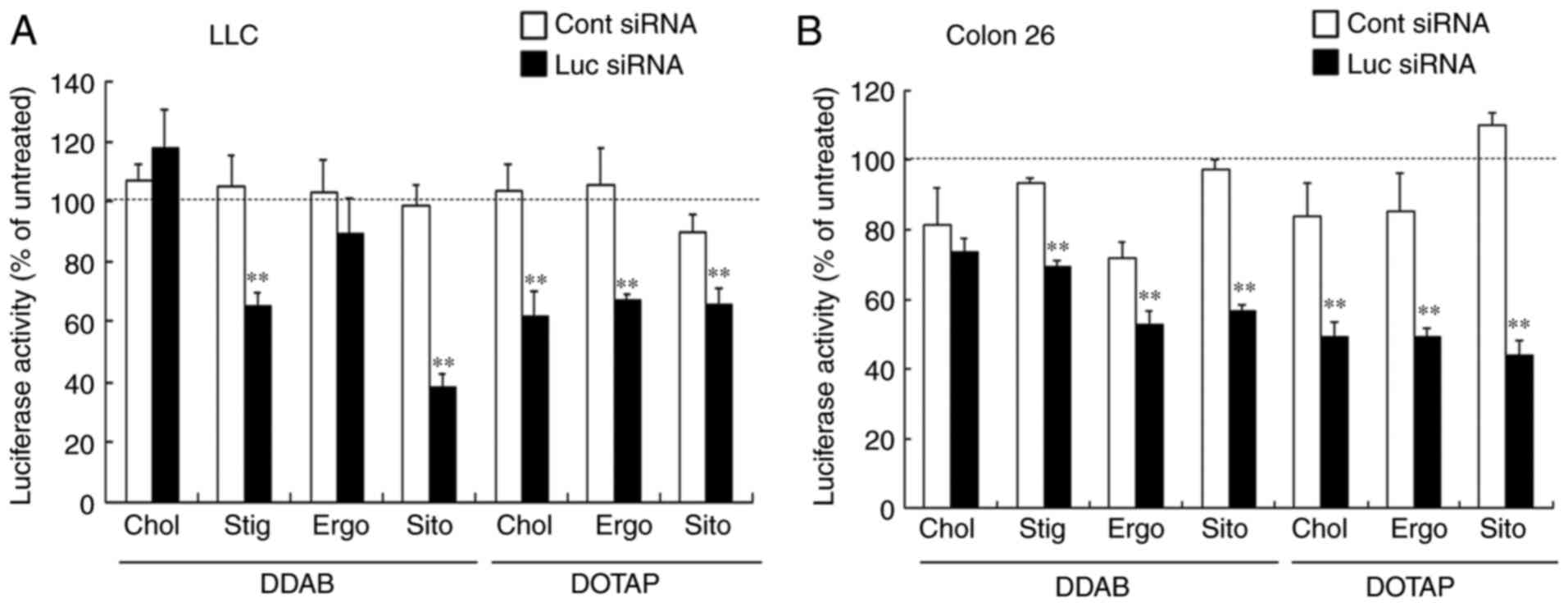 | Figure 3.Effects of sterol derivatives in
cationic liposomes on gene suppression in siRNA lipoplex
transfected LLC-Luc and Colon 26-Luc cells. (A) LLC-Luc or (B)
Colon 26-Luc cells were incubated for 48 h with 50 nM Cont siRNA or
Luc siRNA lipoplexes, then luciferase activity was assayed. Data
are presented as the mean ± standard deviation (n=3). **P<0.01
vs. Cont siRNA. si, short interfering; lipoplex, cationic liposome
complexes; DDAB, dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; Chol,
cholesterol; Stig, stigmasterol; Ergo, ergosterol; Sito,
β-sitosterol; Cont, control; Luc, luciferase. |
Cytotoxicity of siRNA lipoplexes
We measured cell viability 24 h after transfecting
Colon 26-Luc and LLC-Luc cells with siRNA lipoplexes to determine
the effects of sterol derivatives in cationic liposomes on the
cytotoxicity of siRNA lipoplexes. The lipoplexes were not
significantly cytotoxic (>80% cell viability; Fig. 4).
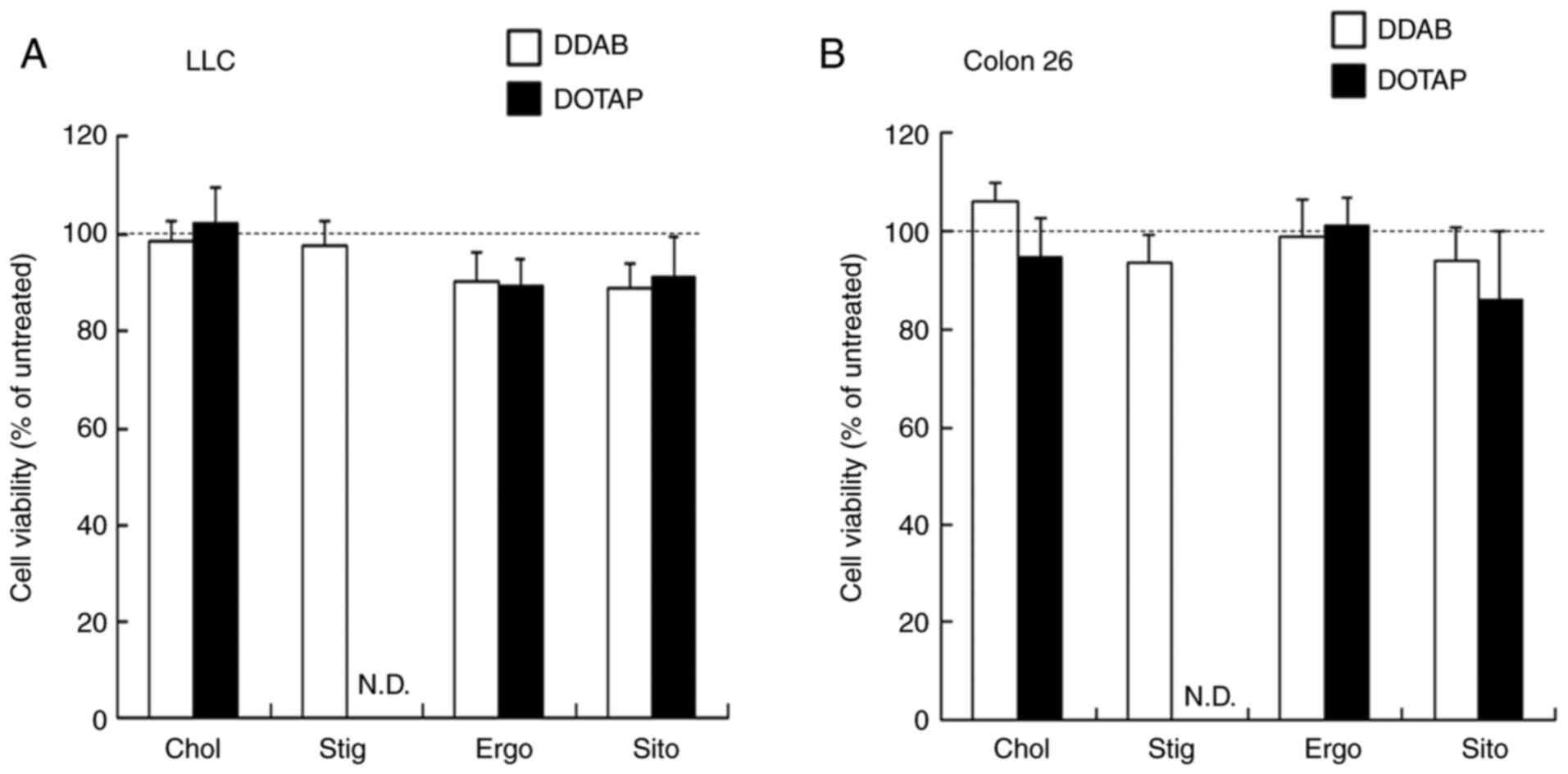 | Figure 4.Effects of sterol derivatives in
cationic liposomes on cell viability 24 h after transfection with
siRNA lipoplexes into LLC-Luc or Colon 26-Luc cells. siRNA
lipoplexes were added to (A) LLC-Luc or (B) Colon 26-Luc cells at
50 nM siRNA. After a 24 h incubation period, cell viability (%) was
measured. Data are presented as the mean ± standard deviation (A,
n=7-8; B, n=6-7). N.D., not determined; DDAB,
dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; Chol,
cholesterol; Stig, stigmasterol; Ergo, ergosterol; Sito,
β-sitosterol; lipoplex, cationic liposome complexes. |
Interaction with erythrocytes and
siRNA lipoplexes
siRNA lipoplexes bound to blood components, such as
erythrocytes, in the systemic circulation after systemic
administration. Furthermore, agglutinates were captured by lung
capillaries (23,24), which are the first microvascular
system through which injected siRNA lipoplexes must pass through.
We mixed siRNA lipoplexes with erythrocyte suspensions to determine
the effects of sterol derivatives in cationic liposomes on their
agglutination. All siRNA lipoplexes agglutinated with erythrocyte
suspensions, regardless of the sterol derivative in the liposomal
formulations (Fig. 5). We did not
detect large differences in the appearance of erythrocyte-siRNA
lipoplex agglutination regardless of the sterol derivatives in DDAB
and DOTAP liposomes.
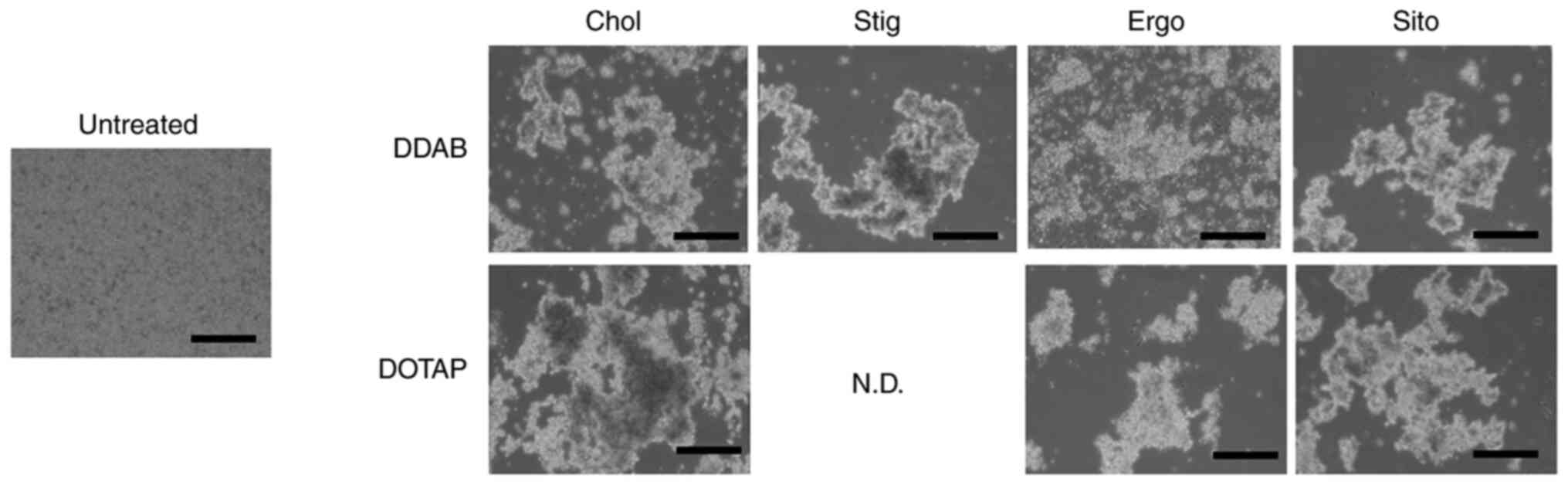 | Figure 5.Effect of sterol derivatives in
cationic liposomes on agglutination of siRNA lipoplexes with
erythrocytes. Lipoplexes carrying 2 µg siRNA were incubated with
erythrocyte suspensions for 15 min at 37°C, then assessed by phase
contrast microscopy. DOTAP/Stig at a molar ratio of 1:1 aggregated
during the preparation of cationic liposomes, so this formulation
was excluded from further study. Scale bar, 200 µm. N.D., not
determined; DDAB, dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; Chol,
cholesterol; Stig, stigmasterol; Ergo, ergosterol; Sito,
β-sitosterol; lipoplex, cationic liposome complexes. |
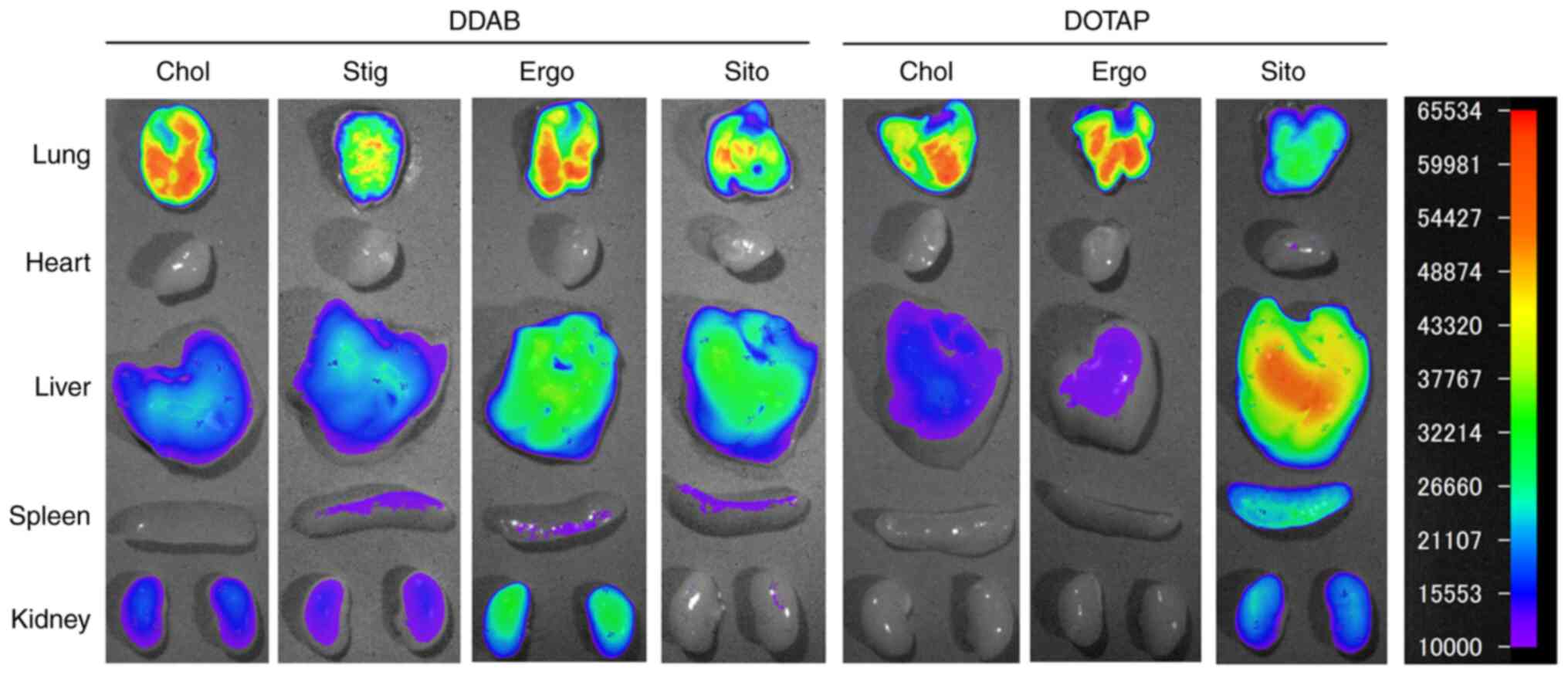
Biodistribution of siRNA after
systemic injection of siRNA lipoplexes
We previously showed that adding Chol to the
liposomal formulation increased siRNA accumulation in the lungs
compared with DOPE (9). To
investigate the effect of sterol derivatives on the biodistribution
of siRNAs after systemic injection of siRNA lipoplexes, mice were
systemically injected with lipoplexes carrying Cy5.5-siRNA and the
biodistribution of siRNAs was assessed 1 h later. Injected
LP-DDAB/Chol, LP-DDAB/Ergo, LP-DOTAP/Chol, and LP-DOTAP/Ergo
lipoplexes induced abundant siRNA accumulation in the lungs
(Fig. 6). The inclusion of Chol or
Ergo into cationic liposomes resulted in stable aggregates of siRNA
lipoplexes with erythrocytes in the blood circulation and efficient
capture by lung capillaries. These results indicated that the
biodistribution of siRNA after systemic injection of siRNA
lipoplexes was largely affected by the sterol derivative in
cationic liposomes.
Gene silencing in the lungs after
systemic injection of siRNA lipoplexes
The Tie2 receptor is primarily expressed on
endothelial cells (25,26), and the gene-silencing effect of
siRNA lipoplexes in the pulmonary endothelium has been evaluated
using the Tie2 gene (3). We
previously showed that injected LP-DDAB/DOPE lipoplexes carrying 50
µg of Tie2 siRNA significantly suppressed Tie2 mRNA levels in the
lungs (79% knockdown vs. Cont siRNA), whereas LP-DOTAP/DOPE did not
(9). We investigated the effects of
sterol derivatives in cationic liposomes on knockdown efficiency in
the pulmonary endothelium after systemic injection of siRNA
lipoplexes. We measured the gene-silencing effect of Tie2 mRNA in
the lungs of mice 48 h given a single systemic injection of
lipoplexes carrying 20 µg Tie2 siRNA (Fig. 7A). Injected LP-DDAB/Chol,
LP-DDAB/Stig, LP-DDAB/Ergo, and LP-DDAB/Sito lipoplexes with Tie2
siRNA significantly suppressed Tie2 mRNA levels (~76, 51, 82 and
49%, respectively, vs. Cont siRNA; Figs. 7A and S1). Injected LP-DOTAP/Chol and
LP-DOTAP/Sito lipoplexes with Tie2 siRNA significantly knocked down
Tie2 mRNA in the lungs by ~ 62 and 62%, respectively, compared with
Cont siRNA, whereas LP-DOTAP/Ergo lipoplexes with Tie2 siRNA did
not (Figs. 7A and S1). Among the cationic liposomes,
LP-DDAB/Chol and LP-DDAB/Ergo exhibited the highest gene silencing
effect in the lungs. Injected LP-DDAB/Chol or LP-DDAB/Ergo
lipoplexes carrying 10 µg of Tie2 siRNA significantly knocked down
Tie2 mRNA expression in mouse lungs by ~ 40% vs. compared with
untreated lungs; Fig. 7B).
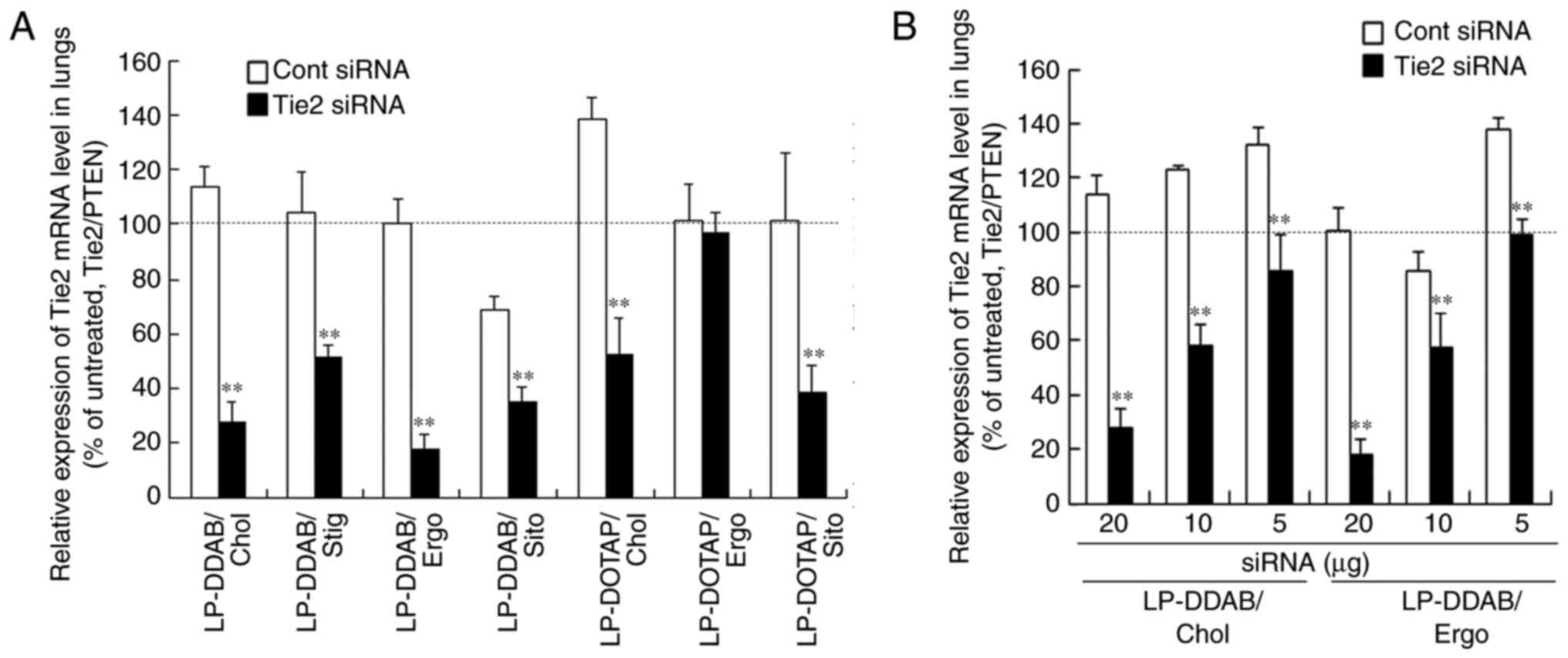 | Figure 7.Effects of sterol derivatives in
cationic liposomes on knockdown of Tie2 mRNA expression in the
lungs of mice systemically injected with siRNA lipoplexes carrying
Tie2 siRNA. (A) Expression of Tie2 mRNA in lungs was quantified
relative to that of PTEN mRNA at 48 h after systemic administration
of LP-DDAB/Chol, LP-DDAB/Stig, LP-DDAB/Ergo, LP-DDAB/Sito,
LP-DOTAP/Chol, LP-DOTAP/Ergo and LP-DOTAP/Sito lipoplexes along
with 20 µg of Cont siRNA or Tie2 siRNA. (B) Tie2 mRNA expression in
the lungs was quantified relative to that of PTEN mRNA at 48 h
after systemic administration of LP-DDAB/Chol and LP-DDAB/Ergo
lipoplexes with 5, 10 or 20 µg of Cont siRNA or Tie2 siRNA.
Expression (%) of Tie2 mRNA was calculated relative to that of
non-transfected lungs. Data are presented as the mean ± standard
deviation (n=3-4). **P<0.01 vs. Cont siRNA. LP; liposome, Chol;
cholesterol, Stig; stigmasterol, Ergo; ergosterol, Sito;
β-sitosterol; DDAB, dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; si,
short interfering; Tie2, angiopoietin-1 receptor; Cont, control;
lipoplex, cationic liposome complexes. |
Neutral helper lipids with membrane fluidity and
rigidity in cationic liposomes affect gene silencing. The silencing
efficiency of siRNA lipoplexes in vivo (Fig. 7A) and in vitro (Fig. 3) did not correlate. Including Chol
in DDAB-based cationic liposomes decreased gene knockdown efficacy
in vitro, but increased accumulation and gene knockdown
efficacy in the lungs. siRNA lipoplexes stabilized with Chol might
form stable siRNA lipoplex-erythrocyte aggregates, that are
efficiently captured by lung capillaries, resulting in abundant
siRNA transfection into the pulmonary endothelium. Regardless of
the sterol derivative, DDAB-based cationic liposomes transfected
in vivo induced significant gene silencing in the lungs. In
contrast, DOTAP-based cationic liposomes elicited significant
gene-silencing effects in the lungs only when Chol or Sito were
included. These findings indicated that the degree of saturation of
alkyl chains and/or linkers between the head group and alkyl chains
in cationic lipids affects the optimal combination of sterol
derivatives and thus the ability of systemically injected siRNA
lipoplexes to knock down genes in the lungs. Among the cationic
liposomes examined herein, LP-DDAB/Chol and LP-DDAB/Ergo knocked
down genes in the lungs with 70–80% efficiency (Figs. 7 and S1, and Table
II).
 | Table II.Summary of gene-silencing effect by
siRNA lipoplexes. |
Table II.
Summary of gene-silencing effect by
siRNA lipoplexes.
| Liposome | Gene-silencing
efficacy in LLC cells | Gene-silencing
efficacy in Colon26 cells | Gene-silencing
efficacy in lungs |
|---|
| LP-DDAB/Chol | − | − | +++ |
| LP-DDAB/Stig | + | − | ++ |
| LP-DDAB/Ergo | − | − | +++ |
| LP-DDAB/Sito | ++ | + | + |
| LP-DOTAP/Chol | + | + | ++ |
| LP-DOTAP/Ergo | + | + | − |
| LP-DOTAP/Sito | − | ++ | ++ |
Side effects in the liver
Injected plasmid DNA (pDNA) lipoplexes containing
the cationic lipid DOTAP induces toxic effects in the liver, but
not in the lungs (27). Therefore,
we assessed GOT and GPT levels in serum after injecting mice with
LP-DDAB/Chol, LP-DDAB/Ergo, and LP-DOTAP/Chol lipoplexes to
evaluate hepatic toxicity (Fig. 8).
None of these siRNA lipoplexes largely increased serum GOT and GPT
levels at 24 h. These results suggested that the systemic
administration of LP-DDAB/Chol, LP-DDAB/Ergo, and LP-DOTAP/Chol
lipoplexes did not induce high side effects in terms of
hepatotoxicity.
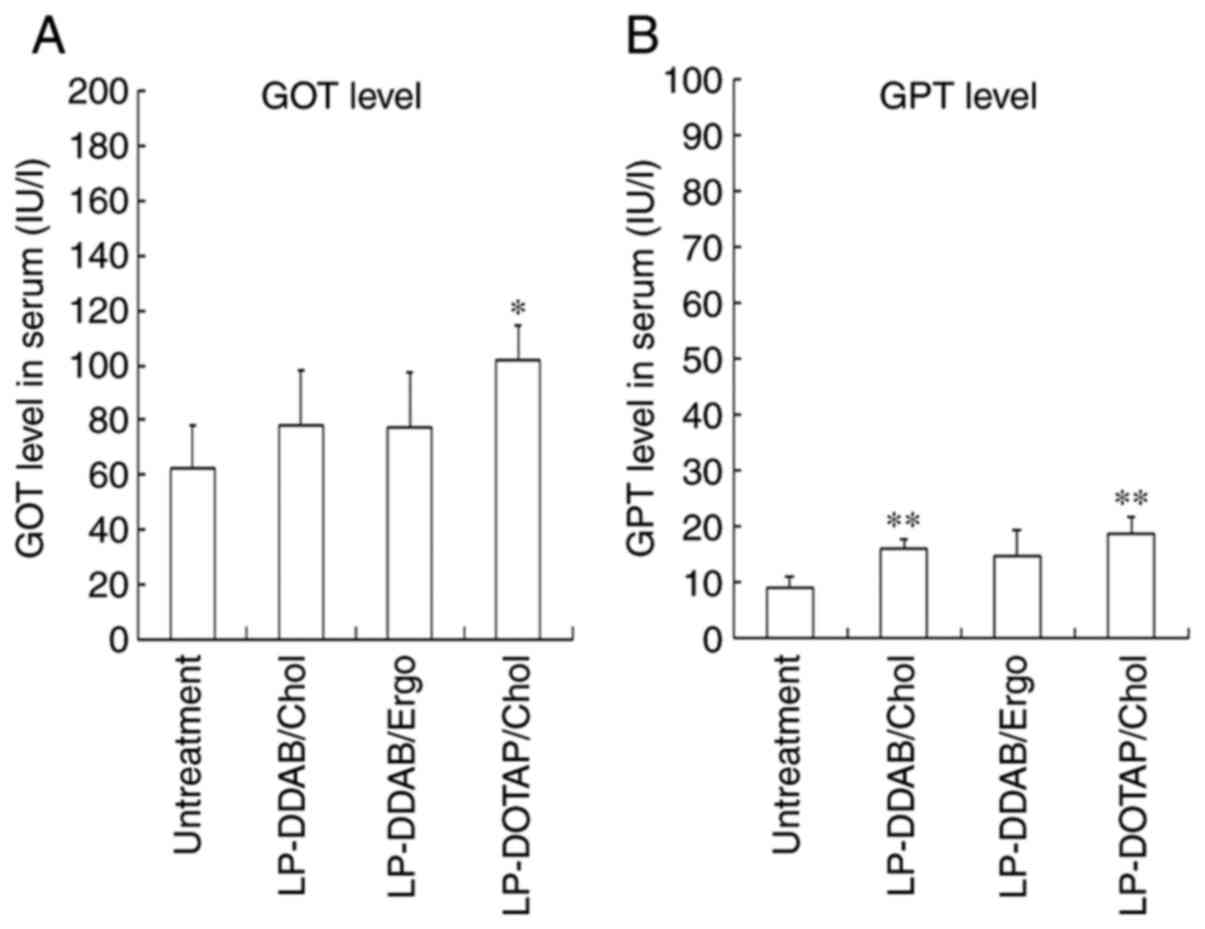 | Figure 8.Liver toxicity after systemic
injection of siRNA lipoplexes into mice. LP-DDAB/Chol, LP-DDAB/Ergo
and LP-DOTAP/Chol lipoplexes with 20 µg Cont siRNA were injected
into the lateral tail veins of mice, after which concentrations of
(A) GOT and (B) GPT in blood were measured 24 h later. Values are
shown as means ± standard deviation (n=4). *P<0.05 and
**P<0.01 vs. untreated control. LP, liposome; Chol, cholesterol;
Ergo, ergosterol; DDAB, dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; si,
short interfering; GOT, aspartate aminotransferase; GPT, alanine
aminotransferase; lipoplex, cationic liposome complexes. |
A single injection of DACC lipoplexes (2.8 mg Tie2
siRNA/kg) comprising the cationic lipid
β-L-arginyl-2,3-L-diaminopropionic
acid-N-palmityl-N-oleyl-amide trihydrochloride
(AtuFECT01), decreases Tie2 mRNA levels by >80% after 3 days
(3). We showed that a single
injection of LP-DDAB/Chol and LP-DDAB/Ergo lipoplexes carrying Tie2
siRNA (1 mg/kg) reduced the expression of Tie2 mRNA in the lungs by
70–80% within 2 days (Fig. 7A),
indicating that LP-DDAB/Chol and LP-DDAB/Ergo lipoplexes carrying
lower doses of siRNA can efficiently knock down the expression of a
targeted gene in the lung endothelium. Among the sterol
derivatives, ergosterol has anti-tumor and anti-angiogenic
activities (28,29). Therefore, LP-DDAB/Ergo might be an
effective siRNA vector for treating lung tumors. Our findings
showed that cationic DDAB-based liposomes combined with sterol
derivatives have potential as vectors for delivering siRNA
therapeutics to the lungs.
We examined the effects of sterol derivatives in
cationic liposomes on siRNA biodistribution and gene knockdown in
the lungs of mice systemically injected with siRNA lipoplexes.
DDAB-based liposomal formulations including Chol or Ergo
effectively silenced genes in the lungs of mice. This study
provides valuable information about the optimal combination of
cationic lipids and sterol derivatives in liposomal formulations
for optimal siRNA delivery to the lungs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and designed the study. HS, TO and KIO
conducted experiments. YH and HS confirm the authenticity of all
the raw data. YH wrote the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee of
Hoshi University approved the animal experiments of the present
study (approval no. 20-018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Merkel OM, Rubinstein I and Kissel T:
siRNA delivery to the lung: What's new? Adv Drug Deliv Rev.
75:112–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujita Y, Takeshita F, Kuwano K and Ochiya
T: RNAi Therapeutic platforms for lung diseases. Pharmaceuticals
(Basel). 6:223–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fehring V, Schaeper U, Ahrens K, Santel A,
Keil O, Eisermann M, Giese K and Kaufmann J: Delivery of
therapeutic siRNA to the lung endothelium via novel Lipoplex
formulation DACC. Mol Ther. 22:811–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garbuzenko OB, Saad M, Betigeri S, Zhang
M, Vetcher AA, Soldatenkov VA, Reimer DC, Pozharov VP and Minko T:
Intratracheal versus intravenous liposomal delivery of siRNA,
antisense oligonucleotides and anticancer drug. Pharm Res.
26:382–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merkel OM and Kissel T: Nonviral pulmonary
delivery of siRNA. Acc Chem Res. 45:961–970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCaskill J, Singhania R, Burgess M,
Allavena R, Wu S, Blumenthal A and McMillan NA: Efficient
biodistribution and gene silencing in the lung epithelium via
intravenous liposomal delivery of siRNA. Mol Ther Nucleic Acids.
2:e962013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuruba R, Wilson A, Gao X and Li S:
Targeted delivery of nucleic-acid-based therapeutics to the
pulmonary circulation. AAPS J. 11:23–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Shimizu S, Yoshiike Y, Taguchi M, Ohno H, Ozaki KI and Onishi H:
Effect of cationic lipid in cationic liposomes on siRNA delivery
into the lung by intravenous injection of cationic lipoplex. J Drug
Target. 27:217–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hattori Y, Tamaki K, Ozaki K, Kawano K and
Onishi H: Optimized combination of cationic lipids and neutral
helper lipids in cationic liposomes for siRNA delivery into the
lung by intravenous injection of siRNA lipoplexes. J Drug Deliv Sci
Technol. 52:1042–1050. 2019. View Article : Google Scholar
|
|
10
|
Hattori Y, Shimizu S, Ozaki KI and Onishi
H: Effect of cationic lipid type in folate-PEG-modified cationic
liposomes on folate receptor-mediated siRNA transfection in tumor
cells. Pharmaceutics. 11:112019. View Article : Google Scholar
|
|
11
|
Hattori Y, Nakamura A, Arai S, Kawano K,
Maitani Y and Yonemochi E: siRNA delivery to lung-metastasized
tumor by systemic injection with cationic liposomes. J Liposome
Res. 25:279–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hattori Y, Arai S, Kikuchi T, Ozaki KI,
Kawano K and Yonemochi E: Therapeutic effect for liver-metastasized
tumor by sequential intravenous injection of anionic polymer and
cationic lipoplex of siRNA. J Drug Target. 24:309–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aleku M, Schulz P, Keil O, Santel A,
Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Röder N,
et al: Atu027, a liposomal small interfering RNA formulation
targeting protein kinase N3, inhibits cancer progression. Cancer
Res. 68:9788–9798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in PEGylated siRNA lipoplexes
on in vitro gene silencing effects and siRNA biodistribution in
mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI
|
|
15
|
Hattori Y, Nakamura T, Ohno H, Fujii N and
Maitani Y: siRNA delivery into tumor cells by lipid-based
nanoparticles composed of hydroxyethylated cholesteryl triamine.
Int J Pharm. 443:221–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato A, Ohtsuki M, Hata M, Kobayashi E and
Murakami T: Antitumor activity of IFN-lambda in murine tumor
models. J Immunol. 176:7686–7694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oku T, Ando Y, Ogura M and Tsuji T:
Development of splice variant-specific monoclonal antibodies
against human alpha3 integrin. Monoclon Antib Immunodiagn
Immunother. 35:12–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oku T, Shimada K, Kenmotsu H, Ando Y,
Kurisaka C, Sano R, Tsuiji M, Hasegawa S, Fukui T and Tsuji T:
Stimulation of peritoneal mesothelial cells to secrete matrix
metalloproteinase-9 (MMP-9) by TNF-alpha: A role in the invasion of
gastric carcinoma cells. Int J Mol Sci. 19:192018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hattori Y, Nakamura A, Hanaya S, Miyanabe
Y, Yoshiike Y, Kikuchi T, Ozaki K and Onishi H: Effect of
chondroitin sulfate on siRNA biodistribution and gene silencing
effect in mice after injection of siRNA lipoplexes. J Drug Deliv
Sci Technol. 41:401–409. 2017. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Z, He B, Ma L, Sun Y, Niu Y and Zeng B:
Recent advances in ergosterol biosynthesis and regulation
mechanisms in Saccharomyces cerevisiae. Indian J Microbio.
57:270–277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benveniste P: Biosynthesis and
accumulation of sterols. Annu Rev Plant Biol. 55:429–457. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eliyahu H, Servel N, Domb AJ and Barenholz
Y: Lipoplex-induced hemagglutination: Potential involvement in
intravenous gene delivery. Gene Ther. 9:850–858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simberg D, Weisman S, Talmon Y, Faerman A,
Shoshani T and Barenholz Y: The role of organ vascularization and
lipoplex-serum initial contact in intravenous murine lipofection. J
Biol Chem. 278:39858–39865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loughna S and Sato TN: Angiopoietin and
Tie signaling pathways in vascular development. Matrix Biol.
20:319–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van der Heijden M, van Nieuw Amerongen GP,
Chedamni S, van Hinsbergh VW and Johan Groeneveld AB: The
angiopoietin-Tie2 system as a therapeutic target in sepsis and
acute lung injury. Expert Opin Ther Targets. 13:39–53. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loisel S, Le Gall C, Doucet L, Ferec C and
Floch V: Contribution of plasmid DNA to hepatotoxicity after
systemic administration of lipoplexes. Hum Gene Ther. 12:685–696.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Yong T, Zhang Y, Su J, Jiao C and
Xie Y: Anti-tumor and Anti-angiogenic Ergosterols from Ganoderma
lucidum. Front Chem. 5:852017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takaku T, Kimura Y and Okuda H: Isolation
of an antitumor compound from Agaricus blazei Murill and its
mechanism of action. J Nutr. 131:1409–1413. 2001. View Article : Google Scholar : PubMed/NCBI
|